Abstract
Benzodiazepine withdrawal symptoms vary from mild anxiety to life-threatening delirium or seizures. In susceptible individuals, such as those with mood disorders, benzodiazepine withdrawal may also precipitate catatonia. A 26-year-old man with schizoaffective disorder (depressed type with catatonia) ran out of lorazepam and presented with catatonia, delirium, and seizures. He was taking olanzapine, venlafaxine, and trazodone for schizoaffective disorder. Lorazepam 2 mg twice daily kept him free of catatonia for 6 months. Besides catatonia and delirium, lorazepam withdrawal also triggered convulsive seizures and nonconvulsive status epilepticus. He was admitted to the intensive care unit where he underwent continuous video-EEG monitoring. Catatonia resolved with lorazepam on day 2. Seizures stopped with levetiracetam, lacosamide, and propofol on day 4. His mental status was normal when he was discharged on day 6. If not immediately recognized and treated, catatonia and delirium can lead to significant morbidity or mortality. Unfortunately, physicians tend to overlook catatonia and delirium, especially if both syndromes are present. At first, we suspected that our patient had ictal catatonia, but video-EEG showed no clear-cut correlation between catatonia, seizures, and epileptiform activity. As with prior observations, the patient’s catatonia was more sensitive to benzodiazepine withdrawal and treatment than his seizures. The efficacy of benzodiazepines in aborting catatonia, seizures, and mixed delirium-catatonia syndromes suggests a key pathogenetic role of abnormal GABA neurotransmission in these brain disorders.
Keywords: catatonia, delirium, seizure, lorazepam, benzodiazepine withdrawal, EEG
Introduction
The Diagnostic and Statistical Manual of Mental Disorders (DSM)—from the first release (DSM-I) to the latest edition (DSM-5)—is the ultimate guidebook for identifying and diagnosing chronic psychiatric disorders and acute neuropsychiatric syndromes.1,2 A major advantage of the DSM is it serves both researchers and clinicians. However, serving 2 “masters” may result in conflict—researchers need detailed and accurate diagnoses, whereas clinicians prefer simpler, shorter, and more practical diagnostic criteria.3 Health care providers must be familiar with the neuropsychiatric syndromes of catatonia and delirium. Both syndromes require early recognition, etiological diagnosis, and timely medical intervention. Unfortunately, both syndromes remain underrecognized and underdiagnosed in the acute care setting.4,5 Table 1 summarizes the current DSM-5 diagnostic criteria for catatonia and delirium.2
Table 1.
The Diagnostic Criteria for Delirium and Catatonia as Listed in the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition.2
| Catatonia associated with another mental disordera (catatonia specifier) |
| A. The clinical picture is dominated by 3 (or more) of the
following symptoms: 1. Stupor (ie, no psychomotor activity; not actively relating to environment) 2. Catalepsy (ie, passive induction of a posture held against gravity) 3. Waxy flexibility (ie, slight, even resistance to positioning by examiner) 4. Mutism (ie, no, or very little, verbal response [not applicable if there is an established aphasia]) 5. Negativism (ie, opposition or no response to instructions or external stimuli) 6. Posturing (ie, spontaneous and active maintenance of a posture against gravity) 7. Mannerism (ie, odd, circumstantial caricature of normal actions) 8. Stereotypy (ie, repetitive, abnormally frequent, non-goal-directed movements) 9. Agitation, not influenced by external stimuli 10. Grimacing 11. Echolalia (ie, mimicking another’s speech) 12. Echopraxia (ie, mimicking another’s movements) |
| Catatonia due to another medical condition |
| A. The same Criterion A for catatonia associated with another
mental disorder (see above) B. There is evidence from the history, physical examination, or laboratory findings that the disturbance is the direct pathophysiological consequence of another medical condition. C. The disturbance is not better explained by another mental disorder (eg, a manic episode). D. The disturbance does not occur exclusively during the course of a delirium. E. The disturbance causes clinically significant distress or impairment in social, occupational, or other important areas of functioning. |
| Delirium |
| A. A disturbance in attention (ie, reduced ability to direct,
focus, sustain, and shift attention) and awareness (reduced
orientation to the environment). B. The disturbance develops over a short period of time (usually hours to a few days) represents a change from baseline attention and awareness, and tends to fluctuate in severity during the course of a day. C. An additional disturbance in cognition (eg, memory deficit, disorientation, language, visuospatial ability, or perception). D. The disturbances in Criteria A and C are not better explained by another preexisting, established, or evolving neurocognitive disorder and do not occur in the context of a severely reduced level of arousal, such as coma. E. There is evidence from the history, physical examination, or laboratory findings that the disturbance is a direct physiological consequence of another medical condition, substance intoxication, or withdrawal (ie, due to a drug of abuse or to a medication), or exposure to a toxin, or is due to multiple etiologies. |
Must meet diagnostic criteria for the specific mental disorder.
