Abstract
Aims:
First, to compare clinical features and biological disease modifying anti-rheumatic drugs (bDMARDs) response in patients with axial spondyloarthritis (axSpA) and axial psoriatic arthritis (axPsA). Second, to identify possible predictors of treatment response in both entities.
Methods:
One-year follow-up, observational, single-center study including all patients with axSpA or axPsA who started bDMARDs therapy. Clinical features were collected at baseline while disease activity was measured at baseline, 6 and 12 months by the Ankylosing Spondylitis Disease Activity Score and the Physician Global Assessment. The frequency of patients achieving inactive disease (ID), low disease activity (LDA), high or very high disease activity and clinical improvement were compared between axSpA and axPsA. Baseline predictor factors for achieving treatment response were identified through regression models, using odds ratio (OR) as an estimate.
Results:
In total, 352 patients were included: 287 (81.5%) axSpA and 65 (18.5%) axPsA. No significant differences at baseline were observed between the two diseases for most of the characteristics. While HLA-B27 positivity was associated with axSpA (OR = 5.4; p < 0.001), peripheral manifestations were associated with axPsA (OR = 4.7; p < 0.001). The frequency of patients with axSpA and axPsA achieving ID/LDA after 6 and 12 months of bDMARDs was comparable: 53% versus 58%, p = 0.5; and 58% versus 60%, p = 0.9, respectively. Both diseases also presented similar clinical improvement. In axSpA and axPsA, male gender seemed to be associated with achieving LDA [OR at 12 months visit = 2.8 (p < 0.01) and 2.7 (p = 0.09)].
Conclusion:
In clinical practice, patients with axSpA and axPsA present numerous similarities, including comparable medium-term clinical response to bDMARDs. Male gender could be a predictor of treatment response in both diseases.Keyword: axial spondyloarthritis, psoriatic arthritis, axial involvement, clinical characteristics
Keywords: axial spondyloarthritis, psoriatic arthritis, axial involvement, clinical characteristics
Background
The spondyloarthritides (SpAs) constitute a group of heterogeneous entities sharing common characteristics, including familiar aggregation, association with HLA-B27, and some clinical features. Traditionally, the term SpA included both psoriatic arthritis (PsA) and axial SpA (axSpA) but nowadays patients with SpA tend to be classified according to their predominant disease manifestation into axSpA and peripheral SpA. In addition, the concept of axSpA involves radiographic axSpA (r-axSpA), also known as ankylosing spondylitis (AS), and non-radiographic axSpA (nr-axSpA); both fall within the disease spectrum.
Clinical manifestations in SpA include enthesitis and joint inflammation, which can affect either axial or peripheral anatomical regions or both. A serious debate exists as to what extent PsA might be regarded a disease similar to other SpAs.1 The majority of patients with PsA present peripheral involvement, even when axial manifestations eventually appear during the course of the disease.2 Whereas this peripheral presentation has been broadly studied, this is less true of axial involvement in PsA. It remains unclear whether the characteristics in patients with axial PsA are similar to those with axSpA, and findings comparing their axial characteristics are scant and inconsistent.3 There is recent evidence that, in more than half of all cases, asymptomatic patients may develop radiological manifestations.4 Although, according to some relevant studies, patients with established PsA present an estimated prevalence of axial involvement between 25% and 70%,5 the possibility of non-radiographic affection in PsA is still unknown. Hence, it is currently unclear whether this form of disease should be classified as axSpA with psoriasis or as PsA with axial involvement, also known as axial PsA (axPsA). In addition, this broad range of axPsA disease prevalence data also shows the existing discrepancies that hamper the drawing of any firm conclusions.6
Previous studies have addressed comparisons of the clinical features of r-axSpA against the entire spectrum of PsA. In general, patients with r-axSpA were found to be more frequently male and younger than those with PsA.7 However, most of these studies were influenced by the predominant peripheral presentation that characterizes PsA. In contrast, the differences between r-axSpA patients and those with axPsA seem less consistent.8 It has been reported that the presence of skin psoriasis and HLA-B27 may influence the patterns of axial disease,7 although studies comparing nr-axSpA and axPsA are few in number.
