Abstract
Background:
When fetal growth begins to differ by maternal glycemic status is not well understood. We examined gestational diabetes (GDM), impaired glucose tolerance (IGT) and early pregnancy glucose levels in relation to fetal growth trajectories.
Methods:
The cohort study included 2,458 pregnant women from the NICHD Fetal Growth Studies-Singletons. Women were enrolled at gestational weeks 8–13 and randomized among four ultrasonogram schedules to capture weekly fetal growth. GDM, IGT, and normal glucose tolerance (NGT) were defined by medical record review. Glucose was measured in a sub-sample at weeks 10–14. Fetal growth trajectories were modeled using linear mixed models with cubic splines.
Findings:
GDM (n=107) was associated with a larger estimated fetal weight (EFW) that started at week 20 and was statistically significant at week 28–40 (at week 37: 3,061g [95% CI: 3,128, 3,170] for women with GDM vs. 2,943g [95% CI: 3,181, 3,397] for women with NGT, adjusted P = 0.02). In addition, glucose at weeks 10–14 were positively associated with EFW starting at week 23 and the association became significant at week 27 (at week 37: 3,073g [95% CI: 2,983, 3,167] in the highest tertile vs. 2,853g [95% CI: 2,755, 2,955] in the lowest tertile, adjusted P < 0.001).
Interpretation:
GDM was associated with a larger fetal size that started at week 20 and became statistically significant at gestational week 28. Efforts to mitigate GDM-related fetal overgrowth may start before 28 gestational weeks, when GDM is typically screened for in the U.S.
Keywords: gestational diabetes, fetal growth, blood glucose, longitudinal study
INTRODUCTION
Maternal glucose levels are positively associated with birth weight1. In particular, infants born to mothers with gestational diabetes (GDM) are more likely to be born large-for-gestational-age (LGA), and to develop obesity and diabetes later in life, contributing to an inter-generational cycle of metabolic risk 2. However, the exact timing during gestation when fetal growth begins to deviate in relation to women’s glycemic status is not completely understood; the answer to this question may inform the optimal timing for hyperglycemia screening and treatment. Further, data on glucose homeostasis in early pregnancy (i.e., 1st trimester to early 2nd trimester) in relation to fetal growth trajectories over pregnancy are lacking.
Studies on GDM and fetal growth have mostly focused on late pregnancy (i.e., late 2nd trimester through 3rd trimester). While some studies found a significant association between GDM and increased fetal size in late pregnancy 3–5, the remaining studies did not observe an association6–9. Most of these studies were limited by infrequent longitudinal fetal measures (e.g., three or fewer) and sporadic coverage across gestation 3,7–9, potentially missing the relevant window for fetal growth alterations. In addition, few studies have been able to identify when fetal growth alterations begin. Further, although metabolic disturbances related to GDM start during the first trimester10, fetal growth was rarely examined in early pregnancy among women later diagnosed with GDM 4,6.
In a multi-racial U.S. population, we examined the associations between maternal glycemic status and fetal growth trajectories throughout gestational weeks 10–40 and how maternal and fetal characteristics may modify these associations. We also evaluated the associations between a random glucose measure in early pregnancy and fetal growth trajectories.
METHODS
Study Design and Population
This study is based on the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Fetal Growth Studies–Singletons (2009–2013), a prospective cohort of 2,802 women recruited from 12 U.S. clinical sites 11. The cohort included 2,334 non-obese women (pre-pregnancy BMI < 30 kg/m2) aged 18–40. Exclusion criteria were having major chronic conditions, lifestyle risk factors (smoking, alcohol and illicit drugs use), past pregnancy complications, and conception by assisted reproductive technology. The cohort also included 468 obese women (pre-pregnancy BMI ≥30 kg/m2). As obesity is associated with chronic conditions, fewer chronic conditions and no lifestyle risk factors were among the exclusion criteria for obese women, yet diabetes remained an exclusion criterion.11 All women underwent ultrasound screening between 8 weeks 0 days and 13 weeks 6 days to ensure valid gestational dating consistent with last menstrual period (LMP) dating. Research approval was obtained from all participating institutions and the participants provided written informed consent. The NICHD Fetal Growth Studies-Singletons is registered with Clinicaltrials.gov, number NCT00912132.
Of the 2,802 women enrolled, 18 were found ineligible after enrollment, 201 did not have medical record abstraction due to study deactivation, and 125 did not have records of a glucose challenge test (GCT) or oral glucose tolerance test (OGTT). After the exclusion of these women, the current study consisted of 2,458 women.
Study documents and data were entered into the Clinical Trial Management System by the participating clinical centers and electronically transferred to the Data Coordinating Center. Ultrasound measurements and images were captured in ViewPoint (GE Healthcare) and electronically transferred to the Study’s imaging data coordination center.
Exposure Assessment
Women were identified as having GDM, impaired glucose tolerance (IGT), or normal glucose tolerance (NGT). GDM was defined by the OGTT results based on the Carpenter-Coustan criteria 12 (at least two values met or exceeded: fasting – 95 mg/dL, 1 hour – 180 mg/dL, 2 hours – 155 mg/dL, 3 hours – 140 mg/dL), and/or by receipt of GDM medications. IGT was defined as having a 2-hour plasma glucose level after OGTT between 140–199 mg/dL 12, but not meeting the criteria for GDM. NGT was defined as having no elevated values in GCT (below 140 mg/dL) or OGTT (below all thresholds: fasting – 95 mg/dL, 1 hour – 180 mg/dL, 2 hours – 140 mg/dL, 3 hours – 140 mg/dL) 12. Women who had any elevated values in OGTT and/or GCT but did not meet the criteria for GDM or IGT were excluded in the final analytical population (n = 213). The mean gestational age of OGTT was 27.5 weeks (SD = 4.1).
Random glucose and HbA1c levels were measured in a sub-study among all women with GDM (n = 107) and randomly selected women with NGT (n = 214) matched on a 1:2 ratio (based on age, race/ethnicity, and the week of blood collection)13. Specifically, 101 women with GDM and 203 women with NGT who had complete data were included in the analysis in the sub-study.
