Abstract
Immune checkpoint inhibitors successfully treat various malignancies by inducing an immune response to tumor cells. However, their use has been associated with a variety of autoimmune disorders, such as diabetes, hepatitis, and pneumonitis. Pulmonary arterial hypertension due to checkpoint inhibitor use has not yet been described. We present a novel case of pulmonary arterial hypertension associated with systemic lupus erythematosus and Sjogren’s syndrome overlap that was induced by therapy with the checkpoint inhibitor durvalumab.
Keywords: autoimmune disease, durvalumab, immunotherapy, programmed death-ligand 1 inhibitor, right heart failure
Case description
A 44-year-old non-smoking African American woman with a history of stage 3 squamous cell lung cancer and recurrent pleural effusions was transferred to our hospital due to acute hypoxic respiratory failure. Two years prior, she was diagnosed with squamous cell lung cancer and was initially treated with cisplatin, etoposide, carboplatin, and paclitaxel. One year later, she was started on the programmed death-ligand 1 (PD-L1) inhibitor durvalumab (10 mg/kg every two weeks for approximately eight months). Over this time, she developed new onset insulin-dependent diabetes, hypothyroidism, and adrenal insufficiency. These phenomena were attributed to autoimmunity induced by durvalumab, which was subsequently stopped (Fig. 1a).
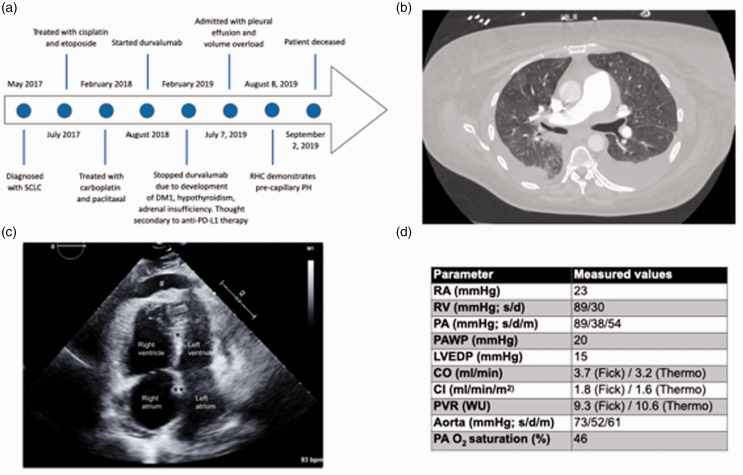
Fig. 1.
Timeline, imaging studies and hemodynamic values. (a) A timeline of the patient’s clinical course. SCLC: small cell lung cancer, DM1: type 1 diabetes mellitus, RHC: right heart catheterization, PH: pulmonary hypertension. (b) A computer tomography image depicting mild diffuse ground-glass opacities as well as pulmonary artery enlargement and bilateral pleural effusions. (c) A two-dimensional-echocardiographic apical four chamber view. Note right atrial and right ventricular enlargement, leftward shift of interventricular septum (*) and interatrial septum (**), compression of left atrium and left ventricle, as well as presence of a pericardial effusion (#). (d) Hemodynamic values during right heart catheterization. RA: right atrium; RV: right ventricle; PA: pulmonary artery; PAWP: PA wedge pressure; LVEDP: left ventricular end-diastolic pressure; CO: cardiac output; CI: cardiac index; PVR: pulmonary vascular resistance; WU: Wood units; s: systole; d: diastole; m: mean. Dobutamine was stopped during RHC measurements.
Two months later, the patient presented with dyspnea, anasarca, bilateral exudative pleural effusions, and a pericardial effusion. Pleural fluid cytology was negative for recurrent lung cancer. She eventually was transferred to our center for further evaluation.
On presentation, the patient was severely volume overloaded and hypoxemic (requiring 5 L O2/min). She exhibited signs of tissue hypoperfusion including a venous lactate level of 3.9 mmol/L. B-type natriuretic peptide level was 628 pg/ml, sodium was 126 mmol/L, and creatinine was 1.5 mg/dl. Chest computed tomography demonstrated mild diffuse ground-glass opacities with otherwise normal lung parenchyma as well as pulmonary artery enlargement and bilateral pleural effusions (Fig. 1b). In addition, right ventricular (RV) enlargement with reflux of contrast into the liver and a pericardial effusion were noted. Echocardiography revealed elevated RV systolic pressure, profound RV dysfunction, and a moderate pericardial effusion without hemodynamic compromise (Fig. 1c). The left atrium and ventricle (LV) were underfilled due to RV failure. There was no evidence of structural left heart disease.
