Abstract
Axitinib, a vascular endothelial growth factor receptor-tyrosine kinase inhibitor, will be used in combination first-line therapies against metastatic renal cell carcinoma (mRCC), but its effects as a first-line monotherapy are unclear. Thus, we aimed to elucidate pretreatment clinical factors that predict the prognosis of patients with mRCC receiving first-line axitinib therapy. We enrolled 63 patients with mRCC treated with axitinib as first-line therapy between Nov. 2003 and Jul. 2018. Progression-free survival (PFS) and overall survival (OS) were assessed using the Wald χ2 statistic in Cox proportional hazards regression. Median patient age was 67 (range: 25–85) years. Seven (11.1%) patients were classified as being at favorable risk, 33 (52.4%) at intermediate risk, and 23 (36.5%) at poor risk according to the International Metastatic Renal Cell Carcinoma Database Consortium (IMDC) risk classification system. Median follow-up duration after axitinib initiation was 14 (range: 1–72) months. Median PFS and OS were 18 months and 65 months, respectively. Cox regression analyses of clinical predictors revealed that high C-reactive protein (CRP) levels were significantly correlated with shorter PFS [hazard ratio (HR), 1.63; 95% confidence interval (CI) 1.7–4.0)], whereas spindle cells and poor IMDC risk scores were related to worse OS (HR, 2.87 and 2.88, respectively; 95% CI 1.4–11.0 and 1.1–8.5, respectively). Thus, patients with mRCC and spindle histology or poor IMDC risk scores had worse OS, and those with high CRP levels had shorter PFS in first-line axitinib treatment. Other therapies might be more suitable for initial management of such patients.
Subject terms: Renal cell carcinoma, Tumour biomarkers
Introduction
An advancement in combination therapy using immune checkpoint inhibitors (ICI) with axitinib, which is a vascular endothelial growth factor receptor (VEGFR)-tyrosine kinase inhibitor (TKI), or anti-cytotoxic T-lymphocyte associated antigen-4 (CTLA-4) antibody would provide a standard first-line therapy in treatment of metastatic renal cell carcinoma (mRCC)1–3. However, immune combination therapy has not shown a clear advantage over VEGFR-TKI therapy in patients with mRCC that have favorable International Metastatic Renal Cell Carcinoma Database Consortium (IMDC) risk scores1–3. In other words, certain patients are good candidates for first-line VEGFR-TKI monotherapy4,5. Additionally, since sunitinib has been the only VEGFR-TKI used as a control drug in these pivotal combination therapy studies, the clinical effects of other VEGFR-TKIs have not been fully evaluated yet in this setting1–3.
Axitinib provides higher selective inhibition of VEGFR 1–3 than do other VEGFR-TKIs and is approved as a second-line therapy for mRCC. This distinctive characteristic might allow for the same or better clinical outcomes as well as effective first-line therapy, although axitinib has not demonstrated improved prognosis over that of sorafenib in first-line settings6. In contrast, axitinib has resulted in superior clinical outcomes, in terms of both survival and safety profile, compared to those of sunitinib in a real-world retrospective cohort7.
Two new combination therapies consist of axitinib and ICIs. The combination is expected to provide a synergistic effect8,9, but information on outcomes of first-line axitinib monotherapy against mRCC in the subset of patients with favorable or intermediate IMDC risk scores is still needed.
The objective of this study was to elucidate pretreatment clinical factors that predict the prognosis of patients with mRCC receiving first-line axitinib therapy. Our results may improve the selection of candidates for combination therapies.
Methods
Eligibility criteria
Patients with histologically proven mRCC, regardless of Eastern Cooperative Oncology Group (ECOG) performance status (PS), were included in this study. One hundred and sixty patients were treated with VEGFR-TKI at the Akita University Hospital (Akita, Japan) between Nov. 2003 and Jul. 2018. This study enrolled 63 of these patients who were treated with axitinib as first-line therapy. Patients who were given axitinib as a presurgical treatment were excluded. This study was approved by our institutional review board (No. 2265). All procedures were performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki.
Primary and secondary endpoints
The primary objective was to assess clinical outcomes of axitinib treatment, including the objective response rate (ORR), progression-free survival (PFS) rate, overall survival (OS) rate, and incidence of adverse events (AEs). The secondary objectives were to identify pretreatment clinical factors that could affect the prognosis of patients with mRCC treated with first-line axitinib.
