Abstract
Background
Several biomarkers of gemcitabine effectiveness have been studied in cancers, but less so in hepatocellular carcinoma (HCC), which is identified as the fifth most common cancer worldwide. Investigation of human equilibrative nucleoside transporter‐1 (HENT‐1) and deoxycytidine kinase (DCK), genes involved in gemcitabine uptake and metabolism, can be beneficial in the selection of potential cancer patients who could be responding to the treatment.
Aim
To study HENT‐1 and DCK gene expression in HCC patients with different protocols of treatment.
Methods
Using real‐time PCR, we analyzed expression levels of HENT‐1 and DCK genes from peripheral blood samples of 109 patients (20 controls & 89 HCC patients) between March 2015 and March 2017. All the 89 HCC patients received the antioxidants selenium (Se) and vitamin E (Vit.E) either alone (45 patients) or in combination with gemcitabine (24 patients) or radiofrequency ablation (RFA) (20 patients).
Results
There was a significant increase in HENT‐1 expression levels in HCC patients treated with Se and Vit.E alone as compared to controls (P ˂ .0001), while there was no significant difference between HCC patients treated with gemcitabine or RFA as compared to controls. In contrast, expression of DCK was significantly increased in all groups of HCC patients as compared to controls (P ˂ .0001).
Conclusions
HENT‐1 and DCK mRNA expressions are important markers of HCC and for GEM effect and GEM sensitivity in patients with HCC. This could be beneficial in the selection of HCC patients sensitive to gemcitabine to avoid subjecting resistant patients to unnecessary chemotherapy.
Keywords: deoxycytidine kinase, gemcitabine, hepatocellular carcinoma, human equilibrative nucleoside transporter‐1
HENT‐1 and DCK expression in HCC groups and healthy control. HENT‐1 and DCK mRNA expressions are important markers of HCC and for GEM effect and GEM sensitivity in patients with HCC.

1. INTRODUCTION
HCC is the fifth most common cancer worldwide and the second most common cause of cancer mortality. 1 HCC is unique in that it largely occurs within an established background of chronic liver disease and cirrhosis. 2 Major causes of cirrhosis in patients with HCC include hepatitis B, hepatitis C, alcoholic liver disease, and possibly nonalcoholic steatohepatitis. 3
Because of the aggressive nature of HCC, which results in an overall poor prognosis and short life expectancy, the appropriate choice of effective treatment options is crucial for positive therapeutic outcome. Although there are multiple treatment options are available for HCC either pharmacological or non‐pharmacological, 4 , 5 , 6 , 7 , 8 , 9 the average life expectancy after diagnosis of clinically apparent HCC is less than 12 months. 10 Therefore, ineffective treatment outcomes, such as those caused by resistance to chemotherapy, directly affect patients’ survival.
Among cancer chemotherapeutic treatments, gemcitabine is widely used as an anti‐neoplastic medication that exerts its cytotoxic effect intracellular with documented broad‐spectrum antitumor activity in almost all major localizations of cancer. 11 Specifically, gemcitabine has been a standard chemotherapy drug for inoperable pancreatic cancer. 12 Because liver is tissue homologous to the gallbladder and pancreas, systemic chemotherapy in advanced or inoperable HCC has drawn lessons from the chemotherapy of other digestive tract cancers. Currently, gemcitabine‐based chemotherapy regimens are used in advanced HCC. 13
If gemcitabine could be appropriately and selectively administered to patients with gemcitabine sensitivity based on the expression of genes in the tumor, maximal chemotherapy efficacy could be achieved without subjecting gemcitabine‐resistant patients to unnecessary side effects. 14 Recent investigations using cell lines or surgical specimens have revealed that the expression of several genes may be predictors of gemcitabine efficacy in gemcitabine‐treated patients.
Such gemcitabine efficacy predictor genes include HENT‐1, the major mediator of gemcitabine uptake in human cells, 15 and DCK, gemcitabine metabolism‐related enzyme. 16 Indeed, 17 and 18 demonstrated that levels of expression of these genes correlated with gemcitabine sensitivity in patients with pancreatic ductal carcinoma.
