Abstract
Background. Vaccination is an effective public health intervention that has contributed to a substantial reduction in the burden of vaccine-preventable diseases. Abridged evidence on incomplete vaccination is not well established in Ethiopia. Therefore, this meta-analysis aimed to estimate the pooled prevalence of incomplete vaccination and its predictors among children aged 12 to 23 months. Methods. Primary studies conducted in Ethiopia were searched. The methodological quality of the included studies was assessed using the Joanna Briggs Institute (JBI) checklist. The analysis was conducted using STATA 14 and RevMan. The presence of statistical heterogeneity was checked using the Cochran Q test, and its level was quantified using I2 statistics. Pooled prevalence and odds ratio (OR) were computed at a 95% confidence interval (CI). Results. The pooled prevalence of incomplete vaccination was 30% (95% CI: 25-35). Maternal illiteracy (OR = 1.96; 95% CI: 1.40, 2.74) and home delivery (OR = 2.78; 95% CI: 2.28, 3.38) were associated factors that increased incomplete vaccination. However, maternal autonomy (OR = 0.54; 95% CI: 0.33, 0.89), maternal knowledge (OR = 0.31; 95% CI: 0.20, 0.47), husband employment (OR = 0.49; 95% CI: 0.35, 0.67), urban residence (OR = 0.61; 95% CI: 0.43, 0.86), ANC visits (OR = 0.30; 95% CI: 0.23, 0.39), postnatal care (OR = 0.39; 95% CI: 0.30, 0.52), and tetanus toxoid vaccine (3+) (OR = 0.42; 95% CI: 0.26, 0.69) were factors that reduced incomplete vaccination. Conclusion. In Ethiopia, 3 out of 10 children have incomplete vaccination. Policies should focus on strengthening and improving women’s education, maternal health knowledge, empowering women, and the utilization of prenatal care can overcome some of the barriers.
Keywords: vaccination, immunization, systematic review, meta-analysis, children, Ethiopia
Background
Vaccination is an effective public health intervention that has contributed to the substantial reduction in the burden of vaccine-preventable diseases (VPDs) worldwide.1–3 Approximately 23 million deaths are averted with the measles vaccine between 2010 and 2018.4 More than half of early childhood deaths are caused by diseases that could be easily prevented or treated with easily affordable interventions, such as administering vaccines.5 The Expanded Program of Immunization (EPI) was launched by the World Health Organization (WHO) in 1974, and Ethiopia has launched in 1980 to vaccinate all children.6,7
The global immunization target is to reach 90% national coverage for all vaccines by 2020.5 The Sustainable Development Goals (SDGs) aimed to ensure maintaining the hard-won gains on vaccination to achieve more, leaving no one behind in all the countries by 2030.2 Vaccination attributes a 24% reduction in mortality rates in under 5 children between 2010 and 2017.2 Despite the significant reductions in the incidence of VPDs, a considerable number of children have incomplete vaccination, which causes marked variation in vaccination coverage worldwide.8,9 For instance, more than 17 million cases and 83 439 deaths attributable to measles occurred worldwide in 2017.10,11 The impact of vaccines extends beyond public health, which affects children’s educational achievements and national economic growth.1 Moreover, children suffer from vaccine-preventable disabilities, impaired growth, and cognitive development. An estimated 24 million people will fall under poverty by 2030, linked with VPDs.2,12
Globally, 86% of infants (116.3 million) received the recommended 3 doses of diphtheria-tetanus-pertussis (DTP) vaccine in 2018.13,14 The WHO and United Nations Children’s Fund (UNICEF) reported that more than 20 million children have not received a full course of basic vaccines worldwide.2,4,5 Of these, more than 60% of unvaccinated and undervaccinated children live in 10 low- and middle-income countries (LMICs), including Ethiopia, which may disproportionately affected by infectious disease, which has been exacerbated due to the fragile nature of the health care system or conflict in regions.5,14 Similarly, approximately 10 million children remain unvaccinated or partially vaccinated in Africa.15,16
As a result of implementing vaccination programs through EPI, a vaccination campaign, and community health expansion programs, under-five mortality reduced to 55 deaths per 1000 live births in Ethiopia in 2019.17 Ethiopia scheduled, single-dose for BCG, 3 doses of diphtheria, tetanus, pertussis, hepatitis B, Haemophilus influenza type B, 2 doses of Rota, 3 doses of the pneumococcal conjugate, 3 doses of polio and one measles vaccine have been given at birth, 6, 10, 14 weeks, and 9 months for measles vaccine for infants.18 Nevertheless, vaccination coverage remains suboptimal, and sporadic outbreaks of VPDs, such as measles, occur in the country.14,19 Only 39% of children had fully vaccinated for the recommended vaccine in 2016.18,20 Likewise, incomplete vaccination of children ranged from 2.9% to 52.9% in Ethiopia.21,22
Existing literature has shown that maternal education, occupation, and residence, fear of side effects, household wealth, place of delivery, and maternal knowledge were the factors associated with incomplete vaccination in children.9,20,22–25 Moreover, it may be related to healthcare services, including access or distance factors, missed opportunities, inadequate supply, and access to prenatal care.26,27 Vaccine hesitancy is defined as a lack of confidence in the safety and effectiveness of vaccines.3,19,28,29 Other contextual factors, such as sociocultural beliefs influencing the behavior of stakeholders, also affect the completion of vaccination.3,25,30,31 However, inconsistency exists between studies concerning the abovementioned factors, and hence, pooled measures of the factors are required to feature the broad picture.
Determining which group of children are less likely to be vaccinated in terms of geographical, cultural, social, and strengthening in-country evidence-based decision-making is important to inform the development of appropriate intervention programs.3 Abridged evidence on incomplete vaccination and its associated factors are not well established in Ethiopia. Therefore, this systematic review and meta-analysis aimed to estimate the pooled prevalence of incomplete vaccination and its associated factors among children in Ethiopia.
Materials and Methods
Protocol Design
This systematic review and meta-analysis methodology was developed according to the Preferred Reporting Items for Systematic Review and Meta-Analysis Protocol (PRISMA-P) 2015 recommendations.32 The necessary items of the PRISMA checklist have been addressed, and the details are found in the additional file (see Additional file 1). Likewise, the protocol of this systematic review and meta-analysis was registered by the International Prospective Register of Systematic Reviews and Meta-Analysis (PROSPERO) and identified with the registration number (CRD42020148729).
Studies Search and Identification
All published and unpublished studies were systematically searched through main electronic databases, including PubMed, African Journal Online, WHO databases (HINARI), and Google Scholar Searches. The search strings emerged from the following keywords (vaccination, immunization, an expanded program of immunization and associated factors, predictors, risk factors, determinants, children, Ethiopia). The search string was prepared according to the requirements of the specified database to identify relevant studies (see Additional file 2).
Eligibility Criteria for the Studies
Studies were included in the systematic review using the following eligibility criteria: studies written in the English language conducted in Ethiopia from 1974 to 2020, published and unpublished available studies, conducted either community or facility settings, observational studies, and survey findings on incompletely vaccinated children aged 12 to 23 months. Nevertheless, fact sheet reports, commentaries, editorial reports, and case reports were not included. Articles not accessed after a minimum of 2 email contacts (every 2 weeks) of the primary authors were excluded.
Selection of the Studies
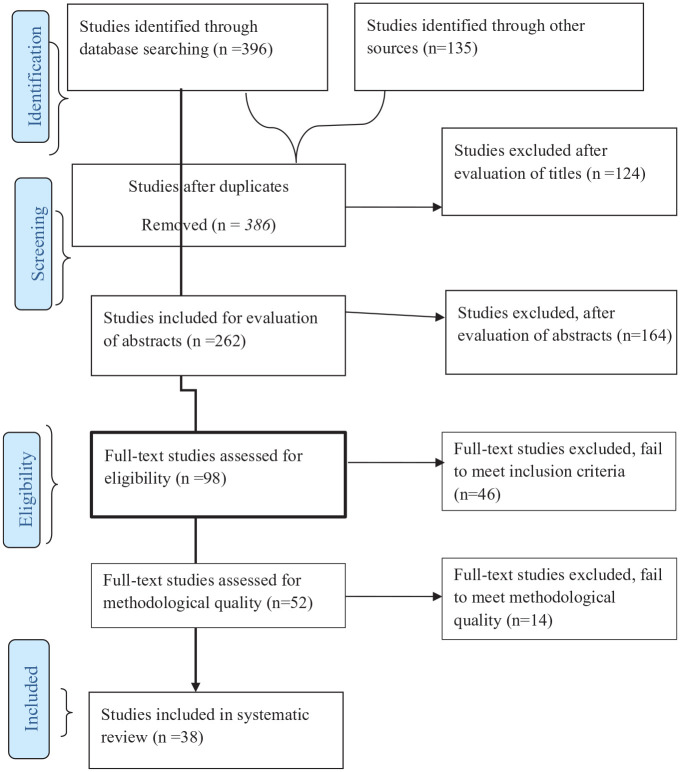
All observational studies, such as cross-sectional, cohort, and case-control studies, were included. AD and GT identified the relevant studies using the search string and applied the filters in the selected main databases. The identified studies were exported into the citation manager (EndNote) to remove duplicates. The 2 authors (AD and AS) independently screened studies based on titles and abstracts. The studies were put into 3 categories, included, excluded, and undecided categories. The 2 authors (AD and AS) again independently assessed the full texts of the included and undecided categories of the studies against the eligibility criteria to decide on their inclusion in the systematic review and meta-analysis. The studies were judged based on the eligibility criteria set forth above. Studies that did not fulfill the eligibility criteria were excluded. In case of any disagreement among reviewers, the third author (SB) consulted for understanding. The selection process was guided by the PRISMA flow diagram (Figure 1).