One of the most relevant aspects pertinent to resolving the conundrum of whether axPsA is, in fact, axSpA with psoriasis or PsA with axial involvement, involves the clinical response to treatment, especially to biological drugs. Elucidating this unknown remains a challenging task, since current advances in therapy have rendered management of SpA a constantly evolving process. In fact, clinical response in the subset of patients with axial disease and psoriasis has yet to be evaluated. Most randomized control trials evaluating the efficacy of new drugs have included patients with axSpA,9,10 the results then being typically extrapolated to those patients with axPsA. Hence, it is not clear whether clinical response to pharmacological treatment between axSpA and axPsA is equivalent. Data comparing the medium-term treatment response to biological disease modifying anti-rheumatic drugs (bDMARDs) in axSpA and axPsA would help clarify this matter.
Aims
To compare disease characteristics and clinical treatment response in patients with axSpA and those with axPsA receiving biological therapy. As a secondary objective, to identify possible predictor factors of clinical response in both diseases after 1 year of bDMARDs therapy.
Methods
Study design and population
For this study, a one-year follow-up dataset from the SpA-Paz cohort was used. This is a prospective, ongoing, observational cohort including patients with axSpA or axPsA who have initiated bDMARDs treatment due to predominantly axial manifestations from 2002 to 2019 at the University Hospital La Paz, Madrid, Spain. axSpA and axPsA were defined in clinical practice according to the prescribing rheumatologist, who based the diagnosis on clinical features and complementary examinations. All patients with a diagnosis of axSpA or axPsA initiating bDMARDs were included in the study, according to the established protocol in the rheumatology unit. Thus, bDMARDs were prescribed for the rheumatological symptoms of axSpA or axPsA, following the treatment guidelines of the Spanish Society of Rheumatology through consensus decisions in clinical sessions including at least 10 expert rheumatologists. The study was approved by the La Paz University Hospital Ethics Committee and all patients provided written informed consent.
Outcome variables
At baseline, demographic information, disease characteristics, concomitant treatment and laboratory tests (including acute-phase reactants) before starting biological drug(s) were collected from the electronic medical record and biologic database. Data for bDMARDs were also collected. For this study, the presence of sacroiliitis according to modified New York criteria in the pelvis radiograph was assessed by two expert rheumatologist readers, who were blinded to the diagnosis of the patient. Clinical disease activity was measured by Ankylosing Spondylitis Disease Activity Score-C-reactive protein (ASDAS-CRP) and Physician Global Assessment-Visual Analog Scale (PhyGA-VAS) range 0–100 mm) at baseline, 6 and 12 months after initiating bDMARDs. In addition, the Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) and Patient Pain Visual Analogue Scale (PtVAS) were collected during the same time periods.
According to ASDAS, disease activity was defined as follows: inactive disease (ID) (ASDAS <1.3), low disease activity (LDA) (ASDAS 1.3–2.1), high disease activity (HDA) (ASDAS 2.1–3.5) and very high disease activity (VHDA) (ASDAS >3.5).11 Clinically important improvement and major improvement were defined by ASDAS as delta-ASDAS ⩾1.1 and ⩾2.0, respectively.
According to collected PhyGA, disease activity cut-offs were established based on the consensus of three expert rheumatologists as follows: Inactive Global Assessment (IGA) with PhyGA <5 mm, Low Global Assessment (LGA) with PhyGA 5–30 mm, High Global Assessment (HGA) with PhyGA >30–60 mm and Very High Global Assessment (VHGA) with PhyGA >60 mm. Clinical improvement by PhyGA was defined as an improvement of at least 30% compared with baseline at the studied time points.
According to BASDAI, disease activity was defined as follows: ID (BASDAI <2), LDA (BASDAI 2–4), HDA (BASDAI 4–6) and VHDA (BASDAI >6).
According to collected PtVAS, disease activity cut-offs were established by a consensus of three expert rheumatologists as follows: IGA with PtVAS <5 mm, LGA with PtVAS 5–30 mm, HGA with PtVAS >30–60 mm and VHGA with PtVAS >60 mm.
Statistical analysis
Demographic, clinical and complementary test information was summarized through descriptive analyses. Categorical variables were described by frequencies and percentages. Continuous variables were described using means and standard deviations (SDs). For comparisons of the two groups (axSpA and axPsA), chi square or the exact Fisher’s test were used for categorical variables and the Student t or Mann–Whitney U for continuous variables, according to the data distributions. In addition, associations between demographic and clinical features and the disease diagnosis (axSpA or axPsA) were analyzed using univariable and multivariable logistic regression models.
