Abstract
Objectives
To evaluate the utility of the 2010 American College of Rheumatology (ACR) adult fibromyalgia criteria for use in adolescents with juvenile fibromyalgia (JFM).
Study design
Participants included 47 adolescent girls diagnosed with JFM (mean age = 15.3 years) and 48 age- and sex-matched adolescents (mean age = 15.0 years) with localized chronic pain (eg, headaches or abdominal pain). A trained examiner administered the Widespread Pain Index and Symptom Severity measures and also completed a manual tender point exam. Clinicians completed a form indicating the presence of active JFM per Yunus and Masi (1985) criteria, the only available and most commonly used measure for JFM. Criterion validity analysis was performed as well as t tests comparing symptoms between JFM and controls.
Results
With the Yunus and Masi criteria used as the gold standard, the 2010 ACR fibromyalgia criteria showed a sensitivity of 89.4% and specificity of 87.5%.
Conclusion
The 2010 ACR measure appears to be a valuable tool for the identification of JFM. However, a slight modification to the 2010 ACR measure and inclusion of a clinical exam is recommended.
At present, 2%−6% of school-age children are estimated to suffer from juvenile fibromyalgia (JFM),1–3 a chronic condition of widespread musculoskeletal pain, fatigue, and poor sleep. Unfortunately, there is significant morbidity among patients with JFM, with associated poor school attendance, difficulties in physical functioning,4 poor social acceptance,5 and comorbid mood disorders.6 Patients often go undiagnosed for years; are referred frequently to subspecialists for symptoms of chronic headaches, chest, or abdominal pain; and undergo costly evaluations that typically reveal normal findings. Reports in adult fibromyalgia (FM) indicate annual costs to be around $4533–$11 049 per patient,7,8 which is a considerable economic burden to the health care system. Emerging evidence suggests that JFM is a condition that frequently continues into adulthood with chronic physical and psychological symptoms,4,9 making it important to correctly identify and treat this condition in adolescence. Yet, JFM is often underdiagnosed from a lack of awareness and lack of agreed-on criteria.
Traditionally, the diagnosis of FM in adults has been based on the 1990 American College of Rheumatology (ACR) Classification Criteria,10 which includes at least a 3-month history of widespread musculoskeletal pain and the presence of 11 of 18 tender point locations. These criteria have been criticized on the basis of 2 major points, including the controversial tender point examination and the limited acknowledgement of associated symptoms that are characteristic of FM (eg, fatigue, irritable bowel syndrome, headaches, and insomnia). In the pediatric population, criteria proposed by Yunus and Masi11 nearly 3 decades ago are the only available criteria to help diagnose FM in children. Although the Yunus and Masi criterion includes assessment of associated symptoms, it also relies on the presence of 5 of 18 tender points.
The manual tender point examination is controversial, given that it is often performed incorrectly or not performed at all in clinical practice.12 Further controversy surrounds the utility of the tender point examination because it is thought to incorporate a degree of subjectivity as well as apprehension or anxiety on the part of the patient.12 There also has been concern that tender points appear to be somewhat arbitrary, do not discriminate types of pain, and can be exclusive such that patients who would otherwise fit the diagnosis of FM but do not suffer from enough tender points are excluded (ie, male patients). The utility of the tender point examination to examine pain sensitivity also has been diminished by newer, more sophisticated techniques that incorporate quantitative sensory testing to quantify pain thresholds, pain tolerance, and conditioned pain modulation in patients with FM.13,14
In 2010, Wolfe et al15 proposed new criteria for the clinical diagnosis of FM in adults. These criteria include a clinician-administered tool for the Widespread Pain Index (WPI) and a Symptom Severity (SS) Scale of key characteristic symptoms as well as associated symptoms of FM (Figure). In a multicenter study, the new criteria recognized 88.1% of the 256 cases with FM defined by the original 1990 ACR classification criteria, thus proving to be an effective tool for classifying FM. Unlike previous criteria, this tool is more comprehensive by including cardinal and additional somatic symptoms of FM, providing a quantifiable cut-off point and severity scores and eliminating the tender point examination. Two other studies have since cross-validated the 2010 ACR criteria in adults with FM.16,17
Figure.