Treatment and follow-up examinations
The following patient characteristics were recorded and laboratory tests were conducted before starting treatment and during therapy at the attending physician’s discretion: complete medical history, physical examination, ECOG PS, blood cell counts with differential and platelet counts, biochemical profile (including electrolytes, renal and hepatic function, coagulation, pancreatic amylase, and lipase), urinalyses, and chest radiography. Toxicity was graded using the Common Terminology Criteria for Adverse Events version 4.0. The size of each tumor was evaluated and measured using computed tomography scans obtained within 4 weeks before starting axitinib. After axitinib treatment was started, the assessment interval was scheduled for individual patients by the attending physicians. Tumor response was evaluated using the Response Evaluation Criteria in Solid Tumors guidelines version 1.1.
Statistical analysis
PFS was defined as the time between the initiation of axitinib treatment and disease progression or death as confirmed by radiological images or obvious clinical manifestation of progressive disease. OS was defined as the time between axitinib initiation and death. The database record was closed upon patient death or the final follow-up. The cut-off value for neutrophil–lymphocyte ratio (NLR), serum albumin level, and C-reactive protein (CRP) level was set as their median values. Data are expressed as means ± standard deviation values, and differences with p-values less than 0.05 were considered statistically significant. The chi-squared test was used to examine differences in categorical data. PFS and OS were stratified using the Kaplan–Meier method. The Cox proportional hazard regression model was used for the analysis of hazard ratio (HR) and 95% confidence interval (CI). Analyses were performed using SPSS version 24.0 statistical software (SPSS, Inc., Chicago, IL, USA).
Ethics approval
This study was approved by the institutional review board. All procedures were performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent for publication
Patients signed informed consent regarding publication of their data.
Results
Patient characteristics
The 63 enrolled patients received at least 2 weeks of axitinib therapy and were assessed for treatment efficacy and toxicity. The median patient age was 67 (range: 25–85) years. All patients were Japanese, and the cohort included 47 (74.6%) men and 16 (25.4%) women. Fifty (79.4%) patients underwent radical nephrectomy before starting axitinib therapy. Fifty-eight (92.1%) patients had clear cell histology, four (6.3%) had papillary, two each (3.0%) had chromophobe and Xp11.2 translocation, and thirteen (20.6%) had spindle histology. Thirty (47.6%) patients had an ECOG PS of 0, 13 (20.6%) had an ECOG PS of 1, and 11 (17.5%) had an ECOG PS of 2 or higher. Under the IMDC risk classification system, 7 (11.1%) patients were classified as being at favorable risk, 33 (52.4%) at intermediate risk, and 23 (36.5%) at poor risk. Eighteen (28.6%) patients had one metastatic site, 19 (30.2%) had two, and 26 (41.3%) had three or more (Table 1).
Table 1.
Baseline demographic and clinical characteristics.
| Total patient number | N = 63 |
|---|---|
| Age | |
| Median year (range) | 67 (25–85) |
| Gender, n (%) | |
| Male | 47 (74.6) |
| Female | 16 (25.4) |
| Nephrectomy, n (%) | |
| Yes | 50 (79.4) |
| Histology, n (%) | |
| Clear cell | 58 (92.1) |
| With spindle component | 13 (20.6) |
| Papillary | 4 (6.3) |
| Chromphobe | 2 (3.2) |
| Xp11.2 translocation | 2 (3.2) |
| Clinical stage at the time of diagnosis with RCC, n (%) | |
| 1 | 17 (27.0) |
| 2 | 6 (9.5) |
| 3 | 8 (12.7) |
| 4 | 32 (50.8) |
| IMDC risk classification, n (%) | |
| Favorable | 7 (11.1) |
| Intermediate | 33 (52.4) |
| Poor | 23 (36.5) |
| Metastatic site, n (%) | |
| 1 | 18 (28.6) |
| 2 | 19 (30.2) |
| 3 | 9 (14.3) |
| 4 ≤ | 17 (27.0) |
| ECOG PS, n (%) | |
| 0 | 39 (61.9) |
| 1 | 13 (20.6) |
| 2 ≤ | 11 (17.5) |
| Site of metastases, n (%) | |
| Lung | 49 (77.8) |
| Lymph node | 37 (58.7) |
| Bone | 21 (33.3) |
| Liver | 12 (19.0) |
| Adrenal gland | 6 (9.5) |
| Brain | 5 (7.9) |
| Opposite kidney | 5 (7.9) |
| Others | 11 (17.5) |
| Time from diagnosis to systemic therapy, n (%) | |
| < 1 year | 31 (49.2) |
| Neutrophil–lymphocyte ration, n (%) | |
| > 2.9 | 31 (49.2) |
| Hemoglobin, n (%) | |
| < Lower limit of normal | 49 (77.8) |
| Platelet count, n (%) | |
| > 34.8 × 104/μL | 11 (17.5) |
| Albumin, n (%) | |
| < 3.7 g/dL | 30 (47.6) |
| Corrected carcium, n (%) | |
| > 10 mg/dL | 4 (6.3) |
| LDH, n (%) | |
| > 1.5 × upper limit of normal | 5 (7.9) |
| CRP, n (%) | |
| > 0.5 mg/dL | 32 (50.8) |
IMDC International Metastatic Renal Cell Carcinoma Database Consortium, ECOG Eastern Cooperative Oncology, PS performa status, LDH lactate dehydrogenase, CRP C-reactive protein.