The frequency that patients achieved each clinical activity status as well as the clinical improvements at 6 and 12 months were calculated, separately for axSpA and axPsA, followed by a comparison using the Fisher test. Only patients with a valid value for ASDAS or PhyGA at these visits were included for the evaluation of each treatment response. Baseline predictive factors for achieving clinical response and clinical improvement were identified using univariable and multivariable binary logistic regression models, inserting the possible predictors as independent variables and the clinical response achievement (by ASDAS or PhyGA, in two separate models) as the outcome. All those variables with a p-value lower than 0.1 in the univariable were included in the multivariable analysis. Odds ratios (ORs) with p-value < 0.05 were used as measures of association. All data were analyzed using SPSS software version 24.
Results
Demographic and clinical characteristics
Three-hundred and fifty-two patients starting a bDMARD were included, of whom 287 (81.5%) had been diagnosed as axSpA and 65 (18.5%) as axPsA, according to the prescribing rheumatologist. Among patients with axSpA, 203 (70.7%) were classified as r-axSpA, whereas 84 (29.3%) were nr-axSpA. The socio-demographic and disease characteristics of patients are shown in Table 1.
Table 1.
Demographic and disease characteristics of patients included in the study. Results are shown as absolute numbers (percentages) or expressed as the mean ± standard deviation.
| Total N = 352 |
axSpA n = 287 (81%) |
axPsA n = 65 (19%) |
p-value | |
|---|---|---|---|---|
| Sex, male | 223 (63.4) | 180 (62.7) | 43 (66.2) | 0.7 |
| Age, years: | ||||
| At diagnosis | 35.9 ± 13.4 | 35.7 ± 13.7 | 36.9 ± 12.1 | 0.9 |
| At starting biologic therapy | 44.4 ± 13.2 | 44.1 ± 13.4 | 45.8 ± 11.6 | 0.3 |
| Disease duration before biologic therapy, years | 8.4 ± 9.2 | 7.9 ± 11.3 | 8.9 ± 9.0 | 0.7 |
| Current smoking habit | 158 (44.9) | 129 (44.9) | 29 (44.6) | 0.9 |
| HLA-B27 positive, n/N | 219/322 (67.8) | 204/281 (72.3) | 16/47 (34.1) | <0.001 |
| CRP, mg/dL | 12.4 ± 17.9 | 12.6 ± 18.9 | 11.1 ± 12.7 | 0.5 |
| Clinical involvement: | ||||
| Only axial | 170 (48.2) | 168 (58.5) | 14 (21.5) | <0.001 |
| Axial and peripheral | 182 (51.7) | 119 (41.5) | 51 (78.5) | |
| Radiographic sacroiliitis, mNY criteria, n/N | 227/341 (64.5) | 203/287 (70.7) | 24/54 (44.4) | <0.001 |
| Psoriasis | 74 (21.3) | 11 (4.2) | 63 (97) | <0.001 |
| Enthesitis, n/N | 85/205 (41.5) | 73/163 (44.8) | 12/42 (28.6) | 0.07 |
| Dactylitis | 10 (2.7) | 7 (2.4) | 3 (4.6) | 0.4 |
| IBD | 9 (2.6) | 8 (2.8) | 1 (1.5) | 0.7 |
| Uveitis | 46 (13.6) | 44 (15.3) | 2 (3.1) | 0.03 |
| ASDAS | 3.3 ± 0.9 | 3.3 ± 1.0 | 3.1 ± 1.0 | 0.1 |
| ASDAS ID | 8 (2.5) | 5 (2.0) | 3 (4.7) |
0.25 |
| ASDAS LDA | 24 (7.6) | 17 (6.8) | 7 (10.9) | |
| ASDAS HDA | 143 (45.4) | 112 (44.6) | 31 (48.4) | |
| ASDAS VHDA | 140 (44.4) | 117 (46.6) | 23 (35.9) | |
| BASDAI (0–10) | 5.9 ± 4.2 | 6.1 ± 4.5 | 5.23 ± 2.1 | 0.1 |
| PGA (0–100) | 63.2 ± 21.8 | 64.1 ± 21.5 | 58.8 ± 23.2 | 0.1 |
| PhyGA (0–100) | 39.1 ± 21.5 | 37.4 ± 13.7 | 44.4 ± 22.6 | 0.02 |
| PhyGA IGA | 2 (0.8) | 2 (1.0) | 0 |
0.13 |
| PhyGA LGA | 75 (28.6) | 58 (29.3) | 17 (26.6) | |
| PhyGA HGA | 115 (43.9) | 92 (46.5) | 23 (35.9) | |
