Abstract
Objective:
Naturalistic studies suggest that expectation of adverse experiences such as pain exerts particularly strong effects on anxious youth. In healthy adults, expectation influences the experience of pain. The current study uses experimental methods to compare the effects of expectation on pain among adults, healthy youth, and youth with an anxiety disorder.
Methods:
Twenty-three healthy adults, 20 healthy youth, and 20 youth with an anxiety disorder underwent procedures in which auditory cues were paired with noxious thermal stimulation. Through instructed conditioning, one cue predicted low pain stimulation and the other predicted high pain stimulation. At test, each cue was additionally followed by a single temperature calibrated to elicit medium pain ratings. We compared cue-based expectancy effects on pain across the three groups, based on cue effects on pain elicited on medium heat trials.
Results:
Across all groups, as expected, participants reported greater pain with increasing heat intensity (β = 2.29, t(41) = 29.94, p < .001). Across all groups, the critical medium temperature trials were rated as more painful in the high-relative to low-expectancy condition (β = 1.72, t(41) = 10.48, p < .001). However, no evidence of between-group differences or continuous associations with age or anxiety were observed.
Conclusions:
All participants showed strong effects of expectancy on pain. No influences of development or anxiety arose. Complex factors may influence associations among anxiety, development, and pain reports in naturalistic studies. Such factors may be identified using experiments that employ more complex, yet controlled manipulations of expectancy or assess neural correlates of expectancy.
Keywords: cued expectancy, pain, thermal heat, pediatric anxiety, conditioning
Introduction
Expectations impact pain perception, and these effects may be particularly robust among youth with anxiety, who frequently present with symptoms of pain. For instance, needle anxiety can cause a relatively painless insertion to be psychologically incapacitating. Most research on pain expectation examines healthy adults, where neuroimaging studies suggest that prefrontal and subcortical pathways mediate effects of expectation on pain reports (1, 2, 3). However, studies in adolescence are important, since most anxiety disorders that afflict adults begin during childhood or adolescence (4, 5). To our knowledge, no studies test directly whether cue-based expectancy effects on pain vary across development or between anxious and non-anxious youth. To overcome this limitation and test the hypothesis that expectation effects on pain relate to age or anxiety, the current study experimentally manipulated effects of expectation on subjective measures of pain among healthy adults, healthy youth, and youth with an anxiety disorder. This extension of prior work can both help characterize potential neurocognitive mechanisms of expectancy and inform clinical targets for treatment of pain in pediatric anxious patients.
Naturalistic studies suggest that expectation exerts particularly strong effects on youth. Evidence for such greater effects emerge in physicians’ reports concerning reactions to medical procedures (6). Adolescents may exhibit particularly large effects of expectancy. These effects are thought to explain uniquely powerful influences from media on diverse behaviors, including smoking (7), eating-related behaviors (8), sexual behaviors (9), suicide contagion (10), and other risk-taking behaviors (see 11 for a meta-analysis).
By directly manipulating expectancy, experimental manipulations more directly quantify effects of expectancy than this naturalistic research. Studies using placebo manipulations provide one well established experimental approach (12). Indeed, evidence suggests that placebo responses may be larger in children and adolescents than in adults (13, 14, 15, 16, 17, 18, 19, 20, 21), although the data are equivocal (22). In studies that include both children and adolescents, some results point to a higher placebo response in children than adolescents (i.e. 13), and others show the opposite pattern (i.e. 15, 16, 17) or no group differences (14). Thus, substantial evidence indicates heightened response to placebo in youth compared to adults. Interestingly, however, no studies use pain to compare age groups on the experimentally induced nocebo response.
Taken together, then, some data suggest greater effects of expectancy in youth than adults. However, no empirical studies experimentally manipulate and directly compare cued expectancy effects on pain among youth and adults. In adults, a large body of work shows predictive cues to differ from placebo effects (23). In particular, pain-based nocebo manipulations isolate the effects of affective learning and expectations on pain while holding constant contextual and social factors. This study draws on this extensive work to address fundamental questions on nocebo response in youth.
As an initial goal, the current study tests the feasibility of a thermal pain paradigm previously used with adults (24) in youth. This is an important goal. Ethical factors complicate attempts to administer pain to youth, and demonstrating the safe conduct of such research would lay the groundwork for many future studies. The second goal was to compare cue-based expectancy effects on pain in youth and adults. As noted above, research demonstrates greater effects of expectancy in youth than adults, but no such research uses pain-based nocebo manipulations.
Finally, beyond age, anxiety represents another factor that could influence expectancy effects on experienced pain. Anxiety is one of the most common psychiatric conditions affecting adolescents (5, 25). Moreover, because most anxiety disorders in adults begin during childhood or adolescence (4), a study of anxiety in youth is relevant to adults. Prior work shows strong associations between anxiety heightened pain experience (26, 27). Levels of anxiety symptoms correlate with ratings of pain intensity, and anxious individuals experience significantly more pain during medical procedures than non-anxious individuals (28, 29). Strong nocebo responses occur in pediatric anxiety disorders (14, 30, 31, 32). Anxious individuals, compared to non-anxious individuals, also rate future negative events as more likely and expect to perform worse on social and cognitive tasks (33).
While we know of no experimental studies of expectancy effects on pain in anxious youth, this line of reasoning suggests that anxiety may interact with expectancy to create heightened pain experience. Thus, the third goal of the current study was to compare the effects of experimentally manipulated expectancy on the experience of pain in anxious and healthy youth. Given prior clinical work, we hypothesized that pediatric patients with an anxiety disorder might be particularly vulnerable to the effects of expectancy on pain experience.
This study compared the effects of expectation on pain among healthy adults, healthy youth, and youth with an anxiety disorder, adapting a previously employed experimental paradigm (24). Conditioned auditory cues elicited expectations for low or high painful thermal stimulation, and cue effects on subjective pain report were assessed using a single level of noxious heat calibrated to elicit medium pain. The primary study aim was to test the feasibility of this aversive paradigm in pediatric populations. The second aim was to test the hypothesis that cue-based expectancies have differential effects on the experience of pain in youth and adults. Finally, the third aim was to examine whether the influence of expectancy effects is heightened in youth with an anxiety disorder relative to typically developing youth.