In order to reach their intracellular targets, many anticancer nucleosides need a specific nucleoside transport (NT) proteins in the plasma membranes. 19 The hENT proteins have around 450 amino acid residues 20 and another eleven predicted transmembrane domains. 21 Most human cells contain the plasma membrane hENT1, and hENT1 abundance may be a factor in the response of nucleoside drugs in some cancers. 22
In the study by Kulsoom et al authors detected that bone marrow and peripheral blood hENT1 expression interrelated with each other and with dCK, CDA, dCMPD, and Topo‐IIa positively in respective bone marrow and peripheral blood samples. These observations might indicate a coordinated regulation of expression of these genes suggesting differential transcriptomic regulation. 23
The current study aimed to determine gemcitabine sensitivity in HCC patients treated with gemcitabine. Following previous successful studies, we targeted expression levels of genes responsible for the uptake HENT‐1 and metabolism DCK of gemcitabine. By examining gene expression levels in peripheral blood samples from HCC patients, an easy non‐invasive method can be used to predict gemcitabine treatment efficacy. No single gene expression level can be used as a predictive marker for gemcitabine sensitivity; however, low levels of HENT‐1 expression could be considered as a negative prognostic factor in HCC patients in Egypt. Also, high levels of DCK expression can be assumed as a good predictive marker for gemcitabine sensitivity.
2. PATIENTS AND METHODS
2.1. Patients
The study is an analytical cross‐sectional study that included 109 consecutive patients consisting of 20 healthy control subjects and 89 patients in whom hepatocellular carcinoma had been identified by liver ultrasonography and/or biopsy and/or computed tomography. The 89 patients were further divided into three groups. The second patient group (HCC control) involved 45 HCC patients who received Se and Vit. E as antioxidants at a dose of 50 and 15 mg/d, respectively, for 4 weeks, while the third patient group (HCC + GEM) consisted of 24 HCC patients who were treated with a combination of gemcitabine (Gemzar, GEM), Se and Vit. E. The dose of gemcitabine was 1000 mg/m2 intravenous infusion for 1 week followed by 3 weeks rest. The remaining patient group (HCC + RFA) was consisted of 20 HCC patients who were treated with Se and Vit.E following radiofrequency ablation (RFA) which was performed percutaneously under ultrasound guidance. Our study was implemented according to the 1976 Declaration of Helsinki and its later amendments and approved by our local Ethic Committee. All patients gave their informed consent to the study.
Both healthy control individuals and patients attended Suez Canal University hospital and Communicable Diseases Research and Training Center (CDRTC) in Ismailia city, Egypt, between March 2015 and March 2017. Subjects were excluded if they had a concomitant extra‐hepatic tumor, HIV, bronchial asthma, diabetes, or received recent HCC treatment with regimens other than gemcitabine (<6 months) (EASL–EORTC Clinical Practice Guidelines: Management of hepatocellular carcinoma, 2012) (Table 1).
Table 1.
Groups’ names and status
| Group name | No. of patients | Status |
|---|---|---|
| Healthy control | 20 | Healthy individuals |
| HCC control | 45 | HCC patients with Se and Vit.E |
| HCC + GEM | 24 | HCC patients with Se and Vit.E + GEM |
| HCC + RFA | 20 | HCC patients with Se and Vit.E + RFA |
2.2. Sample collection
From each patient in this study, a sample of 1 mL of peripheral whole blood was collected and stored in a coded sterile EDTA tube. Freshly collected samples were immediately processed for mRNA extraction.
2.3. Target mRNA
Expressions of HENT‐1 and DCK were examined as genetic predictive markers associated with GEM transport and metabolism, respectively. Specific primers were designed using OLIGO 6.22 software (Molecular Biology Insights, Cascade, CO) (Stam, den Boer et al 2003). The following primers were used for real‐time PCR: HENT‐1– forward primer, 5'‐TGT TTC CAG CCG TGA CT‐3' reverse primer, 5'‐CAG GCC ACA TGA ATA CAG‐3'probe, 5'‐(FAM)‐CAG CAC CTG GGA ACG TTA CTT‐(TAMRA)‐3', DCK – forward primer, 5'‐TGC AGG GAA GTC AAC ATT‐3' reverse primer, 5'‐TCC CAC CAT TTT TCT GAG‐3'probe, 5'‐(FAM)‐TAA ACA ATT GTG TGA AGA TTG GGA AG‐(TAMRA)‐3'.
