Abstract
Objectives:
Evidence-based clinical practice guidelines (CPGs) for the treatment of pneumonia and sepsis have existed for many years with multiple studies suggesting improved patient outcomes. Despite their importance, little is known about variation in emergency department (ED) adherence to these CPGs. Our objectives were to estimate variation in ED adherence across CPGs for pneumonia and sepsis and identify patient, provider, and environmental factors associated with adherence.
Methods:
This was a multicenter retrospective study using standard medical record review methods. The population consisted of consecutive adults hospitalized for pneumonia or sepsis (identified by discharge ICD-9 codes) at five Colorado hospitals (two academic, three community) who were admitted to the hospital from the ED and for whom the ED diagnosed or initiated treatment. The outcome measured was ED adherence to the CPG (primary) and in-hospital mortality (secondary). Hierarchical generalized linear models were used for analysis.
Results:
Among 827 patients, ED care was 57% adherence to CPGs with significant variation in adherence across CPGs (sepsis 50%, pneumonia 64%, p < 0.001). Patients were less likely to receive adherent care if they presented with chief complaints that were associated but not typical of the diagnosis (odds ratio [OR] = 0.6, 95% confidence interval [CI] = 0.4–0.8), received an ED diagnosis that was not specific to the CPG (associated diagnosis OR = 0.3 [95% CI = 0.2–0.5]; unrelated diagnosis OR = 0.4 [95% CI = 0.2–0.6]) or presented to a community hospital (OR = 0.6,95% CI = 0.4–0.9). ED CPG nonadherence was associated with higher in-hospital mortality (OR = 2.4, 95% CI = 1.2–4.8).
Conclusion:
Adherence to ED infectious CPGs for pneumonia and sepsis varies significantly across diseases and types of institutions with significant room for improvement, especially in light of a significant association with in-hospital mortality.
Pneumonia and sepsis are two of the most common reasons for hospital admission and death in the United States, accounting for 2.4 million hospitalizations, 200,000 in-hospital deaths, and $35.8 billion in aggregate hospital costs annually.1 Emergency departments (EDs) play a vital role in providing evidence-based care for the management of pneumonia and sepsis as the initial evaluation and treatment is most often initiated in the ED, and both conditions have clinical practice guidelines (CPGs) relevant to ED management that have been shown to improve mortality, hospital length of stay (LOS), and costs.2–26
While ED guidelines for the treatment of pneumonia and sepsis have existed for more than a decade, both have undergone recent updates. For pneumonia, the recommendations to obtain blood cultures on all patients and administered antibiotics within 6 hours of ED arrival have been retired from the Center for Medicare and Medicaid Services (CMS) Pneumonia Core Measure. The Infectious Disease Society of America (IDSA) and the American Thoracic Society (ATS), however, continue to recommend that guideline concordant antibiotics be given in accordance with a patient’s risk for atypical organisms.27,28 For sepsis, the early management bundles advocated by the Surviving Sepsis Campaign (SSC) have broken down the original 6-hour early resuscitation bundle to two distinct resuscitation bundles with 3- and 6- hour goals, with the 3-hour bundle specifically targeted toward ED management.4 Recognizing the importance and need to improve evidence-based care for patients hospitalized with sepsis, CMS introduced a sepsis core measure (SEP-1) in 2016 that parallels the SSC’s 3-hour bundle and mandates that hospitals publicly report their adherence to the SEP-1 guideline.29
Previous literature on CPG adherence for pneumonia and sepsis does not reflect current guidelines. In addition, previous literature on sepsis CPG adherence using the SSC registries has largely mixed ED and inpatient care, making it difficult to assess guideline adherence specifically initiated in the ED.15,16 Thus, the primary objective of this study was to estimate ED adherence to CPGs for inpatient community acquired pneumonia and sepsis treatment. Secondary objectives were to identify patient, physician, and environmental factors associated with ED adherence and estimate the association between adherence and in-hospital patient outcomes including mortality and hospital LOS.
METHODS
Study Design
We performed a retrospective study using standardized medical record review to identify a large, consecutive patient population to determine variation in ED adherence to CPGs for inpatient community acquired pneumonia treatment and early identification and management of sepsis and septic shock. The institutional review boards at each participating hospital approved the study with a waiver of consent.
Study Setting and Population
This study was performed at five hospitals in Colorado with heterogeneous and diverse practice environments that represent the main types of EDs including: 1) urban academic safety-net hospital, 2) suburban academic tertiary care hospital, and 3) urban and rural community hospitals (Table 1). Each ED was staffed by emergency medicine board-certified or board-eligible physicians at all times.
Table 1.
Characteristics of Study Sites in 2014–2015
| Urban Safety Net | Tertiary Care | Rural Community | Suburban Community | Urban Community | |
|---|---|---|---|---|---|
| Annual adult ED census | 80,000 | 97,000 | 25,000 | 48,000 | 81,000 |
| % ED patients admitted | 15 | 13 | 19 | 16 | 9 |
| Beds in ED | 72 | 76 | 20 | 30 | 77 |
| % Patients seen by residents | 70 | 55 | < 1 | < 1 | 0 |
| % Patients seen by PA/NP | 10 | 30 | 33 | 33 | 11 |
NP = nurse practitioner; PA = physician assistant.
Consecutive patients were identified retrospectively by any hospital discharge ICD-9 codes for pneumonia (481–486.xx) or severe sepsis/septic shock (785.52, 995.92).30,31 Starting on January 1, 2013, investigators initially obtained a list of consecutive patients with these ICD-9 codes from the safety-net and tertiary care hospitals. Sufficient sample sizes were obtained from the safety net hospital after reviewing 4 months of consecutive patient charts (i.e., September 2012 to January 1, 2013) and after reviewing 5 months of consecutive charts at the tertiary care hospital (i.e., August 2012 to January 1, 2013). The study was then expanded to the three community hospitals to increase generalizability. Investigators, similarly, obtained a list of consecutive patients from the three community hospitals starting on January 1, 2015. Sufficient sample sizes were obtained from each of the three community hospitals after reviewing 12 months of consecutive patient charts at each hospital from January 2014 to January 1, 2015. From the initial cohort, each chart was screened by a physician abstractor for inclusion using the following criteria: 1) a discharge diagnosis in the medical record of pneumonia, severe sepsis, or septic shock; 2) admission to the hospital from the ED; and 3) diagnosis or initiated treatment of the disease process in the ED. Pneumonia was present in the ED if definitively identified on imaging by a radiologist or treated in the ED based on documentation of clinical suspicion. Pneumonia was not considered to be present if azithromycin was given for the treatment of a chronic obstructive pulmonary disease (COPD) exacerbation alone. Severe sepsis or septic shock was present in the ED if the patient met all criteria for severe sepsis or septic shock as defined by the SSC while in the ED.4,32 Exclusion criteria were age < 18 years, repeat visits by the same patients, and patients transferred from another facility as the initial management would not have occurred in the included EDs. Additionally, patients were not included in both the pneumonia and the sepsis cohorts. If the patient met criteria for the sepsis cohort due to pneumonia, the patient was included in the sepsis cohort rather than the pneumonia cohort.