Figure 1.
PRISMA flow diagram depicting the selection process of studies for systematic review and meta-analysis.
Outcome Variable
Incomplete vaccination was the primary outcome measure in this study. According to WHO, a child is considered incompletely vaccinated if he/she receives at least one of the following, but not all, (a) 1 dose of Bacille Calmette-Guerin (BCG); (b) 3 doses of oral polio vaccine DPT, hepatitis B vaccines, Hib and (c) 1 dose of measles vaccine, all before attaining 1 year.2–4 However, we found that studies including other vaccines, such as rotavirus and pneumococcal conjugate, have carefully checked the agreement with the abovementioned case definition and were included in the analysis.
Quality Appraisal
Studies were critically evaluated for their methodological rigor and validity of the findings. We used the Joanna Briggs Institute (JBI) critical appraisal checklist for the methodological rigorousness of observational studies. Studies with a positive response score of 5 or more were included. Particular attention was given to a clear statement of the objective of the study, identification of the study subjects, and precise measurement of outcomes of interest and exposure variables as well as documentation of sources of bias or confounding. The 2 authors (AD and AS) independently checked the scientific quality of the studies using the quality assessment tools mentioned above. In the case of uncertainties, we resolved by a joint discussion and consulting the third (SB) and fourth authors (GT) (see Additional file 3).
Data Abstraction
Raw data (frequency) were extracted using a structured data extraction form, which was designed using Microsoft Excel. The 2 authors (AD and SB) abstracted the data systematically. In addition, studies’ characteristics that mainly focused on the author, year, study area, design, objective, sampling, and key findings were summarized in the Microsoft Word Table (Table 1). The first author (AD) contacted the authors of the article and requested details through email in case of missing data, incomplete reports, or any uncertainties.
Table 1.
Describe the Characteristics of Included Studies for Outcome Variables in the Systematic Review and Meta-Analysis.
| Author | Study setting | Objective | Target population | Study design | Sample size | Outcome N(%) | Associated factors |
|---|---|---|---|---|---|---|---|
| Ali et al34 | South Wollo, Amhara | To identify those factors associated with incomplete vaccination | 12–23 months | Cross-sectional | 480 | 37 (7.7%) | Home delivery, no history of TT vaccination, living near the health post, being young maternal, parents with no education and ANC follow-ups |
| Animaw et al35 | Arba Minch, SNNPR | To measured immunization coverage and identified the predictors | 12–23 months | Cross-sectional | 630 | 128 (20.3%) | Mother education, ‘perception of, mothers’ knowledge, and place of delivery |
| Aregawi et al36 | Laelay Adiabo, Tigray | To identify the determinants of defaulting from child immunization completion | 9–23 months | Case-control | 270 | – | >30 minutes to reach the vaccination site, poor participation in women’s developmental groups; no postnatal care, and poor knowledge |
| Asfaw et al37 | Sodo Zurea, SNNPR | To identify determinants of default to full completion of immunization | 12–23 months | Case-control | 344 | – | Maternal education, no postnatal care follow up, maternal knowledge, and maternal favorable attitude |
| CSA38 | Nationwide | Data on vaccination coverage | 12–23 months | Survey | 2004 | 902 (45%) | – |
| CSA17 | Nationwide | Data on vaccination coverage | 12–23 months | Survey | 1028 | 388 (37.7%) | – |
| Debie and Lakew27 | Emerging regions of Ethiopia | To identify the factors associated with the access and continuum of childhood vaccination | 12–23 months | Survey | 642 | 214 (33.4%) | Mothers’ formal education, ANC, health facility-based delivery, and rich wealth |
| Deressa et al21 | Sidama, SNNPR | To assess the vaccination status and its associated factors | 9–24 months | Cross-sectional | 107 | 3 (2.9%) | Mothers age and birth at home |
| Ebot39 | Nationwide | To assess women’s household autonomy and immunization | 12–30 months | Survey | 2941 | 1588 (61%) | Women’s socioeconomic status and household autonomy |
| Etana and Deressa40 | Ambo, Oromia | To assess complete immunization coverage and its associated factors | 12–23 months | Cross-sectional | 536 | 218 (40.7%) | Antenatal care follow-up, born in the health facility, mothers’ knowledge |
| G/Mariam et al41 | Bench Maji, SNNPR | To identify determinants of incomplete vaccination | 12–23 months | Case-control | 312 | – | No ANC, home delivery, having no postnatal care visit, the inconvenient appointment time |
| Girmay and Dadi42 | Sekota Zuria, Amhara | Aimed at bringing data about immunization service coverage and its associated factors | 12–23 months | Cross-sectional | 620 | 96 (15.5%) | Having ANC visit, higher maternal education, mothers’ good knowledge, short distance to the health facility, and born in health facility, 5 and more family size |
| Gualu and Dilie43 | Debre Markos, Amhara | To determine vaccination coverage and associated factors | 12–23 months | Cross-sectional | 288 | 19 (6.6%) | Male birth, wanted pregnancy, ANC follow-ups, a short distance from the vaccination site |
| Hailu et al24 | Wonago SNNPR | To evaluate immunization coverage and identify factors of incomplete vaccination | 6–36 months | Cross sectional | 1119 | 333 (29.8%) | Older mothers’ age, ANC, tetanus-toxoid vaccination, mothers knowing the age and being a female |
| Kassahun et al44 | Lay-Armachiho Amhara | To assess immunization coverage and associated factors | 12–23 months | Cross-sectional | 751 | 163 (21.66%) | Mothers knowledge, tetanus toxoid immunization and Urban residence |
| Kidane and Tekie45 | Tselemti, Tigray | To identify factors influencing urban and rural immunization | 12–13 months | Cross-sectional | 220 | 53 (23.9%) | Residence and mother’s education |
| Kidanne et al46 | Nationwide | To identify factors associated with the timeliness of vaccine doses | 12–23 months | Cross-sectional | 600 | 256 (42.7%) | Children from pastoral areas mothers/caregivers aged 30 or above |
| Kindie Yenit47 | East Gojjam, Amhara | To identify factors associated with incomplete childhood vaccinations | 12–23 months | Case-control | 308 | – | Delivered at home, no ANC visit, misperception on vaccine contraindication, and no Postnatal care visit |
| Kinfe et al63 | Nationwide | To assess individual and community level factors associated with full immunization | 12–23 months | Survey | 1929 | – | Mother’s education, husband employment, mother’s religion, ANC visit, presence of vaccination document, region |
| Lakew et al64 | Nationwide | Identify factors associated with full immunization coverage | 12–23 months | Survey | 1927 | – | Information from vaccination card, received postnatal check-up, women’s awareness, and rich wealth index |
| Legesse and Dechasa48 | Bale Zone, Oromia | To assess complete immunization coverage and its associated factors | 12–23 months | Cross-sectional | 591 | 128 (21.7%) | ANC follow up, being a farmer, the level having a household family income, walking time from home to health facilities, health extension workers, mothers’ knowledge |
| Mekonnen et al49 | Minjar-shenkora, Amhara | To assess the immunization coverage and its factors | 12–23 months | Cross-sectional | 566 | 105 (18.5%) | Being unmarried, traveling time greater than 2 hours on foot |
| Meleko et al50 | Mizan Aman, SNNPR | To assess immunization and factors associated | 12–23 months | Cross-sectional | 322 | 159 (49.4%) | Educational level, place of delivery, maternal health care utilization, knowledge about vaccine |