2.4. mRNA extraction
Extraction of mRNA was performed within few hours of sample collection. Total mRNA was purified from EDTA blood with a fast spin‐column procedure (QIAGEN, Hilden, Germany). Red blood cells were selectively lysed and white cells collected by centrifugation and lysed using highly denaturing conditions. After homogenization using the QIAshredder spin column, the samples were applied to the QIAamp spin column and total RNA bonded to the QIAamp membrane and contaminants were washed away, leaving pure RNA to be eluted in 30‐100 µL RNase‐free water.
Total mRNA concentration was determined by spectrophotometer, and 1 μg total RNA was reverse transcribed using a Turbo‐I First‐Strand cDNA Synthesis Kit (Biomatik Corporation, Canada). Quantification of the target cDNA and an internal reference gene was conducted by quantitative real‐time reverse transcription–polymerase chain reaction (qRT–PCR).
2.5. qRT–PCR
Quantitative real‐time PCR was performed using the Light Cycler Fast start DNA Master hybridization probes (Roche Diagnostics GmbH), according to the manufacturer's instructions. Each reaction was carried out in a total volume of 20 μL in glass capillaries, containing 4 μL of cDNA sample, 25 mmol/L MgCl2, 10% LightCycler Faststart DNA Master hybridization probes buffer (Taq DNA polymerase, reaction buffer, deoxynucleotide triphosphate mix and LightCycler Fast start enzyme), 10 µmol/L each primer, and for the probe reactions, 4 pmol (Flu) and 8 pmol (LC).
The cDNA sample was denatured at 95ºC for 10 minutes and then added to the capillaries. After this, one cycle of melting curve from 70 to 95ºC by a transition rate of 0.2ºC/s with continuous detection of fluorescence was performed. The temperature transition rate for all amplifications was 20 ºC/s. Negative controls were concomitantly run to confirm that the samples were not cross contaminated. Analysis was carried out with the step one machine (Applied Biosystems).
2.6. Statistical analysis
Quantitative data will be expressed in the form of mean ± standard deviation (SD). For differences between more than 2 groups, one‐way analysis of variance (ANOVA) was used for parametric variables and Kruskal‐Wallis test was used for non‐parametric variables to find whether there was a statistically significant difference in between the groups. Qualitative data will be demonstrated through figures of frequency and percentage. Between groups of qualitative data, chi‐squared test (χ 2) or Fisher's exact test was used. Data were analyzed using SPSS/PC + 11.5 (SPSS, Inc, Chicago, IL). Statistical significance was set at P < .05. Demographic and clinical information was obtained from medical records. Charts of different types will be used to illustrate data and relations where appropriate. To identify relations between different variables, Spearman (ρ) correlation was used. Scatter plot graph was used to illustrate relations between several variables, and to produce a line of best fit and R 2 value of regression.
3. RESULTS
3.1. Patient characteristics
Gender distribution of the study population (89 HCC patients and 20 healthy controls) is summarized in (Figure 1) that shows that there was a narrow range of discrepancy in gender distribution and ratio across the study groups.
Figure 1.
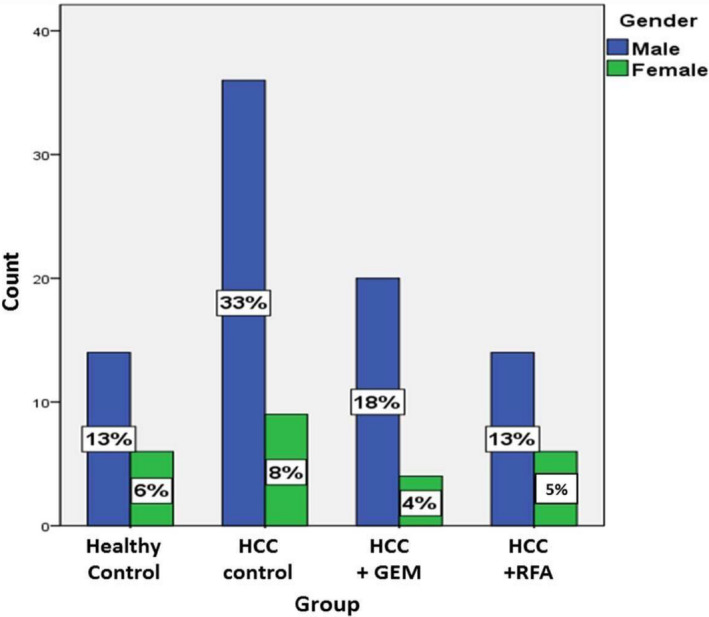
Gender presentation in study groups
Totally among the 89 HCC patients, 70 patients were males (78.65%) and 19 were females (21.35%). There was no significant difference between the groups regarding gender.
