Abstract
Objects:
Inflammation is one of the hallmarks of cancer. Tumor-associated inflammatory response plays a crucial role in enhancing tumorigenesis. This study aimed to establish an effective predictive nomogram based on inflammation factors in patients with advanced non-small cell lung cancer (NSCLC).
Methods:
We retrospectively evaluated 887 patients with advanced NSCLC between November 2004 and December 2015 and randomly divided them into primary (n = 520) and validation cohorts (n = 367). Cox regression analysis was used to identify prognostic factors for building the nomogram. The predictive accuracy and discriminative ability of the nomogram were determined using a concordance index (C-index), calibration plot, and decision curve analysis and were compared to the TNM staging system.
Results:
The nomogram was established using independent risk factors (P < 0.05): age, TNM stage, C reaction protein-to-albumin ratio (CAR), and neutrophils (NEU). The C-index of the model for predicting OS had a superior discrimination power compared to that of the TNM staging system both in the primary [0.711 (95% CI: 0.675-0.747) vs 0.531 (95% CI: 0.488-0.574), P < 0.01] and validation cohorts [0.703, 95% CI: 0.671 -0.735 vs 0.582, 95% CI: 0.545-0.619, P < 0.01]. Decision curves also demonstrated that the nomogram had higher overall net benefits than that of the TNM staging system. Subgroup analyses revealed that the nomogram was a favorable prognostic parameter in advanced NSCLC (P < 0.05). The results were internally validated using the validation cohorts.
Conclusions:
The proposed nomogram with inflammatory factors resulted in an accurate prognostic prediction in patients with advanced NSCLC.
Keywords: nomogram, inflammation factor, advanced NSCLC, prognosis, overall survival
Introduction
Lung cancer remains the most common type of cancer and the leading cause of cancer death in China.1 Non-small cell lung cancer (NSCLC) accounts for approximately 85% of lung cancers.2 A majority of patients are diagnosed with advanced-stage disease, when surgery, the best curative option, is no longer feasible.3 In NSCLC, staging based on the tumor-node-metastasis (TNM) system and histological subtype has been used to determine prognosis and design optimal treatment regimens.4 However, different patients at identical stages undergoing similar treatment regimens often have varied clinical outcomes, suggesting that the current staging system is inadequate for predicting postoperative survival. In such patients with advanced cancers, the TNM staging system has reached its ceiling5; therefore, other factors are needed to assess patient prognosis.
Recently there has been an increased interest in improving NSCLC prognostication using clinical, inflammatory, and molecular biomarkers; however, there remains a lack of reliable, reproducible, and low-cost biomarkers that can be readily incorporated into routine practice to optimally predict prognosis and guide treatment. The host response to malignant tumors consists of changes in the tumor microenvironment as well as systemic alterations. Inflammation is one of the hallmarks of cancer and the tumor-associated inflammatory response has a critical role in enhancing tumorigenesis by inducing tumor-cell growth, angiogenesis, and genome instability.6 Given the importance of this systemic inflammatory response, the study of the markers of systemic inflammation with the intent of developing cost-effective prognostic biomarkers for patients with cancer, including those with lung cancer, is ongoing. One of the widely studied groups of inflammatory markers is derived from elements of the ubiquitously available and inexpensive procedure of whole blood count. The migration and activation of neutrophils (NEUs) can cause inflammation and sensitization directly or indirectly7; thus, a strong correlation between poor clinical outcomes and high neutrophil content, both locally and systemically, has been reported in patients with NSCLC.8,9 The neutrophil (NEU) counts and neutrophil-lymphocyte ratio (NLR) have been evaluated in both localized and advanced NSCLC and appear prognostic in these patient populations.10,11 Additionally, the platelet-to-lymphocyte ratio (PLR) has been introduced as another measurable parameter to determine inflammation and reveal the impact on the clinical outcomes of NSCLC.12,13 Of these indicators, the NLR is most widely studied and has been adopted as a predictor of mortality and morbidity in several cancers.10,14
With regard to biochemical indices, several studies consider the level of C-reactive protein (CRP), an acute-phase reactant excessively produced by the liver during inflammation, to predict patient outcomes in NSCLC.15,16 Apart from CRP, another commonly used inflammatory marker is albumin (ALB), a negative acute phase reactant in patients with cancer, which is inversely correlated with inflammation. However, the CRP-to-ALB ratio (CAR), combining CRP and ALB, has not been widely investigated as a biomarker in NSCLC. Only recently has the CAR been proposed and investigated as a prognostic marker in patients with solid tumors.17,18 There is recent evidence that the CAR predicts long-term outcomes in patients with operable NSCLC.19 However, few previous studies have studied CAR for the prognosis of advanced NSCLC. Considering the small-sized cohort in these studies, the clinical utility of the CAR in NSCLC, especially advanced NSCLC (the commonest subset of NSCLC), remains to be further defined.
Nomograms are currently widely used to predict the overall survival (OS) of patients with cancers, including lung cancer, colorectal cancer, and gastric cancer.14,16,20-22 Furthermore, nomograms have been proven to be effective in precise predictions compared to the traditional TNM staging systems and are, therefore, used to predict OS in several cancers.5,16 The aim of this study was to construct a nomogram integrating inflammation-based factors to estimate the prognosis of patients with advanced NSCLC. We also tested whether the nomogram model could provide a more accurate prediction of patient outcomes compared to those provided by the traditional TNM system.
Materials and Methods
Inclusion and Exclusion Criteria
Inclusion criteria: patients had diagnosed with stage IIIB and IV NSCLC. All patients who fulfilled the inclusion criteria were assessed by a multidisciplinary team comprising a medical oncologist, radiation oncologist, and a thoracic surgeon. Patients were determined to be unsuitable for radical surgery for lung cancer. Exclusion criteria: patients with double primary cancer, those previously treated, patients with clinical evidence of infection or other inflammation within 1 month of commencing therapy, patients who failed to follow up, those without available data on NEU, lymphocyte, and platelet (PLT) counts and biochemical parameters, such as serum ALB and CRP levels.
