Abstract
Two oral medications targeting the prostacyclin pathway are available to treat pulmonary arterial hypertension in the United States: oral treprostinil and selexipag. We compared real-world hospitalization in patients receiving these medications. A retrospective administrative claims study was conducted using the Optum® Clinformatics® Data Mart database. Patients with pulmonary hypertension were identified using diagnostic codes. Cohort inclusion required age ≥ 18 years, first oral treprostinil or selexipag prescription between 1 January 2015 and 30 September 2017 (index date), and continuous enrollment in the prior ≥6 months. Patients who switched index drug were excluded. Follow-up was from index date until the first of end of index drug exposure, end of continuous enrollment, death, or 31 December 2017. Multivariable Cox proportional hazard and Poisson regression were used to compare risk and rate, respectively, of hospitalization associated with oral treprostinil vs. selexipag, adjusting for potential confounders. The study cohort included 99 patients receiving oral treprostinil and 123 receiving selexipag. Mean age was 61 years, and most patients were females (71%). Compared with oral treprostinil, selexipag was associated with a 46% lower risk of all-cause hospitalization (hazard ratio 0.54, 95% confidence interval 0.31, 0.92; P = 0.02), a 47% lower risk of pulmonary hypertension-related hospitalization (hazard ratio 0.53, 95% confidence interval 0.31, 0.93; P = 0.03), a 42% lower all-cause hospitalization rate (rate ratio 0.58, 95% confidence interval 0.39, 0.87; P = 0.01), and a 46% lower pulmonary hypertension-related hospitalization rate (rate ratio 0.54, 95% confidence interval 0.35, 0.82; P = 0.004). This study suggests that selexipag is associated with lower hospitalization risk and rate than oral treprostinil.
Keywords: pulmonary hypertension, selexipag, oral treprostinil, hospitalization, retrospective database analysis
Until recently, available therapies for patients with pulmonary arterial hypertension (PAH) targeting the prostacyclin pathway have been administered via continuous intravenous, subcutaneous infusion, or inhaled routes, all of which are associated with significant administration-related adverse effects and patient burden.1 Oral therapy offers greater convenience and could enable the use of these therapies earlier in the disease evolution.2 Two oral prostacyclin pathway medications, oral treprostinil and selexipag, were approved by the Food and Drug Administration in December 2013 and December 2015, respectively.3
The oral formulation of the prostacyclin analog treprostinil was approved to improve exercise capacity on the basis of the FREEDOM-M randomized, controlled trial (RCT) in patients not on background PAH therapy, which met its primary end point of change from baseline to week 12 in 6-min walk distance (6MWD).4,5 However, this primary end point was not met in the FREEDOM-C1 and FREEDOM-C2 RCTs in patients on background endothelin receptor antagonist (ERA) and/or phosphodiesterase type 5 inhibitor (PDE5i) therapy.6,7
The selective prostaglandin I2 (IP) prostacyclin-receptor agonist selexipag was approved to delay disease progression and reduce the risk of hospitalization for PAH on the basis of the event-driven, placebo-controlled GRIPHON RCT, which included patients not receiving background therapy, patients receiving background monotherapy with an ERA or PDE5i, and patients receiving background dual therapy with an ERA and a PDE5i.8,9 GRIPHON met its primary end point of time to a morbidity event or death from any cause.8 The treatment effect of selexipag on the primary end point was similar in the overall population, the subgroup of patients not receiving PAH-specific treatment at baseline, and the subgroup on background treatment (including patients receiving a combination of an ERA and a PDE5i at baseline).8,10
Shifting of the primary trial end point from a short-term correlate such as 6MWD, as in FREEDOM-M, to a long-term true clinical efficacy measure such as clinical worsening, as in GRIPHON, represents an important evolution in PAH RCT design.11 This shift was recommended at the fourth World Symposium on Pulmonary Hypertension (WSPH) and endorsed at the fifth WSPH.12,13 Clinical worsening in PAH leads to increasingly debilitating symptoms, high morbidity, frequent hospitalizations, and ultimately right-heart failure and premature death.14–17 Thus, delaying clinical worsening is essential to attain the treatment goals for patients with PAH of achieving and maintaining good exercise capacity, quality of life and right-ventricular function, avoiding hospitalization, and improving survival.11,18–20
It has recently been reported that the event-driven, placebo-controlled FREEDOM-EV RCT of oral treprostinil met its primary end point of delayed time to first adjudicated clinical worsening event.21 However, no clinical trials have directly compared oral treprostinil vs. selexipag for clinical worsening (or any other end point). In the absence of head-to-head data, hospitalization data for both agents in real-world use may provide valuable comparative evidence.
PAH-related hospitalization is an important measure of clinical worsening that has been demonstrated to predict increased mortality among patients in clinical practice15,22 and in the long-term, event-driven clinical trials.14 Hospitalization for worsening PAH is a key component of the composite end point of time to clinical worsening recommended at the fourth and fifth WSPH.12,13 Furthermore, hospitalizations in patients with PAH are costly, and readmission is common following discharge.23
Hospitalization data can be extracted from medical administrative claims data, a source of real-world evidence.24 Although RCTs remain the gold standard for the assessment of the efficacy and safety of therapies, real-world evidence is recognized as being an important resource to expand the evidence generated by traditional clinical trials and is increasingly being considered in regulatory decision-making.24–26 Accordingly, the objective of this study was to compare the rate and risk of hospitalization (all-cause and pulmonary hypertension (PH)-related) in patients with PH receiving either oral treprostinil or selexipag, based on a retrospective claims database analysis.
