Abstract
Background:
The rate of osteoarthritis (OA) in patients with a history of previous anterior shoulder instability (ASI) varies within the literature, with the majority of studies investigating rates after surgical stabilization. ASI appears to lead to increased rates of OA, although risk factors for developing OA in cohorts treated nonoperatively and operatively are not well-defined.
Purpose:
To determine the incidence of clinically symptomatic OA and identify potential risk factors for the development of OA in patients younger than 40 years with a known history of ASI.
Study Design:
Case-control study; Level of evidence, 3.
Methods:
An established, geographically based database was used to identify patients in the United States who were younger than 40 years and were diagnosed with ASI between 1994 and 2014. Patient information, including demographic, imaging, and surgical details, was collected. Comparative analysis was performed between groups with and without OA at final follow-up as well as between patients who underwent surgical and nonsurgical management.
Results:
The study population consisted of 154 patients with a mean follow-up of 15.2 years (range, 5.1-29.8 years). The mean age at initial instability event was 20.9 years (95% CI, 19.9-22.0 years). Overall, 22.7% of patients developed clinically symptomatic glenohumeral OA. Multivariate analysis revealed that current or former smokers (odds ratio [OR], 4.3; 95% CI, 1.1-16.5; P = .030), hyperlaxity (OR, 10.1; 95% CI, 1.4-72.4; P = .020), laborer occupation (OR, 6.1; 95% CI, 1.02-36.1; P = .043), body mass index (BMI) (OR, 1.2; 95% CI, 1.03-1.3; P = .012), and age at initial instability (OR, 1.1; 95% CI, 1.02-1.2; P = .013) as potential independent risk factors when accounting for other demographic and clinical variables.
Conclusion:
In a US geographic population of patients younger than 40 years with ASI, approximately one-fourth of patients developed symptomatic OA at a mean follow-up of 15 years from their first instability event. When accounting for differences in patient demographic and clinical data, we noted a potentially increased risk for the development of OA in patients who are current or former smokers, have hyperlaxity, are laborers, have higher BMI, and have increased age at initial instability event. Smoking status, occupation, and BMI are modifiable factors that could potentially decrease risk for the development of symptomatic OA in these patients.
Keywords: anterior shoulder instability, glenohumeral, shoulder, osteoarthritis
Glenohumeral instability is a common musculoskeletal problem in the younger population, particularly in military personnel and athletes.12,17 The majority of shoulder instability episodes and dislocations are in the anterior direction, with reported rates ranging between 74% and 80%.3,13,18 To allow for greater range of motion, the bony anatomic structure of the glenohumeral joint has an inherent degree of laxity that is largely compensated by dynamic and static soft tissue stabilizers. Disruption of these stabilizers can lead to recurrent instability and altered biomechanics.19 Thus, the purpose of both surgical stabilization and nonoperative treatment is to restore or improve shoulder stability in the short term in order to avoid development of potential short- and long-term sequelae, such as recurrent instability and osteoarthritis (OA).
The prognosis of anterior shoulder instability (ASI) has been well-studied in both cohorts treated nonoperatively5,10,16 and operatively.1–7,14,20,24 Neer et al15 were among the first authors to describe glenohumeral arthritis as a consequence of recurrent shoulder instability, and numerous studies since then have identified a high rate of glenohumeral arthropathy.2,3,6,7,16 Hovelius and Saeboe10 reported that 55% of patients who sustained a first-time anterior shoulder dislocation before the age of 40 years displayed some degree of arthropathy on radiographs after 25-year follow-up. Ogawa et al16 performed a preoperative analysis of 282 patients with an anterior dislocation event before age 40 that revealed arthritic changes in 31% of patients on computed tomography scan. Other studies have shown an increase in glenohumeral arthritis after surgical stabilization for ASI.2,3,7,14,20
Although the development of OA has been brought to the forefront, less is known about the potential risk factors for development of OA in patients with ASI. In patients undergoing surgical stabilization for ASI, several risk factors have been identified for the development of glenohumeral arthritis, including older age at the initial dislocation, older age at surgery, increased number of dislocations, increased length of time from the initial dislocation until surgery, increased number of anchors used at surgery, and a more degenerated labrum at the time of surgery.2,3,7,14,20 Patients with primary anterior shoulder dislocation treated nonoperatively were more likely to develop arthropathy if they experienced the primary dislocation when older than 25 years, if they developed persistent recurrence, if the dislocation was due to high-energy sports, or if they had a history of alcohol abuse.10 Information is also lacking about the proportion of patients with evidence of OA on imaging studies who go on to develop arthritic symptoms. Although patients undergoing nonoperative and operative management of anterior shoulder dislocations and instability have been investigated, these cohorts have not been compared longitudinally in a single patient population or included a population-based cohort in the United States. Therefore, the purpose of our study was to (1) identify the incidence of OA in patients undergoing nonoperative or operative treatment for ASI that initially occurred before the age of 40 years and (2) determine risk factors for the development of glenohumeral OA in patients with ASI.
