Abstract
Aim
We sought to examine the association of epicardial adipose tissue (EAT) quantified on chest computed tomography (CT) with the extent of pneumonia and adverse outcomes in patients with coronavirus disease 2019 (COVID-19).
Methods
We performed a post-hoc analysis of a prospective international registry comprising 109 consecutive patients (age 64 ± 16 years; 62% male) with laboratory-confirmed COVID-19 and noncontrast chest CT imaging. Using semi-automated software, we quantified the burden (%) of lung abnormalities associated with COVID-19 pneumonia. EAT volume (mL) and attenuation (Hounsfield units) were measured using deep learning software. The primary outcome was clinical deterioration (intensive care unit admission, invasive mechanical ventilation, or vasopressor therapy) or in-hospital death.
Results
In multivariable linear regression analysis adjusted for patient comorbidities, the total burden of COVID-19 pneumonia was associated with EAT volume (β = 10.6, p = 0.005) and EAT attenuation (β = 5.2, p = 0.004). EAT volume correlated with serum levels of lactate dehydrogenase (r = 0.361, p = 0.001) and C-reactive protein (r = 0.450, p < 0.001). Clinical deterioration or death occurred in 23 (21.1%) patients at a median of 3 days (IQR 1–13 days) following the chest CT. In multivariable logistic regression analysis, EAT volume (OR 5.1 [95% CI 1.8–14.1] per doubling p = 0.011) and EAT attenuation (OR 3.4 [95% CI 1.5–7.5] per 5 Hounsfield unit increase, p = 0.003) were independent predictors of clinical deterioration or death, as was total pneumonia burden (OR 2.5, 95% CI 1.4–4.6, p = 0.002), chronic lung disease (OR 1.3 [95% CI 1.1–1.7], p = 0.011), and history of heart failure (OR 3.5 [95% 1.1–8.2], p = 0.037).
Conclusions
EAT measures quantified from chest CT are independently associated with extent of pneumonia and adverse outcomes in patients with COVID-19, lending support to their use in clinical risk stratification.
Abbreviations: ACE-2, angiotensin-converting enzyme 2; BMI, body mass index; COVID-19, Coronavirus disease 2019; CRP, c-reactive protein; CT, computed tomography; ICU, intensive care unit; EAT, epicardial adipose tissue; GGO, ground glass opacities; HU, Hounsfield unit; RT-PCR, reverse transcription polymerase chain reaction; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2
Keywords: COVID-19, SARS-CoV-2, Computed tomography, Epicardial adipose tissue
Highlights
-
•
Epicardial adipose tissue (EAT) volume and attenuation associate with the quantitative burden of COVID-19 pneumonia.
-
•
An increasing EAT volume or attenuation independently predicts clinical deterioration or death.
-
•
EAT may contribute to the augmented systemic inflammatory response to COVID-19.
-
•
The integration of EAT measurements into COVID-19 risk scores has the potential to enhance outcome prediction.
1. Introduction
Coronavirus disease 2019 (COVID-19) is a global pandemic and public health crisis of catastrophic proportions, with over 40 million confirmed cases worldwide as of October 20th 2020 [1]. Although the majority of patients present with no or mild symptoms, up to 20% develop severe disease requiring hospitalization, with 5–8% subsequently being admitted to the intensive care unit (ICU) [2]. Chest computed tomography (CT) is highly sensitive for the diagnosis of COVID-19 pneumonia [3], and the extent of lung abnormalities reflects the disease stage [[4], [5], [6]]. Obesity has been shown to associate with critical illness, need for ICU admission, and prolonged hospitalization in patients with COVID-19 pneumonia [2,[7], [8], [9], [10], [11], [12]], however the underlying pathophysiologic mechanisms remain unclear [13]. Epicardial adipose tissue (EAT) is an active endocrine organ which modulates the metabolic environment of both the coronary arteries and myocardium [14]. EAT volume measured on CT has been shown to associate with lung function in both healthy individuals and those with chronic lung disease [[15], [16], [17], [18]]. Further, CT-derived EAT attenuation (Hounsfield Units [HU]) has an established association with cardiometabolic risk factors and circulating inflammatory markers [19,20]. COVID-19 induces an immune-mediated systemic inflammatory response [21] and it has been postulated that EAT may transduce this inflammation to the heart [22,23]. Moreover, ectopic intrapulmonary fat reservoirs could potentially facilitate viral infiltration and promote local lung inflammation [24,25]. Few studies have evaluated the relationship of EAT measures with radiological and clinical severity of COVID-19 pneumonia [26,27]. In this post-hoc analysis of a prospective, international, multicenter registry, we sought to examine the association of CT-derived quantitative EAT volume and attenuation with pneumonia burden and adverse outcomes in hospitalized patients with COVID-19.
2. Materials and methods
2.1. Study design
This prospective, international, multicenter registry included centers from North America (Cedars Sinai Medical Center, Los Angeles, USA [n = 17]) and Europe (Centro Cardiologico Monzino [n = 75], and Istituto Auxologico Italiano [n = 17]; both Milan, Italy). Consecutive patients who underwent noncontrast chest CT and had a positive reverse transcription polymerase chain reaction (RT-PCR) test result for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) during index admission between January 10 and April 14, 2020 (Fig. 1 ) were enrolled. Chest CT was performed to aid in the triage of patients with a high clinical suspicion for COVID-19, in the setting of a pending or negative RT-PCR or comorbidities associated with severe illness from COVID-19. The CT images from each patient and the clinical database were fully anonymized and transferred to one coordinating center for core lab analysis. The study was conducted with the approval of local institutional review boards (Cedars-Sinai Medical Center IRB# study 617) and written informed consent was waived for fully anonymized data analysis.
Fig. 1.
Study flowchart.
2.2. Scan protocol and image reconstruction
Noncontrast chest CT scans were performed with different multi-slice CT systems: Aquilion ONE (Toshiba Medical Systems, Otawara, Japan); GE Revolution, GE Discovery CT750 HD, or LightSpeed VCT (GE Healthcare, Milwaukee, WI, USA); and Brilliance iCT (Philips Healthcare, Cleveland, OH, USA). Parameters used for scans included a peak x-ray tube voltage of 120 kV, automatic tube current modulation (300–500 mAs), and slice thickness of 0.625 to 1.25 mm. Images were reconstructed using lung kernels specific for respective scanner vendors. All scans were obtained in the supine position during inspiratory breath-hold.