Method
Participants
Participants were recruited from a database at the National Institute of Mental Health (NIMH) in Bethesda, Maryland. Adult participants who consented and underage participants who verbally assented and whose primary caregivers gave written consent were enrolled. Procedures were approved by the NIMH Institutional Review Board. Participants were compensated for their time. Exclusion criteria were current or recent use of psychoactive medications; current suicidal ideation; lifetime history of mania, psychosis, or pervasive developmental disorder; current diagnosis of Tourette’s Disorder, obsessive compulsive disorder, post-traumatic stress disorder, or conduct disorder; current diagnosis of attention deficit hyperactivity disorder of sufficient severity to require pharmacotherapy; pregnancy; serious medical problems; or IQ <70. All youth participants and their parents completed a structured psychiatric interview, the Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL; 34). HV youth were free of Axis I diagnoses. At a separate visit, participants completed the calibration procedure and heat pain task. Both healthy and anxious youth and their parents also reported on the child’s anxiety symptoms using the Screen for Child Anxiety Related Emotional Disorders (SCARED; 35).
A total of 25 healthy adults, 21 healthy youth, and 27 youth with an anxiety disorder participated. Data were collected over 16 months, between August 2015-December 2016. One healthy youth (4.8%) and three anxious youth (11.1%) discontinued participation when they became anxious (compared with 14% HV and 49% anxious youth in an auditory-based conditioning study; 36). Youth who discontinued did not differ on SCARED parent and child summed total scores from children who completed the procedures (t(39) = 1.48, p = .15). One adult and three anxious youth were excluded for poor reliability during calibration (defined in Procedure section). Twenty-five healthy adults (18F, mean age = 27.87 ± 7.62 years), 20 healthy youth (10F, mean age = 13.35±2.63 years), and 21 youth with an anxiety disorder (12F, Age = 13.58±2.71 years) completed the procedures. From these 66 participants, data from one healthy adult and one anxious youth were excluded, blind to dependent measures, from analyses for having relatives (and thus non-independent data) who completed the task and were included in the sample. Therefore, 23 healthy adults, 20 healthy youth, and 20 anxious youth were included in the final analyses. Of the 20 children who were diagnosed with an anxiety disorder, 15 (75%) were diagnosed with Generalized Anxiety Disorder, 5 (25%) were diagnosed with Social Anxiety Disorder, 17 (85%) were diagnosed with Social Phobia, and 9 (45%) were diagnosed with Specific Phobia (see Table 1 for demographic information).
Table 1.
Demographic characteristics of the adults and children in the analyses.
| Variable | Healthy Adults | Healthy Children | Anxious Children |
|---|---|---|---|
| Total (N) | 23 | 20 | 20 |
| Female (N, %) | 17 (74%) | 10 (50%) | 11 (55%) |
| Age in years (M, SD) | 27.34 (7.33) | 13.35 (2.63) | 13.53 (2.77) |
| Race and Ethnicity | |||
| Hispanic (N, %) | 2 (8.7%) | 2 (10.0%) | 4 (20.0%) |
| White (N, %) | 15 (65.2%) | 10 (50.0%) | 14 (70.0%) |
| African-American (N, %) | 5 (21.7%) | 5 (25.0%) | 3 (15.0%) |
| Asian (N, %) | 1 (4.3%) | 2 (10.0%) | 1 (5.0%) |
| Other (N, %) | 2 (8.7%) | 3 (15.0%) | 2 (10.0%) |
| IQ (M, SD) | 120.52 (13.11) | 112.65 (6.47) | 107.90 (.10) |
| SCARED Child (M, SD) | 10.75 (9.20) | 30.05 (12.85) | |
| SCARED Parent (M, SD) | 4.50 (6.47) | 29.53 (11.11) | |
| LSAS (M, SD) | 11.11 (9.70) |
Procedure
Pain calibration.
For each participant, we first performed an adaptive staircase pain calibration procedure adapted from Atlas et al. (24). We applied thermal stimulation (using a 16×16 ATS stimulator (Medoc LTD, Ramat Yishai, Israel) to eight sites on the volar surface of the nondominant forearm. Participants provided verbal ratings using the FACES pain scale (37) on each trial following heat offset. For all participants, two initial temperatures of 34°C and 36°C were applied on the dominant arm to acclimate them to the procedure. The next four temperatures applied on the non-dominant hand were 38°C, 41°C, 44°C, and 47°C, which provided a linear rating-by-temperature curve. This initial linear function allowed us to select temperatures predicted to elicit ratings of 2 (low pain), 5 (moderate pain), and 7 (one step below maximum tolerable pain on a scale from 0–10). After each trial, we used elicited ratings and linear regression to iteratively fit this linear function. Upon completion of 18 total pseudorandom trials, the overall rating-by-temperature curve allowed us to derive each participant’s dose-response curve for the linear relationship between applied thermal stimulation and reported pain (reliability; slope, intercept, R2). In addition, average residuals were calculated for each skin site, and the four sites with the lowest average residuals were used during the heat expectancy task. For each participant, the linear function fit over the course of the calibration was used to determine appropriate temperatures for level 2 (low pain), level 5 (medium pain), and level 7 (high pain) to be applied during the experimental protocol. Participants were required to have a reliable relationship between stimulus temperature and reported pain (minimum R2 = 0.40) to be eligible for the test phase (24). Participants who did not show a reliable relationship (i.e., R2 < 0.40) were excluded from the study (n=1 healthy adult, n=1 healthy youth, n=3 anxious youth).
Training task.
Participants first completed a training task of 20 trials in which they had to discriminate between two tones that they were told would predict low or high painful stimulation, respectively (low- and high-cues). Tones lasted 2s and were either 500 or 1000 Hz, counterbalanced across participants. They were presented in random order, and participants used the “L” key on a computer keyboard to identify low-pain cues and the “H” key to identify high-pain cues. Participants were required to successfully identify at least 80% of trials to proceed to the heat expectancy task. If needed, participants (n = 6) repeated the training task until they reached 80% accuracy.