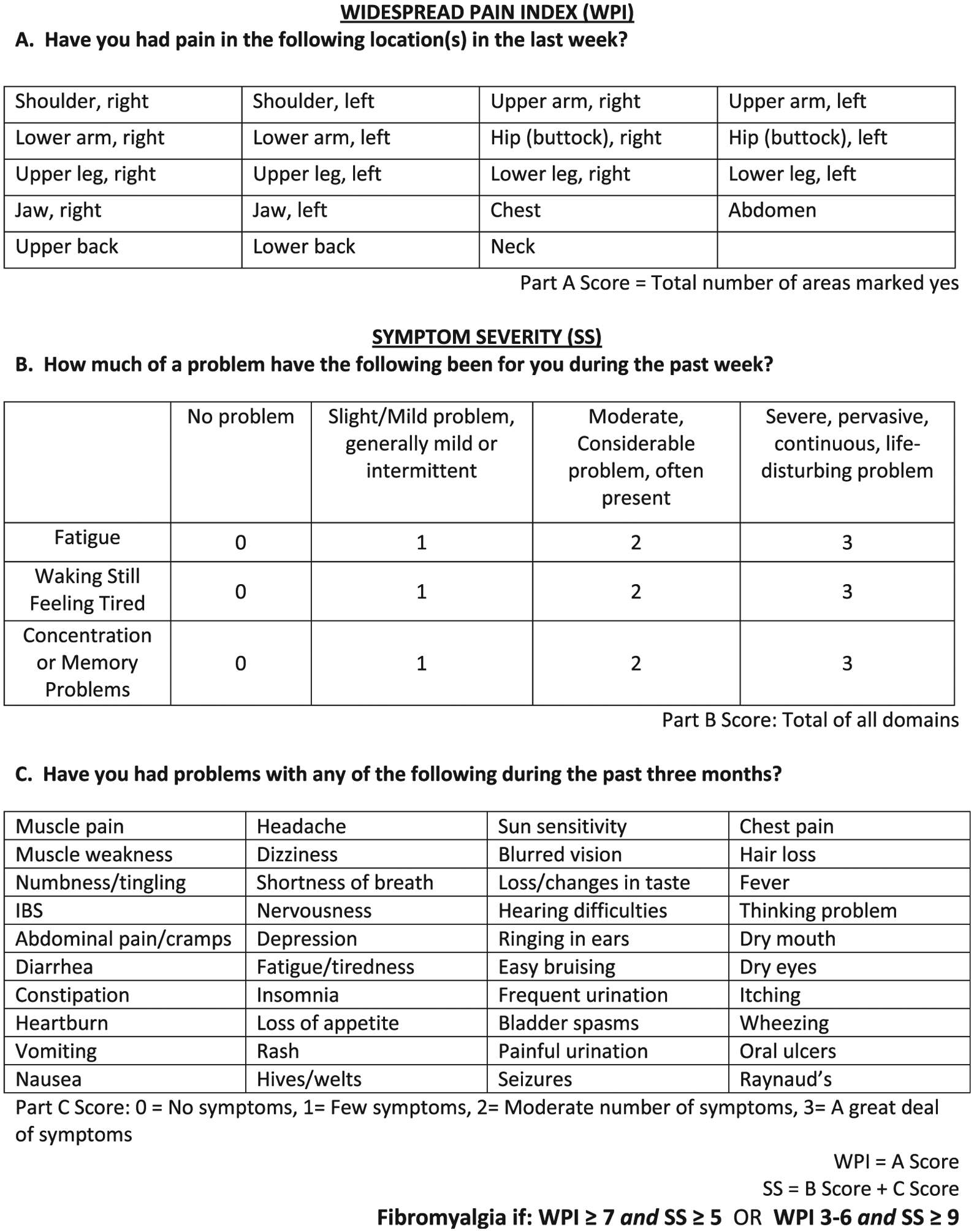
2010 ACR Criteria for FM questionnaires. IBS, irritable bowel syndrome. Adapted from Wolfe et al.15
Currently, there are no such studies for the classification of JFM. Hence, it is a priority to validate the adult FM ACR criteria using a similar, rapidly delivered and scored measure for general and subspecialty clinicians to help make a diagnosis of JFM more efficiently. This could further minimize costs and anxiety with unnecessary referrals and tests. A validated set of criteria for JFM also could accelerate research into this poorly understood condition. Finally, quantifying symptom severity via the WPI and SS index would aid in improved ability to assess changes in response to treatment among patients with JFM.
The primary aim of this study was to assess the criterion validity of the 2010 ACR FM criteria in a sample of patients with JFM. We predicted that the tool would correctly classify ≥80% of patients with JFM and control patients without JFM (localized pain). A secondary aim was to compare the group with JFM with the localized pain controls on number of tender points, pain locations, and cardinal and associated somatic symptoms. We hypothesized that the group with JFM would have significantly greater scores in each domain than controls.