Methods
Patient Population
This study was approved by an institutional review board. Patients who were younger than 40 years at the time of diagnosis of ASI were identified through use of the Rochester Epidemiology Project (REP). The REP is a medical record linkage system that has collected all health care information for residents of southeastern Minnesota and southwestern Wisconsin in the United States between 1966 and the present day.23 The methodology and generalizability of the REP to the US population have been reported in detail.22,23
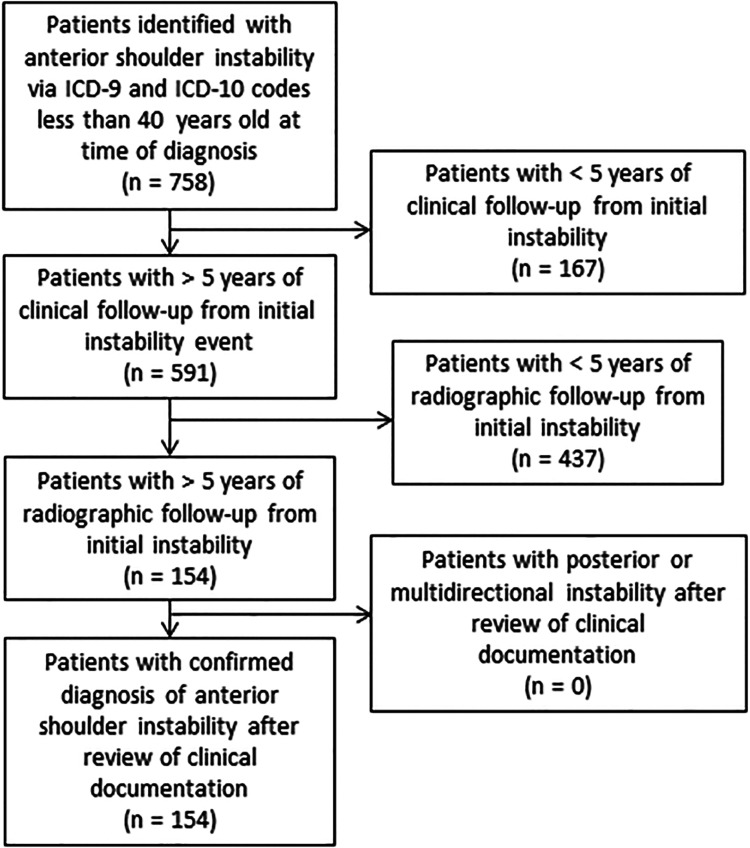
Patients were identified by use of International Classification of Diseases, Ninth Revision and Tenth Revision, codes for shoulder instability (Figure 1). The REP data collected between January 1, 1994, and July 31, 2014, were searched for diagnostic codes relating to ASI events. ASI was then confirmed by review of each patient’s medical record. Patients were considered to have experienced an ASI event if they had a clinical diagnosis as well as imaging or surgical findings supporting the diagnosis, such as an anterior glenohumeral dislocation on radiograph, a Hill-Sachs or bony Bankart lesion on radiograph, or magnetic resonance imaging (MRI) or intraoperative evidence of an anterior-inferior labral tear. Inclusion criteria for the study consisted of (1) 1 or more ASI events confirmed using clinical documentation, (2) age younger than 40 years at the time of initial instability, (3) a minimum of 5 years between the initial instability event and final clinical visit, and (4) radiographic evaluation >5 years from initial instability event. Exclusion criteria consisted of (1) posterior or (2) multidirectional shoulder instability.
Figure 1.
Flowchart showing study patient selection based on inclusion and exclusion criteria. ICD-9, International Classification of Diseases, Ninth Revision; ICD-10, International Classification of Diseases, Tenth Revision.
Demographic, Imaging, and Surgical Information
Demographic information (including age, sex, body mass index [BMI], practice of sports, hyperlaxity, occupational class), imaging findings (Hill-Sachs, bony Bankart, labral pathology, cartilage injury), and procedural information (surgical approach, labral repair, rotator cuff repair, conversion to arthroplasty) was obtained directly from the patient’s medical record (Table 1). Imaging obtained at the initial evaluation and subsequent clinical visits was determined by the treating physician. Radiographic evaluation included at least 2 views of the affected shoulder, but the vast majority of patients were evaluated using 3 or 4 views, which included a combination of anterior-posterior with internal rotation and external rotation, Grashey, scapular-Y, and axillary views. No protocol was in place to obtain baseline MRI. A total of 99 of the 154 patients (64%) underwent MRI of the affected shoulder. Approximately two-thirds of the MRI scans were obtained using intra-articular gadolinium. Noncontrast MRI protocols used T1-weighted, T2-weighted, and proton density sequences to evaluate the bony anatomic features and surrounding soft tissues of the shoulder.
Table 1.