Outcome Assessment
Women received an ultrasonographic exam at enrollment. They were randomized into one of four ultrasonography follow-up schedules: weeks 16, 24, 30, 34 and 38 (group A); weeks 18, 26, 31, 35 and 39 (group B); weeks 20, 28, 32, 36 and 40 (group C); weeks 22, 29, 33, 37 and 41 (group D). Study visits were scheduled within ±1 week of the targeted gestational age to accommodate women’s availability.11 Randomization was performed in permuted blocks and random numbers were generated by a computer-based pseudo-random number generator. This mixed longitudinal randomization scheme captured weekly fetal growth data without exposing individual women to ultrasound weekly (See Figure S1 for the flowchart).
Before data collection, all participating sonographers underwent a multi-day educational program from the study’s central sonology unit in the acquisition of standardized images using study equipment and the performance of standardized measurements, and they were credentialed after the training. At each ultrasonographic examination, fetal biometrics were performed following standard operating procedures and using identical equipment (Voluson E8; GE Healthcare). Fetal biometrics included head circumference (HC, mm), biparietal diameter (BPD, mm), abdominal circumference (AC, mm), humerus length (HL, mm), femur length (FL, mm), and HC/AC ratio. The fetal biometrics showed high levels of reliability for repeated measures performed by the same sonographer and as compared to a gold standard sonographer.14 Reliability of fetal ultrasound measures did not vary by obesity status. The estimated fetal weight (EFW, g) was calculated using a Hadlock formula based on HC, AC, and FL15.
Covariates Assessment
At enrollment and follow-up visits, research nurses interviewed women about their sociodemographic characteristics, lifestyle, and medical, reproductive, and pregnancy histories, followed by anthropometric assessments. Pre-pregnancy BMI was calculated using self-reported weight before pregnancy and height measured at enrollment. Self-reported weights had shown high levels of agreement with weights measured by a technician in a previous study among U.S. women.16 Types of GDM treatment, date and mode of delivery, infant sex, birthweight, and adverse neonatal outcomes were abstracted from medical charts, and gestational age at delivery was calculated based on ultrasound verified LMP and date of delivery. LGA and small-for-gestational-age (SGA) were defined using sex-specific references 17.
Statistical Analysis
Characteristics of women and their neonates were presented by hyperglycemic status (i.e., GDM, IGT, and NGT). Trajectories of EFW and fetal biometrics were modeled using a linear mixed model estimated using a restricted maximum likelihood approach.18 The fetal measures were log-transformed to stabilize variances across gestational age and approximate normal distributions. The model initially included fixed effects of the linear, quadratic, and cubic terms and cubic spline terms of gestational age (3 knots at 25th, 50th, 75th percentiles), as well as a random intercept and random effects of the linear, quadratic, and cubic terms of gestational age. The random effect covariance was unstructured. The random effect cubic term of gestational age was removed to facilitate model convergence. First, to test the overall difference in fetal growth trajectories across glycemic status (i.e., global test), the model was fit adjusting for an a priori list of covariates (race-ethnicity [non-Hispanic white, non-Hispanic black, Hispanic, Asian/Pacific Islander], age, parity, pre-pregnancy BMI, and infant sex [male, female]); another model was fit with the addition of glycemic status and its interaction with gestational age terms. The two models were compared using a likelihood ratio test. Second, the weekly means (log scale fetal measures) were calculated by glycemic status using separate models (the back-transformed values were shown in tables and figures). Third, to compare weekly fetal measures across glycemic status, the weekly means were calculated again adjusting for the list of covariates; differences across glycemic status were calculated (the values were back-transformed into ratios and shown in tables) and tested using Wald tests. Multiple week-by-week comparisons were justified as their confidence intervals (CI) confirms the global test. Visits where the fetal measure was missing were excluded from the analysis. The adjusted covariates had no missing value. This is a secondary study of the NICHD Fetal Growth Study-Singletons. We estimate that this study would have sufficient statistical power to test its primary aims. At week 30, for example, a two-sample t-test have a 97% power (alpha = 0.05) to detect a 15% difference in EFW between women with GDM (n = 107) and NGT (n = 2,020).
In sensitivity analyses, we repeated the analyses excluding 94 women who had miscarriage (n = 1), fetal death (n=4), or infants with karyotype (n = 6) and/or structural abnormalities (n = 85). We also repeated the analysis excluding women with HbA1c > 6.5% (i.e., overt diabetes)19 at the enrollment visit (n = 3). Lastly, we repeated the analyses using an EFW estimated using an alternative Hadlock formula based on BPD, HC, AC, and FL15, and using all women who did not have GDM or IGT (n = 2,233) as the comparison group.
We evaluated potential effect modification by race-ethnicity (non-Hispanic white, Hispanic black, Hispanic, Asian/Pacific Islander), family history of diabetes (yes, no), pre-pregnancy obesity (yes, no), and infant sex (male, female) in stratified analysis. We tested interactions between GDM and potential effect modifiers using the global test. We evaluated the joint status of GDM and selected effect modifiers in relation to fetal growth. We also repeated the primary analyses stratifying women with GDM by GDM severity, as indicated by the type of treatment (i.e., lifestyle vs. medications).
Lastly, in the sub-study, we examined the associations of random glucose levels at weeks 10–14 with fetal growth trajectories. The model adjusted for the same set of covariates as previous models, and it was reweighted to be representative of the full cohort. Only a linear random effect was included to facilitate model convergence. All analyses were implemented using SAS, version 9.4 (SAS Institute Inc) and R, version 3.1.2 (R Development Core Team).