Methods
Data source
Data for this study were obtained from the Optum® Clinformatics® Data Mart healthcare claims database, a longitudinal database of medical and pharmaceutical administrative claims for a privately insured US population. Most of the approximately 82 million unique patients in the database are enrollees in the UnitedHealth Group and have both medical and pharmacy benefits.
All data used in these analyses have been deidentified and are fully compliant with the Health Insurance Portability and Accountability Act Privacy Rules; as such, Institutional Review Board review and approval were not required.
Study design and sample
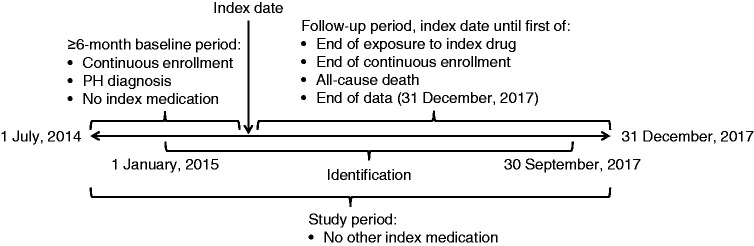
Data were retrieved for the study period commencing 1 July 2014 and ending 31 December 2017 (Fig. 1). A study start date earlier than the launch of selexipag was chosen to capture sufficient data for patients taking oral treprostinil because relatively few patients were prescribed oral treprostinil after the selexipag launch. Any between-group differences in the distribution of prescribing dates should not have had an impact on hospital admission, given the short (2.5-year) study period. The index date was defined as the first date of receipt of oral treprostinil or selexipag within the identification period of 1 January 2015 to 30 September 2017. This allowed for a minimum six-month pre-index and three-month post-index evaluation of each patient's data. The follow-up period for each patient was from their index date until the end of index drug exposure (defined as a gap in therapy of at least 45 days), the end of continuous health-plan enrollment, the date of all-cause death (defined as the first day of the month of death), or the end of data (31 December 2017), whichever came first.
Fig. 1.
Study design. PH: pulmonary hypertension.
Adult patients (at least 18 years of age) were eligible if they had continuous health-plan enrollment for at least six months prior to the index date (termed the baseline period). Because the claims database does not include linked medical charts that would have permitted confirmation of PH diagnoses, patients were ascertained using a claims-based algorithm. Inclusion required at least one medical claim on an outpatient visit or hospitalization in the baseline period with an International Classification of Diseases, Ninth or Tenth Revision, Clinical Modification (ICD-9-CM or ICD-10-CM, respectively) diagnostic code for PH, namely, ICD-9-CM 416.0 (primary PH), 416.8 (other chronic pulmonary heart disease; pulmonary hypertension, secondary), or 416.9 (chronic pulmonary heart disease, unspecified) or ICD-10-CM I27.0 (primary PH), I27.2 (other secondary PH), I27.89 (other specified pulmonary heart diseases), or I27.9 (pulmonary heart disease, unspecified).
To limit the analysis to new users, patients were excluded if they had any use of the index medication in the baseline period. To avoid the crossover effects of therapy, patients with any switching between oral treprostinil and selexipag at any time during the entire study period were also excluded.
Baseline characteristics for each patient were recorded during the baseline period. Demographic variables measured at the index date were age, gender, geographic region, and type of insurance. Clinical comorbidities of interest were the Charlson comorbidity score (calculated based on ICD-9-CM and/or ICD-10-CM codes using the methods of Quan et al.27 with updated weights from Quan et al.28), depression, hypertension, diabetes, chronic obstructive pulmonary disease (COPD), heart failure, hyperlipidemia, obesity, and PAH-related comorbidities (connective tissue diseases (CTDs), congenital heart diseases (CHDs), and portal hypertension). Selected comorbidities were defined based on medical claims using ICD-9-CM and ICD-10-CM codes (see Supplementary Table 1 for diagnostic codes). PAH-related comorbidities were identified by codes used in a previous retrospective database analysis.29 Other selected comorbidities were identified using Medicare Chronic Conditions Data Warehouse algorithms.30
Clinical management variables retrieved were PAH medications, all-cause outpatient visits, all-cause hospitalizations, and PH-related hospitalizations. Other than the index medications (oral treprostinil and selexipag), PAH medications included ERAs (oral ambrisentan, bosentan, and macitentan), PDE5is (oral sildenafil, tadalafil), prostacyclins (subcutaneous, intravenous, and inhaled treprostinil, inhaled iloprost, intravenous epoprostenol), and the oral soluble guanylate cyclase stimulator riociguat. These drugs were identified from pharmacy claims by National Drug Code and from medical claims by Healthcare Common Procedure Coding System codes (see Supplementary Table 1 for drug codes).