Study Protocol
Once the study cohort was obtained, structured medical record abstraction was performed using established, standard methodology.33–35 To maximize validity and reliability of the medical record abstraction process, we used the following established methodologies: 1) physician abstractors, blinded to the purpose of the study, to ensure expert familiarity with medical records and documentation; 2) abstractors trained by the lead author using a set of test cases to standardize approaches; 3) use of a previously developed and refined closed-response data collection instrument (Data Supplement S1, available as supporting information in the online version of this paper, which is available at http://onlinelibrary.wiley.com/doi/10.1111/acem.13639/full); 4) performance of 10 pilot reviews, using actual cases sampled from each hospital but not included for analysis to gain familiarity with each hospital’s medical record system; 5) reabstraction of 15% of randomly selected included cases to estimate interrater reliability of the primary outcome, with the intention of performing reabstraction with adjudication of 100% of the cases if agreement of the 15% is less than K < 0.8; and 6) routine oversight of the abstractor team by the lead author, who was also available throughout the data collection process to address questions and problems that occurred.33,34 Using a structured data abstraction form, abstractors documented the presence of all prespecified variables necessary to assess adherence with each CPG. Using the same data abstraction form, data were collected related to patient, physician, and environmental characteristics that had been shown to be associated with CPG adherence in previous studies on other emergency conditions.36–39
Patient factors included patient demographics, primary health insurance, primary language, infectious disease-related comorbidities, and chief complaint. Patient demographics, insurance, and language were obtained directly from each hospital’s administrative database. Missing data were abstracted directly from the patient’s medical record when available and when unavailable were recorded as missing. All remaining characteristics were obtained directly from the medical record. Infectious disease-related comorbidities included diabetes, acquired immune deficiency syndrome, and iatrogenic immunosuppression (e.g., chemotherapy or other immunosuppressive medication). Patient chief complaints were stratified into three groups based on how typical the complaint was for the diagnosis. Stratification of chief complaints into three groups was defined by the lead and senior author based on frequency and specificity of the chief complaint for the diagnosis. Typical chief complaints for pneumonia included cough, shortness of breath, and fever. The only typical chief complaint for sepsis was fever. Associated chief complaints for pneumonia included chest pain, abdominal pain, flu, upper respiratory infection, congestion, hemoptysis, chills, myalgias, altered mentation, hypoxia, hypotension, tachycardia, and weakness. Associated chief complaints for sepsis included cough, dysuria, abdominal pain, flank pain, back pain, cellulitis, abscess, wound infection, blood infection, vomiting, diarrhea, altered mentation, chills, myalgias, shortness of breath, hypotension, and tachycardia. All other chief complaints were grouped into an “other” category.
Physician factors included the individual ED physician, ED physician’s experience, type of medical degree, and ED diagnosis as well as the admitting hospital unit (i.e., floor vs. intensive care). Patients who were admitted under observation status or admitted to intermediate care units were considered floor admissions. ED physician’s experience was determined as the number of years of independent practice at the time the patient was seen (i.e., years following completion of residency training). ED physician’s medical degree was categorized into MD or DO. Physician’s ED diagnosis was categorized into three groups based on its association with pneumonia or sepsis. If the physician documented pneumonia, sepsis, severe sepsis, or septic shock as the primary ED diagnosis, then the ED diagnosis was designated as “primary.” For patients with pneumonia, if the physician documented COPD, hypoxia, pleural effusion, respiratory failure, or sepsis as the primary diagnosis, then the ED diagnosis of pneumonia was designated as “associated” with the primary diagnosis. Similarly, for patients with severe sepsis, if the physician documented a specific type of infection (e.g., pneumonia, cellulitis, and pyelonephritis) as the primary diagnosis, then the ED diagnosis of sepsis was designated as “associated.” All other primary ED diagnoses were categorized as “other.”
Environmental factors included time of day, day of week, ED occupancy, and hospital. Time of day was categorized into four groups: day (6 am–11:59 am), afternoon (12 pm–5:59 pm), evening (6 pm–11:59 pm), and night (12 am–5:59 am). Day of week was categorized into two groups: weekday (Monday 7 am–Friday 4:59 pm) and weekend (Friday 5 pm–Monday 6:59 am).
Outcome Measures
The primary outcome was ED adherence to the respective CPG for community-acquired pneumonia and severe sepsis/septic shock as written or endorsed by the IDSA/ATS and the SSC.4,27,32 Table 2 describes how adherence was determined for each CPG. Secondary outcomes included hospital LOS and all-cause in-hospital mortality. Hospital LOS was measured in days from time of hospital admission order to time of hospital discharge order.
Table 2.
Description of CPG Measures
| Disease | Quality Measure |
|---|---|
| Community-acquired pneumonia | Guideline-concordant antibiotic selection given risk for atypical infections |
| Severe sepsis/septic shock | 3-hour bundle 1. Lactate measured 2. Blood cultures prior to antibiotics 3. Antibiotics within 3 hours of ED arrival 4. 30 mL/kg bolus of IVF within 3 hours of ED arrival if sBP < 90 or lactate ≥ 4 |
CPG = clinical practice guideline; IVF = intravenous fluids.
Data Management and Statistical Analyses
All data management and statistical analysis were performed using SAS version 9.4. Descriptive statistics were calculated for all variables. Continuous data were reported as medians with interquartile ranges (IQRs) and categorical variables as percentages with 95% confidence intervals (CIs). Prevalence estimates with 95% CIs were used to report adherence with CPGs, and a chi-square test was used to test the a priori hypothesis that a statistically significant difference in adherence existed between the two CPGs. A p-value of <0.05 was considered statistically significant.
Unadjusted logistic regression was used to estimate the association of each patient, physician, and environmental variable with ED adherence to CPGs within the combined cohort and each disease subgroup. Hierarchical generalized linear models were used to estimate adjusted associations between patient, physician, and environmental factors and ED adherence with CPGs within the combined cohort. Adherence for all CPGs was initially modeled as a composite outcome to evaluate for factors associated with ED adherence to pneumonia and sepsis CPGs. Secondary models for each individual CPG were also developed, incorporating additional disease-specific patient factors. Models were developed by first creating a full model followed by dropping variables found to be collinear. Hospital was included as a random effect. Effect modification, using interaction terms, was assessed for sex, primary language, and race/ethnicity by complaint category and included if they contribute significantly to the model (p < 0.05).
