Conspectus
SmI2 was introduced to organic chemistry as a single electron transfer agent in 1977. After ca. 15 years of latency, the scientific community has realized the high potential of this reagent, and its chemistry has started blooming. This versatile reagent has mediated a myriad of new bond formations, cyclizations, and other reactions. Its popularity stems largely from the fact that three different intermediates, radical anions, radicals, and anions, depending on the ligand or additive used, could be obtained. Each of these intermediates could in principle lead to a different product. While these options vastly enrich the repertoire of SmI2, they necessitate a thorough mechanistic understanding, especially concerning how appropriate ligands direct the SmI2 to the desired intermediate. Our first paper on this subject dealt with the reduction of an activated double bond. The results were puzzling, especially the H/D isotope effect, which depended on the order of the reagents addition. This seminal paper was fundamental to an understanding of how the SmI2 works and enabled us to later explain various phenomena. For example, it was found that in a given reaction, when MeOH is used as a proton source, a spiro compound is obtained, while a bicyclic product is obtained when t-BuOH is used. Our contribution culminated in formulating guidelines for the rational use of proton donors in SmI2 reactions.
The need to understand the complexity of the effect of additives on various processes is nicely demonstrated in photoinduced reactions. For example, hexamethylphosphoramide (HMPA) enhances the reduction of anthracene while hampering the reaction of benzyl chloride. The mechanistic understanding gained enabled us also to broaden the scope of photostimulated reactions from substrates reacting by a dissociative electron transfer mechanism to normal reductions, which are difficult to accomplish at the ground state. Harnessing the classical knowledge of proton transfer mechanisms to our SmI2 research enabled us to decipher an old conundrum: why does the combination of water and amine have such an enhancing effect on the reactivity of SmI2, which is not typical of these two when used separately. In our studies on the affinity of ligands to SmI2, we discovered that, in contradistinction to the accepted dogma, SmI2 is much more azaphilic than it is oxophilic. On the basis of the size difference between Sm3+ and Sm2+, we developed a simple diagnostic tool for the nature of the steps following the electron transfer. The reduction of imines showed that substrate affinity to SmI2 plays also a crucial role. In these reactions, new features such as autocatalysis and catalysis by quantum dots were discovered. Several studies of the ligand effect lead to a clear formulation of when an inner sphere or outer sphere electron transfer should be expected. In addition, several reactions where proton-coupled electron transfer (PCET) is the dominant mechanism were identified. Finally, the surprisingly old tool of NMR “shift reagents” was rediscovered and used to directly derive essential information on the binding constants of ligands and substrates to SmI2.
Key References
Amiel-Levy M.; Hoz S.. Guidelines for the Use of Proton Donors in SmI2 Reactions: Reduction of α-cyanostylbene. J. Am. Chem. Soc. 2009, 131, 8280–8284.1 Mechanistic investigation shows what causes ligands such as water, MeOH, and ethylene glycol to route the SmI2 reactions to a different path and mechanism than proton donors such as trifluoroethanol or t-BuOH.
Maity S.; Hoz S.. Deciphering a 20 Year Old Conundrum: The Mechanisms of the Reduction by the Water/Amine/SmI2 Mixture. Chem. - Eur. J. 2015, 21, 18394–18400.2 Here it is shown why and how the combination of two “innocent” additives (water and amine) has such a dramatic effect on the chemistry of SmI2.
Maity S.; Flowers R. A. II; Hoz S.. Aza versus Oxophilicity of SmI2: A Break of a Paradigm. Chem. - Eur. J. 2017, 23, 17070–17077.3 Contrary to the common belief, this paper shows that nitrogen-based ligands have higher affinity to SmI2 than their oxygen analogs. The effect is even more pronounced for Sm3+, resulting in a larger augmentation of the aza ligand effect on the reduction potential of SmI2.
De S.; Gottlieb H. E.; Hoz S.. Quantification of the Interaction of SmI2 with Substrates and Ligands. Chem. - Eur. J. 2020, 26, 6846–6850.4 Although SmI2 was introduced to organic chemistry 30 years ago, while the shift reagents technique was already practically abandoned, we combined the two to enable accurate association constants of ligands and substrates to SmI2 using NMR.
Introduction
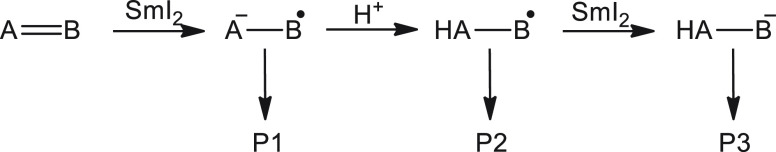
The chemistry of SmI2 displays a rich repertoire of reactions that enable a myriad of synthetic possibilities.5 In the course of reduction of substrate A=B, three different intermediates can be obtained (Scheme 1).6 In principle, each intermediate can lead to a different product. This complexity, which provides great opportunities for the synthetic chemist, also creates a fascinating mechanistic maze.
Scheme 1. Mechanistic Options for Reduction by SmI2.
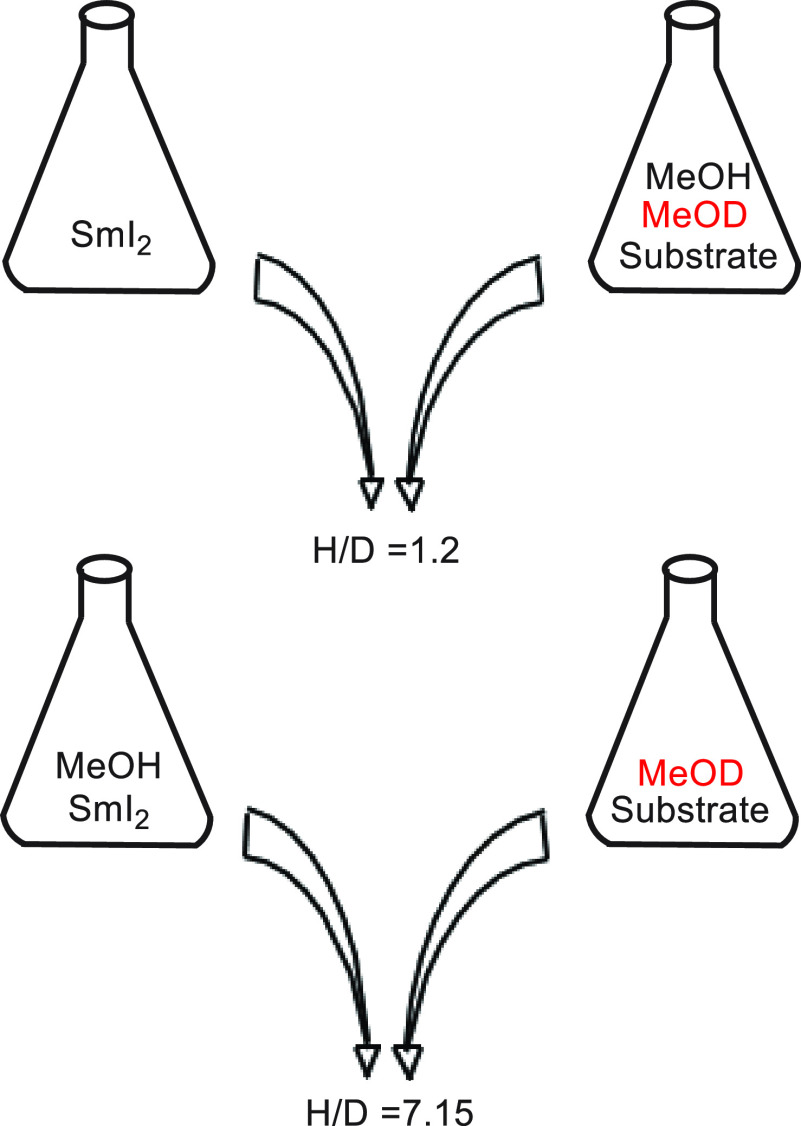
In retrospect, our first report7a on SmI2 was seminal to the eventual unraveling of the mechanistic mysteries of this reagent. However, the results were very puzzling. The H/D product isotope effect for the reaction depicted in eq 1 was determined as described in Figure 1.
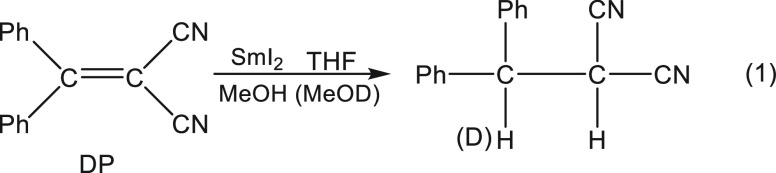 |
1 |
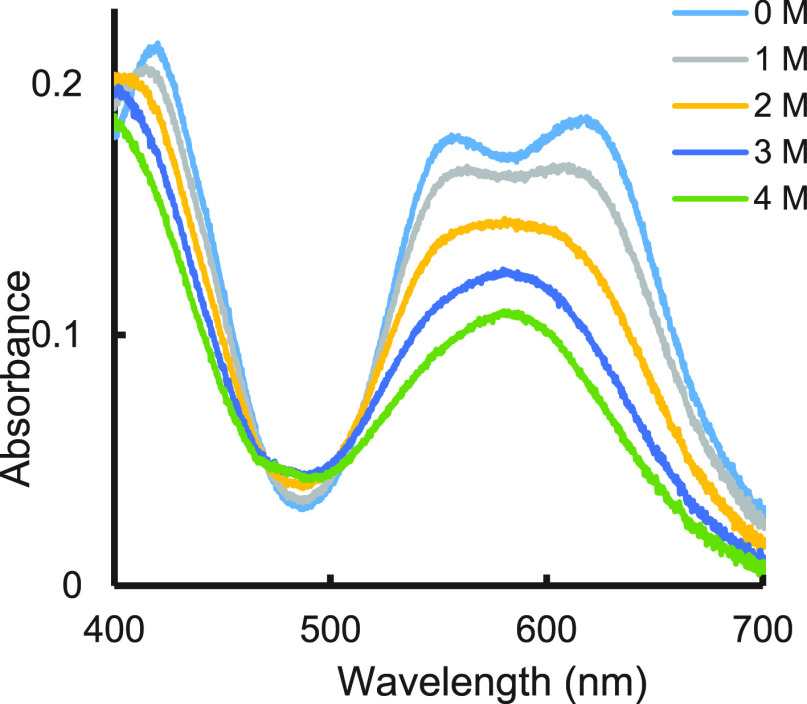
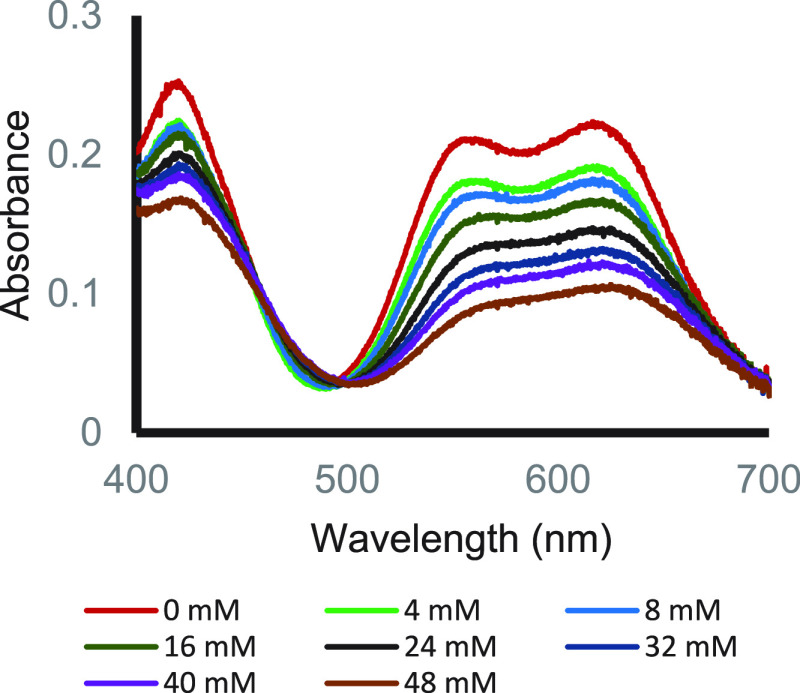
When a THF solution of the substrate containing both MeOH and MeOD (1:1 ratio) was reacted with a THF solution of SmI2, the H/D incorporation ratio into the benzylic carbon was 1.2. However, when the mixing protocol was changed, and methanol was introduced into the SmI2 solution, while MeOD was kept with the substrate solution, the H/D incorporation ratio increased to 7.2. This baffling result, which was confirmed by three generations of postdocs and graduate students, led a referee to suggest that the samarium may insert into the O–H bond to give a hydride that does not exchange its hydrogen. However, we clearly showed that SmI2 forms reversibly a complex with MeOH, which affects its visible spectrum as shown in Figure 2.