Catatonia was originally described by Kahlbaum in 1874 as a syndrome consisting of catalepsy, waxy flexibility, muscle rigidity, mutism, negativism, and autonomic disturbance.6 He also realized that catatonia can be associated, not only with psychiatric disorders, but also with infection, alcoholism, epilepsy, and other medical illnesses. Subsequently, Kraepelin popularized the view of catatonia as a form of schizophrenia.7 This view was adapted in the first 4 editions of the DSM and expanded to include other psychiatric disorders in DSM-IV.8 The original concept of catatonia as a syndrome that is not specific to psychiatric disorders was restored in the DSM-5.2 As much as 9% to 17% of patients in psychiatric facilities and emergency departments meet the diagnostic criteria for catatonia.9 Despite its high incidence, catatonia remains clinically underdiagnosed. A study found that clinicians identified catatonia in only 2% of patients, whereas researchers detected catatonia in 18%.10 The latter used the Bush-Francis Catatonia Rating Scale to detect the presence of catatonia.11 The enduring view of catatonia as a schizophrenia phenotype is one reason this syndrome remains underdiagnosed.12 Catatonia is actually more commonly associated with mood disorders than with schizophrenia.8,9 Medical disorders and drug treatment or withdrawal are also common precipitants of catatonia.8,9,12
Delirium (Latin for derailment) was first used as a medical term by Celsus in the first century AD to describe the “derailment of the mind” during fever or head trauma.13 Historically, various terms and definitions have been associated with the concept of delirium.14 It was not until DSM-III that delirium became a unifying concept for various cognitive disorders due to medical illness and intoxication.15 Impaired consciousness has always been central to the concept of delirium, but in the course of time, the emphasis has shifted from level of consciousness (arousal) to content of consciousness (attention).16 In the DSM-5, delirium was defined exclusively in terms of attention and cognitive features; the element of arousal, which was explicit or implicit in previous DSM editions, was finally dropped.2 Several studies showed that delirium continues to be underdiagnosed.17-21 Delirium can be overlooked because of its variable presentation, waxing and waning course, and overlap with other psychiatric disorders.18 Delirium is especially difficult to recognize in its early stages, or when it is expressed in the hypoactive form.19,20 Many physicians are not aware of the diagnostic criteria and bedside screening tools for delirium.20,21 For example, few physicians use the Confusion Assessment Method, a validated bedside tool for detecting delirium.22
Benzodiazepine withdrawal can trigger delirium and generalized tonic-clonic seizure.23 Although rare, it can also give rise to nonconvulsive status epilepticus (NCSE).24 We present a patient with schizoaffective disorder who presented with benzodiazepine withdrawal delirium, catatonia, and 3 types of seizures: generalized tonic-clonic seizure, focal clonic seizures, and NCSE.
Case Presentation
A 26-year-old man with a 2-year history of schizoaffective disorder (depressed type with catatonia) presented with catatonia, delirium, and seizures. He was taking olanzapine 10 mg, venlafaxine 300 mg, and trazodone 100 mg on a daily basis for at least a year. About a year prior to admission, he fell at work and sustained a concussion, but he was never diagnosed with seizures/epilepsy. He had several hospital visits for catatonia in the past. During his last visit, he was prescribed lorazepam 2 mg bid, which rendered him free of catatonia for 6 months. He ran out of lorazepam 10 days prior to admission. Three days later, he became increasingly withdrawn and less active. After another 3 days, he appeared stiff and assumed abnormal postures. He was brought to the emergency room (ER) after he was found down, face toward the ground, moaning, and soaked with urine. Informants thought he had a convulsion.
On arrival, he was hypotensive (80/50 mm Hg), tachycardic (142-160/min), and diaphoretic, but normothermic (37 °C). Intravenous (IV) fluids increased his blood pressure (132-142/74-90 mm Hg) and decreased his heart rate (118-132/min). He exhibited psychomotor retardation, catalepsy, and waxy flexibility. Response to verbal instructions and noxious stimulation was minimal. He uttered 2 words at most—“hospital” when asked where he was, and “no” when asked if he felt anything unusual. Formal testing of attention and other cognitive function was not done because he did not follow instructions. He kept his eyes closed. When pried open to check his pupils, he shut them tightly. Neurological examination was limited on account of catatonia. Cranial nerve functions were intact. All limbs were rigid and deep tendon reflexes were accentuated (3+). There was bilateral ankle clonus, but Babinski sign was absent. Blood and urine toxicology was negative for substances of abuse, including benzodiazepines. Blood tests were normal, except for elevated creatine kinase (19 370 U/L) and mild hyperammonemia (42 µmol/L). Cerebrospinal fluid analysis was normal, except for mildly increased protein (71.5 mg/dL). Computed tomography of the head and magnetic resonance imaging of the brain were normal. His home medications were put on hold because neuroleptic malignant syndrome (NMS) and toxic serotonin syndrome (TSS) could not be excluded.
