Abstract
Patients with malignancy may present with significant thromboembolic complications including deep vein thrombosis (DVT), pulmonary embolism, arterial thrombosis, nonbacterial thrombotic endocarditis, and stroke due to abnormal coagulation cascades. Although these events are typically recognized later in the disease process, complications of a hypercoagulable state can rarely present as the first manifestation of an occult malignancy. We report a case of a young male who was ultimately found to have an aggressive form of lung adenocarcinoma after the initial presentation of multiple thromboembolic events. DVT and stroke as an initial presentation of an active lung adenocarcinoma in a young patient is extremely rare as patients presenting in a hypercoagulable state usually are older. Though testing for a hypercoagulable state is not recommended for the first unprovoked DVT, clinicians should be prompted to screen for malignancy in the setting of cryptogenic strokes, especially in younger patients with no prior risk factors.
Keywords: lung cancer, thromboembolic events, malignancy-associated hypercoagulable state
Introduction
Thromboembolic events such as deep vein thrombosis (DVT), pulmonary embolism (PE), arterial thrombosis, nonbacterial thrombotic endocarditis, and ischemic strokes can be the initial event leading to the diagnosis of an underlying malignancy; this is due to abnormalities in the coagulation cascade that often accompanies many oncologic diseases. However, these events are typically recognized later in the disease process and rarely present as the first manifestation of an occult malignancy. We report a case of a young male who was ultimately found to have an aggressive form of lung adenocarcinoma after the initial presentation of multiple thromboembolic events.
Case Presentation
A 36-year-old male truck driver with a history of tobacco abuse initially presented to the emergency department with a chief complaint of right lower leg swelling and pain. Lower extremity ultrasound revealed a DVT of the right popliteal and right posterior tibial vein. Initial laboratory examinations showed normal complete blood count—hemoglobin 13.1 g/dL, hematocrit 40.7%, white blood cells 7.91 × 103/µL, platelet count 155 × 103/µL, and normal coagulation studies— activated partial thromboplastin time 29.9 seconds, activated partial thromboplastin time ratio 1.09, prothrombin time 14.1 seconds, and international normalized ratio 1.1. He was discharged home with rivaroxaban, a factor Xa inhibitor.
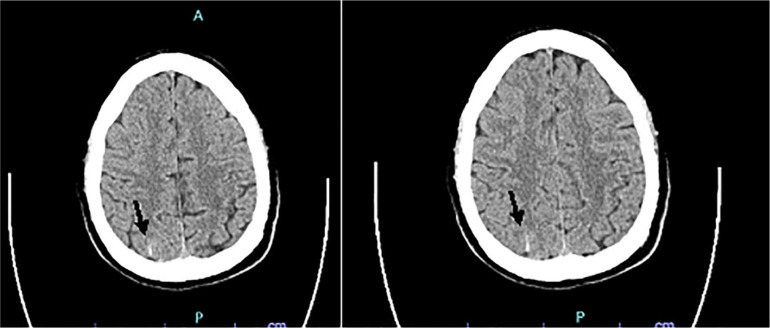
He returned to the hospital 1 day later complaining of facial droop, slurred speech, and right upper extremity weakness. Imaging studies revealed multiple infarctions in the bilateral cerebral hemispheres with perfusion defect most significant in the left middle cerebral artery (MCA; Figure 1). Given the ischemic strokes were suggestive of an embolic event, a transesophageal echocardiogram was performed, which resulted negative for patent foramen ovale, valvular disease, and arrhythmias. Initial hypercoagulable workup showed decreased protein S activity (65% of normal), normal protein C activity (95% of normal), normal antithrombin III levels (97% of normal), negative lupus anticoagulant (lupus anticoagulant not detected; dRVVT ≤45 seconds, negative hexagonal phase phospholipid neutralization, decreased factor VIII (36% of normal), and factor V (Leiden) mutation was not detected. Other pertinent diagnostic studies included negative ANA, ANCA, and anticardiolipin IgG/IgM. During the hospital stay the patient was anticoagulated with enoxaparin 1 mg/kg and once discharged rivaroxaban was resumed.