Heat Expectancy Task.
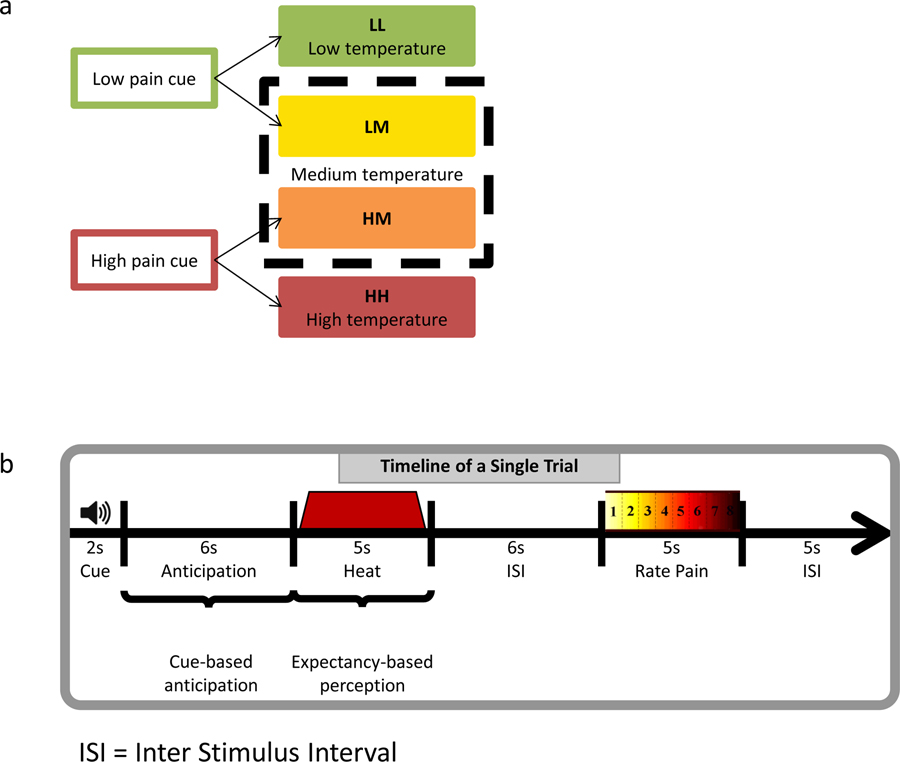
Full details are provided in Atlas et al. (24). In brief, participants underwent 8 blocks of thermal stimulation, with 8 trials per block. The thermode was moved to a new skin site once in the middle of each block, and also between blocks to avoid sensitization or habituation. At the start of each block of the task, participants heard low- and high-pain cues once and were asked, after each tone, “When you hear this tone, how much pain do you expect?” On each trial, participants were first presented with the low- or high-pain cue (2 seconds). Six seconds following cue offset, participants received stimulation calibrated to elicit low pain (VAS = 2), medium pain (VAS = 5), or high pain (VAS = 7). Thermal stimuli lasted 8s (1.5s ramp up from baseline (32°C), 5s at peak destination temperature, 1.5s return to baseline) and was followed by a fixation cross that was presented for an average of 6s (5s/6s/7s random jitter). The words “How painful?” then appeared on the screen for 5s above an eight-point visual analog scale (VAS), and participants then rated the intensity of the preceding stimulus using a computer mouse. Following the rating, an 5s average inter-trial interval (4s/5s/6s jitter) concluded the trial.
As in Atlas et al. (24), participants first underwent a conditioning phase (2 blocks), in which low-pain cues were followed by low-pain stimulation (LL) and high pain stimulation was preceded by high cues (HH). During the subsequent test phase (6 blocks), each cue was equally likely to be followed by the temperatures paired during conditioning, or a single temperature calibrated to elicit medium pain (level 5), thus leading to four conditions presented in a random order: LL, HH, LM (medium heat preceded by low cue), and HM (medium heat preceded by high cue). Comparisons between HM and LM trials measure cue-based expectancy effects, since in these trials the objective heat stimulus was identical but the preceding cues differed.
Measures
Screen for Child Anxiety Related Emotional Disorders (SCARED).
We collected parent and child ratings of anxiety symptoms using the SCARED (35; see Table 1 for details). Items are rated on a 3-point Likert scale ranging from 0 (not true or hardly ever true) to 2 (true or often true). The SCARED produces a total score (41 items; Cronbach’s α = 0.92) and five symptom dimensions (generalized anxiety, social anxiety, panic, separation anxiety, and school anxiety). The SCARED has been shown to be a reliable and valid measure of child and adolescent anxiety symptomatology (38, 39, 40), and higher scores have been associated greater symptom severity and functional impairment (41). Parent and child versions are identical in content, and prior investigations have identified optimal cutoff scores of 25 (child) and 17 (parent) to best identify clinically significant anxiety (38). The instrument has good convergent and divergent validity when compared to formal psychiatric diagnoses (38). We used the sum of the parent total and the child total SCARED scores in our main analyses as we find that the two scores load on the same factor in latent variable approaches, suggesting that they are indicators of the same factor (42).
The State Trait Anxiety Inventory: State Subscale (STAI-State) and State Trait Anxiety Inventory for Children: State Subscale (STAI-C-State).
The STAI-State and STAI-C-State are 20-item questionnaires rated on 4-point intensity scales, developed to assess the dimension of state anxiety in adults and children (43). The STAI-State scale has high internal consistency, with Cronbach’s alpha coefficient ranging between .86 and .95 (44, 45).
Analyses
Self-report measures were submitted to a repeated-measures ansalysis of variance (ANOVA), with Trial Type (LL, LM, HM, HH) as a within-subject factor and Group (Adults, Healthy Youth, Anxious Youth) as a between-subjects factor. Greenhouse-Geisser corrected p-values are reported when the sphericity assumption was violated, while follow-up comparisons were examined with post-hoc paired t-tests. For all analyses, statistical significance was set to α = .05, and all tests were two-sided.
