Abstract
Objective
To evaluate the coronavirus disease 2019 perioperative infection rate and mortality rate of patients undergoing urological surgeries during the early pandemic period in Spain.
Methods
This was a non‐interventional multicenter prospective study carried out from 9 March to 3 May 2020 in two urology departments in Madrid, Spain. Clinical, microbiological and radiological data of patients who underwent surgery were collected from computerized medical records.
Results
A total of 148 patients were included in the study, and 141 were analyzed for nosocomial infection risk, after excluding previous and concomitant severe acute respiratory syndrome coronavirus type 2 infections. Elective surgeries represented 76.6% of the procedures, whereas emergent surgeries represented 23.4%. Preoperative screening was carried out with polymerase chain reaction test in 34 patients, all were negative. A total of 14 patients also had chest X‐ray (not suspicious in all cases). Three patients (2.1%) developed severe acute respiratory syndrome coronavirus type 2 nosocomial infection (symptoms developed between the third day after surgery to the 14th day after hospital discharge). Time from admission to a compatible clinical case was 5.5 days (4–12 days). Two patients underwent surgery with concomitant diagnosis of coronavirus disease. The mortality rate due to severe acute respiratory syndrome coronavirus type 2 infection is 0.7%, and the specific mortality rate in patients undergoing surgery with community‐acquired coronavirus disease 2019 infection was 50% (1/2).
Conclusions
The nosocomial severe acute respiratory syndrome coronavirus type 2 infection rate was low in patients undergoing urological surgical procedures during the peak of the pandemic in Madrid. With appropriate perioperative screening, urological surgical activity can be carried out in safety conditions.
Keywords: COVID‐19, nosocomial infection, SARS‐CoV‐2, surgery, urology
Abbreviations & Acronyms
- BMI
body mass index
- COPD
chronic obstructive pulmonary disease
- COVID‐19
coronavirus disease 2019
- CT
computed tomography
- CVD
cardiovascular disease
- EAU
European Association of Urology
- ERUS
European Association of Urology Robotic Urology Section
- HIS
Hospital Universitario Infanta Sofia
- HRJC
Hospital Universitario Rey Juan Carlos
- HT
hypertension
- ICU
intensive care unit
- PCP
primary care physician
- PCR
polymerase chain reaction
- SARS‐CoV‐2
severe acute respiratory syndrome coronavirus type 2
- SUMMA
Madrid's Medical Urgencies Service
- TURB
transurethral bladder resection
- ULPA
ultra‐low penetration air
- WHO
World Health Organization
Introduction
In December 2019, China reported the first case of unknown etiology pneumonia in Wuhan (Hubei province). 1 On 7 January new SARS‐CoV‐2 was isolated as the pathogenic agent of COVID‐19 and its genome was sequenced. 2 From this time on, the spread of COVID‐19 has increased exponentially worldwide. On 11 March, the WHO declared COVID‐19 as a pandemic 2020. 3 Spain, along with Italy, the UK and France, has been one of the most affected countries in Europe, with more than 377 906 confirmed cases and 28 813 deaths. At the time this article was written, there are currently more than 21.3 million confirmed cases and more than 761 000 deaths worldwide. 4
COVID‐19 has become the biggest threats to healthcare systems worldwide, with important national and regional differences. Medical activity changed dramatically to avoid physical contact and to maintain social distance, while health resources were diverted to treat COVID‐19 patients. Face‐to‐face outpatient appointments and diagnostics procedures were cancelled. Patients with suspected or confirmed malignancies were evaluated in person, and patients with non‐urgent conditions were attended to by telephone and re‐scheduled for later appointments. It had a significant impact on surgery. Elective surgical activity was cancelled, and only urgent and high‐risk oncological interventions were carried out. Furthermore, in some hospitals, even oncological surgeries were on hold for weeks as a result of a lack of resources, such as ventilators, ICU availability, post‐anesthesia recovery rooms or anesthesiologists. Increasing concern about nosocomial SARS‐CoV‐2 infection has influenced delaying non‐urgent surgical procedures, due to higher mortality and postoperative complications rates published in a cohort of patients undergoing surgeries during the incubation period of COVID‐19 infection in China. 5
Fortunately, the number of cases has decreased, and the health system including the urology departments have to adapt their activity to the new COVID‐19 era. The EAU published a guideline to summarize the knowledge and advice regarding urological practice during the COVID‐19 era. 6 Diagnosis, surgical treatments and follow‐up procedures were evaluated from low to high priority, to help staff in decision‐making. Only emergencies and high‐priority surgeries (no deferrable >6 weeks due to risk of clinical harm if postponed) were recommended initially. Delaying surgical activity will have an impact that cannot be assessed yet, not only on the prognosis of oncological patients, but also on the quality of life in patients without malignant conditions.