Variables With Their Respective Source Obtained During Retrospective Reviewa
| Source | Variables |
|---|---|
| Clinical notes | Sex Date of birth Date of initial instability/age at initial instability Body mass index Smoking status Medical history (diabetes, hyperlaxity, seizure disorder) Occupation Direction of instability Number of dislocations Number of subluxations Sports participation Acute trauma Recurrent instability |
| Operative notes | Surgery date Open vs arthroscopic surgery Surgical repairs (eg, rotator cuff, labrum, humeral avulsion of the glenohumeral ligament, biceps tendon) Bony procedures (eg, Latarjet) Reoperation |
| Radiographs | Hill-Sachs lesion Bony Bankart lesion Radiographic views Osteoarthritis grade |
| MRI scans | Posterior-superior labral tear Superior labral anterior-posterior tear Posterior-inferior labral tear Anterior-inferior labral tear Bony Bankart lesion Cartilage injury Hill-Sachs lesion Biceps tendon pathology Rotator cuff tear |
aMRI, magnetic resonance imaging.
Rates of clinically symptomatic OA were obtained for patients undergoing surgical management and patients undergoing nonoperative management. Clinically symptomatic OA was defined as the presence of degenerative changes within the glenohumeral joint that warranted physician consultation with subsequent shoulder radiography. This was confirmed via careful review of clinical documentation to confirm symptoms attributed to shoulder OA in combination with the radiographic evidence. Glenohumeral OA grade was classified on follow-up radiographs through use of a modified Samilson and Preito21 classification that was later used by Buscayret et al.2 In this system, stage 1 consists of glenoid or humeral osteophytes measuring <3 mm in greatest diameter; stage 2 consists of osteophytes measuring between 3 and 7 mm in greatest diameter, with mild glenohumeral joint irregularity; stage 3 consists of osteophytes measuring >7 mm with glenohumeral joint narrowing and sclerosis; and stage 4 consists of complete obliteration of the glenohumeral joint space with or without osteophyte formation.2
Risk Factors for OA
The following potential risks factors for the development of OA were assessed in this study: sex, diabetes mellitus, current or former smoker, laborer occupation, seizure disorders, hyperlaxity, mean age at first instability event, acute trauma at initial instability, number of instability events before diagnosis, number of instability events at final follow-up, recurrent instability, sports practiced at time of instability, baseline radiograph with Hill-Sachs lesion, baseline radiograph with bony Bankart lesion, bony Bankart lesion on MRI scan, Hill-Sachs lesion on MRI scan, labral tear on MRI scan, rotator cuff tear on MRI scan, cartilage injury on MRI scan, initial surgical intervention, and revision surgery.
Statistical Analysis
Data were collected and stored in Microsoft Excel (2010; Microsoft Corp). Through univariate analysis, measures of central tendency and dispersion were calculated for demographic, surgical, and imaging variables. The Student t test or Wilcoxon rank sum test was used for continuous variables, and the chi-square test or Fisher exact test was used for categorical variables. All statistical tests were 2-sided, and P values were considered significant if P < .05. For multivariable analysis using significant or clinically important risk factors, we used multivariable logistic regression models. From these models, we reported odds ratios (OR), 95% CI, and likelihood ratio P values. Relative risk (RR) was reported from the univariate analysis. All analyses were performed using SAS JMP Version 14.0 (SAS Inc).
Results
Demographic, Imaging, and Treatment Data
Of the 758 patients with a diagnosis of ASI initially identified via a database inquiry (Figure 1), 154 patients were eligible for inclusion in the current study. The average age at initial instability event was 20.9 years. The mean clinical follow-up time from the first episode of instability was 15.2 years (range, 5.1-29.8 years). Additional patient characteristics are provided in Table 2. Analysis of imaging results showed significantly higher rates of anterior-inferior labral tears (P = .005) and cartilage injuries (P = .028) on MRI scan in the OA group (Table 3). A total of 84 patients underwent surgical management for ASI. In 2 patients with OA, a total shoulder arthroplasty was required after a previous surgical stabilization procedure.
Table 2.
Patient Characteristics at Final Follow-up (N = 154 patients)a
| Characteristic | |
|---|---|
| Male/female, % | 77/23 |
| Age at initial instability, y, mean (95% CI) | 20.9 (19.9-22.0) |
| Follow-up, y, mean (range) | 15.2 (5.1-29.8) |
| Body mass index, mean | 26.9 |
| Current or former smoker, % | 30 |
| Diabetes, % | 2 |
| Hyperlaxity, % | 17 |
| Seizure disorder, % | 6 |
| Laborer occupation, % | 13 |
| Sports participation, % | 57 |
| Contact athlete, % | 36 |
| Throwing or overhead athlete, % | 16 |
| Recurrent instability after consultation, n (%) | 91 (71) |
| Surgery, n (%) | 84 (55) |
| Open/arthroscopic procedure, % of surgery | 28/72 |
| Latarjet, n (% of surgery) | 11 (13) |
| Reoperation 1, n (% of surgery) | 22 (26) |
| Reoperation 2, n (% of reoperation 1) | 4 (18) |
Table 3.
