Abstract
Following a request from the European Commission, the EFSA Panel on Nutrition, Novel Foods and Food Allergens (NDA) was asked to deliver an opinion on the change in the production process and specifications of lacto‐N‐neotetraose (LNnT) as a novel food (NF) pursuant to Regulation (EU) 2015/2283. The NF is mainly composed of the human‐identical milk oligosaccharide (HiMO) LNnT but also contains lactose, lacto‐N‐triose II (LNT II), para‐lacto‐N‐neo‐hexaose (para‐LNnH) and other related carbohydrates. The NF is produced by fermentation with two genetically modified strains of Escherichia coli BL21. LNnT when chemically synthesised or produced by microbial fermentation using another E. coli strain (K‐12) is already authorised and included in the EU list of NFs. This application is limited to a change in the manufacturing process and specifications while target population, uses and use levels and consequently the anticipated intake do not change. The information provided on the manufacturing process, including the absence of DNA from the producing microorganisms, composition, identity and specifications of the NF do not raise safety concerns. Particularly, the proposed changes in the specifications are limited to a slightly higher ash content and limits for the presence of yeast and moulds, while specifications for methanol and LNnT fructose isomer have been removed. Food supplements are not intended to be used if other foods with the added NF or breast milk for young children are consumed on the same day. The Panel concludes that lacto‐N‐neotetraose (LNnT) as a NF when produced by fermentation with two genetically modified strains of E. coli BL21 is safe under the proposed conditions of use.
Keywords: lacto‐N‐neotetraose, LNnT, human milk oligosaccharide, HiMO, novel food, change in specifications, food for specific groups
1. Introduction
1.1. Background and Terms of Reference as provided by the requestor
On 23 June 2019, the company Jennewein Biotechnologie GmbH submitted a request to the Commission in accordance with Article 10 of Regulation (EU) No 2015/2283 to place on the EU market microbiologically produced lacto‐N‐neotetraose as a novel food.
The microbiologically produced lacto‐N‐neotetraose is intended to be used in a number of foods and in food supplements as defined in Directive 2002/46/EC excluding food supplements for infants. The applicant has not requested data protection according to the provisions of Article 26 of Regulation (EU) 2015/2283.
In accordance with Article 10(3) of Regulation (EU) 2015/2283, the European Commission asks the European Food Safety Authority to provide a scientific opinion on microbiologically produced lacto‐N‐neotetraose as a novel food.
1.2. Interpretation of Terms of reference
The application is referring to a request for changes in the production process and specifications for the microbiologically produced lacto‐N‐neotetraose (LNnT). LNnT is already authorised (Commission Implementing Decision (EU) 2016/375 of March 11, 20161) and included in the European Union (EU) list of authorised novel foods (NFs) (Commission Implementing Regulation (EU) 2017/24702) when chemically synthesised or when produced by fermentation, but with a different strain (K‐123) of E. coli. Therefore, the current assessment is exclusively focused on the proposed changes with regard to the possible impact on the safety and nutritional aspects.
1.3. Additional information
Information on existing evaluations and authorisations.
In 2015, EFSA adopted an opinion on the safety of the LNnT when chemically synthesised (EFSA NDA Panel, 2015a). The Panel concluded that LNnT was safe when added to infant and follow‐on formula and other foods at the proposed uses and use levels. In the same year, the NDA Panel also adopted a statement on the use of LNnT and 2′‐FL (fucosyllactose) in food supplements for children (EFSA NDA Panel, 2015b). The Panel concluded that in children of 1–10 years of age, the combined intakes from all foods in which LNnT and 2′‐FL are intended to be added and from food supplements ‘could result in intake levels which were reported to cause mild gastrointestinal symptoms in adults’.
2. Data and methodologies
2.1. Data
The safety assessment of this NF is based on data supplied in the application and information submitted by the applicant following an EFSA request for supplementary information.
During the assessment, the Panel identified additional data which were not included in the application (Alderman and Stranks, 1967; Hosomi and Takeya, 1989; Miwa et al., 2010; Bode, 2012; Gidrewicz and Fenton, 2014; Thurl et al., 2017).