Antitumor effect
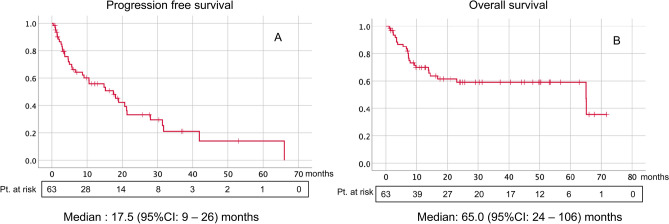
At the time of analysis, 12 patients (19.0%) were still being treated. The reasons for treatment discontinuation were progressive disease (58.7%) and AE (19.0%). An objective response was found in 25 (39.7%) patients (Table 2). The median PFS was 17.5 months (95% CI 9–26), and the overall survival was 65.0 months (95% CI 24–106) (Fig. 1). These survival rates are comparable to the median PFS (12.2 months) and OS (33.0 months) in the Japanese population from a randomized phase III trial10.
Table 2.
Results of axitinib management.
| Initial dose (mg/day) | |
| Median (range) | 10 (6–10) |
| Duration (month) | |
| Median month (range) | 9 (1–67) |
| Current treatment status, n (%) | |
| Continue | 12 (19.0) |
| Discontinuation | 51 (81.0) |
| Cause of discontinuation | |
| PD | 37 (58.7) |
| AE | 12 (19.0) |
| Others | 2 (3.2) |
| Objective response rate, n (%) | |
| CR + PR | 25 (39.7) |
| Best response, n (%) | |
| CR | 2 (3.2) |
| PR | 23 (36.5) |
| SD | 23 (36.5) |
| PD | 12 (19.0) |
| Not assessed | 3 (4.8) |
PD progressive disease, AE adverse event, CR complete response, PR partial response, SD stabel disease.
Figure 1.
Kaplan–Meier curves of progression-free survival (A) and overall survival (B) for the entire study population (N = 63).
Adverse events
By the time of the data cut-off, 29 patients (46.0%) had experienced an AE (Table 3), and 12 (19.0%) had discontinued nivolumab treatment because of AEs. The most common AEs of grade 3 or higher included 3 cases each of anorexia, general fatigue, hypertension, and proteinuria; two cases each of myocardial infarction, liver dysfunction, diarrhea, and pneumonia; and one case each of renal sufficiency, glossitis, shingles, aspiration pneumonia, and artery mural thrombosis (Table 3). There was no relationship between AE development and clinical response (data not shown).
Table 3.
Treatment-related adverse events.
| Total patient number | N = 63 |
|---|---|
| Number of patients with AEs (any grade), n (%) | 29 (45.5) |
| Cause of discontinuation, n (%) | |
| PD | 37 (58.7) |
| AE | 12 (19.0) |
| The detail of AEs (G3 or more), n (%) | 15 (23.8) |
| Anorexia | 3 (4.8) |
| Myocardial infarction | 2 (3.2) |
| Liver dysfunction | 2 (3.2) |
| Diarrhea | 2 (3.2) |
| Pneumonia | 2 (3.2) |
| Renal insufficiency | 1 (1.6) |
| Proteinuria | 1 (1.6) |
| Glossitis | 1 (1.6) |
| Shingles | 1 (1.6) |
| Aspiration pneumonia | 1 (1.6) |
| Artery mural thrombus | 1 (1.6) |
PD progressive disease, AE adverse event, G grade.