Comparison of Patient Imaging Characteristicsa
| Operative (n = 84) | Nonoperative (n = 70) | P Value | Osteoarthritis (n = 35) | No Osteoarthritis (n = 119) | P Value | |
|---|---|---|---|---|---|---|
| Baseline radiograph | ||||||
| Arthritis | 2 (2) | 1 (1) | ≥.999 | 3 (9) | 0 (0) | .001 |
| Hill-Sachs lesion | 27 (32) | 21 (30) | .729 | 13 (37) | 35 (29) | .301 |
| Bony Bankart lesion | 1 (1) | 7 (10) | .024 | 3 (9) | 5 (4) | .375 |
| Baseline MRI scan | ||||||
| Posterior-superior labral tear | 3 (5) | 2 (6) | ≥.999 | 1 (4) | 4 (5) | ≥.999 |
| Superior labral anterior-posterior tear | 18 (28) | 8 (23) | .811 | 8 (35) | 18 (24) | .418 |
| Posterior-inferior labral tear | 8 (3) | 3 (8) | .743 | 1 (4) | 10 (13) | .450 |
| Anterior-inferior labral tear | 54 (84) | 25 (74) | .283 | 23 (100) | 56 (75) | .005 |
| Cartilage injury | 40 (63) | 18 (53) | .394 | 18 (78) | 40 (53) | .028 |
| Biceps tendon pathology | 4 (6) | 2 (6) | ≥.999 | 2 (9) | 4 (5) | .623 |
| Rotator cuff tear | 12 (19) | 11 (33) | .142 | 5 (22) | 18 (24) | .822 |
| Hill-Sachs lesion | 50 (78) | 21 (62) | .088 | 17 (74) | 54 (72) | .860 |
| Bony Bankart lesion | 20 (31) | 8 (24) | .487 | 8 (35) | 20 (27) | .455 |
aData are reported as n (%). Boldface indicates significance (P < .05). MRI, magnetic resonance imaging.
Subgroup Analysis
Of the 154 patients available at final follow-up, symptomatic glenohumeral arthritis was detected in 35 patients (22.7%). There were 3 patients (1.9%) who had evidence of OA on initial radiograph. Rates of OA on most recent radiographs were 28% and 17% in the operative and nonoperative groups (P = .176), respectively. On final radiograph, 20 patients had stage 1 OA, 12 patients had stage 2, and 3 patients had stage 3 based on the modified Samilson and Prieto classification system.2 No patients had stage 4 OA. No significant differences were found in sex, age at first instability episode, length of follow-up, smoking status, diabetes, hyperlaxity, seizure disorders, laborer occupation, sports participation, or recurrent instability between the operative and nonoperative subgroups (Table 4). Baseline imaging was also similar between groups, with the exception being rate of bony Bankart lesions on radiograph (1% operative vs 10% nonoperative; P = .024) (Table 3).
Table 4.
Characteristics of Patients With and Without Surgery
| Operative (n = 84) |
Nonoperative (n = 70) |
P Value | |
|---|---|---|---|
| Male/female, % | 77/23 | 77/23 | ≥.999 |
| Age at initial instability, y, mean (95% CI) | 20.6 (19.3-22.0) | 21.3 (19.6-23.1) | .767 |
| Follow-up, y, mean | 15.3 | 15.1 | .876 |
| Body mass index, mean | 27.6 | 26.0 | .319 |
| Current or former smoker, % | 33 | 27 | .473 |
| Diabetes, % | 1 | 3 | .594 |
| Hyperlaxity, % | 14 | 21 | .380 |
| Seizure disorder, % | 4 | 9 | .305 |
| Laborer occupation, % | 16 | 9 | .226 |
| Sports participation, % | 62 | 50 | .188 |
| Contact athlete, % | 40 | 29 | .176 |
| Throwing or overhead athlete, % | 17 | 16 | .935 |
| Osteoarthritis at final radiograph, % | 28 | 17 | .176 |
Univariate analysis revealed that patients who developed OA versus those who did not were significantly older at the initial instability event (P = .002) (Table 5). In these 2 groups, we found similar rates of diabetes (P = .668), hyperlaxity (P = .878), overhead/throwing athletes (P = .799), recurrent instability (P = .995), surgical management (P = .176), surgical approach (P = .891), Latarjet procedures (P = .432), and first-time reoperations (P = .782). No difference was found in the number of total dislocation events (OA: 2.9 vs non-OA: 3.6; P = .215) or total instability events (OA: 5.6 vs non-OA: 5.2; P = .593). The percentage of patients developing OA based on number of dislocations or instability was 36.8% with 1 event, 23.1% with 2 events, 20.0% with 3 events, 14.3% with 4 events, and 21.7% with >5 events (P = .59). Additionally, patients with OA at final follow-up were significantly more likely to be current or former smokers (P = .004), have a seizure disorder (P = .033), be manual laborers (P = .044), or have higher BMI (P = .007).