Figure 1.
Computed tomography scan of the head showing multiple infarctions in the bilateral cerebral hemispheres—marked by arrows—with perfusion defect most significant in the left middle cerebral artery. Bilateral cerebral infarctions are more often seen as the result of thromboembolic events rather than atherosclerotic disease.
Two weeks later, the patient returned to the emergency department with a sudden episode of headache, chest pain, and confusion. Computed tomography (CT) scan of the brain revealed a small subarachnoid hemorrhage in the right posterior parietal and left frontal lobe (Figure 2). Emergent cerebral angiography revealed a total occlusion of the M2 branch of the right MCA. Repeat echocardiogram revealed a mobile mass attached to the anterior mitral valve leaflet on its atrial side, suspicious for a thrombus. During this admission, the patient developed multiple thromboembolic strokes in the left MCA and bilateral posterior cerebellar arteries. Repeat lower extremity ultrasound showed an acute DVT within the left deep femoral artery. CT angiogram of the chest revealed a PE, multilevel supraclavicular, mediastinal, hilar lymphadenopathy, and a bilobed pulmonary nodule opacity at the posterior basal segment of the left lower lung (Figure 3).
Figure 2.
Computed tomography scan of the brain revealed a small subarachnoid hemorrhage in the right posterior parietal and left frontal lobe—marked by arrows.
Figure 3.
Computed tomography angiogram of the chest revealed a pulmonary embolism (PE), multilevel supraclavicular, mediastinal, hilar lymphadenopathy, and a bilobed pulmonary nodule opacity at the posterior basal segment of the left lower lung.
CT-guided biopsy of a right supraclavicular lymph node showed metastatic carcinoma. Immunohistochemical stains for CK7 and napsin A were positive; CK20 and TTF were focally positive, while CD45, S-100, and SOX-10 were negative, suggesting a lung primary malignancy. Follow-up stains showed PDL-1 expression by immunohistochemistry (high expression at 50%) and ALK/ROS1 gene rearrangement by fluorescence in situ hybridization was positive for ROS1 gene rearrangement. EGFR mutation was also negative. Given his aggressive hypercoagulable state, he was restarted on rivaroxaban. Treatment with crizotonib was initiated as he was a poor candidate for platinum-based chemotherapy. Repeat CT of chest showed improvement in the size of the pulmonary nodule and lymphadenopathy 6 weeks later (Figure 3). The patient remained ventilator dependent with poor neurological status and required bilateral below the knee amputations due to lower extremity gangrene. Given the lack of neurological responsiveness and poor prognosis, hospice was offered; however, his family insisted the patient continue chemotherapy. The patient remained in the hospital for a total of 6 months and ultimately died of cardiac arrest.
Discussion
Provoked Versus Unprovoked DVT
The association between thromboembolic events and underlying malignancies has been known for many years. The most common thromboembolic events seen in these patients include DVT and PE; other less frequently seen complications include arterial events, such as vascular cerebral events or strokes, and myocardial infarctions. These complications have been linked to an increased mortality in cancer patients. In a study by Khorana,1 cancer patients were noted to have a 47-fold elevation of death secondary to venous thromboembolism (VTE; both arterial and venous), being the most common cause of non–cancer-related death, along with infections. Similarly, in their study, cancer patients had a 2.7-fold elevation on the annualized death rate for arterial thrombosis. However, these complications are often seen later in the course of cancer and seldom lead to the initial diagnosis as it was the case with the patient described here.