Sample Collection and Laboratory Analysis
After screening based on the inclusion and exclusion criteria, 887 patients diagnosed with stage IIIB and IV NSCLC at the Sun Yat-Sen University Cancer Center (SYSUCC) between November 2004 and December 2015 were retrospectively reviewed. All patients underwent standard workup, which included systemic imaging including positron emission tomography (PET), PET/computerized tomography (CT), CT and/or bone scan, brain imaging consisting of magnetic resonance imaging (MRI) or CT with contrast, and routine blood work prior to treatment. Patients were treated with definitive chemotherapy with or without radiation. Some of them underwent thoracoscopic pleural biopsy. All patients presented with clinical stage IIIB and IV disease based on the AJCC 7th edition TNM classification and staging system. Patients were followed up at our outpatient department every 3–6 months for the first 2 years, and then annually. The last follow-up was in March 2017.
Our study retrieved the NEU, lymphocyte, PLT counts, and biochemical parameters, including serum ALB and CRP, from the medical files of the enrolled patients. The records indicated that all tests were performed according to the manufacturer’s protocols. The serum levels of CRP and ALB were measured using an Automatic Biochemical Analyzer (Hitachi 7600, Japan), while NEU, lymphocyte, and PLT count values were collected from routine blood examination results and were detected using a Sysmex XS800 analyzer (Sysmex, Japan). All biochemical tests were performed before commencing treatment.
Statistical Analysis
Data were analyzed using SPSS standard version 20.0 (SPSS, Chicago, USA) and R software version 3.6.1 (http://www.R-project.org). For patients who were still alive, the duration of OS was calculated from the diagnosis of malignancy until death due to any cause or until the date of the last follow-up. Cut-off values were determined using the X-tile program of R software. The Kaplan-Meier method was used to estimate OS and calculate the 95% confidence intervals (CIs). Univariate and multivariate analyses to determine differences in survival were performed using the Cox proportional hazards model and expressed as hazard ratios (HRs) and 95% CIs. All variables in the multivariable model were enrolled to construct a prognostic nomogram model using the rms package. Calibration of the nomogram for 1-, 2-, and 3-year OS was executed by comparing the predicted survival and observed survival. The discriminative ability and predictive accuracy of the established nomogram were assessed using the C-index and decision curve and were compared with the traditional TNM staging system. The P-values in comparison of the C-indexes were calculated based on normal approximation using the function, rcorrp.cens, in the Hmisc package. Pearson’s χ2 test was used to analyze the relationship between CAR, NEU and NLR, PLR, CRP, ALB, the clinicopathological baseline characteristics. Two-sided p-values < 0.05 were considered statistically significant.
Results
Patient Characteristics
This study enrolled a total of 887 patients. We randomly divided the patients into primary (n = 520) and validation cohorts (n = 367). The patients’ demographic data and clinical characteristics are listed in Table 1. There were 520 patients in the primary cohort comprising 368 male (70.8%) and 152 female patients (29.2%); the ages of the patients ranged from 25–84 years. Of the enrolled patients, 120 (23.1%) were staged as IIIB and 400 (76.9%) as stage IV. The median OS was 12.6 months (range 0.2–38.6 months). The validation cohort included 230 male (62.7%) and 137 female patients (37.3%) with their age ranging from 25–86 years. Of these, 80 (21.8%) were staged as IIIB and 287 (78.2%) as stage IV. The median OS was 11.4 months (range 0.3–49.9 months). The 1-, 2-, and 3-year OS rates for the primary and validation cohorts were 52.12%, 7.31%, 0.38% and 48.87%, 17.02%, 0.92%, respectively.
Table 1.
Comparison Between Modeling Group and Validation Group by Clinicopathological Characteristics.
| Characteristics | Primary group | Validation group | ||
|---|---|---|---|---|
| No. of patients | % | No. of patients | % | |
| patients | 520 | 367 | ||
| Age | ||||
| ≤58 | 257 | 49.4 | 198 | 54 |
| >58 | 263 | 50.6 | 169 | 46 |
| Gender | ||||
| Female | 152 | 29.2 | 137 | 37.3 |
| Male | 368 | 70.8 | 230 | 62.7 |
| Histology type | ||||
| Adenocarcinoma | 358 | 68.8 | 259 | 70.6 |
| Non-Adenocarcinoma | 162 | 31.2 | 108 | 29.4 |
| Clinical stage | ||||
| III | 120 | 23.1 | 80 | 21.8 |
| IV | 400 | 76.9 | 287 | 78.2 |
| cT Status | ||||
| cT 1+cT2 | 223 | 42.9 | 170 | 46.3 |
| cT3+cT4 | 245 | 47.1 | 168 | 45.8 |
| n.a. | 52 | 10 | 29 | 7.9 |
| cN Status | ||||
| cN0+cN1 | 66 | 12.7 | 37 | 10.1 |
| cN2+cN3 | 389 | 74.8 | 308 | 84 |
| n.a. | 65 | 12.5 | 22 | 5.9 |
| Treatment | ||||
| Radiotherapy | 24 | 4.6 | 7 | 1.9 |
| Chemotherapy | 354 | 68.1 | 261 | 71.4 |
| NEU(×109/L) | ||||
| ≤5.40 | 289 | 55.6 | 182 | 49.6 |
| >5.40 | 231 | 44.4 | 189 | 50.4 |
| NLR | ||||
| ≤2.70 | 180 | 34.6 | 139 | 37.9 |
| >2.70 | 340 | 65.4 | 228 | 62.1 |
| PLR | ||||
| ≤232 | 397 | 76.3 | 265 | 72.2 |
| >232 | 123 | 23.7 | 102 | 27.8 |
| C-reactive protein(mg/L) | ||||
| ≤6.33 | 221 | 42.5 | 153 | 41.7 |
| >6.33 | 299 | 57.5 | 214 | 58.3 |
| Albumin(g/L) | ||||
| ≤40.0 | 252 | 48.5 | 160 | 43.6 |
| >40.0 | 268 | 51.5 | 207 | 56.4 |
NEU, neutrophil; NLR, neutrophil-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio.