Table 5.
Characteristics of Patients With and Without Symptomatic Osteoarthritis at Final Follow-upa
| Osteoarthritis (n = 35) |
No Osteoarthritis (n = 119) |
P Value | |
|---|---|---|---|
| Male/female, % | 83/17 | 76/24 | .493 |
| Age at initial instability, y, mean (95% CI) | 24.4 (21.7-27.1) | 19.9 (18.8-21.0) | .002 |
| Follow-up, y, mean (range) | 16.9 (5.4-28.9) | 14.6 (5.1-29.8) | .070 |
| Body mass index, mean | 29.1 | 26.2 | .007 |
| Current or former smoker, % | 52 | 24 | .004 |
| Diabetes, % | 3 | 2 | .668 |
| Hyperlaxity, % | 18 | 17 | .878 |
| Seizure disorder, % | 14 | 4 | .033 |
| Laborer occupation, % | 23 | 9 | .044 |
| Total dislocation events per patient, n | 2.9 | 3.6 | .215 |
| Total instability events per patient, n | 5.6 | 5.2 | .593 |
| Sports participation, % | 40 | 61 | .032 |
| Contact athlete, % | 20 | 40 | .043 |
| Throwing or overhead athlete, % | 14 | 17 | .799 |
| Recurrent instability after consultation, n (%) | 16 (73) | 75 (71) | .995 |
| Surgery, n (%) | 23 (66) | 61 (51) | .176 |
| Open/arthroscopic procedure, % of surgery | 27/73 | 29/71 | .891 |
| Latarjet procedure, n (% of surgery) | 2 (9) | 9 (15) | .432 |
| Reoperation 1, n (% of surgery) | 5 (22) | 17 (28) | .782 |
| Reoperation 2, n (% of reoperation 1) | 4 (80) | 0 (0) | <.001 |
aBoldface indicates significance (P < .05).
The variables with statistical significance were seizure disorders (RR, 2.6; 95% CI, 1.3-5.1), cartilage injury evident on initial MRI (RR 2.5, 95% CI, 1.004-6.1), current or former smoking status (RR, 2.5; 95% CI, 1.4-4.4), and laborer occupation (RR, 2.1; 95% CI, 1.1-4.0) (Table 6). Fewer athletes and contact athletes were found in the group that developed symptomatic OA, with relative risks of 0.5 (95% CI, 0.3-0.9) and 0.5 (95% CI, 0.2-0.97), respectively.
Table 6.
Univariate Analysis of Relative Risk for the Development of Osteoarthritis in Patients With Anterior Shoulder Instabilitya
| Relative Risk (95% CI) | P Value | |
|---|---|---|
| Seizure disorder | 2.6 (1.3-5.1) | .0310 |
| Cartilage injury evident on initial MRI | 2.5 (1.004-6.1) | .0283 |
| Current or former smoking status | 2.5 (1.4-4.4) | .0031 |
| Laborer occupation | 2.1 (1.1-4.0) | .0399 |
| Athlete | 0.5 (0.3-0.9) | .0245 |
| Contact athlete | 0.5 (0.2-0.97) | .0236 |
aBoldface indicates significance (P < .05). MRI, magnetic resonance imaging.
Multivariate analysis revealed current or former smoking (OR, 4.3; 95% CI, 1.1-16.5; P = .030), hyperlaxity (OR, 10.1; 95% CI, 1.4-72.4; P = .020), laborer occupation (OR, 6.1; 95% CI, 1.02-36.1; P = .043), BMI (OR, 1.2; 95% CI, 1.03-1.3; P = .012), and age at initial instability (OR, 1.1; 95% CI, 1.02-1.2; P = .013) as potential independent risk factors when accounting for sex, smoking status, BMI, seizure disorder, hyperlaxity, diabetes mellitus, laborer occupation, age at initial instability, sports, number of patient-reported dislocations, and number of patient-reported subluxations (Table 7).
Table 7.
Multivariate Analysis of Potential Risk Factors for the Development of Osteoarthritis in Patients With Anterior Shoulder Instabilitya
| Odds Ratio (95% CI) | P Value | |
|---|---|---|
| Current or former smoker | 4.3 (1.1-16.5) | .030 |
| Hyperlaxity | 10.1 (1.4-72.4) | .020 |
| Laborer occupation | 6.1 (1.02-36.1) | .043 |
| Body mass index | 1.2 (1.03-1.3) | .012 |
| Age at initial instability | 1.1 (1.02-1.2) | .013 |
aMultivariate logistic regression was performed, and odds ratios were calculated accounting for sex, smoking status, body mass index, seizure disorder, hyperlaxity, diabetes mellitus, laborer occupation, age at initial instability, sports, number of patient-reported dislocations, and number of patient-reported subluxations. Boldface indicates significance (P < .05).