Sample Size Estimation
In an effort to report estimates with reasonable precision, we chose a priori to include numbers of patients based on an upper 95% confidence limit of 5% (10% total CI). This degree of precision allowed for appropriate statistical separation between estimates across institutions with relatively high and relatively low adherence and allowed for separation of all prevalence estimates from our a priori defined 95% adherence threshold. Thus, using estimates from our preliminary data, we estimated needing a minimum sample size of 350 total patients for each disease process (700 total patients) to achieve the above stated degree of precision. To provide a more balanced sample between the academic and community hospitals, we increased the sample size needed from the community hospitals as the data from the academic hospitals had already been collected prior to adding the community hospitals to the study.
RESULTS
Patient Characteristics
Overall, 827 patients were included in the study including 414 patients with pneumonia and 413 patients with severe sepsis or septic shock. Inter-rater reliability of abstraction of the primary outcome exceeded our predefined threshold (κ > 0.8). Table 3 describes the characteristics of the patients included in the study. The median age was 60 years (IQR = 49–74 years), and 53% were male. Patients were primarily non-Hispanic white (66%), spoke English primarily (91%), and were insured by Medicare (46%). While 60% of pneumonia patients presented with complaints typical of pneumonia, only 14% of sepsis patients presented with complaints typical of sepsis.
Table 3.
Patient Characteristics
| Characteristics | Combined (n = 827) | Pneumonia (n = 414) | Sepsis (n = 413) | |||
|---|---|---|---|---|---|---|
| Age (years) | 60 | (49–74) | 60 | (48–76) | 61 | (49–73) |
| Sex | ||||||
| Male | 435 | (53) | 211 | (51) | 224 | (54) |
| Female | 392 | (47) | 203 | (49) | 189 | (46) |
| Race/ethnicity | ||||||
| Non-Hispanic white | 543 | (66) | 281 | (68) | 262 | (63) |
| Hispanic | 161 | (20) | 65 | (16) | 96 | (23) |
| Non-Hispanic black | 82 | (10) | 46 | (11) | 36 | (9) |
| Other | 41 | (5) | 22 | (5) | 19 | (5) |
| Language | ||||||
| English | 748 | (91) | 382 | (92) | 366 | (89) |
| Spanish | 64 | (8) | 23 | (6) | 41 | (10) |
| Other | 15 | (2) | 9 | (2) | 6 | (2) |
| Primary health insurance | ||||||
| Medicare | 383 | (46) | 197 | (48) | 186 | (45) |
| Medicaid | 160 | (19) | 71 | (17) | 89 | (22) |
| Uninsured | 158 | (19) | 96 | (23) | 62 | (15) |
| Private | 110 | (13) | 42 | (10) | 68 | (17) |
| Other source | 16 | (2) | 8 | (2) | 8 | (2) |
| Comorbidities | ||||||
| HIV/AIDS | 20 | (2) | 16 | (4) | 4 | (1) |
| Congestive heart failure | 101 | (12) | 47 | (11) | 54 | (13) |
| Diabetes | 198 | (24) | 85 | (21) | 113 | (27) |
| End-stage liver disease | 20 | (2) | 3 | (1) | 17 | (4) |
| End-stage renal disease | 20 | (2) | 1 | (0) | 19 | (5) |
| Immunosuppression | 69 | (8) | 34 | (8) | 35 | (9) |
| Chief complaint | ||||||
| Typical for disease | 305 | (37) | 249 | (60) | 56 | (14) |
| Associated with disease | 419 | (51) | 125 | (30) | 294 | (71) |
| Other | 103 | (13) | 40 | (10) | 63 | (15) |
Data are reported as median (IQR) or n (%).
IQR = interquartile range.
Prevalence of Adherence
Overall, the prevalence of adherence to ED infectious CPGs was 57% (95% CI = 54%-61%) (Table 4). Physicians were more adherent to prescribing IDSA-concordant antibiotics to patients with pneumonia (64%, 95% CI = 59%-69%) than completing the SSC’s 3-hour bundle (50%, 95% CI = 45%-55%; p < 0.001). Overall adherence to the SSC’s 3-hour bundle was no different between patients with severe sepsis and septic shock (p = 0.9; Figure 1). However, while the composite adherence to the SSC’s 3-hour bundle was only 50%, completion of individual components of the bundle were markedly better with 92% obtaining a screening lactate, 82% obtaining blood cultures before antibiotics, 72% receiving antibiotics within 3 hours of ED arrival, and 69% of septic shock patients receiving 30 mL/kg IV fluids within 3 hours of ED arrival.
Table 4.
Adherence to ED Infectious CPGs
| Overall | Pneumonia* | Sepsis and Septic Shock† | p-value | |
|---|---|---|---|---|
| Adherence to CPG | 57.2 (53.7–60.6) | 64.0 (59.2–68.6) | 50.4 (45.4–55.3) | <0.001 |
| Hospital type | ||||
| Tertiary care | 65.4 (58.9–71.5) | 68.4 (59.1–76.7) | 62.4 (53.0–71.2) | |
| Community | 52.9 (47.6–58.2) | 56.7 (49.1–64.0) | 49.2 (41.6–56.7) | |
| Safety net | 55.6 (48.9–62.0) | 70.9 (61.8–79.0) | 40.2 (31.2–49.6) | |
Data are reported as % (95% CI).
CPGs = clinical practice guidelines.
Infectious Disease Society of America guideline-concordant antibiotics.
Surviving Sepsis Campaign 3-hour bundle.
Figure 1.