2.3. CT lung analysis
Images were analyzed by two physicians (K.G. and A.L.) with 3 and 8 years of experience in chest CT, respectively, and who were blinded to clinical data. A standard lung window (width: 1500 Hounsfield units [HU]; level: −400 HU) was used. Lung abnormalities were quantified using semi-automated research software (FusionQuant Lung v1.0, Cedars-Sinai Medical Center, Los Angeles, CA, USA). First, both lungs were segmented by a deep learning model based on U-Net architecture [28]. The acquired pulmonary mask and a second deep learning model trained with the Lung Tissue Research Consortium dataset were then used to compute lobe segmentations, with manual adjustments made by the reader as required [29]. The right lung was divided into upper, middle, and lower lobes with respect to the horizontal and oblique fissures; while the left lung was separated into upper and lower lobes by the oblique fissure.
Lung lesions associated with COVID-19 pneumonia were then segmented using a semi-automated brush-like tool; the boundaries of which were delimited by a region-growing algorithm. These included ground glass opacities (GGO), consolidation, or pleural effusion according to the Fleischner Society lexicon (Fig. 2A–B) [30,31]. Adaptive thresholds were used, defined by a fixed window around the HU of the selected voxel. Chronic lung abnormalities such as emphysema or fibrosis were excluded from segmentation, based on correlation with previous imaging and/or a consensus reading. GGO was defined as hazy opacities that did not obscure the underlying bronchial structures or pulmonary vessels; consolidation as opacification obscuring the underlying bronchial structures or pulmonary vessels; and pleural effusion as a fluid collection in the pleural cavity. Total pneumonia volume was calculated by summing the volumes of the GGO and consolidation components. Total pneumonia burden was calculated as: total pneumonia volume / total lung volume × 100% [31].
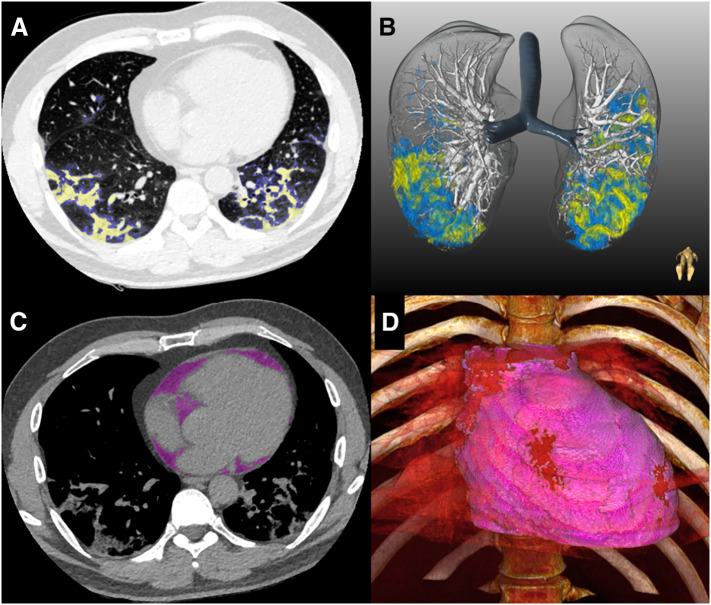
Fig. 2.
Quantitative CT analysis in COVID-19 patient. Semi-automated segmentation of ground-glass opacities (blue) and consolidation (yellow) was performed using axial slices (A) and reconstructed in 3D (B). Epicardial adipose tissue (purple) was segmented using a deep learning algorithm (C) and volume-rendered (D).
The average time taken for full lung and lesion segmentation ranged from 10 to 20 min depending on patient anatomy and the extent of pneumonia. The axial distribution of lung abnormalities was visually classified as peripheral (predominantly outer one-third of the lung), central (predominantly inner two-thirds of the lung), or diffuse (no clear distribution pattern [6]. Difficult cases of visual or quantitative analysis were resolved by consensus. The presence of any breathing artifact was also noted.
2.4. EAT quantification from chest CT
EAT was defined as all adipose tissue enclosed by the visceral pericardium [32,33]. EAT volume and attenuation were quantified using a deep learning algorithm incorporated into research software (QFAT version 2.0; Cedars-Sinai Medical Center). The development and validation of this automated method have been described previously [32,33]. Briefly, the limits of the heart were automatically defined as the pulmonary artery bifurcation (superior limit) to the posterior descending artery (inferior limit) and pericardial contours traced by the algorithm. For ungated CT data in our cohort, the pericardial contours were adjusted if needed. EAT volume (reported in mL) and mean attenuation (reported in HU) were automatically calculated from 3-dimensional fat voxels between the HU limits of −190 and −30 HU (Fig. 2C–D).
2.5. Outcomes and definitions
The primary outcome was a composite of clinical deterioration (intensive care unit [ICU] admission, invasive mechanical ventilation, or vasopressor therapy) or death. The time to the first occurrence of any one of the components was documented. Chronic lung disease included asthma, chronic obstructive pulmonary disease, and/or obstructive sleep apnea. Chronic kidney disease was defined as eGFR <60 mL/min/1.73m2. Immunodeficiency was defined as active cancer treated with chemotherapy or human immunodeficiency virus infection. Patient symptoms were self-reported upon hospital admission. Serum levels of biomarkers were obtained at hospital admission.
2.6. Statistical analysis
Data were tested for normality using the Shapiro–Wilk test. Continuous variables are expressed as mean ± standard deviation or median (interquartile range [IQR]), as appropriate. Categorical variables are presented as absolute numbers (percentage). Continuous variables were compared using the Student's t-test or nonparametric Mann-Whitney U test, as appropriate. Categorical variables were compared using a Chi-square test. Correlations between continuous variables were assessed using the Spearman's rank correlation coefficient. We performed multivariate linear regression to examine the association of EAT measures with total pneumonia burden, adjusted for age, sex, and comorbidities previously shown to associate with severe illness from COVID-19 (diabetes mellitus, hypertension, smoking, chronic lung disease, history of coronary artery disease, and history of heart failure) [34,35]. To evaluate the predictive value of EAT measures for the primary outcome, we performed multivariable logistic regression analysis, adjusted for total pneumonia burden and the above clinical parameters. Quantitative pneumonia burden and EAT volume were not normally distributed and hence normalized with logarithmic adjustment; base-2 log transformation was used as this represented doubling of the variable. We selected the optimum cutoffs for EAT volume by identifying the ROC values that maximized Youden's J statistic (sensitivity + specificity - 1). A 2-sided p-value <0.05 was considered statistically significant. All analyses were performed using Stata 14.0 (StataCorp, College Station, TX, USA).