Figure 1.
Alternate mixing protocols yield markedly different H/D ratios.
Figure 2.
Effect of MeOH concentration on SmI2 visible spectrum.
It is reasonable to assume that the isotope effect on MeOH complexation to SmI2 is negligible. Thus, in the first experiment, MeOH and MeOD complex similarly with SmI2. In the second experiment, however, only the MeOH is complexed to SmI2, and protonation occurs within the complex where methanol is coordinated to Sm3+, which is ion-paired to the radical anion of the substrate. This unimolecular protonation, which is much faster than a bimolecular protonation by MeOD in the bulk, results in a high H/D isotope effect.
This Account aims to shed light on the main mechanistic channels that characterize the SmI2 reactions. We shall begin with the preassociation effect.
Preassociation Effect
The association of the substrate radical anion, the Sm3+, and the proton donor that is complexed to it, has an immense effect on the chemistry, as will be shown in the following sections. This proximity effect is an entropic factor,8 which reflects the higher probability for a unimolecular reaction to occur within a reaction cage, as opposed to the much lower probability for a bimolecular reaction. This may result in rate enhancement of up to 13 orders of magnitude.9
In a SmI2 reaction that includes protonation, there are three relevant preassociations: (1) proton donor with SmI2, (2) SmI2 with the substrate, and (3) proton donor with the substrate.
Preassociation of the Proton Donor with SmI2
As demonstrated above, it is well established that preassociation of the proton donor with SmI2 significantly affects the reaction.10 Following electron transfer, Sm3+I2 complexed with the proton donor is paired to the radical anion of the substrate. The most obvious effect of this preassociation is on the protonation rate. Thus, if protonation of the radical anion is rate determining (eq 2), the proximity of the proton donor to the radical anion renders the protonation highly efficient, diminishing the rate of the back electron transfer. If a back electron transfer takes place, the Coulombic attraction, which keeps the ROH–Sm3+ near the radical anion, is lost, and most probably, the reactants will escape the solvent cage, and no reaction will occur.
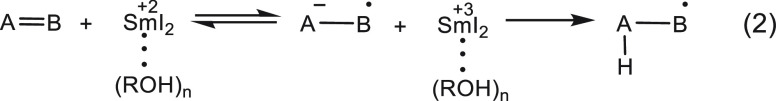 |
2 |
The significance of this proximity is inversely proportional to the lifetime of the radical anion; when the lifetime is shorter, the probability of a bimolecular encounter with a proton donor from the bulk is diminished. Consequently, the need for a neighboring proton donor is increased. This is nicely demonstrated in the following example (eq 3).1
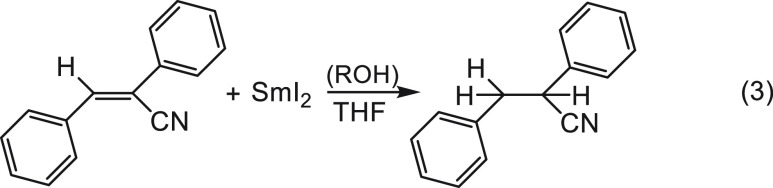 |
3 |
In this system the lifetime of its radical anion is relatively very short. As a result, proton donors that do not complex to SmI2 such as trifluoroethanol (TFE), i-PrOH, or t-BuOH did not lead to any reaction. However, in the presence of water or ethylene glycol, which do coordinate to SmI2, rapid reactions took place (τ1/2 < 1 ms). Thus, because of the low electrophilicity of the substrate, the radical anion in this endothermic reaction has a very short lifetime and can only be successfully trapped by a proton donor that resides in its vicinity.
In order to rule out the possibility that the reaction is a hydride transfer as suggested by a referee or that it is a HAT, transfer of a hydrogen atom,11 we performed an experiment with the less stable cis isomer in which it reacted with SmI2 in the absence of a proton donor. As hydride or H transfer reactions are irreversible, the observed conversion of the cis isomer into the trans isomer (eq 4) proves unequivocally that the reaction course is an electron transfer followed by a proton transfer and rules out the two alternative mechanisms.
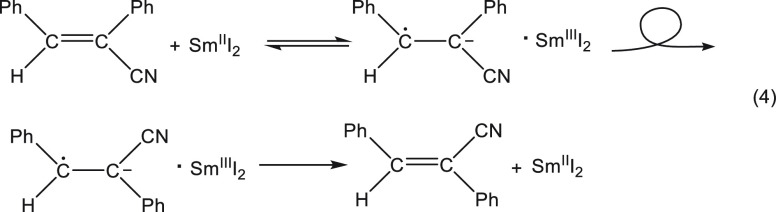 |
4 |
A side issue, which is both very interesting and counterintuitive, was discovered upon comparison of the kinetic and the product isotope effects using MeOH/MeOD. Most chemists will agree that the charge in the radical anion resides mainly on the carbon α to the cyano group as shown in eq 4. This intuitively suggests that protonation will occur on this carbon. However, four repetitive determinations of the product isotope effect showed that the H/D incorporation isotope effect on the α carbon is 4.5 ± 0.2, whereas for the β carbon it is 6.8 ± 0.3. Yet, the kinetic isotope effect was 6.7 ± 0.3, showing that the first protonation, which locks the reaction and prevents its reversal, occurs on the β rather than the α carbon. Based on this and other evidence, it was shown that protonation occurs in such a way that the most stable radical is formed.12
We next used this strategy to broaden the scope of photostimulated SmI2 reactions.13 Irradiation of SmI2 at its λmax (ca. 600 nm) converts it to a powerful electron donor, which enables an electron transfer even to substrates with a very high LUMO (the excitation energy of SmI2 around 600 nm is equivalent to ca. 2.2 eV, which is much more than the 0.72 V increase in reduction potential effected by 4 equiv of hexamethylphosphoramide (HMPA)10). The major deficiency of this procedure is that when applied to reduction of resistant substrates, it yields a high-energy radical anion, which will eagerly give back the additional electron to Sm3+. Therefore, the photostimulated reductions were successful only in cases of dissociative electron transfer reactions such as shown in eqs 5 and 6. In these reactions, the electron transfer is the rate-determining step, as the cleavage of the leaving group is coupled to the electron transfer. However, the fact that we can bring the proton donor to the reaction center enables the trapping of even very short-lived radical anions such as those of naphthalene and diphenylacetylene. Figure 3 displays diode array kinetics of the reduction of naphthalene in the presence of a MeOH in comparison with the reaction in the presence of TFE, which is a stronger proton donor but unlike MeOH does not complex to SmI2. It should be pointed out that in the dark no reaction takes place.
 |
5 |
| 6 |
Figure 3.
Diode array monitoring of the reaction of naphthalene (0.2 M) and SmI2 (2.5 mM) in the presence of (a) MeOH (1 M, 200 s) and (b) TFE (1 M, 800 s). Reproduced with permission from ref (13). Copyright 2010 Wiley-VCH.
The preassociation of the proton donor with SmI2 may lead not only to rate enhancement and the ability to reduce substrates that resist “normal” reduction but also to different products with the same substrate. This is beautifully demonstrated by the elegant work of Procter et al. depicted in eq 7, which was the major stimulus for our work on this topic.14
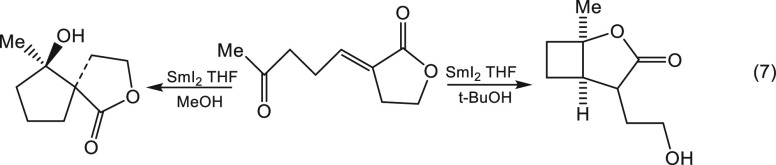 |
7 |
The authors suggest14 that t-BuOH and MeOH differ in the rate of protonation of the radical anion. Our work makes it clear that MeOH, unlike t-BuOH, is capable of complexing SmI2. Thus, MeOH will protonate the radical anion at a much higher rate, not only because of its higher acidity but also because it changes the protonation mechanism from a bimolecular to a unimolecular one.
We turn to another surprising and important facet of the proximity effect on synthetic feasibility. A paper published in 1995 by Cabri15 showed that although the addition of water or amine to a reaction mixture does not affect much the reactions of SmI2, their combination significantly facilitates reaction rates. This discovery lay dormant for seven years until the group of Hilmersson brought it back to the public attention.16 The water/amine combination received a major push by Procter and Szostak17 who, in a long series of papers, got the best out of this “mixture” for synthetic applications. Procter faithfully described the situation in the following words: “the mechanistic details of this process, including the critical role of amine and H2O additives, remained unclear”.17e
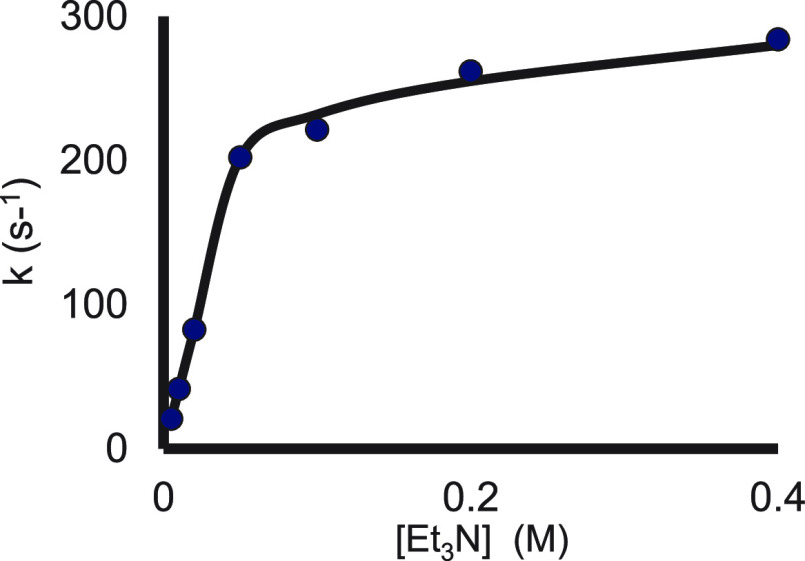
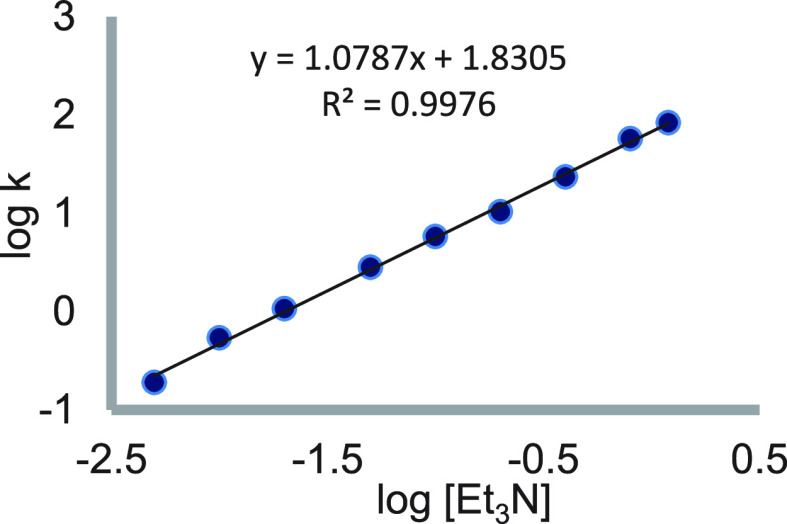
Probing this reaction, we noticed that gradual addition of an amine, such as Et3N, that does not complex to SmI2 results in a rate enhancement, which levels off (e.g., 3-methyl-2-butanone, see Figure 4).2 The kinetic order in the amine before the onset of the plateau is one.