Role of the Funding Source
The study sponsors had no role in study design, the collection, analysis and interpretation of data, the writing of the report, or the decision to submit the paper for publication. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
RESULTS
In total, 107 (4.4%) women had GDM, 118 (4.8%) had IGT, and 2,020 (82.1%) had NGT. Compared to women with NGT, women with GDM and IGT were both more likely to be older, married, overweight or obese, and to have a family history of diabetes; women with GDM were also more likely to be Hispanic or Asian/Pacific Islander and to have lower educational attainment (all Ps < 0.05; Table 1; see Table S1 for additional characteristics). Infants of women with GDM, on average, were born slightly earlier and heavier, more likely to be LGA, less likely to be SGA, and more likely to receive intensive care; infants of women with IGT were also heavier and more likely to be LGA (all Ps < 0.05; Table 1). In the sub-study, the weighted mean of random glucose levels was 84.9 mg/dL (SD = 37.5) and the weighted mean of HbA1c levels was 5.05 (SD = 1.04) at gestational weeks 10–14.
Table 1.
Characteristics of women and their neonates by women’s hyperglycemic status during pregnancy in the NICHD Fetal Growth Studies-Singletons
| Characteristic | NGT (n=2,020) | IGT (n=118) | GDM (n=107) |
|---|---|---|---|
| Women’s Characteristics | |||
| Age (years), mean (SD) | 27.9 (5.5) | 29.5 (5.1) | 30.5 (5.7) |
| Race, n (%) | |||
| Non-Hispanic white | 571 (28.3) | 32 (27.1) | 25 (23.4) |
| Non-Hispanic black | 581 (28.8) | 24 (20.3) | 15 (14.0) |
| Hispanic | 572 (28.3) | 38 (32.2) | 41 (38.3) |
| Asian/Pacific Islander | 296 (14.7) | 24 (20.3) | 26 (24.3) |
| Married or cohabiting, n (%) | 1476 (73.7) | 99 (83.9) | 92 (86.0) |
| Education, n (%) | |||
| <High school | 225 (11.1) | 13 (11.0) | 17 (15.9) |
| High school/GED | 387 (19.2) | 16 (13.6) | 15 (14.0) |
| Some college/associate degree | 584 (28.9) | 41 (34.8) | 47 (43.9) |
| College undergraduate degree | 474 (23.5) | 35 (29.7) | 16 (15.0) |
| Postgraduate degree | 350 (17.3) | 13 (11.0) | 12 (11.2) |
| Parity, n (%) | |||
| 0 | 976 (48.3) | 46 (39.0) | 48 (44.9) |
| 1 | 676 (33.5) | 47 (39.8) | 29 (27.1) |
| 2 | 255 (12.6) | 13 (11.0) | 20 (18.7) |
| 3+ | 113 (5.6) | 12 (10.2) | 10 (9.3) |
| Family history of diabetes, n (%) | 396 (19.6) | 33 (28.0) | 40 (37.4) |
| Pre-pregnant BMI (kg/m2), n (%) | |||
| < 25 | 1187 (58.8) | 55 (46.6) | 37 (34.6) |
| 25–29 | 526 (26.0) | 34 (28.8) | 35 (32.7) |
| ≥30 | 307 (15.2) | 29 (24.6) | 35 (32.7) |
| Neonates’ Characteristics | |||
| Sex, n (%) | |||
| Male | 1038 (51.9) | 54 (45.8) | 54 (50.5) |
| Female | 963 (48.1) | 64 (54.3) | 53 (49.5) |
| Gestational age at birth, weeks (SD) | 39.3 (1.6) | 39.1 (1.5) | 38.9 (1.7) |
| Birthweight (g), mean (SD) | 3328 (501) | 3446 (493) | 3439 (566) |
| Large for gestational age, n (%) | 161 (8.1) | 17 (14.4) | 18 (17.0) |
| Small for gestational age, n (%) | 187 (9.4) | 6 (5.1) | 0 (0.0) |
| Delivery mode, n (%) | |||
| Spontaneous vaginal delivery | 1333 (66.6) | 72 (61.0) | 60 (56.1) |
| Operative vaginal delivery | 96 (4.8) | 3 (2.5) | 6 (5.6) |
| Scheduled C-section without labor | 275 (13.7) | 19 (16.1) | 22 (20.6) |
| C-section after trial of labor | 299 (14.9) | 24 (20.3) | 19 (17.8) |
| Birth injury, n (%) | 13 (0.6) | 0 (0.0) | 2 (1.9) |
| Admission to neonatal intensive care unit, n (%) | 204 (10.2) | 14 (11.9) | 19 (17.8) |
SD – standard deviation; GED – General Educational Development; BMI – body mass index; C-section – cesarean-section.
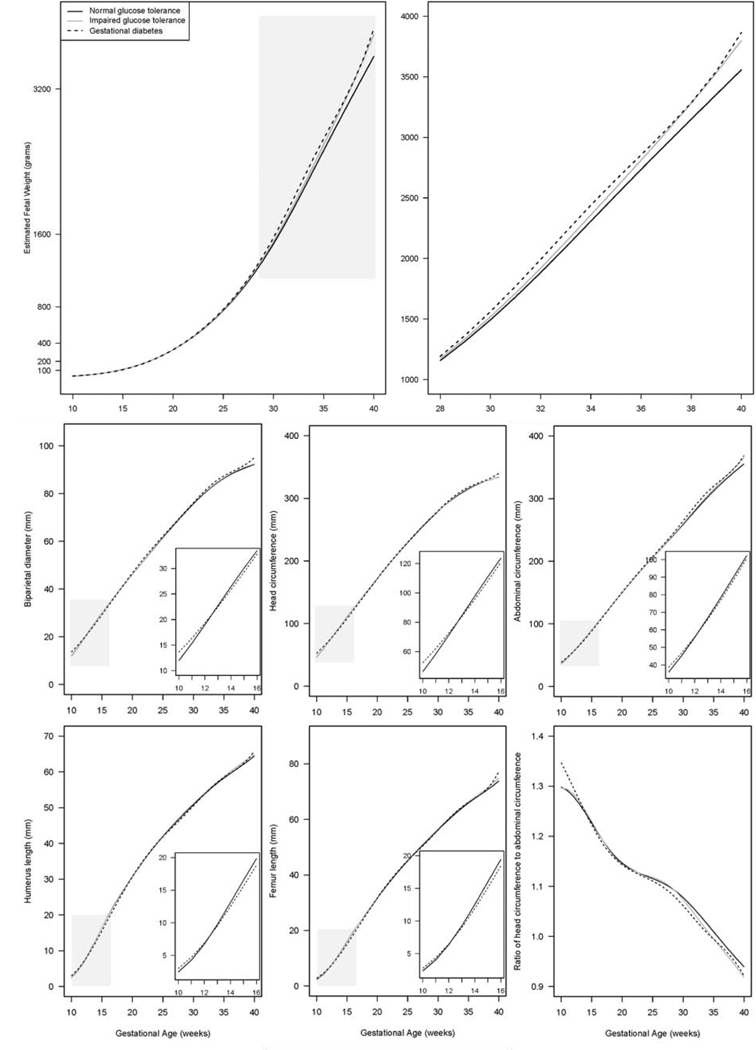
GDM was statistically significantly associated with the EFW trajectory (Figure 1; global P < 0.001). An association between GDM and larger EFW started to emerge at week 20 and became statistically significant at week 28; the significant association persisted through term. As the fetus reached term at week 37, EFW was on average 118 g larger (adjusted P = 0.02) in women with GDM (3,061 g, 95% CI: 2,967, 3,164) than NGT (2,943 g, 95% CI: 2,924, 2,962). IGT was marginally associated with the overall EFW trajectory (Figure 1; global P = 0.06) and significantly associated with a larger EFW at weeks 36–40 (Table 2).