Methods
A convenience sample of pediatric patients, ages 11–17 years with a known diagnosis of active primary JFM as determined by a pediatric rheumatologist, were recruited from a tertiary care rheumatology clinic. The 1985 Yunus and Masi criteria, the most widely used criteria in pediatric clinical practice and research studies, were used to diagnose JFM. Given that symptoms of FM can wax and wane, adolescents with a known diagnosis of JFM who did not have active symptoms (ie, did not meet criteria based on clinician judgment) at time of recruitment were excluded from participation. Additionally, matched (sex, age [within 1 year]) controls with localized pain, including chronic headaches, abdominal pain, and/or limb pain were recruited from the rheumatology, pain management, or behavioral medicine clinics.
Patients were excluded if they did not have a diagnosis of primary FM or had localized pain attributable to another reason, including other inflammatory pain conditions (eg, juvenile idiopathic arthritis, juvenile dermatomyositis, systemic lupus erythematosus).
Potential participants meeting eligibility criteria were identified via medical chart review. Eligible patients and their parents were contacted by a member of the investigative team during a routine clinic appointment with the attending clinician’s permission, screened for eligibility, and invited to participate in the study. The study was approved by the Institutional Review Board. Informed parental consent/permission along with patient assent was obtained at this screening/study visit.
During the study visit, participants were administered the WPI and the SS questionnaire, and a tender point examination was performed as described in this article.
Measures
For each participant, the following demographic information was collected via parental report: sex, age, race/ethnicity, parental education, occupation, and household income. Referring clinicians were asked to complete a questionnaire to confirm active JFM and document which features of the Yunus and Masi criteria were met at time of study visit. If patients with a previous diagnosis of JFM did not have active symptoms at time of recruitment, they were excluded from participation.
The study rheumatologist or a trained examiner administered the WPI and SS questionnaires to the patient with the parent/caregiver present to verify the patient’s report, followed by a standardized 18-site tender point examination. WPI is a questionnaire that identifies pain in the past week occurring in specific musculoskeletal sites, including shoulders, upper arms, lower arms, hips, upper legs, lower legs, jaws, chest, abdomen, upper back, lower back, and neck. Each painful site is given a single point and totaled to provide a final score (range 0–19). SS is a questionnaire composed of 2 components that identifies the degree of severity of cardinal symptoms and the presence or absence of associated symptoms. Cardinal symptoms of fatigue, waking unrefreshed, and cognitive symptoms are addressed on a severity scale from 0–3, with 0 being no problem and 3 being a severe problem. A separate list of 40 different somatic symptoms is indexed and assessed on the basis of the presence or absence in the past week (stable during the past 3 months). This list was derived from the recommendations of the authors of the original criteria.15 Although the authors’ original intent was for clinicians to assess overall number of somatic symptoms rather than use a specific checklist, we elected to list these items for standardization purposes. A score is given to this list based upon the overall frequency of symptoms (0–3, 0 = none at all and 3 = a great deal [≥10] of symptoms). A final score is calculated by adding the total score from the cardinal symptoms and the overall frequency of somatic symptoms (maximum score = 12). According to the adult ACR criteria for FM, a score of SS ≥ 5 and WPI ≥ 7 or a score of SS ≥ 9 and WPI 3–6 indicates a positive diagnosis for FM.
All participants underwent a manual tender point examination via the standard thumb palpation method. A trained research assistant or physician applied up to 4 kg/cm2 of pressure to each of the 18 tender point locations. Examiners (K.B., C.W.) were trained by the same rheumatologist (T.T.) to perform a standard examination with manual palpation by using a maximum of 4 kg/cm2 of pressure. Points were considered positive if the participant reported pain at <4 kg/cm2 of pressure.
Statistical Analyses
Data were entered and analyzed using SPSS Version 20 (IBM, Armonk, New York). Data were first examined for missing components to ensure completeness. Descriptive data for the group with JFM and control group of the WPI, SS index, and tender point counts were computed. The number and proportion of patients in each group who met and did not meet Yunus and Masi and ACR 2010 criteria was computed to establish sensitivity, specificity, and predictive values of the ACR 2010 criteria using the Yunus and Masi 1985 criteria as the gold standard. t tests were conducted to compare the 2 groups on number of pain locations (WPI score), cardinal symptoms score, total number of somatic symptoms endorsed, and number of tender points. The data were examined for skewness and deemed suitable for parametric testing. For descriptive purposes, the somatic symptoms were grouped by body system (musculoskeletal, mood, neurologic, gastrointestinal, chest/respiratory, skin, genitourinary, and other).