Association of Preoperative Serum CAR and NEU Levels With Clinical Characteristics
Patient characteristics and correlations between preoperative CAR and NEU levels and clinicopathological parameters are shown in Table 2. The X-tile program was used to determine the optimal cut-off values for CAR and NEU of OS, which were 0.15 and 5.4 × 109/L respectively. In the primary cohort, the CAR was associated with T status (P = 0.028). Males (P = 0.015) and patients with adenocarcinoma (P = 0.035) had higher preoperative NEU levels. The NEU levels were also associated with N status (P = 0.038), chemotherapy (P = 0.020), and lymphocyte counts (P = 0.040). In the validation cohort, age (>58) (P = 0.025), male (P < 0.001), adenocarcinoma (P < 0.001), T status (T3+T4) (P < 0.001), N status (N2+N3) (P = 0.047) had a higher pre-operative CAR. NEU levels were associated with adenocarcinoma (P = 0.008) and T status (P = 0.001), while CAR and NEU levels were associated with PLT, NLR, PLR, CRP and ALB in both cohorts (P < 0.001).
Table 2.
Correlation Between CAR, NEU and Clinicopathological Variables of NSCLC Patients in Primary Group and Validation Group.
| Characteristics | Primary group | Validation group | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No of patients | CAR | NEU | No of patients | CAR | NEU | |||||||||
| ≤0.15 | >0.15 | P a | ≤5.4 × 109/L | >5.4 × 109/L | P a | ≤0.15 | >0.15 | P a | ≤5.4 × 109/L | >5.4 × 109/L | P a | |||
| Patients | 520 | 221 | 299 | 289 | 231 | 367 | 151 | 216 | 182 | 185 | ||||
| Age | 0.306 | 0.746 | 0.025 | 0.705 | ||||||||||
| ≤58 | 257 | 115(44.7%) | 142(55.3%) | 141(54.9%) | 116(45.1%) | 198 | 92(46.5%) | 106(53.5%) | 100(50.5%) | 98(49.5%) | ||||
| >58 | 263 | 106(40.3%) | 157(59.7%) | 148(56.3%) | 115(43.7%) | 169 | 59(34.9%) | 110(65.1%) | 82(48.5%) | 87(51.5%) | ||||
| Gender | 0.391 | 0.015 | <0.001 | 0.381 | ||||||||||
| Female | 152 | 69(45.4%) | 83(54.6%) | 97(63.8%) | 55(36.2%) | 137 | 74(54.0%) | 63(46.0%) | 72(52.6%) | 65(47.4%) | ||||
| Male | 368 | 152(41.3%) | 216(58.7%) | 192(52.2%) | 176(47.8%) | 230 | 77(33.5%) | 153(66.5%) | 110(47.8%) | 120(52.2%) | ||||
| Histology type | 0.059 | 0.035 | <0.001 | 0.008 | ||||||||||
| Adenocarcinoma | 358 | 162(45.3%) | 196(54.7%) | 210(58.7%) | 148(41.3%) | 108 | 28(25.9%) | 80(74.1%) | 140(54.1%) | 119(45.9%) | ||||
| Non-Adenocarcinoma | 162 | 59(36.4%) | 103(63.6%) | 79(48.8%) | 83(51.2%) | 259 | 123(47.5%) | 136(52.5%) | 42(38.9%) | 66(61.1%) | ||||
| Clinical stage | 0.674 | 0.885 | 0.983 | 0.400 | ||||||||||
| IIIB | 120 | 49(40.8%) | 71(59.2%) | 66(55.0%) | 54(45.0%) | 80 | 33(41.2%) | 47(58.8%) | 43(53.8%) | 37(46.2%) | ||||
| IV | 400 | 172(43.0%) | 228(57.0%) | 223(55.8%) | 177(44.2%) | 287 | 118(41.1%) | 169(58.9%) | 139(48.4%) | 148(51.6%) | ||||
| T Status | 0.028 | 0.109 | <0.001 | 0.001 | ||||||||||
| cT 1+cT2 | 223 | 109(48.3%) | 114(51.1%) | 122(54.7%) | 101(45.3%) | 170 | 90(52.9%) | 80(47.1%) | 100(58.8%) | 70(41.2%) | ||||
| cT3+cT4 | 245 | 95(38.8%) | 150(61.25%) | 131(53.5%) | 114(46.5%) | 168 | 47(28.0%) | 121(72.0%) | 66(39.3%) | 102(60.7%) | ||||
| unknown | 52 | 17(32.7%) | 35(57.5%) | 36(69.2%) | 16(30.8%) | 29 | 14(48.3%) | 15(51.7%) | 16(55.2%) | 13(44.8%) | ||||
| N Status | 0.149 | 0.038 | 0.047 | 0.232 | ||||||||||
| cN0+cN1 | 66 | 32(48.5%) | 34(51.5%) | 39(59.1%) | 27(40.9%) | 37 | 22(59.5%) | 15(40.5%) | 21(56.8%) | 16(43.2%) | ||||
| cN2+cN3 | 389 | 168(43.2%) | 221(56.8%) | 205(52.7%) | 184(47.3%) | 308 | 119 (38.6%) | 189(61.4%) | 147(47.7%) | 161(52.3%) | ||||
| unknown | 65 | 21(32.3%) | 44(67.7%) | 45(69.2%) | 20(30.8%) | 22 | 10(45.5%) | 12(54.5%) | 14(63.6%) | 8(36.4%) | ||||
| M status | 0.674 | 0.885 | 0.983 | 0.400 | ||||||||||
| Yes | 400 | 172(43.0%) | 228(57.0%) | 223(55.8%) | 177(44.2%) | 80 | 33(41.2%) | 47(58.8%) | 43(53.8%) | 37(46.2%) | ||||