To minimize patients’ risk of nosocomial SARS‐CoV‐2 infection, new perioperative management should be considered. There is no consensus on the optimal protocol for preoperative COVID‐19 screening, intraoperative management and postoperative care. In general terms, scientific societies recommend preoperative SARS‐CoV‐2 testing and chest imaging for all patients before elective surgery, ideally carried out within 48–72 h of the procedure. 7 On 16 May 2020, the Spanish Health Ministry published recommendations for safe surgical activity during the COVID‐19 pandemic transition period, including the design of clean COVID‐19 routes for elective surgery patients, carrying out surgical procedures by surgeons who have minimal contact with COVID‐19 patients, determining the SARS‐CoV‐2 status of healthcare workers if it is possible, minimizing hospital stay by surgical day admission and hospital discharge as soon as possible, SARS‐CoV‐2 screening before surgery, SARS‐CoV‐2 transcriptase PCR test 72 h before all elective procedures and reducing postoperative visits. Systematic SARS‐CoV‐2 serology, thoracic CT scan and other specific tests are not recommended, except in suspected cases depending on the clinical context. 8
To the best of our knowledge, the nosocomial SARS‐CoV‐2 transmission rate in urological surgeries has not been published. The primary outcome of the present study was to evaluate COVID‐19 perioperative infection in urological surgeries during the pandemic period in two urology departments in Madrid, Spain. The secondary outcomes were to describe global SARS‐CoV‐2 mortality in urological surgery and specific mortality of patients who underwent surgery with concomitant infection.
Methods
This was a non‐interventional, multicenter, prospective study carried out within two urology departments.
Inclusion criteria
Patients undergoing urological surgeries (elective and urgent) between 9 March and 3 May 2020 in two second‐level hospitals in Madrid, Spain (HRJC and HIS) were included in the present study.
The inclusion period included the higher SARS‐CoV‐2 incidence period in Madrid, beginning on 9 March (1 week before the Spanish government lockdown) and finishing on 3 May (when the government announced the first de‐escalation steps). For 3 weeks in HRJC and 4 weeks in HIS, surgical activity in both hospitals was restricted to urgent and selected high‐risk oncological patients.
Exclusion criteria for nosocomial infection analysis
Patients with preoperative diagnosis of SARS‐CoV‐2 infection based on clinical and radiological features or confirmed by the SARS‐CoV‐2 PCR test or serology test were excluded from the present study. Patients with concomitant SARS‐CoV‐2 infection at the moment of surgical intervention were also excluded.
According to current knowledge, COVID‐19 reinfections have not been shown in patients with previous SARS‐CoV‐2 disease. 9 , 10 To prevent underestimation of the nosocomial infection rate, patients with previous COVID‐19 infection were excluded.
Preoperative SARS‐CoV‐2 screening
Preoperative assessment of COVID‐19 infection was modified in both hospitals during the months of the pandemic, as the knowledge of this new disease increased. Before 9 March, no SARS‐CoV‐2 screening was carried out. When the pandemic was declared and tests were more available, routine SARS‐CoV‐2 screening was carried out before surgery due to the increasing concern of postoperative complications and nosocomial transmission, as well as the potential risk to the healthcare workers.
Two different preoperative protocols for COVID‐19 screening in elective surgeries were used: in HRJC, only the SARS‐CoV‐2 PCR test was carried out, whereas in HIS, chest X‐ray and the SARS‐CoV‐2 PCR test was required. If a positive PCR test was obtained, elective surgery was postponed. All patients undergoing elective surgery signed a specific informed consent acknowledging the risk of COVID‐19 infection.