Moreover, VTEs are often classified into provoked versus unprovoked according to the underlying risk factors present on the patient under study. Risk factors can be transient, such as immobilization after a surgical procedure or persistent such as active cancer. When no transient or persistent provoking risk factors are found in the patient’s history, the VTE is determined to be unprovoked.2 Inherited thrombophilia, a genetic tendency to thrombotic complications in the setting of an imbalance in hemostasis of the coagulation cascade, such as antithrombin deficiency, antithrombin resistance, protein C deficiency, protein S deficiency, and factor V Leiden mutation are commonly associated with the development of VTE.3 These conditions should be investigated in patients to assess the likelihood of recurrence. The population that often benefits from testing are young patients, those with family history of thrombotic events, pregnant females, patients with VTE in unusual sites, and young patients presenting with arterial ischemia and right-to-left shunt. However, the timing of testing is of crucial importance; testing should be performed at least 1 month after the finalizing anticoagulation treatment or 3 months after the initial thrombotic event. On the contrary, common acquired causes of thrombophilia include age more than 65 years, body mass index more than 30 kg/m2, and the presence of antiphospholipid antibodies and lupus anticoagulant.3 Our patient satisfied at least 2 conditions which indicated testing, including age less than 50 years and an arterial event (ischemic stroke of the left MCA). However, the patient’s initial workup was done while inpatient, 2 days after presentation and after 2 doses of rivaroxaban. The above-mentioned caveats could explain why the patient’s protein S activity was below the normal range as active thrombosis can lower the levels of free protein S and therefore lead to an erroneous diagnosis.4
Initial Treatment of Provoked Versus Unprovoked DVTs
The mainstream treatment for VTE consists of anticoagulation. The available choices of anticoagulation consists of vitamin K antagonists (VKAs) such as a warfarin, unfractionated heparin (UFH), low-molecular-weight heparin (LMWH), the indirect factor Xa inhibitor fondaparinux, and direct oral anticoagulants Xa inhibitors, and direct thrombin inhibitors (DOACs) such as apixaban, endoxaban, dabigatran, and rivaroxaban.5,6 Additionally, the use of DOACs for VTE has many advantages, including its availability in oral form, the lack of prothrombin time/international normalized ratio monitoring, rapid onset of action, short half-life, and the lack of prior bridging (with the exception of dabigatran and endoxaban, which require treatment overlap with a parenteral anticoagulant).5 Because of the above-mentioned characteristics, DOACs are not only the gold standard for the treatment of VTE in noncancer patients but are also among the first options for cancer patients who are determined to have a low risk of bleeding.7 Our patient was appropriately treated with a DOAC, rivaroxaban, however, presented shortly after with an arterial thrombosis, an event that should immediately lead to the initiation of a thrombophilia screening.
Hypercoagulable States Workup After DVT/PEs
The most common causes of thrombophilia that are typically tested include genetic conditions such as deficiency of protein C, protein S, antithrombin, or the genetic mutation of factor V Leiden. Other inherited causes of thrombophilia include hyperhomocysteinemia, dysfibrinogenemia, and certain blood groups such as non–O blood groups, mostly blood group B.8,9 Moreover, those patients younger than 50 years with no obvious identified risk factors for the development of a thromboembolic event should be considered for screening of occult cancer, paroxysmal nocturnal hemolysis, and autoimmune conditions as the underlying cause of their hypercoagulable state.8
The current data indicate that patients who are diagnosed with a first nonprovoked VTE in whom cancer screening is determined to be appropriate, a limited screening strategy that includes a complete history, physical examination, basic blood testing, chest radiograph, and age-/sex-specific cancer screening are appropriate. The addition of further imaging studies such as CT scan of the abdomen and pelvis do not lead to a clinical significant increase in the detection rate of underlying malignancy in patients.10,11 However, the detection rate of occult cancer after 1 year of the diagnosis of an unprovoked VTE is 1 in 20 patients.10 In those patients in whom cancer was diagnosed during screening following an unprovoked VTE, colon cancer was the most common malignancy, found in 17% of the patients, followed by lung cancer and pancreatic cancer with 15% and 11%, respectively.10
Malignancy as a Hypercoagulable State
Malignancies are associated with a 4% to 20% increase in VTE and 2% to 5% increase in arterial thrombosis.12 Many pathways have been implicated in the development of venous thrombosis in cancer patients. In a review article by Hisada and Mackman,12 the authors describe the main pathways and biomarkers associated with venous thrombosis in cancer patients. Leukocytosis is present in approximately 30% of cancer patients and the cancer subtypes in which the most frequently seen include colon and lung cancer.12 Neutrophils and monocytes are the cell lines with the greatest contribution to the development of thrombosis. Neutrophils increase the likelihood of thrombotic events by generating neutrophils extracellular traps, which have prothrombotic quality,13 while monocytes have been shown to be procoagulant by the expression of tissue factor.12,14 Similarly, thrombocytosis is often found in laboratory examination of cancer patients. Thrombocytosis is present in approximately 21.6% of patients with lung cancer, and it is most often seen in patients with advance cancer stages.15 Additionally, platelets are of extreme importance in the development of arterial thrombosis and also play a significant role the development of VTE in cancer patients.12