Figure 4.
Rate constants as a function of Et3N concentration in the reaction of SmI2 with 3-methyl-2-butanone in the presence of water. Reproduced with permission from ref (2). Copyright 2015 Wiley-VCH.
Our working hypothesis was that the role of the amine is to deprotonate a water molecule bound to the samarium cation, with subsequent generation of a hydroxide ion next to it. This would reduce the effective charge on the Sm3+ and consequently its ability to accept the electron back from the radical anion. The deprotonation could occur before the electron transfer, in concert with it, or after it. Using the classical tool of physical organic chemistry to distinguish between specific and general base catalysis,18 we showed that the reaction is a classic case of general base catalysis. Namely, the proton is transferred at the rate-determining step. This and other experiments rule out the first and second aforementioned options and leave us with the third option shown in eq 8 (A = substrate, only one water molecule is shown).
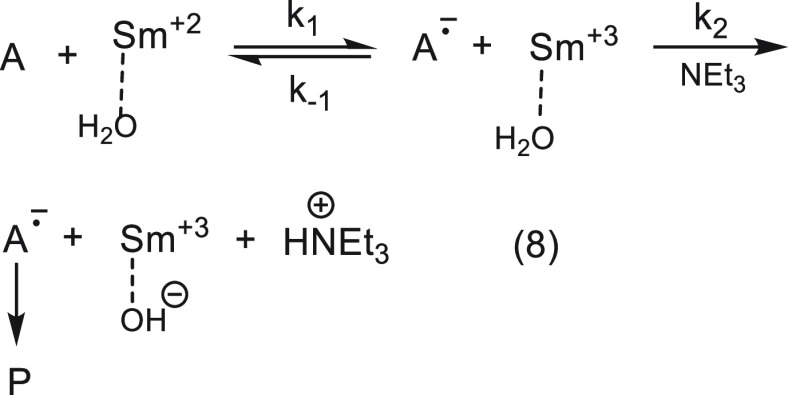 |
8 |
The major conclusion of this section is that the range of substrates that react with SmI2 can be vastly expanded to include resilient substrates by using proton donors that efficiently complex to SmI2. We identified two mechanisms by which a proton donor “riding on the back” of the samarium can trap a short-lived radical anion and prevent the back electron transfer to Sm3+. The first is by an efficient unimolecular protonation of the radical anion within the ion pair.7b The second mechanism is by reducing the affinity of the Sm3+ to accept back the electron from the radical anion by generating a negatively charged hydroxide ion coordinated to it.2
Preassociation of SmI2 with the Substrate
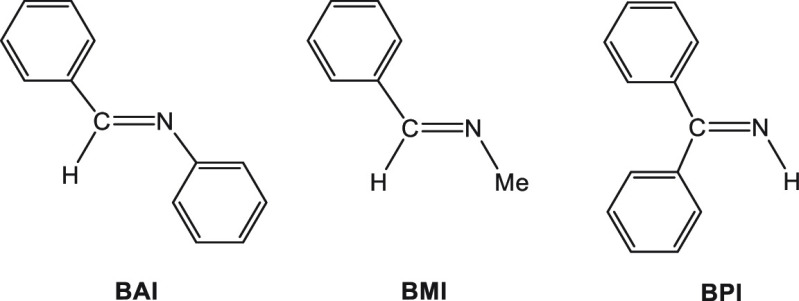
The advantage of uni- over bimolecularity applies here as well, and it is clear that preassociation of SmI2 with the substrate will significantly enhance the rate of electron transfer. This phenomenon was termed “substrate-directable reactions” in a paper published by Procter, Szostak, and Flowers where the association of SmI2 with a carbonyl function was enhanced by a neighboring OH group.19 Similarly, we showed that in the reduction of the imine depicted in Chart 1, the SmI2 preassociates with the lone pair on the imine nitrogen.20
Chart 1. Three Imines Used in the Preassociation Study.
The reactivity order is BPI > BMI > BAI. It does not conform with the electron affinity order of the substrates but rather with the accessibility of the nitrogen lone pair for complexation with SmI2, demonstrating once again the importance of preassociation with the substrate.
It should be emphasized that this binding may not necessarily be to the reaction center but rather to a remote site in the molecule. This is exemplified in the case of 4-styrylpyridine, where the samarium binds to the lone pair of the pyridine nitrogen while the actual reduction takes place on the double bond (eq 9).21
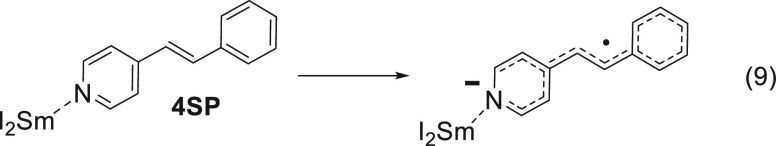 |
9 |
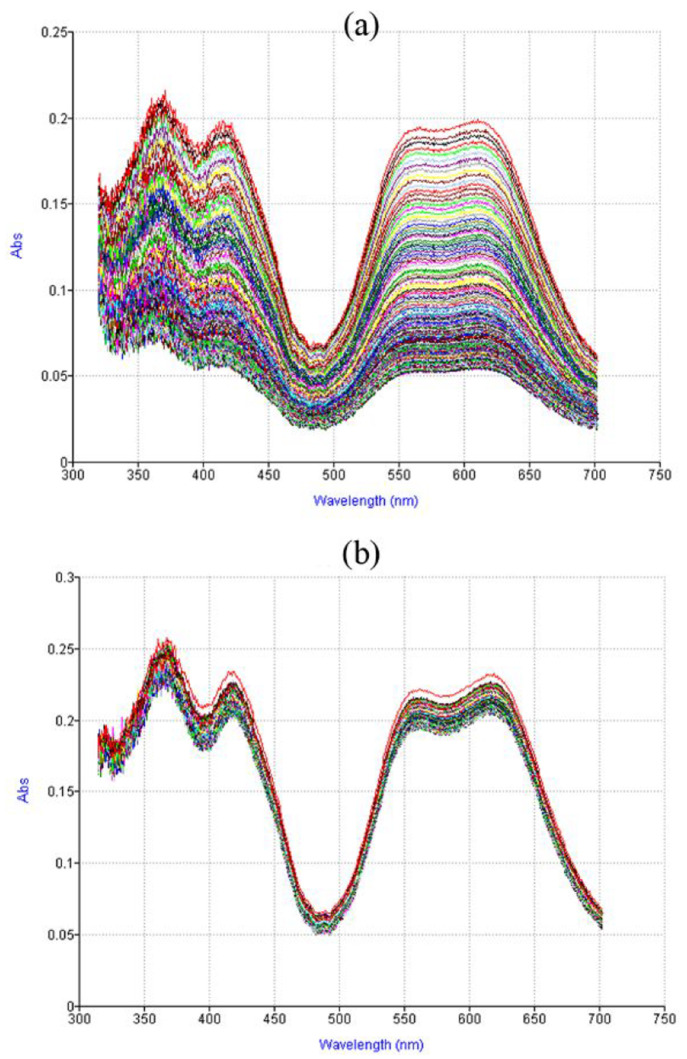
The efficacy of the proximity effect culminates in the reduction of benzene.22 The reduction of benzene is usually achieved by using strong reducing agents.23 In order to position the SmI2 in the vicinity of the benzene ring, we used bidentate ligands (ethanolamine and ethylenediamine) as side arms on the benzene ring. These ligands are known to bind strongly to SmI2, as demonstrated by visible spectroscopy (Figure 5).
Figure 5.
Visible spectra of SmI2 as a function of BNO concentration. Reproduced with permission from ref (22). Copyright 2017 Wiley-VCH.
However, due to the resistance of benzene toward reduction, the proximity itself was insufficient to induce a reduction, and we had to use in addition the water/amine magic mixture in order to execute a reduction. After 24 h, 95% conversion into the two classical products of the Birch reduction24 was observed (eq 10).
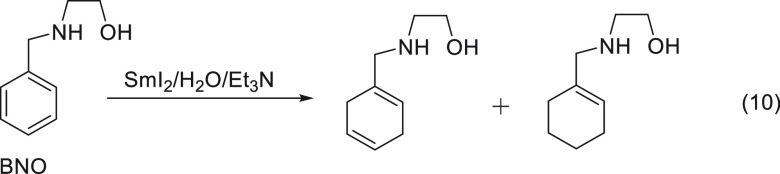 |
10 |
The aforementioned examples show that the association of SmI2 with the substrate opens new vistas for SmI2 reactions.
Preassociation of the Proton Donor with the Substrate
The benefit of preassociation of the proton donor with the substrate is somewhat less commonly encountered or discernible. If it occurs, its effect can be masked by the rate enhancement due to the protonation step. Moreover, hydrogen bonding between a carbonyl oxygen and the proton donor must compete with the hydrogen bonding to the oxygen of THF, which is the common solvent for SmI2 reactions. Therefore, it may be of a low likelihood. Higher probability and visibility of such bonding are with substrates that contain basic nitrogen as shown in the Proton-Coupled Electron Transfer (PCET) section.