| No | 120 | 49(40.8%) | 71(59.2%) | 66(55.0%) | 54(45.0%) | 287 | 118(41.1%) | 169(58.9%) | 139(48.4%) | 148(51.6%) | ||||
| Surgeryb | 0.973 | 0.763 | 0.93 | 0.842 | ||||||||||
| Yes | 21 | 9(42.9%) | 12(57.1%) | 11(52.4%) | 10(47.6%) | 19 | 8(42.1%) | 11(57.9%) | 9(47.4%) | 10(52.6%) | ||||
| No | 499 | 212(42.5%) | 287(57.5%) | 278(55.7%) | 221(44.3%) | 348 | 143(41.1%) | 205(58.9%) | 173(49.7%) | 175(50.3%) | ||||
| Chemotherapy | 0.917 | 0.02 | 0.706 | 0.921 | ||||||||||
| Yes | 354 | 151(42.7%) | 203(57.3%) | 209(59.0%) | 145(41.0%) | 261 | 109(41.8%) | 152(58.2%) | 129(49.4%) | 132(50.6%) | ||||
| No | 166 | 70(42.2%) | 96(57.8%) | 80(48.2%) | 86(51.8%) | 106 | 42(39.6%) | 64(60.4%) | 53(50.0%) | 53(50.0%) | ||||
| Radiotherapy | 0.447 | 0.325 | 0.495 | 0.261 | ||||||||||
| Yes | 24 | 12(50%) | 12(50%) | 11(45.8%) | 13(54.2%) | 7 | 2(28.6%) | 5(71.4%) | 2(28.6%) | 5(71.4%) | ||||
| No | 496 | 209(42.1%) | 287(57.9%) | 278(56.0%) | 218(44.0%) | 360 | 149(41.4%) | 211(58.6%) | 180(50.0%) | 180(50.0%) | ||||
| Lymphocyte (×109/L) | 0.175 | 0.04 | 0.167 | 0.327 | ||||||||||
| ≤1.58 | 258 | 102(39.5%) | 156(60.5%) | 158(60.1%) | 103(39.9%) | 164 | 61(37.2%) | 103(62.8%) | 86(52.4%) | 78(47.6%) | ||||
| >1.58 | 262 | 119(45.4%) | 143(54.6%) | 134(51.1%) | 128(48.9%) | 203 | 90(44.3%) | 113(55.7%) | 96(47.3%) | 107(52.7%) | ||||
| PLT (×109/L) | <0.001 | <0.001 | <0.001 | <0.001 | ||||||||||
| ≤263 | 261 | 132(50.6%) | 129(49.4%) | 169(64.8%) | 92(35.2%) | 179 | 100(55.9%) | 79(44.1%) | 119(66.5%) | 60(33.5%) | ||||
| >263 | 259 | 89(34.4%) | 170(65.6%) | 120(46.3%) | 139(53.7%) | 188 | 51(27.1%) | 137(72.9 | 63(33.5%) | 125(66.5%) | ||||
| NLR | <0.001 | <0.001 | <0.001 | <0.001 | ||||||||||
| ≤2.70 | 180 | 103(57.2%) | 77(42.8%) | 157(87.2%) | 23(12.8%) | 139 | 95(68.3%) | 44(31.7%) | 117(84.2%) | 22(15.8%) | ||||
| >2.70 | 340 | 118(34.7%) | 222(65.3%) | 132(38.8%) | 208(61.2%) | 228 | 56(24.6%) | 172(75.4%) | 65(28.5%) | 163(71.5%) | ||||
| PLR | <0.001 | 0.052 | <0.001 | 0.001 | ||||||||||
| ≤232 | 397 | 190(47.9%) | 207(52.1%) | 230(57.9%) | 167(42.1%) | 265 | 130(49.1%) | 135(50.9%) | 146(55.1%) | 119(44.9%) | ||||
| >232 | 123 | 31(25.2%) | 92(74.8%) | 59(48.0%) | 64(52.0%) | 102 | ||||||||
| 21(20.6%) | 81(79.4%) | 36(35.3%) | 66(64.7%) | |||||||||||
| C-reactive protein(mg/L) | <0.001 | <0.001 | <0.001 | <0.001 | ||||||||||
| ≤6.33 | 221 | 218(98.6%) | 3(1.4%) | 152(68.8%) | 69(32.1%) | 153 | 151(98.7%) | 2(1.3%) | 106(69.3%) | 47(30.7%) | ||||
| >6.33 | 299 | 3(1.0%) | 296(99.0%) | 137(45.8%) | 162(54.2%) | 214 | 0(0%) | 214(100%) | 76(35.5%) | 138(64.5%) | ||||
| Albumin(g/L) | <0.001 | <0.001 | <0.001 | 0.001 | ||||||||||
| ≤40.0 | 252 | 50(19.8%) | 202(80.2%) | 175(65.3%) | 93(34.7%) | 207 | 43(20.8%) | 164(79.2%) | 87(42.0%) | 120(58.0%) | ||||
| >40.0 | 268 | 171(63.8%) | 97(36.2%) | 114(45.2%) | 138(54.8%) | 160 | 108(67.5%) | 52(32.5%) | 95(59.4%) | 65(40.6%) | ||||
a Using Chi-squared test, p < 0.05 was considered statistically significant. b Surgery, thoracoscopic pleural biopsy. CAR, C-reactive protein -to- albumin ratio; NEU, neutrophil; PLT, platelet; NLR, neutrophil-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio.
Univariate Analysis of the OS in the Primary Cohorts
In the univariate analysis, the CAR and NEU levels were found to be associated with OS (P < 0.001) in patients with advanced NSCLC, along with other variables, such as age (P = 0.04), gender (P = 0.026), clinical stage (P = 0.022), radiotherapy (P = 0.013), NLR (P < 0.001), PLR (P = 0.003), CRP (P < 0.001), and ALB (P < 0.001) (Table 3). Moreover, multivariate analyses using the Cox proportional hazard model showed that age (HR = 1.398, 95% CI: 1.048–1.866, P = 0.023), clinical stage (HR = 1.804, 95% CI: 1.227–2.653, P = 0.003), CAR (HR = 2.791, 95% CI: 1.966–3.961, P < 0.001), and NEU (HR = 1.555, 95% CI: 1.130–2.139, P = 0.007) were independent prognostic factors of OS in patients with advanced NSCLC.