Outcomes
Hospitalizations were the only outcomes assessed, as both rates and risks. Hospitalization rate was defined as the number of hospitalizations per person-time. Patients contributed person-time in the study as long as they remained on the index drug, continuously enrolled, and alive. Hospitalization risk was defined as the time to all-cause hospitalization and PH-related hospitalization from an on-treatment analysis with follow-up beginning on the index date and censored at the end of index drug exposure, end of continuous enrollment, all-cause death, or end of data (31 December 2017), whichever occurred earliest. Patients were considered to be on treatment unless they had a ≥45-day gap in therapy. PH-related hospitalization was defined as a hospitalization with a PH diagnosis code at any position on the medical claim.
Statistical analysis
Descriptive statistics for baseline characteristics are reported as counts and percentages for categorical variables and mean and standard deviation for continuous variables. Between-group comparisons were performed using Chi-square or Fisher's exact test for categorical variables and Student's t test for continuous variables.
Kaplan–Meier estimates of survival probabilities were plotted to assess the time to all-cause and PH-related hospitalization. Multivariable Cox proportional hazards regression models were constructed to estimate hazard ratios (HRs) with 95% confidence intervals (CIs) for the risks of first all-cause and PH-related hospitalization in patients using oral treprostinil vs. those using selexipag. The following potential confounders were included as model covariates: age, gender, geographic region, insurance type, Charlson comorbidity score, PAH medication used (ERAs, PDE5is, parenteral and inhaled prostacyclin, and riociguat), specified comorbidities (depression, hypertension, diabetes, COPD, heart failure, hyperlipidemia, and obesity), PAH-associated comorbidities (CTDs, CHDs, and portal hypertension), and the number of hospitalizations and outpatient visits during the baseline period.
Multivariable Poisson regression models were used to calculate rate ratios (RRs) and 95% CIs comparing all-cause hospitalization and PH-related hospitalization rates with oral treprostinil vs. selexipag. These models included the same covariates included in the Cox models and incorporated an offset variable to account for the varying length of person-time.
Sample selection and creation of analytic variables were performed using the Instant Health Data platform (BHE, Boston, MA). Statistical analyses were undertaken with R, version 3.2.1 (R foundation for Statistical Computing, Vienna, Austria).
Results
Patient characteristics
After the application of all eligibility criteria, 222 patients were included in the analysis cohort: 99 treated with oral treprostinil and 123 treated with selexipag (Fig. 2). Notably, these numbers exclude 9 patients in the oral treprostinil group and 14 patients in the selexipag group who took the other drug at some time. Patients were predominantly females (71%), and the gender distribution was similar in both groups (Table 1). Overall, 48% of patients were aged 65 years or older. Although the difference was not statistically significant, the oral treprostinil group, on average, was older than the selexipag group (mean 63.0 vs. 59.5 years, respectively; P = 0.08; Table 1). Most patients resided in the south region of the United States (54%) and were enrolled in Medicare Advantage (66%).
Fig. 2.

Ascertainment of the patient cohort. PH: pulmonary hypertension.
Table 1.
Patient baseline characteristics.
| Characteristic | Oral treprostinil | Selexipag | Study population | P |
|---|---|---|---|---|
| N = 99 | N = 123 | N = 222 | ||
| Age, years, mean (SD) | 63.0 (15.4) | 59.5 (14.5) | 61.1 (14.9) | 0.08 |
| Female, n (%) | 71 (71.7) | 87 (70.7) | 158 (71.2) | 0.99 |
| Geographic region, n (%) | ||||
| Midwest | 21 (21.2) | 18 (14.6) | 39 (17.6) | 0.44 |
| Northeast | 7 (7.1) | 9 (7.3) | 16 (7.2) | |
| South | 54 (54.6) | 66 (53.7) | 120 (54.1) | |
| West | 17 (17.2) | 30 (24.4) | 47 (21.2) | |
| Insurance type, n (%) | ||||
| Commercial | 27 (27.3) | 49 (39.8) | 76 (34.2) | 0.07 |
| Medicare | 72 (72.7) | 74 (60.2) | 146 (65.8) | |
| Charlson comorbidity score, mean (SD) | 3.4 (2.0) | 2.8 (2.1) | 3.1 (2.1) | 0.04 |
| Comorbidities, n (%) | ||||
| COPD | 53 (53.5) | 49 (39.8) | 102 (46.0) | 0.06 |
| Depression | 23 (23.2) | 16 (13.0) | 39 (17.8) | 0.07 |
| Diabetes | 44 (44.4) | 32 (26.2) | 76 (34.2) | 0.01 |
| Heart failure | 53 (53.5) | 64 (52.0) | 117 (52.7) | 0.93 |
| Hyperlipidemia | 55 (55.6) | 52 (42.3) | 107 (48.2) | 0.07 |
| Hypertension | 75 (75.8) | 83 (67.5) | 158 (71.2) | 0.23 |
| Obesity | 28 (28.3) | 28 (22.8) | 56 (25.2) | 0.43 |
| PAH-associated comorbidities, n (%) | ||||
| CTD | 28 (28.3) | 30 (24.4) | 58 (26.1) | 0.62 |
| CHD | 8 (8.1) | 13 (10.6) | 21 (9.5) | 0.69 |
| Portal hypertension | 4 (4.0) | 4 (3.3) | 8 (3.6) | 1.0 |
| PAH medications, n (%) | ||||
| ERAs | 56 (56.6) | 102 (82.9) | 158 (71.2) | <0.0001 |
| PDE5is | 62 (62.6) | 95 (77.2) | 157 (70.7) | 0.03 |
| Inhaled or parenteral prostacyclins | 43 (43.4) | 22 (17.9) | 65 (29.3) | <0.0001 |
| Riociguat | 8 (8.1) | 15 (12.2) | 23 (10.4) | 0.44 |
| Overall PAH medicationsa | 87 (87.9) | 119 (96.8) | 206 (92.8) | 0.02 |
| Number of PH-related hospitalizations, mean (SD) | 0.54 (1) | 0.40 (0.92) | 0.46 (0.96) | 0.30 |
| Number of all-cause hospitalizations, mean (SD) | 0.59 (1.05) | 0.40 (0.92) | 0.48 (0.98) | 0.16 |
| Number of all-cause outpatient visits, mean (SD) | 14.8 (15.0) | 13.5 (13.7) | 14.1 (14.2) | 0.49 |
Note: CHD: congenital heart disease; COPD: chronic obstructive pulmonary disease; CTD: connective tissue disease; ERA: endothelin receptor antagonist; PAH: pulmonary arterial hypertension; PH: pulmonary hypertension; PDE5i: phosphodiesterase type 5 inhibitor; SD: standard deviation.
