Abstract
Extracellular histones released from injured or dying cells following trauma and other severe insults can act as potent damage-associated molecular patterns. In fact, elevated levels of histones are present in human circulation in hyperinflammatory states such as acute respiratory distress syndrome and sepsis. The molecular mechanisms owing to histone-induced pathologies are at the very beginning of elucidating. However, neutralization of histones with antibodies, histone-binding or histone-degrading proteins, and heparan sulfates have shown promising therapeutic effects in pre-clinical acute respiratory distress syndrome and sepsis models. Various cell types undergoing necrosis and apoptosis or activated neutrophils forming neutrophil extracellular traps have been implicated in excessive release of histones which further augments tissue injury and may culminate in multiple organ failure. At the molecular level, an uncontrolled inflammatory cascade has been considered as the major event; however, histone-activated coagulation and thrombosis represent additional pathologic events reflecting coagulopathy. Furthermore, epigenetic regulation and chemical modifications of circulating histones appear to be critically important in their biological functions as evidenced by increased cytotoxicity associated with citrullinated histone. Herein, we will briefly review the current knowledge on the role of histones in acute respiratory distress syndrome and sepsis, and discuss the future potential of anti-histone therapy for treatment of these life-threatening disorders.
Keywords: histones, acute respiratory distress syndrome (ARDS), sepsis, lung injury, endothelial dysfunction, coagulation
Introduction
Histones represent a group of highly positively charged core nuclear chaperone proteins that undergo various post-translational modifications such as acetylation, methylation, phosphorylation, and ubiquitination to regulate gene expression.1 Five sub-types of histones have been described. H2A, H2B, H3, and H4 known as core histones and H1 and H5 as designated as linker histones remain inert in the nucleus, but once released into extracellular space, they act as damage-associated molecular patters (DAMPs) and may exert profound cytotoxic effects.2,3 Acute respiratory distress syndrome (ARDS) and severe sepsis still remain the most common cause of mortality in critically ill patients.4 Recent studies have linked histones to the proposed pathogenesis of these disorders by associating the levels of circulating histones with severity of illness.5,6 Indeed, therapeutic strategies targeting neutralization and degradation of histones have been proven to be effective in animal models of sepsis and inflammatory lung injury.5,7 These observations prompted further studies to better understand the mechanisms of histone release by affected tissues, role in various types of tissue injury, as well as efforts to develop novel anti-histone therapies to mitigate pathological consequences of extracellular histone release.
Histones as DAMPs in ARDS
Histones are integral structural components of chromatin and play a major role in the epigenetic regulation of DNA. Two functional sub-sets of histones form nucleosome complex: core histones, an octameric nucleosome complex comprised of H2A, H2B, H3, and H4, and linker histones, H1 and H5, connect adjacent nucleosomes.2 Despite their functionality, these essential nuclear proteins turn into DAMPs with potent cytotoxic effects when released extracellularly. Histones are released into the extracellular space from dying or activated cells during sepsis, trauma, ARDS, and other acute organ injuries where they act as potent pro-inflammatory mediators.3 It has been suggested that histones may be released extracellularly in a free form or as a part of a DNA-bound nucleosome.8 The most immediate source of extracellular histones is necrotic cell death when intracellular content is released due to the rupture of the plasma membrane. However, apoptotic cells, through membrane blebs and nucleosomes, and neutrophils, by forming neutrophil extracellular traps (NETs), also release histones.9,10 However, potential differences in cytotoxicity of particular histone subtypes as well as differences between biological activities of free and nucleosome-bound histones present in circulation remain incompletely understood. This review will focus on the role of histones in pathogenesis of lung disorders associated with ARDS and sepsis that are characterized by profound endothelial dysfunction.
A number of studies have demonstrated that histones impair endothelial function which ultimately results in the development of ARDS and sepsis. For instance, a study by Xu et al. showed that extracellular histones, mainly H3 and H4 subunits, caused endothelial cell (EC) death that was prevented by activated protein C (APC) through histone cleavage.7 Likewise, treatment with histone complex containing all sub-types of histones directly caused cell death in cultured epithelial and ECs that were also prevented by pre-treatment with APC.11 Further analysis revealed that NET-induced cell death of epithelial and ECs was due to histones present in the NET, and co-treatment with histone-blocking antibodies or histone-binding glycan, polysialic acid, attenuated NET-caused cytotoxicity. These findings reflect evidence that a feedback amplification loop of cell injury exists and results in the compromised epithelial/endothelial vascular barrier leading to an influx of immune cells, cytokines, and other DAMPs into the lung that may play a crucial role in development and increased severity of ARDS. This vicious cycle of histones release, NET formation, and endothelial damage was illustrated by a study where histones present in the plasma collected from severe trauma patients increased permeability and cytokine production in ECs and induced NET formation and myeloperoxidase release in neutrophils.12
A role of histones in the pathogenesis of ARDS and sepsis has been further investigated in various animal model studies. Histones degradation by APC reduced mortality in mice caused by intravenous injection of histone complex.7 In line with the proposed pathological role of extracellular histones, administration of anti-H4 antibody protected animals from hyperinflammation and sepsis caused by lipopolysaccharide (LPS), cecal ligation and puncture (CLP), and tumor necrosis factor-α.7 Furthermore, mice receiving intravenous boluses of histone mix died within two hours due to lung edema and alveolar hemorrhage.12 These findings suggested a critical role of extracellular histones in developing vascular endothelial dysfunction which contributed to histone-induced mortality.