| Negero et al51 | Oromia | To assess immunization and associated factors | 12–23 months | Cross-sectional | 436 | 113 (26.2%) | delivered at home, illiterate mother, poor satisfaction of services, side effects, no ANC |
| Mesfin52 | Yirgalem, SNNPR | To assess incomplete vaccination and associated factors | 12–23 months | Cross-sectional | 473 | 96 (20.0%) | Primary caregivers knowledge, ANC attendance and place of delivery |
| Mohammed and Atomsa22 | Eastern, Oromia | To assess the immunization coverage and its determinants | 12–23 months | Cross-sectional | 694 | 367 (52.9%) | Unaware of the need for immunization, fear of the side reaction, time of immunization wrong perception about the time of immunization |
| Mohamud et al53 | Jigjiga, Somali | To measure the immunization coverage and associated factors | 12–23 months | Cross-sectional | 582 | 221 (38%) | Maternal literacy, tetanus toxoid vaccine, place of delivery and place of residence |
| Negussie et al54 | Sidama, SNNPR | To identify determinant factors of incomplete childhood immunization | 12–23 months | Case-control | 548 | – | Young mothers, a mother’s knowledge about immunization benefits, mother’s negative, perception of vaccine side effects |
| Okwaraji et al55 | Dabat, Amhara | To assess vaccine coverage and factors associated | 12–59 months | Cross-sectional | 775 | 36 (4.6%) | Travel time |
| Porth et al56 | Nationwide | To explores healthcare services utilization or receiving a vaccine | 12–23 months | Survey | 2722 | 1255 (46.1%) | Residence and possession of a vaccination card |
| Tamirat and Sisay57 | Nationwide | To assess full immunization coverage and its determinants among children | 12–23 months | Cross-sectional | 1909 | 744 (39%) | Rural residence, employed, female household head, wealth index [middle and richness primary school, maternal education, ANC follow-ups and delivery at health facilities |
| Tefera et al58 | Worabe, SNNPR | To assess factors associated with full immunization | 12–23 months | Cross-sectional | 484 | 187 (39%) | Fewer ANC visits |
| Tesfaye et al59 | East Gojam, Amhara | To assess vaccination coverage and its predicting factors | 12–23 months | Cross-sectional | 846 | 144 (17%) | Urban residence, having ANC visit, place of delivery, and vaccination site at health institutions |
| Tessema et al60 | Pastoral zones in Ethiopia | To assess vaccination coverage, estimate dropout rates, and identify associated factors | 12–23 months | Cross-sectional | 600 | 121 (21.0%) | Residence, age and education, and maternal occupation |
| Tolera61 | Addis Ababa | To determine full immunization coverage and the predictors that influence the complete | 12–23 months | Cross-sectional | 585 | 140 (24%) | Maternal occupation, postnatal care follow up, knowledge about the objective of vaccination and place of delivery |
| Wado et al62 | SNNPR | To examine the influences of women’s autonomy on the vaccination | 12–24 months | Cross-sectional | 889 | 464 (41%) | Women’s autonomy, mother’s education, use of ANC services, and proximity to a health facility |
| Yismaw et al26 | Gondar, Amhara | To determine incomplete vaccination and associated factors | 12–23 months | Cross-sectional | 301 | 73 (24.3%) | Knowledge of vaccination age of the child, time to reach a health facility |
| Workina et al33 | Jimma, Oromia | To assess reason for incomplete vaccination and associated factors | 12–23 months | Cross-sectional | 267 | 126 (45.5%) | Educational status, marital status, and monthly income |
Data Synthesis and Statistical Analyses
The data were first presented using a narrative synthesis of the included studies. A summary table was prepared to describe the characteristics of the included studies. For those studies that were suitable for quantitative synthesis, a meta-analysis was carried out. The pooled estimate of the outcome variable was conducted using Stata 14 window Version and RevMan v5.3 software for meta-analysis. Subgroup analysis was conducted by the Regional States in the Federal Democratic Republic of Ethiopia. The 2 authors (AS and GT) conducted the meta-analysis. Pooled prevalence and odds ratio (OR) were computed at a 95% confidence interval (CI). The presence of statistical heterogeneity was checked using the Cochran Q test at a P-value of .05, and its level was quantified using the I² statistics, where substantial heterogeneity was assumed if the I² value was >60%. The random-effects model was used to analyze the data, as there was considerable heterogeneity between the included studies. Eggers and Begg’s test was computed to examine the existence of publication bias among the included studies.
Ethical Approval and Informed Consent
This is a systematic review and meta-analysis of the original articles conducted in different parts of the country. Ethical approval and informed consent did not apply to this study since the data were generated from computed pooled analysis. In Ethiopia, most of the research institutions have institutional review boards and therefore the respective studies had prior approval before the actual data collection period.
Results
Search Results
As shown in Figure 1, the online database search identified 531 studies, of which 145 studies were duplicates. The remaining studies were screened for titles and abstracts, which excluded 288 studies from further screening. The full texts of 98 studies were evaluated to ensure the presence of at least one of the primary outcomes, and 46 studies were excluded. The remaining 52 studies underwent a critical appraisal, and 14 studies were excluded from the synthesis due to the relatively poor methodological quality, data inconsistency, and unavailability or incompleteness of the data. The remaining 38 studies17,21,22,24,26,27,33–64 were included in this systematic review and meta-analysis.
Characteristics of the Included Studies
From the 38 included studies in the systematic review and meta-analysis, 33 studies were community-based cross-sectional studies, some were nationwide surveys, and 5 were case-control studies. A total of 30 646 children and 9581 cases with incomplete vaccination were included in the analysis. The smallest (n = 107) and largest (n = 2941) sample sizes were reported in studies conducted in the Southern Nations, Nationalities, and People’s Region (SNNPR) and nationwide survey.21,39 The details of the included studies’ characteristics and descriptions are presented using a table (Table 1).
Reporting Bias
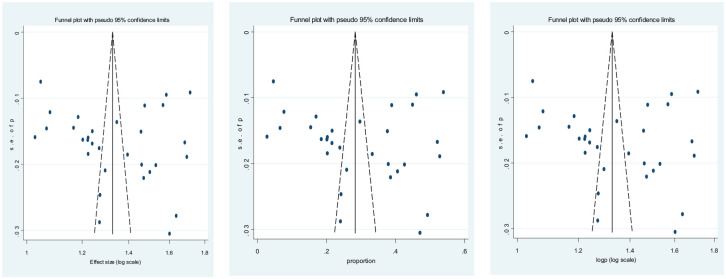
A random-effect model was used to analyze the data to moderate the variability between and within studies. Reporting bias was assessed using both funnel plot illustration (Figure 2). Publication bias was not noticed in the included studies, as evidenced by Egger’s test (P = .362) and Begg’s test continuity corrected (P = .339).
Figure 2.
Funnel plot to visualize reporting bias (n = 31 studies).
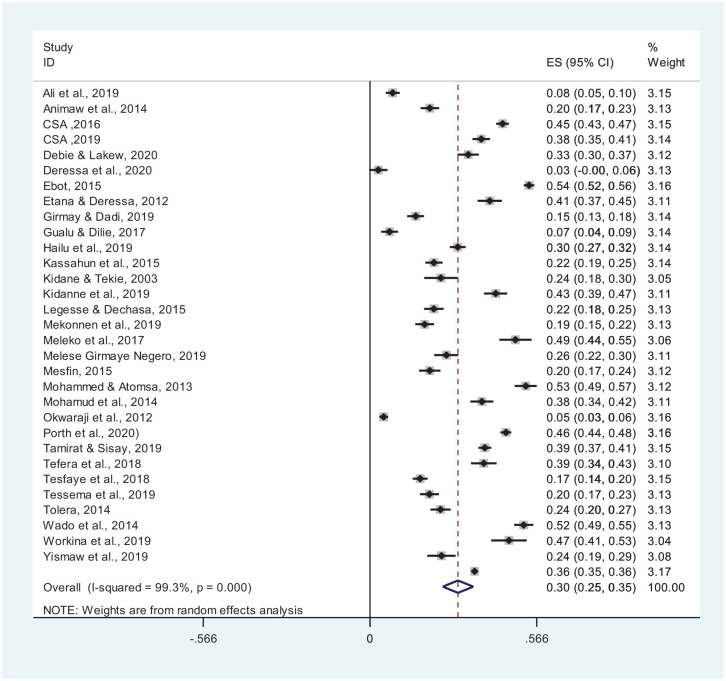
Pooled Prevalence of Incomplete Vaccination
In 31 studies with a sample size of 25 008 and 8878 cases of incomplete vaccination, the pooled prevalence of incomplete vaccination was 30% (95% CI: 25-35) (Figure 3).
Figure 3.
Pooled prevalence of incomplete vaccination in Ethiopia (n = 31 studies).
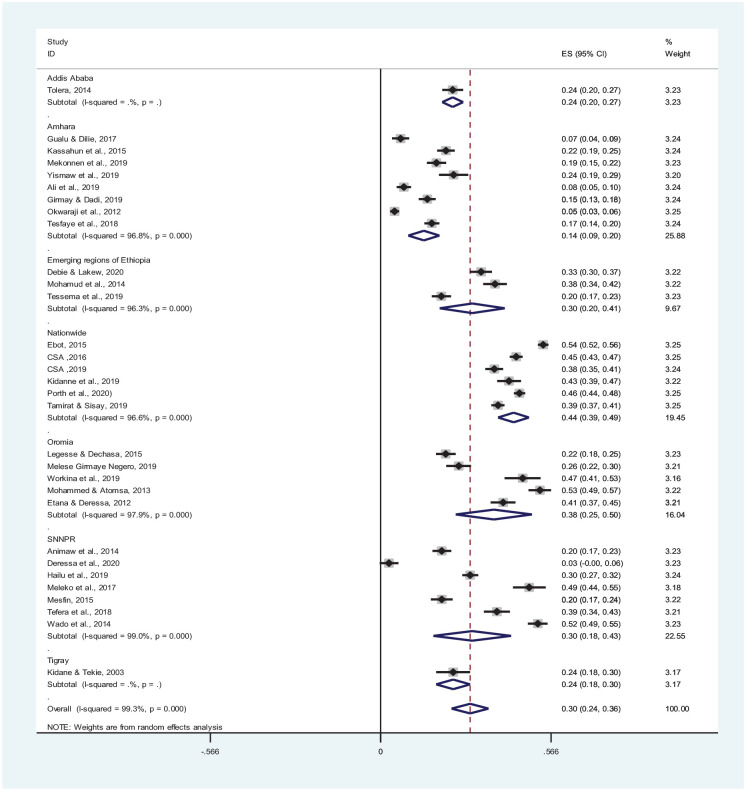
Subgroup analysis of the pooled prevalence of incomplete vaccination for 31 studies was carried out based on regions and nationwide studies in Ethiopia. Only 1 study was included in the capital city, Addis Ababa, which showed a pooled prevalence of 24%. In 6 nationwide studies, the prevalence of incomplete vaccination was 44% (95% CI: 39-49). Likewise, in 5 studies included from Oromia and 8 studies from Amhara Regional State, incomplete vaccination was 38% (95% CI: 25-50) and 14% (95% CI: 9-20), respectively (Figure 4).
