Abstract
The global pandemic of severe acute respiratory syndrome coronavirus 2 has caused significant morbidity and mortality, not only causing devastating lung injury but also has an enormous effect on the cardiovascular system. The hypercoagulable state associated with coronavirus 2019 plays a major role in the disease manifestation. Venous thromboembolism including pulmonary embolism remains the most common thrombotic manifestation of the disease as compared to arterial thrombosis. Nonetheless, the co-occurrence of both venous and arterial thrombosis in a coronavirus 2019 patient, to our knowledge, has rarely been reported. Herein, we are presenting a case of co-occurrence of left ventricular thrombosis with pulmonary embolism in the setting of coronavirus 2019 with successful treatment with apixaban.
Keywords: Coronavirus 2019, severe acute respiratory syndrome coronavirus 2, coagulopathy, pulmonary embolism, left ventricular thrombus
Background
Coronavirus 2019 (COVID-19) which is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has profoundly threatened the health of millions of people worldwide.1 In addition to the classical respiratory symptoms, hypercoagulability-related thrombotic vascular events are a well-known associated complication.2 Pulmonary embolism is the most common thromboembolic event reported in the literature, and it is much more common than the arterial events.3 Herein, we are reporting a very unique and rare case of COVID-19 who presented with left ventricular thrombus with co-occurrence of pulmonary embolism that improved dramatically with anticoagulation therapy, in a short period of time; to our knowledge, we are the first to report such a case in the literature.
Case presentation
A 60-year-old gentleman with a past medical history of heart failure with reduced ejection fraction (HFrEF), epilepsy, and schizophrenia presented to our facility complaining of dry cough and generalized weakness for few days duration. He is an active smoker and has a remote history of cocaine and heroin use, which he has quit 22 years ago. His medications included metoprolol succinate, lisinopril, levetiracetam, and risperidone. He has no prior history of atrial fibrillation, hypertension, myocardial infarction, or thrombosis. Initial vital signs showed a temperature 37.1 C, heart rate 92/min, respiratory rate of 23/min, and oxygen saturation 97% on room air. Physical exam revealed decreased air entry to the lungs bilaterally with diffuse rhonchi. He also had +1 bilateral lower limb pitting edema. Laboratory studies revealed a normal complete blood count and complete metabolic panel. N-terminal (NT)-pro hormone BNP was mildly elevated of 517 pg/mL (0–100 pg/mL). Inflammatory markers were markedly elevated with D-Dimer 64,000 ng/mL (0–500 ng/ml), Ferritin 729.1 ng/ mL (24–336 ng/mL), C-reactive protein 14.4 mg/dL (0.0–0.8 mg/dL), and lactate dehydrogenase 669 U/L (122–222 U/L). Troponins were negative. SARS-CoV-2 real-time polymerase chain reaction (RT-PCR) was positive from a nasopharyngeal swab. The electrocardiograph (ECG) showed a sinus tachycardia with signs of left ventricular hypertrophy. Multifocal opacities were appreciated in the chest X-ray (Figure 1). Computed tomography (CT) angiogram of the chest revealed right upper lobe segmental and subsegmental emboli, suggestions of multiple thrombi in the left ventricle, and multifocal ground-glass opacities (Figure 2). Echocardiogram was done for further evaluation which revealed a large thrombus of a diameter of 3 × 3 cm in the apical region of the left ventricle, and an ejection fraction of 20%–25% with signs of left ventricular hypertrophy (Figure 3), normal right ventricular systolic function with no signs of right heart strain, and no evidence of any thrombus in the right heart chambers or any valvular heart disease. Three months before his presentation, the patient had a two-dimensional (2D) echocardiograph with no evidence of thrombus in the left ventricle, with the same ejection fractions and the signs of left ventricular hypertrophy (Figure 4). He was started on a therapeutic dose of enoxaparin (1 mg/kg) twice daily for 7 days for the pulmonary emboli and the left ventricle thrombus, before transition to apixaban. At the same time, he was also started on guideline-directed medical therapy for the heart failure.
Figure 1.