Regarding age, there was no significant difference between the different studied groups regarding the age.
Concerning etiology, among the 89 HCC patients, 85 (95.5%) patients were HCV‐related HCC and only 4 (4.5%) were HBV‐related HCC indicating that most HCC cases were mainly due to HCV in Suez Canal area, Egypt. There was no significant difference among different HCC patients’ groups (P > .05). However, the prevalence of HBV‐ and HCV‐related HCC among HCC patients in the different studied groups was different (Figure 2).
Figure 2.
Difference in AFP level in study groups (AFP < 400 vs. AFP ≥ 400)
The expression of HENT‐1 was significantly increased in the HCC control group relative to the other HCC groups and healthy controls (P ˂ .0001). Interestingly, HENT‐1 expression was significantly lower in either HCC + GEM or HCC + RFA group (P < .0001) as compared to HCC control group. In addition, when compared to healthy control group, HENT‐1 expression was not significantly increased in either HCC + GEM or HCC + RFA group (P = .161& P = 1.000, respectively) (Figure 3).
Figure 3.
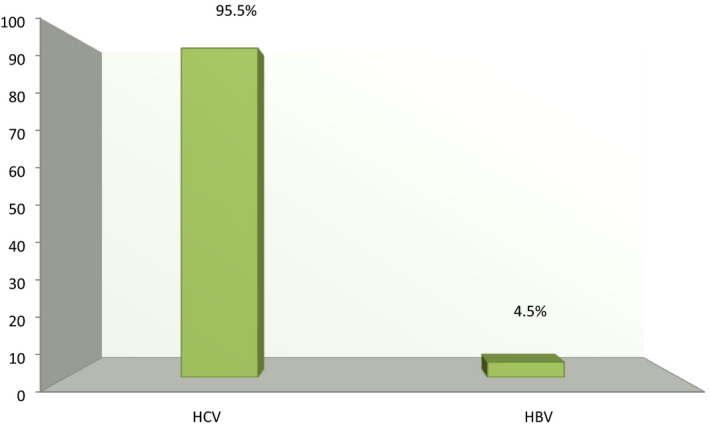
The prevalence of HBV‐ and HCV‐related HCC among HCC patients in the study population
Regarding the expression of DCK, it was found to be significantly increased in HCC control group compared to healthy controls (P ˂ .0001). Similarly, DCK expression was also significantly increased in either HCC + GEM group or HCC + RFA group when compared to the healthy controls (P ˂ .0001). On the other hand, there was no significant difference regarding DCK expression among all HCC groups (P > .05) (Figure 4).
Figure 4.
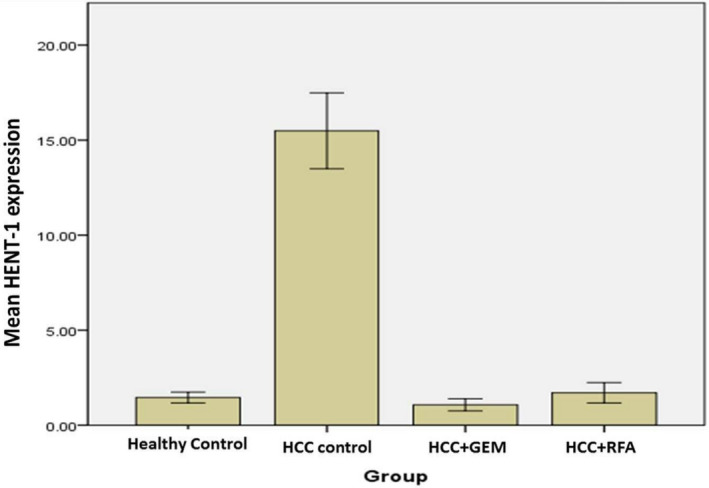
HENT‐1 expression in HCC patient groups and healthy control
4. DISCUSSION
Worldwide, several biomarkers of gemcitabine sensitivity and effectiveness have been widely studied in multiple types of cancer 24 , 25 , 26 but less so in hepatocellular carcinoma. However, no molecular biomarkers have been studied in Egypt to predict gemcitabine chemotherapy effectiveness, and patients are empirically treated until patients improve or deteriorate. Therefore, it is tremendously essential to determine predictive molecular biomarkers of gemcitabine resistance and sensitivity for more effective treatment of these cancer patients (Figure 5).