3. Results
3.1. Patient characteristics
A total of 109 patients (age 64 ± 16 years; 62% male) with laboratory-confirmed COVID-19 who underwent chest CT during their admission were included. The primary outcome occurred in 23 (21.1%) patients: 15 (65.2%) were admitted to ICU, 10 (43.5%) required mechanical ventilation, 10 (43.5%) required vasopressors, and 13 (56.5%) had in-hospital death. The median time from self-reported onset of symptoms to chest CT was 7 days (IQR 5–9 days), and the median time from chest CT to the occurrence of the primary outcome was 3 days (IQR 1–13 days). The remaining patients (n = 86; 78.9%) did not require critical care or had been discharged alive at the time of data collection. Patients who experienced deterioration or died were older and had a greater number of comorbidities (Table 1 ). No significant differences in body mass index (BMI) were observed between the two groups (p = 0.526). Serum inflammatory markers were increased in patients with versus without the primary outcome (Table 1).
Table 1.
Clinical and laboratory characteristics of patients on admission.
| Clinical deterioration or death |
P value | ||
|---|---|---|---|
| Yes (N = 23) | No (N = 86) | ||
| Clinical characteristics | |||
| Age, years | 74 ± 11 | 61 ± 16 | <0.001 |
| Male sex | 16 | 52 | 0.424 |
| Body mass index, kg/m2 | 26.9 ± 5.1, 12 | 26.1 ± 3.7, 68 | 0.526 |
| Hypertension | 18 (78.3) | 40 (46.5) | 0.007 |
| Diabetes mellitus | 12 (52.2) | 11 (12.8) | <0.001 |
| Hyperlipidemia | 14 (60.9) | 23 (26.7) | 0.002 |
| Smoking status | 0.463 | ||
| Former smoker | 4 (17.4) | 10 (11.6) | |
| Current smoker | 0 | 4 (4.7) | |
| History of lung disease | 6 (26.1) | 9 (10.5) | 0.053 |
| History of heart failure | 9 (39.1) | 7 (8.1) | <0.001 |
| History of coronary artery disease | 10 (43.5) | 15 (17.4) | 0.008 |
| Chronic kidney disease | 9 (39.1) | 5 (5.8) | <0.001 |
| Immunodeficiency | 2 (8.7) | 1 (1.2) | 0.050 |
| Symptoms | |||
| Fever | 13 (56.5) | 66 (76.7) | 0.054 |
| Chills | 1 (4.3) | 7 (8.1) | 0.536 |
| Fatigue | 16 (69.6) | 63 (73.3) | 0.724 |
| Dyspnea | 17 (73.9) | 47 (54.7) | 0.096 |
| Dry cough | 14 (60.9) | 40 (45.5) | 0.221 |
| Sputum production | 0 | 5 (5.8) | 0.236 |
| Hemoptysis | 0 | 1 (1.2) | 0.603 |
| Sore throat | 0 | 1 (1.2) | 0.603 |
| Loss of smell | 0 | 7 (8.1) | 0.157 |
| Loss of taste | 0 | 5 (5.8) | 0.236 |
| Muscle/joint pain | 11 (47.8) | 43 (50.0) | 0.853 |
| Headache | 6 (26.1) | 20 (23.3) | 0.777 |
| Nasal congestion | 3 (13.0) | 6 (7.0) | 0.347 |
| Nausea or vomiting | 3 (13.0) | 6 (7.0) | 0.348 |
| Diarrhea | 1 (4.3) | 9 (10.5) | 0.367 |
| Blood biomarkers | |||
| Lymphocytes (%) | 19.5 (14.6–25.2), 13 | 18.5 (13.7–24.0), 79 | 0.562 |
| Lactate dehydrogenase (U/L) | 421 ± 221, 9 | 272 ± 120, 74 | 0.002 |
| C-reactive protein (mg/L) | 213.6 (185.5–245.5), 16 | 14.5 (4.0–51.2), 86 | <0.001 |
| Ferritin (ng/mL) | 918 (795–1450), 13 | 502 (282–659), 34 | 0.016 |
| Prothrombin time (s) | 13.8 (13.3–14.5), 7 | 13.1 (12.3–14.1), 50 | 0.114 |
| D-dimer (μg/mL) | 1.841 (1.24–22.5), 11 | 0.7 (0.5–1.2), 41 | 0.002 |
| Troponin (ng/mL) | 0.11 (0.09–0.37), 13 | 0.02 (0.01–0.06), 42 | <0.001 |
| Creatine kinase-MB (U/L) | 56.5 (28.8–96.8), 8 | 65.0 (37.5–85.8), 72 | 0.795 |
Data are n (%), median (IQR), or mean ± SD, n if fewer patients had laboratory results available than the total study population.
3.2. Chest CT measurements
The median time from chest CT to positive RT-PCR testing was 0 (IQR 0–3) days. Breathing artifacts were observed in 18 (16.5%) cases. The prevalence and distribution of lung abnormalities were comparable between patients with and without the primary outcome, apart from pleural effusions (30.4% vs 9.3%, p = 0.009) being more common in patients with clinical deterioration/death (Table 2 ). Patients with deterioration/death had higher quantitative pneumonia burden compared to those without (19.6% [IQR 9.3–52.1%] vs 5.9% [1.7–11.6%], p < 0.001). EAT volume was higher in patients with versus without the primary outcome (132.2 mL [IQR 88.4–157.6 mL] vs 84.9 mL [IQR 58.3–128.6 mL], p = 0.020; Fig. 3A). Median EAT attenuation in patients with versus without the primary outcome was −68.4 HU (IQR −76.4 – −65.8) vs −73.0 HU (IQR −76.8 – −68.6), with a trend toward statistical significance (p = 0.077; Fig. 3B).
Table 2.