Lung Cancer and VTE
Lung cancer is associated with an increased prevalence of VTE. In patients with newly diagnosed lung cancer, Zhang and colleagues16 described that 13.2% of patients with recently diagnosed lung cancer developed a VTE either within 1 week from admission or 3 months prior to admission. In this study, 6.2% of the patients developed DVT only, 4.9% developed PE only, and 2.1% of patients were found to have both DVT and PE.16 Similarly, Ruiz-Artacho and colleagues17 described that the development of VTE in lung cancer patients typically occurs earlier in the disease course. Fifty percent of the patients in their cohort of lung cancer patients with diagnosed VTE developed the thromboembolic complication within less than 3 months after diagnosis and 32% within the first 30 days.17 Interestingly, elevated CEA levels has been associated with an increased risk for the development of PE.16,18 Of the histologic variants, lung adenocarcinoma is the lung cancer histopathology most associated with the later development of VTE.16 Other important risk factors that were associated with increased prevalence of VTE in patients with lung cancer were the presence of distant metastasis, leukocytosis, and younger age.16
ROS-1 Rearranged Non–Small Cell Lung Cancer (NSCLC) and Thromboembolic Events
The incidence of venous thromboembolic events in patients with non–small cell cancer has been established to be approximately 14% during the course of disease.19 In addition to the adenocarcinoma histologic subtype of lung cancer being associated with a higher incidence of lung cancer, the different genetic mutations often seen in NSCLC, such as EGFR mutation, KRAS mutation, ALK rearrangement, and ROS1 rearrangement, have been associated with and specific incidence of VTE. ROS1 rearrangement occurs approximately in 2% of patients with NSCLC.20 Of the different above-mentioned genetic mutations associated with NSCLC, patients with ROS1 rearrangement NSCLC have been noted to have 3- to 5-fold higher risk of developing VTEs soon before or after the initial diagnosis, with most patients developing the thromboembolic events within 1.8 months of diagnosis.20 The exact mechanism through which this occurs is not completely understood. Some research suggest an increased incidence of inherited thrombophilia in patients with this specific genetic alteration.20
Lung Cancer and Strokes
An increased incidence of strokes, both ischemic and hemorrhagic, has been described in patients with underlying cancer. Graus and colleagues21 initially described that cerebrovascular disease (CVD) was present in 14.6% of patients with cancer. In patients with underlying carcinomas, ischemic strokes were more commonly observed than hemorrhagic strokes, although both have been described.21 The most common underlying etiologies of ischemic/embolic strokes in this population are hypercoagulopathy and other coagulation disorders. Cardioembolic strokes, atherosclerosis, small vessel occlusion, and nonbacterial thrombotic endocarditis has also been mentioned in the literature although in a lesser frequency.22,23 Among the underlying cancers that have been more frequently observed in patients with CVD are pancreas, gastric, and lung cancer.24
In patients with lung cancer, biomarkers such as elevated CEA levels, CA155, and CA199 were independently associated with and increased risk of stroke in patients with no other typical risk factors for the development of CVD such as dyslipidemia or atrial fibrillation. Similar to the timeline of our patient described in this case report, strokes are typically seen within the first 4 months after diagnosis of lung cancer and the distribution of the lesions often involves multiple arterial territories of the brain consistent with a cryptogenic stroke.25 Moreover, the development of CVD in patients with cancer carries an increased mortality rate of approximately 25%.23
Lung Cancer and Nonbacterial Endocarditis
Nonbacterial thrombotic endocarditis (NBTE) is defined as the presence of vegetation on a valvular cardiac structure, consisting of fibrin and platelet deposition in the absence of bacterial colonization or inflammation.26 This entity has an estimated incidence of 0.3% to 9.3% and is more often found in patients with advanced stage malignancy. Recurrent embolization is frequently observed and approximately 50% of the patients with nonbacterial thrombotic endocarditis develop emboli to different organs, most important, the brain, kidneys, and spleen.26 These lesions occur given the interaction between macrophages and malignant cells, which release cytokines leading to the damage of the valvular endothelium, which promotes the further deposition of platelets and subsequent thrombus formation.27 As with any other thromboembolic complications, NBTE is most commonly observed in patients with lung, pancreas, or gastric cancer, adenocarcinoma being the histology most commonly associated with this entity.27
Teaching Points
Recurrent thromboembolic events in young patients with no known underlying medical conditions should prompt clinicians to investigate known causes of VTEs such as malignancy.