Figure 5.
DCK expression in HCC groups and healthy control
In the present study, we investigated the effect of GEM, RFA, Se, and Vit.E on the expression levels of HENT‐1 and DCK in the peripheral blood from different HCC patient groups in Suez Canal University Hospital, and its correlation with clinical characteristics of HCC patients. We found that HENT‐1 expression significantly increased in HCC control group (P ˂ .0001) when compared to the healthy controls and the other treated groups. On the other hand, HENT‐1 expression significantly decreased in HCC patients treated with Se and Vit.E in combination with either GEM or RFA when compared to the HCC control group (P < .0001). It is worth mentioning that the expression levels of HENT‐1 in HCC treated groups (GEM or RFA) were comparable to normal control group levels.
Cellular resistance to gemcitabine can be intrinsic or acquired during gemcitabine treatment. 27 Current data came in line with Jiang et al who have reported a high expression of HENT‐1 in human hepatocellular carcinoma cell membranes. They explained that the expression of HENT‐1 in human hepatocellular carcinoma cells could have a potential clinical value for clinical chemotherapy strategies. 28
The HENT‐1 protein has been previously estimated as a prognostic biomarker in gemcitabine‐treated pancreatic cancer patients as shown by earlier studies 14 , 15 , 29 Elsaleh et al 2009. They reported that higher HENT‐1 protein expression levels were related to the increased overall survival and disease‐free survival in pancreatic cancer patients under gemcitabine treatment. Also, it was reported by Candelaria 24 that downregulation of genes coding for nucleoside transporters and drug metabolism responsible for uptake and metabolic activation of gemcitabine is related to acquired tumor resistance against this agent.
Indeed, the present study revealed that HENT‐1 expression decreased after gemcitabine treatment and RFA suggesting decreased drug uptake and bad prognosis. In addition, the RFA‐induced reduction in HENT‐1 expression currently observed in HCC patients could make them unresponsive to subsequent GEM treatment post exposure to RFA.
Regarding DCK protein expression, 30 reported a positive correlation between DCK expression and overall survival, disease‐free survival, and reduced mortality in patients with pancreatic ductal adenocarcinoma. 30 Moreover, it was reported by Kerr 31 that decreased DCK expression levels caused gemcitabine resistance in bladder cancer cell lines. 31 In addition, 32 showed that DCK protein expression, using 5 heterotopic transplantation tumor models including several types of malignancies, was closely associated with gemcitabine chemosensitivity. They also proposed that transcriptional regulation in DCK gene and protein expression can be an applicable prognostic marker of gemcitabine response 32 .
HENT‐1 expression is regarded as a possible predictive biomarker for gemcitabine response in some cancers. Gemcitabine is a key chemotherapeutic drug used for the treatment biliary tract cancer (BTC). 33 showed that increased HENT‐1 expression is associated with a stronger toxic effect of gemcitabine on BTC cell lines. The study suggested that increased intratumoral HENT‐1 is a potential biomarker predicting better therapeutic effects of gemcitabine on advanced BTC patients. 33 , 34 proved that downregulating the transcription of hENT1 in pancreatic adenocarcinoma was found to promote gemcitabine resistance. 34
High tumoral hENT1 expression on IHC with 10D7G2 is a strong and reproducible prognostic marker for improved outcome among gemcitabine‐treated patients with PBC.