| PhyGA VHGA | 70 (26.7) | 46 (23.2) | 24 (37.5) | |
| Concomitant therapy: | 193 (52.4) | 145 (50.5) | 48 (73.8) | 0.001 |
| Only MTX | 66 (20.7) | 36 (13.9) | 30 (46.2) | <0.001 |
| Only SFZ | 82 (25.6) | 73 (28.2) | 9 (13.8) | 0.03 |
| Prednisone use | 32 (9.5) | 20 (7.7) | 12 (20) | 0.004 |
ASDAS, Ankylosing Spondylitis Disease Activity Score; axPsA, axial psoriatic arthritis; axSpA, axial spondyloarthritis; BASDAI, Bath Ankylosing Spondylitis Disease Activity Index; CRP, C-reactive protein; HDA, high disease activity; HGA, High Global Assessment; IBD, inflammatory bowel disease; ID, inactive disease; IGA, Inactive Global Assessment; LDA, low disease activity; LGA, Low Global Assessment; mNY, modified New York; MTX, methotrexate; PGA, Patient Global Assessment; PhyGA, Physician Global Assessment; SFZ, sulfasalazine; VHDA, very high disease activity; VHGA, Very High Global Assessment.
In total, 74 patients in our cohort had psoriasis at any time during the follow-up (11 within axSpA and 63 within axPsA group). The 11 patients with psoriasis in the axSpA group (n = 287) developed it after the diagnosis of the rheumatic disease and therefore were included in this diagnostic group. In the group of patients with axPsA (n = 65), 63 had psoriasis when the rheumatic diagnosis was made and two had a family history of psoriasis.
Biological therapies initiated included adalimumab in 21.3% of cases, certolizumab in 1.1%, etanercept in 26.1%, golimumab in 12.8%, infliximab in 36.6% and secukinumab in 2.0%.
No significant differences at baseline were observed between axSpA and axPsA for most of the characteristics evaluated in the study. Interestingly, gender, smoking habit, age at diagnosis, age at starting biologic therapy and disease duration beforehand did not differ between the two diseases. Similarly, there were no differences in clinical features such as enthesitis, dactylitis or inflammatory bowel disease. In addition, no significant differences were observed regarding the degree of disease activity, as measured either by ASDAS, BASDAI, C-reactive protein (CRP) or patient global assessment.
However, a few differences between the two groups in some relevant characteristics were observed. axSpA patients more often presented uveitis (15.3 versus 3.1%, p = 0.03), were more frequently HLA-B27 positive (72.3 versus 34.1%, p < 0.001) and had a higher percentage of radiographic sacroiliitis (70.7 versus 30.7%, p < 0.001) compared with those with axPsA. In contrast, axPsA patients showed more peripheral involvement (78.5 versus 41.5%, p = 0.004) and a slightly worse PhyGA (44.4 versus 37.4, p = 0.02). Regarding concomitant treatment, axSpA patients were more often prescribed sulfasalazine (28.2 versus 13.8%, p = 0.003), whereas methotrexate (13.9 versus 46.2%, p < 0.001) and prednisone (7.7 versus 20%, p < 0.01) were less frequently administered to axSpA patients. After running multivariate analyses, HLA-B27 positivity (OR = 5.4; p < 0.001) was independently associated with axSpA, while the presence of peripheral manifestations was associated with axPsA (OR = 4.7; p < 0.001).
In total, data to assess ASDAS and PhyGA clinical response were available for 289 and 228 patients at 6 months and 242 and 212 patients at 12 months, respectively.