Table 3.
Univariate and Multivariate COX Regression Analyses for Overall Survival in Patients With Non-Small Cell Lung Cancer.
| Variables | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| HR | CI | P | HR | CI | P a | |
| Age | 1.341 | 1.012-1.778 | 0.04 | 1.398 | 1.048-1.866 | 0.023 |
| Gender | 1.431 | 1.034-1.980 | 0.026 | 1.348 | 0.964-1.883 | 0.081 |
| Histology type | 0.792 | 0.585-1.071 | 0.135 | - | - | - |
| Clinical stage | 1.518 | 1.045-2.206 | 0.022 | 1.804 | 1.227-2.652 | 0.003 |
| Surgeryb | 1.587 | 0.903-2.789 | 0.132 | - | - | - |
| Chemotherapy | 0.79 | 0.597-1.047 | 0.104 | - | - | - |
| Radiotherapy | 0.348 | 0.129-0.939 | 0.013 | 0.393 | 0.144-1.071 | 0.068 |
| NEU | 1.965 | 1.490-2.592 | ≤ 0.001 | 1.555 | 1.130-2.139 | 0.007 |
| Lymphocyte | 0.965 | 0.733-1.269 | 0.797 | - | - | - |
| NLR | 1.906 | 1.382-2.628 | ≤ 0.001 | 1.247 | 0.858-1.812 | 0.247 |
| PLT | 1.31 | 0.995-1.726 | 0.054 | - | - | - |
| PLR | 1.608 | 1.191-2.171 | 0.003 | 1.2 | 0.872-1.651 | 0.263 |
| C-reaction protein | 3.218 | 2.353-4.400 | ≤ 0.001 | 1.979 | 0.298-13.167 | 0.48 |
| Albumin | 1.781 | 1.350-2.351 | ≤ 0.001 | 1.081 | 0.797-1.465 | 0.618 |
| CAR | 3.214 | 2.351-4.394 | ≤ 0.001 | 2.791 | 1.966-3.961 | <0.001 |
a P < 0.05 was considered statistically significant. b Surgery, thoracoscopic pleural biopsy. CI = confidence interval; HR = hazard ratio. CAR, C-reaction protein -to- albumin ratio; NEU, neutrophil; PLT, platelet; NLR, neutrophil-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio.
Prognostic Nomogram Model for OS
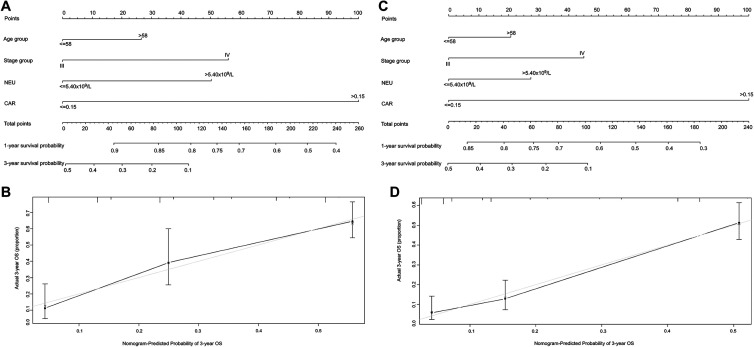
According to the Cox proportional hazard model, variables, such as age > 58, clinical stage IV, CAR ≥ 0.15, and NEU > 5.4×109/L, were poor prognostic factors for OS. Thus, age, clinical stage, CAR, and NEU were included in the nomogram (Figure 1). The predictive accuracies for OS in patients with NSCLC between the nomogram model and conventional TNM staging systems were compared by calculating the Harrell’s C-index (Table 4). In the primary cohort, the nomogram model achieved a C-index of 0.711 (95% CI, 0.675–0.747) (Figure 1A), which was significantly higher than that achieved for the TNM staging system (0.531, 95% CI, 0.488–0.574, P < 0.01). This result was also confirmed in the validation cohort. The C-index of the nomogram model (0.703, 95% CI: 0.671–0.735) (Figure 1C) was higher than that of the TNM staging system (0.582, 95% CI: 0.545–0.619, P < 0.01). Calibration curves for the probability of survival at 3-years showed optimal agreement between the prediction established in the 2 nomograms and the actual observation (Figure 1B, 1D).
Figure 1.
Nomogram showing results of the prognostic model using age, clinical stage, CAR, and NEU characteristics predicting OS in the primary cohort (A). Nomogram model predicting the 1- and 3-year OS of patients with NSCLC in the validation cohort (C). Calibration curves predicting patient OS at 3 years in the primary cohort (B) and validation cohort (D). Total points projected on the bottom scales indicate the probability of 1- and 3-year survival.
Table 4.
The C-index of Nomogram Model and TNM Stage for Prediction of OS in the Primary Cohort and Validation Cohort.
| Variables | Primary cohort | Validation cohort | ||
|---|---|---|---|---|
| C-index(95%CI) | P | C-index(95%CI) | P | |
| Nomogram Model | 0.711(0.675-0.747) | 0.703(0.671-0.735) | ||
| TNM stage | 0.531(0.488-0.574) | 0.582(0.545-0.619) | ||
| Nomogram Model vs TNM stage | < 0.01 | < 0.01 | ||
* Nomogram Model: including 4 risk factors (age, stage, CAR, NEU). C-index = concordance index; CI = confidence interval. P < 0.05 was considered statistically significant.
Decision Curve Analysis for 3-Year Survival Predictions
The results of the decision curve analysis in the primary and validation cohorts at 3 years is presented in Figure 2. Compared to the traditional TNM staging system, the 2 nomogram models that were established had higher overall net benefits than the traditional TNM staging systems across a wide range of threshold probabilities.
Figure 2.
Decision curve analysis for 3-year survival predictions in the primary cohort (A) and validation cohort (B). In the decision-curve analysis, the y-axis indicates net benefit. The straight line represents the assumption that all patients will die, while the horizontal line represents the assumption that no patients will die.