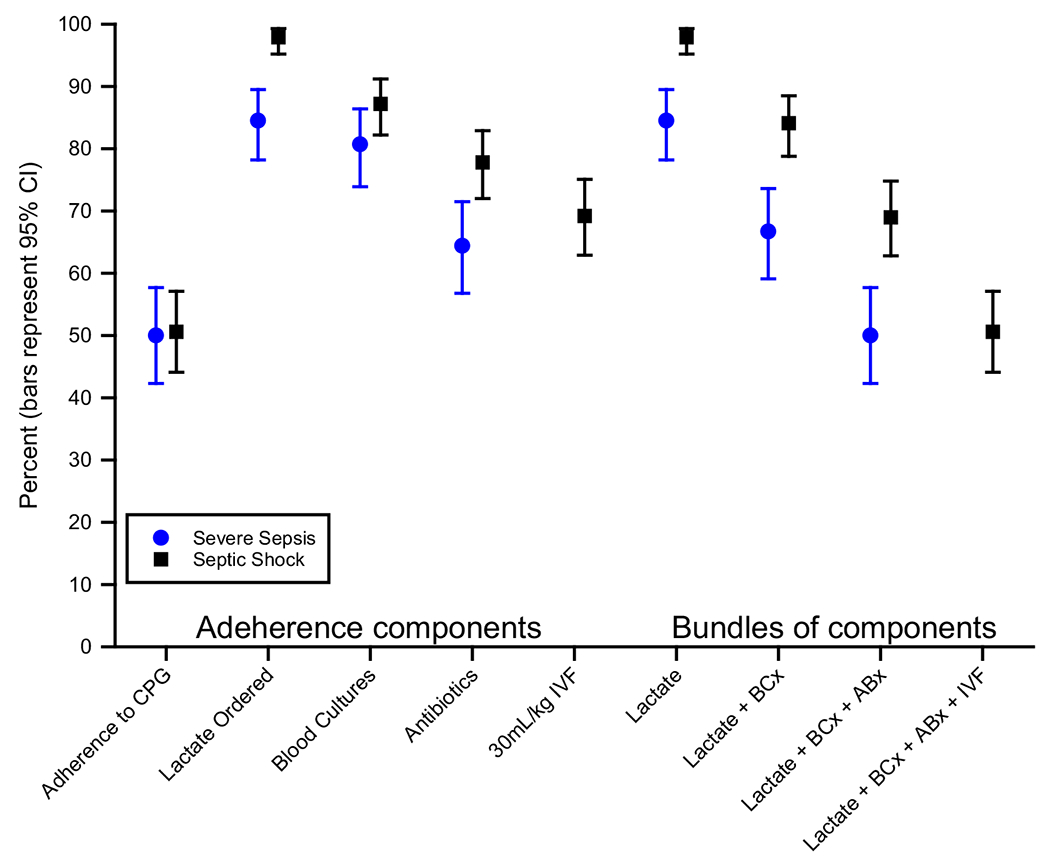
ED adherence to Surviving Sepsis Campaign’s 3-hour bundle and its components. CPG = clinical practice guideline; IVF = intravenous fluids; BCx = blood cultures; ABx = antibiotics.
Patient, Physician, and Environmental Variables Associated With Adherence
Table 5 shows the results of our adjusted multivariable analysis for the combined cohort. Patients were more likely to receive adherent care in the ED if they presented with chief complaints that were typical for the diagnoses and if the primary diagnosis in the ED was specific to the CPG. When patients presented with symptoms that were associated but not typical for the disease, the odds of receiving adherent care in the ED were 0.6 (95% CI = 0.4–0.8). When the primary ED diagnosis was associated but not specific to the CPG, the odds of receiving adherent care were 0.3 (95% CI = 0.2–0.5) and 0.4 (95% CI = 0.2–0.6) for other primary diagnoses. Finally, patients, who presented to a community hospital, were less likely to receive adherent care than patients who presented to an academic tertiary care hospital (adjusted odds ratio [AOR] = 0.6, 95% CI = 0.4–0.9).
Table 5.
Multivariable Model of Adherence to ED Infectious CPGs, Adjusting for Clustering by Hospital
| Combined Cohort (N = 827) | n | (%) | Adherence, n (%) | Adjusted OR | (95% CI) | |
|---|---|---|---|---|---|---|
| Age (years) | 60 | (49–74) | 60 | (48–75) | 1.00 | (0.99–1.02) |
| Sex | ||||||
| Male | 435 | (53) | 250 | (57) | [Ref] | |
| Female | 392 | (47) | 223 | (57) | 0.94 | (0.70–1.26) |
| Language | ||||||
| English | 748 | (90) | 424 | (57) | [Ref] | |
| Spanish | 64 | (8) | 38 | (59) | 1.15 | (0.64–2.06) |
| Other | 15 | (2) | 11 | (73) | 2.19 | (0.63–7.60) |
| Insurance | ||||||
| Medicare | 383 | (46) | 217 | (57) | [Ref] | |
| Medicaid | 160 | (19) | 89 | (56) | 1.05 | (0.65–1.69) |
| Uninsured | 158 | (19) | 101 | (64) | 1.19 | (0.72–1.97) |
| Commercial | 110 | (13) | 57 | (12) | 0.81 | (0.50–1.32) |
| Other source | 16 | (2) | 9 | (56) | 0.85 | (0.29–2.44) |
| Chief complaint | ||||||
| Typical for disease | 305 | (37) | 203 | (67) | [Ref] | |
| Associated with disease | 419 | (24) | 211 | (50) | 0.60 | (0.42–0.84) |
| Other | 103 | (12) | 59 | (57) | 0.85 | (0.52–1.39) |
| Primary ED diagnosis | ||||||
| Pneumonia or sepsis | 532 | (64) | 179 | (66) | [Ref] | |
| Associated with disease | 197 | (24) | 115 | (42) | 0.34 | (0.24–0.49) |
| Other | 98 | (12) | 60 | (39) | 0.38 | (0.23–0.60) |
| Admitting hospital unit | ||||||
| Floor | 508 | (61) | 293 | (56) | [Ref] | |
| ICU | 319 | (39) | 180 | (58) | 1.15 | (0.84–1.59) |
| Provider experience (years) | 9 | (5–15) | 9 | (5–15) | 1.01 | (0.99–1.02) |
| Degree | ||||||
| MD | 682 | (82) | 396 | (58) | [Ref] | |
| DO | 145 | (18) | 77 | (53) | 0.99 | (0.64–1.51) |
| Hospital type | ||||||
| Tertiary care | 234 | (28) | 153 | (65) | [Ref] | |
| Community | 359 | (43) | 190 | (53) | 0.61 | (0.41–0.91) |
| Safety net | 234 | (28) | 130 | (56) | 0.77 | (0.50–1.17) |
| Time of day | ||||||
| Morning (6 am–11:59 am) | 245 | (30) | 150 | (61) | [Ref] | |
| Afternoon (noon–5:59 pm) | 300 | (36) | 160 | (53) | 0.79 | (0.54–1.13) |
| Evening (6 pm–11:59 pm) | 190 | (30) | 108 | (57) | 0.92 | (0.61–1.40) |
| Night (midnight–5:59 am) | 92 | (11) | 55 | (60) | 1.10 | (0.65–1.85) |
| Day of week | ||||||
| Weekday | 516 | (62) | 303 | (59) | [Ref] | |
| Weekend (Fri 6 pm to Mon 6 am) | 311 | (38) | 170 | (55) | 0.82 | (0.60–1.11) |
Data are reported as median (IQR) or n (%).
CPG = clinical practice guidelines; ICU = intensive care unit; IQR = interquartile range.
Table 6 shows the results of our adjusted multivariable analysis for the sepsis cohort. Patients were significantly more likely to receive all components of the SSC’s 3-hour bundle in the ED if they presented at night (AOR = 2.5, 95% CI = 1.2–5.3) compared to morning hours. Patients were significantly less likely to receive all components of the SSC’s 3-hour bundle in the ED if their infectious source was abdominal (AOR = 0.3, 95% CI = 0.1–0.8) or soft tissue (AOR= 0.4, 95% CI = 0.2–0.9) compared to a respiratory source; if they had fluid-sensitive comorbidities such as end-stage renal disease on hemodialysis, congestive heart failure, or end-stage liver disease (AOR = 0.6, 95% CI = 0.3–0.99); were diagnosed by the ED physicians with a specific infection rather than severe sepsis or septic shock (AOR = 0.5, 95% CI = 0.3–0.9); were admitted to a general medical or surgical floor (AOR = 0.5, 95% CI = 0.3–0.9) rather than an intensive care unit; or presented to a community or safety-net hospital (AOR = 0.4, 95% CI = 0.2–0.7, respectively) rather than a quaternary care hospital.