Overall PAH medication use includes ERAs, PDE5is, parenteral or inhaled prostacyclins, and riociguat.
Compared with the selexipag group, the oral treprostinil group had a significantly higher mean Charlson comorbidity score and a numerically higher prevalence of several selected comorbidities (Table 1). ERAs (71%) and/or PDE5is (71%) were the most common PAH mediations received prior to index drug initiation. Overall PAH medication use at baseline was less common in the oral treprostinil group than in the selexipag group (88% vs. 97%, respectively; P = 0.02). Oral treprostinil recipients were more likely than selexipag recipients to take parenteral or inhaled prostacyclins at baseline (43% vs. 18%, respectively; P < 0.0001) but less likely to take ERAs (57% vs. 83%, respectively; P < 0.0001) and/or PDE5is (63% vs. 77%, respectively; P = 0.03).
Exposure to index drug
The median follow-up was 0.59 years (interquartile range (IQR) 0.27–1.01 years) for patients treated with oral treprostinil and 0.61 years (IQR 0.30–0.98 years) for patients treated with selexipag. The most common reason for censoring was discontinuing index therapy (oral treprostinil 45% and selexipag 38%).
Hospitalization
Over a total of 162.05 person-years of follow-up among the 222 study participants, there were 147 all-cause hospitalizations and 134 PH-related hospitalizations. Overall, 79 patients experienced at least one hospitalization from any cause and 75 patients experienced at least one hospitalization associated with PH.
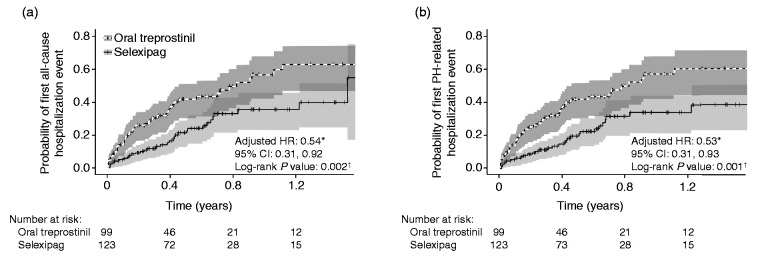
Based on the Kaplan–Meier analysis, patients treated with selexipag had lower risk for all-cause hospitalization (log rank P = 0.002) and lower risk for PH-related hospitalization (log rank P = 0.001) compared with patients on oral treprostinil (Fig. 3). After controlling for potentially confounding baseline characteristics, treatment with selexipag was associated with a 46% lower hazard of all-cause hospitalization (P = 0.02) and a 47% lower hazard of PH-related hospitalization (P = 0.03) relative to treatment with oral treprostinil (Table 2).
Fig. 3.
Kaplan–Meier curves with 95% confidence intervals of (a) first all-cause hospitalization and (b) first PH-related hospitalization in patients receiving oral treprostinil or selexipag. CI: confidence interval; HR: hazard ratio; PAH: pulmonary arterial hypertension; PH: pulmonary hypertension. *Reference group: oral treprostinil; Cox proportional hazard models were adjusted for age, gender, geographic region, insurance type, Charlson comorbidity score, PAH medication, PAH-associated comorbidities, other selected comorbidities, and baseline hospitalization and outpatient visits. †Log rank test.
Table 2.
Number of patients with a hospitalization event by treatment group (N = 222).
| Hospitalization | Patients, n (%) |
Unadjusted HR (95% CI)a | Adjusted HR (95% CI)a,b | |
|---|---|---|---|---|
| Oral treprostinil (N = 99) | Selexipag (N = 123) | |||
| All-cause | 47 (47.5) | 32 (26.0) | 0.5 (0.32, 0.78) | 0.54 (0.31, 0.92) |
| PH-related | 46 (46.5) | 29 (23.6) | 0.45 (0.28, 0.72) | 0.53 (0.31, 0.93) |
Note: CI: confidence interval; HR: hazard ratio; PAH: pulmonary arterial hypertension; PH: pulmonary hypertension.
Reference group: oral treprostinil.
Cox proportional hazards model adjusted for age, gender, geographic region, insurance type, Charlson comorbidity score, PAH medication used in baseline period, specified comorbidities, PAH-associated comorbidities, and baseline hospitalization and outpatient visits.