After aggressive diuresis, right heart catheterization (RHC) was performed. This demonstrated severe precapillary pulmonary hypertension (PH) and RV failure (Fig. 1d). Infection, liver disease, congenital heart disease, pulmonary embolism, and illicit drugs were ruled out. The patient did not exhibit signs or symptoms of sleep-disordered breathing. There was no family history of PH. Hemoglobin was 7.3 mg/dl, platelets were 124 per microliter, and haptoglobin was <6 mg/dl. Serologic workup revealed positive antinuclear antibody (ANA) of 1:1280 with a speckled pattern, positive SS-A antibody >8 U, and positive rheumatoid factor of 116 IU/ml. Rheumatology was consulted and a diagnosis of systemic lupus erythematosus (SLE)/Sjogren’s syndrome overlap constellation was made based on sicca syndrome, recurrent pleural/pericardial effusions, hemolytic anemia, thrombocytopenia, positive ANA, positive SS-A, and high-titer positive rheumatoid factor. Treatment for SLE/Sjogren’s syndrome was initiated with prednisone and hydroxychloroquine for the duration of her hospitalization. Given the temporal relationship between symptom onset and durvalumab therapy without signs of connective tissue disease (CTD) or pulmonary arterial hypertension (PAH) prior to starting durvalumab, and given the lack of alternative causes for PAH development, the patient was diagnosed with PAH associated with SLE/Sjogren’s syndrome overlap induced by PD-L1 inhibitor therapy.
The patient was treated with high-flow oxygen, dobutamine, norepinephrine, furosemide, intravenous treprostinil, and sildenafil for severe PAH with RV failure and hypoxemic respiratory failure. Unfortunately, she exhibited refractory RV failure with cardiogenic shock, and ultimately support was withdrawn.
Discussion
To the best of our knowledge, this is the first report of checkpoint inhibitor-associated PAH. Programmed cell death protein 1 (PD-1) and its ligand PD-L1 limit activation of self-reactive T cells and protect against autoimmunity.1 Immune checkpoint inhibitors represent a new generation of cancer therapeutics that, through inhibition of immune checkpoints such as PD-1 or PD-L1, exhibit anti-tumor immunity by inducing removal of malignant cells. Their strong therapeutic potential has led to extensive and increasing clinical use. However, the associated increase in T-cell activation can cause excessive inflammation with development of immune-related adverse events such as pneumonitis, thyroiditis, neuropathy, and hepatitis.2 Furthermore, as checkpoint inhibitor use has increased, mounting evidence points towards the propensity of these drugs to lead to development of autoimmune conditions such as sicca syndrome, inflammatory myopathy, vasculitis, and lupus nephritis. These autoimmune phenomena are due to removal of PD-1-mediated inhibition of immune checkpoints that maintain self-tolerance and normally prevent autoimmunity.3 In patients with preexisting autoimmune disease, checkpoint inhibitor use has also been associated with worsening control or flare of the underlying autoimmune condition.4 In this patient, development of autoimmune conditions such as hypothyroidism and diabetes necessitated cessation of durvalumab. However, autoimmune phenomena can occur up to almost one year after checkpoint inhibitor use, suggesting that the patient’s development of SLE/Sjogren’s syndrome overlap was due to checkpoint inhibitor use as well.5 It is unclear whether she developed a CTD de novo or whether she had subclinical disease unmasked by durvalumab use. However, in either scenario, checkpoint inhibitor therapy led to the development of uncontrolled CTD.
Current evidence suggests that the PD-1/PD-L1 pathway may play a role in PAH development. PD-L1 expression is reduced in lungs of rats with experimental PAH. Regulatory T-cells (Tregs) protect from PAH development by increasing PD-L1 expression, and blocking PD-1/PD-L1 signaling abrogates Treg-mediated protection from experimental PAH.6 These data suggest that inhibition of PD-1/PD-L1 signaling in patients indeed may increase the risk of PAH development. PD-1/PD-L1 inhibition-induced PAH may be mediated by loss of protective Treg signaling and/or by activation of potentially pathogenic self-reactive T cells.7
While the patient presented with volume overload, LV disease and group 2 PH were ruled out by echocardiography and RHC. While ground-glass opacities are not typically thought to be associated with PAH, they have in fact been identified in >40% of PAH patients.8 They are typically seen in severe PAH when a dilated RV leads to LV compression with subsequent pulmonary venous congestion. We suspect this was the case in our patient. Another potential explanation for ground-glass opacity development would be pulmonary veno-occlusive disease, but the patient did not exhibit septal lines or lymphadenopathy, making this entity less likely. While ground-glass opacities could also result from checkpoint inhibitor-induced pneumonitis (indicating group 3 PH), lung parenchyma looked otherwise normal, and the severity of hemodynamic alterations was beyond those expected with group 3 PH.9
While multiple cardiovascular autoimmune phenomena have been associated with immune checkpoint inhibitor therapy, to the best of our knowledge, no invasively confirmed cases of pre-capillary PH due to immune checkpoint inhibitor therapy have been described, thus making our report both clinically relevant and novel. While there is one reported case of pulmonary arterial vasculopathy and RV dysfunction,10 this patient had RV myocarditis and pulmonary vasculitis, thus presenting with an etiology and phenotype that are distinct from a typical PH phenotype with subsequent RV failure. In addition, no hemodynamic data were available in that case, making PH classification difficult. In another case report, also without invasive hemodynamics, PH was associated with left ventricular myocarditis, suggesting a post-capillary etiology of PH.11 This is the first report with a hemodynamically confirmed pre-capillary phenotype.