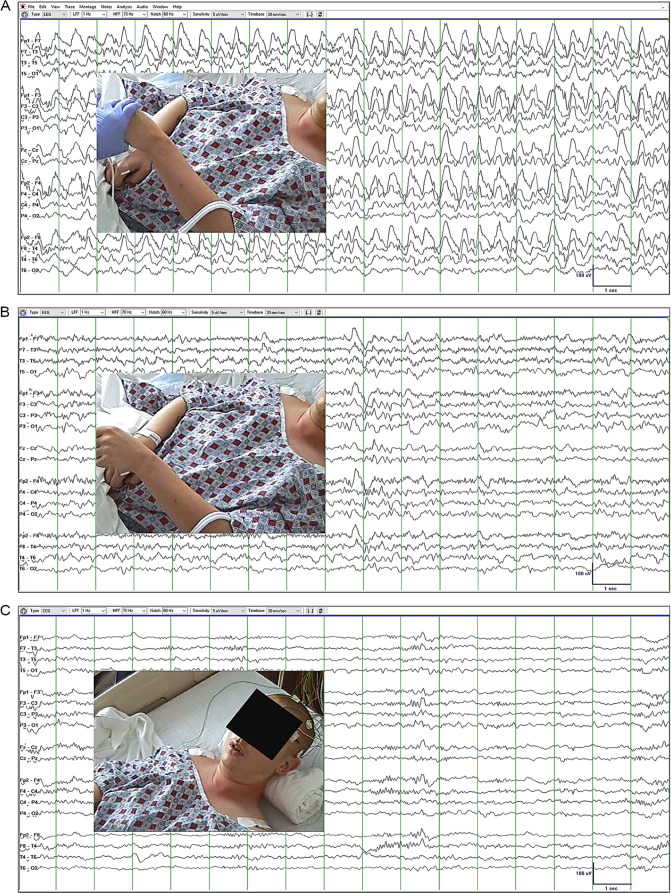
Electroencephalography (EEG) was performed in the ER (Figure 1). During EEG acquisition, the patient maintained a fixed rigid posture with hands fisted, forearms pronated and slightly flexed, and legs and ankles slightly extended. When his arm was raised against gravity, he maintained the posture, consistent with waxy flexibility (Figure 1A and B). EEG showed 25- to 115-second episodes of high-voltage generalized rhythmic delta activity (GRDA) alternating with 12- to 72-second episodes of non-GRDA background activity with low-voltage beta, alpha, and theta rhythms and rare delta waves. Minor fluctuations in GRDA amplitude and frequency was noted with 2.5 Hz as average frequency. Lorazepam 2 mg was injected IV, and within 10 minutes, GRDA was replaced by low-voltage semirhythmic theta and intermittent rhythmic beta activity (Figure 1C). Moreover, catalepsy and waxy flexibility vanished and the patient opened his eyes, answered simple questions (eg, full name, location) and followed simple commands (count 1-10, close your eyes). Altogether, these findings convinced us that the patient was in NCSE (this is explained in greater detail under Discussion).
Figure 1.
Electroencephalography (EEG) recorded in the emergency room. Catatonia did not correlate with EEG findings. (A) Waxy flexibility (posture maintained after left arm was raised) was detected, while high-voltage generalized rhythmic delta activity (GRDA) was present in the EEG. (B) Waxy flexibility was also detected in the absence of GRDA with the EEG showing low-voltage beta, alpha, and theta rhythms with rare delta waves. (C) Waxy flexibility and GRDA resolved completely and the patient started answering simple questions within 10 minutes of lorazepam injection.
In the ER, the patient also had 10- to 20-second episodes of rhythmic right leg jerking, consistent with focal clonic seizures. Several episodes were witnessed by the medical staff, but all occurred after the EEG was recorded. Levetiracetam 4000 mg IV was loaded followed by 1500 mg IV q12h. Catatonia recurred prompting administration of lorazepam 2 mg IV q8h PRN. Catatonia resolved after he received a total of 8 mg of lorazepam. Focal clonic seizures still occurred but were less frequent. Because of fluctuations in mental status, with periods of deep somnolence and stupor, the patient was intubated for airway protection. Propofol IV infusion was started at 5 µg/kg/min. He was admitted to the intensive care unit where continuous video-EEG monitoring was performed over 3 days.
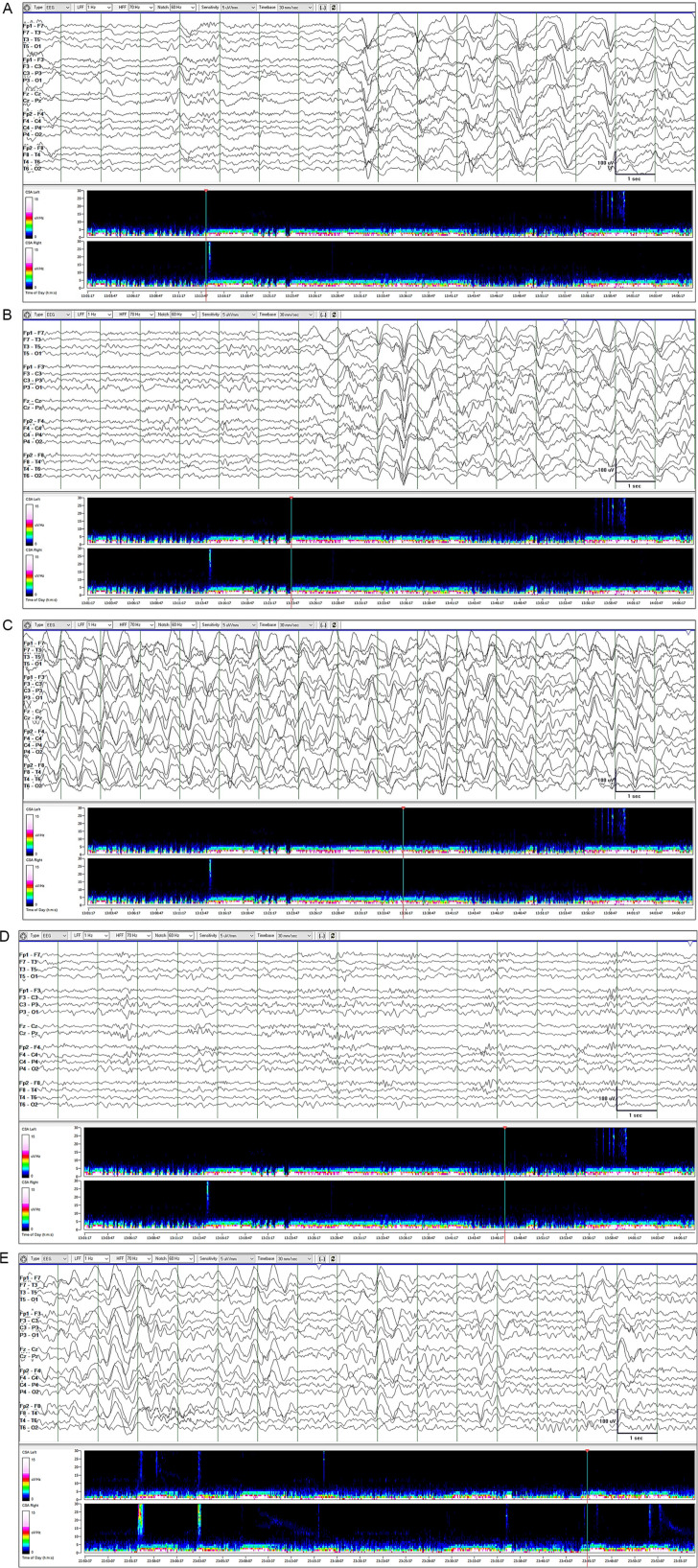
Continuous EEG monitoring with video and compressed spectral array trending initially showed long periods of GRDA (2-42 minutes) punctuated by shorter non-GRDA periods (0.5-11 minutes; Figure 2). As a rule, GRDA was not associated with any clinical change (Figure 2A). However, the onset of a few GRDA episodes coincided with right lower limb jerking or focal motor seizure (Figure 2B). Even though GRDA persisted for several minutes, limb jerking (when present) occurred only within the first 10 to 20 seconds of GRDA. There was no change in the EEG, such as appearance of focal epileptiform discharges, during leg jerking. The same GRDA was seen whether the patient was not moving or his leg was jerking. GRDA showed some fluctuation in amplitude, sharpness, and frequency from 1.5 to 2.5 Hz (Figure 2A-C). Propofol was gradually titrated toward a maximum rate of 50 µg/kg/min. Lacosamide was also started: 400 mg IV load, then 200 mg q12h. The duration of GRDA and non-GRDA episodes progressively increased and decreased, respectively (Figure 2D). GRDA completely disappeared after 4 days of levetiracetam, lacosamide, and propofol therapy. Propofol was tapered and discontinued on day 4. There was no recurrence of GRDA, seizures, and catatonia. Serum creatine kinase levels showed a downward trend from day 1 (19 370 U/L) to day 6 (617 U/L). On day 6, mental status examination was normal and the patient was discharged on levetiracetam 1500 mg bid and lacosamide 200 mg bid. He was seen in clinic 4 weeks later. He remained free of catatonia symptoms but continued experiencing focal motor seizures of the right leg 3 to 4 times a week. At that point, extended-release valproate 500 mg bid was added to his regimen. We learned via phone call, 4 weeks later, that the focal motor seizures stopped completely.