Discussion
The major finding in this population-based study is that symptomatic glenohumeral arthritis was detected in 22.7% of patients at an average of 15 years after initial instability event. When we compared rates of OA between patients undergoing operative (28%) and nonoperative (17%) management for ASI, no significant difference was found. Furthermore, multivariate analysis revealed increased odds for developing symptomatic OA in current or former smokers (OR, 4.3) as well as patients with hyperlaxity (OR, 10.1), laborer occupation (OR, 6.1), higher BMI (OR, 1.2), and increased age at initial instability event (OR, 1.1).
The rate of OA in our young patient population with ASI was 22.7% at final follow-up. The most prominent study investigating the natural history of anterior shoulder dislocations was performed in Sweden by Hovelius and Rahme.9 Their 25-year radiographic analysis of 229 shoulders revealed 57% of shoulders with arthropathy. That study is a very impressive epidemiologic study but differs from our study in that it followed all patients after primary anterior shoulder dislocation, which included asymptomatic individuals, and was performed in the Swedish population. Other studies have reported preoperative rates of radiographic shoulder OA ranging from 11% to 25%.3,16 It can be presumed that these studies included symptomatic shoulder OA because those patients went on to have surgical intervention for their shoulder instability. The natural history of glenohumeral arthritis in patients without shoulder instability has been studied as well, with similar rates (16%-18%) found in patients with mean ages ranging from 65 to 72 years. The comparison of these results portrays the accelerated progression of OA in young patients with ASI, as similar rates can be found in patients with “normal” shoulders 2 and 3 times their age. Our reported rate of symptomatic OA as well as those previously reported in the literature are important for surgeon-patient discussions with regard to potential outcomes and prognosis of ASI. Patients should be counseled, even those well under the age of 40, that approximately 20% of patients with ASI in this age group will potentially develop symptomatic glenohumeral arthritis within 15 years.
The development of OA in patients with shoulder instability after various surgical stabilization procedures has been thoroughly investigated. Hurley et al,11 in a systematic review of 822 patients who underwent Latarjet procedure for ASI, showed that 38% of patients had arthritic changes at a mean 16-year follow-up. Outcomes analysis after a modified Bankart repair showed a 14% increase in arthritis at 2 years.1 Rates of arthritis in patients undergoing arthroscopic Bankart repair vary, with one study reporting 21.8% at 8-year follow-up7 and another study reporting that 69% of patients had some degree of OA at 13-year follow-up.20 Fabre et al6 performed open Bankart repair in 49 patients, with 69% having signs of OA at a final follow-up of 28 years. Although the studies are difficult to compare given their heterogeneity, no single procedure has been shown to be superior in preventing or reducing OA, and rates of OA vary greatly depending on the procedure, follow-up time, and patient population. The present study included 84 patients who underwent a surgical stabilization procedure; 13% of those procedures were Latarjet procedures, and 72% were arthroscopic stabilizations. The main goal of operative and nonoperative management in ASI is to improve shoulder stability with both dynamic and static stabilizers and, thus, prevent recurrence. When the shoulder is restored closer to its native biomechanical state, there is a theoretical reduction in abnormal micromotion and less altered joint loading. Surgical stabilization procedures have been shown to improve stability of the glenohumeral joint but do not completely restore it to its native state.19 Despite operative interventions, rates of glenohumeral arthritis remain very high and increase over time.1 The present study did not focus heavily on specific surgical techniques and the development of OA, but our heterogeneous surgically treated subgroup still presented similar rates of OA to those reported in the literature. Furthermore, we found no statistically significant difference in the rates of symptomatic glenohumeral OA in patients in the present study treated with surgical stabilization and those who were treated nonoperatively. These findings raise important questions as to why similar rates were found in our patient population and what factors (eg, number of instability events; time to surgery; additional cartilaginous, bony, and soft tissue injury) may warrant earlier surgical intervention to prevent development of glenohumeral OA.
When accounting for both demographic and clinical variables, the present study demonstrated a potentially increased risk for the development of OA in patients who are current or former smokers, have hyperlaxity, are laborers, have higher BMI, and have increased age at the initial instability event. Older age at the initial instability event or dislocation is a commonly reported risk factor in the literature,2,3,7,8,20 although 1 study showed no significant correlation with age.6 Medical comorbidities, such as obesity and diabetes, and other modifiable risk factors, such as smoking status and occupation, were not previously shown to contribute to the development in OA. In the present study, patients with ASI were 1.12 times more likely to develop symptomatic OA with each additional BMI unit. Smoking was more prevalent in the group with OA compared with the group that did not develop OA. Multivariate analysis revealed that current or former smokers with ASI were 4.3 times more likely to develop symptomatic OA than were nonsmokers. Patients with a laborer occupation, another modifiable risk factor, were found be 6.1 times more likely to develop symptomatic OA in the setting of ASI than were patients who were nonlaborers. To our knowledge, BMI, smoking status, and occupation have yet to be reported in the literature as potential risk factors for OA. These are modifiable risk factors that may negatively affect patient outcomes and should be discussed when patients are counseled about their diagnosis and prognosis.