Figure 4.
Forest plot for the subgroup analysis of incomplete vaccination among children in Ethiopia (n = 31 studies).
Factors Associated with Incomplete Vaccination
In this meta-analysis, maternal education, maternal knowledge, maternal decision making, urban residence, husband employment, place of delivery, antenatal care (ANC) (at least one visit), postnatal care, and tetanus toxoid vaccine (3+) were statistically associated with incomplete vaccination. However, maternal age, marital status, maternal occupation, distance to vaccination centers, wealth status, maternal attitude, and fear of side effects were not statistically associated with incomplete vaccination. To calculate the effect sizes, the random-effects model was implemented when there was heterogeneity among the included studies with a consideration of I2 of more than 60%.
Maternal Education
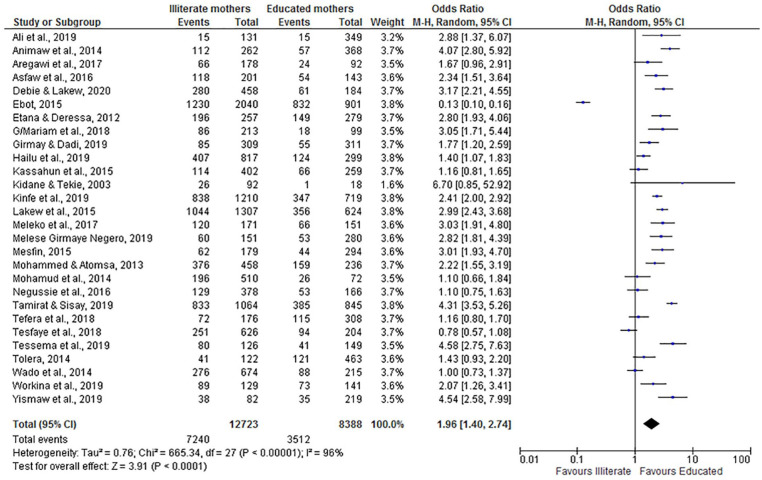
The overall adjusted odds ratio (OR = 1.96; 95% CI: 1.40, 2.74) indicated that children from illiterate women were nearly 2 times more likely to have incomplete vaccination compared with children of educated mothers. Despite the heterogeneity of the studies, the findings showed a statistically significant association. We used a random-effect model for the analysis because the I2 value was 96% (Figure 5).
Figure 5.
The influence of maternal education on incomplete vaccination in Ethiopia (n = 28 studies).
Maternal Age, Occupation, and Marital Status
This systematic review revealed that no significant association between maternal age (OR = 1.23; 95% CI: 0.88, 1.72) and marital status (OR = 0.71; 95% CI: 0.34, 1.51) with incomplete vaccination. Similarly, maternal occupation showed no statistical association with incomplete vaccination (OR = 0.93; 95% CI: 0.66, 1.31). We assumed a random effect model for the analysis because the I2 statistics indicated the presence of heterogeneity (91%), (93%), and (94%) respectively.
Maternal Knowledge
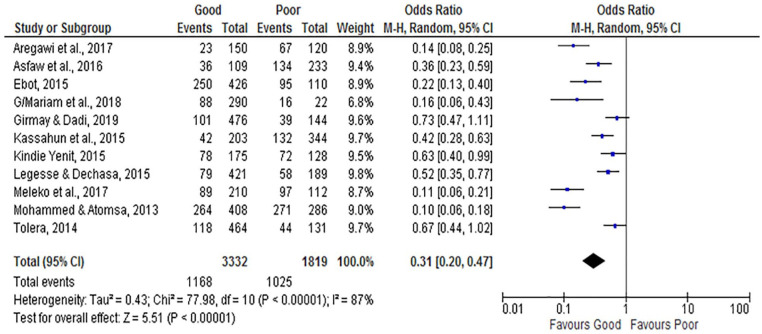
The overall analysis of studies showed that maternal knowledge of vaccination is associated with incomplete vaccination. Knowledgeable women about vaccination were less likely to incompletely vaccinate their infants (OR = 0.31; 95% CI: 0.20, 0.47) compared to non-knowledgeable women. The random-effect model was assumed for the analysis because the I2 value was 87% (Figure 6).
Figure 6.
The influence of maternal knowledge on incomplete vaccination in Ethiopia (n = 11 studies).
Maternal Autonomy
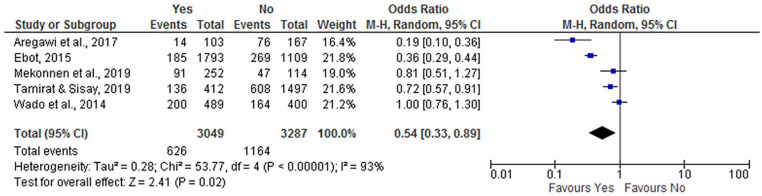
This analysis result revealed that women’s decision-making power had an association with incomplete vaccination, where autonomous women were less likely to have incompletely immunized children (OR = 0.54; 95% CI: 0.33, 0.89) compared to non-autonomous women. The random-effect model was used for the analysis, as the I2 test result was 93% (Figure 7).
Figure 7.
The influence of maternal decision-making power on incomplete vaccination in Ethiopia (n = 5 studies).
Place Residence
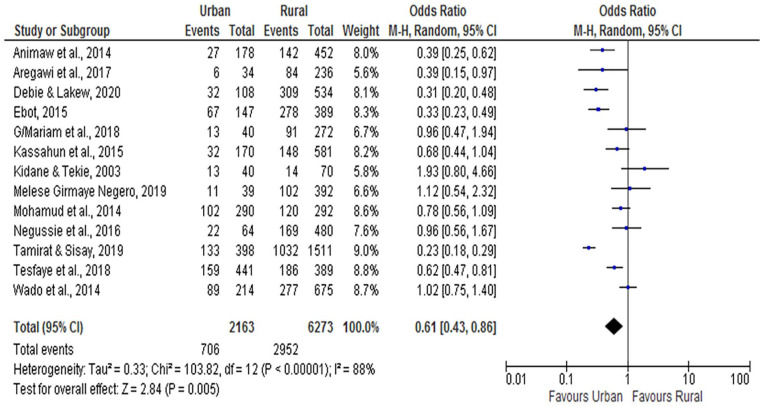
As per the factor analysis of the included studies, the place of residence was significantly associated with incomplete vaccination. We found that urban dwellings were less likely to be incompletely immunized (OR = 0.61; 95% CI: 0.43, 0.86) compared to rural children. The random-effect model was used for the analysis, as the I2 test result was 88% (Figure 8).
Figure 8.
The influence of place of residence on incomplete vaccination in Ethiopia (n = 13 studies).
Perinatal Care-Related Factors
Antenatal Care
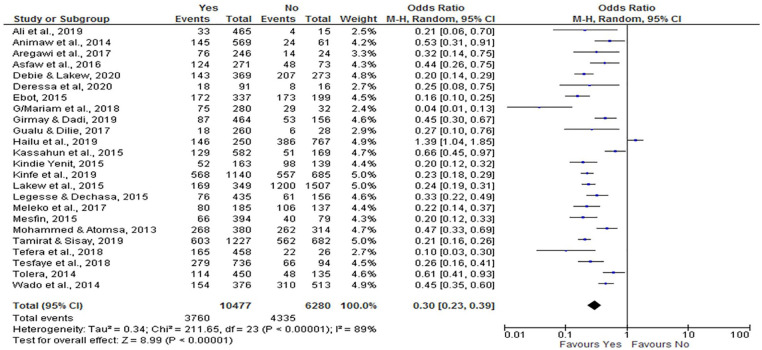
From this review, ANC (at least one visit) utilization has a negative association with incomplete vaccination. Women who attended ANC were less likely to have incompletely immunized children (OR = 0.30; 95% CI: 0.23, 0.39) compared to those who did not initiate ANC follow-up. We analyzed a random effect model because the I2 value was 89% (Figure 9).
Figure 9.
The influence of antenatal care follow-up on incomplete vaccination in Ethiopia (n = 24 studies).