Aza- versus Oxophilicity
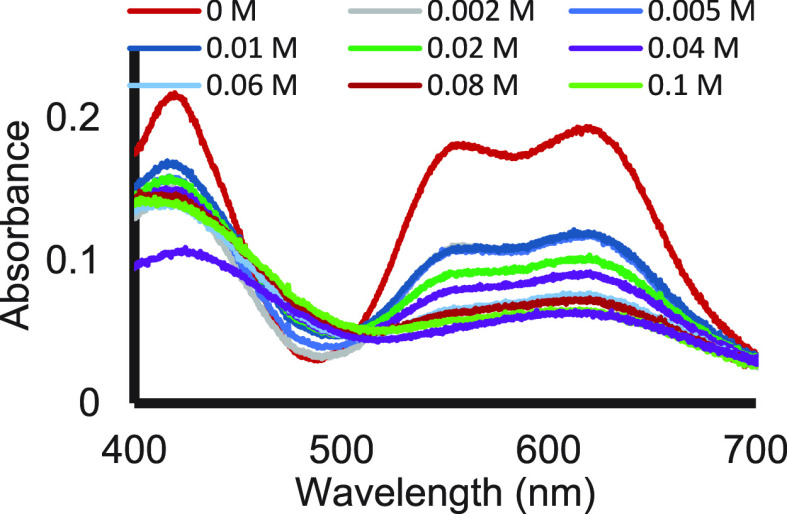
It is well established that SmI2 is an oxophilic reagent. To our surprise, however, we recently discovered that its affinity for nitrogen compounds is much higher than that for their oxygen-based analogs.3 This was shown using several diagnostic tools. Complexation to SmI2 is easily detected by visible spectroscopy. In Figure 6, it is shown that 0.1 M pyrrolidine, the nitrogen analog of THF, is sufficient to fully displace all the solvent THF molecules from the coordination sphere despite the 2 orders of magnitude higher (12.3 M) concentration of THF.
Figure 6.
Visible spectra of SmI2 as a function of pyrrolidine concentration. Reproduced with permission from ref (3). Copyright 2017 Wiley-VCH.
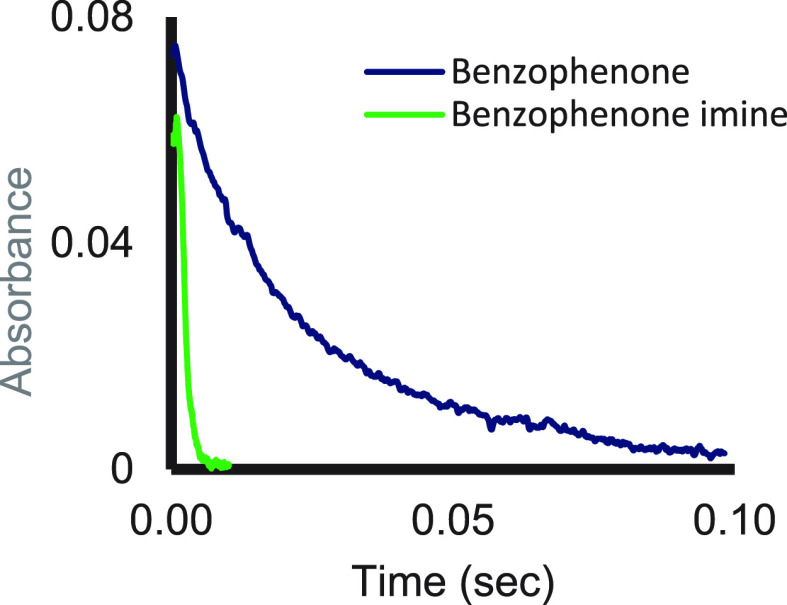
Similarly, for ethylene glycol (EG) to reach spectral saturation, concentrations ten times higher than its nitrogen analog ethylenediamine are needed. The higher azaphilicity was also demonstrated by comparing the kinetics of benzophenone and its aza analog. Under the same conditions, benzophenone imine reacts in nearly the dead time of the stopped-flow spectrometer whereas the reduction of benzophenone itself is about 30 times slower despite its much higher reduction potential (Figure 7).25
Figure 7.
Kinetic traces for the reactions of SmI2 with benzophenone and benzophenone imine. Reproduced with permission from ref (3). Copyright 2017 Wiley-VCH.
The higher azaphilicity was demonstrated for mono- and bidentate ligands, cyclic and acyclic molecules, and crown ethers, as well as for both sp3 and sp2 hybridized systems. It is more pronounced for Sm3+ suggesting that it is governed by the same factors that control basicity. As Sm3+ is harder than Sm2+, it bears a higher resemblance to a proton, and therefore the more basic nitrogen compounds show a larger affinity to it. This is in fact the origin of the larger enhancing effect of nitrogen ligands on the reduction potential of SmI2 relative to their oxygen analogs.
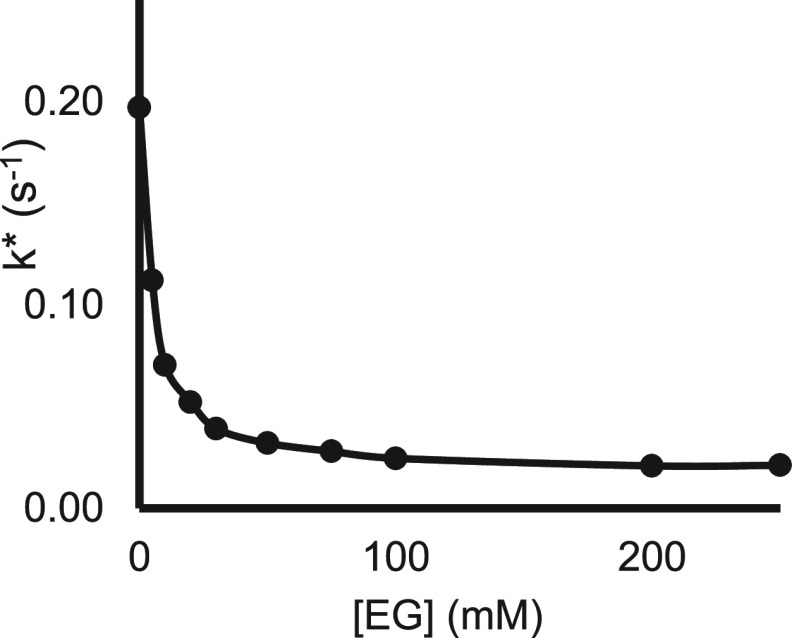
Optimal Coordination Level
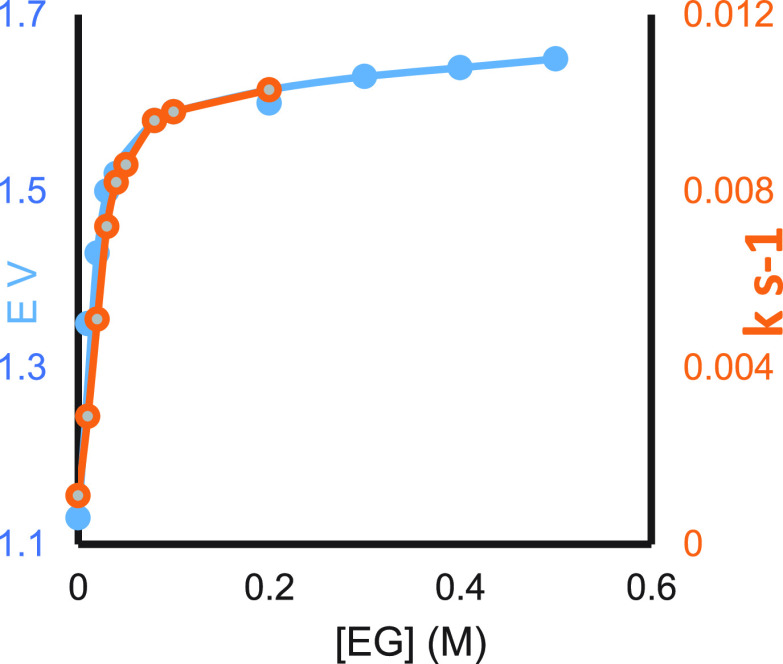
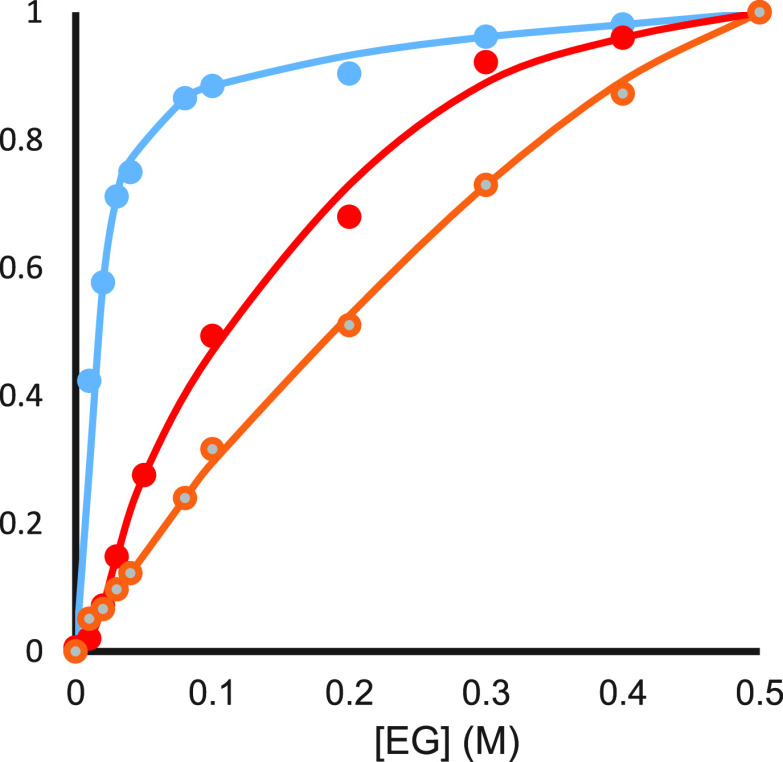
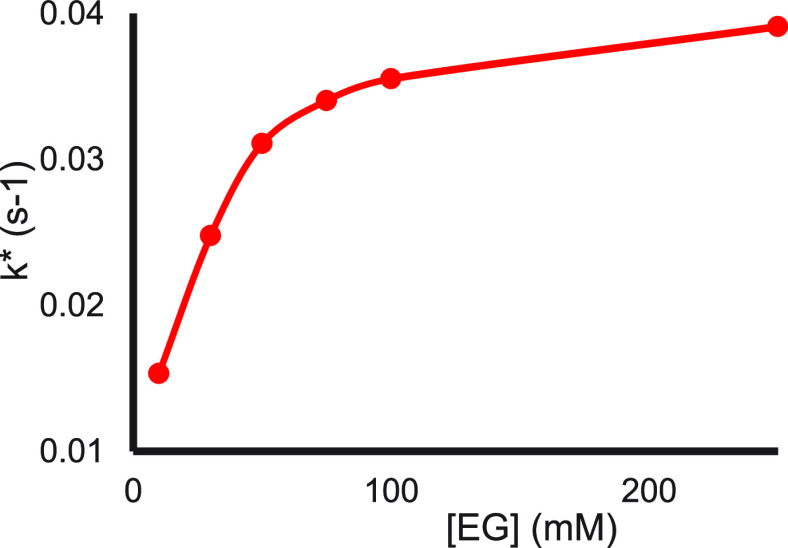
Figure 8 shows the effect of EG on the reduction potential of SmI2 and on the rate constants for the benzyl chloride reaction.26 As can be seen, the two graphs coincide. However, with anthracene and cyclohexanone, the graphs are separate, and the one for cyclohexanone is closer to the reduction potential curve than the one for anthracene (Figure 9).
Figure 8.
Reduction potential (blue) and rate constant (orange) of SmI2 for benzyl chloride as a function of EG concentration.
Figure 9.
Normalized SmI2 reduction potentials (blue), rate constants with anthracene (orange), and rate constants with cyclohexanone (red) as a function of EG concentration.