Figure 2.
Continuous electroencephalography (EEG) monitoring in the intensive care unit. EEG is shown with compressed spectral array trend map. (A) GRDA onset was usually not associated with clinical change. (B) GRDA onset coincided with onset of right lower limb jerking (focal motor seizure) on 5 occasions. (B) GRDA can last as long as 30 minutes with minor fluctuations in amplitude, sharpness, and frequency from 1.5 Hz to 2.5 Hz. When present, right lower limb jerking lasted only 10 to 20 seconds. (D) Non-GRDA epochs were initially shorter than GRDA epochs. (E) GRDA epoch length progressively decreased and non-GRDA epoch length progressively increased with up-titration of antiepileptic drug dosage. GRDA eventually disappeared.
Discussion
Catatonia and delirium are medical emergencies that require immediate recognition and treatment.2 Unfortunately, identifying delirium and catatonia is not always simple, especially if both are present. This was the case with our patient. An intensive care unit–based study found that about a third of patients with delirium have catatonia and patients with more signs of catatonia are more likely to have delirium.25 It has been pointed out that some of the ICSD-5 diagnostic criteria for delirium2 (Table 1) can paradoxically result in the underdiagnosis of delirium.16 For example, diagnosing delirium is forbidden in the presence of an arousal disorder that precludes assessment of attention and cognition. Similar constraints can occur if both catatonia and delirium are present since psychomotor dysfunction can hamper cognitive testing necessary to diagnose delirium. This was most likely the reason for the delayed diagnosis of delirium in our patient. On the other hand, the delayed diagnosis of catatonia in our patient was most likely due to underrecognition of catatonic postures and delayed testing for catatonic features, such as waxy flexibility. It is important to know whether delirium, catatonia, or both are present because different syndromes often require different treatment strategies.25-27 Benzodiazepines can exacerbate delirium, but are often used to treat catatonia. Antipsychotics are often used to treat delirium, but these agents can exacerbate catatonia or precipitate malignant catatonia. Low-dose benzodiazepines can be effective in patients with delirium and catatonia, but not in patients with delirium only.
We explored some reasons why physicians tend to overlook catatonia and delirium. The fact that the same precipitating factor (eg, benzodiazepine withdrawal) can give rise to catatonia and delirium can confuse the physician.28 Besides benzodiazepine withdrawal, antidopaminergic or serotonergic drugs may have been a factor in triggering delirium and catatonia in our patients. The severe form of catatonia, known as malignant catatonia,29 appears to be pathophysiologically related to NMS and TSS.30 Thus, distinguishing these 3 forms of severe catatonia can be challenging.31 Some experts suggested that NMS, TSS, or both, are forms of drug-induced malignant catatonia.32 Drug-induced catatonia is often associated with psychotropic drugs (eg, haloperidol, fluphenazine, risperidone, clozapine), but nonpsychotropic agents (eg, steroids, disulfiram, ciprofloxacin) and substances of abuse (eg, cocaine, phencyclidine, cannabis, LSD, mescaline) may also trigger catatonia.33 Because NMS/TSS could be the reason for catatonia and delirium in our patient, his home medications (olanzapine, venlafaxine, trazodone) were put on hold. Realizing that he ran out of lorazepam 10 days prior to admission, we changed our working diagnosis to lorazepam withdrawal. This is strongly supported by the absence of benzodiazepines in blood and urine samples.