Administrative and scientific requirements for NF applications referred to in Article 10 of Regulation (EU) 2015/2283 are listed in the Commission Implementing Regulation (EU) 2017/24694.
A common and structured format on the presentation of NF applications is described in the EFSA guidance on the preparation and presentation of an NF application (EFSA NDA Panel, 2016). As indicated in this guidance, it is the duty of the applicant to provide all of the available (proprietary, confidential and published) scientific data, (including both data in favour and not in favour) that are pertinent to the safety of the NF.
This NF application does not include a request for the protection of proprietary data.
2.2. Methodologies
The assessment follows the methodology set out in the EFSA guidance on NF applications (EFSA NDA Panel, 2016) and the principles described in the relevant existing guidance documents from the EFSA Scientific Committee. The legal provisions for the assessment are laid down in Article 11 of Regulation (EU) 2015/2283 and in Article 7 of the Commission Implementing Regulation (EU) 2017/2469.
This assessment concerns only the risks that might be associated with consumption of the NF under the proposed conditions of use and is not an assessment of the efficacy of the NF with regard to any claimed benefit.
3. Assessment
3.1. Introduction
The NF primary constituent is LNnT, one of the most abundant oligosaccharides within the complex fraction of human milk oligosaccharides (HMO). The NF is obtained by microbial fermentation. The NF is proposed to be used in foods for infants and young children (including infant formulae (IF) and follow‐on formulae (FOF)), foods for special medical purposes, total diet replacements for weight control, table‐top sweeteners, food supplements, beverages and in a variety of other foods (e.g. dairy products, bakery wares). The target population is the general population, except for the use as food supplement which is intended for individuals above 1 year of age.
The applicant indicated that, according to Regulation (EU) 2015/2283, this NF falls under the following categories:
‘food with a new or intentionally modified molecular structure, where that structure was not used as, or in, a food within the Union before 15 May 1997′; and
‘food consisting of, isolated from or produced from microorganisms, fungi or algae’.
3.2. Identity of the NF
The NF is a white‐ to ivory‐coloured powder constituted by LNnT, a tetrasaccharide consisting of d‐galactose, N‐acetyl‐d‐glucosamine, d‐galactose and d‐glucose, which is produced by fermentation with two genetically modified derivatives of E. coli BL21 (DE3).
LNnT is characterised by the chemical formula: C26H45NO21, molecular mass: 707.63 Da; CAS No: 13007‐32‐4; IUPAC Name: β‐D‐galactopyranosyl‐(1→4)‐2‐acetamido‐2‐deoxy‐β‐D‐glucopyranosyl‐(1→3)‐β‐D‐galactopyranosyl‐(1→4)‐D‐glucopyranose (Figure 1).
Figure 1.
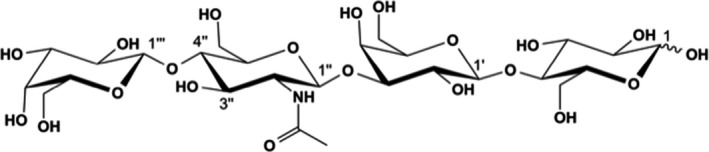
Structure of LNnT
The structure of LNnT has been confirmed by 13C and 1H mono‐ and two‐dimensional NMR spectroscopy and by LC‐MS/MS spectrometry and compared to a commercially available standard. In particular, the structure was unequivocally demonstrated by double‐quantum filtered 1H‐1H‐correlation spectroscopy (COSY) spectra, phase‐sensitive 1H‐13C‐heteronuclear single quantum correlation (HSQC) spectra and phase‐sensitive 1H‐13C‐heteronuclear multiple bond correlation (HMBC) spectra. LNnT differs from LNT only by the type of glycosidic bond connecting the terminal galactose and N‐acetylglucosamine: by a β‐(1→4) bond in LNnT, by a β‐(1→3) bond in LNT. The clear distinction between LNnT and LNT was possible on the basis of the 13C NMR chemical shifts of the carbons C‐3’’ and C‐4’’ of N‐acetylglucosamine, which are specific for the β‐(1→4) bond between the terminal galactose and N‐acetylglucosamine as well as by the coupling of H‐1’’’ of galactose with the C‐4’’ of N‐acetylglucosamine in LNnT which is different from the H‐1’’’ C‐3’’ coupling in LNT, as observed by the HMBC spectra.