Table 6.
Multivariable Model of ED Adherence to SSC’s 3-Hour Bundle, Adjusted for Clustering by Hospital
| Sepsis Cohort (n = 413) | Overall | Adherence | Adjusted | |||
|---|---|---|---|---|---|---|
| Age (years) | 61 | (49–73) | 63 | (50–75) | 1.0 | (1.0–1.0) |
| Sex | ||||||
| Male | 224 | (54) | 116 | (52) | [Ref] | (0.6–1.4) |
| Female | 189 | (46) | 92 | (49) | 0.9 | |
| Race/ethnicity | ||||||
| Non–Hispanic white | 262 | (63) | 136 | (52) | [Ref] | (0.7–2.2) |
| Hispanic | 96 | (23) | 48 | (50) | 1.2 | (0.3–1.7) |
| Non–Hispanic black | 36 | (9) | 16 | (44) | 0.8 | (0.3–2.5) |
| Other | 19 | (5) | 8 | (42) | 0.9 | |
| Comorbidities | ||||||
| Fluid sensitive* | 85 | (21) | 38 | (45) | 0.6 | (0.3–0.99) |
| SIRS | ||||||
| 2 | 136 | (33) | 61 | (45) | [Ref] | |
| 3 | 198 | (48) | 103 | (52) | 1.4 | (0.8–2.2) |
| 4 | 79 | (19) | 44 | (56) | 1.6 | (0.8–2.9) |
| ED source | ||||||
| Respiratory | 137 | (33) | 78 | (57) | [Ref] | |
| Urinary | 110 | (27) | 54 | (49) | 0.6 | (0.4–1.1) |
| Skin/soft tissue | 42 | (10) | 16 | (38) | 0.4 | (0.2–0.9) |
| Abdominal | 34 | (8) | 12 | (35) | 0.3 | (0.1–0.8) |
| Other | 12 | (3) | 6 | (50) | 0.5 | (0.1–1.9) |
| Unknown | 78 | (19) | 42 | (54) | 0.6 | (0.4–1.1) |
| Primary ED diagnosis | ||||||
| Sepsis/severe/shock | 199 | (48) | 119 | (60) | [Ref] | |
| Specific infection | 161 | (39) | 66 | (41) | 0.5 | (0.3–0.9) |
| Other | 53 | (13) | 23 | (43) | 0.6 | (0.3–1.2) |
| Admitting hospital unit | ||||||
| ICU | 144 | (35) | 54 | (38) | [Ref] | |
| Floor | 269 | (65) | 154 | (57) | 0.5 | (0.3–0.9) |
| Hospital type | ||||||
| Tertiary care | 117 | (28) | 73 | (62) | [Ref] | |
| Community | 179 | (43) | 88 | (49) | 0.4 | (0.2–0.7) |
| Safety net | 117 | (28) | 47 | (40) | 0.4 | (0.2–0.7) |
| Time of day | ||||||
| Morning (6 am–11:59 am) | 109 | (26) | 52 | (48) | [Ref] | |
| Afternoon (noon–5:59 pm) | 150 | (36) | 68 | (45) | 1.0 | (0.6–1.8) |
| Evening (6 pm–11:59 pm) | 106 | (26) | 57 | (54) | 1.2 | (0.7–2.2) |
| Night (midnight–5:59 am) | 48 | (12) | 31 | (65) | 2.5 | (1.2–5.3) |
Data are reported as n (%) or OR (95% CI).
ICU = intensive care unit; IQR = interquartile range; SIRS = systemic inflammatory response syndrome; SSC = Surviving Sepsis Campaign.
Congestive heart failure, end-stage renal disease on hemodialysis, and end-stage liver disease.
Unadjusted associations between ED adherence to infectious disease CPGs and all patient, provider, and environmental variables for the combined cohort and each disease subgroup are provided in Data Supplement S1 (Tables S1–S3). Adjusted multivariable analysis for the pneumonia cohort is also presented in Data Supplement S1 (Table S4).
Secondary Outcomes
In the combined cohort, 40 (4.8%) patients died during the index hospitalization, 95% of whom were patients in the sepsis cohort. Adjusted for patient age, sex, admitting disease, admitting hospital unit, and ED CPG adherence, the odds of in-hospital mortality were significantly increased in patients who did not receive adherent CPG care in the ED (AOR =2.4, 95% CI = 1.2–4.8; Table 7). The median hospital LOS for pneumonia patients receiving guideline adherent care in the ED was 1 day shorter than pneumonia patients receiving nonadherent care in the ED. In contrast, the median hospital LOS was 1 day longer for sepsis patient receiving guideline adherent ED care than sepsis patients receiving nonadherent care in the ED (Table 8).
Table 7.
Multivariable Model of In-hospital Mortality, Adjusted for Clustering by Hospital
| n (%) | In-hospital Mortality, n (%) | Adjusted OR (95% CI) | |
|---|---|---|---|
| Age (years), mean (±SD) | 60 (±18) | 64 (±13) | 1.02 (1.00–1.04) |
| Sex | |||
| Male | 435 (53) | 23 (58) | Ref |
| Female | 392 (47) | 17 (42) | 0.9 (0.5–1.8) |
| Admitting disease | |||
| Pneumonia | 414 (50) | 2 (5) | Ref |
| Severe sepsis/shock | 413 (50) | 38 (95) | 9 (2–38) |
| Admitting hospital unit | |||
| Floor | 508 (61) | 5(15) | Ref |
| ICU | 319 (39) | 34 (85) | 5.4 (2.1–14) |
| Guideline adherent ED care | |||
| Yes | 473 (57) | 15 (38) | Ref |
| No | 354 (43) | 25 (63) | 2.4 (1.2–4.8) |
ICU = intensive care unit.
Table 8.
Hospital LOS and Adherence to ED Infectious Clinical Practice Guideline
| Overall (N = 827) | Adherence (n = 473) | Nonadherence (n = 354) | Median Difference | |||||
|---|---|---|---|---|---|---|---|---|
| Hospital LOS (days) | 5 | (3–9) | 5 | (3–8) | 5 | (3–9) | 0 | (−0.7 to 0.7) |
| Disease type | ||||||||
| Pneumonia (n = 414) | 5 | (3–7) | 4 | (3–6) | 5 | (3–7) | −1.0 | (−1.5 to −0.5) |
| Sepsis (n = 413) | 7 | (4–12) | 7 | (4–13) | 6 | (4–11) | 1.0 | (0.3 to 1.7) |
Data are reported as median (95% CI).