Results
A total of 105 eligible patients were approached, and 97 (92.4%) agreed to participate. Eight patients who were approached declined to participate primarily because of heightened pain at time of contact and/or time constraints in the day. One patient with JFM had missing data, and another patient with JFM was discovered during data examination to have not met criteria for active JFM and, therefore, both were excluded from final analysis. The final sample (N = 95) consisted of 47 patients with JFM (mean age = 15.3, SD = 1.43) and 48 matched controls (mean age = 15.0, SD = 1.48) with localized pain. Participants were 100% female and 94.8% Caucasian. Mean “average pain score (0–10) in the past week” at time of recruitment was 6.3 (SD 1.6) and 5.8 (SD 2.0) for JFM and matched controls, respectively. Matched controls had back pain (n = 18), abdominal pain (n = 11), extremity pain (n = 9), complex regional pain syndrome (n = 5), headache (n = 1), pelvic pain (n = 1), or a combination of 2 of the aforementioned (n = 3).
Diagnostic Utility of 2010 ACR Criteria in JFM
When the Yunus and Masi criteria were used as the gold standard, the overall sensitivity and specificity of the ACR 2010 criteria for diagnosing JFM were 89.4% and 87.5%, respectively (Table I). The positive predictive value was 87.5%, and the negative predictive value 89.4%. Positive and negative likelihood ratios were 7.14 and 0.12, respectively.
Table I.
Number of JFM and matched controls meeting the 2010 ACR FM criteria
| 2010 ACR criteria met | JFM (N = 47) | Matched control (N = 48) |
|---|---|---|
| Yes | 42 | 6 |
| No | 5 | 42 |
Comparison of Clinical Characteristics
There was a significant difference in the total number of painful regions (WPI), cardinal symptoms severity score, symptom severity index totals, and the total number of tender points, with greater scores in the group with JFM compared with the control participants with chronic localized pain (Table II). Table III summarizes the group differences across clinical characteristics. Results demonstrate a greater frequency and severity of symptoms among the group with JFM compared with matched controls, with the majority of participants in both groups reporting primarily musculoskeletal, mood, and neurologic symptoms. The reported frequency of specific somatic symptoms (Table IV; available at www.jpeds.com) varied between JFM and controls. Indeed, there were certain somatic symptoms, including Raynauds, diarrhea, oral ulcers, photosensitivity, alopecia, taste changes, rashes, hearing difficulties, seizures, hives or welts, painful urination, fevers, bladder spasms, wheezing, and vomiting, that were endorsed by <25% of patients with JFM (as well as control participants).
Table II.
t test comparisons of total number of tender points, pain locations, and symptoms between JFM and matched localized pain controls
| JFM, Mean (SD) | Matched controls, Mean (SD) | t | Sig. | |
|---|---|---|---|---|
| Tender points | 11.83 (3.94) | 2.81 (2.80) | 12.83 | .000 |
| Widespread pain | 10.91 (3.53) | 2.94 (2.16) | 13.27 | .000 |
| Cardinal symptoms | 5.74 (2.15) | 3.00 (2.14) | 6.23 | .000 |
| Somatic symptoms | 14.40 (5.47) | 6.88 (4.29) | 7.45 | .000 |
Sig., significance.
Table III.
Frequency of symptoms reported by participants (“in the past week”)
| JFM (n = 47) | Matched controls (n = 48) | |
|---|---|---|
| Cardinal symptoms | Number (%) | Number (%) |
| Fatigue | ||
| No problem | 1 (2.1) | 14 (29.2) |
| Slight/mild problem | 11 (23.4) | 11 (22.9) |
| Moderate, considerable problem | 20 (42.6) | 17 (35.4) |
| Severe, pervasive, continuous | 15 (31.9) | 6 (12.5) |
| Waking unrefreshed | ||
| No problem | 4 (8.5) | 11 (22.9) |
| Slight/mild problem | 6 (12.8) | 13 (27.1) |
| Moderate, considerable problem | 15 (31.9) | 14 (29.2) |
| Severe, pervasive, continuous | 22 (46.8) | 10 (20.8) |
| Cognitive symptoms | ||
| No problem | 11 (23.4) | 39 (81.3) |
| Slight/mild problem | 11 (23.4) | 8 (16.7) |
| Moderate, considerable problem | 14 (29.8) | 1 (2.1) |
| Severe, pervasive, continuous | 11 (23.4) | 0 (0.0) |
| SS index* | ||
| Musculoskeletal | 80.9% | 45.9% |
| Mood | 54.3% | 21.9% |
| Neurologic | 43.4% | 21.1% |
| Gastrointestinal | 35.1% | 16.5% |
| Other | 34.0% | 21.7% |
| Pulmonary | 31.2% | 7.6% |
| Skin | 20.4% | 6.7% |
| Genitourinary | 14.0% | 4.9% |
A total of 40 clinical symptoms grouped by the following system categories: gastrointestinal (abdominal pain, diarrhea, constipation, irritable bowel symptoms, heartburn, loss of appetite, loss or change in taste, oral ulcers, dry mouth, vomiting); genitourinary (painful urination, bladder spasms, frequent urination); mood (depression, nervousness); musculoskeletal (muscle pain, muscle weakness); neurologic (headaches, numbness, dizziness, thinking problems, ringing in ears, hearing difficulties, seizures); other (fevers, tiredness, insomnia, hair loss, dry eyes, blurry vision, Raynaud); pulmonary (wheezing, shortness of breath, chest pain); skin (hives/welts, rashes, sun sensitivity, easy bruising, itching).