Multivariate analysis
The following 6 variables exhibited significance in univariate selective analysis of clinically relevant factors (Table 4) and were included in the multivariate model: poor IMDC risk score, not having undergone nephrectomy, spindle histology, NLR of 2.9 or more, CRP level of 0.5 or more, and 4 or more metastatic organs (Table S1 in the Supplementary Appendix). Cox regression analyses for clinical predictors revealed that high CRP value was significantly correlated with shorter PFS (HR, 1.63; 95% CI 1.7–4.0), whereas spindle histology and poor IMDC risk score were correlated with worse OS (HR, 2.87 and 2.88, respectively; 95% CI 1.4–11.0 and 1.1–8.5, respectively) (Table 4).
Table 4.
Prognostic values of clinical variables for predicting progression-free survival and overall survival in 63 patients with metastatic renal cell carcinoma analyzed by using univariate and multivariate Cox proportional hazards regression models.
| Risk factor | Risk category | Univariate | Multivariate | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| PFS | OS | PFS | OS | ||||||||
| HR | p | HR | p | HR | 95% CI | p | HR | 95% CI | p | ||
| IMDC risk classification | Poor | 4.53 | 0.033 | 14.28 | < 0.001 | 1.72 | – | 0.189 | 2.88 | 1.10–8.47 | 0.030 |
| Nephrectomy | Not performed | 1.51 | 0.219 | 6.22 | 0.013 | 0.24 | – | 0.622 | |||
| Spindle histology | Positive | 5.16 | 0.023 | 10.71 | 0.001 | 1.96 | – | 0.162 | 2.87 | 1.40–11.00 | 0.020 |
| NLR | 2.9 or more | 3.51 | 0.061 | 8.72 | 0.003 | 1.83 | – | 0.176 | |||
| CRP | 0.5 or more | 4.87 | 0.027 | 12.50 | 0.002 | 1.63 | 1.70–4.02 | 0.031 | 3.07 | 0.97–7.75 | 0.058 |
| Metastatic organ | 4 or more | 5.22 | 0.022 | 7.30 | 0.007 | 2.21 | – | 0.137 | 1.20 | – | 0.274 |
Statistically significant factors are shown in bold.
PFS progression-free survival, OS overall survival, HR Hazard ratio, CI confidence interval, IMDC International metastatic renal cell carcinoma database consortium, NLR neutrophil lymphocyte ratio, CRP C reactive protein.
Discussion
This study focused on patients that were treated with axitinib as a first-line therapy and as a representative the VEGFR-TKI regimen. The clinical outcomes of these patients were reported, and prognostic factors for these patients were elucidated. The median OS of all patients was 65.0 months, which was comparable with the findings of subgroup analysis in a pivotal study on sunitinib10 and a first-line axitinib regimen7 in Japanese patients with mRCC. In addition, we identified CRP expression level as a potential prognostic factors of PFS and poor IMDC risk score and spindle histology as potential prognostic factors of OS. Patients that did not exhibit any of these adverse prognostic factors received long-term benefits from axitinib as a first-line treatment and showed better OS. These findings indicate that axitinib is effective and well-tolerated as a first-line treatment among patients with mRCC.
The results of this study were comparable to those of previous studies on first-line VEGFR-TKIs11,12. In our study and in a phase II trial of sunitinib, the median OS rates were 65.0 months and 33.1 months, respectively; the median PFS rates were 17.5 months and 12.2 months, respectively; and the ORRs were 39.7% and 52.0%, respectively10. The median duration of axitinib treatment was longer (9.0 months) in our study than in the phase II trial of sunitinib (6.0 months). The median OS in our study was better than those of other retrospective cohort studies (median OS: 65.0 to 33.2 months). The most common AEs of grade 3 or more that led to treatment discontinuation were anorexia, general fatigue, hypertension, and proteinuria. Although the most common AE (of any grade) in the first-line axitinib phase III trial (AXIS trial) was hypertension13, our treatment was not interrupted due to hypertension.