Western blot analysis of bronchoalveolar lavage (BAL) collected from mice undergoing LPS, immunoglobulin G, or complement component 5 a (C5a)-induced lung injury showed the presence of histones H3 and H4.13 In fact, the release of extracellular histones into BAL was documented in several mouse models: hydrochloric acid-induced ARDS,14 LPS-induced sepsis, or lung injury caused by complement factor C5a.13 Histone release into BAL of C5a-treated mice required the presence of activated neutrophils and was dependent on the expression of C5a receptors, C5aR and C5L2, by neutrophils. Other studies reported inflammation and lung dysfunction caused by direct intratracheal injection of histone complex in rats13 and vascular barrier disruption, infiltration of inflammatory cells into the alveolar space, and robust release of pro-inflammatory cytokines and chemokines in histone-injected mice.12 It was found that histones bind to pulmonary and hepatic EC immediately after administration in mice, and endothelial damage is the earliest pathology that culminates in multiple organ failure.
Analysis of extracellular histone levels in the samples from clinically ill patients revealed striking results. Histone H4 was detected by western blotting in the BAL fluid (BALF) samples from mechanically-ventilated ARDS patients, but was undetectable in BAL from healthy volunteers.13 The plasma levels of histones in ARDS patients detected by ELISA assays were in the range of 29–60 µg/ml compared to 0.37–1.16 µg/ml range in healthy controls. Consistently, extracellular levels of histones were much higher in BALF samples from ARDS patients (50–96 µg/ml) compared to healthy controls (1–3 µg/ml).5 Similarly, circulating histones in patients with trauma-associated lung injury were in the range of 10–230 µg/ml, as compared to blood collected from healthy donors with median 2.3 µg/ml plasma histone.12 Patients with 50 µg/ml or greater histone levels in sera developed respiratory failure. These clinical findings have been recapitulated in cell culture models, where a similar histone dose (50 µg/ml) caused detrimental effects on cultured EC demonstrated by increased endothelial permeability and greater cell death rates. Circulating histone levels also correlated with activation of coagulation in trauma patients as evidenced by increased expression of soluble thrombomodulin.12 Interestingly, BAL from ARDS patients induced epithelial and EC death by upregulating the expression of inflammatory cytokines that can be blocked by heparin or anti-histone H4 antibody.5 These findings reveal a strong link between elevated extracellular histones and ARDS severity which supports the idea of using circulating histones as a diagnostic biomarker. Published studies also strongly suggest that extracellular histones released during a primary insult such as infection or mechanical injury triggers endothelial damage that remains a major factor affecting severity of ARDS and sepsis (Fig. 1). It is also important to note that cell-damaging effects by extracellular histones may lead to release of other DAMPs from affected cells, as illustrated by release of high-mobility group protein box 1 following histone administration in mice.15 Such synergistic effects of DAMPs may ultimately lead to multiple organ failure.
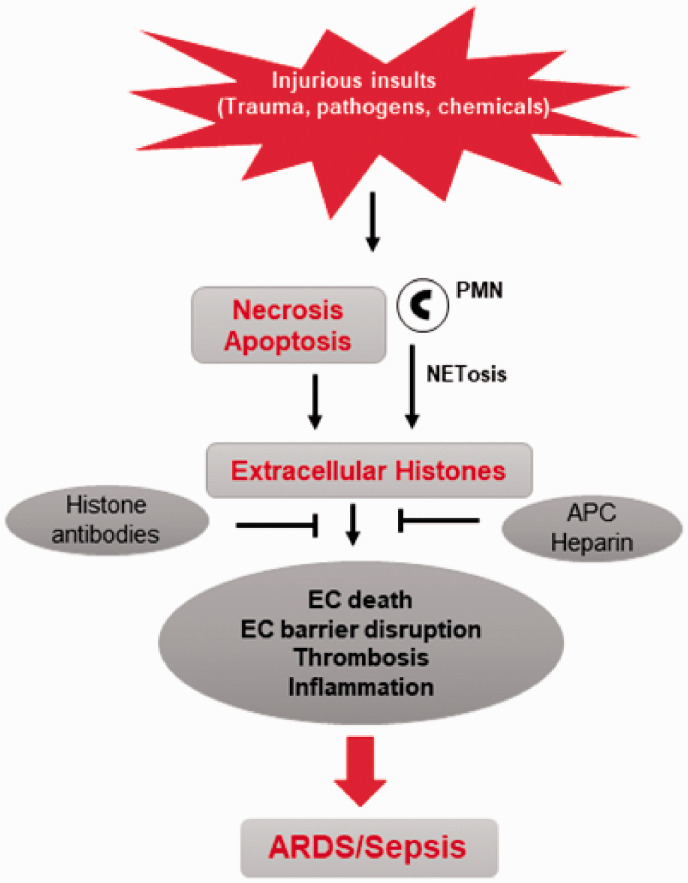
Fig. 1.