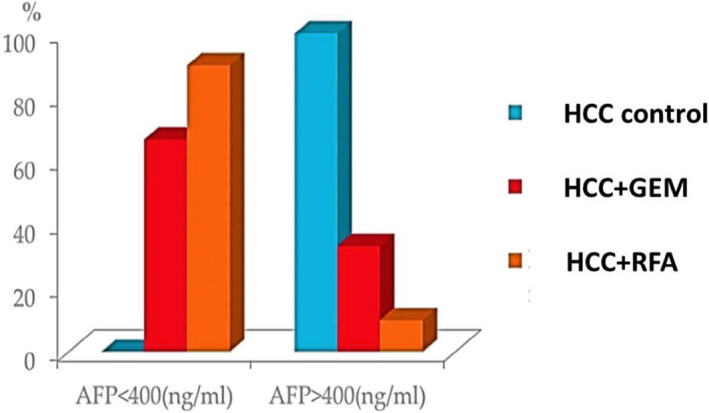
Moreover, it was found that 100% of HCC control patients have alpha fetoprotein (AFP) levels greater than 400 ng/mL, while only 33.3% and 10% of HCC patients treated with either GEM or RFA produced AFP greater than or equal to 400 ng/mL (P ˂ .001), respectively. This comes in agreement with Patrikidou et al who reported that, in advanced hepatocellular carcinoma (HCC) patients treated with GEM and oxaliplatine, a decrease in AFP level of ≥25% was observed in 25% of patients, and a decrease of ≥50% was detected in 20% of patients. 35 The decrease in AFP level is also indicative of antitumor efficacy, as it is considered one of the most important biomarkers correlated with better overall survival and progression‐free survival. Patrikidou and his colleagues suggested that this chemotherapy protocol had stabilized the disease, regardless of the fact it was for a short period. 35 From the previous results, we observed that serum levels of the tumor marker AFP were significantly decreased in HCC patients treated with either GEM or RFA compared to HCC controls, suggesting a better prognosis mediated by GEM and RFA treatment.
5. CONCLUSION
In the current study, we found that DCK expression levels were significantly increased in the HCC control group when compared to healthy controls (P ˂ .0001). Paradoxically, either GEM or RFA has maintained the high level of DCK expression detected in HCC control group, suggesting an improved gemcitabine sensitivity and good prognosis. This can be theoretically true, but as DCK is dependent in its activity on HENT‐1, increased DCK expression could be of no value in case of decreased HENT‐1 expression.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
AUTHORS' CONTRIBUTIONS
Fadia M Attia participated in study design, the sequence alignment and drafting of article, and carried out the laboratory work, carried out the design of the study, and participated by acquisition of data performed the statistical analysis. Sara Fathy carried out the analysis and interpretation of data and helped to draft the article. Mona Elazab carried out the analysis and interpretation of data and helped to draft the article. Adel Hassan carried out the study conception and design, coordination, and drafted the article. Fawzy Attia carried out the study conception and design, coordination, and drafted the article. Gehan Ibrahium participated in study design and coordination and helped to draft the article. Maha Anani participated in study design, the sequence alignment and drafting of article and carried out the laboratory work, carried out the design of the study, and participated by acquisition of data performed the statistical analysis.
ETHICAL APPROVAL
The ethics of this article was approved by the Suez Canal University Ethical Committee.
CONSENT FOR PUBLICATION
We transfer copyright of our article to your journal.
ACKNOWLEDGMENTS
We gratefully acknowledge our anonymous referee for their assistance/comments/support.
Attia F, Fathy S, Anani M, et al. Human equilibrative nucleoside transporter‐1 and deoxycytidine kinase can predict gemcitabine effectiveness in Egyptian patients with Hepatocellular carcinoma. J Clin Lab Anal. 2020;34:e23457 10.1002/jcla.23457
Funding information
The authors declare that they did not receive any fund from any authority.
DATA AVAILABILITY STATEMENT
The data can be publically available at the Faculty of Medicine, Suez Canal University.