We also used custom Matlab software (MathWorks; code available at https://github.com/canlab) to implement a linear mixed-effects model, as in Atlas et al. (24). At the first level of the multilevel model, regression coefficients for the effects of stimulation temperature, cue type (H vs L), and their interaction on pain reports were estimated for each individual. To get precise estimates of expectancy effects of pain, we then conducted additional analyses of cue effects restricted to trials with medium heat stimulation. The second level of each mixed-effects model assessed the significance of these coefficients across individuals, treating the participant as a random variable, and treating between-subjects factors (Group, Age) as fixed. We conducted two types of across-subject comparisons: One measured effects of age / developmental group irrespective of patient status, and one measured effects of anxiety within the youth participants. We also modeled age and SCARED scores as continuous variables in a subsequent analysis.
Results
Calibration.
We first examined the relationship between administered temperature and reported pain ratings for each group during calibration. We compared the temperatures that elicited ratings of low pain (2 on the VAS), medium pain (5 on the VAS) and high pain (7 on the VAS), which were then used in the main task. The average temperatures corresponding to low pain for adults, healthy youth, and anxious youth were: M = 38.41°C (SD =.28), M = 37.85°C (SD = 1.94), and M = 38.48°C (SD = 2.59), respectively. Temperatures corresponding to medium pain for adults/healthy youth/anxious youth were: M = 42.26°C (SD = 2.07); M = 42.00°C (SD = 1.75); M = 42.03°C (SD = 2.76). Temperatures corresponding to high pain for adults/healthy youth/anxious youth were: M = 44.44°C (SD = 2.30); M = 44.80°C (SD = 1.85); M = 44.50°C (SD = 2.76). No group differences in temperature emerged for the different pain levels, ps > .63.
Cue-based expectations across groups.
To measure explicit expectations at baseline, we conducted a 2 (cue: Low, High) × 3 (group: Adults, Healthy Youth, Anxious Youth) ANOVA examining self-reported expectancies collected after instructions but before conditioning. Results revealed that, across groups, all participants expected high pain in response to the high cue (adults/healthy youth/anxious youth: M = 7.87 (SD = .70); M = 7.21 (SD = .98); M = 7.12 (SD = .89)) and low pain in response to the low cue (adults/healthy youth/anxious youth: M = 1.94 (SD = .46); M = 2.59 (SD = .94); M = 2.45 (SD = .87)). However, we also observed a significant Group × Cue interaction that reflected developmental differences, F(2,60) = 6.26, p < .010, ηp2 = .173. That is, follow-up contrasts revealed that for high pain cues, Adults expected to experience higher pain levels than both Healthy Youth t(41) = 2.57, p = .014 and Anxious Youth t(41) = 3.08, p = .004. For low pain cues, adults expected to experience lower pain levels than Healthy Youth t(41) = 2.93, p = .006 and Anxious Youth t(41) = 2.43, p = .020. Healthy and anxious youth did not differ in their average ratings for either high- or low-pain cues.
During the subsequent six test runs, there was no main effect of time on the difference between cue-related expectancies (high-low) (p = .80), indicating that expectancies remained stable throughout the experiment and participants were not aware that some trials featured medium temperature in later runs. No interactions with Group emerged (p = .49), indicating that expectancies did not change over time as a function of group.
Cue-based expectancy effects on pain.
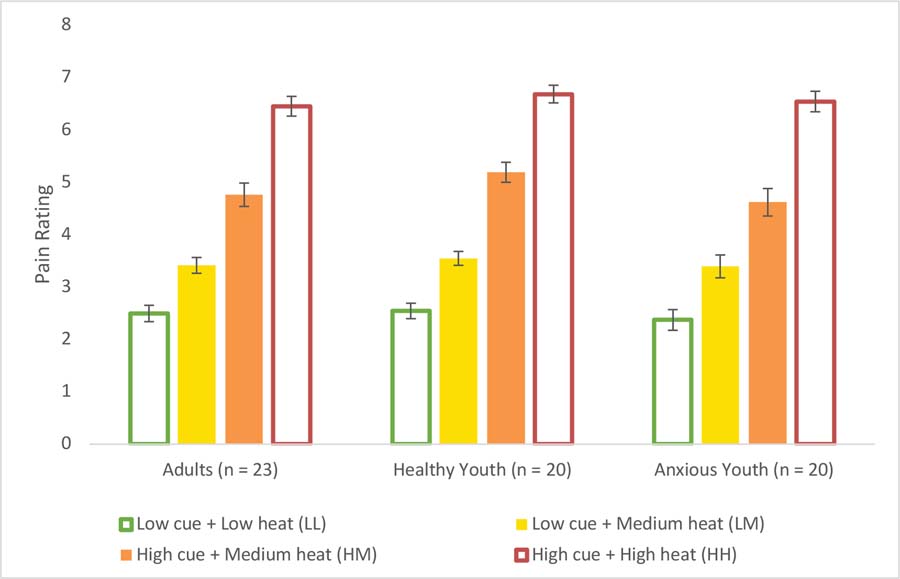
We examined the effect of cue-based expectancy on reported pain for each trial type (LL, LM, HM, HH). A repeated measures ANOVA yielded a significant main effect of trial type, F(3,180) = 438.32, p < .001, ηp2 = .880. Follow-up contrasts revealed that across participants, the critical medium temperature was rated as more painful in the high (M = 4.85 (SD = 1.05)) relative to low-expectancy (M = 3.45 (SD = .77)) condition, t(62) = 10.88, p < .001. Moreover, follow-up comparisons between the LL (M = 2.47 (SD = .76)) and LM (M = 3.45 (SD = .77)), and between the HM (M = 4.85 (SD = 1.05)) and HH (M = 6.55 (SD = .84) conditions were also significant (all ps ≤ .001) indicating that overall, participants reported greater pain with increasing heat level. No trial type-by-group interaction emerged, p = .66. No sex differences were observed (t(57) = −0.092, p = .93).