Lung cancer, in particular, lung adenocarcinoma, is highly associated with multiple hypercoagulable conditions such as DVT, PE, CVD, and NBTE.
The development of DVT, PE, CVD, and NBTE in patients with underlying malignancy is often associated with a poor prognosis and is frequently seen in late stages of the disease process.
ROS-1 rearrangement associated NSCLC have an increased risk of VTE when compared with other genetic profiles.
Further research is needed to understand the pathophysiologic process behind the hypercoagulable state associated with hematological and solid malignancies and regarding possible preventive strategies for patients with newly diagnosed disease.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethical Approval: Our institution does not require ethical approval for reporting individual cases or case series.
Informed consent: Informed consent for patient information to be published in this article was not obtained because it was not required by our institution.
ORCID iDs: Gliceida M. Galarza Fortuna  https://orcid.org/0000-0002-1084-953X
https://orcid.org/0000-0002-1084-953X
Adam Jacobs  https://orcid.org/0000-0002-9732-9771
https://orcid.org/0000-0002-9732-9771
References
- 1. Khorana AA. Venous thromboembolism and prognosis in cancer. Thromb Res. 2010;125:490-493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kearon C, Ageno W, Cannegieter SC, Cosmi B, Geersing GJ, Kyrle PA; Subcommittees on Control of Anticoagulation, and Predictive and Diagnostic Variables in Thrombotic Disease. Categorization of patients as having provoked or unprovoked venous thromboembolism: guidance from the SSC of ISTH. J Thromb Haemost. 2016;14:1480-1483. [DOI] [PubMed] [Google Scholar]
- 3. Colucci G, Tsakiris DA. Thrombophilia screening: universal, selected, or neither? Clin Appl Thromb Hemost. 2017;23:893-899. [DOI] [PubMed] [Google Scholar]
- 4. D’Angelo A, Vigano-D’Angelo S, Esmon CT, Comp PC. Acquired deficiencies of protein S. Protein S activity during oral anticoagulation, in liver disease, and in disseminated intravascular coagulation. J Clin Invest. 1988;81:1445-1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bartholomew JR. Update on the management of venous thromboembolism. Cleve Clin J Med. 2017;84:39-46. [DOI] [PubMed] [Google Scholar]
- 6. Serhal M, Barnes GD. Venous thromboembolism: a clinician update. Vasc Med. 2019;24:122-131. [DOI] [PubMed] [Google Scholar]
- 7. Antunes LF. New oral anticoagulants (NOACs) are the gold standard invenous thromboembolism. Rev Port Cir Cardiotorac Vasc. 2020;27:33-37. [PubMed] [Google Scholar]
- 8. Colucci G, Tsakiris DA. Thrombophilia screening revisited: an issue of personalized medicine. J Thromb Thrombolysis. 2020;49:618-629. doi: 10.1007/s11239-020-02090-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Baudouy D, Moceri P, Chiche O, et al. B blood group: a strong risk factor for venous thromboembolism recurrence. Thromb Res. 2015;136:107-111. [DOI] [PubMed] [Google Scholar]
- 10. Van Es N, Le Gal G, Otten HM, et al. Screening for occult cancer in patients with unprovoked venous thromboembolism. Ann Intern Med. 2017;167:410-417. [DOI] [PubMed] [Google Scholar]