Figure 1.
Geometric means of fetal biometrics by gestational age in women with normal glucose tolerance, impaired glucose tolerance, and gestational diabetes, in the NICHD Fetal Growth Studies-Singletons
The shaded areas are enlarged in separate plots.
Table 2.
Geometric means of estimated fetal weight by gestational week GW) and ratios of the geometric means across the comparison groups in women with normal glucose tolerance (NGT), impaired glucose tolerance (IGT), and gestational diabetes (GDM) in the NICHD Fetal Growth Studies-Singletons
| GW | Geometric means, g (95% CI) | Ratios of geometric means (95% CI)a | |||
|---|---|---|---|---|---|
| NGT (n=2,020) | IGT (n=118) | GDM (n=107) | IGT: NGT | GDM: NGT | |
| 10 | 35 (35, 36) | 34 (32, 36) | 38 (36, 41) | 0.97 (0.91, 1.03) | 1.09 (1.02, 1.17) |
| 12 | 55 (55, 55) | 54 (53, 56) | 55 (54, 57) | 0.99 (0.96, 1.02) | 1.01 (0.98, 1.04) |
| 14 | 88 (87, 88) | 88 (85, 90) | 86 (84, 88) | 1.00 (0.97, 1.03) | 0.98 (0.95, 1.01) |
| 16 | 140 (139, 140) | 140 (136, 144) | 137 (134, 141) | 1.00 (0.97, 1.03) | 0.98 (0.96, 1.01) |
| 18 | 218 (217, 219) | 218 (213, 224) | 217 (211, 223) | 1.00 (0.98, 1.03) | 1.00 (0.97, 1.03) |
| 20 | 327 (325, 329) | 327 (319, 336) | 328 (319, 337) | 1.00 (0.98, 1.03) | 1.00 (0.98, 1.03) |
| 22 | 470 (468, 473) | 472 (461, 484) | 474 (462, 487) | 1.00 (0.98, 1.03) | 1.01 (0.98, 1.04) |
| 24 | 653 (650, 656) | 658 (642, 674) | 661 (644, 679) | 1.01 (0.98, 1.03) | 1.01 (0.99, 1.04) |
| 26 | 879 (875, 884) | 889 (868, 911) | 897 (873, 922) | 1.01 (0.99, 1.04) | 1.02 (0.99, 1.05) |
| 28 | 1156 (1150, 1162) | 1173 (1145, 1202) | 1193 (1161, 1226) | 1.01 (0.99, 1.04) | 1.03 (1.00, 1.06) |
| 30 | 1492 (1485, 1500) | 1518 (1482, 1555) | 1562 (1520, 1605) | 1.02 (0.99, 1.04) | 1.05 (1.02, 1.08) |
| 32 | 1883 (1873, 1893) | 1919 (1872, 1968) | 1994 (1938, 2051) | 1.02 (0.99, 1.05) | 1.06 (1.03, 1.09) |
| 34 | 2307 (2294, 2321) | 2359 (2299, 2420) | 2443 (2373, 2515) | 1.02 (1.00, 1.05) | 1.06 (1.03, 1.09) |
| 36 | 2734 (2718, 2751) | 2811 (2737, 2887) | 2858 (2771, 2947) | 1.03 (1.00, 1.06) | 1.05 (1.01, 1.08) |
| 38 | 3149 (3128, 3170) | 3281 (3188, 3376) | 3287 (3181, 3397) | 1.04 (1.01, 1.07) | 1.04 (1.01, 1.08) |
| 40 | 3556 (3518, 3595) | 3799 (3622, 3984) | 3862 (3664, 4072) | 1.07 (1.02, 1.12) | 1.09 (1.03, 1.15) |
| Global | 0.06 | <0.001 | |||
| P | |||||
Adjusted for race/ethnicity (white, black, Asian, Hispanic), age, parity, pre-pregnancy BMI (kg/m2) and infant sex (male, female).CI – confidence interval; NICHD - Eunice Kennedy Shriver National Institute of Child Health and Human Development.
GDM was also statistically significantly associated with trajectories of all fetal biometrics (Figure 1; global Ps ≤ 0.001). In early pregnancy, GDM was associated with transitory changes in all fetal biometrics except for HC/AC ratio (i.e., BPD, HC, AC, FL, HL), characterized by larger size at weeks 10–11 and a smaller size around weeks 14–16. In late pregnancy, GDM was generally associated with larger fetal AC, BPD, and HC, and smaller HC/AC ratio (Table S2). IGT was only significantly associated with smaller HC/AC ratio at weeks 33–39 (global P = 0.04; Table S2).