To avoid the possible release of small viral particles through intraoperative smoke during laparoscopic or robotic procedures, intelligent integrated flow systems and ULPA filter were used since the publication of the EAU ERUS guidelines on 25 March. 11
Data collection and SARS‐CoV‐2 status definitions
Postoperative follow up was carried out for 14 days after hospital discharge. The Madrid government’s HORUS computer platform was used to collect patient data. All healthcare consultations in Madrid’s region are registered on this platform, including hospital urgent and non‐urgent consultations, primary care, and SUMMA (urgent and emergent medical out of hospital care) evaluations.
Although there is no consensus in the literature on the length of the incubation period of SARS‐CoV‐2, for the present study, the preoperative and nosocomial infection definitions are based on data published in the WHO guidelines (2–14 days). 12 Postoperative COVID‐19 infection is defined as the disease developing between hospital admission and 14 days after hospital discharge. In the present study, postoperative infection is distinguishable in two clinical profiles: (i) patients with concomitant infection; and (ii) patients with nosocomial infection. Concomitant infection is defined as the patient develops disease during their hospital stay, is preoperatively diagnosed or disease developed from admission to the second hospitalization day. If no previous hospital or primary care consultations were registered in HORUS, it is accepted that those patients had contact with virus outside hospital. Nosocomial infection is considered when symptoms appeared between the third day from hospital admission and 14th day after hospital discharge.
SARS‐CoV‐2 nosocomial infection included those confirmed by serology/PCR test (confirmed case) and/or high clinical suspicion (possible case). If a possible case had a negative result or serology/PCR test was not carried out, but there was high clinical suspicion due to symptoms and radiological test (CT scan or chest X‐ray), it was included on statistical analysis as a COVID‐19 case. A negative PCR test was not an exclusion criterion, because the SARS‐CoV‐2 PCR’s sensitivity values have not been clearly articulated in the literature so far (60% PCR sensitivity has been published). 13 , 14
Statistical analysis
Statistical analysis was carried out with Stata version 12.0 (StataCorp, College Station, TX, USA). Quantitative variables were described as the mean and standard deviation. Qualitative variables were described as the relative frequency and percentage. For nosocomial rate calculation, patients with past or concomitant SARS‐CoV‐2 infection were excluded.
This work was reviewed favorably and approved by the Research Committee of HRJC.
Results
Nosocomial SARS‐CoV‐2 infection
From 9 March 2020 to 3 May 2020, 148 patients underwent surgery in two urology departments. Figure 1 shows the flowchart of the inclusion process for nosocomial SARS‐CoV‐2 infection rate analysis.
Fig. 1.
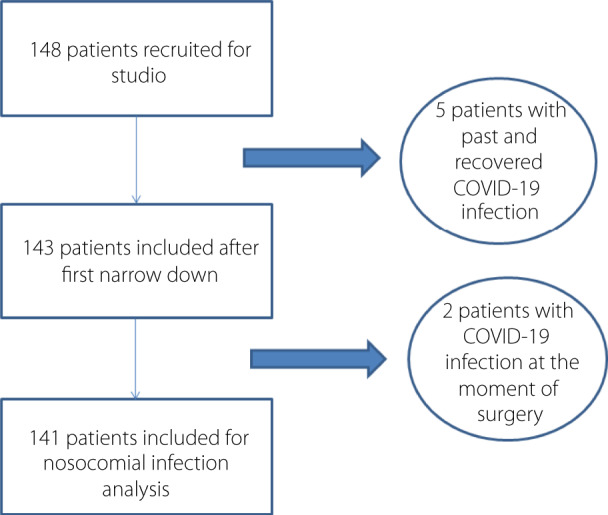
Flowchart of the inclusion process for nosocomial SARS‐CoV‐2 infection rate analysis.
Five patients were excluded for preoperative COVID‐19 infection. Two patients were excluded for presenting COVID‐19 infection at the time of surgery. Finally, 141 patients were included in the statistical analysis. HRJC included 96 patients (68.1%) and HIS included 45 patients (31.9%).
Table 1 shows the baseline characteristics of the study cohort.
Table 1.