Benzodiazepine withdrawal catatonia has been reported several times in the literature.34-44 Some early reports did not use the term catatonia to describe the motor phenomena.34 Withdrawal catatonia tends to occur after chronic use of benzodiazepine with a typical onset of 3 to 7 days after discontinuation and a duration of 3 to 10 days.35 Most cases of withdrawal catatonia occur after several years of benzodiazepine use, but cases after only 6 months of use (as in our patient) have also been reported.35 Although both delirium and catatonia are often present, pure withdrawal catatonia without delirium can occur if the patient has a predisposition to catatonia, for example, schizophrenia.36 It appears that adults, especially the elderly, have the highest risk of benzodiazepine withdrawal catatonia.37-43 There is, however, a recent report of a 9-year-old child who developed catatonia after midazolam withdrawal.44 Treatment with a benzodiazepine, usually lorazepam, was often successful in suppressing catatonia, even if the dose administered is low. In our patient, catatonia resolved with lorazepam on day 2, focal motor seizures stopped with levetiracetam and low-dose propofol on day 3, NCSE/GRDA disappeared with levetiracetam, lacosamide, and high-dose propofol on day 4, and mental status normalized on day 6.
The occurrence of a seizure during the catatonic period is not simply a matter of coincidence. Kahlbaum described the association between catatonia and seizures in his original monograph.16 In the series of Barnes et al, 4 of 25 patients with catatonia also had generalized seizures/epilepsy.45 Primavera et al found that seizures occurred during the catatonic periods in 4 of 29 patients with acute catatonic syndrome.46 These incidence rates are higher than those of an age-matched group in the general population. In some reports of concurrent catatonia and seizures, catatonia was considered as a seizure semiology itself (ictal catatonia),47-50 but in other reports, catatonia and seizures were viewed as distinct phenomena.51-56 Our patient was clearly predisposed to recurrent catatonia on account of schizoaffective disorder. A positive history of head injury also suggested that he was developing a predisposition for seizures (epilepsy). Although benzodiazepine withdrawal can explain his seizures, an alternative (and equally plausible) view is that his seizures were new-onset seizures due to epilepsy. The fact that he continued having focal seizures a month after his last benzodiazepine dose favors this view. In fact, focal seizures stopped only after valproate was added to levetiracetam and lacosamide during his clinic visit.
Did our patient have ictal catatonia? At first, we thought that video-EEG data would easily answer this question. We were wrong. Catatonia resolved completely on day 2 and the only pre-intubation video-EEG record available was a 30-minute record prior to lorazepam injection and a 20-minute record after lorazepam injection. Accurate clinical assessment of catatonia, delirium, and status epilepticus was no longer possible after intubation and propofol infusion. Moreover, the patient’s EEG did not unequivocally show “ictal” patterns, such as electrographic seizures with typical spatiotemporal evolution. Is it reasonable (at least in this particular case) to interpret GRDA as an EEG expression of an underlying ictal activity? A recent study of periodic and rhythmic EEG patterns in critically ill patients showed that GRDA is not associated with increased seizure risk.57 However, GRDA was detected in our patient prior to intubation and propofol infusion, at a time when he was neither comatose nor critically ill. More important, criteria A4b of the Modified Salzburg Consensus Criteria for NCSE was fulfilled in our patient—rhythmic activity >0.5 Hz with fluctuation (1.5-2.5 Hz GRDA in our patient), lasting ≥10 seconds (25-105 seconds in our patient), and reactivity to antiepileptic drug within 10 minutes after IV administration (clinical improvement and complete resolution of GRDA within 10 minutes of lorazepam injection in our patient).58 It is therefore not unreasonable to assume that GRDA reflects ongoing ictal activity, at least in our patient. With this premise in mind, we examined the relationship between catatonic symptoms (catalepsy and waxy flexibility) and the appearance of GRDA in the EEG prior to the administration of lorazepam (Ativan challenge). GRDA periods lasted 25 to 115 seconds and alternated with non-GRDA periods that lasted 12 to 72 seconds. Before lorazepam was given, we did not detect any gross fluctuation in catalepsy and waxy flexibility between GRDA and non-GRDA periods. There was complete resolution of GRDA, waxy flexibility, and catalepsy (patient started responding) within 10 minutes of lorazepam injection. In summary, although we found no evidence to support ictal catatonia, we cannot exclude the possibility that our patient had ictal catatonia.
Catatonia is a syndrome of impaired psychomotor function, meaning neural processes involved in planning, initiating, executing, or modulating volitional movements are not working properly.59 In reality, the neural mechanisms of catatonia are not yet fully understood.8,60 Fink and Taylor proposed a seizure-like model of catatonia to explain the overlap between seizures and catatonia, the higher risk of seizures in patients with catatonia, and the efficacy of anti-seizure drugs (notably benzodiazepines) and electroconvulsive therapy in suppressing seizures and catatonia.61 The threshold concept used to explain seizure generation can help us understand catatonia. Even if our patient was predisposed to seizures due to a chronically low seizure threshold (epilepsy), he did not have seizures until his seizure threshold was further reduced by lorazepam withdrawal. Applying the threshold concept to the patient’s catatonia, we can say that his catatonia threshold was depressed by schizoaffective disorder. Treatment with lorazepam increased his catatonia threshold rendering him free of catatonia for 6 months until he developed catatonia again because he was not able to get refills for lorazepam.