Limitations
The present study is not without limitations. The results were based on analysis after retrospective review of patients with ASI and, thus, were subject to inherent biases of that process. This limitation includes recall bias from patient-reported dislocations and instability events. Our patient population was relatively small, but this limitation was due to geographic constraints and a lack of adequate clinical and/or radiographic follow-up. Furthermore, our definition of symptomatic OA assumed that patients who consulted a physician and underwent shoulder radiography, in the presence of OA, were experiencing shoulder pain as a result of glenohumeral OA. There is a possibility that patients had a different reason for consulting a physician, but we decided it was fair to assume that most patients had symptomatic OA. Additionally, the study population was quite heterogeneous, specifically with regard to surgical treatment. The study involved multiple surgeons and treatment centers, and the choice of surgical versus nonsurgical treatment was based on surgeon preference and experiences. No protocol was in place regarding treatment or postoperative rehabilitation, which may have altered patient outcomes. Last, the included patient cohort may have been subject to sampling bias, but our inclusion criteria were chosen to investigate patients with appropriate length of radiographic and clinical follow-up to allow ample time for them to develop and be evaluated for symptomatic glenohumeral OA.
Conclusion
In this US community-based population of patients younger than 40 years with ASI, approximately one-fourth of patients developed symptomatic OA at a mean follow-up of 15 years from their first index instability event. When accounting for differences in patient demographic and clinical data, we found a potentially increased risk for the development of OA in patients who are current or former smokers, have hyperlaxity, are laborers, have higher BMI, and have increased age at the initial instability event. Smoking status, occupation, and BMI are modifiable factors that could potentially decrease the risk for the development of symptomatic OA in these patients.
Footnotes
Final revision submitted May 2, 2020; accepted May 22, 2020.
One or more of the authors has declared the following potential conflict of interest or source of funding: A.J.K. has received research support from Aesculap/B.Braun, Arthritis Foundation, Ceterix, Exactech, Gemini Medical, and Histogenics; consulting fees from Arthrex, JRF Ortho, and Vericel; and royalties from Arthrex; he is a board or committee member for the Musculoskeletal Transplantation Foundation and has stock/stock options in Responsive Arthroscopy. D.L.D. has received research support from Arthrex and is a member of the NBA/GE Strategic Advisory Board; her spouse receives royalties from and owns stock in Sonex Health and Tenex Health. J.S.-S. has received research support from Stryker; educational support from Arthrex; consulting fees from Acumed, Exactech, Stryker, Tornier, and Wright Medical; speaking fees from Acumed, Stryker, and Wright Medical; nonconsulting fees from Merck Sharp & Dohme; and royalties from Elsevier, Oxford University Press, and Stryker. C.L.C. has received educational support from Arthrex and hospitality payments from Zimmer Biomet. AOSSM checks author disclosures against the Open Payments Database (OPD). AOSSM has not conducted an independent investigation on the OPD and disclaims any liability or responsibility relating thereto.
Ethical approval for this study was obtained from Mayo Clinic (study ID: 16-007084).
References
- 1. Boileau P, Fourati E, Bicknell R. Neer modification of open Bankart procedure: what are the rates of recurrent instability, functional outcome, and arthritis? Clin Orthop Relat Res. 2012;470(9):2554–2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Buscayret F, Edwards TB, Szabo I, et al. Glenohumeral arthrosis in anterior instability before and after surgical treatment: incidence and contributing factors. Am J Sports Med. 2004;32(5):1165–1172. [DOI] [PubMed] [Google Scholar]
- 3. Cameron ML, Kocher MS, Briggs KK, Horan MP, Hawkins RJ. The prevalence of glenohumeral osteoarthrosis in unstable shoulders. Am J Sports Med. 2003;31(1):53–55. [DOI] [PubMed] [Google Scholar]
- 4. Cheung EV, Sperling JW, Hattrup SJ, Cofield RH. Long-term outcome of anterior stabilization of the shoulder. J Shoulder Elbow Surg. 2008;17(2):265–270. [DOI] [PubMed] [Google Scholar]