ASDAS – clinical response
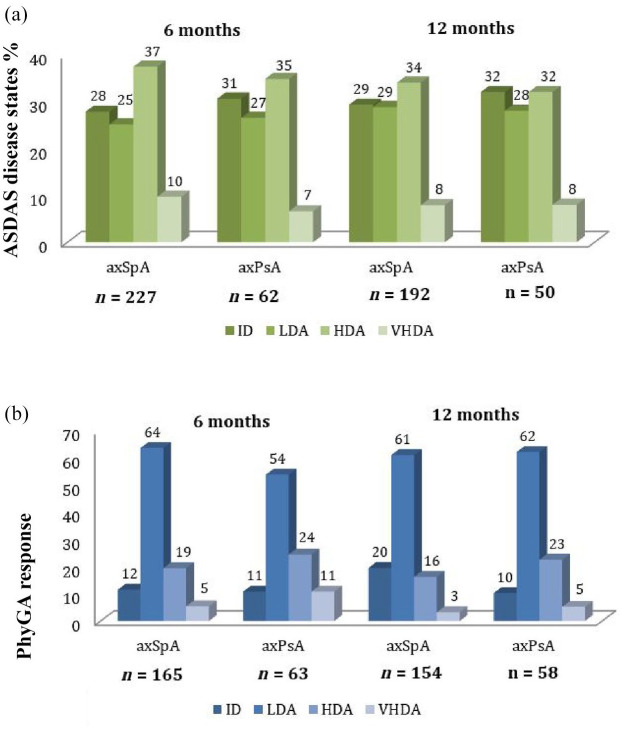
Based on ASDAS, no statistically significant differences in clinical efficacy were observed after 6 and 12 months of bDMARDs treatment. As shown in Figure 1(a), the observed percentage of patients with axSpA and axPsA achieving the recommended treatment goal (ID/LDA) at both visits was comparable: 53% versus 58% at 6 months (p = 0.5); and 58% versus 60% at 12 months (p = 0.9). The percentage of patients achieving clinical improvement is shown in Table 2. Major clinical improvement was comparable between axSpA and axPsA at 6 months (28.1 versus 26.7%, p = 0.8) and 12 months (50 versus 57%, p = 0.4). No significant differences were found in terms of clinical improvement criteria.
Figure 1.
Clinical response at 6 and 12 months in axSpA and axPsA. (a) Response rates (in percentages) by ASDAS. No statistically significant differences were found among any of the intervals between axSpA and axPsA. (b) Response rates (in percentages) as measured by PhyGA. No statistically significant differences between axSpA and axPsA were found among any of the intervals.
ASDAS, Ankylosing Spondylitis Disease Activity Score; axPsA, axial psoriatic arthritis; axSpA, axial spondyloarthritis; HDA, high disease activity; HGA, High Global Assessment; ID, inactive disease; IGA, Inactive Global Assessment; LDA, low disease activity; LGA, Low Global Assessment; PhyGA, Physician Global Assessment; VHDA, very high disease activity. VHGA: Very High Global Assessment.
Table 2.
Clinical improvement in axSpA and axPsA after 6 and 12 months, according to ASDAS and PhyGA.
| 6 months | 12 months | |||||
|---|---|---|---|---|---|---|
| axSpA | axPsA | p-value | axSpA | axPsA | p-value | |
| Clinically important improvement ASDAS, n/N (%) | 118/227 (52) | 29/62 (47) | 0.6 | 109/192 (57) | 25/50 (50) | 0.4 |
| Major improvement ASDAS, n/N (%) | 64/227 (28) | 16/62 (26) | 0.8 | 62/192 (32) | 16/50 (32) | 0.4 |
| Clinical improvement PhyGA, n/N (%) | 108/165 (65) | 42/63 (67) | 0.9 | 100/154 (65) | 43/58 (74) | 0.2 |
Clinically important improvement ASDAS, delta-ASDAS ⩾1.1; major improvement ASDAS, delta-ASDAS ⩾2.0; clinical improvement PhyGA, improvement of at least 30% compared with baseline at the studied time points.
ASDAS, Ankylosing Spondylitis Disease Activity Score; axPsA, axial psoriatic arthritis; axSpA, axial spondyloarthritis; PhyGA, Physician Global Assessment.
In addition, separate analyses for BASDAI and PtVAS for disease activity as secondary outcomes were also performed. The results of these analysis were consistent with the ASDAS results (see Supplemental Material Figure S1 and Figure S2 online).
PhyGA – clinical response
PhyGA response rates at 6 and 12 months are shown in Figure 1(b). Similarly to ASDAS response, no statistically significant differences were found for any disease activity interval. Interestingly, the percentage of patients with axSpA and axPsA achieving LGA at 6 months and 12 months also proved comparable: 64% versus 54%, p = 0.2 and 61% versus 62%, p = 0.9, respectively. Accordingly, clinical improvement by PhyGA was also similar between axSpA and axPsA at 6 months (65.5 versus 64.6%, p = 0.9) and 12 months (64.9 versus 74.1%, p = 0.2) (Table 2).