In summary, we report a novel case of PAH associated with SLE/Sjogren’s syndrome overlap induced by checkpoint inhibitor therapy. While no clear diagnostic criteria exist for checkpoint inhibitor-induced autoimmune disease, the close temporal relationship between durvalumab use and CTD and PAH development and the absence of other potential causes for PAH make checkpoint inhibitor-induced CTD the most likely explanation for this patient’s PAH. With the increasing use of checkpoint inhibitors and the well-known contribution of immune dysregulation and autoimmunity to PAH pathogenesis, these therapies may represent an increasingly prevalent novel risk factor for PAH development.
Acknowledgements
We thank all the health care professionals who participated in the care of this patient.
Footnotes
Authors’ contributions: MG, TL – patient care, manuscript writing; CB, DL, KM, SS – patient care, manuscript editing.
Conflict of interest: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: TL has received consultancy fees from Bayer and Altavant Sciences as well as research reagents from Eli Lilly & Company. He is the site PI for a clinical trial funded by Complexa, Inc.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iD: Matthew Glick https://orcid.org/0000-0002-8110-106X
References
- 1.Francisco LM, Sage PT, Sharpe AH. The pd-1 pathway in tolerance and autoimmunity. Immunol Rev 2010; 236: 219–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stucci S, Palmirotta R, Passarelli A, et al. Immune related adverse events during anticancer immunotherapy: pathogenesis and management (Review). Oncol Lett 2017; 14: 5671–5680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tocut M, Brenner R, Zandman-Goddard G. Autoimmune phenomena and disease in cancer patients treated with immune checkpoint inhibitors. Autoimmun Rev 2018; 17: 610–616. [DOI] [PubMed] [Google Scholar]
- 4.Cappelli LC, Shah AA, Bingham CO. Cancer immunotherapy-induced rheumatic diseases emerge as new clinical entities. RMD Open 2016; 2: e000321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Corrigendum KV. Current diagnosis and management of immune related adverse events (irAEs) induced by immune checkpoint inhibitor therapy. Front Pharmacol 2017; 8: 49. [DOI] [PMC free article] [PubMed]
- 6.Tamosiuniene R, Manouvakhova O, Mesange P, et al. Dominant role for regulatory T cells in protecting females against pulmonary hypertension. Circul Res 2018; 122: 1689–1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Taraseviciene-Stewart L, Nicolls MR, Kraskauskas D, et al. Absence of T cells confers increased pulmonary arterial hypertension and vascular remodeling. Am J Respir Crit Care Med 2007; 175: 1280–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rajaram S, Swift AJ, Condliffe R, et al. CT features of pulmonary arterial hypertension and its major subtypes: a systematic CT evaluation of 292 patients from the ASPIRE Registry. Thorax 2014; 70: 382–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nathan SD, Barbera JA, Gaine SP, et al. Pulmonary hypertension in chronic lung disease and hypoxia. Eur Respir J 2019; 53: 1801914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sanzgiri P, Koppikar S, Krishna BA, et al. Toxicity to immune checkpoint inhibitors presenting as pulmonary arterial vasculopathy and rapidly progressing right ventricular dysfunction. Am J Cancer Case Reports 2019; 7: 1–7. [Google Scholar]
- 11.Baldetti L, Melillo F, Beneduce A, et al. Combined checkpoint inhibitor-associated myocarditis and pulmonary vasculitis mimicking acute pulmonary embolism. Eur Heart J Cardiovasc Imaging 2019; 20: 243. [DOI] [PubMed] [Google Scholar]