Performance of the Nomogram Model in Stratifying Risk
Based on the prediction of the nomograms, patients in the primary cohort were divided into three groups, namely, low-risk (score: 0–198), intermediate-risk (score: 198–286), and high-risk groups (score: ≥ 286) (Table 5). Results suggested that patients with higher scores corresponded to worse prognoses. In the 2 cohorts, the survival probabilities for 1, 2, and 3 years in the low-risk group were 67.07%, 24.45%, 1.16%, and 70.50%, 21.65%, 7.91%, respectively. The survival probabilities of the 2 cohorts in the intermediate-risk group were 53.30%, 5.29%, 0%, and 38.66%, 7.56%, 0% for 1, 2, and 3 years, respectively. The survival probabilities of the 2 cohorts in the high-risk group were 28.33%, 1.68%, 0%, and 26.61%, 2.75%, 0% for 1, 2, and 3 years, respectively. Patients with advanced NSCLC were divided into different risk subgroups after applying the cutoff values. Kaplan-Meier curves showed that these subgroups were significantly associated with OS outcomes in stage III and stage IV in the primary (P < 0.001, P = 0.001) and validation cohorts (P = 0.003, P < 0.001) (Figure 3).
Table 5.
Point Assignment and Prognostic Score of the Nomogram Model.
| Variable and prognostic score | Score | Primary cohort | Validation cohort | Estimated 1-year OS (%) | Estimated 2-Year OS (%) | Estimated 3-year OS (%) |
|---|---|---|---|---|---|---|
| Age group points | ||||||
| ≤58 | 50 | 44 | 50 | |||
| >58 | 71 | 70 | 71 | |||
| Stage group | ||||||
| III | 23 | 15 | 23 | |||
| IV | 68 | 71 | 68 | |||
| CAR group points | ||||||
| ≤0.15 | 0 | 0 | 0 | |||
| >0.15 | 100 | 100 | 100 | |||
| NEU(×109/L) | ||||||
| ≤5.40 | 45 | 35 | 45 | |||
| >5.40 | 72 | 85 | 72 | |||
| Primary cohort | 0-198 | 67.05 | 24.45 | 1.16 | ||
| 198-286 | 53.30 | 5.29 | 0 | |||
| ≥ 286 | 28.33 | 1.68 | 0 | |||
| Validation cohort | 0-198 | 70.50 | 21.65 | 7.91 | ||
| 198-286 | 38.66 | 7.56 | 0 | |||
| ≥ 286 | 26.61 | 2.75 | 0 |
Figure 3.
Risk group stratification within III and IV stage of patients with NSCLC in the primary and validation cohorts. Kaplan-Meier curves of OS according to the score predicted OS shown in the nomogram.
Discussion
Lung cancers are aggressive, have a high incidence globally, and are associated with mortality.2 Patients in similar stages of NSCLC may have different outcomes; additionally, the survival of individual patients is remarkably heterogeneous.23 In most patients with stage III-IV lung cancer, chemotherapy, radiotherapy, and targeted therapy are the available treatment options.24 An important factor influencing treatment decisions is the expected prognosis; however, clinicians are often inaccurate in their survival predictions.25 Therefore, other factors are needed to assess prognosis in patients. Our study aimed to compare the relative prognostic values of existing and routinely available inflammatory variables in patients with NSCLC. Our study investigated these parameters in patients with advanced NSCLC undergoing chemoradiotherapy or chemotherapy in an attempt to clarify the optimal use of these biomarkers and enable prompt and easy evaluation with regard to cancer prognosis.
To the best of our knowledge, the present study represents the first and single largest advanced NSCLC cohort to investigate and compare the prognostic value of a wide set of circulating biomarkers of inflammatory response. The biochemical “cross-talk” between inflammatory cells and the growing neoplastic clone is of great pathogenic and prognostic importance.26 On a general level, markers derived from the blood are representative of inflammation that occurs both at local and systemic levels during cancer, which may be useful in studying the association between inflammation and carcinogenesis. Furthermore, determining the levels of these markers is a relatively inexpensive process. They are routinely measured in daily clinical practice and readily provide objective information to help medical practitioners estimate patient prognosis.In this study, we established a nomogram based on CAR, NEU, and clinical characteristics to predict the survival of patients with advanced NSCLC. Univariable analysis showed that age, gender, clinical stage, radiotherapy, NEU, NLR, PLR, CRP, ALB, and CAR are associated with the OS of patients with advanced NSCLC. Using multivariable analysis, we identified age, clinical stage, CAR, and NEU as independent prognostic factors in patients with advanced NSCLC. Subsequently, we established an effective predictive nomogram model for these patients, which included age, clinical stage, CAR, and NEU. The C-index of our model predicted OS with an accuracy of 0.711 (95% CI: 0.675–0.747), which was a significantly better prediction than that of the TNM staging system (0.531, 95% CI: 0.488–0.574) (P < 0.01). Moreover, in the validation cohort, the C-index of the nomogram model (0.703, 95% CI: 0.671–0.735) was higher than that of the TNM staging system (0.582, 95% CI: 0.545–0.619, P < 0.01). Additionally, either the established nomogram or the validated nomogram model had a higher overall net benefit than the TNM staging system at 3 years. Based on our model, patients were divided into 3 risk groups. Each group had a distinct survival outcome, and the high-risk group had the shortest OS among the 3 risk groups. Therefore, the nomogram model is a reliable tool to predict outcomes in patients with advanced NSCLC.