Predictive factors of LDA achievement
In the axSpA group, the univariable analysis revealed that achieving LDA (by ASDAS) was associated with male gender (OR = 3.5, p < 0.001) at 6 months and with male gender (OR = 2.8, p = 0.001) and HLA-B27 positivity (OR = 2.3, p = 0.01) at 12 months. In the multivariable analysis, all of these variables remained independently associated with LDA: male gender at 6 months (OR = 3.7, p < 0.001) and 12 months (OR = 2.7, p < 0.01) and HLA-B27 positivity at 12 months (OR = 2.6, p < 0.01).
In the axPsA group, the univariate analysis showed that male patients tended to achieve LDA more frequently at 6 months (OR = 3.0, p = 0.05) and at 12 months (OR = 2.8, p = 0.09), although this association was not statistically significant. In the multivariable analyses, none of the factors was significantly associated either with clinical improvement or with LDA in patients with axPsA.
Finally, the same predictors were identified when secondary outcomes (BASDAI and PtVAS for disease activity) were employed, male gender being a predictor of clinical response in axSpA at 6 months (OR = 1.8; p = 0.04) and 12 months (OR = 2.8; p = 0.001); and in axPsA at 6 months (OR = 6.6; p = 0.01) and 12 months (OR = 20.4; p = 0.006). In addition to these, CRP was also identified as a predictor for achieving clinical response in the multivariable analysis at 6 months (OR = 1.12; p = 0.01) and 12 months according to BASDAI (OR = 1.17; p = 0.03) in axPsA.
Discussion
Some researchers have suggested the possibility that axPsA is simply a presentation of axSpA with psoriasis, while others insist that the two be classified as different diseases. Hereby, the definition of axial disease remains a challenge. In fact, despite the frequent axial involvement (defined as a high BASDAI) in some pivotal studies for new drugs used in PsA, there are not specific outcomes for this subgroup of patients.12 The present study investigated and compared disease characteristics and clinical bDMARDs treatment response in patients with axSpA versus those with axPsA in clinical practice. In addition, it also evaluated the predictive factors of clinical response after 1 year of bDMARDs treatment in both diseases of the SpA spectrum.
First, it is remarkable that numerous similarities between axSpA and axPsA were evident in our cohort. In fact, no differences were observed in such relevant characteristics as gender, smoking habit, age at diagnosis, age at starting biologic therapy or disease duration. Nor did disease activity at the different time points studied show any significant differences between axSpA and axPsA. Nevertheless, some differences regarding clinical features were apparent. AxSpA patients presented less peripheral involvement and were more frequently HLA-B27 positive compared with those with axPsA. This is in accordance with published data, which showed that r-axSpA patients are more likely to be male, present more severe spinal disease and have a higher prevalence of HLA-B27 compared with those with axPsA.13 In addition, previous data showed that the disease presented by approximately 25% of patients with PsA mainly consisted of axial manifestations, with peripheral arthritis being the most common.2 In our cohort, patients with axPsA presented exclusively axial manifestations in 21.5% of cases, which is similar to that reported by another study despite subtle differences in the definition of disease.
Second, our study investigated bDMARD clinical response at 6 and 12 months in both diseases based on two different measures, ASDAS and PhyGA. The use of these indices strengthens the validity of our comparisons since most of the previous studies used BASDAI, whose measurement properties have been shown to be inferior to those of ASDAS. Indeed, PhyGA is an interesting outcome measure captured by our study and one that can be used as a comparator for future research. Both patient groups presented comparable clinical response using these indices. Previous studies suggested some similarities in self-reported measures of health status and disease activity in r-axSpA and PsA.14,15 However, there is no reliable information regarding clinical outcomes of r-axSpA compared with those of axPsA. Moreover, comparisons of two indexes that include patient-based and physician-based reported outcomes make this comparison more sensitive in terms of obtaining a clearer picture of the actual disease status. Furthermore, these resemblances were present not only in disease activity status, but also in clinical response, both for patient-based and physician-based reported measures.
In addition, another aspect that was addressed in our study was an examination of the predictive factors of clinical response in both diseases. Male gender and HLA-B27 appeared as a predictor of good clinical outcomes in axSpA. Although they were not statistically significant in the case of axPsA, the lower tendency probably reflects an insufficient sample size for this group. Together with the results observed for clinical features and treatment response to bDMARDs, the fact that there is a common main predictive factor in both forms of presentation also supports the hypothesis that they are similar diseases with subtle differences.