Expectancy effects across development.
The second study goal was to test the hypothesis that adults and children differ in cue-induced expectancy effects on pain. When we examined effects across all trials, we found strong effects of temperature on reported pain across participants (β = 2.29, t(41) = 29.94, p < .001, dz = 1.70 (adults); 1.51 (healthy youth); 1.51 (anxious youth)), but effects did not vary between adults and healthy youth, both in terms of average pain rating (p = .98) and temperature effects on pain (p = .93). We then examined ratings and cue effects on medium heat trials and found strong expectancy-based modulation (β = 1.72, t(41) = 10.48, p < .001), but again no evidence of group mean differences between adults and healthy youth in average pain ratings (p = .92) or cue effects (p = .97), as shown in Figure 2. Similarly, we found no developmental differences on average pain, heat effects, or cue-based expectancy effects on pain when we modeled age as a continuous variable in our healthy volunteers (all ps > .51).
Figure 2.
Mean pain ratings by group
Anxiety and expectancy-based pain modulation.
The third goal was to test the hypothesis that anxiety symptoms would influence expectancy effects on pain. To test this hypothesis, we compared anxious and healthy youth. Results revealed significant differences on the STAI-C-State between healthy (M = 27.75 (SD = 4.45)) and anxious (M = 32.11 (SD = 4.82)) youth, t(37) = 2.94, p < .010, indicating greater baseline state anxiety in patients than healthy youth. However, no group differences emerged in the change between STAI pre-and post-procedure (p = .14). As illustrated in Figure 2, we observed significant effects of heat level on reported pain within our youth participants (β = 2.27, t(38) = 29.35, p < .001), but we found no group differences in average pain rating (p = .19) or temperature effects on pain (p = .93). Next, we examined ratings and cue effects on medium heat trials. Again, we found strong cue-based modulation (β = 1.50, t(38) = 9.38, p < .001), without any evidence of group differences in average pain ratings (p = .14) or cue effects on pain (p = .19). Finally, we examined the effect of SCARED sum (parent and child) total scores as a continuous predictor across all youth. There was no correlation between cue-induced expectation (i.e., the mean difference between pain ratings on HM and LM trials) and the SCARED total composite score (r =.07, p = .69).
Discussion
This study reports the first experimental evidence for substantial cue-induced effects on pain experience across development, i.e. in healthy and anxious youth aged 8 to 18 years, as well as healthy adults. The study had three objectives. The first was to test the feasibility of a heat pain conditioning task in youth. The second was to test the hypothesis that cue-based expectancies have differential effects on the experience of pain in youth than adults. The third was to examine whether youth with an anxiety disorder are more influenced by expectancies than typically developing youth.
Three main findings emerged. First, the task was tolerated by most youth. Thus, the current task can be used successfully in pediatric non-anxious and anxious samples from the age of 8 years, adding to the limited repertoire of methods for eliciting conditioned fear in youth (46, 47). Second, contrary to our predictions, all participants, regardless of age, showed strong effects of expectancy on pain, with no age-associated differences in the magnitude of these effects. Third, also contrary to our predictions, associations between expectancy and pain experience were equivalent between anxious and non-anxious youth.
Based on self-report data, our results demonstrated that overall, participants across age and anxiety symptoms showed robust expectancy effects on the experience of thermal pain. The magnitude of this effect (dz range = 1.51–1.70) was consistent with previous studies in healthy adults (dz = 2.03; 24). Importantly, our findings highlight the safety and potential utility of using this paradigm to extend the existing literature on the effects of informational cues on pain experience to youth with and without an anxiety diagnosis. Such future research might probe subtler differences in cognitive influences on pain experience that may be present in implicit measures.
Naturalistic studies suggest that developmental factors modulate the effects of expectancy on subsequent pain experience (6, 9, 10). It has also been suggested that children and adolescents generally show larger placebo responses than adults (13, 14, 16, 19, 20). In our experimental approach, we manipulated cue-based expectancy effects on pain experimentally. We failed to find age effects. Both youth and adults rated medium-temperature thermal heat as more painful when it was preceded by a low pain cue than a high pain cue, with no modulation by age. Possibly, procedural and task-specific differences might have contributed to this null finding. First, suggestibility appears to increase steadily from an early age, peak between 9 and 12 years, and decline thereafter (48). Because the mean age of our participants was 13 years, they may have been older than the peak age for susceptibility. Furthermore, our task design involves associative learning, which reduces the psychosocial components of expectancy which might be required for suggestibility to influence outcomes. Second, inconsistencies with previous findings may have arisen due to different types of pain stimuli; our stimuli involved painful thermal heat, while clinical reports highlight anticipatory anxiety for painful medical procedures such as venipuncture, immunization or preoperative procedures (6). Third, based on recommendations from Human Subjects IRB to ensure that procedures were not unduly aversive, we took great care to make sure that children felt safe (i.e., by repeatedly checking in, speaking slowly, allowing breaks between runs, and applying lower maximal temperatures). This might have reduced anxiety and diminished its potential effect on the findings, accounting for some of the null expected effects.
While we did not collect neural measures in this task, our behavioral data allow for speculation about the neural underpinnings that might explain why youth and adults both exhibited equally robust expectancy effects on pain experience. Previous studies of expectancy-based pain modulation, cognitive control, and emotion regulation implicate the dorsolateral prefrontal cortex (DLPFC) as playing a critical role in downregulating affective responses. Consistent with this, studies of placebo analgesia suggest a causal role for the DLPFC, including work using transcranial magnetic stimulation (49) and studies linking expectancy-based processing to the integrity of frontal cortex in Alzheimer’s Disease (50). Contrary to these findings, our data suggest that cue-based pain modulation does not depend on the maturation of prefrontal cortex, as we found robust modulation throughout development. We suggest that this cue-based modulation might instead depend on subcortical processes linked with learning and endogenous pain modulation (3). Future studies should directly measure whether expectation-related pain modulation in children relies on dissociable neural pathways and processing strategies relative to adults.
