Abstract
Streptococcus pneumoniae (the pneumococcus) has wall teichoic acid (WTA) and lipoteichoic acid (LTA) expressing the Forssman antigen (FA). Two lectins, Dolichos biflorus agglutinin (DBA) and Helix pomatia agglutinin (HPA), are known to bind FA. To determine the molecular structure targeted by these two lectins, different pneumococcal strains were studied for DBA/HPA binding with flow cytometry and fluorescence microscopy. Genetic experiments were used to further examine the lectins’ molecular target. Twelve strains were positive for DBA binding while three were negative. Super resolution microscopy showed that DBA stained only the subcapsular area of pneumococci. The three DBA non-binders showed no phosphorylcholine esterase (Pce) activity in vitro, whereas 10 DBA binders displayed Pce activity (the remaining two strains were DBA binders with no Pce activity in vitro). The pce gene sequence for 10 representative strains revealed two functional pce alleles, the previously recognized “allele A” and a newly-discovered “allele B” (with 12 additional nucleotides). Isolates with allele B showed no Pce activity in vitro but did bind to DBA, indicating allele B Pce is functional in vivo. Genetic transfer experiments confirmed that either allele is sufficient (and necessary) for DBA binding. The three DBA non-binders had various mutations that affected Pce function. Observations with HPA were identical to those with DBA. We show that DBA and HPA bind only to the WTA/LTA of pneumococcal isolates with a functional Pce enzyme. A newly-discovered Pce variant (allele B) is functional in vivo but nonfunctional when assayed in vitro.
Keywords: Dolichos biflorus agglutinin, Helix pomatia agglutinin, teichoic acid, Streptococcus pneumoniae, phosphorylcholine esterase
1. INTRODUCTION
The Gram-positive bacteria have a structurally complex cell surface that is critical to their survival. The cell surface has a thick mesh-like cell wall structure formed with peptidoglycan (PG) as well as various surface proteins, capsular polysaccharide, and other glycopolymers such as teichoic acid. These cell surface structures are also involved in several aspects of bacteria-host interaction, including adhesion to host cells, induction of inflammation, antibody binding, and activation of complement [1]. The PG meshwork, which is the target for many antibiotics [2], is critical for bacterial survival as it provides protection against osmotic lysis, maintains cellular structural integrity, and defines the shape of the cell.
Reflecting its critical importance, synthesis of the PG meshwork has been extensively studied with many bacterial species, including S. pneumoniae (the pneumococcus). Pneumococcus has emerged as a model organism because of its medical importance and genetic flexibility. Genetic and biochemical studies have shown that the synthesis of PG begins with a structural unit that comprises a disaccharide of N-acetyl glucosamine (GlcNAc) and N-acetyl muramic acid (MurNAc), with an attached pentapeptide, synthesized by a series of Mur ligases [3]. After translocation across the cell membrane, the PG structural units are assembled by transglycosylation of the disaccharides to form linear glycan chains of various lengths, which are then crosslinked via transpeptidation of the short peptide chains [2,3]. With the development of super resolution microscopy and specific PG markers, we are beginning to resolve the molecular architecture and dynamics of PG synthesis [4].
The PG meshwork is further modified by recruiting other glycopolymers such as capsule or teichoic acid (TA). Teichoic acids are phosphate-rich polymers that can comprise up to half of the cell wall mass [5] and provide a layer, or ‘continuum’, of anionic charge important for bacterial survival [1,5]. Genetic and biochemical studies have shown that pneumococcal TA has 5–8 repeating units of GalNAc-GalNAc-ribitol-Glc-AATGal that are decorated with phosphorylcholine (PC) [6] at one or both of the GalNAc residues, added via two phosphorylcholine transferases, LicD1 and LicD2 [7,8]. The PC-decorated TA repeating units are polymerized, transported out of the cytoplasm, and are then either covalently attached to the cell wall via linkage to the MurNAc residue of PG (wall teichoic acid, WTA) or attached to the cell membrane via a diacylglycerol-containing glycolipid anchor (lipoteichoic acid, LTA) by the LCP family of transferases [1,5,9,10]. Pneumococcal WTA and LTA can have their associated PC residues removed from the terminal repeating unit by phosphorylcholine esterase (Pce) [7,11,12], which is encoded by the pce gene. Removal of these terminal PC residues creates the terminal disaccharide (GalNAcα1→3GalNAcβ1) [13,14] that is identical to the non-reducing end of the Forssman antigen (GalNAcα1→3GalNAcβ1→3Galα1→4Galβ1→4Glcβ1→1Ceramide) [15].