Characteristics of lung abnormalities on chest computed tomography
| Clinical deterioration or death |
P value | ||
|---|---|---|---|
| Yes (N = 23) | No (N = 86) | ||
| Lung abnormality | |||
| Only ground-glass opacities | 0 (0.0) | 21 (24.4) | 0.008 |
| Only consolidation | 0 (0.0) | 1 (1.2) | 0.603 |
| Ground-glass opacities and consolidation | 22 (95.7) | 58 (67.4) | 0.007 |
| Pleural effusion | 7 (30.4) | 8 (9.3) | 0.009 |
| Emphysema | 3 (13.0) | 5 (5.8) | 0.238 |
| Fibrosis | 2 (8.7) | 3 (3.5) | 0.289 |
| None | 1 (4.3) | 6 (7.0) | 0.648 |
| Total pneumonia volume (mL) | 1040.1 (339.7–2100.7) | 282.6 (85.6–520.5) | <0.001 |
| Total pneumonia burden (%) | 19.6 (9.3–52.1) | 5.9 (1.7–11.6) | <0.001 |
| Lateralitya | |||
| Unilateral | 1 (4.5) | 10 (12.5) | 0.289 |
| Right | 1 (4.5) | 6 (7.5) | |
| Left | 0 (0.0) | 4 (5.0) | |
| Bilateral | 21 (95.5) | 70 (87.5) | |
| Lobar distributiona | |||
| Right upper lobe | 21 (26.3) | 65 (81.3) | 0.105 |
| Right medial lobe | 20 (25.0) | 64 (80.0) | 0.235 |
| Right lower lobe | 22 (27.5) | 73 (91.3) | 0.151 |
| Left upper lobe | 20 (25.0) | 68 (85.0) | 0.476 |
| Left lower lobe | 21 (26.3) | 72 (90.0) | 0.424 |
| Lobar involvementa | |||
| 1 lobe | 0 (0.0) | 5 (6.3) | 0.478 |
| 2 lobes | 1 (4.5) | 5 (6.3) | |
| 3 lobes | 1 (4.5) | 7 (8.8) | |
| 4 lobes | 1 (4.5) | 9 (11.3) | |
| 5 lobes | 19 (86.4) | 54 (67.5) | |
| Axial distributiona | |||
| Central | 0 (0.0) | 0 (0.0) | 0.046 |
| Peripheral | 6 (27.3) | 41 (51.3) | |
| Diffuse | 16 (72.7) | 39 (48.8) | |
calculated for patients with presence of COVID-19 pneumonia (n = 102).
Fig. 3.
Differences in epicardial adipose tissue volume (A) and attenuation (B) in patients with and without clinical deterioration or death.
3.3. EAT and extent of COVID-19 pneumonia
In bivariate analysis, EAT volume was positively correlated with total pneumonia burden (r = 0.29, p = 0.006). In multivariable linear regression adjusted for clinical parameters, the total pneumonia burden was independently associated with EAT volume (β = 10.6, 95% CI 3.4–17.8, p = 0.005) and attenuation (β = 5.2, 95% CI 1.5–8.9, p = 0.004; Table 3 )
Table 3.
Multivariable associations of clinical variables and epicardial adipose tissue with total pneumonia burden (%).
| β-coefficient | Standard Error | 95% CI | P value | |
|---|---|---|---|---|
| Age | 0.1 | 0.2 | −0.3–0.5 | 0.552 |
| Male sex | −9.3 | 4.4 | −18.0 to −0.7 | 0.035 |
| Diabetes mellitus | 8.9 | 5.6 | −9.9–8.4 | 0.116 |
| Hypertension | −0.8 | 4.6 | −2.3–20.0 | 0.863 |
| Smoking history | −5.2 | 4.1 | −13.4–3.0 | 0.210 |
| Chronic lung disease | −2.7 | 5.6 | −13.8–8.5 | 0.637 |
| History of heart failure | 1.2 | 6.6 | −12.0–14.3 | 0.862 |
| Presence of coronary artery disease | −1.5 | 5.6 | −12.7–9.6 | 0.785 |
| Epicardial adipose tissue volume (mL)a | 10.6 | 3.6 | 3.4–117.8 | 0.005 |
| Epicardial adipose tissue attenuation (HU)b | 5.2 | 1.9 | 1.5–8.9 | 0.004 |
Odds ratios are per 2-fold increase/doubling of the variable.
Odds ratios are per 5 HU increase.
3.4. EAT as a predictor of clinical deterioration in COVID-19 patients
In multivariable logistic regression analysis, EAT volume (OR 5.1 [95% CI 1.8–14.1] per doubling p = 0.011) and EAT attenuation (OR 3.4 [95% CI 1.5–7.5] per 5 Hounsfield unit increase, p = 0.003) were independent predictors of clinical deterioration or death, as were total pneumonia burden (OR 2.5, 95% CI 1.4–4.6, p = 0.002), chronic lung disease (OR 1.3 [95% CI 1.1–1.7], p = 0.011), and history of heart failure (OR 3.5 [95% 1.1–8.2], p = 0.037) (Table 4 ).
Table 4.
Association of clinical and CT parameters with the risk of clinical deterioration or death in univariable and multivariable⁎ logistic regression analysis.
| Univariable analysis |
Multivariable analysis |
|||
|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | |
| Age | 1.1 (1.0–1.2) | 0.001 | – | |
| Male sex | 1.7 (0.62–4.9) | 0.292 | – | |
| Diabetes mellitus | 5.1 (1.8–14.5) | 0.002 | – | |
| Hypertension | 1.2 (1.0–1.3) | 0.006 | – | |
| Smoking history | 0.9 (0.3–2.4) | 0.782 | – | |
| Chronic lung disease | 1.1 (0.9–1.2) | 0.136 | 1.3 (1.1–1.7) | 0.011 |
| History of heart failure | 6.8 (1.5–14.6) | <0.001 | 3.5 (1.1–8.2) | 0.037 |
| History of coronary artery disease | 4.1 (1.3–13.1) | 0.017 | – | |
| Epicardial adipose tissue volume (mL)a | 2.9 (1.2–7.2) | 0.020 | 5.1 (1.8–14.1) | 0.011 |
| Epicardial adipose tissue attenuation (HU)b | 1.3 (0.9–1.8) | 0.114 | 3.4 (1.5–7.5) | 0.003 |
| Total pneumonia burden (%)a | 2.3 (1.5–3.5) | <0.001 | 2.5 (1.4–4.6) | 0.002 |
All variables entered into multivariable logistic regression with backward stepwise selection at a Wald p-value of 0.1. The final model containing statistically significant variables is shown.