Table IV.
Frequency of somatic symptoms endorsed by JFM and control participants (“in the past week”)
| Somatic symptoms | JFM (n = 47), n (%) | Controls (n = 48), n (%) |
|---|---|---|
| Muscle pain | 45 (95.7) | 29 (60.4) |
| Headaches | 43 (91.5) | 25 (52.1) |
| Tiredness | 43 (91.5) | 35 (72.9) |
| Muscle weakness | 31 (66) | 15 (31.3) |
| Abdominal pain | 31 (66) | 20 (41.7) |
| Nausea | 30 (63.8) | 18 (37.5) |
| Nervousness | 29 (61.7) | 12 (25) |
| Dizziness | 28 (59.6) | 15 (31.3) |
| Thinking problems | 25 (53.2) | 4 (8.3) |
| Irritable bowel symptoms | 25 (53.2) | 7 (14.6) |
| Chest pain | 24 (51.1) | 6 (12.5) |
| Insomnia | 23 (48.9) | 20 (41.7) |
| Easy bruising | 22 (46.8) | 7 (14.6) |
| Depression | 22 (46.8) | 9 (18.8) |
| Ringing in ears | 21 (44.7) | 9 (18.8) |
| Numbness | 21 (44.7) | 14 (29.2) |
| Dry mouth | 19 (40.4) | 6 (12.5) |
| Loss of appetite | 18 (38.3) | 12 (25) |
| Heartburn | 17 (36.2) | 5 (10.4) |
| Blurry vision | 16 (34) | 5 (10.4) |
| Shortness of breath | 16 (34) | 5 (10.4) |
| Frequent urination | 15 (31.9) | 5 (10.4) |
| Constipation | 15 (31.9) | 6 (12.5) |
| Dry eyes | 12 (25.5) | 4 (8.3) |
| Itching | 12 (25.5) | 5 (10.4) |
| Raynaud | 10 (21.3) | 6 (12.5) |
| Diarrhea | 9 (19.1) | 6 (12.5) |
| Oral ulcers | 8 (17) | 1 (2.1) |
| Sun sensitivity | 7 (14.9) | 0 (0) |
| Hair loss | 6 (12.8) | 1 (2.1) |
| Loss or change in taste | 6 (12.8) | 2 (4.2) |
| Rashes | 6 (12.8) | 4 (8.3) |
| Hearing difficulties | 5 (10.6) | 4 (8.3) |
| Wheezing | 4 (8.5) | 0 (0) |
| Vomiting | 4 (8.5) | 4 (8.3) |
| Bladder spasms | 3 (6.4) | 1 (2.1) |
| Fevers | 2 (4.3) | 2 (4.2) |
| Painful urination | 3 (6.4) | 1 (2.1) |
| Hives/welts | 1 (2.1) | 0 (0) |
| Seizures | 0 (0) | 0 (0) |
Discussion
In this preliminary validation study, the 2010 ACR FM Criteria appear to have very good sensitivity (89.4%) and specificity (87.5%) for use in diagnosing JFM in adolescents. In comparison, the initial validation study for adults with FM correctly classified 88.1% of patients with FM.15 At face value, the 2010 ACR criteria offer a simplified means of diagnosing a complex condition like FM. In comparison with the previous classification criteria—either the Yunus and Masi11 or the 1990 ACR Criteria10—the 2010 questionnaire based criteria clearly has several advantages.
First, it eliminates the need for the tender point examination, which has been criticized for its subjectivity and inconsistencies for clinical use,12 including applicability toward male patients, who generally appreciate less pain to pressure. Unfortunately, we were unable to recruit male patients for this study, given a low prevalence (<5%) in our tertiary care clinic. It is also important to note that the tender point examination alone in this study appears to differentiate JFM from controls, with controls reporting only an average of 2–3 tender points and participants with JFM averaging 11, reflecting the presence of generalized pain sensitivity of patients with JFM. Nonetheless, results of this study suggest that conducting a manual 18-point tender point examination may not be necessary to obtain accurate classification of JFM.