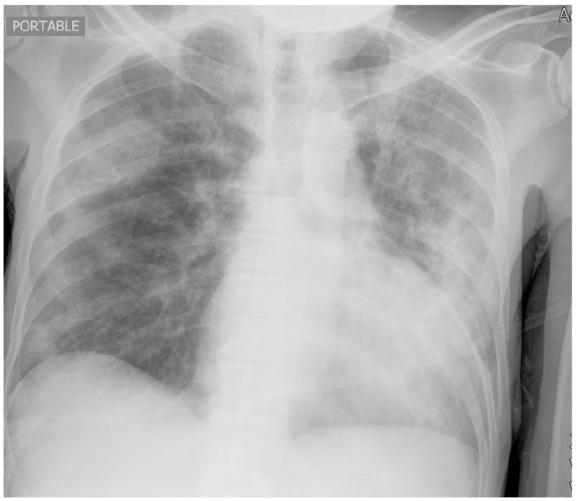
Chest X-ray showed multifocal opacities bilaterally.
Figure 2.
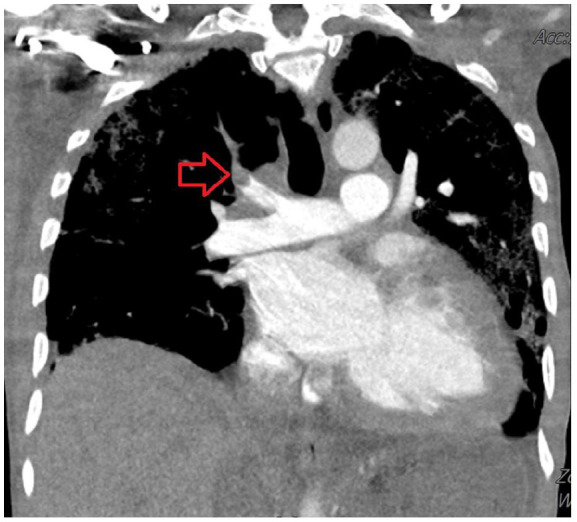
CT angiogram of the chest showed right upper lobe segmental and subsegmental emboli.
Figure 3.
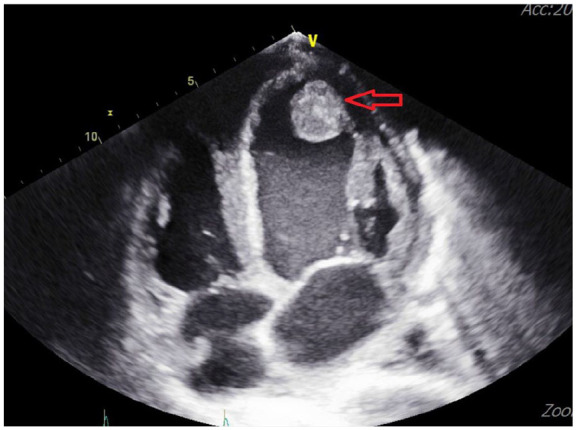
Echocardiogram revealed a large thrombus of a diameter of 3 cm in the apical region of the left ventricle.
Figure 4.
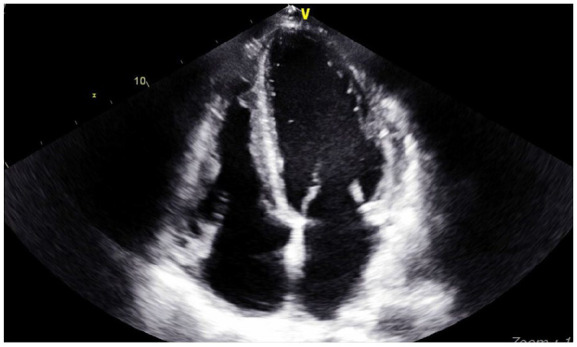
Echocardiogram 3 months prior to his presentation with no signs of left ventricular thrombus.