Baseline characteristics of study cohort
| Variable | Mean | Standard deviation |
|---|---|---|
| Age (years) | 60.7 | 17.7 |
| Relative frequency | Percentage (%) | |
|---|---|---|
| Sex | ||
| Male | 116/141 | 82.3 |
| Female | 25/141 | 17.7 |
| Hospitals | ||
| HRJC | 96/141 | 82.3 |
| HIS | 45/141 | 17.7 |
| HT | 63/141 | 44.7 |
| Diabetes mellitus | 32/141 | 22.7 |
| Dyslipidemia | 61/141 | 43.2 |
| CVD | 35/141 | 24.8 |
| Respiratory disease | 31/141 | 22.0 |
| Smoker status | ||
| Non‐smoker | 65/141 | 46.1 |
| Smoker | 31/141 | 22.0 |
| Ex‐smoker | 45/141 | 31.9 |
| Immunosuppression | 3/141 | 2.1 |
| Oncological active disease | 38/141 | 27.0 |
| Pregnancy | 1/141 | 0.7 |
| BMI | ||
| Normal (18.5–24.9) | 36/120 | 30.0 |
| Overweight (25–29.9) | 60/120 | 50.0 |
| Obesity grade I (30–34.9) | 22/120 | 18.4 |
| Obesity grade II (35–39.9) | 1/120 | 0.8 |
| Obesity grade III (>40) | 1/120 | 0.8 |
More frequent risk factors of the severity and mortality of SARS‐CoV‐2 infection in our cohort were: male sex (82.3%, n = 116), obesity (70%, n = 60) including 20% of patients with BMI >30 (n = 24), smoking history (past or active) (53.9%, n = 76), HT (44.7%, n = 63), oncological active disease (27%, n = 38) and respiratory disease (22%, n = 31).
Preoperative SARS‐CoV‐2 screening was carried out in 24.1% of patients (n = 34); all of them had a PCR test (negative test all cases) and just 14 patients also had a chest X‐ray, with no COVID‐19 infection‐compatible results. SARS‐CoV‐2 serology or chest CT scan were not used for preoperative screening or diagnosis.
Table 2 shows the surgical characteristics.
Table 2.
Surgical characteristics
| Variable | Mean | Standard deviation |
|---|---|---|
| Surgical time (min) | 66.3 | 66.2 |
| Postoperative ICU stay (days) | 2 | 1.4 |
| Hospital stay (days) | 2.2 | 4.3 |
| Relative frequency | Percentage (%) | |
|---|---|---|
| Surgery | ||
| Scheduled | 108/141 | 76.6 |
| Urgent | 33/141 | 23.4 |
| Surgical procedure | ||
| Minor surgery | 31/141 | 22.0 |
| Endoscopic procedure | 80/141 | 56.7 |
| Open surgery | 13/141 | 9.2 |
| Laparoscopic surgery | 7/141 | 5.0 |
| Robot‐assisted surgery | 10/141 | 7.1 |
| Anesthetic procedure | ||
| Local | 22/141 | 15.6 |
| Sedation | 9/141 | 6.4 |
| Epidural | 52/141 | 36.9 |
| Non‐intubated general anesthesia | 11/141 | 7.8 |
| Intubated general anesthesia | 47/141 | 33.3 |
| Postoperative ICU stay | 2/141 | 1.4 |
Elective surgeries represented 76.6% (n = 108) of the procedures carried out, whereas 23.4% of the cases were urgent surgeries (n = 33). The most frequent type of procedure carried out was endoscopic surgery (56.7%, n = 80).
SARS‐CoV‐2 postoperative infection was diagnosed in three patients, all of whom met the nosocomial infection criteria, which means a 2.1% ratio of nosocomial infection. The time from admission to diagnosis was 5.5 days (range 4–12 days).
Table 3 describes three nosocomial SARS‐CoV‐2 patient characteristics of the study cohort.
Table 3.
Characteristics of patients with SARS‐CoV‐2 nosocomial infection
| Age (years) | Sex | Risk factors | Preoperative screening | Surgery | ICU | Hospital stay | Incubation period | COVID‐19 admission | Evolution | |
|---|---|---|---|---|---|---|---|---|---|---|
| Case 1 | 70 | Female | HT | No | TURB | No | 1 day | 4 days | Yes (3 days) |
Favorable No ICU |
| Case 2 | 80 | Male |
Gender Age HT CVD COPD Smoker Obesity |
Yes Negative PCR |
PNC | No | 1 day | 12 days | No |
Favorable Follow up by PCP |
| Case 3 | 40 | Male | Sex | No | Vasectomy | No | None | 6 days | No |
Favorable Follow up by PCP |
Mortality
Two patients underwent surgery with concomitant SARS‐CoV‐2 infection. One of them died as a result of COVID‐19 complications (respiratory distress syndrome), the other patient overcame the infection without complications.