The core motor circuits of the frontal lobe and basal ganglia are the most likely sites of dysfunction in catatonia, but the locus of dysfunction can also be in the circuits of the thalamus, parietal lobe, cerebellum, and limbic cortex.62,63 Abnormal γ-aminobutyric acid (GABA), dopamine, glutamate, and serotonin (5-HT1) neurotransmission have all been implicated in catatonia. The dramatic response of catatonia to benzodiazepines suggests a crucial role of abnormal GABA neurotransmission in catatonia.64,65 A study revealed decreased GABAA receptor density in the left sensorimotor cortex of patients with catatonia.66 The induction of catatonia by dopamine receptor antagonists suggests that hypoactivity of dopaminergic neurotransmission can give rise to catatonia.67 Catatonia was also linked to serotonin hyperactivity at the 5-HT1A receptor and hypoactivity at the 5-HT2A receptor,68 as well as to glutamate hypoactivity at the NMDA receptor.69 The differential susceptibility of neurotransmitter systems to drugs, drug withdrawal, and other factors may be the reason for the interindividual variability in the clinical expression of catatonia, delirium, and seizures.
Conclusion
Not only can benzodiazepine withdrawal precipitate delirium or seizures, it can also trigger catatonia in patients with mood disorder. Delirium or catatonia can be elusive, especially if both are present. Patients with delirium or catatonia may also be at risk for seizures. Although ictal catatonia has been reported, the presence of both catatonia and seizures can be explained by a common mechanism (eg, impaired GABA neurotransmission) reducing both seizure and catatonia thresholds. In patients with a predisposition for both catatonia (eg, mood disorder) and seizures (ie, epilepsy), the catatonia threshold appears to be more sensitive than the seizure threshold to benzodiazepine withdrawal. Enhancement of GABA neurotransmission with benzodiazepines can effectively abort seizures, catatonia, and perhaps mixed delirium-catatonia syndromes with prominent hyperkinetic features or autonomic instability. NMS and TSS should always be in the differential diagnosis of benzodiazepine withdrawal catatonia and delirium, especially if the patients is taking antidopaminergic or serotonergic drugs. Differentiating malignant catatonia from NMS and TSS is not always easy. The safest approach is to immediately stop antidopaminergic and serotonergic drugs and start empiric treatment with lorazepam. Video-EEG recording should always be considered.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethics Approval: Our institution does not require ethical approval for reporting a case.
Informed Consent: Informed consent for patient information to be published in this article was not obtained because this is a case report and our ethics committee does not require institutional review board approval.
ORCID iD: Edward C. Mader Jr  https://orcid.org/0000-0003-4516-2305
https://orcid.org/0000-0003-4516-2305
References
- 1. Wikipedia Contributors. Diagnostic and Statistical Manual of Mental Disorders. Wikipedia, The Free Encyclopedia; 2020. [Google Scholar]
- 2. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Association; 2013. [Google Scholar]
- 3. Kendler KS, Parnas JS. Philosophical Issues in Psychiatry II: Nosology. Oxford University Press; 2012. [Google Scholar]
- 4. La Mantia MA, Messina FC, Jhanji S, et al. Emergency medical service, nursing, and physician providers’ perspectives on delirium identification and management. Dementia. 2017;16:329-343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Anand S, Paliwal VK, Singh LS, et al. Why do neurologists miss catatonia in neurology emergency? A case series and brief literature review. Clin Neurol Neurosurg. 2019;184:105375. [DOI] [PubMed] [Google Scholar]
- 6. Kahlbaum KL. Catatonia or the Tension Insanity. A Clinical Form of Mental Illness (1874). Arts & Boeve; 2000. [Google Scholar]
- 7. Kraepelin E. Dementia Praecox and Paraphrenia. Thoemmes Press; 1919. [Google Scholar]
- 8. Sienaert P, Dhossche DM, Gazdag G. Adult catatonia: etiopathogenesis, diagnosis and treatment. Neuropsychiatry. 2013;41:391-399. [Google Scholar]
- 9. Fink M, Shorter E, Taylor MA. Catatonia is not schizophrenia: Kraepelin’s error and the need to recognize catatonia as an independent syndrome in medical nomenclature. Schizophr Bull. 2010;36:314-320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. van der Heijden FM, Tuinier S, Arts NJ, Hoogendoorn MLC, Kahn RS, Verhoeven WMA. Catatonia: disappeared or under-diagnosed? Psychopathology. 2005;38:3-8. [DOI] [PubMed] [Google Scholar]
- 11. Bush G, Fink M, Petrides G, Dowling F, Francis A. Catatonia. I. Rating scale and standardized examination. Acta Psychiatr Scand. 1996;93:129-136. [DOI] [PubMed] [Google Scholar]
- 12. Shorter E, Fink M. The Madness of Fear: A History of Catatonia. Oxford University Press; 2018. [Google Scholar]
- 13. Mendez MF, Padilla CR. Delirium. Bradley Neurol Clin Pract. 2016;2:23-33. [Google Scholar]