The product identity was verified for five independently manufactured batches of the NF. The LNnT in the NF produced by the microbial fermentation described in the production process has been shown to be structurally identical to its naturally occurring counterpart present in human milk oligosaccharides by mono‐ and two‐dimensional NMR and is therefore considered as a HiMO (human identical milk oligosaccharide).
3.3. Production process
According to the information provided by the applicant, the NF is produced in a facility that is ISO:9001 and FSSC 22000 certified.
The NF is produced by a two‐step fed‐batch fermentation process using two genetically modified strains derived from the host strain Escherichia coli BL21 (DE3). These strains are the ‘Production strain’ E. coli BL21 (DE3) PS‐LNnT‐JBT and the ‘Degradation strain’ E. coli BL21 (DE3) DS‐LNnT‐JBT. The production strain has been modified to effectively synthesise LNnT, while the degradation strain is equipped with enzymes to degrade intermediate carbohydrate by‐products and remaining substrates in order to facilitate the production process. Several carbohydrates are used as carbon and energy sources for the cultivation of both strains and lactose is added as a substrate for the production of the LNnT by the production strain. At the end of the fermentation process, the bacteria are removed from the final product by several filtration steps. Methods for verification of the absence of Enterobacteriaceae (microbial family including E. coli species) in 10 g of the final product are applied (ISO 21528‐1: 2017‐09).
The production strain E. coli BL21 (DE3) PS‐LNnT‐JBT is a genetically modified derivative of the host strain E. coli BL21 (DE3) (F− ompT hsdSB (rB −mB −) gal dcm DE3). The E. coli BL21 (DE3) strain was developed for T7 RNA polymerase‐based gene expression by introducing a lambda prophage containing a T7 RNA polymerase and it is widely used in laboratories around the world. E. coli BL21 (DE3) is considered to be non‐pathogenic and unlikely to survive in host tissues or to cause disease (Chart et al., 2000). The genome sequence of E. coli BL21 (DE3) revealed the absence of genes encoding invasion factors, adhesion molecules and enterotoxins associated with virulence (Jeong et al., 2009).
The host strain has been deposited at the German Collection of Microorganisms and Cell Cultures (DSMZ) under DSM No. 33523. The production strain of LNnT E. coli BL21 (DE3) PS‐LNnT‐JBT and the degradation strain of LNnT E. coli BL21 (DE3) dS‐LNnT‐JBT have been deposited at DSMZ in Braunschweig, Germany. A detailed description of the genetic modification steps applied to obtain both the production strain and the degradation strain has been provided by the applicant. No residual DNA from both strains was detected in five independent batches of the NF using polymerase chain reaction (PCR) amplification of five antimicrobial resistance genes introduced during the genetic modification. Upon EFSA's request, the applicant provided data to demonstrate the validity of the method in accordance with the EFSA Guidance of microorganisms used as production organisms (EFSA FEEDAP Panel, 2018).
The Panel considers that the production process is sufficiently described and does not raise safety concerns.
3.4. Compositional data
The NF contains LNnT as primary ingredient (≥ 93% w/w dry matter), the remaining is a mixture of substances including lactose, lacto‐N‐triose II (LNT II), para‐lacto‐N‐neo‐hexaose (para‐LNnH) and other carbohydrates. LNT II is a neutral trisaccharide and minor component of breast milk resulting from the hydrolysis of LNnT (Hosomi and Takeya, 1989; Miwa et al., 2010) and para‐LNnH is another minor component present in human milk as difucosylated (Thurl et al., 2017). Lactose is the most prevalent compound in human breast milk (approximately 7 g/100 mL) (Bode, 2012; Gidrewicz and Fenton, 2014).5
With regard to the physico‐chemical properties, the NF can be described as white to ivory‐coloured powder readily soluble in aqueous solutions (minimum solubility 500 g/L, 25°C).
The microbiological quality of the batches of the NF has been assessed for non‐pathogenic microorganisms (bacteria, yeasts and moulds as general hygiene indicators) and for selected food‐borne pathogens.