Table 4 describes patients with concomitant SARS‐CoV‐2 infection.
Table 4.
Characteristics of patients who underwent surgery with concomitant SARS‐CoV‐2 infection
| Age (years) | Sex | Risk factors | SARS‐CoV‐2 diagnosis | Surgery | Hospital stay | ICU admission | Evolution | |
|---|---|---|---|---|---|---|---|---|
| Case 1 | 44 | Male |
HT Sex Smoker Obesity |
Clinical Negative PCR |
Ureteral stent | 6 days | No |
Favorable Completely recovery |
| Case 2 | 56 | Female | Obesity | Positive PCR | Urgent open nephrectomy | 10 days | Yes | Died of SARS‐CoV‐2 |
The global mortality rate due to SARS‐CoV‐2 infection in patients who underwent either elective or urgent surgical interventions was 0.7% (1/148). The specific mortality rate for patients with concomitant infection at surgical intervention was 50% (1/2).
Discussion
The present article reports for the first time a prospective cohort of patients who contracted nosocomial SARS‐CoV‐2 infection after elective or urgent urological surgery. SARS‐CoV‐2, like influenza and other respiratory pathogens, can be spread through aerosols, and possibly also respiratory droplets, such as coughing and sneezing. 15 For patients in whom contamination necessarily took place in hospital, the mechanism of viral spread is not clearly defined. It could be related to accidental contact with other COVID‐19 patients, outside visitors or contamination from caregivers. In our urology departments, just two staff had SARS‐CoV‐2 pneumonia (both from the HIS department), they were infected at the beginning of the pandemic and they were out of healthcare activity for more than 4 weeks. In HRJC, no one from the staff members had COVID‐19 symptoms, and all members had negative serology (immunoglobulin M and immunoglobulin G) in the prevalence institutional study. To minimize nosocomial viral spread, several steps were implemented: increasing hand hygiene (hydro‐alcoholic gel, repeated hand washing), mandatory face mask use, design circuits for treating COVID‐19 patients, restriction of hospital visitors at first and later prohibition of visitors with a few exceptions, improving health workers training and, finally, implementing preoperative screening.
There is no consensus about optimal preoperative screening for COVID‐19. In our sample, a systematic preoperative PCR test was not carried out at the beginning of our study, which is why just 34 of our sample had a PCR test carried out. The availability of the SARS‐CoV‐2 test was erratic and mostly reserved for urgent care patients. During the following weeks, COVID‐19 test availability and knowledge of the disease increased; a high rate of respiratory complications in postoperative patients were published, high mortality was described in surgical patients and there was high concern about intraoperative virus spread in the operating room. Scientific societies published their recommendations in preoperative care, suggesting that the PCR test be carried out in all patients before surgery. 6 , 8 , 11 , 16 There was no consensus about screening with chest X‐ray, also exposed in the present study, because only one urology department used it routinely. No dissociation between PCR and chest X‐ray was observed in preoperative screening.
There is little evidence of SARS‐CoV‐2 nosocomial infection in surgery departments. Luong‐Nguyen et al. described nosocomial infection in 4.9% of 301 patients operated in three general surgery departments of university hospitals in France. 17 We reported a nosocomial infection rate of 2.1% in urological surgery patients, which is concordant with the results of Luong‐Nguyen et al.
In the present study, two patients with nosocomial COVID‐19 were diagnosed by a PCP based on clinical and radiological findings, not confirmed with a PCR test. Two factors were probably involved in the absence of a nasopharyngeal PCR test in these cases: (i) lack of testing capacity at the beginning of the COVID‐19 pandemic; and (ii) changes of COVID‐19 diagnosis criteria due to the intrinsic dynamic nature of the pandemic. During the highest incidence period of the COVID‐19 pandemic, in patients with mild SARS‐CoV‐2 symptoms without complications, the diagnosis was made by compatible symptoms and epidemiological positive history (such as hospitalization) without a nasopharyngeal PCR test.