Upon discharge, he was transitioned to a therapeutic dose of apixaban (10 mg twice daily for 7 days and followed by 5 mg twice daily). Six weeks later, echocardiography was repeated and showed a significant reduction in the size of the left ventricular thrombus from 3 × 3 cm to 1 × 1 cm (Figure 5), and the patient was recommended to continue the treatment to complete at least 6 months duration, where another echocardiogram will be done to re-assess the situation A full hypercoagulability workup showed mildly elevated anticardiolipin antibodies IgM 57. Other hypercoagulable tests including dilute Russell’s viper venom time (dRVVT) screening for lupus anticoagulant, homocysteine levels, paroxysmal nocturnal hemoglobinuria screening, Protein C and S activity, antithrombin mutations, and factor V Leiden and prothrombin genotyping were unremarkable. Autoimmune workup including antinuclear antibody, antineutrophil cytoplasmic antibody, anti-double-stranded DNA antibody, rheumatoid factor, anti-cyclic citrullinated peptide, anti-histone antibodies, Sjogren antibody, myeloperoxidase antibodies, proteinase 3 autoantibodies, complement levels, Smith antibody, and RNP antibody were also within normal limit. Tumor markers were also negative. Although the history of HFrEF with severe low ejection fraction can be a risk factor for left ventricular thrombosis; however, he was not in acute decompensated heart failure upon his presentation. Moreover, his previous echocardiogram, which was done 3 months before his presentation had no evidence of left ventricular thrombus. The timing with COVID-19 infection, and with the co-occurrence of pulmonary embolism, this raises the suspicion of a hypercoagulable state secondary to COVID-19.
Figure 5.
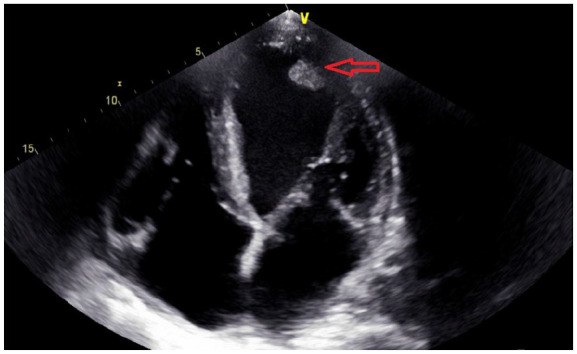
Repeated echocardiogram after 6 weeks post discharge which showed a significant reduction in the size of the left ventricular thrombus from 3 cm to 1 cm.
Discussion
COVID-19 which is caused by the SARS-CoV-2 has resulted in a major global health crisis since December 2019.4 Hypercoagulable state, either in the form of venous or arterial thromboembolism, is an emerging sequelae of COVID-19 and has been linked to a poor prognosis.5 Multiple theories have evolved in trying to explain the COVID-19 coagulopathy, they include a severely intensified inflammatory response that leads to thrombo-inflammation via cytokine storm, complement activation, endothelins, as well as direct activation of the coagulation cascade by SARS-CoV-2 itself.6–8 Other theories suggest that severe pneumonia can cause hypoxia, which may lead to high blood viscosity and can promote thrombosis.9 In addition, it has been reported in some cases that COVID-19 patients may have positive antiphospholipid antibodies that may play a role in hypercoagulable state, as seen in our patient.10,11
Venous thromboembolism including pulmonary embolism is the most common thrombotic manifestation of COVID-19. Kaminetzky et al.12 mentioned in his cohort study that 37.1% of the COVID-19 patients were positive for pulmonary embolism demonstrated by the computerized tomography of the pulmonary arteries (CTPA). Chi et al.13 in a meta-analysis has reported an incidence of 23.9% hospitalized patients with COVID-19 who developed venous thromboembolism despite receiving anticoagulation, and the mortality was further estimated to be 21.3%. It is more common in critically ill patients with severe pneumonia with acute respiratory distress syndrome (ARDS) and patients who were hemodynamically unstable.14 Critically ill patients with COVID-19 admitted to intensive care unit were predicted to have a 2.5 times greater risk for venous thromboembolism than those who were admitted to the regular ward (30.4% vs 13.0%).13 In contrast, the incidence of arterial thrombosis is much less than the venous thromboembolism,15 and only a few cases were reported in the literature with left ventricle thrombosis associated with COVID-19, most of them presented with myocardial infarction.16 To our knowledge, we are the first to report a case of COVID-19 associated with both left ventricle thrombus and pulmonary embolism without myocardial infarction and was hemodynamically stable.
Left ventricle thrombus is a rare and life-threatening entity.17 Acute myocardial infarction remains the most common cause for his situation; patients who present with anterior ST-elevation myocardial infarctions or large infarction with an ejection fraction of less than 30% are at the highest risk for this complication.18 In addition to the acute myocardial infarction, severe left ventricular systolic dysfunction secondary to non-ischemic cardiomyopathies (especially when they present in the acute decompensated phase),19 myocarditis, and hypercoagulable state can be considered as well causes for true left ventricular thrombus.18,20 Herein, we are presenting a case of a patient who has HFrEF without acute decompensated heart failure and a previous echocardiograph without any evidence of left ventricle thrombus, who presented with left ventricular thrombus and multiple pulmonary emboli in the setting of COVID-19.