Crude rates of all-cause and PH-related hospitalization per 100 person-years were 113.9 (95% CI 92.6, 140.2) and 103.7 (95% CI 83.5, 128.9), respectively, for oral treprostinil, and 69.1 (95% CI 53.5, 89.3) and 63.2 (95% CI 48.3, 82.6), respectively, for selexipag. After controlling for baseline characteristics, selexipag was associated with a 42% lower all-cause hospitalization rate (P = 0.01) and 46% lower PH-related hospitalization rate (P = 0.004) vs. oral treprostinil (Fig. 4 and Table 3).
Fig. 4.
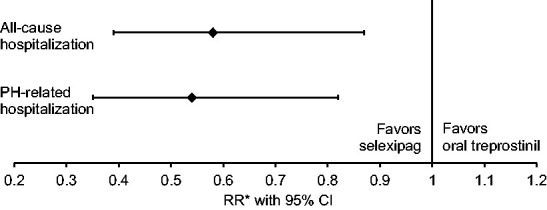
Relative rate of all-cause and PH-related hospitalization with selexipag vs. oral treprostinil. CI: confidence interval; PAH: pulmonary arterial hypertension; PH: pulmonary hypertension; RR: rate ratio. *Reference group: oral treprostinil; Poisson regression models with an offset variable for all-cause and PH-related hospitalization were adjusted for age, gender, geographic region, insurance type, Charlson comorbidity score, PAH medication, PAH-associated comorbidities, other selected comorbidities, and baseline hospitalization and outpatient visits.
Table 3.
Number of hospitalizations by treatment groups (N = 222).
| Hospitalization | Person-years |
Number of hospitalizations |
Rate ratio (95% CI)a |
|||
|---|---|---|---|---|---|---|
| Oral treprostinil | Selexipag | Oral treprostinil | Selexipag | Unadjusted | Adjustedb | |
| All-cause | 78.1 | 83.9 | 89 | 58 | 0.61 (0.43, 0.84) | 0.58 (0.39, 0.87) |
| PH-related | 78.1 | 83.9 | 81 | 53 | 0.61 (0.43, 0.86) | 0.54 (0.35, 0.82) |
Note: CI: confidence interval; PAH: pulmonary arterial hypertension; PH: pulmonary hypertension.
Reference group: oral treprostinil.
Poisson model with offset variable adjusted for age, gender, geographic region, insurance type, Charlson comorbidity score, PAH medication in baseline period, specified comorbidities, PAH-associated comorbidities, and baseline hospitalization and outpatient visits.
Discussion
These results provide real-world evidence suggesting the two available oral agents targeting the prostacyclin pathway may yield different clinical outcomes, specifically all-cause and PH-related hospitalizations. Notably, PH-related hospitalizations accounted for 91% of all hospitalizations in the cohort.
Clinical guidelines current during the study period gave a Class I (Evidence Level B) recommendation to selexipag for patients with PAH in World Health Organization (WHO) Functional Class (FC) II or III and a Class IIb (Evidence Level B) recommendation to oral treprostinil for patients in WHO FC III.18 It is unknown to what extent prescribers may have selected different patients for treatment with oral treprostinil vs. selexipag in accordance with these recommendations because WHO FC was not recorded in the database. However, compared with patients treated with selexipag, those treated with oral treprostinil tended to be older and have more comorbidities. Although these baseline differences would be expected to bias the analysis results, even after controlling for these potential confounders the differences in the risk and rate of hospitalization in favor of selexipag remained large and statistically significant. Notably, these results should not be influenced by the prevalence of CTD, which was similar between groups (28.3% for oral treprostinil vs. 24.4% for selexipag; P = 0.62), and for which the models controlled.
Results of the event-driven FREEDOM-EV trial of oral treprostinil have recently been reported.21 Oral treprostinil met the primary end point of time to first adjudicated clinical worsening event (death, hospitalization due to worsening PAH, initiation of inhaled/infused prostacyclin, disease progression, or unsatisfactory long-term clinical response) with delay in disease progression accounting for the majority of the treatment effect vs. placebo. The primary-end point HR for oral treprostinil vs. placebo was 0.74 (95% CI 0.56, 0.97; P = 0.0275).
Notably, whereas hospitalization due to PAH as the first clinical worsening event was similar between groups in FREEDOM-EV (oral treprostinil 10.1%, placebo 10.2%), hospitalization for worsening of PAH was an important driver of the treatment effect in GRIPHON (selexipag 13.6%, placebo 18.7%).8,21 The observed difference in hospitalizations in the present real-world study appears directionally consistent with this finding and thus the smaller reduction vs. placebo in primary-end point events for oral treprostinil in FREEDOM-EV (26%) compared with selexipag in GRIPHON (40%; HR 0.60, 99% CI 0.46, 0.78; P < 0.001).8,21 However, no firm conclusions can be drawn from these observations because no analysis comparing the results with oral treprostinil in FREEDOM-EV vs. the results with selexipag in GRIPHON with adjustment for baseline patient characteristics in the two trials has been conducted.