Limitations
While the present study contributes to the literature on cognitive effects on pain experience by extending prior work in adults to typically developing and anxious youth, several limitations should be noted. First, the sample sizes were modest, which may have limited our ability to observe age-related group differences in the magnitude of expectancy effects. However, despite modest sample sizes, the study acquired a considerable amount of data from 40 heat pain sessions in children, as well as over 20 more in adults. Previous work using aversive exposures in pediatric anxiety in our lab shows large effects (i.e. 1.0 to 0.80) for robust psychological phenomena associated with anxiety (i.e. 51, 52, 53). If the heat pain procedures in the current study were to generate comparable effects, the current study would have adequate statistical power (> 0.80) to detect them. We note that although the study is sufficiently powered to examine the different cue conditions, the study is underpowered to detect medium effect sizes for group differences (20/group enables detection of about Cohen’s d = 0.9, which is a large effect size). Second, our study design also did not include an adult group with anxiety disorders, thus precluding a more complete examination of both factors studied and their interaction. Third, data from this study are cross-sectional, thus limiting our ability to make any inferences about developmental trajectories of associative learning and potentially reflecting other confounding factors such as cohort effects. Future longitudinal studies on cue effects on pain-evoked responses are warranted. A further limitation relevant to sample selection is that informed-consent screening may have biased the sample of participating children, such that those consenting to experience pain may also have been those less anxious about this aversive prospect, thus potentially reducing our ability to detect some between-group differences. Future research might continue to try and include as many participants as possible so as to maximize the range of relevant demographic and child characteristics. Individual characteristics may additionally moderate expectancy effects (i.e. 54, 55, although see 56, 57). Future work might thus also examine effects of potentially relevant trait-level factors, such as fear of pain, negative outcome expectancies or optimism. Lastly, other limitations relate to methodological constraints of data acquisition. While we obtained trial-by-trial measurement of subjective pain experience, only self-report data were collected during this initial study. Future research would benefit from collecting continuous neural measures across both calibration and testing sessions to more comprehensively assess pain modulation.
In conclusion, the present results suggest that the robust modulation of pain experience by anticipatory cues, previously described in adults (24), is also observed in healthy and anxious youth, with large effect sizes. Complex factors may influence associations among anxiety, development, and pain reports in naturalistic studies. Such factors may be identified using experiments that employ more complex manipulations of expectancy or that assess the neural correlates of pain expectancy. Future studies should investigate pain modulation paradigms that dissociate prefrontal and subcortical contributions where stronger age-related differences may be detected.
Figure 1.
Experimental design. a. Conditions of interest. During the first two blocks of the task, low-pain cues always preceded low-pain stimulation (LL) and high-pain cues preceded high-pain stimulation (HH). During the subsequent six test blocks, trials were evenly divided between these conditions and trials in which each predictive cue was followed by a stimulus calibrated to elicit medium pain [high cue plus medium pain (HM); low cue plus medium pain (LM)].
b. Trial structure. Each trial consisted of an auditory predictive cue followed by an anticipatory delay and 5 s of noxious thermal stimulation. After a 5 s average interstimulus interval, participants reported trial-by-trial perceived pain using a visual analog scale.
Acknowledgments
Supported by National Institute of Mental Health Intramural Research Program Project number ZIAMH00278 and National Center for Complementary and Integrative Health Project number ZIAAT000032.
Footnotes
Conflict of Interest: The authors declare no conflict of interest related to this work.
References
- 1.Benedetti F, Mayberg HS, Wager TD, Stohler CS, & Zubieta JK (2005). Neurobiological mechanisms of the placebo effect. Journal of Neuroscience, 25, 10390–10402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ploghaus A, Becerra L, Borras C, & Borsook D (2003). Neural circuitry underlying pain modulation: expectation, hypnosis, placebo. Trends in cognitive sciences, 7, 197–200. [DOI] [PubMed] [Google Scholar]
- 3.Wager TD, & Atlas LY (2015). The neuroscience of placebo effects: connecting context, learning and health. Nature Reviews Neuroscience, 16, 403–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pine DS, Cohen P, Gurley D, Brook J, & Ma Y (1998). The risk for early-adulthood anxiety and depressive disorders in adolescents with anxiety and depressive disorders. Archives of General Psychiatry, 55, 56–64. [DOI] [PubMed] [Google Scholar]
- 5.Beesdo K, Knappe S, & Pine DS (2009). Anxiety and anxiety disorders in children and adolescents: developmental issues and implications for DSM-V. Psychiatric Clinics of North America, 32, 483–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Racine NM, Riddell RRP, Khan M, Calic M, Taddio A, & Tablon P (2016). Systematic review: predisposing, precipitating, perpetuating, and present factors predicting anticipatory distress to painful medical procedures in children. Journal of Pediatric Psychology, 41, 159–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gidwani PP, Sobol A, DeJong W, Perrin JM, & Gortmaker SL (2002). Television viewing and initiation of smoking among youth. Pediatrics, 110, 505–508. [DOI] [PubMed] [Google Scholar]