Analyses were performed by high‐performance anion exchange chromatography coupled to pulsed amperometric detection (HPAEC‐PAD), by the applicant, using ‘in‐house’ validated methods (carbohydrates content) and by certified external international laboratories applying standard methods (e.g. ISO).
Information was provided on the accreditation of the laboratories that conducted the analyses presented in the application.
In order to confirm that the manufacturing process is reproducible and adequate to produce on a commercial scale a product with the required characteristics, the applicant provided analytical information for five independent batches of the NF (Table 1).
Table 1.
Batch‐to‐batch analysis for five batches of NF
| Parameter (Unit) | Batch number | ||||
|---|---|---|---|---|---|
| 10916019 | 10916029 | 10916039 | 10916049 | 10916059 | |
| Physico‐chemical properties | |||||
| Appearance | Powder to agglomerate | ||||
| Colour | White to ivory‐coloured | ||||
| pH (20°C, 5% solution) | 6.9 | 6.8 | 6.4 | 6.9 | 6.9 |
| Composition | |||||
| LNnT (% dry matter) | 93.5 | 95.9 | 96.1 | 93.6 | 95.8 |
| Lactose (% dry matter) | < 0.5 | < 0.5 | < 0.5 | < 0.5 | < 0.5 |
| LNT II (% dry matter) | 1.2 | 1.7 | 1.6 | 1.3 | 1.0 |
| Para‐LNnH (% dry matter) | 1.6 | < 0.5 | < 0.5 | 1.1 | < 0.5 |
| Sum of saccharides (% dry matter)a | 96.3 | 97.6 | 97.7 | 96.0 | 96.8 |
| Sum of other saccharides (% dry matter) | 3.6 | 2.4 | 2.3 | 4.0 | 3.2 |
| Water content (%) | 6.3 | 7.8 | 8.6 | 8.6 | 7.1 |
| Protein content (μg/g) | 1.8 | 0.5 | 0.7 | 1.1 | 1.0 |
| Total ash (%) | 0.04 | 0.07 | 0.14 | 0.10 | 0.16 |
| Lead (mg/kg) | < 0.01 | < 0.01 | < 0.01 | 0.014 | 0.013 |
| GMO detectionb | Not detected | Not detected | Not detected | Not detected | Not detected |
| Microbiological parameters | |||||
| Standard Plate Count (CFU/g) | < 10 | < 10 | < 10 | < 10 | < 10 |
| Yeasts and moulds (CFU/g) | < 20 | < 20 | < 20 | < 20 | < 20 |
| Bacillus cereus (CFU/g) | < 10 | < 10 | < 10 | < 10 | < 10 |
| Enterobacteriaceae (in 10 g) | n.d. | n.d. | n.d. | n.d. | n.d. |
| Listeria monocytogenes (in 25 g) | n.d. | n.d. | n.d. | n.d. | n.d. |
| Salmonella spp. (in 25 g) | n.d. | n.d. | n.d. | n.d. | n.d. |
| Cronobacter spp. (in 10 g) | n.d. | n.d. | n.d. | n.d. | n.d. |
| Residual endotoxins (EU/mg) | 0.940 | < 0.005 | < 0.005 | < 0.005 | < 0.005 |
LNnT: lacto‐N‐neotetraose; LNT II: lacto‐N‐triose II; para‐LNnH: para‐lacto‐N‐neo‐hexaose n.d.: not detected; CFU, colony‐forming unit; EU: endotoxin unit; GMO: genetically modified organisms.
Lacto‐N‐neotetraose, lactose, lacto‐N‐triose II, para‐Lacto‐N‐neohexaose.
microbial DNA < 10 ng DNA/1 g NF.
The Panel considers that the information provided on the composition is sufficient for characterising the NF.
3.4.1. Stability
The applicant performed stability tests with three independently produced batches of the NF. The tests were carried out at ambient temperature for at least 24 months. The batches were analysed for the LNnT content that resulted to be constant over time (≥ 99.7% of the original content).
In addition, the Panel noted that with identical (LNnT synthetically produced) or similar HiMO (LNT, the regioisomer of LNnT, obtained by microbial fermentation), the stability of the respective NFs was at least 24 months (EFSA NDA Panel, 2015a, 2019).