Mechanisms of histone release and its role in induction of ARDS. Injurious insults such as trauma and bacterial/viral pathogens induce the release of histones to the extracellular space as result of apoptosis, NETosis, or necrotic cell death. Histones released into circulation contribute to severity of ARDS/sepsis by various mechanisms, including direct endothelial cell death, coagulation, thrombosis, and production of inflammatory cytokines. Histone-blocking antibodies and other histone-neutralizing molecules, such as APC and heparin that bind or degrade histones, inhibit their cytotoxic effects and promote tissue recovery.
EC: endothelial cell; PMN: polymorphonuclear leukocytes; NET: neutrophil extracellular traps; APC: activated protein C; ARDS: acute respiratory distress syndrome.
Mechanisms of histone-induced pathologies
Involvement of toll-like receptors
Endothelial dysfunction and microvascular thrombosis represent the best known pathological events in histone-induced tissue injury and organ failure (Fig. 2). Until now, most of the studies have shown that the involvement of toll-like receptors (TLRs), especially TLR4 and TLR2, is important in mediating toxic effects of histones.16,17 Both TLR2 and TLR4 were activated in histone-challenged mice and also associated with elevated plasma levels of inflammatory cytokines.17 Interestingly, in the model of sterile chemical injury caused by injection of concanavalin A in mice, inflammation and lethality associated with elevation of extracellular histones was dependent on TLR2/4 signaling, since TLR2 or TLR4 knockout mice were resistant to this type of injury.17 Furthermore, blocking of TLR2 and TLR4 with respective monoclonal antibodies in platelets attenuated thrombin generation.16 However, the role of TLRs as a sole mechanism of histone-induced endothelial dysfunction and ensuing lung diseases is doubtful. It is plausible that histones, as potent DAMPs, partially act through TLR-mediated pathways, but their cellular effects are not entirely limited to TLRs activation, as evidenced by failure of TLR4 and TLR2 antibodies to protect against histone-induced endothelial damage driven by increased calcium influx.12 More recent studies provide increasing evidence of TLR-independent mechanisms of histone-induced cytotoxicity, as TLR inhibitors failed to rescue histone-induced apoptosis and autophagy in cultured EC.18 Extracellular histones induce secretion of IL-1β associated with activation of NLR family pyrin domain containing 3 (NLRP3) inflammasome19 and EC permeability,18,20 suggesting the existence of other pathways in histone-induced inflammation. These findings warrant further investigation to identify TLR-independent pathways of cellular dysfunction caused by circulating histones.
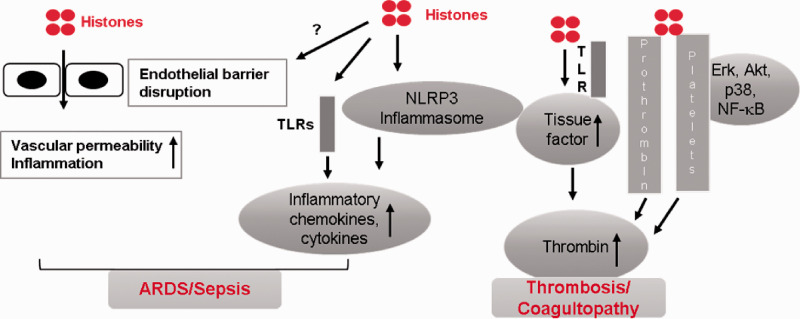
Fig. 2.
Mechanisms of histone-induced toxicity leading to ARDS. Histones stimulate the release of inflammatory chemokines and cytokines via TLR-dependent pathways or by activating NLRP3 inflammasome. Additionally, histones cause endothelial barrier disruption via yet-to-be-investigated mechanisms leading to increased vascular permeability and exacerbation of inflammatory response. Histones also increase thrombin generation by acting through TLRs or by directly binding to prothrombin and platelets. Multiple intracellular signaling pathways involving stress-induced kinases and other signaling kinases mediate histone-induced platelet activation and subsequent thrombin production.
ARDS: acute respiratory distress syndrome; TLR: toll-like receptor.