Two prognostic factors of OS were identified in our study. Poor IMDC risk score14,15 and spindle histology16, which have already been identified as markers of poor prognosis in second-line axitinib treatment cohorts, were shown to also be prognostic markers in the first-line axitinib setting. Although VEGFR-TKI monotherapy is still considered usable in all IMDC risk categories by both the European Association of Urology and the National Comprehensive Cancer Network treatment guidelines for mRCC17,18, the efficacy of VEGFR-TKIs does not satisfy us. In clinical practice, ICI combination therapy would be a better choice19, regardless of its complicating immune-related adverse events20. Moreover, since patients with mRCC and spindle histology received only a small benefit to survival in our study, ICI combination therapy seems a better strategy21,22. Specifically, combination therapy consisting of anti-CTLA-4 (ipilimumab) and anti-PD-1 (nivolumab) may be an optimal choice to treat this formidable disease23. The ratio of spindle cells in tumors is also a known predictive factor of treatment outcome16,21. Patients with 25% or more spindle cells might have worse prognoses. In contrast, those with relatively small proportions of spindle cells might expect better clinical courses, even with VEGFR-TKI monotherapy16. However, of the three patients with less than 10% spindle cells in our study, two died within one year of initiation of systemic treatment. Consequently, patients with mRCC and poor IMDC risk scores or spindle features would be better treated with first-line ICI combination therapy even if they have contraindicated co-morbidities, such as autoimmune disorders or organ transplantations24.
In previous studies, several prognostic markers for axitinib treatment have been suggested, such as NLR25, serum albumin level26, serum lactate dehydrogenase level26, and serum CRP level27, among others. However, the model-development cohorts for these studies consisted of heterogeneous populations that included patients who had received a variety of treatments, including several VEGFR-TKIs. CRP value was related to poor PFS but not related to OS in our study. This might be caused by an interaction between NLR and CRP as an inflammatory representation, and both CRP and NLR were left over after the Cox hazard model. However, we still expect that CRP is a predictor of good prognosis in patients with mRCC28,29.
Our study is limited by its retrospective design, selection bias, unmeasured confounding factors, changes in clinical practice patterns over time, and the fact that the cohort was previously treated with VEGFR-TKI monotherapy before the immunotherapy era. Nevertheless, this study provides additional data that will help clinicians identify patients with mRCC who would receive long-term benefits from axitinib. Further studies on cohorts treated with immune-oncology therapies and longer follow-up times will be needed to validate our model.
Conclusions
Our findings show that patients with mRCC and spindle histology or poor IMDC risk scores had worse OS and that high CRP levels predicted shorter PFS in first-line axitinib treatment. ICI combination therapies might be more suitable for initial management of such patients.
Supplementary information
Acknowledgements
We are grateful to Ms. Mitobe for assistance with data collection.
Abbreviations
- ICI
Immune checkpoint inhibitors
- CTLA-4
Cytotoxic T-lymphocyte associated antigen-4
- mRCC
Metastatic renal cell carcinoma
- VEGFR
Vascular endothelial growth factor receptor
- TKI
Tyrosine kinase inhibitor
- IMDC
International Metastatic Renal Cell Carcinoma Database Consortium
- ECOG
Eastern Cooperative Oncology Group
- PS
Performance status
- ORR
Objective response rate
- PFS
Progression-free survival
- OS
Overall survival
- AE
Adverse event
- NLR
Neutrophil–lymphocyte ratio
- CRP
C reactive protein
- HR
Hazard ratio
- CI
Confidence interval
Author contributions
K.N.: study design, manuscript drafting. M.K.: study design, manuscript drafting. Y.M.: data acquisition and analysis. Y.S.: data acquisition and analysis. M.T.: data acquisition and analysis. S.K.: data acquisition and analysis. R.Y.: data acquisition and analysis. A.K.: data acquisition and analysis. T.N.: data acquisition and analysis. M.S.: data acquisition. S.N.: data acquisition. H.N.: data acquisition and analysis. T.H.: data acquisition and analysis, critical manuscript revision for important intellectual content.All authors reviewed the manuscript.
Funding
This work was supported by Grants-in-Aid for Scientific Research, Japan (grant no. 17K11121).
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Competing interests
Dr. Habuchi has received honoraria from Novartis Pharma, Pfizer Co., GlaxoSmithKline, and Ono Pharma Co. No other author has any conflicts of interest to declare.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-020-77135-6.