- 11. Carrier M, Lazo-Langner A, Shivakumar S, et al. ; SOME Investigators. Screening for occult cancer in unprovoked venous thromboembolism. N Engl J Med. 2015;373:697-704. [DOI] [PubMed] [Google Scholar]
- 12. Hisada Y, Mackman N. Cancer-associated pathways and biomarkers of venous thrombosis. Blood. 2017;130:1499-1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Brinkmann V, Reichard U, Goosmann C, et al. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532-1535. [DOI] [PubMed] [Google Scholar]
- 14. Gregory SA, Morrissey JH, Edgington TS. Regulation of tissue factor gene expression in the monocyte procoagulant response to endotoxin. Mol Cell Biol. 1989;9:2752-2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Maráz A, Furák J, Varga Z, Kahan Z, Tiszlavicz L, Hideghety K. Thrombocytosis has a negative prognostic value in lung cancer. Anticancer Res. 2013;33:1725-1729. [PubMed] [Google Scholar]
- 16. Zhang Y, Yang Y, Chen W, et al. Prevalence and associations of VTE in patients with newly diagnosed lung cancer. Chest. 2014;146:650-658. [DOI] [PubMed] [Google Scholar]
- 17. Ruiz-Artacho P, Trujillo-Santos J, López-Jiménez L, et al. ; RIETE Investigators. Clinical characteristics and outcomes of patients with lung cancer and venous thromboembolism. TH Open. 2018;2:e210-e217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Guo J, Deng QF, Xiong W, et al. Comparison among different presentations of venous thromboembolism because of lung cancer. Clin Respir J. 2019;13:574-582. [DOI] [PubMed] [Google Scholar]
- 19. Chiari R, Ricciuti B, Landi L, et al. ROS1-rearranged non–small-cell lung cancer is associated with a high rate of venous thromboembolism: analysis from a phase II, prospective, multicenter, two-arms trial (METROS). Clin Lung Cancer. 2020;21:15-20. [DOI] [PubMed] [Google Scholar]
- 20. Alexander M, Pavlakis N, John T, et al. A multicenter study of thromboembolic events among patients diagnosed with ROS1-rearranged non-small cell lung cancer. Lung Cancer. 2020;142:34-40. [DOI] [PubMed] [Google Scholar]
- 21. Graus F, Rogers LR, Posner JB. Cerebrovascular complications in patients with cancer. Medicine (Baltimore). 1985;64:16-35. [DOI] [PubMed] [Google Scholar]
- 22. Dardiotis E, Aloizou AM, Markoula S, et al. Cancer-associated stroke: pathophysiology, detection and management. Int J Oncol. 2019;54:779-796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Grazioli S, Paciaroni M, Agnelli G, et al. Cancer-associated ischemic stroke: a retrospective multicentre cohort study. Thromb Res. 2018;165:33-37. [DOI] [PubMed] [Google Scholar]
- 24. Navi BB, Iadecola C. Ischemic stroke in cancer patients: a review of an underappreciated pathology. Ann Neurol. 2018;83:873-883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Xie X, Chen L, Zeng J, et al. Clinical features and biological markers of lung cancer-associated stroke. J Int Med Res. 2016; 44:1483-1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Asopa S, Patel A, Khan OA, Sharma R, Ohri SK. Non-bacterial thrombotic endocarditis. Eur J Cardiothorac Surg. 2007;32:696-701. [DOI] [PubMed] [Google Scholar]
- 27. el-Shami K, Griffiths E, Streiff M. Nonbacterial thrombotic endocarditis in cancer patients: pathogenesis, diagnosis, and treatment. Oncologist. 2007;12:518-523. [DOI] [PubMed] [Google Scholar]