- 8.Ata R, Ludden A, & Lally M (2007). The Effects of Gender and Family, Friend, and Media Influences on Eating Behaviors and Body Image During Adolescence. Journal of Youth & Adolescence, 36, 1024. [Google Scholar]
- 9.Brown JD, & Witherspoon EM (2002). The mass media and American adolescents’ health. Journal of Adolescent Health, 31, 153–170. [DOI] [PubMed] [Google Scholar]
- 10.Gould MS (2001). Suicide and the media. Annals of the New York Academy of Sciences, 932, 200–224. [DOI] [PubMed] [Google Scholar]
- 11.Fischer P, Greitemeyer T, Kastenmüller A, Vogrincic C, & Sauer A (2011). The effects of risk-glorifying media exposure on risk-positive cognitions, emotions, and behaviors: A meta-analytic review. Psychological Bulletin, 137, 367–390. [DOI] [PubMed] [Google Scholar]
- 12.Colloca L, Flaten MA, Meissner K Placebo and pain: from bench to bedside. Oxford, UK: Elsevier; 2013. [Google Scholar]
- 13.Bridge JA, Birmaher B, Iyengar S, Barbe RP, & Brent DA (2009). Placebo response in randomized controlled trials of antidepressants for pediatric major depressive disorder. American Journal of Psychiatry, 166, 42–49. [DOI] [PubMed] [Google Scholar]
- 14.Cohen D, Deniau E, Maturana A, Tanguy ML, Bodeau N, Labelle R, … & Guile JM (2008). Are child and adolescent responses to placebo higher in major depression than in anxiety disorders? A systematic review of placebo-controlled trials. PloS one, 3(7), e2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Emslie GJ, Rush AJ, Weinberg W,A, Kowatch RA, Hughes C,W, Carmody T, & Rintelmann J (1997). A double-blind, randomized, placebo-controlled trial of fluoxetine in children and adolescents with depression. Arch Gen Psychiatry, 54(11), 1031–1037. [DOI] [PubMed] [Google Scholar]
- 16.Emslie GJ, Heiligenstein JH, Wagner KD, Hoog SL, Ernest DE, Brown E, … Jacobson JG (2002). Fluoxetine for Acute Treatment of Depression in Children and Adolescents: A Placebo-Controlled, Randomized Clinical Trial. Journal of the American Academy of Child & Adolescent Psychiatry, 41, 1205–1215. [DOI] [PubMed] [Google Scholar]
- 17.Loder E, Goldstein R, & Biondi D (2005). Placebo effects in oral triptan trials: the scientific and ethical rationale for continued use of placebo controls. Cephalalgia, 25, 124–131. [DOI] [PubMed] [Google Scholar]
- 18.Mayes TL, Tao R, Rintelmann JW, Carmody T, Hughes CW, Kennard BD, … & Emslie GJ (2007). Do children and adolescents have differential response rates in placebo-controlled trials of fluoxetine? CNS Spectrums, 12, 147–154. [DOI] [PubMed] [Google Scholar]
- 19.Krummenacher P, Kossowsky J, Schwarz C, Brugger P, Kelley JM, Meyer A, & Gaab J (2014). Expectancy-induced placebo analgesia in children and the role of magical thinking. The Journal of Pain, 15, 1282–1293. [DOI] [PubMed] [Google Scholar]
- 20.Weimer K, Gulewitsch MD, Schlarb AA, Schwille-Kiuntke J, Klosterhalfen S, & Enck P (2013). Placebo effects in children: a review. Pediatric Research, 74, 96–102. [DOI] [PubMed] [Google Scholar]
- 21.Winner P, Lewis D, Visser WH, Jiang K, Ahrens S, & Evans JK (2002). Rizatriptan 5 mg for the Acute Treatment of Migraine in Adolescents: A Randomized, Double-Blind, Placebo-Controlled Study. Headache, 42, 49–55. [DOI] [PubMed] [Google Scholar]
- 22.Waschbusch DA, Pelham WE Jr, Waxmonsky J, & Johnston C (2009). Are there placebo effects in the medication treatment of children with attention-deficit hyperactivity disorder? Journal of Developmental & Behavioral Pediatrics, 30, 158–168. [DOI] [PubMed] [Google Scholar]
- 23.Atlas LY, & Wager TD (2013). Expectancies and beliefs: insights from cognitive neuroscience Oxford handbook of cognitive neuroscience. Oxford University Press, Oxford, NY, 359–381. [Google Scholar]
- 24.Atlas LY, Bolger N, Lindquist MA, & Wager TD (2010). Brain Mediators of Predictive Cue Effects on Perceived Pain. Journal of Neuroscience, 30, 12964–12977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Costello EJ, Mustillo S, Erkanli A, Keeler G, & Angold A (2003). Prevalence and development of psychiatric disorders in childhood and adolescence. Archives of General Psychiatry, 60, 837–844. [DOI] [PubMed] [Google Scholar]
- 26.McWilliams LA, Cox BJ, & Enns MW (2003). Mood and anxiety disorders associated with chronic pain: an examination in a nationally representative sample. Pain, 106, 127–133. [DOI] [PubMed] [Google Scholar]
- 27.Mcwilliams LA, Goodwin RD, & Cox BJ (2004). Depression and anxiety associated with three pain conditions: results from a nationally representative sample. Pain, 111, 77–83. [DOI] [PubMed] [Google Scholar]
- 28.Kain ZN, Mayes LC, Caldwell-Andrews AA, Karas DE, & McClain BC (2006). Preoperative anxiety, postoperative pain, and behavioral recovery in young children undergoing surgery. Pediatrics, 118, 651–658. [DOI] [PubMed] [Google Scholar]
- 29.Means-Christensen AJ, Roy-Byrne PP, Sherbourne CD, Craske MG, & Stein MB (2008). Relationships among pain, anxiety, and depression in primary care. Depression and Anxiety, 25, 593–600. [DOI] [PubMed] [Google Scholar]
- 30.Hartley CA, & Phelps EA (2012). Anxiety and decision-making. Biological Psychiatry, 72, 113–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Research Unit of Pediatric Psychopharmacology (RUPP) Anxiety Study Group, (2001). Fluvoxamine for the treatment anxiety disorders in children and adolescents. New England Journal of Medicine, 344, 1279–1285. [DOI] [PubMed] [Google Scholar]
- 32.Walkup JT, Albano AM, Piacentini J, Birmaher B, Compton SN, Sherrill JT, … & Iyengar S (2008). Cognitive behavioral therapy, sertraline, or a combination in childhood anxiety. New England Journal of Medicine, 359, 2753–2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eysenck MW, & Derakshan N (1997). Cognitive biases for future negative events as a function of trait anxiety and social desirability. Personality and Individual differences, 22, 597–605. [Google Scholar]