References
- 1.Motzer RJ, Tannir NM, McDermott DF, et al. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N. Engl. J. Med. 2018;378:1277–1290. doi: 10.1056/NEJMoa1712126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rini BI, Plimack ER, Stus V, et al. Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N. Engl. J. Med. 2019;380:1116–1127. doi: 10.1056/NEJMoa1816714. [DOI] [PubMed] [Google Scholar]
- 3.Motzer RJ, Penkov K, Haanen J, et al. Avelumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N. Engl. J. Med. 2019;380:1103–1115. doi: 10.1056/NEJMoa1816047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Numakura K, Tsuchiya N, Kagaya H, et al. Clinical effects of single nucleotide polymorphisms on drug-related genes in Japanese metastatic renal cell carcinoma patients treated with sunitinib. Anticancer Drugs. 2017;28:97–103. doi: 10.1097/CAD.0000000000000425. [DOI] [PubMed] [Google Scholar]
- 5.Numakura K, Fujiyama N, Takahashi M, et al. Clinical implications of pharmacokinetics of sunitinib malate and N-desethyl-sunitinib plasma concentrations for treatment outcome in metastatic renal cell carcinoma patients. Oncotarget. 2018;9:25277–25284. doi: 10.18632/oncotarget.25423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hutson TE, Lesovoy V, Al-Shukri S, et al. Axitinib versus sorafenib as first-line therapy in patients with metastatic renal-cell carcinoma: A randomised open-label phase 3 trial. Lancet Oncol. 2013;14:1287–1294. doi: 10.1016/S1470-2045(13)70465-0. [DOI] [PubMed] [Google Scholar]
- 7.Konishi S, Hatakeyama S, Tanaka T, et al. Comparison of axitinib and sunitinib as first-line therapies for metastatic renal cell carcinoma: A real-world multicenter analysis. Med. Oncol. 2018;36:6. doi: 10.1007/s12032-018-1231-3. [DOI] [PubMed] [Google Scholar]
- 8.Bai X, Yi M, Jiao Y, et al. Blocking TGF-beta signaling to enhance the efficacy of immune checkpoint inhibitor. Onco Targets Ther. 2019;12:9527–9538. doi: 10.2147/OTT.S224013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Uemura M, Tomita Y, Miyake H, et al. Avelumab plus axitinib vs sunitinib for advanced renal cell carcinoma: Japanese subgroup analysis from JAVELIN Renal 101. Cancer Sci. 2020;111:907–923. doi: 10.1111/cas.14294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tomita Y, Shinohara N, Yuasa T, et al. Overall survival and updated results from a phase II study of sunitinib in Japanese patients with metastatic renal cell carcinoma. Jpn. J. Clin. Oncol. 2010;40:1166–1172. doi: 10.1093/jjco/hyq146. [DOI] [PubMed] [Google Scholar]
- 11.Motzer RJ, Hutson TE, Tomczak P, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N. Engl. J. Med. 2007;356:115–124. doi: 10.1056/NEJMoa065044. [DOI] [PubMed] [Google Scholar]
- 12.Motzer RJ, Hutson TE, Cella D, et al. Pazopanib versus sunitinib in metastatic renal-cell carcinoma. N. Engl. J. Med. 2013;369:722–731. doi: 10.1056/NEJMoa1303989. [DOI] [PubMed] [Google Scholar]
- 13.Rini BI, Escudier B, Tomczak P, et al. Comparative effectiveness of axitinib versus sorafenib in advanced renal cell carcinoma (AXIS): A randomised phase 3 trial. Lancet. 2011;378:1931–1939. doi: 10.1016/S0140-6736(11)61613-9. [DOI] [PubMed] [Google Scholar]
- 14.Konishi S, Hatakeyama S, Numakura K, et al. Validation of the IMDC prognostic model in patients with metastatic renal-cell carcinoma treated with first-line axitinib: A multicenter retrospective study. Clin. Genitourin Cancer. 2019;17:e1080–e1089. doi: 10.1016/j.clgc.2019.07.006. [DOI] [PubMed] [Google Scholar]
- 15.Ueda K, Suekane S, Hirano T, et al. Efficacy of axitinib as second-line treatment in locally advanced and metastatic renal cell carcinoma. Anticancer Res. 2018;38:5387–5392. doi: 10.21873/anticanres.12868. [DOI] [PubMed] [Google Scholar]