A second advantage of the 2010 criteria is the fact that, unlike the 1990 ACR criteria, it includes an assessment of severity of key somatic symptoms in FM: fatigue, unrefreshing sleep, and cognitive symptoms. In this study, participants with JFM had greater severity across all 3 symptoms than localized pain controls. Additionally, patients can endorse the presence of few to several other somatic symptoms in the 2010 ACR criteria, which is more consistent with the clinical symptomatology of FM rather than being limited to pain and tender points alone. In practice and in clinical research, this broad scoring system would allow for assessing improvement across the spectrum of symptoms rather than relying only on report of musculoskeletal pain or tenderness alone as a measure of efficacy in treating JFM. Indeed, patients with JFM in this study clearly had greater rates of somatic symptoms in general, reporting nearly twice as many concerns. In particular, neurologic, sleep, musculoskeletal, and abdominal symptoms were noted more frequently than among their localized pain peers. However, we found that some items were very rarely endorsed by youth with JFM (eg, bladder spasms, loss or change in taste, itching, hives/welts, painful urination, Raynaud, rashes, diarrhea, hearing difficulties, numbness, fevers, and seizures).
Lastly, the ease in which the 2010 ACR questionnaires are given is a great advantage over previous criteria. This questionnaire was administered in less than 5 minutes and therefore could be administered readily in the clinical setting. As a brief and user-friendly tool, the 2010 ACR criteria could therefore also serve as a screening tool, the results of which could help guide referral patterns and prevent excessive anxiety surrounding multiple referrals and tests. Furthermore, limiting delays to time to diagnosis can allow for earlier implementation of appropriate counseling and therapies to prevent additional morbidity associated with the condition. Such interventions might include general education, improvements in sleep hygiene, reduction of disability via cognitive behavioral therapy,18,19 and participation in targeted physical exercise programs that have been shown to improve quality of life.20
We recognize that in 2011, a modified version of the 2010 ACR Criteria was suggested.12 This version allowed for a completely self-reported questionnaire of widespread pain and modified the symptom severity score to include severity of cardinal symptoms of fatigue, unrefreshing sleep, and cognitive symptoms on a 0–3 scale as well as the sum of the presence or absence of headache, abdominal pain/cramps, and/or depression in the past 6 months for a 4-item SS score of 0–12. Interestingly, the modified version is fairly similar to the Yunus and Masi criteria for JFM but without the tender point examination. Several studies have evaluated the modified 2010 Criteria with respect to adult FM and it has shown to be useful.21–24 However, in a recently published study (2015), Jones et al25 compared the 1990, 2010, and modified 2010 ACR Criteria and found that the modified (self-reported) version identified a greater number of patients with FM (a 4-fold increase) than the other criteria. Yet sensitivity (64%) and specificity (78%) were poor to fair. Interestingly, these authors found that the original 2010 Criteria had a sensitivity of 55% and specificity of 99%, with lowest prevalence rates possibly attributable to recruiting bias. Given our study was initiated before these modifications, we were unable to assess the effectiveness of this modified version.
In this study of female adolescents with JFM, we noted several features of the measure that could be improved for greater applicability to a younger population and would require relatively small modifications that would not compromise the integrity of the measure. First, pediatric patients tend to be very literal with respect to “pain in the past week” and would include acute injuries (eg, sprained ankle) unless guided otherwise. Hence, extending the time frame of the WPI to “in the past 3 months” and specifying that the pain be persistent (“every day or almost every day”) would enhance their understanding of the nature of chronic or recurrent pain. For the somatic symptoms, several items rarely were endorsed by the youth (<10%), and some were not understood and/or were redundant and we would recommend removing these items (muscle pain, fatigue/tiredness, chest pain, fever, diarrhea, wheezing, Raynaud phenomenon, hives/welts, vomiting, oral ulcers, loss of/change in taste, seizures, rash, sun sensitivity, hearing difficulties, hair loss, painful urination, and bladder spasms) from the list of somatic symptoms and utilize only the most useful 22 remaining items. Furthermore, it may be useful to include lightheadedness/balance problems along with dizziness and to add items pertaining to tenderness to touch and sensitivity to loud sounds, bright lights, or strong smells. These items were suggested in the recent article by Bennett et al22 and adding these items would not require any change to the categorical scoring system of the symptom severity index.
The main limitations to this study are the small sample size of only female patients with primary JFM and recruitment from within 1 tertiary care center. These may limit generalizability to male patients with JFM (who generally tend to report symptoms less often than females), patients with JFM secondary to another disease condition (eg, juvenile arthritis), and those who present to primary care clinics in the community setting. Controls with localized pain also were recruited primarily from pain clinics and behavioral medicine and thus may not have been as thoroughly screened for more widespread FM type symptoms leading to a misdiagnosis of primarily localized pain. Studies involving sufficiently powered sample sizes based upon different geographic regions inclusive of community practices would address this limitation. Also, testing the utility of these criteria in patients with secondary JFM would be useful.