However, when interpreting the results of this study, several limitations need to be considered. First, this was an observational study, which implies that it could be affected by selection and information bias, as well as loss to follow-up. Some data that might have been useful, such as the performance of magnetic resonance imaging or the quantification of the extent of psoriasis at baseline, were not assessed in all patients by the clinician and therefore could not be included in the study. In this regard, missing data from a heterogeneous cohort hinders the analysis. However, our study includes a representative sample of the usual patient population in routine clinical practice, which enables a more precise comparison on physician’s diagnosis and treatment effectiveness. On the other hand, not all of the patients in our cohort completed the 12-month follow-up period, which explains the lack of data at 12 months. There are also missing data regarding the outcome measures (in particular PhyGA). Another limitation of the study is that the number of included patients in the regression analysis for the axPsA group is low as compared with the axSpA group. Finally, the definitions used for axSpA and axPsA involved subjective judgments by the clinicians, since there is no gold standard for diagnosis. However, one of the strengths of our study is that the diagnoses of axSpA and axPsA were also based on the consensus of at least 10 expert rheumatologists, who confirmed the diagnosis using the information on the clinical report and complementary examinations before biological therapy was initiated.
In conclusion, patients with axSpA and axPsA present important similarities, including similar clinical response to biological therapy within the first year of treatment. In addition, male gender could be a predictive factor of good clinical response in both diseases. Nevertheless, these findings need to be confirmed and further studies should be carried out to elucidate the different presentations across the SpA spectrum. In this regard, the emergence of magnetic resonance imaging, combined with new data analysis techniques, may yield great insights towards resolving these important questions.
Supplemental Material
Supplemental material, SUPPLEMENTARY_MATERIAL_14SEP2020 for Axial spondyloarthritis and axial psoriatic arthritis: similar or different disease spectrum? by Diego Benavent, Chamaida Plasencia-Rodríguez, Karen Franco-Gómez, Romina Nieto, Irene Monjo-Henry, Diana Peiteado, Alejandro Balsa and Victoria Navarro-Compán in Therapeutic Advances in Musculoskeletal Disease
Acknowledgments
The authors thank the Spanish Society of Rheumatology (SER) for the English editing service.
Footnotes
Conflict of interest statement: DB received grants/speaker/research supports from Roche and Abbvie. CP received grants/speaker/research supports from Pfizer, Sanofi, Novartis, Roche and Lilly. RN received grants/speaker/research supports from Novartis, Sanofi Genzyme, Pfizer and Montpellier. IM received grants/research supports from Novartis and speaker’s fees from AbbVie, UCB, Roche and Novartis. DP received grants/research supports from Abbvie, Lilly, MSD and Roche, and had participation in company sponsored speaker’s bureau from Abbvie, Novartis, Lilly, Roche, and MSD. AB received grant/research support, fees for consultancies or as a speaker for Abbvie, Pfizer, Novartis, BMS, Nordic, Sanofi, Sandoz, Lilly, UCB, Roche. VN: consultancy/speaker/research grants from: Abbvie, BMS, Lilly, MSD, Novartis, Pfizer, Roche, UCB.
Ethics approval: The study is attached to the project approved by the ethics committee from La Paz University Hospital with approval code PI-1479.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: D. Benavent  https://orcid.org/0000-0001-9119-5330
https://orcid.org/0000-0001-9119-5330
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Diego Benavent, Rheumatology Service, Hospital Universitario la Paz-IdiPaz, Paseo de la Castellana 261, Madrid, 28046, Spain.
Chamaida Plasencia-Rodríguez, Rheumatology Service, Hospital Universitario la Paz-IdiPaz, Madrid, Spain.
Karen Franco-Gómez, Rheumatology Service, Hospital Universitario la Paz-IdiPaz, Madrid, Spain.
Romina Nieto, Rheumatology Department, Hospital Provincial de Rosario, Santa Fe, Argentina.
Irene Monjo-Henry, Rheumatology Service, Hospital Universitario la Paz-IdiPaz, Madrid, Spain.
Diana Peiteado, Rheumatology Service, Hospital Universitario la Paz-IdiPaz, Madrid, Spain.
Alejandro Balsa, Rheumatology Service, Hospital Universitario la Paz-IdiPaz, Madrid, Spain.
Victoria Navarro-Compán, Rheumatology Service, Hospital Universitario la Paz-IdiPaz, Madrid, Spain.