Histone-induced coagulopathy
Extracellular histones exhibit potent procoagulant activity causing microvascular thrombosis, one of the main clinical manifestations associated with ARDS and sepsis. Involvement of circulating histones in activation of coagulation is complex. Treatment with exogenous histones stimulates the expression of tissue factor in vascular EC via TLR4- and TLR2-dependent mechanisms.21 Administration of histones also enhanced the production of plasma thrombin by impairing anti-coagulant cascades, specifically by inhibiting thrombomodulin-dependent activation of protein C.22 Histone H4 binding to prothrombin causes autoactivation of the latter and leads to thrombin generation.23 Furthermore, histones appear to activate platelets by converting them into procoagulant phenotypes and triggering the generation of thrombin; this process is mediated by TLR2 and TLR4.17 Moreover, histones are shown to directly bind to platelets, induce their aggregation, and cause acute thrombocytopenia in mice.24,25 Histone-induced activation of platelets and generation of thrombin is mediated by activation of extracellular signal-regulated kinase (ERK), Akt, p38, and nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) signaling pathways.26 Heparin, APC, albumin, and recombinant thrombomodulin exhibited potent inhibitory effects on histone-induced coagulation and thrombosis, suggesting their potential as prototype therapeutics preventing histone-dependent endothelial damage and coagulopathy in sepsis and ARDS. In support of this notion, a recent study has suggested that serum histone H3 levels and platelet counts could serve as markers of coagulopathy in septic patients at high risk for death.27
Histone-induced endothelial injury
Increased endothelial permeability leading to an acute inflammatory response due to accumulation of neutrophils and development of pulmonary edema are hallmarks of ARDS and sepsis.28 EC represent a primary target of histone-induced cytotoxicity, as illustrated by a study showing the role of histones in sepsis. Histone-induced EC death was associated with rapid and robust increase in intracellular Ca2+ levels.7 Later studies verified that extracellular histones cause EC damage via their direct interaction with cell membrane and membrane barrier breach causing calcium influx.12 Exogenous histones were found to directly bind to and activate microvascular EC and stimulate the production of proinflammatory cytokines.12,29 Extracellular histones induced autophagy and apoptosis which were mediated by mammalian target of rapamycin-dependent signaling pathway.18 As an alternative mechanism of histone-induced endothelial damage, other studies showed that histone H3 disrupted endothelial monolayer integrity by targeting the breakdown of intercellular adherens junctions and prompting cytoskeletal remodeling with enhanced actin stress fibers formation.20 The authors also founded that histone H3-induced endothelial permeability was not associated with activation of Rho GTPase but was inhibited by cAMP elevating agent forskolin.20
Effects of extracellular histones on other cells
Besides vascular EC, histones target other cells that directly impact endothelial function. NETs are not only the prominent source of circulating histones but can themselves be stimulated by circulating histones and result in the release of myeloperoxidases12 causing generation of reactive oxygen species and further promoting inflammation. As result, NET formation by histone-activated neutrophils may further escalate epithelial and EC death.11 Other circulating cells are also impacted by histones toxicity. For example, extracellular histones have been shown to induce apoptosis in red blood cells by excessive reactive oxygen species generation and calcium influx.30 Morphological analysis of platelets from trauma patients revealed their shape transformation into procoagulant “balloons” phenotype with cell surface coated by histone H4. Likewise, histone H4 treatment of healthy platelets converted them into procoagulant form with increased expression of histone H4 on their cell surface and robust production of H4-containing microparticles.31 In addition, cellular histone H1 release during brain trauma caused neuronal cell death by inducing mitochondrial damage and apoptosis via activation of microglia.32 The brain–lung axis of systemic injury implies that injurious substances released during brain injury can have deleterious effects on lung function. Studies in systemic vasculature showed that histone H4 binds to and induces membrane lysis of smooth muscle cells causing arterial damage and inflammation.33 Collectively, these findings emphasize a fundamental role of circulating histones in propagation of cell damage, inflammation, and barrier dysfunction in various scenarios of organ injury.
Anti-histone therapy in ARDS
Use of antibodies against histone H4 or APC therapy demonstrated reduction of mortality in mice challenged with different sepsis-inducing agents.7 These results provided a strong evidence that neutralization or degradation of histones could be a potential therapeutic approach to treat sepsis, ARDS, and other inflammatory diseases. A plethora of studies in the recent years have substantiated the efficacy of anti-histone treatments in various animal models of lung injury and sepsis. The study by Lv et al. showed that treatment of ECs with histone H4 antibody or heparin protects against ARDS patients BALF-induced cell death.5 Heparin protected against histone-induced toxicity in vitro and in vivo by direct binding to histones, a phenomenon completely independent off heparin anticoagulant activity.34 This feature of heparin was discovered using anti-thrombin affinity chromatography of purified unfractionated heparin. The obtained heparin fraction lacked anticoagulant activity, but was still able to protect against H3-induced EC death and improve the survival of mice with sterile inflammation caused by concanavalin A challenge and mediated by histones.34 Similarly, heparin or its acetylated form ameliorates acid aspiration-induced lung injury associated with increased levels of circulating histone H4.35 Heparin increased survival of rats with multiple organ dysfunction induced by histone H3.36 Heparin also increased survival of mice challenged with concanavalin A, as well as in animal models of LPS- and CLP-induced sepsis.34 C1-esterase inhibitor (C1NH), an endogenous protein that inhibits serine proteases and targets coagulation pathways, has been shown to bind to extracellular histones in vivo and protect against histone-induced lung injury in mice.37 A number of proteins and synthetic chemicals including human C-reactive protein, thrombomodulin, albumin, and polysialic acid also attenuated histone toxicity.25,38–40 Similarly, pentraxin PTX3, a soluble pattern-recognition molecule with immune function, protects against histone-induced EC death during sepsis.41 The formation of PTX3–NET complexes in sepsis patients and the improved survival of PTX3 overexpressing mice from LPS- or CLP-induced sepsis underscores the importance of this molecule against histone-induced diseases.42–44 Recently, an anionic phosphoglycoprotein, osteopontin, has been shown to strongly bind to histones and attenuate their cytotoxicity.45 Higher levels of osteopontin had been detected in BAL from ARDS patients, likely as a self-protecting response. Accordingly, exposure of osteopontin knockout mice to histone or LPS led to development of more severe lung injury.45 A clinical trial of heparin in severe sepsis patients has demonstrated to be safe for use,46 while recombinant protein C was not effective in septic patients.47