One potential explanation for the observed results is that achieving a clinically effective treprostinil dose via the oral route has proven to be challenging in clinical practice.31 Compared with prostanoids, selexipag has greater selectivity for the IP receptor, leading to reduced gastrointestinal intolerance, which has been a dose-limiting side effect with oral treprostinil.32,33 A recent Delphi panel on the use of oral treprostinil suggested aggressive antidiarrheal medication to manage these adverse effects but failed to reach consensus on optimal dosing strategies.34 In FREEDOM-EV, oral treprostinil was dosed three times a day (TID);21 although this could help reduce the intensity of adverse effects to allow increasing the dose to a therapeutic level,34 TID dosing could introduce adherence issues compared with a drug with less frequent dosing.35,36
The primary limitation of this study is that whereas the index drugs are indicated solely for specified patients with PAH (not other forms of PH), there is no unique ICD-9-CM or ICD-10-CM code for PAH.37 Both coding systems have a diagnostic code for primary PH, which corresponds to idiopathic PAH in the current PAH classification,38–40 but they have no codes that differentiate the other PAH subgroups from non-PAH forms of PH. Consequently, patients were identified based on diagnostic codes for PH, potentially including an unknown percentage of non-PAH patients. Any resulting off-label use of index medication should be minimal, since drugs targeting the prostacyclin pathway are typically reserved for high-risk patients and have inherent side effects, which may make them more difficult to initiate and therefore less likely to be used outside their labels than ERAs and PDE5is, as has been previously observed in clinical practice for patients with chronic thromboembolic PH.41,42
Based on diagnosis codes, COPD prevalence in the study cohort was 46%. High prevalence of COPD diagnosis codes has also been reported in other retrospective database analyses of PAH patients.43 One potential explanation is that some undeterminable percentage of these COPD diagnosis codes could have been registered for exclusion of COPD in the differential diagnosis of PAH. Many patients are labeled with a diagnosis of COPD on their pathway to PAH diagnosis, and the code is often not removed or changed. It is unlikely that a significant percentage of patients in this cohort did not have true PAH because insurers typically require prescribers to provide a statement that PAH is not caused by lung disease if a patient with PAH has a current COPD diagnosis. The COPD category in our study is increased by inclusion of diagnosis codes for chronic bronchitis and bronchiectasis. The high COPD prevalence also likely reflects the fact that more than half of the cohort (54%) came from southern states that have high smoking rates (ranging from 12.5% to 23.1% in the most recently published state-level data from the Centers for Disease Control, for the years 2014–201544) relative to many other regions of the United States.
It should be noted that the multivariable Cox proportional hazard and Poisson models in this study adjusted for several demographic and clinical characteristics that could have impacted its findings, including age, gender, comorbidities, and the number of hospitalizations and outpatient visits. However, the results may be influenced by unmeasured confounders that have been demonstrated to predict outcomes in PAH, such as WHO FC, exercise capacity, right ventricular function and structure, hemodynamics, and brain natriuretic peptide and other biomarkers.45 These variables are not captured in claims databases, so identifying them in a retrospective analysis would require review of individually linked patient charts, which was not possible in our study.
This study excluded patients with any switching between oral treprostinil and selexipag, in order to provide a straightforward analysis of the treatment effect. Because switching medications is typically attributable to lack of effectiveness and/or intolerable side effects, this exclusion criterion may have selected for patients with a more favorable response to their index drug compared with the general population of patients receiving oral treprostinil and selexipag. A limitation of claims data is that they do not provide the reason for switching or discontinuing the medication of interest.
Other study limitations include ascertainment of comorbidities and PH diagnoses from administrative claims data collected for insurance payments not research, which are not clinically validated, may be subject to coding error, and do not provide information on whether prescriptions were filled and taken as prescribed. Because this study included only commercially insured US patients, results may not be generalizable to patients with different coverage or residing outside of the United States. Finally, like all retrospective database studies, it is not possible to draw firm conclusions regarding causality of the associations found in this study.
In conclusion, in this real-world study, selexipag was associated with a lower risk and rate of all-cause and PH-related hospitalization compared with oral treprostinil. Controlled studies are recommended to confirm these findings.
Supplemental Material
Supplemental material, sj-pdf-1-pul-10.1177_2045894020911831 for Comparative effectiveness of oral prostacyclin pathway drugs on hospitalization in patients with pulmonary hypertension in the United States: a retrospective database analysis by John W. McConnell, Yuen Tsang, Janis Pruett and William Drake III in Pulmonary Circulation
Acknowledgments
Medical writing and editorial support were provided by W. Mark Roberts, PhD, Montréal, Québec, Canada, funded by Actelion Pharmaceuticals US, Inc.
Contributorship: Y.T. and J.P. were involved in study design. Y.T. acquired and analyzed the data. All authors participated in study conception, data interpretation, drafting the manuscript, and/or revising it critically for important intellectual content; approved the final version for publication; and take public responsibility for appropriate portions of the content.
Ethical approval: This study is a retrospective claims database analysis and thus does not contain any experiments on human or animal subjects for which ethical approval is required.
Guarantor: Y.T. is the guarantor for this article.