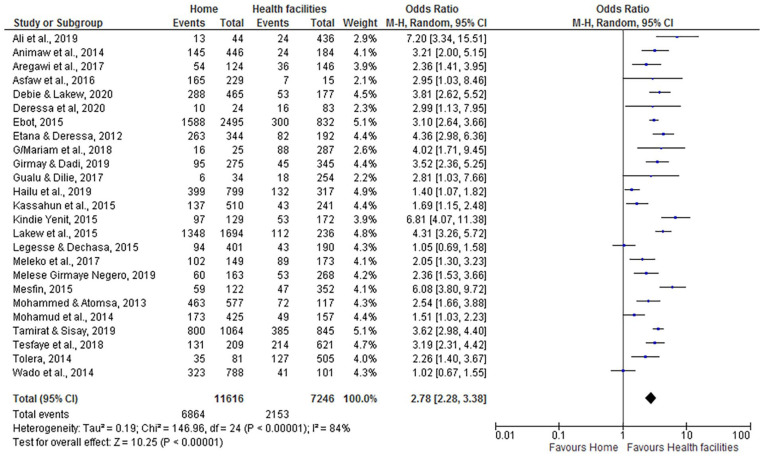
Place of Delivery
According to this systematic review and meta-analysis, women who gave birth at home were nearly 3 times more likely to have incompletely immunized children (OR = 2.78; 95% CI: 2.28, 3.38) than women who delivered at health facilities. We applied a random effect model for the meta-analysis because the I2 value was 84% (Figure 10).
Figure 10.
The influence of place of delivery on incomplete vaccination in Ethiopia (n = 25 studies).
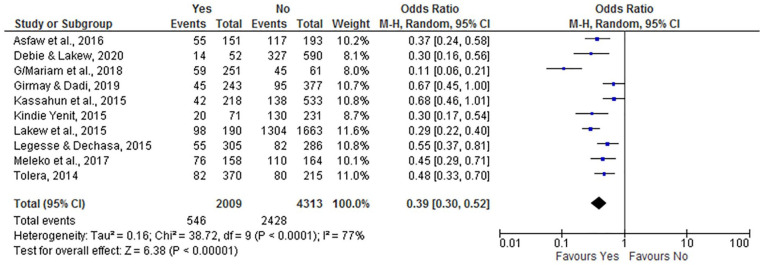
Postnatal Care
Postnatal care utilization showed a negative association with incomplete vaccination. Women who attended postnatal care were less likely to have incompletely immunized infants (OR = 0.39; 95% CI: 0.30, 0.52) compared to those who did not have utilized postnatal care. We analyzed a random effect model because the I2 value was 77% (Figure 11).
Figure 11.
The influence of maternal postnatal care on incomplete vaccination in Ethiopia (n = 10 studies).
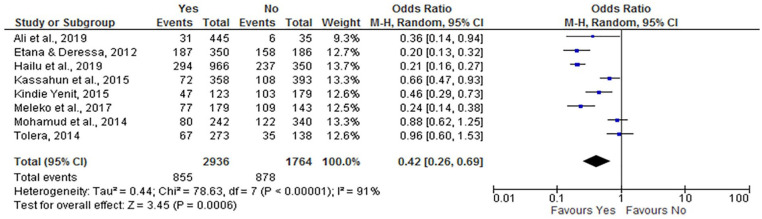
Tetanus Toxoid Vaccine
There was a significant association between tetanus toxoid vaccination of mothers and incomplete child vaccination. Women who took the tetanus toxoid vaccine were less likely to have incompletely vaccinated children (OR = 0.42; 95% CI: 0.26, 0.69) compared with women who did not take the vaccine. As a result of significant heterogeneity, a random effect model was used because the I2 value was 91% (Figure 12).
Figure 12.
The influence of TT3 on incomplete vaccination in Ethiopia (n = 8 studies).
Wealth Status
Monthly average family wealth status was not significantly associated with childhood vaccination. It was demonstrated that monthly average family low wealth status was not associated with incomplete vaccination (OR = 1.78; 95% CI: 0.99, 3.20) compared to women whose average family wealth was medium and high. The random-effect model was assumed for the analysis because the I2 value was 97%.
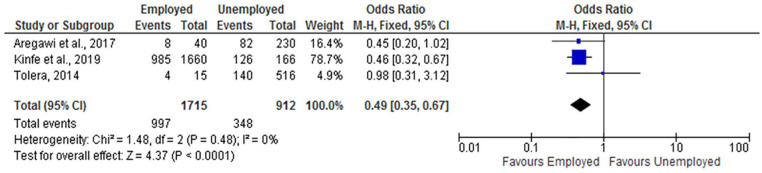
Husband Employment
The results of the review indicated that husband employment was significantly associated with childhood vaccination status. Infants from employed fathers were less likely to be incompletely vaccinated (OR = 0.49; 95% CI: 0.35, 0.67) compared with children from an unemployed father (Figure 13).
Figure 13.
The influence of husband employment status on incomplete vaccination in Ethiopia (n = 3 studies).
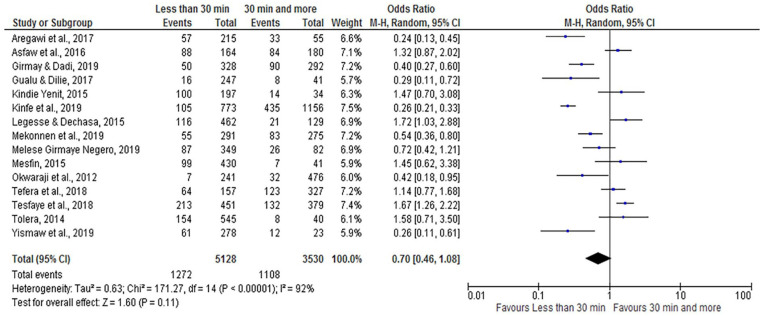
Time Taken to Reach Vaccination Centers
According to this meta-analysis, the time taken to reach vaccination centers was not significantly associated with incomplete vaccination (OR = 0.70; 95% CI: 0.46, 1.08). The random-effect model was assumed for the analysis because the I2 value was 92% (Figure 14).
Figure 14.
The influence of time to reach vaccination centers on incomplete vaccination in Ethiopia (n = 15 studies).
Mother’s Attitude
As evidenced in this meta-analysis, maternal attitude was not associated with incomplete vaccination (OR = 0.82; 95% CI: 0.37, 1.83). Moreover, there was no association between fear of side effects and incomplete vaccination (OR = 1.36; 95% CI: 0.57, 3.22). We assumed a random effect model for the analysis because the I2 statistics indicated the presence of heterogeneity (91%) and (90%), respectively.
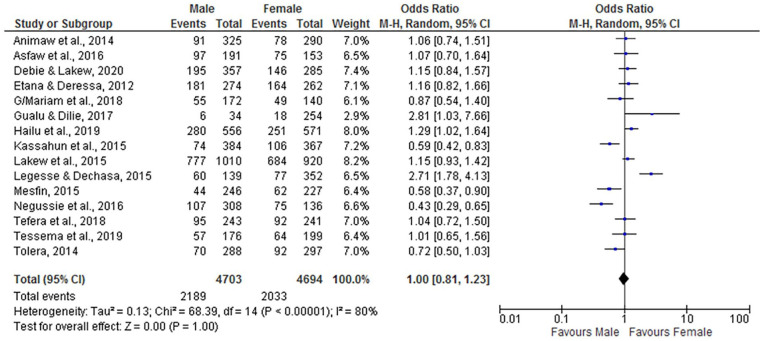
Child Sex
According to this meta-analysis, there is no association between child sex (being male or female) and incomplete vaccination (OR = 1.00; 95% CI: 0.81, 1.23). The random-effect model was assumed for the analysis because the I2 value was 80% (Figure 15).
Figure 15.
The influence of child sex on incomplete vaccination in Ethiopia (n = 15 studies).
Discussion
In Ethiopia, an evidence-based understanding of the barriers to incomplete vaccination and addressing the root causes is critical to improving childhood immunization, which subsequently reduces child mortality. Hence, designing and implementing tailored interventions are essential to ensure that children are vaccinated fully and are safe from VPDs. Without such a systematic approach, millions of children will continue to die from VPDs. For instance, only 39% to 43.3% of children 12 to 23 months are fully vaccinated in Ethiopia with all recommended vaccine doses.17,38 The country is unable to achieve the WHO target of vaccination coverage of 90% by 2020.5 This likely translates to insufficient herd immunity against many VPDs.65 This systematic review and meta-analysis estimated the pooled prevalence of incomplete vaccination and identified its key barriers in Ethiopia.
The overall pooled magnitude of incomplete vaccination among children in Ethiopia was 30% (95% CI: 25-35). This finding is similar to the findings of studies carried out in Australia (35%),66 India (32%),67 and global routine vaccination coverage in 2017 (30%).68 However, the present finding is lower than studies in Pakistan (46%),69 Aurangabad (37.76%),70 and the 2016 and 2019 EDHS (45%),38 (37.7%)17 in Ethiopia. The variations highlight the gradient of vaccination system performance across Ethiopia, given diverse religious, sociocultural, or health service coverage and performance differences.71 Moreover, it might be related to variations in access to preventive care services and perceptions of the importance of vaccination between populations of different countries.
In this meta-analysis, maternal education, maternal knowledge, maternal autonomy, urban residence, husband employment, place of delivery, ANC follow-up, postnatal care, and tetanus toxoid vaccine (3+) were found to be significantly associated with incomplete vaccination. The importance of maternal education and knowledge in children’s health is universally recognized.72 Accordingly, children of less-educated mothers are more likely to be incompletely vaccinated. Knowledgeable women about vaccination are less likely to incompletely vaccinate their infants. This finding is supported by studies conducted in Togo,73 India,67 Indonesia,74 Pakistan,69,75 northern Ethiopia,26 Sub-Saharan Africa,76 and a systematic review of LMICs and across the world.77–80 This could be because women with a better educational background are more likely to be knowledgeable about the benefits of full vaccine doses. It is also possible that better-educated mothers are more flexible, receptive to new ideas, and make confident decisions about their families’ health, including vaccination.