In our nomogram, the CAR and TNM staging system contributed the most in predicting OS in patients with advanced NSCLC. CRP is a sensitive indicator of inflammation and responds quickly to changes in clinical situation.27 CRP levels are indicative of tumor-associated inflammatory responses, which are accompanied by the up-regulation of cytokines and inflammatory mediators, inhibition of apoptosis, induction of angiogenesis, stimulation of DNA damage, immunosuppression, and remodeling of the extracellular matrix, thus promoting tumor growth and metastasis.28 Increased level of CRP has been documented in patients with NSCLC and is associated with poor outcomes.16 Albumin is a negative acute-phase protein because its level reduces during injury and sepsis.29 Fan et al suggested that hypoalbuminemia was associated with worse survival in both operable and inoperable patients without elevated levels of CRP.30 Studies show that albumin levels tend to fall in patients with elevated levels of CRP; this phenomenon is common across different tumor types.31 An abnormal CAR has been previously associated with death in patients with operable NSCLC.18 A recent study also revealed that the CAR is an independent predictor of death in patients with stage IV NSCLC receiving palliative chemotherapy.13 Therefore, the TNM staging system and age are important prognostic factors in patients with lung cancer. Moreover, there is a correlation between poor clinical outcomes and high NEU counts in predicting OS in NSCLC, which has been reported both locally and systemically in patients with NSCLC,8,9 suggesting that NEU and inflammation play important roles in carcinogenesis. Regarding the mechanism, some studies report that NEUs recruited into the tumor stroma exert pro-tumorigenic effects and facilitate tumorigenesis, promote tumor growth and metastasis, stimulate tumor angiogenesis, and mediate immunosuppression.32
Although the nomogram in this study could precisely predict survival in patients with advanced NSCLC, our study has some limitations. Firstly, the study was a retrospective design; therefore, pre-treatment blood tests could not be performed at a defined baseline time point. Thus, the prognostic significance of systematic inflammatory biomarkers in patients with NSCLC remains to be confirmed using prospective and clinical validation studies in the future. Secondly, ours was a single-center study. The data utilized in the study were collected from a single institution; thus, clinical and survival comparison might be influenced by selection bias owing to the differences in patient populations. Therefore, our results need to be further verified using multi-center studies, which would help validate our nomogram model. Despite these limitations, the established nomogram is an effective tool to predict the OS in patients with advanced NSCLC and could potentially help clinicians make individualized treatment decisions.
Conclusion
We established and validated a nomogram model factoring age, clinical stage, CAR, and NEU for predicting survival in patients with advanced NSCLC. It shows a better level of prediction ability than that of the traditional TNM staging system. Our model is a simple, precise, and easy-to-use scoring system, which could help clinicians estimate the survival of patients with advanced NSCLC.
List of abbreviations
- NSCLC
non-small cell lung cancer
- CRP
C-reaction protein
- ALB
albumin
- TNM
tumor-node-metastasis
- NEU
neutrophils
- NLR
neutrophil-lymphocyte ratio
- PLR
platelet-to-lymphocyte ratio
- CAR
CRP-to-ALB ratio
- PLT
platelet
- OS
overall survival
- HRs
hazard ratios
Footnotes
Authors’ Note: NX and STX considered and designed the study. ZLH and SX collected the data and conducted the statistics. YYZ and other authors help to collected the data. All authors participated in the reviewed of the manuscript and approved the final manuscript. The only record of contacting subjects for identification and research is the informed consent document, signed informed consent poses an undue threat to the subject’s privacy. This study is based on retrospective analysis, exemption from ethical approval and informed consent will not adversely affect the subject’s rights and health. Therefore, this study is exempt from ethical approval and informed consent.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by a grant by the Science and technology project of Xiamen City (NO. 3502Z20184004), the Nosocomial project of Xiamen Branch, Zhongshan Hospital, Fudan University (No. 2019ZSXMYS02), Science and technology project of Henan Province (NO. LHGJ20190637).
ORCID iD: Ning Xue,  https://orcid.org/0000-0003-4748-851X
https://orcid.org/0000-0003-4748-851X
References
- 1. Chen W. Cancer statistics: updated cancer burden in China. Chin J Cancer Res. 2015;27(1):1 doi:10.3978/j.issn.1000-9604.2015.02.07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017;67(1):7–30. doi:10.3322/caac.21387 [DOI] [PubMed] [Google Scholar]
- 3. Arrieta O, Guzman-de Alba E, Alba-Lopez LF, et al. National consensus of diagnosis and treatment of non-small cell lung cancer [in Spanish]. Rev Invest Clin. 2013;65(suppl 1):S5–84. Consenso nacional de diagnostico y tratamiento del cancer de pulmon de celulas no pequenas. [PubMed] [Google Scholar]
- 4. Detterbeck FC, Postmus PE, Tanoue LT. The stage classification of lung cancer: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143(5 suppl):e191S–e210S. doi:10.1378/chest.12-2354 [DOI] [PubMed] [Google Scholar]
- 5. Wang Y, Li J, Xia Y, et al. Prognostic nomogram for intrahepatic cholangiocarcinoma after partial hepatectomy. J Clin Oncol. 2013;31(9):1188–1195. doi:10.1200/JCO.2012.41.5984 [DOI] [PubMed] [Google Scholar]
- 6. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi:10.1016/j.cell.2011.02.013 [DOI] [PubMed] [Google Scholar]
- 7. Liu J, Pang Z, Wang G, et al. Advanced role of neutrophils in common respiratory diseases. J Immunol Res. 2017;2017:6710278 doi:10.1155/2017/6710278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ilie M, Hofman V, Ortholan C, et al. Predictive clinical outcome of the intratumoral CD66b-positive neutrophil-to-CD8-positive T-cell ratio in patients with resectable nonsmall cell lung cancer. Cancer. 2012;118(6):1726–1737. doi:10.1002/cncr.26456 [DOI] [PubMed] [Google Scholar]