- 14. Adamis D, Treloar A, Martin FC, Macdonald AJD. A brief review of the history of delirium as a mental disorder. Hist Psychiatry. 2007;18(72 pt 4):459-469. [DOI] [PubMed] [Google Scholar]
- 15. Lipowski ZJ. Acute Confusional States. Oxford University Press; 1990:229-238. [Google Scholar]
- 16. European Delirium Association; American Delirium Society. The DSM-5 criteria, level of arousal and delirium diagnosis: inclusiveness is safer. BMC Med. 2014;12:141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pae CU, Marks DM, Han C, Patkar AA, Masand P. Delirium: underrecognized and undertreated. Curr Treat Options Neurol. 2008;10:386-395. [DOI] [PubMed] [Google Scholar]
- 18. Han JH, Zimmerman EE, Cutler N, et al. Delirium in older emergency department patients: recognition, risk factors, and psychomotor subtypes. Acad Emerg Med. 2009;16:193-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chan PKY. Clarifying the confusion about confusion: current practices in managing geriatric delirium. BC Med J. 2011;53:409-416. [Google Scholar]
- 20. Taylor D, Lewis S. Delirium. J Neurol Neurosurg Psychiatry. 1993;56:742-751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ritter SR, Cardoso AF, Lins MM, Zoccoli TLV, Freitas MPD, Camargos EF. Underdiagnosis of delirium in the elderly in acute care hospital settings: lessons not learned. Psychogeriatrics. 2018;18:268-275. [DOI] [PubMed] [Google Scholar]
- 22. Inouye SK, van Dyck CH, Alessi CA, et al. Clarifying confusion: the confusion assessment method: a new method for detection of delirium. Ann Intern Med. 1990;113:941-948. [DOI] [PubMed] [Google Scholar]
- 23. Pétursson H. The benzodiazepine withdrawal syndrome. Addiction. 1994;89:1455-1459. [DOI] [PubMed] [Google Scholar]
- 24. Olnes MJ, Golding A, Kaplan PW. Nonconvulsive status epilepticus resulting from benzodiazepine withdrawal. Ann Intern Med. 2003;139:956-958. [DOI] [PubMed] [Google Scholar]
- 25. Wilson JE, Carlson R, Duggan MC, et al. Delirium and catatonia in critically ill patients: the DeCat prospective cohort investigation. Crit Care Med. 2017;45:1837-1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Oldham MA, Lee HB. Catatonia vis-a-vis delirium: the significance of recognizing catatonia in altered mental status. Gen Hosp Psychiatry. 2015;37:554-559. [DOI] [PubMed] [Google Scholar]
- 27. Francis A, Lopez-Canino A. Delirium with catatonic features: a new subtype? Psychiatric Times. 2009;29:32-36. [Google Scholar]
- 28. Hauser P, Devinsky O, De Bellis M, Theodore WH, Post RM. Benzodiazepine withdrawal delirium with catatonic features: occurrence in patients with partial seizure disorders. Arch Neurol. 1989;46:696-699. [DOI] [PubMed] [Google Scholar]
- 29. Singerman B, Raheja R. Malignant catatonia—a continuing reality. Ann Clin Psychiatry. 1994;6:259-266. [DOI] [PubMed] [Google Scholar]
- 30. Tormoehlen LM, Rusyniak DE. Neuroleptic malignant syndrome and serotonin syndrome. Handb Clin Neurol. 2018;157:663-675. [DOI] [PubMed] [Google Scholar]
- 31. Azzam PN, Gopalan P. Catatonia, neuroleptic malignant syndrome, and serotonin syndrome. Psychosom Med. 2015:275. doi: 10.1093/med/9780199329311.003.0014 [DOI] [Google Scholar]
- 32. Fink M, Taylor MA. The many varieties of catatonia. Eur Arch Psychiatry Clin Neurosci. 2001;251(suppl 1):I8-I13. [DOI] [PubMed] [Google Scholar]
- 33. Duggal HS, Singh I. Drug induced catatonia. Drugs Today (Barc). 2005;41:599-607. [DOI] [PubMed] [Google Scholar]
- 34. Rapport DJ, Covington EC. Motor phenomena in benzodiazepine withdrawal. Hosp Community Psychiatry. 1989;40:1277-1279. [DOI] [PubMed] [Google Scholar]
- 35. Rosebush PI, Mazurek MF. Catatonia after benzodiazepine withdrawal. J Clin Psychopharmacol. 1996;16:315-319. [DOI] [PubMed] [Google Scholar]
- 36. Deuschle M, Lederbogen F. Benzodiazepine withdrawal-induced catatonia. Pharmacopsychiatry. 2001;34:41-42. [DOI] [PubMed] [Google Scholar]
- 37. Glover SG, Escalona R, Bishop J, Saldivia A. Catatonia associated with lorazepam withdrawal. Psychosomatics. 1997;38:148-150. [DOI] [PubMed] [Google Scholar]
- 38. Brown M, Freeman S. Clonazepam withdrawal-induced catatonia. Psychosomatics. 2009;50:289-292. [DOI] [PubMed] [Google Scholar]
- 39. Parameswaran R, Moore K, Hannan T, Austin M. Catatonia associated with temazepam withdrawal. Aust N Z J Psychiatry. 2011;45:1006-1007. [DOI] [PubMed] [Google Scholar]
- 40. Amos JJ. Lorazepam withdrawal-induced catatonia. Ann Clin Psychiatry. 2012;24:170-171. [PubMed] [Google Scholar]
- 41. Sivakumar T, Yadav A, Sood M, Khandelwal SK. Lorazepam withdrawal catatonia: a case report. Asian J Psychiatr. 2013;6:620-621. [DOI] [PubMed] [Google Scholar]
- 42. Lebin LG, Cerimele JM. Recurrent benzodiazepine withdrawal catatonia in an older adult. Am J Psychiatry. 2017;174:1001-1002. [DOI] [PubMed] [Google Scholar]
- 43. Iyengar S, Bornmann C, Abdelmalak F, LaRocca T. Catatonia due to alprazolam withdrawal. BMJ Case Rep. 2018;11:e227175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Duncan-Azadi CR, Johnson PN, Gormley A. Case report of midazolam withdrawal induced catatonia in a 9-year-old patient. A A Case Rep. 2017;8:242-245. [DOI] [PubMed] [Google Scholar]