The Panel considers that the available data provided sufficient information with respect to the stability of the NF for 24 months when protected from moisture and stored at room temperature.
3.4.2. Stability under the intended conditions of use
No data on stability under the intended conditions of use were provided. The Panel considers that the stability is similar to that of chemically synthesised LNnT which is already authorised and for which the stability as an ingredient in IF, FOF, baby foods and other food products has been documented (EFSA NDA Panel, 2015a).
The Panel considers that the already available information is sufficient with respect to the stability of the NF in the food matrices.
3.5. Specifications
The specifications of the NF as proposed by the applicant are indicated in Table 2.
Table 2.
Specifications of the NF as proposed by the applicant
| Description: Lacto‐N‐neotetraose (LNnT) is a white‐ to ivory‐coloured powder that is produced by a microbiological process | |||
|---|---|---|---|
| Source: A genetically modified strain of Escherichia coli BL21 (DE3) | |||
| Parameter | Specification | Analytical Method | |
| LNnT (% w/w dry matter) | ≥ 80.0 | HPAEC/PAD | |
| D‐Lactose (% w/w dry matter) | ≤ 10.0 | HPAEC/PAD | |
| Lacto‐N‐triose II (% w/w dry matter) | ≤ 3.0 | HPAEC/PAD | |
| Para‐lacto‐N‐neohexaose (% w/w dry matter) | ≤ 5.0 | HPAEC/PAD | |
| Sum of saccharides (lacto‐N‐neotetraose, lactose, lacto‐N‐triose II, para‐lacto‐N‐neohexaose) (% w/w dry matter) | ≥ 92 | HPAEC/PAD | |
| pH (20°C, 5% solution) | 4.0–7.0 | Eur. Ph. 9.2 2.2.3 | |
| Water (% w/w) | ≤ 9.0 | Karl‐Fisher titration | |
| Ash (% w/w) | ≤ 1.0 | ASU L 06.00‐4 | |
| Residual protein (% w/w) | ≤ 0.01 | Nanoquant (modified Bradford) | |
| Microbiological Parameters | |||
| Aerobic mesophilic bacteria total count | ≤500 CFU/g | ISO 4833‐2‐1:2014 | |
| Yeasts and moulds | ≤ 50 CFU/g | ISO 21527‐2:2008 | |
| Residual endotoxins | ≤ 10 EU/mg | Eur. Ph. 2.6.14 | |
LNnT: lacto‐N‐neo‐tetraose; CFU: colony‐forming units; EU: endotoxin units; Eur. Ph.: European Pharmacopoeia; HPAEC: high‐performance anion exchange chromatography; PAD: pulsed amperometric detection; ISO: international organization for standardization.
It is noted that only few differences are proposed for LNnT produced by a genetically modified strain of E. coli BL21 (DE3) compared to the current specifications (EU list of NFs),6 intended for LNnT when produced with E. coli K‐12. More specifically, the following differences have been proposed: a slightly higher limit for ash content (from ≤ 0.4% to ≤ 1%), a slightly higher level for the presence of yeasts and moulds (from ≤ 10 CFU/g for each type of microorganism to ≤ 50 CFU/g for the combination of the two) while levels of methanol (residual solvent) and of LNnT fructose isomer are not included. The Panel noted that specifications allow up to 10% lactose in the NF while the content reported in the representative batches is lower than 0.5%.
The Panel considers that the information provided on the specifications of the NF is sufficient.
3.6. History of use of the NF and of its source
3.6.1. History of use of the NF
The NF does not have a history of use.
However, LNnT is already included in the list of NFs7 when chemically sinthesised or when produced by fermentation using a different strain (K‐12) of E. coli. It is authorised to be added to infant and follow‐on formulae, to a variety of foods as well as to food supplements for individuals above 1 year of age.
3.7. Proposed uses and use levels and anticipated intake
3.7.1. Target population
The target population proposed by the applicant is the general population, except for food supplements for which the target population is individuals above 1 year of age.
3.7.2. Proposed uses and use levels
The intended uses and use levels for the NF are the ones already authorised for LNnT manufactured by chemical synthesis or by fermentation with E. coli K‐12 and included in the EU list of NFs.