Similar results were found for other ligands and substrates. The variance in behavior is indicative of the nature of the rate-determining step. When the electron transfer is rate determining, as for benzyl chloride, the reduction potential and reaction rate constant curves overlap. However, when protonation is rate determining (anthracene and cyclohexanone), a larger concentration of ligand is needed to reach the leveling off region. The faster is the protonation, the more the curve will resemble that of the reduction potential. In general, protonation on oxygen is much faster than on carbon.18 If this is correct for the radical anions of cyclohexanone and anthracene as well, then it is clear why the curve for cyclohexanone is closer to that of the reduction potential than that of anthracene.
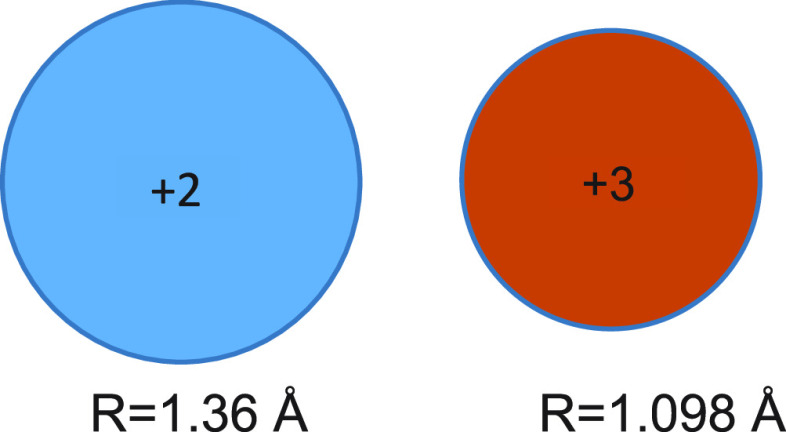
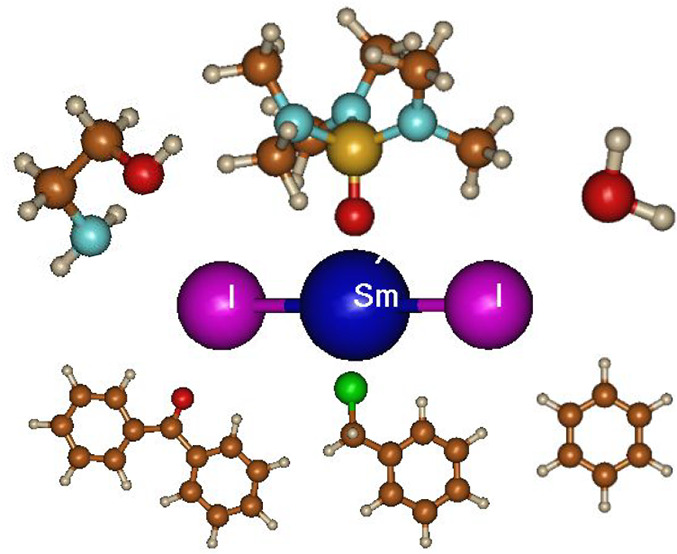
An interesting question is why does the concentration of ligand needed for reaching maximum reduction potential differ from the concentration needed for the maximal protonation rate? The answer is that different tasks need different coordination levels. Ligands increase the reduction potential of SmI2, as the energy gain by coordination to Sm3+ is larger than that obtained by coordination to Sm2+. As the surface area of Sm2+ is larger by more than 50% than that of Sm3+ (Figure 10)27 and as the reduction potential is affected by coordination to Sm3+, it is superfluous to maintain on Sm2+ more ligand molecules than Sm3+ can accommodate. Therefore, the plateau is achieved early. However, for reactions involving protonation, the more proton donor molecules are in the vicinity of the radical anion, the greater the probability for protonation. Therefore, ligand saturation of the larger Sm2+ is beneficial, and the plateau is achieved at higher ligand concentrations.
Figure 10.
Relative sizes of Sm3+ and Sm2+.
Proton-Coupled Electron Transfer (PCET)
In this Account, we shall use the term PCET in its original context. Namely, we shall refer only to cases where the proton and electron are transferred simultaneously to and from two different sites.28 In recent years, PCET was also used to denote a hydrogen atom transfer (HAT). It would seem that the HAT mechanism cannot be very common in SmI2 reactions, as most of the reactions are accelerated by the water/amine system.15−17 Catalysis by this magic mixture is a clear indication of an electron transfer in a pre-equilibrium step, which immediately rules out a HAT mechanism. In addition, HAT cannot operate in the reduction of functional groups for which an inner sphere electron transfer was proven.
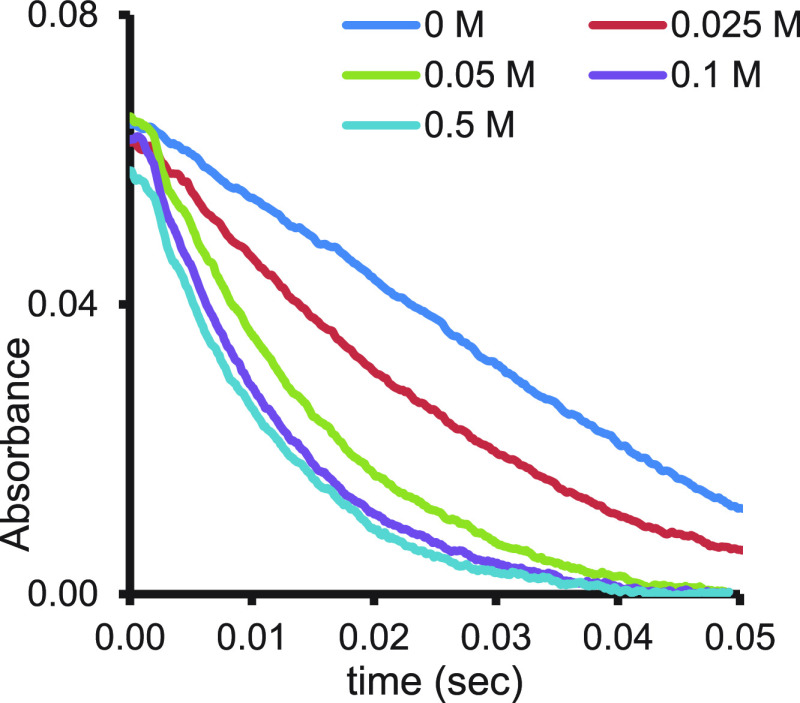
The first proven PCET mechanism in the chemistry of SmI2 was found in the water/amine reaction of benzyl chloride.2 As mentioned before, increasing the concentration of the amine induces rate enhancement, which eventually levels off as the electron transfer becomes rate determining (Figure 4). However, with benzyl chloride no such leveling off is observed, and the rate order in the amine remains one up to the highest amine concentration used (Figure 11).
Figure 11.
Amine kinetic order in the reaction of benzyl chloride (10 mM) with SmI2 (1 mM) and water (50 mM). Reproduced with permission from ref (2). Copyright 2015 Wiley-VCH.
The reduction mechanism of benzyl chloride is different from that of common substrates, as the expulsion of the leaving group is concomitant with the electron transfer.29 Therefore, if electron transfer occurs in the rate-determining step and the deprotonation by the amine is also rate determining (the order in the amine is unity, and the proton transfer is general base catalyzed), it implies that the deprotonation and electron transfer occur simultaneously in the rate-determining step. The electron is transferred to the benzyl chloride, while the proton is transferred to the amine (eq 11). Hence, this is a clear case of the classical PCET mechanism.
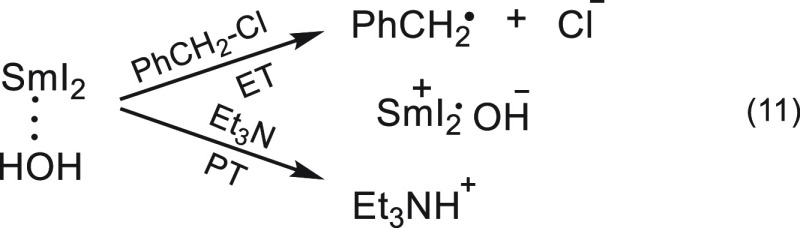 |
11 |
In the two examples below, the PCET mechanism is not fully proven but is highly likely. In the aforementioned case of 4-styrylpyridine (eq 9),21 the reaction is catalyzed both by TFE (Figure 12), which does not bind to SmI2, and by MeOH that binds to it. Catalysis is probably due to hydrogen bonding to the pyridine nitrogen lone pair, which increases its electrophilicity. The mechanistic options for this reaction are shown in Figure 13.
Figure 12.
Kinetic traces showing the effect of TFE concentration on the reaction of SmI2 (0.5 mM) with 4SP (5 mM).
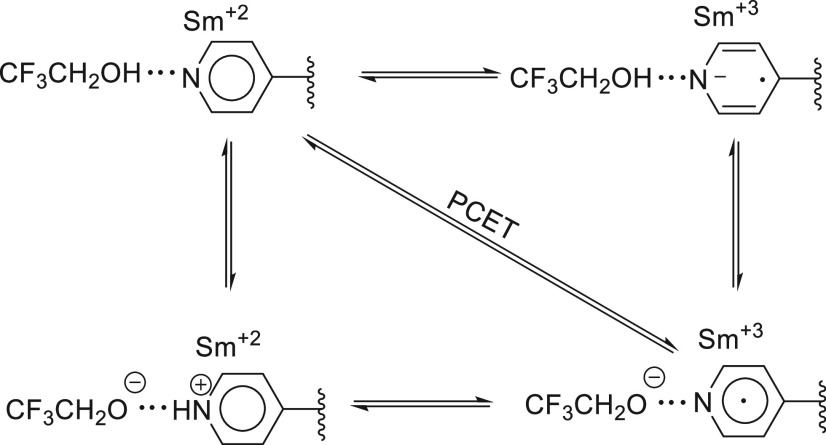
Figure 13.
Schematic presentation of possible reaction mechanisms of 4SP.
Motion along the periphery of the square diagram implies a stepwise mechanism, whereas motion along the diagonal implies a concerted PCET mechanism. The likelihood of this latter mechanism is high due to the hydrogen bond, which positions the proton in the optimal position for proton transfer. As proton transfer between two heteroatoms is very fast, it is highly likely that the proton transfer from the alcohol to the nitrogen will be synchronous with the electron transfer. It should be noted that the catalysis by proton donors competes with the catalytic effect resulting from coordination of the SmI2 to the nitrogen lone pair. The relative effect depends on the binding equilibrium constants, the concentrations ratios, and the individual rate constants.
A similar case is encountered in the mesolytic cleavage of benzyl halides substituted by groups such as p-CN and p-CO2Me. In these cases, we have shown30 that, in accordance with the Bunnett and Rossi rule,31 these systems will undergo an inverse mesolytic cleavage (eq 12b).
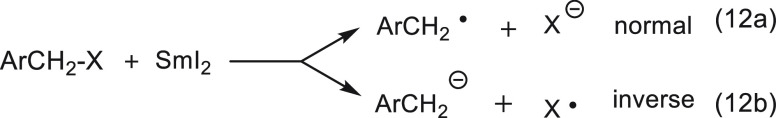 |
12 |
The halide thus departs as a radical, while the negative charge remains on the benzylic system.
The said activating groups are capable of hydrogen bonding to proton donors. Added methanol was indeed found to catalyze only the reactions of the p-CN and the p-CO2Me derivatives and, in line with the inverse cleavage mechanism, did not affect the rate of the other substituted benzyl halides. Thus, in the first step, the MeOH forms a hydrogen bond with a sigma lone pair on these activating groups. When the electron is transferred to the π system of the ring, a concomitant tightening of the hydrogen bond in the orthogonal sigma system takes place to form the covalent N–H bond that neutralizes the negative charge on the ring (eq 13 for p-CN). Departure of a Br radical and aromatization of the para quinoidic structure lead eventually to formation of the corresponding toluene.