Role of citrullination in histones cytotoxicity
Epigenetic post-translational modification of nuclear histones is a classical mechanism of histone-mediated control of cell genome which is out of the scope of this review. However, extracellular histone modification and citrullination has been detected in those inducing cell death and inflammation. Specifically, elevated levels of citrulinated histones had been proposed as a biomarker for certain pathologies including sepsis and advanced cancer.48–50 Inhibition of peptidylarginine deiminase, the enzyme responsible for citrullination of histone, has been found to be protective against LPS-induced lung injury in mice.51 Likewise, Cl-amidine, a pharmacological inhibitor of peptidylarginine deiminase, protects mice from septic shock by inhibiting citrullination of histone H3 and NET formation.52,53 A recent study further substantiated the role of citrullinated histone in lung injury by demonstrating that citrullinated histone H3 peptide is a more potent inducer of endothelial permeability than unmodified H3 and only the former causes NET formation.54 Monoclonal antibody against citrullinated H3 was also effective against LPS-induced ALI with suppression of inflammation and increased survival in mice.54 Future studies should focus on unraveling the mechanisms of citrullination-induced enhanced toxicity of histones and whether histone citrullination is a common phenomenon in various pathological conditions which may make citrullinated histones an ideal prognostic biomarker.
Summary and future perspective
Although a substantial effort has been made to understand the precise role and mechanisms of histone-induced pathological cascades leading to ARDS, the knowledge is still limited, and further studies are required to elaborate on many missing links. It is now widely accepted that vascular endothelium is the primary target of histones but how these DAMPs interact with EC and stimulate them to initiate or propagate inflammation is not completely understood. Both TLR-dependent and TLR-independent pathways are known to be involved in mediating endothelial damage-derived lung dysfunction caused by histones. However, an engagement of other receptors has not been yet fully evaluated. Recent findings suggest that EC death and barrier disruption are also associated with histone-induced injury, and these processes remain to be evaluated. As such, efficacy of apoptotic, necrotic, necroptotic, and authophagic inhibitors in repressing histone cytotoxicity remain to be investigated. Likewise, precise mechanisms of histone-induced endothelial permeability await further characterization due to complexity and diversity of mechanisms regulating endothelial barrier function. A recent report suggests that LPS-induced endothelial barrier disruption can be attenuated by treatment with heparin and is associated with microtubule stabilization.55 Given the major role of microtubule cytoskeleton in dynamic regulation of many cell functions including permeability and inflammation, this mechanism warrants further investigation. Other studies have linked altered calcium signaling to histone-induced endothelial dysfunction,7,12 and a recent study has shown that histone treatment causes vascular dysfunction with the loss of endothelium-mediated dilation of mesenteric arteries by increasing calcium signals in ECs.56 These reports indicate the importance of understanding the exact role of calcium flux in histone-derived cytotoxicity.
Another un-answered question of histone-induced toxicity is why various histone subunits exhibit different degree of harmful effects? Histones form an octamer composed of two copies of each subunit: H2A, H2B, H3, and H4. However, among these four variants, H3 and H4 subunits show potent cytotoxic effects in vitro, while H2A and H2B appear to be neutral (Xu et al.7 and our unpublished data). It is quite intriguing that only a subtle difference in histone subunit structure, with H3/H4 being arginine-rich and the rest being lysine-rich,57 impacts their biological activities to such a great extent. Another interesting question is to determine whether individual histone subunits mimic intact octamer in their biological function and at which form they prevail in circulation? There is a gap in our knowledge on whether released histones may be re-internalized back to cells. Thus, existing data indicate a stunning complexity of cellular effects exhibited by extracellular histones, which appear to be associated with histone protein structure, positive charge, and receptor-independent electrostatic interactions with negatively charged cell surface molecules, as well as activation of receptor-mediated signaling cascades causing barrier dysfunction, inflammation, and other deleterious effects.
Detection of extracellular histones in BAL and plasma samples from ARDS patients5,12,13 brings to light a new opportunity for monitoring the histone levels of these DAMPs which could be potentially employed as diagnostic and prognostic biomarker of disease. However, to successfully achieve these goals, a daunting challenge lies in the substantial improvement of the detection methods. Conventional ELISA and Western blotting techniques to measure histone levels in patient samples so far need to be replaced with more sensitive and real-time monitoring assays such as detection by probes in situ. A careful analysis of histone subtypes also needs to be done because of the sharp variation in their toxicity. Additionally, more advanced detection assays/kits still need to be developed for analysis of modified histones, since some histone modifications, such as citrullination, could considerably enhance histone toxicity54,58 and should be accounted for. As histones serve as an integral part of circulating nucleosomes, analysis of nucleosome levels and their correlation with disease severity could be another potential approach to utilize these complexes as biomarkers.