Excluding women with a pregnancy loss or fetal abnormalities/death, excluding women with GDM who had HbA1c > 6.5% at weeks 10–14, using the alternative formula for EFW, or using all women who did not have GDM or IGT as the comparison group did not alter the primary results materially.
The associations between GDM and EFW were statistically significantly modified by women’s family history of diabetes (Table 3; P-interaction ≤ 0.001), but not by maternal obesity status, maternal race-ethnicity, or fetal sex. Compared with NGT without a family history of diabetes, GDM without a family history of diabetes was only associated with a larger fetal size beginning at week 24 (global P < 0.001). In contrast, GDM with a family history of diabetes was only associated with a transitorily larger size at weeks 10–11 and smaller size at weeks 14–17 (global P < 0.001). Compared to non-obese women with NGT, GDM alone without pre-pregnancy obesity was significantly associated with larger EFW during the 3rd trimester. Women who were obese before pregnancy and developed GDM in pregnancy appeared to have the largest EFW (Figure S2). Lastly, types of GDM treatment was not significantly associated with the EFW trajectory (Figure S3).
Table 3.
Geometric means of estimated fetal weight by gestational week (GW) and ratios of the geometric means across the comparison groups in women with normal glucose tolerance (NGT) and gestational diabetes (GDM), with or without a family history of diabetes (FH), in the NICHD Fetal Growth Studies-Singletons
| GW | Geometric means, g (95% CI) | Ratios of geometric means (95% CI)a | |||||
|---|---|---|---|---|---|---|---|
| NGT without FH (n=1624) | GDM without FH (n=67) | NGT with a FH (n=396) | GDM with a FH (n=40) | GDM without FH: NGT without FH | NGT with FH: NGT without FH | GDM with a FH: NGT without FH | |
| 10 | 35 (35, 36) | 34 (31, 37) | 34 (33, 36) | 45 (40, 51) | 0.99 (0.91, 1.07) | 0.98 (0.94, 1.02) | 1.28 (1.15, 1.43) |
| 12 | 55 (55, 55) | 54 (52, 56) | 55 (54, 55) | 55 (53, 58) | 1.01 (0.97, 1.05) | 1.00 (0.98, 1.01) | 1.00 (0.95, 1.04) |
| 14 | 88 (87, 88) | 87 (84, 90) | 88 (87, 89) | 82 (79, 85) | 1.02 (0.98, 1.06) | 1.00 (0.99, 1.02) | 0.92 (0.88, 0.96) |
| 16 | 139 (139, 140) | 139 (135, 144) | 140 (138, 142) | 133 (128, 137) | 1.02 (0.98, 1.06) | 1.01 (0.99, 1.02) | 0.94 (0.90, 0.98) |
| 18 | 218 (216, 219) | 217 (211, 224) | 218 (215, 220) | 213 (207, 220) | 1.02 (0.98, 1.06) | 1.00 (0.99, 1.01) | 0.97 (0.93, 1.01) |
| 20 | 327 (325, 329) | 327 (317, 337) | 326 (323, 330) | 323 (313, 333) | 1.02 (0.98, 1.06) | 1.00 (0.99, 1.01) | 0.98 (0.94, 1.02) |
| 22 | 470 (468, 473) | 473 (459, 488) | 469 (464, 474) | 464 (450, 477) | 1.03 (0.99, 1.06) | 1.00 (0.99, 1.01) | 0.97 (0.94, 1.01) |
| 24 | 653 (649, 656) | 664 (645, 683) | 651 (644, 658) | 640 (622, 659) | 1.04 (1.00, 1.07) | 1.00 (0.99, 1.01) | 0.97 (0.93, 1.01) |
| 26 | 879 (874, 884) | 905 (879, 932) | 878 (868, 887) | 862 (835, 889) | 1.05 (1.01, 1.09) | 1.00 (0.99, 1.01) | 0.97 (0.93, 1.01) |
| 28 | 1156 (1150, 1163) | 1206 (1172, 1242) | 1155 (1142, 1168) | 1147 (1111, 1184) | 1.06 (1.03, 1.10) | 1.00 (0.99, 1.01) | 0.98 (0.94, 1.02) |
| 30 | 1492 (1484, 1501) | 1573 (1530, 1618) | 1490 (1473, 1507) | 1515 (1466, 1565) | 1.08 (1.04, 1.11) | 1.00 (0.99, 1.01) | 1.00 (0.96, 1.05) |
| 32 | 1883 (1871, 1894) | 1998 (1941, 2057) | 1880 (1857, 1903) | 1943 (1875, 2014) | 1.08 (1.04, 1.12) | 1.00 (0.99, 1.01) | 1.02 (0.97, 1.07) |
| 34 | 2307 (2292, 2322) | 2453 (2382, 2526) | 2305 (2276, 2335) | 2363 (2274, 2454) | 1.08 (1.04, 1.12) | 1.00 (0.99, 1.02) | 1.01 (0.96, 1.06) |
| 36 | 2732 (2713, 2751) | 2899 (2811, 2991) | 2738 (2701, 2776) | 2725 (2612, 2844) | 1.08 (1.04, 1.12) | 1.00 (0.99, 1.02) | 0.99 (0.94, 1.04) |
| 38 | 3144 (3121, 3168) | 3330 (3222, 3441) | 3165 (3118, 3212) | 3155 (3010, 3307) | 1.08 (1.04, 1.12) | 1.01 (0.99, 1.03) | 0.99 (0.94, 1.05) |
| 40 | 3550 (3508, 3593) | 3765 (3555, 3988) | 3583 (3496, 3671) | 3904 (3603, 4231) | 1.08 (1.02, 1.15) | 1.01 (0.98, 1.04) | 1.08 (0.99, 1.18) |
| Global P | <0.001 | 0.73 | <0.001 | ||||
Adjusted for race/ethnicity (white, black, Asian, Hispanic), age, parity, pre-pregnancy BMI (kg/m2) and infant sex (male, female). CI – confidence interval; NICHD - Eunice Kennedy Shriver National Institute of Child Health and Human Development
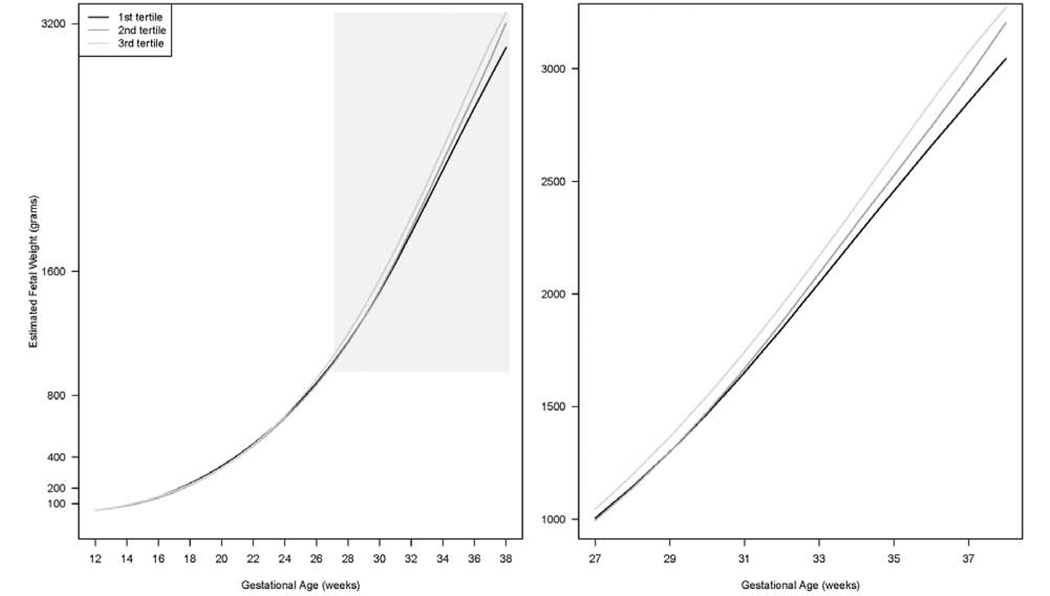