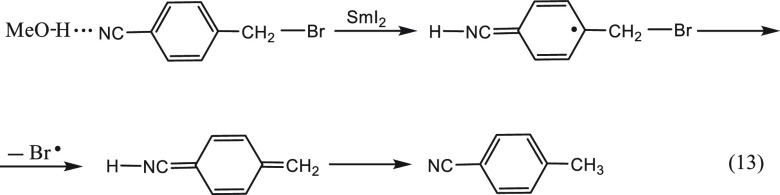 |
13 |
Inner and Outer Sphere Electron Transfer
The issue of inner versus outer sphere electron transfer in the reactions of SmI2 was the subject of several publications.13,32,33 Whether the electron is transferred by an inner or an outer sphere mechanism depends on the mode by which the substrate stabilizes the negative charge of the radical anion. We showed that the Coulombic attraction between the Sm3+ and a localized negative charge in the radical anions of benzophenones contributes tens of kilocalories per mole to the stability of the system.30 Thus, when the charge is localized on an electronegative atom of the substrate, having the Sm3+ in its close vicinity is energetically highly beneficial, leading to an inner sphere electron transfer mechanism. However, in cases where the negative charge is stabilized by delocalization, as in arenes, close interaction with the Sm3+ is counterproductive as it causes charge localization. Therefore, an outer sphere electron transfer mechanism is preferred in these cases. Consequently, an intensive coordination of ligands to SmI2 may prevent the association of the Sm3+ with an electronegative atom on the radical anion and force an outer sphere electron transfer mechanism in cases where an inner sphere is preferred. This is nicely exemplified in the photocatalyzed reactions of benzyl chloride and anthracene with EG as an additive.34 Increasing the concentration of the ligand reduces the rate for benzyl chloride but enhances the rate for anthracene (cf. Figures 14 and 15)
Figure 14.
Pseudo-first-order rate constants for the photoinduced reaction of benzyl chloride as a function of the concentration of ethylene glycol. Reproduced with permission from ref (34). Copyright 2019 Wiley-VCH.
Figure 15.
Pseudo-first-order rate constants for the photoinduced reaction of anthracene as a function of the concentration of EG. Reproduced with permission from ref (34). Copyright 2019 Wiley-VCH.
In the case of benzyl chloride, a voluminous coordination sphere around the Sm3+ interferes with its ability to stabilize the negative charge on the departing chloride ion, leading to rate retardation. The reaction of anthracene, however, is enhanced because the stabilization of Sm3+ by the ligand hampers the electron from hopping back to the Sm3+, and at the same time provides proton donors for the protonation of the radical anion.
To conclude this section, in photoinduced reactions, when ligand addition causes rate retardation, the reaction proceeds by inner sphere electron transfer. On the other hand, a rate enhancement upon ligand addition indicates with high likelihood an outer sphere electron transfer.
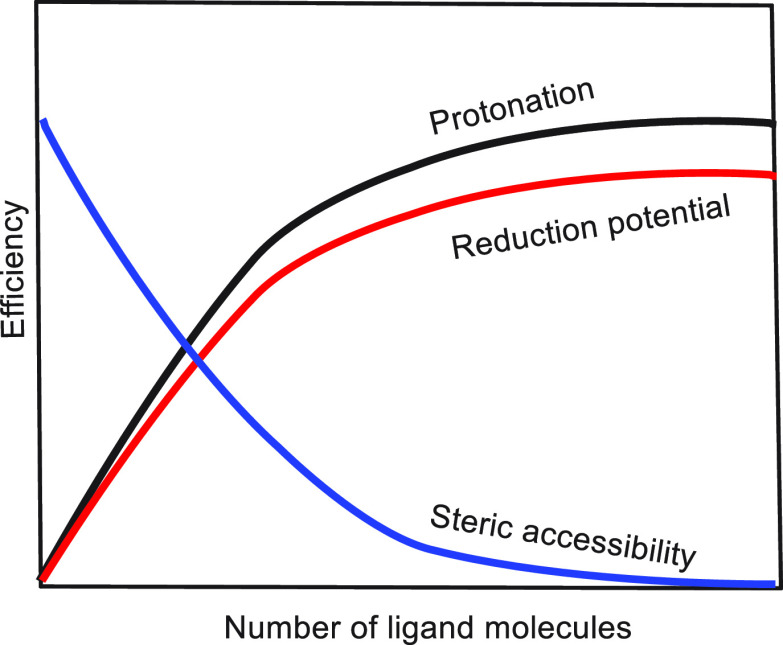
In this respect, it is interesting to analyze ground state reactions in a similar manner.35 The contributions of the various factors to the ground state reaction rate as a function of ligand concentration are shown schematically in Figure 16.
Figure 16.
Effect of coordination number on factors that affect the reaction rate.
Addition of small concentrations of a ligand that is capable of protonation and enhancement of the SmI2 reduction potential may result in rate enhancement since, not considering the relative slopes, there are two rate enhancing factors (reduction potential and protonation) vs only one rate retarding factor (steric accessibility). With HMPA that has only one rate enhancing factor, if a rate retardation is observed at low HMPA concentrations it is clear that preassociation is energetically very important and the reaction is of an inner sphere mechanism as demonstrated for imine and 4SP substrates.20,21 4SP resembles stilbene, which normally reacts by an outer sphere mechanism. However, as the SmI2 binds to the nitrogen lone pair, the electron transfer proceeds by an inner sphere mechanism, and adding low concentrations of HMPA significantly retards the SmI2 coordination to the lone pair, thereby significantly reducing the reaction rate.
Quantitative Determination of Equilibrium Constants of Substrates and Ligands with SmI2
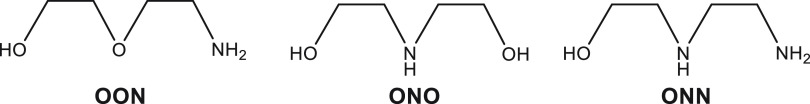
We used two methods to determine the equilibrium constants for the complexation of a ligand to SmI2. One is based on cyclic voltammetry and the other on NMR. The first method was used to determine the equilibrium constants for tridentate ligands shown in Chart 2, as well as the contribution of each added ligand to the reduction potential.35 This was enabled as the CV gave several peaks, each corresponding to a different coordination number (Figure 17). The three successive equilibrium constants for ONO, for example, are 209 ± 32, 444 ± 95, and 313 ± 120 M–1, and the corresponding reduction potentials are 1.55, 1.82, and 2.05 V, respectively.
Chart 2. Tridentate Ligands.
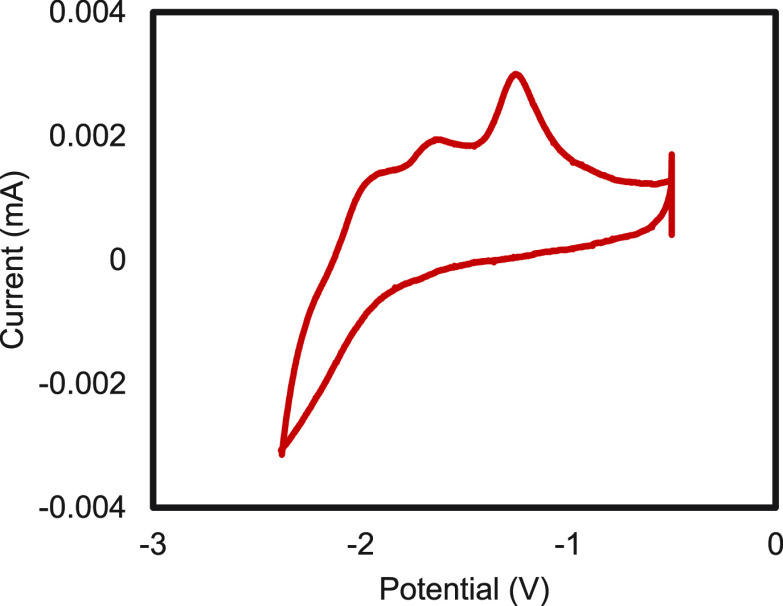
Figure 17.
Cyclic voltammogram of SmI2 (2 mM) in THF in the presence of ONN (1.5 mM).
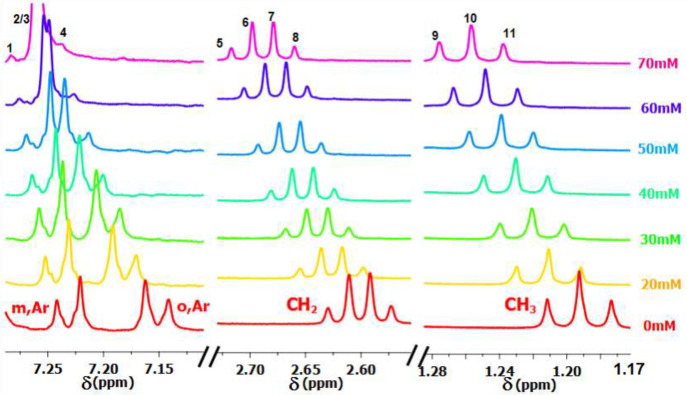
The NMR method, which is much more accurate than the CV, was based on the nearly abandoned concept of lanthanides as shift reagents.36 We used it for the determination of the equilibrium constants for binding ligands and substrates.4Figure 18 shows the effect of various SmI2 concentrations on the chemical shift of p-chloroethylbenzene.
Figure 18.
1H NMR spectra of p-chloroethylbenzene with SmI2 concentration.
The equilibrium constant for the ligation of the first molecule of HMPA to SmI2 was found to be ca. 2500 M–1, compared with 103 M–1 for EG and only 4 M–1 for MeOH.
A unique mechanistic insight was gained by analyzing the equilibrium constants with arenes and haloarenes. The data shows that SmI2 binds to the benzene ring rather than the chlorine atom despite the availability of lone pairs on the latter. Thus, in the case of benzyl chloride, the data suggests that the reaction takes place in two steps. In the first step, the SmI2 binds to the aromatic nucleus, and in the second step, it migrates to the chlorine atom to assist in its departure (eq 14).