Odds ratios are per 2-fold increase/doubling of the variable.
Odds ratios are per 5 HU increase.
3.5. Correlation of EAT with serum biomarkers and clinical variables
Bivariate correlations between CT parameters, serum biomarkers, and clinical variables are presented in Table 5 . EAT volume had a moderate correlation with BMI and serum levels of lactate dehydrogenase and CRP; and a weak correlation with creatine kinase-MB levels and total pneumonia volume and burden. EAT attenuation was inversely correlated with lactate dehydrogenase levels.
Table 5.
Correlation matrix between investigated variables.
| EAT volume | EAT attenuation | Age | Male gender | Body Mass Index | Lymphocytes | Lactate dehydrogenase | C-reactive protein | Ferritin | Prothrombin time | D-dimer | Troponin | Creatine kinase | Total pneumonia volume | Total pneumonia burden | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EAT volume | 1.000 | ||||||||||||||
| EAT attenuation | −0.672⁎ | 1.000 | |||||||||||||
| Age | 0.378⁎ | −0.036 | 1.000 | ||||||||||||
| Male gender | 0.221⁎ | 0.130 | 0.126 | 1.000 | |||||||||||
| Body mass index | 0.369⁎ | −0.193 | 0.047 | 0.092 | 1.000 | ||||||||||
| Lymphocytes | −0.157 | 0.065 | −0.282⁎ | −0.096 | −0.041 | 1.000 | |||||||||
| Lactate dehydrogenase | 0.361⁎ | −0.293⁎ | 0.141 | 0.107 | 0.044 | −0.095 | 1.000 | ||||||||
| C-reactive protein | 0.450⁎ | −0.152 | 0.400⁎ | 0.130 | 0.212 | −0.125 | 0.482⁎ | 1.000 | |||||||
| Ferritin | 0.005 | 0.064 | −0.120 | 0.132 | 0.061 | −0.015 | 0.181 | 0.198 | 1.000 | ||||||
| Prothrombin time | 0.181 | −0.144 | 0.277† | 0.016 | −0.113 | 0.211 | 0.167 | 0.365⁎ | −0.051 | 1.000 | |||||
| D-dimer | 0.180 | 0.206 | 0.270 | 0.243 | 0.003 | 0.057 | −0.032 | 0.409⁎ | 0.165 | 0.209 | 1.000 | ||||
| Troponin | 0.118 | 0.076 | 0.201 | −0.136 | 0.043 | 0.034 | 0.202 | 0.391⁎ | 0.048 | 0.052 | 0.390† | 1.000 | |||
| Creatine kinase-MB | 0.167 | −0.150 | 0.030 | 0.104 | 0.115 | −0.054 | 0.185 | 0.092 | 0.029 | 0.067 | 0.232 | 0.196 | 1.000 | ||
| Total pneumonia volume | 0.297⁎ | 0.018 | 0.319⁎ | 0.133 | 0.193 | −0.053 | 0.313⁎ | 0.372⁎ | 0.346⁎ | 0.156 | 0.468⁎ | 0.265 | −0.086 | 1.000 | |
| Total pneumonia burden | 0.286⁎ | 0.022 | 0.336⁎ | 0.002 | 0.244 | −0.066 | 0.319⁎ | 0.388⁎ | 0.285† | 0.163 | 0.467† | 0.233 | −0.061 | 0.967⁎ | 1.000 |
Correlation is significant at the 0.01 level.
Correlation is significant at the 0.05 level.
4. Discussion
In this international multicenter study of patients with COVID-19, we examine the relationship between EAT quantified from chest CT with the extent of pneumonia and adverse outcomes in patients with COVID-19. We demonstrate that: (1) EAT volume and attenuation associate with the quantitative burden of COVID-19 pneumonia; and (2) an increasing EAT volume or attenuation independently predict clinical deterioration or death.
Obesity has been identified as a risk factor for hospitalization and mechanical ventilation in acute respiratory illnesses such as H1N1 influenza [36], and a similar trend is emerging in patients with COVID-19. Evidence shows that any degree of obesity (BMI ≥30 kg/m2) associates with more advanced disease including respiratory and multiorgan failure in COVID-19, and morbid obesity (BMI ≥40 kg/m2) confers a significantly worse prognosis compared to normal weight [[7], [8], [9], [10], [11]]. Although traditionally used to define obesity, BMI ≥30 kg/m2 remains an indirect marker of excess body fat and does not account for the substantial regional and phenotypic variation in fat depots [14]. Several reports have shown abdominal visceral fat area quantified on CT to associate with critical illness in patients with COVID-19 [26,37], however data on thoracic fat depots are lacking. It is established that CT-derived EAT measures correlate strongly with abdominal visceral adiposity and metabolic risk factors [38] and associate with coronary atherosclerosis [39]. Prior studies utilizing manual measurements of EAT thickness on chest CT of COVID-19 patients have failed to show an association of this metric with extent of pneumonia or clinical course [26,27]. By contrast, our fully automated, three-dimensional measurement of EAT provided a more comprehensive assessment of this visceral fat depot, with the resultant EAT volume associating with pneumonia burden. Furthermore, EAT attenuation quantified by this method was predictive of clinical deterioration or death, independently of cardiovascular risk factors and coronary artery disease. This is consistent with a recent report showing EAT attenuation to be higher in patients with clinically severe or critical COVID-19 compared to those with mild or moderate disease [27].