In the sub-study, continuous glucose levels at weeks 10–14 were significantly associated with overall EFW trajectory and increasing tertiles of glucose generally corresponded with increasingly larger EFW in late pregnancy (Figure 2; global Ps < 0.01). The difference emerged at week 23 and became statistically significant starting from week 27. At week 37, EFW was on average 220 g larger (adjusted P < 0.001) in women in the third tertile (> 87 mg/dL; 3,073 g, 95% CI: 2,983, 3,167) than in the first tertile of glucose levels (≤ 80 mg/dL; 2,853 g, 95% CI: 2,755, 2,955).
Figure 2.
Geometric means of estimated fetal weight by gestational age according to tertiles of glucose levels at gestational weeks 10–14 in the weighted subsample of women with GDM (n = 101) and NGT (n = 203) within the NICHD Fetal Growth Studies-Singletons
The shaded areas are enlarged in separate plots. The trajectories were not plotted for weeks 10–11 and 39–40 due to the limited numbers of observations at these gestational weeks.
DISCUSSION
In this study, GDM was associated with a larger EFW which started to emerge at week 20 and became statistically significant at week 28; the association persisted through term. GDM was also associated with transitory alterations in all fetal biometrics (but not EFW) around weeks 10–16. In addition, women’s glucose levels at weeks 10–14 were statistically significantly associated with a larger EFW from week 27 through term.
Although an association between GDM and larger fetal size in late pregnancy has been reported in previous studies, our study has provided the most precise estimate of the timing of fetal growth alteration, on a weekly basis. Previously, Brand et al found a significant association between GDM and larger fetal size starting from week 24 among 10,705 women (832 women with GDM) in the UK, and the association was consistent across European and South Asian women.4 Sovio et al found GDM to be significantly associated with fetal HC > 90th percentile at week 28 among 4,069 majority European women (171 women with GDM) in the UK.3 Macaulay et al found GDM to be significantly associated with larger fetal AC starting at 16–18 weeks among 741 women (83 women with GDM) in South Africa.5 However, fetal growth was estimated in four-week intervals in Brand et al, and measured and compared at two time-points in Sovio et al and every five weeks in Macauly et al. In addition, our study is the first among the U.S. population. Four other studies from Norway, the Netherlands, Greece, and China 6–9 reported no significant association of GDM with late pregnancy fetal growth, despite significant or suggestive associations with higher birth weight or LGA risk. These null findings may be partly due to insufficient longitudinal fetal measures (2–3 measures) 7–9, small sample size (< 40 women with GDM) 9, or imprecise fetal growth models (linear instead of curve-linear) 9.
In our study, an association between GDM and higher EFW started to emerge at 20 weeks and became statistically significant at week 28. Of note, the difference persisted through term despite standard clinical treatment following GDM diagnosis. The increased fetal growth in women with GDM led to > 100 g increase in birthweight and two times the risk of LGA, which was of clinical significance. Further, IGT was also associated with a marginally different EFW trajectory characterized by a larger EFW at weeks 36–40 and an increased birthweight, consistent with the idea that milder degrees of hyperglycemia also contribute to increased fetal growth.1 While a previous study has demonstrated an association between pre-pregnancy obesity and increased fetal size in the 3rd trimester,20 the current study uniquely contributed to the understanding of the association of GDM and early pregnancy glucose levels independent of pre-pregnancy BMI.
The association between GDM and increased fetal size in late pregnancy is biologically plausible. Both fetal hyperinsulinemia triggered by maternal hyperglycemia 21 and higher maternal triglyceride and free fatty acids 22 have been implicated as potential mechanisms. Further, sub-clinical alterations in glucose homeostasis can arise as early as the first trimester10. It has been hypothesized that maternal hyperinsulinemia in early pregnancy may contribute to fetal hyperinsulinemia through increased placental growth and increased glucose transfer23, leading to increased fetal growth in late pregnancy even in the context of normoglycemia 24. These mechanisms may help to explain our finding of a persistently larger fetal size despite standard clinical treatment for GDM. Indeed, our study demonstrated for the first time that irrespective of later GDM diagnosis, increasing tertiles of glucose levels as early as weeks 10–14 corresponded with larger fetal size in late pregnancy.