In summary, the results of this study suggest that the 2010 ACR Criteria can be applied to a female pediatric population with JFM. We have suggested some minor modifications to suit the developmental level of the patients that can be made without compromising the integrity of the measure and plan to test this in future studies. At this time, we would recommend that the WPI and SS tools be administered by a clinician or health care professional as originally designed, because younger patients may need reminders of the time frame for symptoms and clarification of the meaning of some of the somatic symptoms. We would also emphasize that a standard physical examination and history should be conducted as part of clinical practice to ensure other conditions (eg, thyroid dysfunction, systemic lupus erythematosus, juvenile idiopathic arthritis, sleep disorders) are ruled out. The absence of a tender point examination does not appear to be problematic for the classification of JFM in adolescents based on this study. As a rapidly delivered and scored tool, the ACR 2010 measure could ultimately help clinicians more efficiently make a diagnosis of JFM, better define a population with JFM for clinical research, and ultimately help patients with JFM access needed treatments and resources sooner.
Acknowledgments
We would like to acknowledge our clinical faculty for their care of our participants, including Kenneth Goldschneider, MD, FAAP, Alexandra Szabova, MD, John B. Rose, MD (Cincinnati Children’s Hospital Medical Center and University of Cincinnati College of Medicine), Jennifer Huggins, MD, Hermine Brunner, MD, MSc, MBA, Esi-Morgan Dewitt, MD, MSCE, Rina Mina, MD, MSc, Daniel Lovell, MD, MPH, Alexei Grom, MD (Cincinnati Children’s Hospital Medical Center and University of Cincinnati College of Medicine), Janalee Taylor, MSN, APRN, CNS (Cincinnati Children’s Hospital Medical Center), as well as Daniel Strotman, BA (Cincinnati Children’s Hospital Medical Center) for his statistical contributions.
Supported by the National Institutes of Health/National Institute of Arthritis and Musculoskeletal and Skin Diseases (K24 AR056687 [to S.K.-Z.]). The authors declare no conflicts of interest.
Glossary
- ACR
American College of Rheumatology
- FM
Fibromyalgia
- JFM
Juvenile fibromyalgia
- SS
Symptom Severity
- WPI
Widespread Pain Index
References
- 1.Gedalia A, Press J, Klein M, Buskila D. Joint hypermobility and fibromyalgia in schoolchildren. Ann Rheum Dis 1993;52:494–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gerloni V, Ghirardinin M, Fantini F. Assessment of nonarticular tenderness and prevalence of primary fibromyalgia syndrome in healthy Italian schoolchildren [abstract]. Arthritis Rheum 1998;41:1405. [Google Scholar]
- 3.Mikkelsson M, Salminen JJ, Kautiainen H. Non-specific musculoskeletal pain in preadolescents. Prevalence and 1-year persistence. Pain 1997;73:29–35. [DOI] [PubMed] [Google Scholar]
- 4.Kashikar-Zuck S, Parkins IS, Ting TV, Verkamp E, Lynch-Jordan A, Passo M, et al. Controlled follow-up study of physical and psychosocial functioning of adolescents with juvenile primary fibromyalgia syndrome. Rheumatology 2010;49:2204–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kashikar-Zuck S, Lynch AM, Graham TB, Swain NF, Mullen SM, Noll RB. Social functioning and peer relationships of adolescents with juvenile fibromyalgia syndrome. Arthritis Rheum 2007;57:474–80. [DOI] [PubMed] [Google Scholar]
- 6.Kashikar-Zuck S, Parkins IS, Graham TB, Lynch AM, Passo M, Johnston M, et al. Anxiety, mood, and behavioral disorders among pediatric patients with juvenile fibromyalgia syndrome. Clin J Pain 2008;24:620–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wassem R, Hendrix TJ. Direct and indirect costs of fibromyalgia to patients and their families. J Orthop Nursing 2003;7:26–32. [Google Scholar]
- 8.Berger A, Sadosky A, Dukes EM, Edelsberg J, Zlateva G, Oster G. Patterns of healthcare utilization and cost in patients with newly diagnosed fibromyalgia. Am J Manag Care 2010;16:S126–37. [PubMed] [Google Scholar]