- 34.Kaufman J, Birmaher B, Brent D, Rao UMA, Flynn C, Moreci P, … & Ryan N (1997). Schedule for affective disorders and schizophrenia for school-age children-present and lifetime version (K-SADS-PL): initial reliability and validity data. Journal of the American Academy of Child & Adolescent Psychiatry, 36, 980–988. [DOI] [PubMed] [Google Scholar]
- 35.Birmaher B, Khetarpal S, Brent D, Cully M, Balach L, Kaufman J, & Neer SM (1997). The screen for child anxiety related emotional disorders (SCARED): scale construction and psychometric characteristics. Journal of the American Academy of Child & Adolescent Psychiatry, 36, 545–553. [DOI] [PubMed] [Google Scholar]
- 36.Britton JC, Grillon C, Lissek S, Norcross MA, Szuhany KL, Chen G, … Pine DS (2013). Response to Learned Threat: An fMRI Study in Adolescent and Adult Anxiety. American Journal of Psychiatry, 170, 1195–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hicks C,L, von Baeyer C,L, Spafford P,A, van Korlaar I, & Goodenough B (2001). The Faces Pain Scale-Revised: toward a common metric in pediatric pain measurement. Pain, 93, 173–183. [DOI] [PubMed] [Google Scholar]
- 38.Birmaher B, Brent DA, Chiappetta L, Bridge J, Monga S, & Baugher M (1999). Psychometric Properties of the Screen for Child Anxiety Related Emotional Disorders (SCARED): A Replication Study. Journal of the American Academy of Child & Adolescent Psychiatry, 38, 1230–1236. [DOI] [PubMed] [Google Scholar]
- 39.Hale WW, Crocetti E, Raaijmakers QA, & Meeus WH (2011). A meta-analysis of the cross-cultural psychometric properties of the Screen for Child Anxiety Related Emotional Disorders (SCARED). Journal of Child Psychology and Psychiatry, 52, 80–90. [DOI] [PubMed] [Google Scholar]
- 40.Monga S, Birmaher B, Chiappetta L, Brent D, Kaufman J, Bridge J, & Cully M (2000). Screen for child anxiety-related emotional disorders (SCARED): Convergent and divergent validity. Depression and Anxiety, 12, 85–91. [DOI] [PubMed] [Google Scholar]
- 41.DeSousa DA, Salum GA, Isolan LR, & Manfro GG (2013). Sensitivity and specificity of the Screen for Child Anxiety Related Emotional Disorders (SCARED): a community-based study. Child Psychiatry & Human Development, 44, 391–399. [DOI] [PubMed] [Google Scholar]
- 42.Kircanski K, Zhang S, Stringaris A, Wiggins JL, Towbin KE, Pine DS, … & Brotman MA (2017). Empirically derived patterns of psychiatric symptoms in youth: A latent profile analysis. Journal of Affective Disorders, 216, 109–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Spielberger CD, Gorsuch RL, & Lushene RE (1970). Manual for the state-trait anxiety inventory.
- 44.Rossi V, & Pourtois G (2012). Transient state-dependent fluctuations in anxiety measured using STAI, POMS, PANAS or VAS: a comparative review. Anxiety, Stress & Coping, 25, 603–645. [DOI] [PubMed] [Google Scholar]
- 45.Spielberger CD, & Sydeman SJ (1994). State-Trait Anxiety Inventory and State-Trait Anger Expression Inventory.
- 46.Michalska KJ, Shechner T, Hong M, Britton JC, Leibenluft E, Pine DS, & Fox NA (2016). A developmental analysis of threat/safety learning and extinction recall during middle childhood. Journal of Experimental Child Psychology, 146, 95–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shechner T, Britton JC, Ronkin EG, Jarcho JM, Mash JA, Michalska KJ, … & Pine DS (2015). Fear conditioning and extinction in anxious and nonanxious youth and adults: examining a novel developmentally appropriate fear-conditioning task. Depression and anxiety, 32, 277–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Morgan AH, & Hilgard ER (1973). Age differences in susceptibility to hypnosis. International journal of clinical and experimental Hypnosis, 21, 78–85. [Google Scholar]
- 49.Krummenacher P, Candia V, Folkers G, Schedlowski M, & Schönbächler G (2010). Prefrontal cortex modulates placebo analgesia. PAIN, 148, 368–374. [DOI] [PubMed] [Google Scholar]
- 50.Benedetti F, Arduino C, Costa S, Vighetti S, Tarenzi L, Rainero I, & Asteggiano G (2006). Loss of expectation-related mechanisms in Alzheimer’s disease makes analgesic therapies less effective. Pain, 121, 133–144. [DOI] [PubMed] [Google Scholar]
- 51.Pine DS, Klein RG, Coplan JD, Papp LA, Hoven CW, Martinez J, … & Gorman JM (2000). Differential carbon dioxide sensitivity in childhood anxiety disorders and nonill comparison group. Archives of General Psychiatry, 57, 960–967. [DOI] [PubMed] [Google Scholar]
- 52.Shechner T, Britton JC, Pérez-Edgar K, Bar-Haim Y, Ernst M, Fox NA, … & Pine DS (2012). Attention biases, anxiety, and development: toward or away from threats or rewards? Depression and Anxiety, 29, 282–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Roberson-Nay R, Klein DF, Klein RG, Mannuzza S, Moulton JL, Guardino M, & Pine DS (2010). Carbon dioxide hypersensitivity in separation-anxious offspring of parents with panic disorder. Biological Psychiatry, 67, 1171–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Almarzouki AF, Brown CA, Brown RJ, Leung MH, & Jones AK (2017). Negative expectations interfere with the analgesic effect of safety cues on pain perception by priming the cortical representation of pain in the midcingulate cortex. PloS One, 12(6), e0180006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Geers AL, Wellman JA, Fowler SL, Helfer SG, & France CR (2010). Dispositional optimism predicts placebo analgesia. The Journal of Pain, 11, 1165–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kaptchuk TJ, Kelley JM, Deykin A, Wayne PM, Lasagna LC, Epstein IO, … & Wechsler ME (2008). Do “placebo responders” exist? Contemporary Clinical Trials, 29, 587–595. [DOI] [PubMed] [Google Scholar]
- 57.Horing B, Weimer K, Muth ER, & Enck P (2014). Prediction of placebo responses: a systematic review of the literature. Frontiers in Psychology, 5, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]