DISCUSSION
Our results suggest considerable variation in guideline adherence for two of the most prevalent and deadly infectious diseases encountered in the ED, with only 64% of patients with community-acquired pneumonia and 50% with sepsis receiving recommended therapy in the ED. To our knowledge, this is the only study to examine differences in adherence to contemporaneous guidelines for pneumonia and sepsis treatment in multiple, diverse ED settings in the United States. Similar to our findings among cardiovascular and cerebrovascular ED guideline adherence, chief complaint and primary ED diagnosis were significantly associated with ED adherence, such that the more straightforward the complaint and diagnosis, the more likely ED care was to be adherent to the relevant guideline.40 While the random effect of hospital was small, accounting for only 1% of the variability in ED adherence, hospital type was significantly associated with adherence with community EDs less likely to adhere to infectious disease guidelines compared to an academic, tertiary care hospital. Finally, and perhaps most importantly, our results showed a significant association between guideline-adherent care in the ED and in-hospital mortality. After patient age, sex, admitting disease, and acuity of illness were adjusted for, patients who did not receive guideline-adherent care in the ED were 2.4 times more likely to die in the hospital compared to patients who did receive guideline-adherent care.
Since CMS retired its pneumonia core measure in 2014, little has been written on ED adherence to IDSA/ATS recommended antibiotic administration for patients admitted for community-acquired pneumonia. While the CMS pneumonia core measure was criticized for the lack of evidence related to blood cultures and timing of antibiotics,41,42 the recommendation for appropriate antibiotic therapy is supported by the literature, which suggests decreased mortality and hospital LOS when patients are administered guideline-recommended antibiotics.43–45 Additionally, it is important that patients receive appropriate therapy without being exposed to unnecessarily broad therapy, which can result in increased resistance and other adverse effects.46,47 This is particularly relevant now that the updated IDSA/ATS guidelines for hospital-acquired and ventilator-associated pneumonia recently removed the concepts of health care–associated pneumonia from the guideline given new evidence that contact with the health care system alone is less important than underlying patient characteristics for predicting risk of multidrug-resistant organisms.48–53 Whether health care–associated pneumonia is differentiated from community acquired pneumonia by the IDSA/ATS guidelines for community acquired pneumonia remains unclear at this time. The IDSA and ATS are actively updating their guideline on community-acquired pneumonia with a projected release in the fall 2018. If the concept of health care–associated pneumonia is removed from the guideline, antimicrobial treatment of immunocompetent patients with pneumonia who present to the ED from the community will be greatly simplified, likely leading to improved adherence and antimicrobial stewardship.
For sepsis, most previous literature on adherence to the SSC’s resuscitation bundle did not differentiate adherence to components at 3 and 6 hours. While the 3-hour bundle is frequently initiated and completed in the ED, the 6-hour components are more likely completed in the inpatient setting, making it difficult to assess composite guideline adherence from larger studies.14–16,21 However, a handful of studies in the past few years have begun to report adherence to the SSC 3-hour bundle and the CMS SEP-1 guideline. The IMPreSS study by Rhodes et al.54 showed that adherence to the SSC 3-hour bundle was poor with overall adherence within their multicenter, international cohort being only 19% and rising to only 29% among the subset of North American participating hospitals. Our data are similar to Venkatesh et al.,55 who recently showed that among U.S. EDs participating in the American College of Emergency Physician’s Emergency Quality Network Sepsis Initiative, overall adherence with the CMS SEP-1 was 54%. Both of these studies as well as ours suggest significant room for improvement in early ED sepsis care. Importantly, the SSC released a new “hour-1” bundle in May 2018, which replaced both the 3-and 6-hour bundles.56 The impact of the new SSC recommendation on ED sepsis care is likely to be limited in the United States given its current discordance with the CMS SEP-1 guideline. Moreover, the SSC hour-1 bundle has been highly criticized, particularly within the emergency medicine community, given the low-quality evidence to support these recommendations as well as the risk of over treating some sepsis patients and diverting attention away from nonsepsis patients.57
LIMITATIONS
The use of discharge ICD-9 codes to identify ED patients is limited because discharge diagnoses may not be relevant to the reasons for admission from the ED. Consequently, using discharge ICD-9 codes was coupled with direct chart review to ensure that the sample only represented patients with the diagnoses of interest, who were admitted to the hospital from the ED specifically for these diagnoses. However, we may have missed some sepsis patients who were not coded as such. Additionally, including hospital admission as an inclusion criterion may have excluded patients who died in the ED, had an unknown disposition, or were discharged from the ED. Limiting patients to those who were admitted helped limited chart reviews to patient who were most likely to truly have the disease and in whom the guideline recommended care could actually have been enacted. Missing documentation within the medical chart is a known limitation to medical record abstraction. Missing documentation could have affected our estimates of adherence especially in the pneumonia subgroup where details related to a patient’s immune competence could have been missing. Although we abstracted a comprehensive list of potential patient, physician, and environmental factors that have been shown to be associated with CPGs in other studies, additional variables may have been left out of the model, a known limitation of retrospective analyses. We used admitting hospital unit as a proxy for illness severity. The use of a more robust illness severity score may have resulted in a more specific variable for illness severity.
CONCLUSIONS
Adherence to ED infectious clinical practice guidelines for community-acquired pneumonia and sepsis varies significantly across diseases and institutions with significant room for improvement, especially in light of a significant association with in-hospital mortality.
Supplementary Material
Acknowledgments
The authors acknowledge Erica Ashley Morse, MD, and Michael Susalla, MD, who both contributed to data acquisition.
Supported, in part, by the Agency for Healthcare Research and Quality (F32HS022400), the Emergency Medicine Foundation (EMF), and the Department of Emergency Medicine at the University of Colorado School of Medicine to Dr. Trent ST reports grant money to AHRQ and EMF to conduct research conceived and written by ST from Denver Health. ZJ reports no conflicts of interest. EH reports grant money to NHLBI to conduct research conceived and written by EH from Denver Health. AG reports grant money to NHLBI to conduct research conceived and written by AG from the University of Colorado. JH reports grant money to NIAID and NIDA to conduct research conceived and written by JH from Denver Health.
Footnotes
Presented, in part, at the Society for Academic Emergency Medicine Annual Meeting, Orlando, Florida, May 2017.
Supporting Information
The following supporting information is available in the online version of this paper available at http://onlinelibrary.wiley.com/doi/10.1111/acem.13639/full Data Supplement S1. Supplemental material.