Mechanistically, EAT may exert a direct, local effect on the neighboring lungs and/or contribute to the augmented systemic inflammatory response to COVID-19 [40,41]. An increasing EAT volume has been shown to associate with reduced lung function in healthy individuals [15,16] and disease severity in those with chronic lung conditions [18,42]. The close proximity of EAT to the pulmonary artery potentially enables direct diffusion of inflammatory mediators into the pulmonary circulation [43], which may then exert vasocrine or paracrine effects on the lung tissue. This local inflammation could partly explain the association of EAT measures with quantitative burden of COVID-19 pneumonia in our study. Certainly, it is established that EAT has a pathophysiological influence on adjacent structures such as the coronary arteries and myocardium, promoting atherosclerosis and fibrosis [44]. The release of proinflammatory cytokines from EAT into the general circulation may contribute to the systemic inflammatory state in COVID-19; systemic inflammation, in turn, promotes accumulation of EAT, creating a positive feedback loop [18,[45], [46], [47]]. EAT inflammation may be reflected by a higher CT attenuation of this fat depot [48]. Furthermore, angiotensin-converting enzyme 2 (ACE-2), used by SARS-CoV-2 to enter host cells, is expressed in several different cell-lines including adipocytes [49]. Given that ACE-2 upregulation is seen in adipocytes of patients with obesity and diabetes, EAT may serve as an important viral reservoir [50]. Finally, visceral fat accumulation, associated with reduced testosterone levels especially in older males, may influence the pathophysiology of COVID-19 through dysregulation of the growth hormone (GH)–insulin-like growth factor 1 (IGF1) axis [51]. It has been recently hypothesized that GH insufficiency may contribute to gender-related differences in COVID-19 [52]. Indeed, our study showed an association of EAT, age, and male gender with the extent of pneumonia, underscoring the complex interplay between the endocrine and immune systems.
The integration of EAT volume measurements into clinical risk scores for patients with COVID-19 has the potential to enhance in-hospital outcome prediction. Our deep learning-based method of EAT quantification from routine chest CT is rapid (<3 min per case); however more studies are required to determine whether this approach is applicable to different COVID-19 populations. Anti-inflammatory agents have shown promising results in hospitalized patients with COVID-19 [53,54], and their effects in obese versus normal-weight patients or patients with high versus low EAT volumes should be explored in future studies.
Our study has several limitations. First, different patient profiles and treatment protocols between countries may have resulted in heterogeneity in COVID-19 pneumonia severity or in-hospital outcomes. Second, chest CT indications and acquisition protocols were not standardized across centers. Third, our sample size was relatively small and larger studies are needed to validate our findings. Fourth, BMI measurements were not obtained in all patients and thus we were unable to adjust for the potentially confounding effect of this obesity measure in our multivariable models. Finally, levels of serum biomarkers such as CRP, lactate dehydrogenase, and troponin were not uniformly available and thus not included in our risk prediction models to assess the independent effects of systemic inflammation or myocardial injury on outcomes; further studies are needed to assess these relationships.
5. conclusion
EAT parameters quantified from chest CT are independently associated with extent of pneumonia and adverse outcomes in patients with COVID-19, lending support to its use in clinical risk stratification.
Funding
Funded by COVID-19 supplementary funding, Cedars-Sinai Medical Center, and the National Heart, Lung and Blood Institute [1R01HL133616].
CRediT authorship contribution statement
Kajetan Grodecki: Conceptualization, Methodology, Formal analysis, Investigation, Data curation, Writing - original draft, Visualization. Andrew Lin: Conceptualization, Methodology, Formal analysis, Investigation, Data curation, Writing - original draft, Visualization. Aryabod Razipour: Investigation, Data curation, Writing - review & editing. Sebastien Cadet: Formal analysis, Software, Writing - review & editing. Priscilla A. McElhinney: Investigation, Data curation, Writing - review & editing. Cato Chan: Resources, Data curation, Writing - review & editing. Barry D. Pressman: Resources, Data curation, Writing - review & editing. Peter Julien: Resources, Data curation, Writing - review & editing. Pal Maurovich-Horvat: Resources, Data curation, Writing - review & editing. Nicola Gaibazzi: Resources, Data curation, Writing - review & editing. Udit Thakur: Resources, Data curation, Writing - review & editing. Elisabetta Mancini: Resources, Data curation, Writing - review & editing. Cecilia Agalbato: Resources, Data curation, Writing - review & editing. Robert Menè: Resources, Data curation, Writing - review & editing. Gianfranco Parati: Resources, Data curation, Writing - review & editing. Franco Cernigliaro: Resources, Data curation, Writing - review & editing. Nitesh Nerlekar: Resources, Data curation, Writing - review & editing. Camilla Torlasco: Resources, Data curation, Writing - review & editing. Gianluca Pontone: Resources, Data curation, Writing - review & editing. Piotr J. Slomka: Software, Resources, Data curation, Writing - review & editing. Damini Dey: Conceptualization, Methodology, Formal analysis, Software, Resources, Writing - original draft, Writing - review & editing, Supervision, Funding acquisition.
Declaration of competing interest
The authors have no conflicts of interest to disclose.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.metabol.2020.154436.
Appendix A. Supplementary data
Supplementary material
References
- 1.World Health Organization COVID-19 Situation Report - 139 https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports Retreived 20 October 2020.