Our study also observed a statistically significant association between GDM and transitory alterations in early pregnancy fetal growth, characterized by larger size at weeks 10–11 and smaller size at weeks 14–16, although its clinical implications were unclear. Only one previous study 4 covered such an early gestational period, which found a similar transitorily association at weeks 12–16. A smaller fetal size between weeks 7–14 has been commonly identified among women with type 1 diabetes 6,25. Further analyses in our study suggest that this pattern was primarily due to an interaction between GDM and a family history of diabetes. Subclinical metabolic alterations prior to GDM diagnosis has been linked to oxidative and proinflammatory stress 23. In addition, a family history of diabetes has been related to cardiometabolic dysfunctions 26,27 and placenta vascular perfusion abnormalities 28. We speculate that the two factors may synergistically affect early fetal growth. Alternatively, a higher genetic risk of type 2 diabetes in the mothers (indicated by GDM and a family history of diabetes) inherited by the fetus may lead to decreased insulin-mediated intrauterine growth 29. Future studies are needed to validate this finding.
Our study has several unique strengths. First, it provides the most precise estimate of the timing of GDM-related fetal growth alterations so far, enabled by a median of six fetal measures covering every gestational week starting at week 10 and the longitudinal modeling approach. Second, to our knowledge, it is the first study of GDM and fetal growth in the U.S. The multi-racial population contributes to good generalizability to the U.S. population. Third, the study implemented a standardized protocol across the 12 study centers and used experienced and credentialed sonographers, producing high-quality ultrasound measures 14. Lastly, the unique data on glucose levels in early pregnancy in a subsample allowed us to explore the potential effects of glucose on fetal growth before the diagnosis of GDM. Despite these unique strengths, the fetal ultrasound measures were still subjective to measurement errors reasonable for such measures, and we cannot eliminate residual confounding due to the observational nature of the study, although we have controlled for major known confounders.
In conclusion, in this longitudinal study of a multi-racial/ethnic cohort of U.S. women, GDM was associated with a larger EFW which started to emerge at week 20 and became statistically significant at week 28; the association persisted through term despite standard clinical treatment. GDM was also associated with transitory alterations in fetal growth in early pregnancy, due to an interaction with women’s family history of diabetes. Further, higher glucose levels in early pregnancy were significantly related to a larger fetal size in late pregnancy. If confirmed, these findings suggest that it may be helpful to initiate efforts to monitor glycemic status to mitigate accelerated fetal growth before 28 weeks of gestation, when GDM is typically screened for in the U.S.
Supplementary Material
RESEARCH IN CONTEXT.
Evidence before this study
We searched PubMed for human, observational studies published any time on gestational diabetes (GDM) and fetal growth using search terms “gestational diabetes AND (fetal growth OR fetal ultrasound)”. We identified a total of seven studies that examined GDM in relation to at least two ultrasonographic measures of fetal growth during pregnancy. Despite the established association between GDM and large-for-gestational-age birth, four of the seven studies did not find an association between GDM and increased fetal growth. Further, none of the studies identified the precise gestational week when fetal growth begins to deviate in pregnancies complicated by GDM.
Added value of this study
To our knowledge, this is the first study to identify the precise gestational week when fetal growth starts to differ by maternal glycemia status in pregnancy. In a multi-racial population of pregnant women in the U.S., we observed an association between GDM and larger estimated fetal weight (EFW) that started to emerge at gestational week 20 and became significant at week 28; the association persisted through term despite standard clinical treatment. We also observed transitory deviations in EFW between weeks 10–17 related to GDM with a family history of diabetes. The study also provides the first evidence that irrespective of GDM status, higher glucose levels measured as early as weeks 10–14 were related to a larger EFW in the second half of pregnancy.
Implications of all the available evidence
Efforts to mitigate GDM-related fetal overgrowth should be initiated earlier than 28 gestational weeks, when GDM is typically screened for in the U.S.
Acknowledgments:
This research was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development intramural funding and included American Recovery and Reinvestment Act funding via contract numbers HHSN275200800013C, HHSN275200800002I, HHSN27500006, HHSN275200800003IC, HHSN275200800014C, HHSN275200800012C, HHSN275200800028C, HHSN275201000009C, and HHSN275201000001Z.