Conflict of interest: J.W.M. has received research grants and personal fees from Actelion, Arena Pharmaceuticals, Bayer Pharmaceuticals, Liquidia Pharmaceuticals, Reata Pharmaceuticals, and United Therapeutics and research grants from Eiger Pharmaceuticals and Bellerophon. Y.T. is an employee of Actelion Pharmaceuticals US, Inc., a Janssen Pharmaceutical Company of Johnson & Johnson, and holds stock in Johnson & Johnson. J.P. and W.D. were employees of Actelion Pharmaceuticals US, Inc. when this research was conducted, and hold stock in Johnson & Johnson.
Funding: This research was sponsored by Actelion Pharmaceuticals US, Inc., the manufacturer of selexipag. The sponsor was involved in data analysis and interpretation, funded medical writing and editorial support to the authors, and was involved in the decision to publish the finished manuscript.
Supplemental material: Supplemental material for this article is available online.
References
- 1.Farber HW, Gin-Sing W. Practical considerations for therapies targeting the prostacyclin pathway. Eur Respir Rev 2016; 25: 418–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burger CD, D'Albini L, Raspa S, et al. The evolution of prostacyclins in pulmonary arterial hypertension: from classical treatment to modern management. Am J Manag Care 2016; 22: S3–S15. [PubMed] [Google Scholar]
- 3.El Yafawi R, Wirth JA. What is the role of oral prostacyclin pathway medications in pulmonary arterial hypertension management?. Curr Hypertens Rep 2017; 19: 97. [DOI] [PubMed] [Google Scholar]
- 4.Jing ZC, Parikh K, Pulido T, et al. Efficacy and safety of oral treprostinil monotherapy for the treatment of pulmonary arterial hypertension: a randomized, controlled trial. Circulation 2013; 127: 624–633. [DOI] [PubMed] [Google Scholar]
- 5.United Therapeutics Corp. Orenitram® (treprostinil) prescribing information. Research Triangle Park: United Therapeutics Corp., 2017.
- 6.Tapson VF, Torres F, Kermeen F, et al. Oral treprostinil for the treatment of pulmonary arterial hypertension in patients on background endothelin receptor antagonist and/or phosphodiesterase type 5 inhibitor therapy (the FREEDOM-C study): a randomized controlled trial. Chest 2012; 142: 1383–1390. [DOI] [PubMed] [Google Scholar]
- 7.Tapson VF, Jing ZC, Xu KF, et al. Oral treprostinil for the treatment of pulmonary arterial hypertension in patients receiving background endothelin receptor antagonist and phosphodiesterase type 5 inhibitor therapy (the FREEDOM-C2 study): a randomized controlled trial. Chest 2013; 144: 952–958. [DOI] [PubMed] [Google Scholar]
- 8.Sitbon O, Channick R, Chin KM, et al. Selexipag for the treatment of pulmonary arterial hypertension. N Engl J Med 2015; 373: 2522–2533. [DOI] [PubMed] [Google Scholar]
- 9.Actelion Pharmaceuticals US, Inc. Uptravi® (selexipag) product information. South San Francisco: Actelion Pharmaceuticals US, Inc., 2017.
- 10.Coghlan JG, Channick R, Chin K, et al. Targeting the prostacyclin pathway with selexipag in patients with pulmonary arterial hypertension receiving double combination therapy: insights from the randomized controlled GRIPHON study. Am J Cardiovasc Drugs 2018; 18: 37–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Galiè N, Channick RN, Frantz RP, et al. Risk stratification and medical therapy of pulmonary arterial hypertension. Eur Respir J 2019; 53: 1801889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McLaughlin VV, Badesch DB, Delcroix M, et al. End points and clinical trial design in pulmonary arterial hypertension. J Am Coll Cardiol 2009; 54: S97–S107. [DOI] [PubMed] [Google Scholar]
- 13.Gomberg-Maitland M, Bull TM, Saggar R, et al. New trial designs and potential therapies for pulmonary artery hypertension. J Am Coll Cardiol 2013; 62: D82–D91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McLaughlin VV, Hoeper MM, Channick RN, et al. Pulmonary arterial hypertension-related morbidity is prognostic for mortality. J Am Coll Cardiol 2018; 71: 752–763. [DOI] [PubMed] [Google Scholar]
- 15.Frost AE, Badesch DB, Miller DP, et al. Evaluation of the predictive value of a clinical worsening definition using 2-year outcomes in patients with pulmonary arterial hypertension: a REVEAL Registry analysis. Chest 2013; 144: 1521–1529. [DOI] [PubMed] [Google Scholar]
- 16.Nickel N, Golpon H, Greer M, et al. The prognostic impact of follow-up assessments in patients with idiopathic pulmonary arterial hypertension. Eur Respir J 2012; 39: 589–596. [DOI] [PubMed] [Google Scholar]
- 17.Galiè N, Simonneau G, Barst RJ, et al. Clinical worsening in trials of pulmonary arterial hypertension: results and implications. Curr Opin Pulm Med 2010; 16: S11–S19. [DOI] [PubMed] [Google Scholar]
- 18.Galiè N, Humbert M, Vachiery JL, et al. 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J 2016; 37: 67–119. [DOI] [PubMed] [Google Scholar]
- 19.Gaine S, Chin K, Coghlan G, et al. Selexipag for the treatment of connective tissue disease-associated pulmonary arterial hypertension. Eur Respir J 2017; 50: 1602493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McLaughlin VV, Archer SL, Badesch DB, et al. ACCF/AHA 2009 expert consensus document on pulmonary hypertension. A report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association developed in collaboration with the American College of Chest Physicians, American Thoracic Society, Inc., and the Pulmonary Hypertension Association. J Am Coll Cardiol 2009; 53: 1573–1619. [DOI] [PubMed] [Google Scholar]