- 9.Kashikar-Zuck S, Cunningham N, Sil S, Bromberg MH, Lynch-Jordan AM, Strotman D, et al. Long-term outcomes of adolescents with juvenile-onset fibromyalgia in early adulthood. Pediatrics 2014;133:e592–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wolfe F, Smythe HA, Yunus MB, Bennett RM, Bombardier C, Goldenberg DL, et al. The American College of Rheumatology 1990 Criteria for the Classification of Fibromyalgia. Report of the Multicenter Criteria Committee. Arthritis Rheum 1990;33:160–72. [DOI] [PubMed] [Google Scholar]
- 11.Yunus MB, Masi AT. Juvenile primary fibromyalgia syndrome. A clinical study of thirty-three patients and matched normal controls. Arthritis Rheum 1985;28:138–45. [DOI] [PubMed] [Google Scholar]
- 12.Harth M, Nielson WR. The fibromyalgia tender points: use them or lose them? A brief review of the controversy. J Rheum 2007;34:914–22. [PubMed] [Google Scholar]
- 13.Desmeules J, Cedraschi C, Rapiti E, Baumgartner E, Finckh A, Cohen P, et al. Neurophysiologic evidence for a central sensitization in patients with fibromyalgia. Arthritis Rheum 2003;48:1420–9. [DOI] [PubMed] [Google Scholar]
- 14.Smith BW, Tooley EM, Montague EQ, Robinson AE, Cosper CJ, Mullins PG. Habituation and sensitization to heat and cold pain in women with fibromyalgia and healthy controls. Pain 2008;140:420–8. [DOI] [PubMed] [Google Scholar]
- 15.Wolfe F, Clauw DJ, Fitzcharles MA, Goldenberg DL, Katz RS, Mease P, et al. The American College of Rheumatology preliminary diagnostic criteria for fibromyalgia and measurement of symptom severity. Arthritis Care Res 2010;62:600–10. [DOI] [PubMed] [Google Scholar]
- 16.Bidari A, Hassanzadeh M, Ghavidel Parsa B, Kianmehr N, Kabir A, Pirhadi S, et al. Validation of the 2010 American College of Rheumatology preliminary diagnostic criteria for fibromyalgia in an Iranian population. Rheumatol Int 2013;33:2999–3007. [DOI] [PubMed] [Google Scholar]
- 17.Kim SM, Lee SH, Kim HR. Applying the ACR preliminary diagnostic criteria in the diagnosis and assessment of fibromyalgia. Korean J Pain 2012;25:173–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Degotardi PJ, Klass ES, Rosenberg BS, Fox DG, Gallelli KA, Gottlieb BS. Development and evaluation of a cognitive-behavioral intervention for juvenile fibromyalgia. J Pediatr Psychol 2006;31:714–23. [DOI] [PubMed] [Google Scholar]
- 19.Kashikar-Zuck S, Ting TV, Arnold LM, Bean J, Powers SW, Graham TB, et al. Cognitive behavioral therapy for the treatment of juvenile fibromyalgia: a multisite, single-blind, randomized, controlled clinical trial. Arthritis Rheum 2012;64:297–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stephens S, Feldman BM, Bradley N, Schneiderman J, Wright V, Singh-Grewal D, et al. Feasibility and effectiveness of an aerobic exercise program in children with fibromyalgia: results of a randomized controlled pilot trial. Arthritis Rheum 2008;59:1399–406. [DOI] [PubMed] [Google Scholar]
- 21.Bennett RM, Friend R, Marcus D, Bernstein C, Han BK, Yachoui R, et al. Criteria for the Diagnosis of Fibromyalgia: Validation of the Modified 2010 Preliminary American College of Rheumatology Criteria and the Development of Alternative Criteria. Arthritis Care Res 2014;66:1364–73. [DOI] [PubMed] [Google Scholar]
- 22.Bidari A, Hassanzadeh M, Parsa BG, Kianmehr N, Kabir A, Pirhadi S, et al. Validation of the 2010 American College of Rheumatology preliminary diagnostic criteria for fibromyalgia in an Iranian population. Rheumatol Int 2013;33:2999–3007. [DOI] [PubMed] [Google Scholar]
- 23.Segura-Jiménez V, Aparicio VA, Ávarez-Gallardo IC, Soriano-Maldonado A, Estévez-López F, Delgado-Fernández M, et al. Validation of the modified 2010 American College of Rheumatology diagnostic criteria for fibromyalgia in a Spanish population. Rheumatology 2014;53:1803–11. [DOI] [PubMed] [Google Scholar]
- 24.Usui C, Hatta K, Aratani S, Yagishita N, Nishioka K, Kanazawa T, et al. The Japanese version of the modified ACR Preliminary Diagnostic Criteria for Fibromyalgia and the Fibromyalgia Symptom Scale: reliability and validity. Mod Rheumatol 2013;23:846–50. [DOI] [PubMed] [Google Scholar]
- 25.Jones GT, Atzeni F, Beasley M, Flüß E, Sarzi-Puttini P, Macfarlane GJ. The prevalence of fibromyalgia in the general population–a comparison of the American College of Rheumatology 1990, 2010 and modified 2010 classification criteria. Arthritis Rheumatol 2015;67:568–75. [DOI] [PubMed] [Google Scholar]