3.7.3. Anticipated intake of the NF
Considering that the same uses and use levels as per the authorised LNnT are proposed (see Section 3.7.2), the Panel considers that a new assessment of the intake is not needed. The NF would be used at the same extent as the authorised LNnT.
3.7.4. Precautions and restrictions of use
The applicant has proposed the use of the NF as food supplement for individuals above 1 year of age, as already authorised. Food supplements are not intended to be used if other foods with added LNnT (as well as breast milk for young children) are consumed on the same day.
3.8. Nutritional information
The NF is mainly composed of the non‐digestible oligosaccharide LNnT.
The Panel considers that consumption of the NF at the proposed use levels is not nutritionally disadvantageous.
3.9. Allergenicity
The protein content in the NF is low (≤ 0.01%), as indicated in the specifications (Table 2).
The Panel considers that the likelihood of allergenic reactions to the NF is low.
4. Discussion
The NF is a powdered mixture mainly composed of LNnT, but also containing D‐lactose and other saccharides such as para‐lacto‐N‐hexaose, lacto‐N‐triose II and a small fraction of other carbohydrates. The NF is obtained by microbial fermentation with two genetically modified strains of E. coli BL21.
The applicant intends to add the NF to a variety of foods, comprising foods for infants and young children (including infant and follow‐on formulae), foods for special medical purposes and food supplements. The target population is the general population except for food supplements, for which the target population is individuals above 1 year of age. Food supplements are not intended to be used if other foods with added LNnT (as well as breast milk for young children) are consumed on the same day.
LNnT produced by synthesis or by microbial fermentation using another E. coli strain (K‐12) is already authorised and included in the EU Union list of NFs. The Panel notes that the current application is limited to a change in the manufacturing process and specifications while uses and use levels, and consequently the anticipated intake, will not change.
The information provided on the manufacturing process, including the absence of DNA from the producing microorganisms, composition, identity and specifications of the NF, does not raise safety concerns.
Particularly, the proposed changes in the specifications are limited to a slightly higher ash content and limits for the presence of yeast and moulds, while specifications for methanol and LNnT fructose isomer have been removed.
The Panel considers that the proposed changes of the specifications do not raise safety concerns.
5. Conclusions
The Panel concludes that lacto‐N‐neotetraose (LNnT) as a NF when produced by fermentation with two genetically modified strains of E. coli BL21 is safe under the proposed conditions of use.
6. Steps taken by EFSA
Letter from the European Commission with the request for a scientific opinion on microbiologically produced lacto‐N‐neotetraose as a novel food. Ref. Ares(2020)315926, dated 17/01/2020.
On 17/01/2020, a valid application on lacto‐N‐neotetraose, which was submitted by Jennewein Biotechnologie GmbH, was made available to EFSA by the European Commission through the Commission e‐submission portal (NF 2019/1152) and the scientific evaluation procedure was initiated.
On 15/04/2020, EFSA requested the applicant to provide additional information to accompany the application and the scientific evaluation was suspended.
On 18/06/2020, additional information was provided by the applicant through the Commission e‐submission portal.
On 30/06/2020 and 21/07/2020, EFSA requested the applicant to provide further clarifications to the additional information provided.
On 14/07/2020 and 07/08/2020, clarifications were provided by the applicant through the Commission e‐submission portal and the scientific evaluation was restarted.
During its meeting on 22/10/2020, the NDA Panel, having evaluated the data, adopted a scientific opinion on the safety of lacto‐N‐neotetraose produced by derivative strains of E.coli BL21 as a NF pursuant to Regulation (EU) 2015/2283.
Abbreviations
- CAS
Chemical Abstracts Services
- CFU
colony‐forming units
- COSY
correlation spectroscopy
- Da
dalton
- DSMZ
German Collection of Microorganisms and Cell Cultures (Deutsche Sammlung von Mikroorganismen und Zellkulturen)
- EU
endotoxin units
- Eur.Ph.