- 16.Beuselinck B, Lerut E, Wolter P, et al. Sarcomatoid dedifferentiation in metastatic clear cell renal cell carcinoma and outcome on treatment with anti-vascular endothelial growth factor receptor tyrosine kinase inhibitors: A retrospective analysis. Clin. Genitourin Cancer. 2014;12:e205–e214. doi: 10.1016/j.clgc.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 17.Albiges L, Powles T, Staehler M, et al. Updated European association of urology guidelines on renal cell carcinoma: Immune checkpoint inhibition is the new backbone in first-line treatment of metastatic clear-cell renal cell carcinoma. Eur. Urol. 2019;76:151–156. doi: 10.1016/j.eururo.2019.05.022. [DOI] [PubMed] [Google Scholar]
- 18.Motzer RJ, Jonasch E, Michaelson MD, et al. NCCN guidelines insights: Kidney cancer, version 2.2020. J. Natl. Compr. Cancer Netw. 2019;17:1278–1285. doi: 10.6004/jnccn.2019.0054. [DOI] [PubMed] [Google Scholar]
- 19.Escudier B, Motzer RJ, Tannir NM, et al. Efficacy of nivolumab plus ipilimumab according to number of IMDC risk factors in CheckMate 214. Eur. Urol. 2020;77:449–453. doi: 10.1016/j.eururo.2019.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Numakura K, Horikawa Y, Kamada S, et al. Efficacy of anti-PD-1 antibody nivolumab in Japanese patients with metastatic renal cell carcinoma: A retrospective multicenter analysis. Mol. Clin. Oncol. 2019;11:320–324. doi: 10.3892/mco.2019.1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Debien V, Thouvenin J, Lindner V, et al. Sarcomatoid dedifferentiation in renal cell carcinoma: From novel molecular insights to new clinical opportunities. Cancers. 2019;12:99. doi: 10.3390/cancers12010099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.George G, et al. Salvage ipilimumab associated with a significant response in sarcomatoid renal cell carcinoma. J. Immunother. Cancer. 2020;8:e000584. doi: 10.1136/jitc-2020-000584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tolay S, Nair R, McIntosh AF, et al. Dramatic response to concurrent anti-PD-1 therapy and radiation in resistant tumors with sarcomatoid differentiation. Oncologist. 2019;24:e49–e52. doi: 10.1634/theoncologist.2018-0205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johnson DB, Sullivan RJ, Menzies AM. Immune checkpoint inhibitors in challenging populations. Cancer. 2017;123:1904–1911. doi: 10.1002/cncr.30642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ishihara H, Kondo T, Yoshida K, et al. Effect of systemic inflammation on survival in patients with metastatic renal cell carcinoma receiving second-line molecular-targeted therapy. Clin. Genitourin. Cancer. 2017;15:495–501. doi: 10.1016/j.clgc.2017.01.018. [DOI] [PubMed] [Google Scholar]
- 26.Tamura K, Matsushita Y, Watanabe H, et al. Feasibility of the ACL (albumin, C-reactive protein and lactate dehydrogenase) model as a novel prognostic tool in patients with metastatic renal cell carcinoma previously receiving first-line targeted therapy. Urol. Oncol. 2020;38:6.e9–6.e16. doi: 10.1016/j.urolonc.2019.07.013. [DOI] [PubMed] [Google Scholar]
- 27.Konishi S, Hatakeyama S, Tanaka T, et al. C-reactive protein/albumin ratio is a predictive factor for prognosis in patients with metastatic renal cell carcinoma. Int. J. Urol. 2019;26:992–998. doi: 10.1111/iju.14078. [DOI] [PubMed] [Google Scholar]
- 28.Yasuda Y, Saito K, Yuasa T, et al. Early response of C-reactive protein as a predictor of survival in patients with metastatic renal cell carcinoma treated with tyrosine kinase inhibitors. Int. J. Clin. Oncol. 2017;22:1081–1086. doi: 10.1007/s10147-017-1166-2. [DOI] [PubMed] [Google Scholar]
- 29.Nakayama T, Saito K, Kumagai J, et al. Higher serum c-reactive protein level represents the immunosuppressive tumor microenvironment in patients with clear cell renal cell carcinoma. Clin. Genitourin. Cancer. 2018;16:e1151–e1158. doi: 10.1016/j.clgc.2018.07.027. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.