REFERENCES
- 1. Parkin DM. Global cancer statistics in the year 2000. Lancet Oncol. 2001;2(9):533‐543. [DOI] [PubMed] [Google Scholar]
- 2. Omata M, Lesmana LA, Tateishi R, et al. Asian Pacific Association for the Study of the Liver consensus recommendations on hepatocellular carcinoma. Hepatol Int. 2010;4(2):439‐474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fattovich G, Giustina G, Schalm SW, et al. Occurrence of hepatocellular carcinoma and decompensation in western European patients with cirrhosis type B. The EUROHEP Study Group on Hepatitis B Virus and Cirrhosis. Hepatology. 1995;21(1):77‐82. [DOI] [PubMed] [Google Scholar]
- 4. Chiang DY, Villanueva A, Hoshida Y, et al. Focal gains of VEGFA and molecular classification of hepatocellular carcinoma. Cancer Res. 2008;68(16):6779‐6788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Forner A, Llovet JM, Bruix J, et al. Chemoembolization for intermediate HCC: is there proof of survival benefit? J Hepatol. 2012;56(4):984‐986. [DOI] [PubMed] [Google Scholar]
- 6. Mazzaferro V, Regalia E, Doci R, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334(11):693‐699. [DOI] [PubMed] [Google Scholar]
- 7. Sangro B, Iñarrairaegui M, Bilbao JI. Radioembolization for hepatocellulr carcinoma. J Hepatol. 2012;56(2):464‐473. [DOI] [PubMed] [Google Scholar]
- 8. Shah RP, Brown KT, Sofocleous CT. Arterially directed therapies for hepatocellular carcinoma. AJR Am J Roentgenol. 2011;197(4):W590‐602. [DOI] [PubMed] [Google Scholar]
- 9. Wilhelm S, Carter C, Lynch M, et al. Discovery and development of sorafenib: a multikinase inhibitor for treating cancer. Nat Rev Drug Discov. 2006;5(10):835‐844. [DOI] [PubMed] [Google Scholar]
- 10. Seeff LB. Introduction: The burden of hepatocellular carcinoma. Gastroenterology. 2004;127(5 Suppl 1):S1‐4. [DOI] [PubMed] [Google Scholar]
- 11. Valle J, Wasan H, Palmer DH, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010;362(14):1273‐1281. [DOI] [PubMed] [Google Scholar]
- 12. Bria E, Milella M, Gelibter A, et al. Gemcitabine‐based combinations for inoperable pancreatic cancer: have we made real progress? A meta‐analysis of 20 phase 3 trials. Cancer. 2007;110(3):525‐533. [DOI] [PubMed] [Google Scholar]
- 13. Ge S, Huang D. Systemic therapies for hepatocellular carcinoma. Drug Discov Ther. 2015;9(5):352‐362. [DOI] [PubMed] [Google Scholar]
- 14. Eto K, Kawakami H, Kuwatani M, et al. Human equilibrative nucleoside transporter 1 and Notch3 can predict gemcitabine effects in patients with unresectable pancreatic cancer. Br J Cancer. 2013;108(7):1488‐1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Farrell JJ, Elsaleh H, Garcia M, et al. Human equilibrative nucleoside transporter 1 levels predict response to gemcitabine in patients with pancreatic cancer. Gastroenterology. 2009;136(1):187‐195. [DOI] [PubMed] [Google Scholar]
- 16. Maréchal R, Mackey JR, Lai R, et al. Deoxycitidine kinase is associated with prolonged survival after adjuvant gemcitabine for resected pancreatic adenocarcinoma. Cancer. 2010;116(22):5200‐5206. [DOI] [PubMed] [Google Scholar]
- 17. Ashida R, Nakata B, Shigekawa M, et al. Gemcitabine sensitivity‐related mRNA expression in endoscopic ultrasound‐guided fine‐needle aspiration biopsy of unresectable pancreatic cancer. J Exp Clin Cancer Res. 2009;28:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Itoi T, Sofuni A, Fukushima N, et al. Ribonucleotide reductase subunit M2 mRNA expression in pretreatment biopsies obtained from unresectable pancreatic carcinomas. J Gastroenterol. 2007;42(5):389‐394. [DOI] [PubMed] [Google Scholar]
- 19. Baldwin SA, Mackey JR, Cass CE, Young JD. Nucleoside transporters: molecular biology and implications for therapeutic development. Molecular Med Today. 1999;5(5):216‐224. [DOI] [PubMed] [Google Scholar]