Recently, the American Society of Hematology recommended that prophylactic dose of low molecular weight heparin be given to all patients with COVID-19 who did not have a contraindication for it. If there is an evidence of PE/DVT, the patients should be on therapeutic anticoagulation. The empiric use of a therapeutic dose of anticoagulation in COVID-19 patients without PE/DVT remains controversial. The International Society of Thrombosis and Haemostasis (ISTH) interim guidance, on the other hand, recommends monitoring of coagulation parameters such as D-dimer, fibrinogen, prothrombin time, and platelet count in assisting the use of anticoagulation in hospitalized patients with COVID-19.21 The worsening of coagulation parameters may warrant a more aggressive critical care support and for potential consideration of therapeutic anticoagulation.21 All patients who started on therapeutic anticoagulation should be given a minimum course of 3 months of the regimen.22 Despite the absence of clinical trials regarding the use of direct oral anticoagulant agents (DOACs) in case of left ventricular thrombus, some reports encourage the use of these agents.23,24 It has been shown that the DOACs including apixaban have superior effectiveness in preventing stroke and systemic embolism, lower incidence of major bleeding, rapid onset, better pharmacokinetic/pharmacodynamic profiles, free from frequent laboratory monitoring, fewer food and drug interactions and improved cost effectiveness in comparison to vitamin K antagonists (VKAs).25–27 In our case, we started the patient on enoxaparin, then discharged home on apixaban with significant decrease in the size of the thrombus on the repeated echocardiogram 6 weeks later.
Conclusion
We are presenting an unique case of a patient with co-occurrence of COVID-19, pulmonary embolism, and left ventricular thrombus without acute myocardial infarction or severe pulmonary disease, who responded dramatically to medical therapy. This case highlights that COVID-19 patients presenting with a hypercoagulable state with a very high D-Dimer levels are at high risk of developing pulmonary embolism and possibly arterial thrombosis. In light of the rarity and the lack of a standardized guideline for the management of this presentation, we recommend a multidisciplinary approach for this group of patients. In our case, we used the apixaban with tremendous decrease in the size of the thrombus within 6 weeks.
Footnotes
Author contributions: I.F. and K.H.C. contributed to the conception and design, acquisition, analysis, and interpretation of data, as well as participated in drafting and revision of the manuscript. R.A. actively participated in analysis of data, drafting, and revision of manuscript. G.G., J.S., and A.S. contributed to idea design, data analysis, and critically revised the manuscript and as well as approved the final submission of manuscript.
Consent for publication: Patient has given written informed consent to publish the case including publication of images
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical approval: Our institution does not require ethical approval for reporting individual cases or case series.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Informed consent: Written informed consent was obtained from the patient(s) for their anonymized information to be published in this article.
Availability of data and materials: Data have been presented in the text.