This review further revealed that women’s decision-making power has an association with incomplete vaccination, where autonomous women were less likely to have incompletely immunized children. This finding is in line with several other studies62,39,81,82 concluded that childhood vaccine decision-making begins prenatally. Women’s participation in health care decision-making enables women to decide independently, and in particular, it helps to reduce the vaccine dropout rate.36,81
The husband’s employment status was significantly associated with childhood vaccination. Infants from employed fathers were less likely to be incompletely vaccinated. This might be because employed husbands could have better knowledge and exposure to vaccination-related information from their workplace. It may also be related to the husband’s earnings that eases transport or indirect expenses related to vaccination.64
As per this analysis, place of residence was significantly associated with incomplete vaccination. Mothers who lived in urban areas were less likely to have incomplete vaccination of their children. This finding was supported by studies performed in the emerging regions of Ethiopia.27 This might be explained by urban resident mothers who might have better information and recognize the importance of vaccination. However, this finding is contrary to studies in Sub-Saharan Africa and India, which reported that children from urban areas were more likely to be partially immunized than those from rural areas.67,76 This might be the presence of underserved children living in urban slums with limited access to vaccination services.
Furthermore, ANC follow-up, place of delivery, postnatal care, and tetanus toxoid vaccine (3+) were associated with incomplete vaccination. Accordingly, women who attended and received at least one ANC visit, postnatal care, and tetanus toxoid vaccine are less likely to have incomplete vaccination of their children. However, women who gave birth at home were nearly 3 times more likely to have partially immunized children. Similar findings were reported in other studies in India,67 Pakistan,75 Senegal,71 Philippines,83 Tigray, northern Ethiopia,36 a systematic review across the globe77 and in LMICs.84 The positive impacts of ANC visits and postnatal care on the completion of infants’ vaccination can be explained by the fact that mothers have more opportunities to receive messages on the benefits of childhood vaccination that encourage them to fully vaccinate their children. Prenatal care visits establish communication and build trust between healthcare providers and mothers, which may affect mothers’ immunity-related service-seeking behaviors.84–86
This systematic review had some limitations. First, the majority of the included studies were cross-sectional and prone to confounding. Second, we included data obtained using maternal recall, and vaccination record cards may introduce recall biases. Thirdly, the fact that the current meta-analysis is carried-out despite the presence of heterogeneity across the included studies might have influenced the effect estimates of the study. Finally, many of the data were concentrated in Amhara, Oromia, and Southern nation, national, and people regional states. The review also has strengths. The review considered pertinent and comprehensive databases for the literature search. Subgroup analysis was also conducted to appreciate the regional variations in the overall burden of incomplete vaccination. The review also considered both published and unpublished literature.
Conclusion
In this review and meta-analysis, 3 in every 10 children had incomplete vaccination, which is a public health concern in the country. Maternal education, knowledge, decision-making power, urban residence, husband employment, ANC visits, home delivery, postnatal care, and tetanus toxoid vaccine were identified as factors associated with incomplete vaccination. Increasing women’s education and improving maternal health knowledge and empowering women in decision making would provide an approach to reduce partial immunization. Regular vaccination outreach campaigns and integration of immunization with other services may improve childhood vaccination. Strengthening the interaction between healthcare workers and mothers and improving the quality of prenatal and postnatal care services reduce the rate of incomplete vaccination.
Supplemental Material
Supplemental material, Additional_file_1 for Incomplete Vaccination and Its Predictors among Children in Ethiopia: A Systematic Review and Meta-Analysis by Assefa Desalew, Agumasie Semahegn, Simon Birhanu and Gezahegn Tesfaye in Global Pediatric Health
Supplemental material, Additional_file_2 for Incomplete Vaccination and Its Predictors among Children in Ethiopia: A Systematic Review and Meta-Analysis by Assefa Desalew, Agumasie Semahegn, Simon Birhanu and Gezahegn Tesfaye in Global Pediatric Health
Supplemental material, Additional_file_3 for Incomplete Vaccination and Its Predictors among Children in Ethiopia: A Systematic Review and Meta-Analysis by Assefa Desalew, Agumasie Semahegn, Simon Birhanu and Gezahegn Tesfaye in Global Pediatric Health
Footnotes
Authors’ Contributions: AD* and AS initiated and formulated this meta-analysis. AD conducts activities from initiation to finalization of the manuscript. AD, GT, SB, and AS build-up the search strategies, meta-analysis, and interpretation of the findings. All authors read thoroughly and approved the manuscript.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Assefa Desalew  https://orcid.org/0000-0001-6065-0708
https://orcid.org/0000-0001-6065-0708
Availability of data and materials: All data generated or analyzed during this review are included in this manuscript and its supplementary information files.
Supplemental Material: Supplemental material for this article is available online.
References
- 1. World Health Organization (WHO). Global Vaccine Action Plan Regional Vaccine Action Plans 2016 Progress Reports. WHO; 2016. [Google Scholar]
- 2. World Health Organization (WHO). Immunization Agenda 2030: A Global Strategy to Leave No One Behind. WHO; 2019:1-29. [Google Scholar]
- 3. World Health Organization (WHO). GVAP 2011-2020, Review and Lessons Learned; Strategic Advisory Group of Experts on ImmunizationVaccination [Internet]. WHO; 2020:1-44. Accessed May 2, 2020. https://apps.who.int/iris/bitstream/handle/10665/329097/WHO-IVB-19.07-eng.pdf [Google Scholar]
- 4. Patel MK, Dumolard L, Nedelec Y, et al. Progress toward regional measles elimination — worldwide, 2000–2018. Morb Mortal Wkly Rep. 2019;68:1105-1111. doi: 10.15585/mmwr.mm6848a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. World Health Organization (WHO). National Immunization Coverage Score Cards Estimates for 2018 [Internet]. World Health Organization; 2018:24 Accessed May 2, 2020. http://www.who.int/immunization/monitoring_surveillance/data/en [Google Scholar]
- 6. Chen SI, Norman BA, Rajgopal J, Assi TM, Lee BY, Brown ST. A planning model for the WHO-EPI vaccine distribution network in developing countries. IIE Trans. 2014;46:853-865. doi: 10.1080/0740817X.2013.813094 [DOI] [Google Scholar]
- 7. Federal Ministry of Health Ethiopia (FMoH). Ethiopia National Expanded Program on Immunization, Comprehensive Multi - Year Plan 2016 – 2020. FMoH; 2015:1-115. [Google Scholar]
- 8. Clark A, Sanderson C. Timing of children’s vaccinations in 45 low-income and middle-income countries: an analysis of survey data. Lancet. 2009;373:1543-1549. doi: 10.1016/S0140-6736(09)60317-2 [DOI] [PubMed] [Google Scholar]
- 9. Rainey JJ, Watkins M, Ryman TK, Sandhu P, Bo A, Banerjee K. Reasons related to non-vaccination and under-vaccination of children in low and middle-income countries: findings from a systematic review of the published literature, 1999–2009. Vaccine. 2011;29:8215-8221. doi: 10.1016/j.vaccine.2011.08.096 [DOI] [PubMed] [Google Scholar]
- 10. Larson HJ, de Figueiredo A, Xiahong Z, et al. The state of vaccine confidence 2016: global insights through a 67-country survey. EBioMedicine. 2016;12:295-301. doi: 10.1016/j.ebiom.2016.08.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Roth GA, Abate D, Abate KH, et al. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1736-1788. doi: 10.1016/S0140-6736(18)32203-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chang AY, Riumallo-Herl C, Perales NA, et al. The equity impact vaccines may have on averting deaths and medical impoverishment in developing countries. Health Aff. 2018;37:316-324. 10.1377/hlthaff.2017.0861 [DOI] [PubMed] [Google Scholar]
- 13. World Health Organization (WHO). Immunization Coverage. Fact Sheet. WHO; 2019:1. [Google Scholar]
- 14. World Health Organization (WHO). Annex To the Global Vaccine Action Plan Review and Lessons Learned Report [Internet]. World Health Organization; 2018. Accessed May 18, 2020. http://apps.who.int/bookorders [Google Scholar]
- 15. Madhi SA, Rees H. Special focus on challenges and opportunities for the development and use of vaccines in Africa. Hum Vaccines Immunother. 2018;14:2335-2339. doi: 10.1080/21645515.2018.1522921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. World Health Organization (WHO). Meeting of the Strategic Advisory Group of Experts on immunization, October 2009 - conclusions and recommendations. Biologicals. 2010;38:170-177. doi: 10.1016/j.biologicals.2009.12.007 [DOI] [PubMed] [Google Scholar]
- 17. CSA. Ethiopia Mini Demographic and Health Survey. CSA; 2019. [Google Scholar]
- 18. ICF and (CSA) [Ethiopia]. Ethiopia Demographic and Health Survey 2016: Key Indicators Report. CSA and ICF; 2016. [Google Scholar]
- 19. Gilbert NL, Gilmour H, Wilson SE, Cantin L. Determinants of non-vaccination and incomplete vaccination in Canadian toddlers. Hum Vaccines Immunother. 2017;13:1447-1453. doi: 10.1080/21645515.2016.1277847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Adedokun ST, Uthman OA, Adekanmbi VT, Wiysonge CS. Incomplete childhood immunization in Nigeria: a multilevel analysis of individual and contextual factors. BMC Public Health. 2017;17:1-10. doi: 10.1186/s12889-017-4137-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Deressa AT, Desta MS, Belihu TM. Vaccination status and associated factors among street children 9 – 24 months old in Sidama Region, Ethiopia. Ann Glob Health. 2020;86:1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mohammed H, Atomsa A. Assessment of child immunization coverage and associated factors. Sci Technol Res. 2013;7522:36-41. [Google Scholar]