- 5. Dickens JF, Rue JP, Cameron KL, et al. Successful return to sport after arthroscopic shoulder stabilization versus nonoperative management in contact athletes with anterior shoulder instability: a prospective multicenter study. Am J Sports Med. 2017;45(11):2540–2546. [DOI] [PubMed] [Google Scholar]
- 6. Fabre T, Abi-Chahla ML, Billaud A, Geneste M, Durandeau A. Long-term results with Bankart procedure: a 26-year follow-up study of 50 cases. J Shoulder Elbow Surg. 2010;19(2):318–323. [DOI] [PubMed] [Google Scholar]
- 7. Franceschi F, Papalia R, Del Buono A, et al. Glenohumeral osteoarthritis after arthroscopic Bankart repair for anterior instability. Am J Sports Med. 2011;39(8):1653–1659. [DOI] [PubMed] [Google Scholar]
- 8. Haas M, Plachel F, Wierer G, et al. Glenoid morphology is associated with the development of instability arthropathy. J Shoulder Elbow Surg. 2019;28(5):893–899. [DOI] [PubMed] [Google Scholar]
- 9. Hovelius L, Rahme H. Primary anterior dislocation of the shoulder: long-term prognosis at the age of 40 years or younger. Knee Surg Sports Traumatol Arthrosc. 2016;24(2):330–342. [DOI] [PubMed] [Google Scholar]
- 10. Hovelius L, Saeboe M. Neer award 2008: arthropathy after primary anterior shoulder dislocation—223 shoulders prospectively followed up for twenty-five years. J Shoulder Elbow Surg. 2009;18(3):339–347. [DOI] [PubMed] [Google Scholar]
- 11. Hurley ET, Jamal MS, Ali ZS, et al. Long-term outcomes of the Latarjet procedure for anterior shoulder instability: a systematic review of studies at 10-year follow-up. J Shoulder Elbow Surg. 2019;28(2):e33–e39. [DOI] [PubMed] [Google Scholar]
- 12. Kawasaki T, Ota C, Urayama S, et al. Incidence of and risk factors for traumatic anterior shoulder dislocation: an epidemiologic study in high-school rugby players. J Shoulder Elbow Surg. 2014;23(11):1624–1630. [DOI] [PubMed] [Google Scholar]
- 13. Kraeutler MJ, McCarty EC, Belk JW, et al. Descriptive epidemiology of the MOON shoulder instability cohort. Am J Sports Med. 2018;46(5):1064–1069. [DOI] [PubMed] [Google Scholar]
- 14. Krueger D, Kraus N, Pauly S, Chen J, Scheibel M. Subjective and objective outcome after revision arthroscopic stabilization for recurrent anterior instability versus initial shoulder stabilization. Am J Sports Med. 2011;39(1):71–77. [DOI] [PubMed] [Google Scholar]
- 15. Neer CS II, Watson KC, Stanton FJ. Recent experience in total shoulder replacement. J Bone Joint Surg Am. 1982;64(3):319–337. [PubMed] [Google Scholar]
- 16. Ogawa K, Yoshida A, Ikegami H. Osteoarthritis in shoulders with traumatic anterior instability: preoperative survey using radiography and computed tomography. J Shoulder Elbow Surg. 2006;15(1):23–29. [DOI] [PubMed] [Google Scholar]
- 17. Owens BD, Dawson L, Burks R, Cameron KL. Incidence of shoulder dislocation in the United States military: demographic considerations from a high-risk population. J Bone Joint Surg Am. 2009;91(4):791–796. [DOI] [PubMed] [Google Scholar]
- 18. Owens BD, Duffey ML, Nelson BJ, et al. The incidence and characteristics of shoulder instability at the United States Military Academy. Am J Sports Med. 2007;35(7):1168–1173. [DOI] [PubMed] [Google Scholar]
- 19. Peltz CD, Baumer TG, Mende V, et al. Effect of arthroscopic stabilization on in vivo glenohumeral joint motion and clinical outcomes in patients with anterior instability. Am J Sports Med. 2015;43(11):2800–2808. [DOI] [PubMed] [Google Scholar]
- 20. Plath JE, Aboalata M, Seppel G, et al. Prevalence of and risk factors for dislocation arthropathy: radiological long-term outcome of arthroscopic Bankart repair in 100 shoulders at an average 13-year follow-up. Am J Sports Med. 2015;43(5):1084–1090. [DOI] [PubMed] [Google Scholar]
- 21. Samilson RL, Prieto V. Dislocation arthropathy of the shoulder. J Bone Joint Surg Am. 1983;65(4):456–460. [PubMed] [Google Scholar]
- 22. St Sauver JL, Grossardt BR, Yawn BP, et al. Data resource profile: the Rochester Epidemiology Project (REP) medical records-linkage system. Int J Epidemiol. 2012;41(6):1614–1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. St Sauver JL, Grossardt BR, Yawn BP, Melton LJ III, Rocca WA. Use of a medical records linkage system to enumerate a dynamic population over time: the Rochester Epidemiology Project. Am J Epidemiol. 2011;173(9):1059–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zaffagnini S, Marcheggiani Muccioli GM, Giordano G, et al. Long-term outcomes after repair of recurrent post-traumatic anterior shoulder instability: comparison of arthroscopic transglenoid suture and open Bankart reconstruction. Knee Surg Sports Traumatol Arthrosc. 2012;20(5):816–821. [DOI] [PubMed] [Google Scholar]