ORCID iD: Kok Hoe Chan  https://orcid.org/0000-0002-2550-1122
https://orcid.org/0000-0002-2550-1122
References
- 1. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. J Am Med Assoc 2020; 323(11): 1061–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 2020; 395(10223): 507–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Han H, Yang L, Liu R, et al. Prominent changes in blood coagulation of patients with SARS-CoV-2 infection. Clin Chem Lab Med 2020; 58(7): 1116–1120. [DOI] [PubMed] [Google Scholar]
- 4. Li B, Yang J, Zhao F, et al. Prevalence and impact of cardiovascular metabolic diseases on COVID-19 in China. Clin Res Cardiol 2020; 109(5): 531–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Guan W, Ni Z, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020; 382(18): 1708–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Varga Z, Flammer AJ, Steiger P, et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet 2020; 395(10234): 1417–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Campbell CM, Kahwash R. Will complement inhibition be the new target in treating COVID-19-related systemic thrombosis? Circulation 2020; 141(22): 1739–1741. [DOI] [PubMed] [Google Scholar]
- 8. Oudkerk M, Büller HR, Kuijpers D, et al. Diagnosis, prevention, and treatment of thromboembolic complications in COVID-19: report of the national institute for public health of the Netherlands. Radiology 2020; 297(1): E216–E222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gupta N, Zhao YY, Evans CE. The stimulation of thrombosis by hypoxia. Thromb Res 2019; 181: 77–83. [DOI] [PubMed] [Google Scholar]
- 10. Hossri S, Shadi M, Hamarsha Z, et al. Clinically significant anticardiolipin antibodies associated with COVID-19. J Crit Care 2020; 59: 32–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhang Y, Xiao M, Zhang S, et al. Coagulopathy and antiphospholipid antibodies in patients with Covid-19. N Engl J Med 2020; 382(17): e38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kaminetzky M, Moore W, Fansiwala K, et al. Pulmonary embolism on CTPA in COVID-19 patients. Radiol Cardiothorac Imaging 2020; 2(4): e200308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chi G, Lee JJ, Jamil A, et al. Venous thromboembolism among hospitalized patients with COVID-19 undergoing thromboprophylaxis: a systematic review and meta-analysis. J Clin Med 2020; 9(8): 2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cui S, Chen S, Li X, et al. Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J Thromb Haemost 2020; 18(6): 1421–1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Abou-Ismail MY, Diamond A, Kapoor S, et al. The hypercoagulable state in COVID-19: incidence, pathophysiology, and management. Thromb Res 2020; 194: 101–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Solheim S, Seljeflot I, Lunde K, et al. Frequency of left ventricular thrombus in patients with anterior wall acute myocardial infarction treated with percutaneous coronary intervention and dual antiplatelet therapy. Am J Cardiol 2010; 106(9): 1197–1200. [DOI] [PubMed] [Google Scholar]
- 17. Lee JM, Park JJ, Jung HW, et al. Left ventricular thrombus and subsequent thromboembolism, comparison of anticoagulation, surgical removal, and antiplatelet agents. J Atheroscler Thromb 2013; 20(1): 73–93. [DOI] [PubMed] [Google Scholar]
- 18. Delewi R, Nijveldt R, Hirsch A, et al. Left ventricular thrombus formation after acute myocardial infarction as assessed by cardiovascular magnetic resonance imaging. Eur J Radiol 2012; 81(12): 3900–3904. [DOI] [PubMed] [Google Scholar]
- 19. Satish M, Vukka N, Apala D, et al. Left ventricular thrombus after acute decompensated heart failure in the setting of ischemic cardiomyopathy. Cureus 2019; 11(4): 4537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pahuja M, Ainapurapu B, Abidov A. Large left ventricular thrombus in a patient with systemic and venous thromboembolism secondary to protein C and S deficiency. Case Rep Cardiol 2017; 2017: 7576801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Thachil J, Tang N, Gando S, et al. ISTH interim guidance on recognition and management of coagulopathy in COVID-19. J Thromb Haemost 2020; 18(5): 1023–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. COVID-19 Coagulopathy Hematologyorg, https://www.hematology.org/covid-19/covid-19-and-coagulopathy (accessed 25 July 2020).
- 23. Nagamoto Y, Shiomi T, Matsuura T, et al. Resolution of a left ventricular thrombus by the thrombolytic action of dabigatran. Heart Vessels 2014; 29(4): 560–562. [DOI] [PubMed] [Google Scholar]
- 24. Mano Y, Koide K, Sukegawa H, et al. Successful resolution of a left ventricular thrombus with apixaban treatment following acute myocardial infarction. Heart Vessels 2016; 31(1): 118–123. [DOI] [PubMed] [Google Scholar]
- 25. Miller CS, Grandi SM, Shimony A, et al. Meta-analysis of efficacy and safety of new oral anticoagulants (dabigatran, rivaroxaban, apixaban) versus warfarin in patients with atrial fibrillation. Am J Cardiol 2012; 110(3): 453–460. [DOI] [PubMed] [Google Scholar]
- 26. Mekaj YH, Mekaj AY, Duci SB, et al. New oral anticoagulants: their advantages and disadvantages compared with vitamin K antagonists in the prevention and treatment of patients with thromboembolic events. Ther Clin Risk Manag 2015; 11: 967–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ghaffarpasand E, Tehrani MD, Marszalek J, et al. Non-vitamin K antagonist oral anticoagulants for the treatment of intracardiac thrombosis. J Thromb Thrombolysis 2018; 46(3): 332–338. [DOI] [PubMed] [Google Scholar]