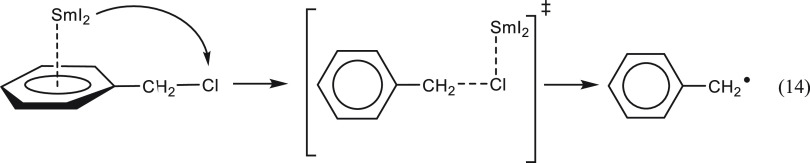 |
14 |
Epilogue
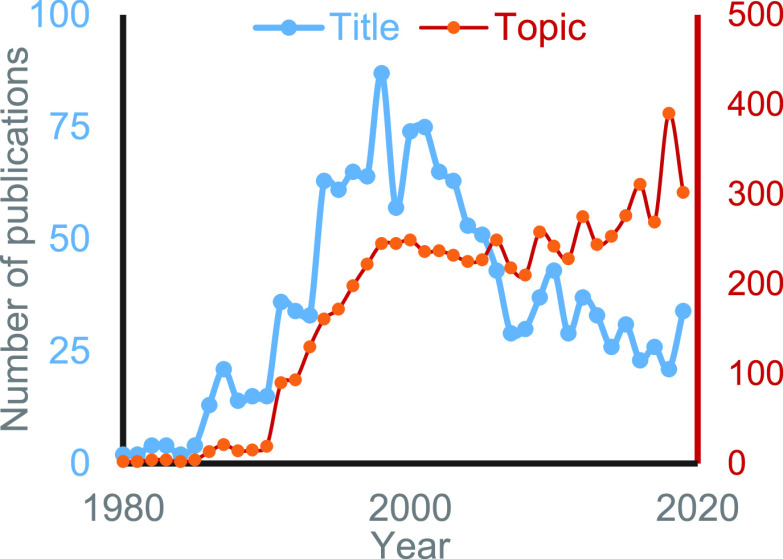
SmI2 has now entered its fifth decade in organic chemistry. A plot of the publications per year in this field during this period as a function of SmI2-related words in the Title or Topic (Figure 19) is very telling and informative.37 Looking at the early years of SmI2 chemistry, one can see that it took a whole decade until the scientific community realized the huge potential of this reagent. Following this latency period, the annual number of papers exploring the scope and limitations of this reagent, rose within less than a decade by more than 10-fold. In the subsequent decade, the field seems to have attained a measure of maturity. Interestingly, if we follow the annual number of publications in which SmI2 appears in the “Title”, there is a decline around the year 2000. This presumably reflects the fact that SmI2 had become pretty much of a conventional reagent, much like NaBH4. However, its overall use, as evaluated by the “Topic” search (Figure 19), seems to remain stable, with a slight increase as more and more people join the “users” group.38
Figure 19.
Annual number of publications on SmI2 based on WOS searches (by Title or Topic).
Regarding studies of the reaction mechanism, it is true that “there is always room for more.” Nevertheless, it seems that the basic forces underlying the chemistry of SmI2 are now largely understood. The contribution of our laboratory to this understanding is firmly based on the foundation laid by those who preceded us in this field. I owe a debt as well, to my contemporaries who supplied a constant flow of novel facts and mechanistic insight, which provided the stimuli and support for our work. We are grateful to them all.
Biography
Shmaryahu Hoz was born in Jerusalem. He received a B.Sc. in Chemistry and Physics and a M.Sc. in Chemistry from the Hebrew University and a Ph.D. in Chemistry from Bar Ilan University. After postdoctoral studies at UCSC with Prof. Joseph F. Bunnett, he joined the Chemistry Department at Bar Ilan University in 1975. Professor Hoz held several administrative positions at Bar Ilan University. He served as the Head of the Department of Chemistry and subsequently as Vice President for Research. His research interests are physical organic chemistry, the chemistry of SmI2, computational nanotechnology, and the effects of electric fields on structure and reactivity.
The author declares no competing financial interest.
References
- Amiel-Levy M.; Hoz S. Guidelines for the Use of Proton Donors in SmI2 Reactions: Reduction of α-cyanostylbene. J. Am. Chem. Soc. 2009, 131, 8280–8284. 10.1021/ja9013997. [DOI] [PubMed] [Google Scholar]
- Maity S.; Hoz S. Deciphering a 20 Year Old Conundrum: The Mechanisms of the Reduction by the Water/Amine/SmI2 Mixture. Chem. - Eur. J. 2015, 21, 18394–18400. 10.1002/chem.201503104. [DOI] [PubMed] [Google Scholar]
- Maity S.; Flowers R. A. II; Hoz S. Aza versus Oxophilicity of SmI2: A Break of a Paradigm. Chem. - Eur. J. 2017, 23, 17070–17077. 10.1002/chem.201703394. [DOI] [PubMed] [Google Scholar]
- De S.; Gottlieb H. E.; Hoz S. Quantification of the Interaction of SmI2 with Substrates and Ligands. Chem. - Eur. J. 2020, 26, 6846–6850. 10.1002/chem.201905233. [DOI] [PubMed] [Google Scholar]
- a Molander G. A.; Harris C. R. Sequencing Reactions with Samarium(II) Iodide. Chem. Rev. 1996, 96, 307–338. 10.1021/cr950019y. [DOI] [PubMed] [Google Scholar]; b Edmonds D. J.; Johnston D.; Procter D. J. Samarium(II)-Iodide-Mediated Cyclizations in Natural Product. Chem. Rev. 2004, 104, 3371–3404. 10.1021/cr030017a. [DOI] [PubMed] [Google Scholar]; c Nicolaou K. C.; Ellery S. P.; Chen J. S. Samarium Diiodide Mediated Reactions in Total Synthesis. Angew. Chem., Int. Ed. 2009, 48, 7140–7165. 10.1002/anie.200902151. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Szostak M.; Procter D. J. Concise Syntheses of Strychnine and Englerin A: The Power of Reductive Cyclizations Triggered by Samarium Iodide. Angew. Chem., Int. Ed. 2011, 50, 7737–7739. 10.1002/anie.201103128. [DOI] [PubMed] [Google Scholar]; e Procter D. J.; Flowers R. A. II; Skrydstrup T.. Organic Synthesis using Samarium Diiodide: A Practical Guide; Royal Society of Chemistry Publishing, 2010. [Google Scholar]; f Szostak M.; Fazakerley N. J.; Parmar D.; Procter D. J. Cross-Coupling Reactions Using Samarium(II) Iodide. Chem. Rev. 2014, 114, 5959–6039. 10.1021/cr400685r. [DOI] [PubMed] [Google Scholar]; g Chciuk T. V.; Flowers R. A. II In Science of Synthesis; Marek I., Ed.; Georg Thieme Verlag KG: Stuttgart, 2016; p 177. [Google Scholar]; h Kern N.; Plesniak M. P.; McDouall J. J. W.; Procter D. J. Enantioselective cyclizations and cyclization cascades of samarium ketyl radicals. Nat. Chem. 2017, 9, 1198–1204. 10.1038/nchem.2841. [DOI] [PubMed] [Google Scholar]; i Huang H.-M.; McDouall J. J. W.; Procter D. J. Radical Anions from Urea-type Carbonyls: Radical Cyclizations and Cyclization Cascades. Angew. Chem., Int. Ed. 2018, 57, 4995–4999. 10.1002/anie.201800667. [DOI] [PubMed] [Google Scholar]
- Yella R.; Hoz S. Channeling the SmI2 Reactions to the Radical Path: Radicals Resisting Reduction by SmI2. Org. Lett. 2014, 16, 3876–3879. 10.1021/ol501490f. [DOI] [PubMed] [Google Scholar]
- a Yacovan A.; Hoz S.; Bilkis I. Reactions of SmI2 with Olefins: Mechanism and Complexation Effect on Chemoselectivity. J. Am. Chem. Soc. 1996, 118, 261–262. 10.1021/ja950937d. [DOI] [Google Scholar]; b For the most recent utilization of this concept see:Li H.; Hou Y.; Liu C.; Lai Z.; Ning L.; Szostak R.; Szostak M.; An J. Pentafluorophenyl Esters: Highly Chemoselective Ketyl Precursors for the Synthesis of α,α-Dideuterio Alcohols Using SmI2 and D2O as a Deuterium Source. Org. Lett. 2020, 22, 1249–1253. 10.1021/acs.orglett.9b04383. [DOI] [PubMed] [Google Scholar]
- Page M. I.; Jencks W. P. Entropic Contributions to Rate Accelerations in Enzymic and Intramolecular Reactions and the Chelate Effect. Proc. Natl. Acad. Sci. U. S. A. 1971, 68, 1678–1683. 10.1073/pnas.68.8.1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Kirby A. J. Adv. Phys. Org. Chem. 1980, 17, 183–278. 10.1016/S0065-3160(08)60129-X. [DOI] [Google Scholar]; b Mandolini L. Adv. Phys. Org. Chem. 1986, 22, 1–111. 10.1016/S0065-3160(08)60167-7. [DOI] [Google Scholar]
- Flowers R. A. Mechanistic Studies on the Roles of Cosolvents and Additives in Samarium(II)-Based Reductions. Synlett 2008, 1427–1439. 10.1055/s-2008-1078414. [DOI] [Google Scholar]
- Shi S.; Szostak R.; Szostak M. Proton-coupled electron transfer in the reduction of carbonyls using SmI2–H2O: implications for the reductive coupling of acyl-type ketyl radicals with SmI2–H2O. Org. Biomol. Chem. 2016, 14, 9151–9157. 10.1039/C6OB01621A. [DOI] [PubMed] [Google Scholar]
- Rozental E.; Hoz S. Intrinsic Barrier for Protonation of Radical Anions. Tetrahedron 2009, 65, 10945–10949. (ab initio calculations show that indeed, when the radical resides on the α carbon it is more stable by 9.7 kcal/mol than if it resides on the β carbon; therefore, protonation of the radical anion is preferred on the carbon β to the cyano group1) 10.1016/j.tet.2009.10.092. [DOI] [Google Scholar]
- Amiel-Levy M.; Hoz S. Broadening the Scope of Photostimulated SmI2 Reductions. Chem. - Eur. J. 2010, 16, 805–809. 10.1002/chem.200902198. [DOI] [PubMed] [Google Scholar]
- Hutton T. K.; Muir K. W.; Procter D. Switching between Novel Samarium(II)-Mediated Cyclizations by a Simple Change in Alcohol Cosolvent. Org. Lett. 2003, 5, 4811–4814. 10.1021/ol0358399. [DOI] [PubMed] [Google Scholar]
- Cabri W.; Candiani I.; Colombo M.; Franzoi L.; Bedeschi A. Non-Toxic Ligands in Samarium Diiodide-Mediated Cyclizations. Tetrahedron Lett. 1995, 36, 949–952. 10.1016/0040-4039(94)02398-U. [DOI] [Google Scholar]
- a Dahlen A.; Hilmersson G. Instantaneous SmI2–H2O-mediated reduction of dialkyl ketones induced by amines in THF. Tetrahedron Lett. 2002, 43, 7197–7200. 10.1016/S0040-4039(02)01673-8. [DOI] [Google Scholar]; b Dahlen A.; Hilmersson G. Selective reduction of carboncarbon double and triple bonds inconjugated olefins mediated by SmI2/H2O/amine in THF. Tetrahedron Lett. 2003, 44, 2661–2664. 10.1016/S0040-4039(03)00369-1. [DOI] [Google Scholar]; c Dahlen A.; Petersson A.; Hilmersson G. Diastereoselective intramolecular SmI2–H2O–amine mediated couplings. Org. Biomol. Chem. 2003, 1, 2423–2426. 10.1039/B305428D. [DOI] [PubMed] [Google Scholar]; d Dahlen A.; Hilmersson G. Chem. - Eur. J. 2003, 9, 1123–1128. 10.1002/chem.200390129. [DOI] [PubMed] [Google Scholar]; e Dahlen A.; Hilmersson G.; Knettle B. W.; Flowers R. A. II Rapid SmI2-Mediated Reductions of Alkyl Halides and Electrochemical Properties of SmI2/H2O/Amine. J. Org. Chem. 2003, 68, 4870–4875. 10.1021/jo034173t. [DOI] [PubMed] [Google Scholar]; f Dahlen A.; Sundgren A.; Lahmann M.; Oscarson S.; Hilmersson G. SmI2/Water/Amine Mediates Cleavage of Allyl Ether Protected Alcohols: Application in Carbohydrate Synthesis and Mechanistic Considerations. Org. Lett. 2003, 5, 4085–4088. 10.1021/ol0354831. [DOI] [PubMed] [Google Scholar]; g Dahlen A.; Hilmersson G. Mechanistic Study of the SmI2/H2O/Amine-Mediated Reduction of Alkyl Halides: Amine Base Strength (pKBH+) Dependent Rate. J. Am. Chem. Soc. 2005, 127, 8340–8347. 10.1021/ja043323u. [DOI] [PubMed] [Google Scholar]; h Ankner T.; Hilmersson G. Instantaneous Deprotection of Tosylamides and Esters with SmI2/Amine/Water. Org. Lett. 2009, 11, 503–506. 10.1021/ol802243d. [DOI] [PubMed] [Google Scholar]