The third case with nosocomial SARS‐CoV‐2 infection had a negative nasopharyngeal PCR test, but compatible symptoms and chest X‐ray. It is consistent with data published previously; SARS‐CoV‐2 PCR test sensitivity is not well established, and it is affected by the sampling technique and timing within the disease course. 14 Fang et al. suggested that findings from chest X‐ray might precede positive results of a PCR test. 13
In the present study, nosocomial SARS‐CoV‐2 infection did not seem to be related with postoperative ICU or hospitalization length. A low number of events (nosocomial infection) makes it difficult to carry out a multivariate analysis to explore variables associated with nosocomial infection.
In our cohort, two patients with preoperative COVID‐19 infection required urgent surgery. One had an emphysematous pyelonephritis with concomitant SARS‐CoV‐2 pneumonia, and died due to severe respiratory complications in the ICU. The other patient underwent a ureteral stent placement due to complicated renal colic with mild COVID‐19 symptoms, he had favorable evolution. The prognosis for SARS‐CoV‐2 infections after surgery is not yet clear; however, high mortality rates have been reported. Li et al. in a thoracic surgery department and Luong‐Nguyen et al. in a digestive surgery department described 38.5% (5/13) and 13.3% (2/13) mortality rates, respectively, in patients with concomitant SARS‐CoV‐2 infection and surgical intervention. 17 , 18 Recent data published in Lancet by the COVIDSurg Collaborative group showed a 23.7% mortality rate (230/969). 19 We report a higher mortality rate (50%), which could be explained by the low number of patients requiring surgery with concomitant COVID‐19 infection.
The present study had some limitations. Patients with no preoperative SARS‐CoV‐2 screening were included, and by doing this, patients with the asymptomatic form of SARS‐CoV‐2 infection resolved before surgery, leading to a possible underestimation of the nosocomial infection rate. To minimize the impact of this problem, patients with suspected COVID‐19 infection before intervention were excluded. In the same way, not carrying out a SARS‐CoV‐2 test (PCR and/or serological tests) 14 days after hospital discharge could underestimate the nosocomial SARS‐CoV‐2 infection rate due to possible asymptomatic nosocomial infections. Nevertheless, the aim of the present study was to determine clinically relevant nosocomial infections, which could eventually alter patient prognosis or survival.
Despite the limitations, this is the first analysis of nosocomial SARS‐CoV‐2 infection in urology departments in patients who underwent urological procedures at the peak of the COVID‐19 pandemic – with an 11.9% (95% CI 10.3–13.3) infection rate in Madrid according to a Spanish seroprevalence study 20 – showing a low nosocomial infection rate in real‐life conditions. These results contribute to reducing concern of nosocomial infection and the impact on surgical activity. More studies should be carried out to evaluate optimal screening and perioperative management to reduce nosocomial infection and postoperative complications related to COVID‐19, and to improve safety for healthcare workers.
In conclusion, the nosocomial SARS‐CoV‐2 infection rate was low in patients undergoing urological surgeries, even at the highest incidence period of the COVID‐19 pandemic. Based on the present results, with the appropriate perioperative prevention steps, urological surgical activity could be carried out under safe conditions. The postoperative mortality of patients with concomitant SARS‐CoV‐2 infection is high and surgery should be postponed in this situation, when possible.
Conflict of interest
None declared.