- 2.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fang Y., Zhang H., Xie J., Lin M., Ying L., Pang P. Sensitivity of chest CT for COVID-19: comparison to RT-PCR. Radiology. 2020;296:E115–E117. doi: 10.1148/radiol.2020200432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang Y., Dong C., Hu Y., Li C., Ren Q., Zhang X. Temporal changes of CT findings in 90 patients with COVID-19 pneumonia: a longitudinal study. Radiology. 2020;296:E55–E64. doi: 10.1148/radiol.2020200843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bernheim A., Mei X., Huang M., Yang Y., Fayad Z.A., Zhang N. Chest CT findings in coronavirus disease-19 (COVID-19): relationship to duration of infection. Radiology. 2020;295:200463. doi: 10.1148/radiol.2020200463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Colombi D., Bodini F.C., Petrini M., Maffi G., Morelli N., Milanese G. Well-aerated lung on admitting chest CT to predict adverse outcome in COVID-19 pneumonia. Radiology. 2020;296:E86–E96. doi: 10.1148/radiol.2020201433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Razieh C., Zaccardi F., Davies M.J., Khunti K., Yates T. Body mass index and the risk of COVID-19 across ethnic groups: analysis of UK biobank. Diabetes Obes Metab. 2020 doi: 10.1111/dom.14125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cai Q., Chen F., Wang T., Luo F., Liu X., Wu Q. Obesity and COVID-19 severity in a designated Hospital in Shenzhen, China. Diabetes Care. 2020;43:1392–1398. doi: 10.2337/dc20-0576. [DOI] [PubMed] [Google Scholar]
- 9.Simonnet A., Chetboun M., Poissy J., Raverdy V., Noulette J., Duhamel A. High prevalence of obesity in severe acute respiratory syndrome Coronavirus-2 (SARS-CoV-2) requiring invasive mechanical ventilation. Obesity (Silver Spring) 2020;28:1195–1199. doi: 10.1002/oby.22831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lighter J., Phillips M., Hochman S., Sterling S., Johnson D., Francois F. Obesity in patients younger than 60 years is a risk factor for COVID-19 hospital admission. Clin Infect Dis. 2020;71:896–897. doi: 10.1093/cid/ciaa415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goyal P., Choi J.J., Pinheiro L.C., Schenck E.J., Chen R., Jabri A. Clinical characteristics of Covid-19 in New York City. N Engl J Med. 2020;382:2372–2374. doi: 10.1056/NEJMc2010419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rebelos E., Moriconi D., Virdis A., Taddei S., Foschi D., Nannipieri M. Letter to the editor: importance of metabolic health in the era of COVID-19. Metabolism. 2020;108:154247. doi: 10.1016/j.metabol.2020.154247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stefan N., Birkenfeld A.L., Schulze M.B., Ludwig D.S. Obesity and impaired metabolic health in patients with COVID-19. Nat Rev Endocrinol. 2020;16:341–342. doi: 10.1038/s41574-020-0364-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fitzgibbons T.P., Czech M.P. Epicardial and perivascular adipose tissues and their influence on cardiovascular disease: basic mechanisms and clinical associations. J Am Heart Assoc. 2014;3 doi: 10.1161/JAHA.113.000582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kwack W.G., Kang Y.-S., Jeong Y.J., Oh J.Y., Cha Y.K., Kim J.S. Association between thoracic fat measured using computed tomography and lung function in a population without respiratory diseases. J Thorac Dis. 2019;11:5300–5309. doi: 10.21037/jtd.2019.11.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hickson D.A., Liu J., Bidulescu A., Burchfiel C.M., Taylor H.A., Petrini M.F. Pericardial fat is associated with impaired lung function and a restrictive lung pattern in adults: the Jackson heart study. Chest. 2011;140:1567–1573. doi: 10.1378/chest.11-0258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zagaceta J., Zulueta J.J., Bastarrika G., Colina I., Alcaide A.B., Campo A. Epicardial adipose tissue in patients with chronic obstructive pulmonary disease. PloS one. 2013;8:e65593. doi: 10.1371/journal.pone.0065593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Long B.D., Stojanovska J., Brown R.K.J., Attili A.K., Jackson E.A., Ognenovski V. Increased epicardial fat volume is independently associated with the presence and severity of systemic sclerosis. Acad Radiol. 2017;24:1473–1481. doi: 10.1016/j.acra.2017.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Franssens B.T., Nathoe H.M., Leiner T., van der Graaf Y., Visseren F.L. Relation between cardiovascular disease risk factors and epicardial adipose tissue density on cardiac computed tomography in patients at high risk of cardiovascular events. Eur J Prev Cardiol. 2017;24:660–670. doi: 10.1177/2047487316679524. [DOI] [PubMed] [Google Scholar]
- 20.Goeller M., Achenbach S., Marwan M., Doris M.K., Cadet S., Commandeur F. Epicardial adipose tissue density and volume are related to subclinical atherosclerosis, inflammation and major adverse cardiac events in asymptomatic subjects. J Cardiovasc Comput Tomogr. 2018;12:67–73. doi: 10.1016/j.jcct.2017.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Coperchini F., Chiovato L., Croce L., Magri F., Rotondi M. The cytokine storm in COVID-19: an overview of the involvement of the chemokine/chemokine-receptor system. Cytokine Growth Factor Rev. 2020;53:25–32. doi: 10.1016/j.cytogfr.2020.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Malavazos A.E., Goldberger J.J., Iacobellis G. Does epicardial fat contribute to COVID-19 myocardial inflammation? Eur Heart J. 2020;41:2333. doi: 10.1093/eurheartj/ehaa471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim I.-C., Han S. Epicardial adipose tissue: fuel for COVID-19-induced cardiac injury? Eur Heart J. 2020;41:2334–2335. doi: 10.1093/eurheartj/ehaa474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Watanabe M., Risi R., Tuccinardi D., Baquero C.J., Manfrini S., Gnessi L. Obesity and SARS-CoV-2: A population to safeguard. Diabetes Metab Res Rev. 2020 doi: 10.1002/dmrr.3325. [DOI] [PubMed] [Google Scholar]
- 25.Malavazos A.E., Corsi Romanelli M.M., Bandera F., Iacobellis G. Targeting the adipose tissue in COVID-19. Obesity. 2020;28:1178–1179. doi: 10.1002/oby.22844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Watanabe M., Caruso D., Tuccinardi D., Risi R., Zerunian M., Polici M. Visceral fat shows the strongest association with the need of intensive Care in Patients with COVID-19. Metabolism. 2020;154319 doi: 10.1016/j.metabol.2020.154319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Iacobellis G., Secchi F., Capitanio G., Basilico S., Schiaffino S., Boveri S. Epicardial Fat Inflammation in severe COVID-19. Obesity. 2020 doi: 10.1002/oby.23019. [DOI] [PubMed] [Google Scholar]