References
- 1. Feld J, Chandran V, Haroon N, et al. Axial disease in psoriatic arthritis and ankylosing spondylitis: a critical comparison. Nat Rev Rheumatol 2018; 14: 363–371. [DOI] [PubMed] [Google Scholar]
- 2. Chandran V, Tolusso DC, Cook RJ, et al. Risk factors for axial inflammatory arthritis in patients with psoriatic arthritis. J Rheumatol 2010; 37: 809–815. [DOI] [PubMed] [Google Scholar]
- 3. Fernández-Sueiro JL. The challenge and need of defining axial psoriatic arthritis. J Rheumatol 2009; 36: 2633–2634. [DOI] [PubMed] [Google Scholar]
- 4. Chandran V, Barrett J, Schentag CT, et al. Axial psoriatic arthritis: update on a longterm prospective study. J Rheumatol 2009; 36: 2744–2750. [DOI] [PubMed] [Google Scholar]
- 5. Gladman DD. Axial disease in psoriatic arthritis. Curr Rheumatol Rep 2007; 59: 1193–1195. [DOI] [PubMed] [Google Scholar]
- 6. Helliwell P, Coates L, Chandran V, et al. Qualifying unmet needs and improving standards of care in psoriatic arthritis. Arthritis Care Res 2014; 66: 1759–1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jadon DR, Sengupta R, Nightingale A, et al. Axial disease in psoriatic arthritis study: defining the clinical and radiographic phenotype of psoriatic spondyloarthritis. Ann Rheum Dis 2017; 76: 701–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chandran V. Psoriatic spondylitis or ankylosing spondylitis with psoriasis: same or different? Curr Opin Rheumatol 2019; 31: 329–334. [DOI] [PubMed] [Google Scholar]
- 9. van Der Heijde D, Dijkmans B, Geusens P, et al. Efficacy and safety of infliximab in patients with ankylosing spondylitis: results of a randomized, placebo-controlled trial (ASSERT). Arthritis Rheum 2005; 52: 582–591. [DOI] [PubMed] [Google Scholar]
- 10. Landewé R, Braun J, Deodhar A, et al. Efficacy of certolizumab pegol on signs and symptoms of axial spondyloarthritis including ankylosing spondylitis: 24-week results of a double-blind randomised placebo-controlled phase 3 study. Ann Rheum Dis 2014; 73: 39–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Machado PM, Landewé R, van Der Heijde D; Assessment of SpondyloArthritis international Society (ASAS). Ankylosing Spondylitis Disease Activity Score (ASDAS): 2018 update of the nomenclature for disease activity states. Ann Rheum Dis 2018; 77: 1539–1540. [DOI] [PubMed] [Google Scholar]
- 12. Ritchlin C, Rahman P, Kavanaugh A, et al. Efficacy and safety of the anti-IL-12/23 p40 monoclonal antibody, ustekinumab, in patients with active psoriatic arthritis despite conventional non-biological and biological anti-tumour necrosis factor therapy: 6-month and 1-year results of the phase 3, multicentre, double-blind, placebocontrolled, randomised PSUMMIT 2 trial. Ann Rheum Dis 2014; 73: 990–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Feld J, Ye JY, Chandran V, et al. Is axial psoriatic arthritis distinct from ankylosing spondylitis with and without concomitant psoriasis? Rheumatology (Oxford) 2020; 59: 1340–1346. [DOI] [PubMed] [Google Scholar]
- 14. Lindström U, Bremander A, Haglund E, et al. Back pain and health status in patients with clinically diagnosed ankylosing spondylitis, psoriatic arthritis and other spondyloarthritis: a cross-sectional population-based study. BMC Musculoskelet Disord 2016; 17: 106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pérez Alamino R, Maldonado Cocco JA, Citera G, et al. Differential features between primary ankylosing spondylitis and spondylitis associated with psoriasis and inflammatory bowel disease. J Rheumatol 2011; 38: 1656–1660. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, SUPPLEMENTARY_MATERIAL_14SEP2020 for Axial spondyloarthritis and axial psoriatic arthritis: similar or different disease spectrum? by Diego Benavent, Chamaida Plasencia-Rodríguez, Karen Franco-Gómez, Romina Nieto, Irene Monjo-Henry, Diana Peiteado, Alejandro Balsa and Victoria Navarro-Compán in Therapeutic Advances in Musculoskeletal Disease