Funding: National Institutes of Health
Footnotes
Clinicaltrials.gov identifier: NCT00912132
Declaration of Interest: None
DATA SHARING: The data, along with a set of guidelines for researchers applying for the data, will be posted to a data-sharing site, the NICHD/DIPHR Biospecimen Repository Access and Data Sharing [https://brads.nichd.nih.gov, date last accessed: 1/8/2020] (BRADS).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.HAPO Study Cooperative Research Group. Hyperglycemia and adverse pregnancy outcomes. New England Journal of Medicine 2008; 358(19): 1991–2002. [DOI] [PubMed] [Google Scholar]
- 2.McIntyre HD, Catalano P, Zhang C, Desoye G, Mathiesen ER, Damm P. Gestational diabetes mellitus. Nature reviews Disease primers 2019; 5(1): 47. [DOI] [PubMed] [Google Scholar]
- 3.Sovio U, Murphy HR, Smith GC. Accelerated Fetal Growth Prior to Diagnosis of Gestational Diabetes Mellitus: A Prospective Cohort Study of Nulliparous Women. Diabetes care 2016; 39(6): 982–7. [DOI] [PubMed] [Google Scholar]
- 4.Brand JS, West J, Tuffnell D, et al. Gestational diabetes and ultrasound-assessed fetal growth in South Asian and White European women: findings from a prospective pregnancy cohort. BMC medicine 2018; 16(1): 203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Macaulay S, Munthali RJ, Dunger DB, Norris SA. The effects of gestational diabetes mellitus on fetal growth and neonatal birth measures in an African cohort. Diabetic medicine : a journal of the British Diabetic Association 2018; 35(10): 1425–33. [DOI] [PubMed] [Google Scholar]
- 6.Hammoud NM, Visser GH, Peters SA, Graatsma EM, Pistorius L, de Valk HW. Fetal growth profiles of macrosomic and non-macrosomic infants of women with pregestational or gestational diabetes. Ultrasound in obstetrics & gynecology : the official journal of the International Society of Ultrasound in Obstetrics and Gynecology 2013; 41(4): 390–7. [DOI] [PubMed] [Google Scholar]
- 7.Kintiraki E, Goulis DG, Mameletzi S, et al. Large- and small-for-gestational-age neonates born by women with gestational diabetes mellitus diagnosed by the new IADPSG criteria: a case-control study of 289 patients and 1 108 controls. Experimental and clinical endocrinology & diabetes : official journal, German Society of Endocrinology [and] German Diabetes Association 2013; 121(5): 262–5. [DOI] [PubMed] [Google Scholar]
- 8.Li J, Chen YP, Dong YP, et al. The impact of umbilical blood flow regulation on fetal development differs in diabetic and non-diabetic pregnancy. Kidney & blood pressure research 2014; 39(4): 369–77. [DOI] [PubMed] [Google Scholar]
- 9.Sletner L, Jenum AK, Yajnik CS, et al. Fetal growth trajectories in pregnancies of European and South Asian mothers with and without gestational diabetes, a population-based cohort study. PloS one 2017; 12(3): e0172946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Catalano PM. Trying to understand gestational diabetes. Diabetic medicine : a journal of the British Diabetic Association 2014; 31(3): 273–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grewal J, Grantz KL, Zhang C, et al. Cohort Profile: NICHD Fetal Growth Studies–Singletons and Twins. International Journal of Epidemiology 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.American Diabetes Association. 2. Classification and diagnosis of diabetes. Diabetes care 2017; 40(Supplement 1): S11–S24. [DOI] [PubMed] [Google Scholar]
- 13.Zhu Y, Mendola P, Albert PS, et al. Insulin-like growth factor axis and gestational diabetes mellitus: a longitudinal study in a multiracial cohort. Diabetes 2016; 65(11): 3495–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hediger ML, Fuchs KM, Grantz KL, et al. Ultrasound Quality Assurance for Singletons in the National Institute of Child Health and Human Development Fetal Growth Studies. Journal of ultrasound in medicine : official journal of the American Institute of Ultrasound in Medicine 2016; 35(8): 1725–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hadlock FP, Harrist RB, Sharman RS, Deter RL, Park SK. Estimation of fetal weight with the use of head, body, and femur measurements--a prospective study. American journal of obstetrics and gynecology 1985; 151(3): 333–7. [DOI] [PubMed] [Google Scholar]
- 16.Rimm EB, Stampfer MJ, Colditz GA, Chute CG, Litin LB, Willett WC. Validity of self-reported waist and hip circumferences in men and women. Epidemiology 1990; 1(6): 466–73. [DOI] [PubMed] [Google Scholar]
- 17.Duryea EL, Hawkins JS, McIntire DD, Casey BM, Leveno KJ. A revised birth weight reference for the United States. Obstetrics & Gynecology 2014; 124(1): 16–22. [DOI] [PubMed] [Google Scholar]
- 18.Bates D, Mächler M, Bolker B, Walker S. Fitting Linear Mixed-Effects Models Using lme4. Journal of Statistical Software 2015; 67(1): 1–48. [Google Scholar]
- 19.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes care 2012; 35 Suppl 1: S64–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang C, Hediger ML, Albert PS, et al. Association of Maternal Obesity With Longitudinal Ultrasonographic Measures of Fetal Growth: Findings From the NICHD Fetal Growth Studies-Singletons. JAMA pediatrics 2018; 172(1): 24–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kc K, Shakya S, Zhang H. Gestational diabetes mellitus and macrosomia: a literature review. Annals of nutrition & metabolism 2015; 66 Suppl 2: 14–20. [DOI] [PubMed] [Google Scholar]
- 22.Schaefer-Graf UM, Graf K, Kulbacka I, et al. Maternal lipids as strong determinants of fetal environment and growth in pregnancies with gestational diabetes mellitus. Diabetes care 2008; 31(9): 1858–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Desoye G. The Human Placenta in Diabetes and Obesity: Friend or Foe? The 2017 Norbert Freinkel Award Lecture. Diabetes care 2018; 41(7): 1362–9. [DOI] [PubMed] [Google Scholar]
- 24.Desoye G, Nolan CJ. The fetal glucose steal: an underappreciated phenomenon in diabetic pregnancy. Diabetologia 2016; 59(6): 1089–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pedersen JF, Molsted-Pedersen L, Mortensen HB. Fetal growth delay and maternal hemoglobin A1c in early diabetic pregnancy. Obstetrics and gynecology 1984; 64(3): 351–2. [PubMed] [Google Scholar]
- 26.Goldfine AB, Beckman JA, Betensky RA, et al. Family history of diabetes is a major determinant of endothelial function. Journal of the American College of Cardiology 2006; 47(12): 2456–61. [DOI] [PubMed] [Google Scholar]
- 27.Axelsen M, Smith U, Eriksson JW, Taskinen MR, Jansson PA. Postprandial hypertriglyceridemia and insulin resistance in normoglycemic first-degree relatives of patients with type 2 diabetes. Annals of internal medicine 1999; 131(1): 27–31. [DOI] [PubMed] [Google Scholar]
- 28.Shargorodsky M, Kovo M, Schraiber L, Bar J. Does a First-Degree Family History of Diabetes Impact Placental Maternal and Fetal Vascular Circulation and Inflammatory Response? The Journal of clinical endocrinology and metabolism 2017; 102(9): 3375–80. [DOI] [PubMed] [Google Scholar]
- 29.Horikoshi M, Beaumont RN, Day FR, et al. Genome-wide associations for birth weight and correlations with adult disease. Nature 2016; 538(7624): 248–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.