- 28.Hofmanninger J., Prayer F., Pan J., Röhrich S., Prosch H., Langs G. Automatic lung segmentation in routine imaging is primarily a data diversity problem, not a methodology problem. Eur Radiol Exp. 2020;4:50. doi: 10.1186/s41747-020-00173-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Negahdar M., Beymer D. SPIE; 2019. Lung tissue characterization for emphysema differential diagnosis using deep convolutional neural networks. [Google Scholar]
- 30.Hansell D.M., Bankier A.A., MacMahon H., McLoud T.C., Müller N.L., Remy J. Fleischner society: glossary of terms for thoracic imaging. Radiology. 2008;246:697–722. doi: 10.1148/radiol.2462070712. [DOI] [PubMed] [Google Scholar]
- 31.Grodecki K., Lin A., Cadet S., McElhinney P.A., Razipour A., Chan C. Quantitative burden of COVID-19 pneumonia on chest CT predicts adverse outcomes: a post-hoc analysis of a prospective international registry. Radiology: Cardiothoracic Imaging. 2020;2 doi: 10.1148/ryct.2020200389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Commandeur F., Goeller M., Razipour A., Cadet S., Hell M.M., Kwiecinski J. Fully automated CT quantification of epicardial adipose tissue by deep learning: a multicenter study. Radiol Artif Intell. 2019;1 doi: 10.1148/ryai.2019190045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eisenberg E., McElhinney P.A., Commandeur F., Chen X., Cadet S., Goeller M. Deep learning-based quantification of epicardial adipose tissue volume and attenuation predicts major adverse cardiovascular events in asymptomatic subjects. Circ Cardiovasc Imaging. 2020;13 doi: 10.1161/CIRCIMAGING.119.009829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cummings M.J., Baldwin M.R., Abrams D., Jacobson S.D., Meyer B.J., Balough E.M. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet. 2020;395:1763–1770. doi: 10.1016/S0140-6736(20)31189-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dietz W., Santos-Burgoa C. Obesity and its implications for COVID-19 mortality. Obesity (Silver Spring) 2020;28:1005. doi: 10.1002/oby.22818. [DOI] [PubMed] [Google Scholar]
- 37.Petersen A., Bressem K., Albrecht J., Thieß H.M., Vahldiek J., Hamm B. The role of visceral adiposity in the severity of COVID-19: highlights from a unicenter cross-sectional pilot study in Germany. Metabolism. 2020;110:154317. doi: 10.1016/j.metabol.2020.154317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rosito G.A., Massaro J.M., Hoffmann U., Ruberg F.L., Mahabadi A.A., Vasan R.S. Pericardial fat, visceral abdominal fat, cardiovascular disease risk factors, and vascular calcification in a community-based sample: the Framingham heart study. Circulation. 2008;117:605–613. doi: 10.1161/CIRCULATIONAHA.107.743062. [DOI] [PubMed] [Google Scholar]
- 39.Lin A., Dey D., Wong D.T.L., Nerlekar N. Perivascular adipose tissue and coronary atherosclerosis: from biology to imaging phenotyping. Curr Atheroscler Rep. 2019;21:47. doi: 10.1007/s11883-019-0817-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ryan P.M., Caplice N.M. Is adipose tissue a reservoir for viral spread, immune activation, and cytokine amplification in coronavirus disease 2019? Obesity. 2020;28:1191–1194. doi: 10.1002/oby.22843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moore J.B., June C.H. Cytokine release syndrome in severe COVID-19. Science. 2020;368:473. doi: 10.1126/science.abb8925. [DOI] [PubMed] [Google Scholar]
- 42.Kiraz K., Gökdeniz T., Kalaycıoglu E., Börekçi A., Akyol S., Baykan A.O. Epicardial fat thickness is associated with severity of disease in patients with chronic obstructive pulmonary disease. Eur Rev Med Pharmacol Sci. 2016;20:4508–4515. [PubMed] [Google Scholar]
- 43.Iacobellis G., Corradi D., Sharma A.M. Epicardial adipose tissue: anatomic, biomolecular and clinical relationships with the heart. Nat Clin Pract Cardiovasc Med. 2005;2:536–543. doi: 10.1038/ncpcardio0319. [DOI] [PubMed] [Google Scholar]
- 44.Packer M. Epicardial adipose tissue may mediate deleterious effects of obesity and inflammation on the myocardium. J Am Coll Cardiol. 2018;71:2360. doi: 10.1016/j.jacc.2018.03.509. [DOI] [PubMed] [Google Scholar]
- 45.Lima-Martinez M.M., Campo E., Salazar J., Paoli M., Maldonado I., Acosta C. Epicardial fat thickness as cardiovascular risk factor and therapeutic target in patients with rheumatoid arthritis treated with biological and nonbiological therapies. Arthritis. 2014;2014:782850. doi: 10.1155/2014/782850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang X., Guo Z., Zhu Z., Bao Y., Yang B. Epicardial fat tissue in patients with psoriasis:a systematic review and meta-analysis. Lipids Health Dis. 2016;15:103. doi: 10.1186/s12944-016-0271-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lo J., Abbara S., Rocha-Filho J.A., Shturman L., Wei J., Grinspoon S.K. Increased epicardial adipose tissue volume in HIV-infected men and relationships to body composition and metabolic parameters. AIDS. 2010;24:2127–2130. doi: 10.1097/QAD.0b013e32833c055a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Iacobellis G., Mahabadi A.A. Is epicardial fat attenuation a novel marker of coronary inflammation? Atherosclerosis. 2019;284:212–213. doi: 10.1016/j.atherosclerosis.2019.02.023. [DOI] [PubMed] [Google Scholar]
- 49.Turner A.J., Hiscox J.A., Hooper N.M. ACE2: from vasopeptidase to SARS virus receptor. Trends Pharmacol Sci. 2004;25:291–294. doi: 10.1016/j.tips.2004.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ryan P.M., Caplice N.M. Is adipose tissue a reservoir for viral spread, immune activation, and cytokine amplification in coronavirus disease 2019? Obesity (Silver Spring) 2020;28:1191–1194. doi: 10.1002/oby.22843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ferrante E., Malavazos A.E., Giavoli C., Ermetici F., Coman C., Bergamaschi S. Epicardial fat thickness significantly decreases after short-term growth hormone (GH) replacement therapy in adults with GH deficiency. Nutr Metab Cardiovasc Dis. 2013;23:459–465. doi: 10.1016/j.numecd.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 52.Lubrano C., Masi D., Risi R., Balena A., Watanabe M., Mariani S. Is growth hormone insufficiency the missing link between obesity, male gender, age, and COVID-19 severity? Obesity (Silver Spring) 2020;28:2038–2039. doi: 10.1002/oby.23000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.RECOVERY Collaborative Group Dexamethasone in hospitalized patients with Covid-19 — Preliminary ReportNew England. J Med. 2020 doi: 10.1056/NEJMoa2021436. [DOI] [Google Scholar]
- 54.Guaraldi G., Meschiari M., Cozzi-Lepri A., Milic J., Tonelli R., Menozzi M. Tocilizumab in patients with severe COVID-19: a retrospective cohort study. The Lancet Rheumatology. 2020;2:e474–e484. doi: 10.1016/S2665-9913(20)30173-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material