- 23. Abadura SA, Lerebo WT, Kulkarni U, Mekonnen ZA. Individual and community-level determinants of childhood full immunization in Ethiopia: a multilevel analysis of Global health. BMC Public Health. 2015;15:1-10. doi: 10.1186/s12889-015-2315-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hailu S, Astatkie A, Johansson KA, Lindtjørn B. Low immunization coverage in Wonago district, southern Ethiopia: a community-based cross-sectional study. PLoS One. 2019;14:1-18. doi: 10.1371/journal.pone.0220144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Glatman-Freedman A, Nichols K. The effect of social determinants on immunization programs. Hum Vaccines Immunother. 2012;8:293-301. doi: 10.4161/hv.19003 [DOI] [PubMed] [Google Scholar]
- 26. Yismaw AE, Assimamaw NT, Bayu NH, Mekonen SS. Incomplete childhood vaccination and associated factors among children aged 12-23 months in Gondar city administration, Northwest, Ethiopia 2018. BMC Res Notes. 2019;12:1-7. doi: 10.1186/s13104-019-4276-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Debie A, Lakew AM. Factors associated with the access and continuum of vaccination services among children aged 12-23 months in the emerging regions of Ethiopia: evidence from the 2016 Ethiopian demography and health survey. Ital J Pediatr. 2020;46:1-11. doi: 10.1186/s13052-020-0793-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dubé E, Laberge C, Guay M, Bramadat P, Roy R, Bettinger J. H_Dube_2013_Vaccine hesitancy overview. Hum Vaccines Immunother. 2013;9:1763-1773. doi: 10.4161/hv.24657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Luman ET, McCauley MM, Shefer A, Chu SY. Maternal characteristics associated with vaccination of young children. Pediatrics. 2003;111:1215-1218. [PubMed] [Google Scholar]
- 30. Atun RA, Menabde N, Saluvere K, Jesse M, Habicht J. Introducing a complex health innovation-primary health care reforms in Estonia (multimethods evaluation). Health Policy (N Y). 2006;79:79-91. doi: 10.1016/j.healthpol.2005.12.005 [DOI] [PubMed] [Google Scholar]
- 31. Atun RA, Kyratsis I, Jelic G, Rados-Malicbegovic D, Gurol-Urganci I. Diffusion of complex health innovations - implementation of primary health care reforms in Bosnia and Herzegovina. Health Policy Plan. 2007;22:28-39. doi: 10.1093/heapol/czl031 [DOI] [PubMed] [Google Scholar]
- 32. Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Workina A, Seid SS, Moga TT. Reason for incomplete vaccination and associated factors among children aged 12-23 months in Serbo Town, Jimma Zone, Oromia Region, Southwest Ethiopia. Adv Res J Multi-Disciplinary Discov. 2019;32:79-84. [Google Scholar]
- 34. Ali Y, Mekonnen FA, Molla Lakew A, Wolde HF. Poor maternal health service utilization associated with incomplete vaccination among children aged 12-23 months in Ethiopia. Hum Vaccines Immunother. 2019;16:1202-1207. doi: 10.1080/21645515.2019.1670124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Animaw W, Taye W, Merdekios B, Tilahun M, Ayele G. Expanded program of immunization coverage and associated factors among children age 12–23 months in Arba Minch town and Zuria. BMC Public Health. 2014;14:464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Aregawi HG, Gebrehiwot TG, Abebe YG, Meles KG, Wuneh AD. Determinants of defaulting from completion of child immunization in Laelay Adiabo District, Tigray Region, Northern Ethiopia: a case-control study. PLoS One. 2017;12:1-13. doi: 10.1371/journal.pone.0185533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Asfaw AG, Koye DN, Demssie AF, Zeleke EG, Gelaw YA. Determinants of default to fully completion of immunization among children aged 12 to 23 months in south Ethiopia: unmatched case-control study. Pan Afr Med J. 2016;23:1-8. doi: 10.11604/pamj.2016.23.100.7879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. CSA. 2016 Ethiopia Demographic and Health Survey (EDHS) Introduction and Methodology. CSA; 2016. Accessed April 15, 2020. http://www.ethiodemographyandhealth.org/Measure_DHS_Ethiopia2016.pdf [Google Scholar]
- 39. Ebot JO. “Girl power!”: the relationship between women’s autonomy and children’s immunization coverage in Ethiopia. J Health Popul Nutr. 2015;33:1-9. doi: 10.1186/s41043-015-0028-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Etana B, Deressa W. Factors associated with complete immunization coverage in children aged 12–23 months in Ambo Woreda, Central Ethiopia. BMC Public Health. 2012;12:1. doi: 10.1186/1471-2458-12-566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. G/Mariam M, Ayele G, Shegaze M, Wassihun B. Determinants of incomplete immunization among children age 12-23 months in Southwest Ethiopia. Res Square. 2019;1-21. doi: 10.21203/rs.2.13436/v1 [DOI] [Google Scholar]
- 42. Girmay A, Dadi AF. Full immunization coverage and associated factors among children aged 12-23 months in a hard-to-reach areas of Ethiopia. Int J Pediatr. 2019; 2019:1-8. doi: 10.1155/2019/1924941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Gualu T, Dilie A. Vaccination coverage and associated factors among children aged 12–23 months in Debre Markos Town, Amhara Regional State, Ethiopia. Adv Public Health. 2017;2017:1-6. doi: 10.1155/2017/5352847 [DOI] [Google Scholar]
- 44. Kassahun MB, Biks GA, Teferra AS. Level of immunization coverage and associated factors among children aged 12-23 months in Lay Armachiho District, North Gondar Zone, Northwest Ethiopia: a community-based cross-sectional study. BMC Res Notes. 2015;8:1-10. doi: 10.1186/s13104-015-1192-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kidane T, Tekie M. Factors influencing child immunization coverage in a rural district of Ethiopia, 2000. Ethiop J Health Dev. 2003;17:105-110. [Google Scholar]
- 46. Kidanne L, Solomon M, Bisrat F, et al. Child vaccination timing, intervals, and missed opportunities in pastoral and semi-pastoral areas in Ethiopia. Ethiop J Health Dev. 2019;33(Special issue):16-23. [Google Scholar]
- 47. Yenit MK, Assegid S, Abrha H. Factors associated with incomplete childhood vaccination among children 12-23 months of age in Machakel Woreda, East Gojjam Zone: a case-control study. J Pregnancy Child Health. 2015;2:180. doi: 10.4172/2376-127x.1000180 [DOI] [Google Scholar]
- 48. Legesse E, Dechasa W. An assessment of child immunization coverage and its determinants in Sinana District, Southeast Ethiopia. BMC Pediatr. 2015;15:1-14. doi: 10.1186/s12887-015-0345-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Mekonnen AG, Bayleyegn AD, Ayele ET. Immunization coverage of 12-23 months old children and its associated factors in Minjar-Shenkora district, Ethiopia: a community-based study. BMC Pediatr. 2019;19:1-8. doi: 10.1186/s12887-019-1575-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Meleko A, Geremew M, Birhanu F. Assessment of child immunization coverage and associated factors with full vaccination among children aged 12–23 months at Mizan Aman Town, Bench Maji Zone, Southwest Ethiopia. Int J Pediatr. 2017;2017:1-11. doi: 10.1155/2017/7976587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Negero MG, Dechassa W, Kassaye M. Immunization incompletion among 12 - 23 months old children and associated factors in Wayu-Tuka District, Western Ethiopia: a community based study. EC Paediatr. 2019; 8:114-127. [Google Scholar]
- 52. Mesfin M. Incomplete vaccination and associated factors among children aged 12-23 months in Yirgalem Town, South Ethiopia. Unpublished thesis. 2015:1-80. http://scholar.googleusercontent.com/scholar. Accessed May 2, 2020. [Google Scholar]
- 53. Mohamud AN, Feleke A, Worku W, Kifle M, Sharma HR. Immunization coverage of 12-23 months old children and associated factors in Jigjiga District, Somali National Regional State, Ethiopia. BMC Public Health. 2014;14:1-9. doi: 10.1186/1471-2458-14-865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Negussie A, Kassahun W, Assegid S, Hagan AK. Factors associated with incomplete childhood immunization in Arbegona district, southern Ethiopia: a case-control study. BMC Public Health. 2016;16:1-9. doi: 10.1186/s12889-015-2678-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Okwaraji YB, Mulholland K, Schellenberg JRMA, Andarge G, Admassu M, Edmond KM. The association between travel time to health facilities and childhood vaccine coverage in rural Ethiopia. A community-based cross-sectional study. BMC Public Health. 2012;12:1. doi: 10.1186/1471-2458-12-476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Porth JM, Wagner AL, Teklie H, Abeje Y, Moges B, Boulton ML. Vaccine non-receipt and refusal in Ethiopia: the expanded program on immunization coverage survey, 2012. Vaccine. 2020;37:2106-2121. doi: 10.1016/j.vaccine.2019.02.045 [DOI] [PubMed] [Google Scholar]
- 57. Tamirat KS, Sisay MM. Full immunization coverage and its associated factors among children aged 12-23 months in Ethiopia: further analysis from the 2016 Ethiopia demographic and health survey. BMC Public Health. 2019;19:1-7. doi: 10.1186/s12889-019-7356-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Tefera YA, Wagner AL, Boulton ML. Predictors and barriers to full vaccination among children in Ethiopia. Vaccine. 2018;6:1-11. doi: 10.3390/vaccines6020022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Tesfaye TD, Temesgen WA, Kasa AS. Vaccination coverage and associated factors among children aged 12–23 months in Northwest Ethiopia. Hum Vaccines Immunother. 2018;14:2348-2354. doi: 10.1080/21645515.2018.1502528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Tessema F, Kidanne L, Bisrat F, et al. Child vaccination coverage and dropout rates in pastoral and semi-pastoral regions in Ethiopia: CORE Group Polio Project implementation areas. Ethiop J Health Dev. 2019;33(Special issue):3-9. [Google Scholar]
- 61. Tolera D. Assessment of magnitude and factors associated with full immunization coverage in children aged 12- 23 months in Addis Ketema sub-city, Addis Ababa, Ethiopia. Unpublished thesis. 2014.