- 9. Carus A, Gurney H, Gebski V, et al. Impact of baseline and nadir neutrophil index in non-small cell lung cancer and ovarian cancer patients: assessment of chemotherapy for resolution of unfavourable neutrophilia. J Transl Med. 2013;11:189 doi:10.1186/1479-5876-11-189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cedres S, Torrejon D, Martinez A, et al. Neutrophil to lymphocyte ratio (NLR) as an indicator of poor prognosis in stage IV non-small cell lung cancer. Clin Transl Oncol. 2012;14(11):864–869. doi:10.1007/s12094-012-0872-5 [DOI] [PubMed] [Google Scholar]
- 11. Takahashi Y, Horio H, Hato T, et al. Prognostic significance of preoperative neutrophil-lymphocyte ratios in patients with stage I non-small cell lung cancer after complete resection. Ann Surg Oncol. 2015;22 Suppl 3:S1324–S1331. doi:10.1245/s10434-015-4735-5 [DOI] [PubMed] [Google Scholar]
- 12. Unal D, Eroglu C, Kurtul N, Oguz A, Tasdemir A. Are neutrophil/lymphocyte and platelet/lymphocyte rates in patients with non-small cell lung cancer associated with treatment response and prognosis? Asian Pac J Cancer Prev. 2013;14(9):5237–5242. [DOI] [PubMed] [Google Scholar]
- 13. Kos M, Hocazade C, Kos FT, et al. Prognostic role of pretreatment platelet/lymphocyte ratio in patients with non-small cell lung cancer. Wien Klin Wochenschr. 2016;128(17-18):635–640. doi:10.1007/s00508-015-0724-8 [DOI] [PubMed] [Google Scholar]
- 14. Li Y, Jia H, Yu W, et al. Nomograms for predicting prognostic value of inflammatory biomarkers in colorectal cancer patients after radical resection. Int J Cancer. 2016;139(1):220–231. doi:10.1002/ijc.30071 [DOI] [PubMed] [Google Scholar]
- 15. Heikkila K, Ebrahim S, Lawlor DA. A systematic review of the association between circulating concentrations of C reactive protein and cancer. J Epidemiol Community Health. 2007;61(9):824–833. doi:10.1136/jech.2006.051292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zeng Q, Xue N, Dai D, et al. A nomogram based on inflammatory factors C-reactive protein and fibrinogen to predict the prognostic value in patients with resected non-small cell lung cancer. J Cancer. 2017;8(5):744–753. doi:10.7150/jca.17423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Liu Z, Jin K, Guo M, et al. Prognostic value of the CRP/Alb ratio, a novel inflammation-based score in pancreatic cancer. Ann Surg Oncol. 2017;24(2):561–568. doi:10.1245/s10434-016-5579-3 [DOI] [PubMed] [Google Scholar]
- 18. Zhang Y, Zhou GQ, Liu X, et al. Exploration and validation of c-reactive protein/albumin ratio as a novel inflammation-based prognostic marker in nasopharyngeal carcinoma. J Cancer. 2016;7(11):1406–1412. doi:10.7150/jca.15401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhang F, Ying L, Jin J, et al. The C-reactive protein/albumin ratio predicts long-term outcomes of patients with operable non-small cell lung cancer. Oncotarget. 2017;8(5):8835–8842. doi:10.18632/oncotarget.13053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wen J, Yang Y, Liu P, et al. Development and validation of a nomogram for predicting survival on the base of modified lymph node ratio in breast cancer patients. Breast. 2017;33:14–22. doi:10.1016/j.breast.2017.01.017 [DOI] [PubMed] [Google Scholar]
- 21. Liao Y, Wang X, Zhong P, Yin GF, Fan XM, Huang CL. A nomogram for the prediction of overall survival in patients with stage II and III non-small cell lung cancer using a population-based study. Oncol Lett. 2019;18(6):5905–5916. doi:10.3892/ol.2019.10977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Liao Y, Fan XM, Wang X. Effects of different metastasis patterns, surgery and other factors on the prognosis of patients with stage IV non-small cell lung cancer: a Surveillance, Epidemiology, and End Results (SEER) linked database analysis. Oncol Lett. 2019;18(1):581–592. doi:10.3892/ol.2019.10373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nesbitt JC, Putnam JB, Jr, Walsh GL, Roth JA, Mountain CF. Survival in early-stage non-small cell lung cancer. Ann Thorac Surg. 1995;60(2):466–472. [DOI] [PubMed] [Google Scholar]
- 24. Hagerty RG, Butow PN, Ellis PM, et al. Communicating with realism and hope: incurable cancer patients’ views on the disclosure of prognosis. J Clin Oncol. 2005;23(6):1278–1288. doi:10.1200/JCO.2005.11.138 [DOI] [PubMed] [Google Scholar]
- 25. Glare P, Virik K, Jones M, et al. A systematic review of physicians’ survival predictions in terminally ill cancer patients. BMJ. 2003;327(7408):195–198. doi:10.1136/bmj.327.7408.195 12881260 [Google Scholar]
- 26. Bremnes RM, Al-Shibli K, Donnem T, et al. The role of tumor-infiltrating immune cells and chronic inflammation at the tumor site on cancer development, progression, and prognosis: emphasis on non-small cell lung cancer. J Thorac Oncol. 2011;6(4):824–833. doi:10.1097/JTO.0b013e3182037b76 [DOI] [PubMed] [Google Scholar]
- 27. Harrison M. Erythrocyte sedimentation rate and C-reactive protein. Aust Prescr. 2015;38(3):93–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420(6917):860–867. doi:10.1038/nature01322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sonoda A, Ohnishi S, Nakao S, et al. Factors affecting serum albumin in the perioperative period of colorectal surgery: a retrospective study. BMC Res Notes. 2015;8:638 doi:10.1186/s13104-015-1632-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fan H, Shao ZY, Xiao YY, et al. Comparison of the Glasgow Prognostic Score (GPS) and the modified Glasgow Prognostic Score (mGPS) in evaluating the prognosis of patients with operable and inoperable non-small cell lung cancer. J Cancer Res Clin Oncol. 2016;142(6):1285–1297. doi:10.1007/s00432-015-2113-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. McMillan DC. An inflammation-based prognostic score and its role in the nutrition-based management of patients with cancer. Proc Nutr Soc. 2008;67(3):257–262. doi:10.1017/S0029665108007131 [DOI] [PubMed] [Google Scholar]
- 32. Zhang X, Zhang W, Yuan X, Fu M, Qian H, Xu W. Neutrophils in cancer development and progression: roles, mechanisms, and implications (Review). Int J Oncol. 2016;49(3):857–867. doi:10.3892/ijo.2016.3616 [DOI] [PubMed] [Google Scholar]