The other major area of investigation is on elucidating the molecular mechanisms of histone cytotoxicity. A comprehensive knowledge of the signaling pathways that mediate histone-induced diseases will help identify novel molecular targets that can be developed into therapeutics against histone toxicity. Current studies are focussed on the identification of several proteins that bind and degrade histones. Further analysis of mechanisms of binding of histones with target proteins will assist in developing optimal candidates that are more efficient and effective in neutralizing histone toxicity in vivo. Finally, exploring the role of extracellular histones in other lung diseases would be also valuable in developing therapeutics against these diseases. For instance, epigenetic regulation and histone modifications have already been implicated in pulmonary hypertension (PH),59 thus it would be interesting to investigate if extracellular histones play any role in advanced stages of PH. One can speculate that histone-induced deregulation of EC-dependent pulmonary vascular tone and augmented coagulation could directly impact the onset and severity of PH. Furthermore, histone-induced endothelial activation and thrombosis has been observed in cerebral malaria and histones from malarial parasite causes endothelial dysfunction.60,61 These findings clearly indicate that pathological effects of extracellular histones are not limited to ARDS and sepsis, but could potentially be implicated in a broader range of cardiopulmonary diseases.
Acknowledgements
The authors wish to thank Fahid Alghanim, MD (Pulmonary and Critical Care, University of Maryland School of Medicine) for valuable comments during revision of the manuscript.
Footnotes
Author contributions: P.K. drafted the article and made appropriate revisions; K.G.B. designed the review outline and edited the content; A.A.B. designed the review outline, revised the manuscript, and approved the version to be published.
Conflict of interest: The author(s) declare that there is no conflict of interest.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by National Institutes of Health grants GM122940 for K.G.B. and HL107920, HL130431, and GM114171 for A.A.B.
ORCID iD: Pratap Karki https://orcid.org/0000-0002-3087-5516
References
- 1.Berger SL. Histone modifications in transcriptional regulation. Curr Opin Genet Dev 2002; 12: 142–148. [DOI] [PubMed] [Google Scholar]
- 2.Felsenfeld G, Groudine M. Controlling the double helix. Nature 2003; 421: 448–453. [DOI] [PubMed] [Google Scholar]
- 3.Chen R, Kang R, Fan XG, et al. Release and activity of histone in diseases. Cell Death Dis 2014; 5: e1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Auriemma CL, Zhuo H, Delucchi K, et al. Acute respiratory distress syndrome-attributable mortality in critically ill patients with sepsis. Intensive Care Med 2020; 46: 1222–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lv X, Wen T, Song J, et al. Extracellular histones are clinically relevant mediators in the pathogenesis of acute respiratory distress syndrome. Respir Res 2017; 18: 165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xu Z, Huang Y, Mao P, et al. Sepsis and ARDS: the dark side of histones. Mediators Inflamm 2015; 2015: 205054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu J, Zhang X, Pelayo R, et al. Extracellular histones are major mediators of death in sepsis. Nat Med 2009; 15: 1318–1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marsman G, Zeerleder S, Luken BM. Extracellular histones, cell-free DNA, or nucleosomes: differences in immunostimulation. Cell Death Dis 2016; 7: e2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu D, Ingram A, Lahti JH, et al. Apoptotic release of histones from nucleosomes. J Biol Chem 2002; 277: 12001–12008. [DOI] [PubMed] [Google Scholar]
- 10.Brinkmann V, Reichard U, Goosmann C, et al. Neutrophil extracellular traps kill bacteria. Science 2004; 303: 1532–1535. [DOI] [PubMed] [Google Scholar]
- 11.Saffarzadeh M, Juenemann C, Queisser MA, et al. Neutrophil extracellular traps directly induce epithelial and endothelial cell death: a predominant role of histones. PLoS One 2012; 7: e32366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abrams ST, Zhang N, Manson J, et al. Circulating histones are mediators of trauma-associated lung injury. Am J Respir Crit Care Med 2013; 187: 160–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bosmann M, Grailer JJ, Ruemmler R, et al. Extracellular histones are essential effectors of C5aR- and C5L2-mediated tissue damage and inflammation in acute lung injury. FASEB J 2013; 27: 5010–5021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang Y, Wen Z, Guan L, et al. Extracellular histones play an inflammatory role in acid aspiration-induced acute respiratory distress syndrome. Anesthesiology 2015; 122: 127–139. [DOI] [PubMed] [Google Scholar]
- 15.Kawai C, Kotani H, Miyao M, et al. Circulating extracellular histones are clinically relevant mediators of multiple organ injury. Am J Pathol 2016; 186: 829–843. [DOI] [PubMed] [Google Scholar]