- 21.White RJ, Jerjes-Sanchez C, Bohns Meyer GM, et al. Combination therapy with oral treprostinil for pulmonary arterial hypertension. A double-blind, placebo-controlled study. Am J Respir Crit Care Med 2020; 201: 707–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Burger CD, Long PK, Shah MR, et al. Characterization of first-time hospitalizations in patients with newly diagnosed pulmonary arterial hypertension in the REVEAL registry. Chest 2014; 146: 1263–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Burke JP, Hunsche E, Regulier E, et al. Characterizing pulmonary hypertension-related hospitalization costs among Medicare Advantage or commercially insured patients with pulmonary arterial hypertension: a retrospective database study. Am J Manag Care 2015; 21: s47–s58. [PubMed] [Google Scholar]
- 24.Spitzer E, Cannon CP, Serruys PW. Should real-world evidence be incorporated into regulatory approvals?. Expert Opin Drug Saf 2018; 17: 1155–1159. [DOI] [PubMed] [Google Scholar]
- 25.Dreyer NA. Advancing a framework for regulatory use of real-world evidence: when real is reliable. Ther Innov Regul Sci 2018; 52: 362–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klonoff DC. The new FDA Real-World Evidence Program to support development of drugs and biologics. J Diabetes Sci Technol 2020; 14: 345–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care 2005; 43: 1130–1139. [DOI] [PubMed] [Google Scholar]
- 28.Quan H, Li B, Couris CM, et al. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol 2011; 173: 676–682. [DOI] [PubMed] [Google Scholar]
- 29.Sikirica M, Iorga SR, Bancroft T, et al. The economic burden of pulmonary arterial hypertension (PAH) in the US on payers and patients. BMC Health Serv Res 2014; 14: 676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chronic Conditions Data Warehouse. Condition categories, www.ccwdata.org/web/guest/condition-categories (2018, accessed March 2018).
- 31.Chin KM, Ruggiero R, Bartolome S, et al. Long-term therapy with oral treprostinil in pulmonary arterial hypertension failed to lead to improvement in important physiologic measures: results from a single center. Pulm Circ 2015; 5: 513–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pugliese SC, Bull TM. Clinical use of extended-release oral treprostinil in the treatment of pulmonary arterial hypertension. Integr Blood Press Control 2016; 9: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Coons JC, Miller T. Extended-release oral treprostinil in the management of pulmonary arterial hypertension: clinical evidence and experience. Ther Adv Respir Dis 2018; 12: 1753466618766490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rahaghi FF, Feldman JP, Allen RP, et al. Recommendations for the use of oral treprostinil in clinical practice: a Delphi consensus project pulmonary circulation. Pulm Circ 2017; 7: 167–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Coleman CI, Limone B, Sobieraj DM, et al. Dosing frequency and medication adherence in chronic disease. J Manag Care Pharm 2012; 18: 527–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Srivastava K, Arora A, Kataria A, et al. Impact of reducing dosing frequency on adherence to oral therapies: a literature review and meta-analysis. Patient Prefer Adherence 2013; 7: 419–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Link J, Glazer C, Torres F, et al. International Classification of Diseases coding changes lead to profound declines in reported idiopathic pulmonary arterial hypertension mortality and hospitalizations: implications for database studies. Chest 2011; 139: 497–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Simonneau G, Robbins IM, Beghetti M, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol 2009; 54: S43–S54. [DOI] [PubMed] [Google Scholar]
- 39.Simonneau G, Gatzoulis MA, Adatia I, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol 2013; 62: D34–D41. [DOI] [PubMed] [Google Scholar]
- 40.Simonneau G, Montani D, Celermajer DS, et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J 2019; 53: 1801913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jensen KW, Kerr KM, Fedullo PF, et al. Pulmonary hypertensive medical therapy in chronic thromboembolic pulmonary hypertension before pulmonary thromboendarterectomy. Circulation 2009; 120: 1248–1254. [DOI] [PubMed] [Google Scholar]
- 42.Pepke-Zaba J, Ghofrani HA, Hoeper MM. Medical management of chronic thromboembolic pulmonary hypertension. Eur Respir Rev 2017; 26: 160107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Burger CD, Ozbay AB, Lazarus HM, et al. Treatment patterns and associated health care costs before and after treatment initiation among pulmonary arterial hypertension patients in the United States. J Manag Care Spec Pharm 2018; 24: 834–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Odani S, Armour BS, Graffunder CM, et al. State-specific prevalence of tobacco product use among adults – United States, 2014-2015. MMWR Morb Mortal Wkly Rep 2018; 67: 97–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kanwar M, Raina A, Lohmueller L, et al. The use of risk assessment tools and prognostic scores in managing patients with pulmonary arterial hypertension. Curr Hypertens Rep 2019; 21: 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-pul-10.1177_2045894020911831 for Comparative effectiveness of oral prostacyclin pathway drugs on hospitalization in patients with pulmonary hypertension in the United States: a retrospective database analysis by John W. McConnell, Yuen Tsang, Janis Pruett and William Drake III in Pulmonary Circulation