European pharmacopeia
- FEEDAP
Panel on Additives and Products or Sub‐stances used in Animal Feeds
- FL
fucosyl‐lactose
- FOF
follow‐on formula
- FSSC
Food Safety System certification
- HiMO
human identical milk oligosaccharide
- HMBC
heteronuclear multiple bond correlation
- HMO
human milk oligosaccharide
- HPAEC
high‐performance anion exchange chromatography
- HSQC
heteronuclear single quantum coherence
- IF
infant formula
- ISO
International Organization for Standardization
- IUPAC
International Union of Pure and Applied Chemistry
- LC
liquid chromatography
- LNnT
lacto‐N‐neotetraose
- LNT
lacto‐N‐tetraose
- LNT II
lacto‐N‐triose II
- MS
mass spectrometry
- NDA
EFSA Panel on Nutrition, Novel Foods and Food Allergens
- NF
novel food
- NMR
nuclear magnetic resonance
- PAD
pulsed amperometric detection
- Para‐LNnH
para‐lacto‐N‐neo-hexaose
- PCR
Polymerase chain reaction
- RH
relative humidity
Suggested citation: EFSA NDA Panel (EFSA Panel on Nutrition, Novel Foods and Food Allergens) , Turck D, Castenmiller J, De Henauw S, Hirsch‐Ernst KI, Kearney J, Maciuk A, Mangelsdorf I, McArdle HJ, Naska A, Pelaez Carmen, Pentieva K, Siani A, Thies F, Tsabouri S, Vinceti M, Cubadda F, Frenzel T, Heinonen M, Marchelli R, Neuhäuser‐Berthold M, Poulsen M, Maradona MP, Schlatter JR, van Loveren H, Colombo P and Knutsen HK, 2020. Scientific Opinion on the safety of lacto‐N‐neotetraose (LNnT) produced by derivative strains of E. coli BL21 as a novel food pursuant to Regulation (EU) 2015/2283. EFSA Journal 2020;18(11):6305, 11 pp. 10.2903/j.efsa.2020.6305
Requestor European Commission
Question number: EFSA‐Q‐2019‐00448
Panel members: Dominique Turck, Jacqueline Castenmiller, Stefaan De Henauw, Karen Ildico Hirsch‐Ernst, John Kearney, Helle Katrine Knutsen, Alexandre Maciuk, Inge Mangelsdorf, Harry J McArdle, Androniki Naska, Carmen Pelaez, Kristina Pentieva, Alfonso Siani, Frank Thies, Sophia Tsabouri and Marco Vinceti.
Adopted: 22 October 2020
Notes
Commission Implementing Decision (EU) 2016/375 of 11 March 2016 authorising the placing on the market of lacto‐N‐neotetraose as a novel food ingredient under Regulation (EC) No 258/97 of the European Parliament and of the Council. OJ L 70, 16.3.2016, p. 22–26.
Commission Implementing regulation (EU) 2017/2470 of 20 December 2017 establishing the Union list of novel foods in accordance with Regulation (EU) 2015/2283 of the European Parliament and of the Council on novel foods. OJ L 351, 30.12.2017, p. 72.
Commission Implementing regulation (EU) 2019/1314 of 2 August 2019 authorising the change of the specifications of the novel food Lacto‐N‐neotetraose produced with Escherichia coli K‐12 under Regulation (EU) 2015/2283 of the European Parliament and of the Council and amending Commission Implementing Regulation (EU) 2017/2470. OJ L 205, 5.8.2019, p. 4–6.
Commission Implementing Regulation (EU) 2017/2469 of 20 December 2017 laying down administrative and scientific requirements for applications referred to in Article 10 of Regulation (EU) 2015/2283 of the European Parliament and of the Council on novel foods. OJ L 351, 30.12.2017, pp. 64–71.
A minor fraction not identified with HPAEC‐PAD was analysed by LC‐MS/MS revealing the presence of small amounts of saccharides (e.g. glucose‐isomers of LNnT). The presence of these constituents is related to the manufacturing process and a similar carbohydrate pattern was noted in the production of LNnT by E. coli K‐12 (Röhrig, 2016).
Union list of authorised novel foods COMMISSION IMPLEMENTING REGULATION (EU) 2017/2470 of 20 December 2017 establishing the Union list of novel foods in accordance with Regulation (EU) 2015/2283 of the European Parliament and of the Council on novel foods. OJ L 351, 30.12.2017, pp. 72–201.