- 16.Semeraro F, Ammollo CT, Morrissey JH, et al. Extracellular histones promote thrombin generation through platelet-dependent mechanisms: involvement of platelet TLR2 and TLR4. Blood 2011; 118: 1952–1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu J, Zhang X, Monestier M, et al. Extracellular histones are mediators of death through TLR2 and TLR4 in mouse fatal liver injury. J Immunol 2011; 187: 2626–2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ibanez-Cabellos JS, Aguado C, Perez-Cremades D, et al. Extracellular histones activate autophagy and apoptosis via mTOR signaling in human endothelial cells. Biochim Biophys Acta Mol Basis Dis 2018; 1864: 3234–3246. [DOI] [PubMed] [Google Scholar]
- 19.Allam R, Darisipudi MN, Tschopp J, et al. Histones trigger sterile inflammation by activating the NLRP3 inflammasome. Eur J Immunol 2013; 43: 3336–3342. [DOI] [PubMed] [Google Scholar]
- 20.Meegan JE, Yang X, Beard RS, Jr, et al. Citrullinated histone 3 causes endothelial barrier dysfunction. Biochem Biophys Res Commun 2018; 503: 1498–1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang X, Li L, Liu J, et al. Extracellular histones induce tissue factor expression in vascular endothelial cells via TLR and activation of NF-kappaB and AP-1. Thromb Res 2016; 137: 211–218. [DOI] [PubMed] [Google Scholar]
- 22.Ammollo CT, Semeraro F, Xu J, et al. Extracellular histones increase plasma thrombin generation by impairing thrombomodulin-dependent protein C activation. J Thromb Haemost 2011; 9: 1795–1803. [DOI] [PubMed] [Google Scholar]
- 23.Barranco-Medina S, Pozzi N, Vogt AD, et al. Histone H4 promotes prothrombin autoactivation. J Biol Chem 2013; 288: 35749–35757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fuchs TA, Bhandari AA, Wagner DD. Histones induce rapid and profound thrombocytopenia in mice. Blood 2011; 118: 3708–3714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lam FW, Cruz MA, Leung HC, et al. Histone induced platelet aggregation is inhibited by normal albumin. Thromb Res 2013; 132: 69–76. [DOI] [PubMed] [Google Scholar]
- 26.Carestia A, Rivadeneyra L, Romaniuk MA, et al. Functional responses and molecular mechanisms involved in histone-mediated platelet activation. Thromb Haemost 2013; 110: 1035–1045. [DOI] [PubMed] [Google Scholar]
- 27.Ito T, Totoki T, Yokoyama Y, et al. Serum histone H3 levels and platelet counts are potential markers for coagulopathy with high risk of death in septic patients: a single-center observational study. J Intensive Care 2019; 7: 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matthay MA, Zemans RL. The acute respiratory distress syndrome: pathogenesis and treatment. Annu Rev Pathol 2011; 6: 147–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang Y, Guan L, Yu J, et al. Pulmonary endothelial activation caused by extracellular histones contributes to neutrophil activation in acute respiratory distress syndrome. Respir Res 2016; 17: 155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yeung KW, Lau PM, Tsang HL, et al. Extracellular histones induced eryptotic death in human erythrocytes. Cell Physiol Biochem 2019; 53: 229–241. [DOI] [PubMed] [Google Scholar]
- 31.Vulliamy P, Gillespie S, Armstrong PC, et al. Histone H4 induces platelet ballooning and microparticle release during trauma hemorrhage. Proc Natl Acad Sci U S A 2019; 116: 17444–17449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gilthorpe JD, Oozeer F, Nash J, et al. Extracellular histone H1 is neurotoxic and drives a pro-inflammatory response in microglia. F1000Res 2013; 2: 148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Silvestre-Roig C, Braster Q, Wichapong K, et al. Externalized histone H4 orchestrates chronic inflammation by inducing lytic cell death. Nature 2019; 569: 236–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wildhagen KC, Garcia de Frutos P, Reutelingsperger CP, et al. Nonanticoagulant heparin prevents histone-mediated cytotoxicity in vitro and improves survival in sepsis. Blood 2014; 123: 1098–1101. [DOI] [PubMed] [Google Scholar]
- 35.Zhang Y, Zhao Z, Guan L, et al. N-acetyl-heparin attenuates acute lung injury caused by acid aspiration mainly by antagonizing histones in mice. PLoS One 2014; 9: e97074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Iba T, Hashiguchi N, Nagaoka I, et al. Heparins attenuated histone-mediated cytotoxicity in vitro and improved the survival in a rat model of histone-induced organ dysfunction. Intensive Care Med Exp 2015; 3: 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wygrecka M, Kosanovic D, Wujak L, et al. Antihistone properties of C1 esterase inhibitor protect against lung injury. Am J Respir Crit Care Med 2017; 196: 186–199. [DOI] [PubMed] [Google Scholar]