References
- 1.HCUPnet, Healthcare Cost and Utilization Project: Agency for Healthcare Research and Quality. Available from: https://hcupnet.ahrq.gov/. Accessed Aug 1, 2018. [PubMed]
- 2.Dean NC, Silver MP, Bateman KA, James B, Hadlock CJ, Hale D. Decreased mortality after implementation of a treatment guideline for community-acquired pneumonia. Am J Med 2001;110:451–7. [DOI] [PubMed] [Google Scholar]
- 3.Capelastegui A, Espana PP, Quintana JM, et al. Improvement of process-of-care and outcomes after implementing a guideline for the management of community-acquired pneumonia: a controlled before-and-after design study. Clin Infect Dis 2004;39:955–63. [DOI] [PubMed] [Google Scholar]
- 4.Rhodes A, Evans LE, Alhazzani W, et al. Surviving Sepsis Campaign: international guidelines for management of sepsis and septic shock: 2016. Intensive Care Med 2017;43:304–77. [DOI] [PubMed] [Google Scholar]
- 5.Hauck LD, Adler LM, Mulla ZD. Clinical pathway care improves outcomes among patients hospitalized for community-acquired pneumonia. Ann Epidemiol 2004;14:669–75. [DOI] [PubMed] [Google Scholar]
- 6.Marrie TJ, Lau CY, Wheeler SL, Wong CJ, Vandervoort MK, Feagan BG. A controlled trial of a critical pathway for treatment of community-acquired pneumonia. CAPITAL Study Investigators. Community-Acquired Pneumonia Intervention Trial Assessing Levofloxacin. JAMA 2000;283:749–55. [DOI] [PubMed] [Google Scholar]
- 7.Frei CR, Attridge RT, Mortensen EM, et al. Guideline-concordant antibiotic use and survival among patients with community-acquired pneumonia admitted to the intensive care unit. Clin Ther 2010;32:293–9. [DOI] [PubMed] [Google Scholar]
- 8.Frei CR, Restrepo MI, Mortensen EM, Burgess DS. Impact of guideline-concordant empiric antibiotic therapy in community-acquired pneumonia. Am J Med 2006;119: 865–71. [DOI] [PubMed] [Google Scholar]
- 9.Bodi M, Rodriguez A, Sole-Violan J, et al. Antibiotic prescription for community-acquired pneumonia in the intensive care unit: impact of adherence to Infectious Diseases Society of America guidelines on survival. Clin Infect Dis 2005;41:1709–16. [DOI] [PubMed] [Google Scholar]
- 10.Menendez R, Ferrando D, Valles JM, Vallterra J. Influence of deviation from guidelines on the outcome of community-acquired pneumonia. Chest 2002;122:612–7. [DOI] [PubMed] [Google Scholar]
- 11.Meehan TP, Weingarten SR, Holmboe ES, et al. A statewide initiative to improve the care of hospitalized pneumonia patients: the Connecticut Pneumonia Pathway Project. Am J Med 2001;111:203–10. [DOI] [PubMed] [Google Scholar]
- 12.Yealy DM, Auble TE, Stone RA, et al. Effect of increasing the intensity of implementing pneumonia guidelines: a randomized, controlled trial. Ann Intern Med 2005;143: 881–94. [DOI] [PubMed] [Google Scholar]
- 13.Orrick JJ, Segal R, Johns TE, Russell W, Wang F, Yin DD. Resource use and cost of care for patients hospitalised with community acquired pneumonia: impact of adherence to Infectious Diseases Society of America guidelines. Pharmacoeconomics 2004;22:751–7. [DOI] [PubMed] [Google Scholar]
- 14.Levy MM, Artigas A, Phillips GS, et al. Outcomes of the Surviving Sepsis Campaign in intensive care units in the USA and Europe: a prospective cohort study. Lancet Infect Dis 2012;12:919–24. [DOI] [PubMed] [Google Scholar]
- 15.Levy MM, Dellinger RP, Townsend SR, et al. The Surviving Sepsis Campaign: results of an international guideline-based performance improvement program targeting severe sepsis. Crit Care Med 2010;38:367–74. [DOI] [PubMed] [Google Scholar]
- 16.Levy MM, Rhodes A, Phillips GS, et al. Surviving Sepsis Campaign: association between performance metrics and outcomes in a 7.5-year study. Crit Care Med 2015;43:3–12. [DOI] [PubMed] [Google Scholar]
- 17.Jeon K, Shin TG, Sim MS, et al. Improvements in compliance with resuscitation bundles and achievement of end points after an educational program on the management of severe sepsis and septic shock. Shock 2012;37:463–7. [DOI] [PubMed] [Google Scholar]
- 18.Gao F, Melody T, Daniels DF, Giles S, Fox S. The impact of compliance with 6-hour and 24-hour sepsis bundles on hospital mortality in patients with severe sepsis: a prospective observational study. Crit Care 2005;9:R764–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Crowe CA, Mistry CD, Rzechula K, Kulstad CE. Evaluation of a modified early goal-directed therapy protocol. Am J Emerg Med 2010;28:689–93. [DOI] [PubMed] [Google Scholar]
- 20.Miller RR 3rd, Dong L, Nelson NC, et al. Multicenter implementation of a severe sepsis and septic shock treatment bundle. Am J Respir Crit Care Med 2013;188:77–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Zanten AR, Brinkman S, Arbous MS, et al. Guideline bundles adherence and mortality in severe sepsis and septic shock. Crit Care Med 2014;42:1890–8. [DOI] [PubMed] [Google Scholar]
- 22.Nguyen HB, Corbett SW, Steele R, et al. Implementation of a bundle of quality indicators for the early management of severe sepsis and septic shock is associated with decreased mortality. Crit Care Med 2007;35:1105–12. [DOI] [PubMed] [Google Scholar]
- 23.Girardis M, Rinaldi L, Donno L, et al. Effects on management and outcome of severe sepsis and septic shock patients admitted to the intensive care unit after implementation of a sepsis program: a pilot study. Crit Care 2009;13:R143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nguyen HB, Kuan WS, Batech M, et al. Outcome effectiveness of the severe sepsis resuscitation bundle with addition of lactate clearance as a bundle item: a multinational evaluation. Crit Care 2011;15:R229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jones AE, Focht A, Horton JM, Kline JA. Prospective external validation of the clinical effectiveness of an emergency department-based early goal-directed therapy protocol for severe sepsis and septic shock. Chest 2007;132:425–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Micek ST, Roubinian N, Heuring T, et al. Before-after study of a standardized hospital order set for the management of septic shock. Crit Care Med 2006;34:2707–13. [DOI] [PubMed] [Google Scholar]
- 27.Mandell LA, Wunderink RG, Anzueto A, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis 2007;44 Suppl 2:S27–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.American Thoracic Society, Infectious Diseases Society of America. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med 2005;171:388–416. [DOI] [PubMed] [Google Scholar]