- 62. Wado YD, Afework MF, Hindin MJ. Childhood vaccination in rural southwestern Ethiopia: the nexus with demographic factors and women’s autonomy. Pan Afr Med J. 2014;17:1-6. doi: 10.11694/pamj.supp.2014.17.1.3135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Lakew Y, Bekele A, Biadgilign S. Factors influencing full immunization coverage among 12-23 months of age children in Ethiopia: evidence from the national demographic and health survey in 2011. BMC Public Health. 2015;15:1-8. doi: 10.1186/s12889-015-2078-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Kinfe Y, Gebre H, Bekele A. Factors associated with full immunization of children 12–23 months of age in Ethiopia: a multilevel analysis using the 2016 Ethiopia Demographic and Health Survey. PLoS One. 2019;14:1-14. doi: 10.1371/journal.pone.0225639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Kim TH, Johnstone J, Loeb M. Vaccine herd effect. Scand J Infect Dis. 2011;43:683-689. doi: 10.3109/00365548.2011.582247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Lawrence GL, Hull BP, MacIntyre CR, McIntyre PB. Reasons for incomplete immunization among Australian children: a national survey of parents. Aust Fam Physician. 2004;33:568-571. [PubMed] [Google Scholar]
- 67. Francis MR, Nohynek H, Larson H, et al. Factors associated with routine childhood vaccine uptake and reasons for non-vaccination in India: 1998–2008. Vaccine. 2018; 36:6559-6566. doi: 10.1016/j.vaccine.2017.08.026 [DOI] [PubMed] [Google Scholar]
- 68. Vanderende K, Gacic-Dobo M, Diallo MS, Conklin LM, Wallace AS. Global routine vaccination coverage — 2017. Morb Mortal Wkly Rep. 2018;67:1261-1264. doi: 10.15585/MMWR.MM6745A2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Riaz A, Husain S, Yousafzai MT, et al. Reasons for non-vaccination and incomplete vaccinations among children in Pakistan. Vaccine. 2018;36:5288-5293. doi: 10.1016/j.vaccine.2018.07.024 [DOI] [PubMed] [Google Scholar]
- 70. Ingale A, Dixit JV, Deshpande D. Reasons behind incomplete immunization: a cross-sectional study at Urban Health Centre of Government Medical College, Aurangabad. Natl J Community Med. 2013;4:353-356. http://njcmindia.org/uploads/4-2_353-356.pdf%0Ahttp://www.njcmindia.org/home/abstrct/438/Apr_-_June [Google Scholar]
- 71. Mbengue MAS, Sarr M, Faye A, et al. Determinants of complete immunization among senegalese children aged 12-23 months: evidence from the demographic and health survey. BMC Public Health. 2017;17:1-9. doi: 10.1186/s12889-017-4493-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Jaca A, Mathebula L, Iweze A, Pienaar E, Wiysonge CS. A systematic review of strategies for reducing missed opportunities for vaccination. Vaccine. 2018;36:2921-2927. doi: 10.1016/j.vaccine.2018.04.028 [DOI] [PubMed] [Google Scholar]
- 73. Ekouevi DK, Gbeasor-Komlanvi FA, Yaya I, et al. Incomplete immunization among children aged 12-23 months in Togo: a multilevel analysis of individual and contextual factors. BMC Public Health. 2018;18:952. doi: 10.1186/s12889-018-5881-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Holipah H, Maharani A, Kuroda Y. Determinants of immunization status among 12- to 23-month-old children in Indonesia (2008-2013): a multilevel analysis. BMC Public Health. 2018;18:1-11. doi: 10.1186/s12889-018-5193-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Noh JW, Kim YM, Akram N, et al. Factors affecting complete and timely childhood immunization coverage in Sindh, Pakistan; a secondary analysis of cross-sectional survey data. PLoS One. 2018;13:1-15. doi: 10.1371/journal.pone.0206766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Wiysonge CS, Uthman OA, Ndumbe PM, Hussey GD. Individual and contextual factors associated with low childhood immunization coverage in Sub-Saharan Africa: a multilevel analysis. PLoS One. 2012;7:e37905. doi: 10.1371/journal.pone.0037905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Tauil MC, Sato APS, Waldman EA. Factors associated with incomplete or delayed vaccination across countries: a systematic review. Vaccine. 2016;34:2635-2643. doi: 10.1016/j.vaccine.2016.04.016 [DOI] [PubMed] [Google Scholar]
- 78. Smith LE, Amlôt R, Weinman J, Yiend J, Rubin GJ. A systematic review of factors affecting vaccine uptake in young children. Vaccine. 2017;35:6059-6069. doi: 10.1016/j.vaccine.2017.09.046 [DOI] [PubMed] [Google Scholar]
- 79. Favin M, Steinglass R, Fields R, Banerjee K, Sawhney M. Why children are not vaccinated: a review of the grey literature. Int Health. 2012;4:229-238. doi: 10.1016/j.inhe.2012.07.004 [DOI] [PubMed] [Google Scholar]
- 80. Mazige FM, Kalwani JD, Kakoko DCV. Social determinants of immunization services uptake in developing countries: a systematic review. Pan Afr Med J. 2016;24:1-10. doi: 10.11604/pamj.2016.24.197.960527583065 [DOI] [Google Scholar]
- 81. Singh K, Haney E, Olorunsaiye C. Maternal autonomy and attitudes towards gender norms: associations with childhood immunization in Nigeria. Matern Child Health J. 2013;17:837-841. doi: 10.1007/s10995-012-1060-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Jung M. The effect of maternal decisional authority on children’s vaccination in East Asia. PLoS One. 2018;13:1-11. doi: 10.1371/journal.pone.0200333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Malecosio SO, Celis MJLD, Delicana KB, et al. Vaccination coverage and factors associated with incomplete childhood vaccination among children aged 12-59 months in Miagao, Iloilo, Philippines. Int J Community Med Public Health. 2020;7:2492. doi: 10.18203/2394-6040.ijcmph20202971 [DOI] [Google Scholar]
- 84. Hajizadeh M. Socioeconomic inequalities in child vaccination in low/middle-income countries: what accounts for the differences? J Epidemiol Community Health. 2018;72:719-725. doi: 10.1136/jech-2017-210296 [DOI] [PubMed] [Google Scholar]
- 85. Dixit P, Dwivedi LK, Ram F. Strategies to improve child immunization via antenatal care visits in India: a propensity score matching analysis. PLoS One. 2013;8:e66175. doi: 10.1371/journal.pone.0066175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Rowe R, Calnan M. Trust relations in health care—the new agenda. Eur J Public Health. 2006;16:4-6. doi: 10.1093/eurpub/ckl003 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Additional_file_1 for Incomplete Vaccination and Its Predictors among Children in Ethiopia: A Systematic Review and Meta-Analysis by Assefa Desalew, Agumasie Semahegn, Simon Birhanu and Gezahegn Tesfaye in Global Pediatric Health
Supplemental material, Additional_file_2 for Incomplete Vaccination and Its Predictors among Children in Ethiopia: A Systematic Review and Meta-Analysis by Assefa Desalew, Agumasie Semahegn, Simon Birhanu and Gezahegn Tesfaye in Global Pediatric Health
Supplemental material, Additional_file_3 for Incomplete Vaccination and Its Predictors among Children in Ethiopia: A Systematic Review and Meta-Analysis by Assefa Desalew, Agumasie Semahegn, Simon Birhanu and Gezahegn Tesfaye in Global Pediatric Health