Commission Implementing Regulation (EU) 2018/1023 of 23 July 2018 correcting Implementing Regulation (EU) 2017/2470 establishing the Union list of novel foods. Official Journal of the European Union L 187, 1–133.
References
- Alderman G and Stranks MH, 1967. The iodine content of bulk herd milk in summer in relation to estimated dietary iodine intake of cows. Journal of the Science of Food and Agriculture, 18, 151–153. [DOI] [PubMed] [Google Scholar]
- Bode L, 2012. Human milk oligosaccharides: every baby needs a sugar mama. Glycobiology, 22, 1147–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chart H, Smith HR, La Ragione RM and Woodward MJ, 2000. An investigation into the pathogenic properties of Escherichia coli strains BLR, BL21, DH5α and EQ1. Journal of Applied Microbiology, 89, 1048–1056. [DOI] [PubMed] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), 2018. Guidance on the characterisation of microorganisms used as feed additives or as production organisms. EFSA Journal 2018;16(3):5206, 24 pp. 10.2903/j.efsa.2018.5206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies), 2015a. Scientific opinion on the safety of lacto‐N‐neotetraose as a novel food ingredient pursuant to Regulation (EC) No 258/97. EFSA Journal 2015;13(7):4183, 32 pp. 10.2903/j.efsa.2015.4183 [DOI] [Google Scholar]
- EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies), 2015b. Statement on the safety of lacto‐N‐neotetraose and 2’‐O‐fucosyllactose as novel food ingredients in food supplements for children. EFSA Journal 2015;13(11):4299, 11 pp. 10.2903/j.efsa.2015.4299 [DOI] [Google Scholar]
- EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies), 2016. Guidance on the preparation and presentation of an application for authorisation of a novel food in the context of Regulation (EU) 2015/2283. EFSA Journal 2016;14(11):4594, 24 pp. 10.2903/j.efsa.2016.4594 [DOI] [Google Scholar]
- EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies), 2019. Scientific Opinion on the safety of lacto‐N‐tetraose (LNT) as a novel food pursuant to Regulation (EU) 2015/2283. EFSA Journal 2019;17(12):5907, 27 pp. 10.2903/j.efsa.2019.5907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gidrewicz D and Fenton TR, 2014. A systematic review and meta‐analysis of the nutrient content of preterm and term breast milk. BMC Pediatrics, 14, 216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosomi O and Takeya A, 1989. The relationship between the (b1‐3) N‐acetylglucosaminytransferase and the presence of oligosaccharides containing lacto‐N‐triose II structure in bovine and human milk. Japanese Journal of Veterinary Sciences, 51, 1–6. [DOI] [PubMed] [Google Scholar]
- Jeong H, Barbe V, Lee CH, Vallenet D, Yu DS, Choi S‐H, Couloux A, Lee S‐W, Yoon SH, Cattolico L, Hur C‐G, Park H‐S, Ségurens B, Kim SC, Oh TK, Lenski RE, Studier FW, Daegelen P and Kim JF, 2009. Genome Sequences of Escherichia coli B strains REL606 and BL21(DE3). Journal of Molecular Biology, 394, 644–652. 10.1016/j.jmb.2009.09.052 [DOI] [PubMed] [Google Scholar]
- Miwa M, Horimoto T, Kiyohara M, Katayama T, Kitaoka M, Ashida H and Yamamoto K, 2010. Cooperation of b‐galactosidase and b‐N‐acethylhexosaminidase from bifidobacterial in assimilation of human milk oligosaccharides with type 2 structure. Glycobiology, 20, 1402–1409. [DOI] [PubMed] [Google Scholar]
- Röhrig CH, 2016. GRAS notice (GRN) No. 659; GRAS exemption claim for LNnT.
- Thurl S, Munzert M, Boehm G, Matthews C and Stahl B, 2017. Systematic review of the concentrations of oligosaccharides in human milk. Nutrition Reviews, 75, 920–933. [DOI] [PMC free article] [PubMed] [Google Scholar]