- 38.Zlatina K, Saftenberger M, Kuhnle A, et al. Polysialic acid in human plasma can compensate the cytotoxicity of histones. Int J Mol Sci 2018; 19: 1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Abrams ST, Zhang N, Dart C, et al. Human CRP defends against the toxicity of circulating histones. J Immunol 2013; 191: 2495–2502. [DOI] [PubMed] [Google Scholar]
- 40.Nakahara M, Ito T, Kawahara K, et al. Recombinant thrombomodulin protects mice against histone-induced lethal thromboembolism. PLoS One 2013; 8: e75961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Daigo K, Nakakido M, Ohashi R, et al. Protective effect of the long pentraxin PTX3 against histone-mediated endothelial cell cytotoxicity in sepsis. Sci Signal 2014; 7: ra88. [DOI] [PubMed] [Google Scholar]
- 42.Dias AA, Goodman AR, Dos Santos JL, et al. TSG-14 transgenic mice have improved survival to endotoxemia and to CLP-induced sepsis. J Leukoc Biol 2001; 69: 928–936. [PubMed] [Google Scholar]
- 43.Daigo K, Yamaguchi N, Kawamura T, et al. The proteomic profile of circulating pentraxin 3 (PTX3) complex in sepsis demonstrates the interaction with azurocidin 1 and other components of neutrophil extracellular traps. Mol Cell Proteomics 2012; 11: M111 015073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Daigo K, Takamatsu Y, Hamakubo T. The protective effect against extracellular histones afforded by long-pentraxin PTX3 as a regulator of NETs. Front Immunol 2016; 7: 344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kasetty G, Papareddy P, Bhongir RKV, et al. Osteopontin protects against lung injury caused by extracellular histones. Mucosal Immunol 2019; 12: 39–50. [DOI] [PubMed] [Google Scholar]
- 46.Levi M, Levy M, Williams MD, et al. Prophylactic heparin in patients with severe sepsis treated with drotrecogin alfa (activated). Am J Respir Crit Care Med 2007; 176: 483–490. [DOI] [PubMed] [Google Scholar]
- 47.Marti-Carvajal AJ, Sola I, Lathyris D, et al. Human recombinant activated protein C for severe sepsis. Cochrane Database Syst Rev 2012: CD004388. [DOI] [PubMed]
- 48.Thalin C, Lundstrom S, Seignez C, et al. Citrullinated histone H3 as a novel prognostic blood marker in patients with advanced cancer. PLoS One 2018; 13: e0191231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang Y, Li M, Stadler S, et al. Histone hypercitrullination mediates chromatin decondensation and neutrophil extracellular trap formation. J Cell Biol 2009; 184: 205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pan B, Alam HB, Chong W, et al. CitH3: a reliable blood biomarker for diagnosis and treatment of endotoxic shock. Sci Rep 2017; 7: 8972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liang Y, Pan B, Alam HB, et al. Inhibition of peptidylarginine deiminase alleviates LPS-induced pulmonary dysfunction and improves survival in a mouse model of lethal endotoxemia. Eur J Pharmacol 2018; 833: 432–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhao T, Pan B, Alam HB, et al. Protective effect of Cl-amidine against CLP-induced lethal septic shock in mice. Sci Rep 2016; 6: 36696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Biron BM, Chung CS, O'Brien XM, et al. Cl-amidine prevents histone 3 citrullination and neutrophil extracellular trap formation, and improves survival in a murine sepsis model. J Innate Immun 2017; 9: 22–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Deng Q, Pan B, Alam HB, et al. Citrullinated histone H3 as a therapeutic target for endotoxic shock in mice. Front Immunol 2019; 10: 2957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mu S, Liu Y, Jiang J, et al. Unfractionated heparin ameliorates pulmonary microvascular endothelial barrier dysfunction via microtubule stabilization in acute lung injury. Respir Res 2018; 19: 220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Collier DM, Villalba N, Sackheim A, et al. Extracellular histones induce calcium signals in the endothelium of resistance-sized mesenteric arteries and cause loss of endothelium-dependent dilation. Am J Physiol Heart Circ Physiol 2019; 316: H1309–H1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Camerini-Otero RD, Sollner-Webb B, Felsenfeld G. The organization of histones and DNA in chromatin: evidence for an arginine-rich histone kernel. Cell 1976; 8: 333–347. [DOI] [PubMed] [Google Scholar]
- 58.Li Y, Liu B, Fukudome EY, et al. Identification of citrullinated histone H3 as a potential serum protein biomarker in a lethal model of lipopolysaccharide-induced shock. Surgery 2011; 150: 442–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gamen E, Seeger W, Pullamsetti SS. The emerging role of epigenetics in pulmonary hypertension. Eur Respir J 2016; 48: 903–917. [DOI] [PubMed] [Google Scholar]
- 60.Moxon CA, Alhamdi Y, Storm J, et al. Parasite histones mediate leak and coagulopathy in cerebral malaria. bioRxiv. Epub ahead of print 2019. http://biorxiv.org/content/early/2019/03/20/563551.
- 61.Gillrie MR, Lee K, Gowda DC, et al. Plasmodium falciparum histones induce endothelial proinflammatory response and barrier dysfunction. Am J Pathol 2012; 180: 1028–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]