- 29.The Joint Commission. Specifications Manual for National Hospital Inpatient Quality Measures 2017. [updated 11/02/17]. Available from: https://www.jointcommission.org/specifications_manual_for_national_hospital_inpatient_quality_measures.aspx. Accessed Aug 1, 2018. [Google Scholar]
- 30.Drahos J, Vanwormer JJ, Greenlee RT, Landgren O, Koshiol J. Accuracy of ICD-9-CM codes in identifying infections of pneumonia and herpes simplex virus in administrative data. Ann Epidemiol 2013;23:291–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jolley RJ, Sawka KJ, Yergens DW, Quan H, Jette N, Doig CJ. Validity of administrative data in recording sepsis: a systematic review. Crit Care 2015;19:139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dellinger RP, Levy MM, Carlet JM, et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock: 2008. Crit Care Med 2008;36:296–327. [DOI] [PubMed] [Google Scholar]
- 33.Gilbert EH, Lowenstein SR, Koziol-McLain J, Barta DC, Steiner J. Chart reviews in emergency medicine research: where are the methods? Ann Emerg Med 1996;27:305–8. [DOI] [PubMed] [Google Scholar]
- 34.Kaji AH, Schriger D, Green S. Looking through the retro-spectoscope: reducing bias in emergency medicine chart review studies. Ann Emerg Med 2014;64:292–8. [DOI] [PubMed] [Google Scholar]
- 35.von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet 2007;370:1453–7. [DOI] [PubMed] [Google Scholar]
- 36.Pham JC, Kelen GD, Pronovost PJ. National study on the quality of emergency department care in the treatment of acute myocardial infarction and pneumonia. Acad Emerg Med 2007;14:856–63. [DOI] [PubMed] [Google Scholar]
- 37.Mikkelsen ME, Gaieski DF, Goyal M, et al. Factors associated with nonadherence to early goal-directed therapy in the ED. Chest 2010;138:551–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Halm EA, Atlas SJ, Borowsky LH, et al. Understanding physician adherence with a pneumonia practice guideline: effects of patient, system, and physician factors. Arch Intern Med 2000;160:98–104. [DOI] [PubMed] [Google Scholar]
- 39.Meurer WJ, Majersik JJ, Frederiksen SM, Kade AM, Sandretto AM, Scott PA. Provider perceptions of barriers to the emergency use of tPA for acute ischemic stroke: a qualitative study. BMC Emerg Med 2011;11:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Trent SA, Johnson MA, Morse EA, Havranek EP, Haukoos JS. Patient, provider, and environmental factors associated with adherence to cardiovascular and cerebrovascular clinical practice guidelines in the emergency department. Am J Emerg Med 2018;36:1397–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yu KT, Wyer PC. Evidence-based emergency medicine/critically appraised topic. Evidence behind the 4-hour rule for initiation of antibiotic therapy in community-acquired pneumonia. Ann Emerg Med 2008;51:651–62, 62.e1–2. [DOI] [PubMed] [Google Scholar]
- 42.Wilson KC, Schunemann HJ. An appraisal of the evidence underlying performance measures for community-acquired pneumonia. Am J Respir Crit Care Med 2011;183:1454–62. [DOI] [PubMed] [Google Scholar]
- 43.Micek ST, Kollef KE, Reichley RM, Roubinian N, Kollef MH. Health care-associated pneumonia and community-acquired pneumonia: a single-center experience. Antimicrob Agents Chemother 2007;51:3568–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Venditti M, Falcone M, Corrao S, Licata G, Serra P. Study Group of the Italian Society of Internal Medicine. Outcomes of patients hospitalized with community-acquired, health care-associated, and hospital-acquired pneumonia. Ann Intern Med 2009;150:19–26. [DOI] [PubMed] [Google Scholar]
- 45.Gattarello S, Borgatta B, Sole-Violan J, et al. Decrease in mortality in severe community-acquired pneumococcal pneumonia: impact of improving antibiotic strategies (2000–2013). Chest 2014;146:22–31. [DOI] [PubMed] [Google Scholar]
- 46.Dryden M, Hand K, Davey P; BSAC Council. Antibiotics for community-acquired pneumonia. J Antimicrob Chemother 2009;64:1123–5. [DOI] [PubMed] [Google Scholar]
- 47.Dellit TH, Owens RC, McGowan JE Jr, et al. Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin Infect Dis 2007;44:159–77. [DOI] [PubMed] [Google Scholar]
- 48.Chalmers JD, Rother C, Salih W, Ewig S. Healthcare-associated pneumonia does not accurately identify potentially resistant pathogens: a systematic review and meta-analysis. Clin Infect Dis 2014;58:330–9. [DOI] [PubMed] [Google Scholar]
- 49.Gross AE, Van Schooneveld TC, Olsen KM, et al. Epidemiology and predictors of multidrug-resistant community-acquired and health care-associated pneumonia. Antimicrob Agents Chemother 2014;58:5262–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yap V, Datta D, Metersky ML. Is the present definition of health care-associated pneumonia the best way to define risk of infection with antibiotic-resistant pathogens? Infect Dis Clin North Am 2013;27:1–18. [DOI] [PubMed] [Google Scholar]
- 51.Jones BE, Jones MM, Huttner B, et al. Trends in antibiotic use and nosocomial pathogens in hospitalized veterans with pneumonia at 128 medical centers, 2006–2010. Clin Infect Dis 2015;61:1403–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Valles J, Martin-Loeches I, Torres A, et al. Epidemiology, antibiotic therapy and clinical outcomes of healthcare-associated pneumonia in critically ill patients: a Spanish cohort study. Intensive Care Med 2014;40:572–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kalil AC, Metersky ML, Klompas M, et al. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 clinical practice guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis 2016;63:e61–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rhodes A, Phillips G, Beale R, et al. The Surviving Sepsis Campaign bundles and outcome: results from the International Multicentre Prevalence Study on Sepsis (the IMPreSS study). Intensive Care Med 2015;41:1620–8. [DOI] [PubMed] [Google Scholar]
- 55.Venkatesh AK, Slesinger T, Whittle J, et al. Preliminary performance on the new CMS Sepsis-1 national quality measure: early insights from the Emergency Quality Network (E-QUAL). Ann Emerg Med 2018;71 10–5.e1. [DOI] [PubMed] [Google Scholar]
- 56.Levy MM, Evans LE, Rhodes A. The Surviving Sepsis Campaign Bundle: 2018 update. Crit Care Med 2018;46:997–1000. [DOI] [PubMed] [Google Scholar]
- 57.Palliln DJ, Spiegel R. The Surviving Sepsis Campaign: A Rush to Judgment: NEJM Journal Watch Emergency Medicine. 2018. Available at: https://www.jwatch.org/na46999/2018/08/03/surviving-sepsis-campaign-rush-judgment. Accessed August 3, 2018. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