- 45. Barnes MP, Saunders M, Walls TJ, Saunders I, Kirk CA. The syndrome of Karl Ludwig Kahlbaum. Neurol Neurosurg Psychiatry. 1986;49:991-996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Primavera A, Fonti A, Novello P, et al. Epileptic seizures in patients with acute catatonic syndrome. J Neurol Neurosurg Psychiatry. 1994;57:1419-1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lim JA, Yagnik PR, Schraeder PA, Wheeler S. Ictal catatonia as a manifestation of nonconvulsive status epilepticus. J Neurol Neurosurg Psychiatry. 1986;49:833-836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kanemoto K, Miyamoto T, Abe R. Ictal catatonia as a manifestation of de novo absence status epilepticus following benzodiazepine withdrawal. Seizure. 1999;8:364-366. [DOI] [PubMed] [Google Scholar]
- 49. Gaete G, Velásquez Á. Ictal catatonia presentation as a non-convulsive status epilepticus: a case report [in Spanish]. Rev Med Chil. 2017;145:126-130. [DOI] [PubMed] [Google Scholar]
- 50. Tan AH, Low SC, Tan CY, Lim KS, Tan CT, Lim SY. “Ictal catatonia”: rare but not to be missed! Parkinsonism Relat Disord. 2016;32:137-139. [DOI] [PubMed] [Google Scholar]
- 51. Gunduz A, Benbir G, Bayar R. Postictal catatonia in a schizophrenic patient and electroconvulsive treatment. J ECT. 2008;24:166-167. [DOI] [PubMed] [Google Scholar]
- 52. Sahaya K, Lardizabal D. Catatonia in encephalitis and nonconvulsive seizures: a case report and review of the literature. Epilepsy Behav. 2010;17:420-425. [DOI] [PubMed] [Google Scholar]
- 53. Verbraeken R, Luykx JJ. Persistent catatonia following epileptic seizures: a case report and systematic literature search. BMC Psychiatry. 2018;18:350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Suzuki K, Miura N, Awata S, et al. Epileptic seizures superimposed on catatonic stupor. Epilepsia. 2006;47:793-798. [DOI] [PubMed] [Google Scholar]
- 55. Swartz CM, Bottum KM, Salazar LS., Jr. Suppression of catatonia-like signs by lorazepam in nonconvulsive status epilepticus without seizure termination. Am J Geriatr Psychiatry. 2002;10:348-350. [PubMed] [Google Scholar]
- 56. Bharadwaj B, Ayyanar S, Mahadevan J, Rajkumar RP. Non-convulsive status epilepticus presenting with catatonia and suicidal behavior. Int J Nutr Pharmacol Neurol Dis. 2013;3:153-155. [Google Scholar]
- 57. Ruiz AR, Vlachy J, Lee JW, et al. Association of periodic and rhythmic electroencephalographic patterns with seizures in critically ill patients. JAMA Neurol. 2017;74:181-188. [DOI] [PubMed] [Google Scholar]
- 58. Leitinger M, Beniczky S, Rohracher A, et al. Salzburg consensus criteria for non-convulsive status epilepticus–approach to clinical application. Epilepsy Behav. 2015;49:158-163. [DOI] [PubMed] [Google Scholar]
- 59. Walther S, Stegmayer K, Wilson JE, Heckers S. Structure and neural mechanisms of catatonia. Lancet Psychiatry. 2019;6:610-619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Rasmussen SA, Mazurek MF, Rosebush PI. Catatonia: our current understanding of its diagnosis, treatment, and pathophysiology. World J Psychiatry. 2016;6:391-398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Fink M, Taylor M. Catatonia. A Clinician’s Guide to Diagnosis and Treatment. Cambridge University Press; 2003. [Google Scholar]
- 62. Northoff G. Neuroimaging and neurophysiology. In: Caroff SN, Mann SC, Francis A, Fricchione GL. eds. Catatonia. From Psychopathology to Neurobiology. American Psychiatric Press; 2004. [Google Scholar]
- 63. Ellul P, Choucha W. Neurobiological approach of catatonia and treatment perspectives. Front Psychiatry. 2015;6:182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Fricchione G, Cassem N, Hooberman D, Hobson D. Intravenous lorazepam in neuroleptic-induced catatonia. J Clin Psychopharmacol. 1983;3:338-342. [PubMed] [Google Scholar]
- 65. Bush G, Fink M, Petrides G, et al. Catatonia. II. Treatment with lorazepam and electroconvulsive therapy. Acta Psychiatr Scand. 1996;93:137-143. [DOI] [PubMed] [Google Scholar]
- 66. Northoff G, Steinke R, Czcervenka C, et al. Decreased density of GABA-A receptors in the left sensorimotor cortex in akinetic catatonia: investigation of in vivo benzodiazepine receptor binding. J Neurol Neurosurg Psychiatry. 1999;67:445-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Mann SC, Caroff SN, Fricchione G, et al. Central dopamine hypoactivity and the pathogenesis of neuroleptic malignant syndrome. Psychiatric Ann. 2000;30:363-374. [Google Scholar]
- 68. Carroll BT. The universal field hypothesis of catatonia and neuroleptic malignant syndrome. CNS Spectr. 2000;5:26-33. [DOI] [PubMed] [Google Scholar]
- 69. Northoff G, Eckert J, Fritze J. Glutamatergic dysfunction in catatonia? Successful treatment of three acute akinetic catatonic patients with the NMDA antagonist amantadine. J Neurol Neurosurg Psychiatry. 1997;62:404-406. [DOI] [PMC free article] [PubMed] [Google Scholar]