References
- 1. Naspro R, Da Pozzo LF. Urology in the time of corona. Nat. Rev. Urol. 2020; 17: 251–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hui DS, Azhar EI, Madani TA et al. The continuing 2019‐nCov epidemic threat of novel coronavirus to global health – the latest 2019 novel coronavirus outbreak in Wuhan, China. Int. J. Infect. Dis. 2020; 91: 264–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. WHO Director‐General’s opening remarks at the media briefing on COVID‐19–11 March 2020. 2020. [Cited 14 May 2020.] Available from URL: https://www.who.int/dg/speeches/detail/who‐director‐general‐s‐opening‐remarks‐at‐the‐media‐briefing‐on‐covid‐19‐‐‐11‐march‐2020
- 4. World Health Organization . Coronavirus disease (COVID‐19) pandemic. 2020. [Cited 20 Aug 2020.] Available from URL: https://www.who.int/emergencies/diseases/novel‐coronavirus‐2019/situation‐reports/
- 5. Lei S, Jiang F, Su W et al. Clinical characteristics and outcomes of patients undergoing surgeries during the incubation period of COVID‐19 infection. EClinicalMedicine 2020; 21: 100331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. EAU Guidelines Office Rapid Reaction Group . An organisation‐wide collaborative effort to adapt the EAU guidelines recommendations to the COVID era. [Cited 18 May 2020.] Available from URL: https://uroweb.org/guideline/covid‐19‐recommendations/?type=archive
- 7. Steward JE, Kitley WR, Schimdt CM et al. Urologic surgery and COVID‐19: How the pandemic is changing the way we operate. J. Endourol. 2020; 34: 541–9. [DOI] [PubMed] [Google Scholar]
- 8. Spanish Health Ministry . Recomendaciones para la programación de cirugía en condiciones de seguridad durante el periodo de transición de la pandemia COVID‐19. May 16th version. [Cited 18 May 2020.] Available from URL: https://www.mscbs.gob.es/profesionales/saludPublica/ccayes/alertasActual/nCov‐China/documentos/200517‐DOCUMENTO_CIRUGIA‐FINAL_(2).pdf
- 9. Kang H, Wang Y, Tong Z, Liu X. Retest positive for SARS‐CoV‐2 RNA of “recovered” patients with COVID‐19: persistence, sampling issues, or re‐infection? J. Med. Virol. 2020; 10.1002/jmv.26114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Law SK, Leung AWN, Xu C. Is reinfection possible after recovery from COVID‐19? Hong Kong Med. J. 2020; 26: 264–5. [DOI] [PubMed] [Google Scholar]
- 11. Mottrie A. ERUS (EAU Robotic Urology Section) guidelines during COVID‐19 emergency. 2020. [Cited 28 May 2020.] Available from URL: https://uroweb.org/eau‐robotic‐urology‐section‐erus‐guidelines‐during‐covid‐19‐emergency/
- 12. World Health Organization . Home care for patients with COVID‐19 presenting with mild symptoms and management of their contacts: interim guidance. 2020. [Cited 17 May 2020.] Available from URL: https://www.who.int/publications‐detail/home‐care‐for‐patients‐with‐suspected‐novel‐coronavirus‐(ncov)‐infection‐presenting‐with‐mild‐symptoms‐and‐management‐of‐contacts
- 13. Fang Y, Zhang H, Xie J et al. Sensitivity of chest CT for COVID19: comparison to RT‐PCR. Radiology 2020; 19: 200432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mayo Clinic . False‐negative COVID‐19 test results may lead to false sense of security. ScienceDaily. [Cited 17 May 2020.] Available from URL: https://www.sciencedaily.com/releases/2020/04/200409144805.htm
- 15. Islam S, Rahman KM, Sun Y et al. Examining the current intelligence on COVID‐19 and infection prevention and control strategies in health settings: a global analysis. Cambridge Coronavirus Collection 2020. 10.1017/ice.2020.237. [DOI] [PMC free article] [PubMed]
- 16. Wong SC‐Y, Kwong RT‐S, Wu TC et al. Risk of nosocomial transmission of coronavirus disease 2019: an experience in a general ward setting in Hong Kong. J. Hosp. Infect. 2020; 105: 119–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Luong‐Nguyen M, Hermand H, Abdalla S et al. Nosocomial infection with SARS‐Cov‐2 within departments of digestive surgery. J. Visc. Surg. 2020; 10.1016/j.jviscsurg.2020.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Li Y‐K, Peng S, Li L‐Q et al. Clinical and transmission characteristics of Covid‐19 – a retrospective study of 25 cases from a single thoracic surgery department. Curr. Med. Sci. 2020; 40: 295–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. COVIDSurg Collaborative . Mortality and pulmonary complications in patients undergoing surgery with perioperative SARS‐CoV‐2 infection: an international cohort study. Lancet 2020; 10.1016/S0140-6736(20)31182-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Estudio . ENE‐COVID: Informe Final Estudio nacional del sero‐epidemiología de la infección por SARS‐CoV‐2 en España. 2020. [Cited 9 Aug 2020.] Available from URL: https://www.mscbs.gob.es/ciudadanos/ene‐covid/docs/ESTUDIO_ENE‐COVID19_INFORME_FINAL.pdf