- a Szostak M.; Spain M.; Procter D. J. Electron Transfer Reduction of Carboxylic Acids Using SmI2 H2O Et3N. Org. Lett. 2012, 14, 840–843. 10.1021/ol203361k. [DOI] [PubMed] [Google Scholar]; b Szostak M.; Spain M.; Procter D. J. Selective Synthesis of α, α-Dideuterio Alcohols by the Reduction of Carboxylic Acids Using SmI2 and D2O as Deuterium Source under SET Conditions. Org. Lett. 2014, 16, 5052–5055. 10.1021/ol502404e. [DOI] [PubMed] [Google Scholar]; c Szostak M.; Sautier; Spain M.; Procter D. J. Electron Transfer Reduction of Nitriles Using SmI2–Et3N–H2O: Synthetic Utility and Mechanism. Org. Lett. 2014, 16, 1092–1095. 10.1021/ol403668e. [DOI] [PubMed] [Google Scholar]; d Szostak M.; Spain M.; Eberhart A. J.; Procter D. J. Highly Chemoselective Reduction of Amides (Primary, Secondary, Tertiary) to Alcohols using SmI2/Amine/H2O under Mild Conditions. J. Am. Chem. Soc. 2014, 136, 2268–2271. 10.1021/ja412578t. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Szostak M.; Spain M.; Procter D. J. On the Role of Pre- and Post-Electron-Transfer Steps in the SmI2/Amine/H2O-Mediated Reduction of Esters: New Mechanistic Insights and Kinetic Studies. Chem. - Eur. J. 2014, 20, 4222–4226. 10.1002/chem.201400295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell R. P.The Proton in Chemistry, 2nd ed.; Chapman and Hall, London, 1973. [Google Scholar]
- Szostak M.; Spain M.; Choquette K. A.; Flowers R. A. II; Procter D. J. Substrate-Directable Electron Transfer Reactions. Dramatic Rate Enhancement in the Chemoselective Reduction of Cyclic Esters Using SmI2–H2O: Mechanism, Scope, and Synthetic Utility. J. Am. Chem. Soc. 2013, 135, 15702–15705. 10.1021/ja4078864. [DOI] [PubMed] [Google Scholar]
- Rao C. N.; Hoz S. Autocatalysis and Surface Catalysis in the Reduction of Imines by SmI2. J. Am. Chem. Soc. 2011, 133, 14795–14803. 10.1021/ja205885q. [DOI] [PubMed] [Google Scholar]
- Yella R.; Hoz S. Reduction of 4-Styrylpyridine by SmI2: An Inner Sphere Electron Transfer Case Where the Binding Site Differs from the Reaction Center. Org. Lett. 2013, 15, 5262–5265. 10.1021/ol402484j. [DOI] [PubMed] [Google Scholar]
- Maity S.; Hoz S. Pushing SmI2 Reactions towards the Limit- Entropy Driven Reduction of a Benzene Ring. ChemistrySelect 2017, 2, 2499–2502. 10.1002/slct.201700214. [DOI] [Google Scholar]
- a Birch A. J. Reduction by dissolving metals. Part I. J. Chem. Soc. 1944, 430–436. 10.1039/jr9440000430. [DOI] [Google Scholar]; b Zimmerman H. E. Orientation in Metal Ammonia Reductions. Tetrahedron 1961, 16, 169–176. 10.1016/0040-4020(61)80067-7. [DOI] [Google Scholar]
- a Birch A. J. Reduction by dissolving metals. Part II. J. J. Chem. Soc. 1945, 809–813. 10.1039/jr9450000809. [DOI] [Google Scholar]; b Birch A. J.; Mukherji S. M. Reduction by dissolving metals. Part VI. Some applications in synthesis. J. Chem. Soc. 1949, 2531–2536. 10.1039/jr9490002531. [DOI] [Google Scholar]
- a Zhan S.; Hawley M. D. J. Electrochemical properties of benzophenone imine and the use of the corresponding anion radical as a strong electrogenerated base. J. Electroanal. Chem. Interfacial Electrochem. 1991, 319, 275–290. 10.1016/0022-0728(91)87084-H. [DOI] [Google Scholar]; b Tsierkezos N. G. J. Investigation of the Electrochemical Reduction of Benzophenone in Aprotic Solvents Using the Method of Cyclic Voltammetry. J. Solution Chem. 2007, 36, 1301–1310. 10.1007/s10953-007-9188-4. [DOI] [Google Scholar]
- Maity S.; Nimkar A.; Hoz S. Task-Dependent Coordination Levels of SmI2. J. Org. Chem. 2019, 84, 1994–1998. 10.1021/acs.joc.8b02989. [DOI] [PubMed] [Google Scholar]
- a Rare Earth Coordination Chemistry, Fundamentals and Applications; Huang C., Ed.; Wiley: Singapore, 2010. [Google Scholar]; b Lanthanide and Actinide Chemistry; Cotton S., Ed.; Wiley: U.K., 2007. [Google Scholar]
- a Costentin C.; Robert M.; Saveant J.-M. Concerted Proton-Electron Transfers: Electrochemical and Related Approaches, Cyrille Costentin. Acc. Chem. Res. 2010, 43, 1019–1029. 10.1021/ar9002812. [DOI] [PubMed] [Google Scholar]; b DiLabio G. A.; Ingold K. U. A Theoretical Study of the Iminoxyl/Oxime Self-Exchange Reaction. A Five-Center, Cyclic Proton-Coupled Electron Transfer. J. Am. Chem. Soc. 2005, 127, 6693–6699. 10.1021/ja0500409. [DOI] [PubMed] [Google Scholar]; c Hammes-Schiffer S.; Stuchebrukhov A. A. Theory of Coupled Electron and Proton Transfer Reactions. Chem. Rev. 2010, 110, 6939–6960. 10.1021/cr1001436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Jensen H.; Daasbjerg K. Co-solvent effects on the indirect reduction of alkyl and benzyl halides: experimental evidence of a link between electron transfer and SN1-like processes. J. Chem. Soc. Perkin Trans. 2 2000, 1251–1257. 10.1039/a908970e. [DOI] [Google Scholar]; b Andrieux C. P.; Le Gorande A.; Saveant J.-M. Electron Transfer and Bond Breaking. Examples of Passage from a Sequential to a Concerted Mechanism in the Electrochemical Reductive Cleavage of Arylmethyl Halides. J. Am. Chem. Soc. 1992, 114, 6892–6904. 10.1021/ja00043a039. [DOI] [Google Scholar]
- Yitzhaki O.; Hoz S. Reversed Electron Apportionment in Mesolytic Cleavage:The Reduction of Benzyl Halides by SmI2. Chem. - Eur. J. 2015, 21, 9242–9248. 10.1002/chem.201500519. [DOI] [PubMed] [Google Scholar]
- Rossi R. A.; Bunnett J. F. The Sense of Cleavage of Substituted Benzenes on Reaction with Solvated Electrons, as Determined by a Product Criterion. J. Am. Chem. Soc. 1974, 96, 112–117. 10.1021/ja00808a018. [DOI] [Google Scholar]
- Farran H.; Hoz S. Quantifying the Electrostatic Driving Force behind SmI2 Reductions. Quantifying the Electrostatic Driving Force behind SmI2 Reductions. Org. Lett. 2008, 10, 4875–4877. 10.1021/ol8019692. [DOI] [PubMed] [Google Scholar]
- a Dahlen A.; Nilsson A.; Hilmersson G. Estimating the Limiting Reducing Power of SmI2/H2O/Amine and YbI2/H2O/Amine by Efficient Reduction of Unsaturated Hydrocarbons. J. Org. Chem. 2006, 71, 1576–1580. 10.1021/jo052268k. [DOI] [PubMed] [Google Scholar]; b Prasad E.; Flowers R. A. Reduction of Ketones and Alkyl Iodides by SmI2 and Sm(II)-HMPA Complexes. Rate and Mechanistic Studies. J. Am. Chem. Soc. 2002, 124, 6895–6899. 10.1021/ja026074n. [DOI] [PubMed] [Google Scholar]; c Enemaerke R. J.; Daasbjerg K.; Skrydstrup T. Is samarium diiodide an inner- or outer-sphere electron donating agent?. Chem. Commun. 1999, 343–344. 10.1039/a809277j. [DOI] [Google Scholar]; d Miller R. S.; Sealy J. M.; Shabangi M.; Kuhlman M. L.; Fuchs J. R.; Flowers R. A. Reactions of SmI2 with Alkyl Halides and Ketones: Inner-Sphere vs Outer-Sphere Electron Transfer in Reactions of Sm(II) Reductants. J. Am. Chem. Soc. 2000, 122, 7718–7722. 10.1021/ja001260j. [DOI] [Google Scholar]; e Enemaerke R. J.; Hertz T.; Skrydstrup T.; Daasbjerg K. Evidence for Ionic Samarium(II) Species in THF/HMPA Solution and Investigation of Their Electron-Donating Properties. Chem. - Eur. J. 2000, 6, 3747–3754. . [DOI] [PubMed] [Google Scholar]
- Nimkar A.; Maity S.; Flowers R. A.; Hoz S. Contrasting Effect of Additives on Photoinduced Reactions of SmI2. Chem. - Eur. J. 2019, 25, 10499. 10.1002/chem.201901997. [DOI] [PubMed] [Google Scholar]
- Nimkar A.; Maity S.; Hoz S. Coordination of Tridentate Ligands to SmI2: Cooperativity and Incremental Effect on Reduction Potential and on Reactivity. Pure Appl. Chem. 2020, 92, 85–96. 10.1515/pac-2019-0213. [DOI] [Google Scholar]
- a Evans D. F.; Wyatt M. J. Direct observation of free and complexed substrate in a lanthanide shift reagent system. J. Chem. Soc., Chem. Commun. 1972, 312–313. 10.1039/c39720000312. [DOI] [Google Scholar]; b Cockerill A. F.; Davies G. L. O.; Harden R. C.; Rackham D. M. Lanthanide Shift Reagents for Nuclear Magnetic Resonance Spectroscopy. Chem. Rev. 1973, 73, 553–588. 10.1021/cr60286a001. [DOI] [Google Scholar]; c Mayo B. C. Lanthanide shift reagents in nuclear magnetic resonance spectroscopy. Chem. Soc. Rev. 1973, 2, 49–74. 10.1039/cs9730200049. [DOI] [Google Scholar]; d Reuben J.; Elgavish G. A. In Handbook on the Physics and Chemistry of Rare Earths; Gschneidner K. A., Eyring L., Eds.; Elsevier: Amsterdam, 1979; Vol. 4, pp 483–514. [Google Scholar]
- Based on data gathered from the “Web of Science”, searches for appearance of SmI2, samarium iodide, samarium diiodide, and Sm(II) in the “Title” or “Topic”.
- I thank Professors D. Procter and R. Flowers for sharing their thoughts with me on this matter.