- 20. Rlteel MW, Yaof SY, Ng AM, Mackeyt JR, Cass CE, Young JD. Molecular cloning, functional expression and chromosomal localization of a cDNA encoding a human Na+/nucleoside cotransporter (hCNT2) selective for purine nucleosides and uridine. Mol Membr Biol. 1998;15(4):203‐211. [DOI] [PubMed] [Google Scholar]
- 21. Mackey JR, Yao SY, Smith KM, et al. Gemcitabine transport in xenopus oocytes expressing recombinant plasma membrane mammalian nucleoside transporters. J Nat Cancer Institute, [online]. 1999;91(21):1876‐1881. [DOI] [PubMed] [Google Scholar]
- 22. Zhang J, Visser F, King KM, Baldwin SA, Young JD, Cass CE. The role of nucleoside transporters in cancer chemotherapy with nucleoside drugs. Cancer Metastasis Rev. 2007;26(1):85‐110. [DOI] [PubMed] [Google Scholar]
- 23. Kulsoom B, Shamsi TS, Afsar NA. (2018). Gene expression of hENT1, dCK, CDA, dCMPD and topoisomerase IIα as an indicator of chemotherapy response in AML treated with cytarabine and daunorubicin. [online] Cancer Management and Research. [DOI] [PMC free article] [PubMed]
- 24. Candelaria M, de la Cruz‐Hernandez E, Taja‐Chayeb L. DNA Methylation‐Independent Reversion of Gemcitabine Resistance by Hydralazine in Cervical Cancer Cells. PLoS One. 2012;7(3):e29181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Garcia‐Manteiga J, Molina‐Arcas M, Casado FJ, Mazo A, Pastor‐Anglada M. Nucleoside transporter profiles in human pancreatic cancer cells: role of hCNT1 in 2',2'‐difluorodeoxycytidine‐ induced cytotoxicity. Clin Cancer Res. 2003;9(13):5000‐5008. [PubMed] [Google Scholar]
- 26. Nakano Y, Tanno S, Koizumi K, et al. Gemcitabine chemoresistance and molecular markers associated with gemcitabine transport and metabolism in human pancreatic cancer cells. Br J Cancer. 2007;96(3):457‐463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Andersson R, Aho U, Nilsson BI, et al. Gemcitabine chemoresistance in pancreatic cancer: molecular mechanisms and potential solutions. Scand J Gastroenterol. 2009;44(7):782‐786. [DOI] [PubMed] [Google Scholar]
- 28. Jiang X‐X, Fu H‐Q, Chao Y‐H, Liu Z‐Y. Expression characteristics of ENT1 in human hepatocellular carcinoma cells. World Chinese J Digestol. 2011;19(20):2104. [Google Scholar]
- 29. Spratlin J. The absence of human equilibrative nucleoside transporter 1 is associated with reduced survival in patients with gemcitabine‐treated pancreas adenocarcinoma. Clin Cancer Res. 2004;10(20):6956‐6961. [DOI] [PubMed] [Google Scholar]
- 30. Xiong J, Altaf K, Ke N, et al. dCK Expression and Gene Polymorphism With Gemcitabine Chemosensitivity in Patients With Pancreatic Ductal Adenocarcinoma: A Strobe‐Compliant Observational Study. Medicine (Baltimore). 2016;95(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kerr M. (2014). "Deoxycytidine kinase expression underpins response to gemcitabine in bladder cancer". Clin cancer Res. 20(21): 5435‐5445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kroep JR, Loves WJP, van der Wilt CL, et al. Pretreatment deoxycytidine kinase levels predict in vivo gemcitabine sensitivity. Mol Cancer Ther. 2002;1(6):371‐376. [PubMed] [Google Scholar]
- 33. Kim J, Kim H, Lee J‐C, et al. Human equilibrative nucleoside transporter 1 (hENT1) expression as a predictive biomarker for gemcitabine chemotherapy in biliary tract cancer. PLoS One. 2018;13(12):e0209104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sun Q, Wenyan XU, Ji S, et al. Role of hepatocyte nuclear factor 4 alpha in cell proliferation and gemcitabine resistance in pancreatic adenocarcinoma. Cancer Cell Int. 2019; 19:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Patrikidou A, Sinapi I, Regnault H, et al. Gemcitabine and oxaliplatin chemotherapy for advanced hepatocellular carcinoma after failure of anti‐angiogenic therapies. Invest New Drugs. 2014;32(5):1028‐1035. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data can be publically available at the Faculty of Medicine, Suez Canal University.