To further investigate TA synthesis, and its attachment to the PG meshwork at a single-molecule level, it would be useful to have a specific and sensitive marker for TA. Two lectins, Dolichos biflorus agglutinin (DBA) and Helix pomatia agglutinin (HPA), are known to bind the Forssman antigen (FA) [16] and may react with WTA/LTA. Though the lectins have been used previously to identify streptococci [17–19], including pneumococci [20,21], the molecular targets of the two lectins have remained unclear. Consequently, we have elucidated the molecules targeted by DBA and HPA by investigating their interaction with many S. pneumoniae strains.
2. MATERIALS AND METHODS
2.1. Bacterial strains and growth conditions
A total of 15 pneumococcus strains expressing 14 different serotypes, and one non-encapsulated strain (R36A), were selected for this study from our laboratory collection (see Figure 1). Pneumococcal strains were grown in Todd-Hewitt broth supplemented with 0.5% yeast extract (THY broth; both, BD, Sparks, MD) at 37°C to an OD600 of 0.5–0.6. Sterile 80% glycerol was added to the pneumococcal culture (16% final concentration) before aliquoting (0.5 ml) and storage at −80°C for future use. For transformant screening, kanamycin (100 μg/ml) and spectinomycin (100 μg/ml) were added to the THY agar plates. Escherichia coli strains were grown in Luria-Bertani broth (BD) overnight at 37°C with shaking, before plasmid DNA extraction.
2.2. Detecting Pce enzyme activity in vitro
Pce activity of a pneumococcal isolate was determined with a colorimetric assay using p-nitrophenylphosphorylcholine (p-NPPC; Cayman Chemical, Ann Arbor, MI) as the artificial substrate, as previously described [11,12,22]. Briefly, a frozen aliquot of pneumococcus was thawed, washed twice with PBS by centrifugation, and lysed (final concentration 0.1% sodium deoxycholate, 0.01% SDS, 150 mM sodium citrate, pH 7.5) for an initial period of 10 min at 37°C, followed by further incubation after the addition of p-NPPC (final concentration 3 mM) in potassium phosphate buffer (final concentration 50 mM, pH 8.0) at 37°C. The OD405 of the supernatant was measured at 1, 2, 3, 4, 5, 6, 7, 8, and 24 h. Pce enzyme activity was defined as the average slope of the OD405 over the first 8 hours, normalized to the OD600 value of the strain’s working stock. The assay was performed three times; one representative run is reported.
2.3. Visualizing DBA and HPA binding with flow cytometry
A frozen aliquot of pneumococcus was thawed, washed with PBS by centrifugation, and diluted (1 in 40) with HBC (Hanks balanced buffer supplemented with 2.2 mM CaCl2 and 0.5% bovine serum albumin). Fifty microliters of diluted bacteria were then incubated with an equal volume of 100 μg/ml biotin-conjugated DBA (Vector Laboratories, Burlingame, CA) or HPA (EY Laboratories, San Mateo, CA) for 30 min on ice. After incubation, bacteria were washed once with HBC and then incubated with 5 μg/ml fluorescein-conjugated streptavidin (Vector Laboratories) for 30 min on ice. Bacteria were then washed with HBC and re-suspended in 125 μl of HBC for flow cytometric analysis on a BD Accuri C6 Plus flow cytometer (BD Biosciences, San Jose, CA). Flow cytometric data were analyzed with FCS Express 6 software (De Novo Software, Glendale, CA) using a curve-smoothing feature.
2.4. Visualizing DBA and HPA staining with microscopy
A frozen aliquot of pneumococcus was thawed, washed by centrifugation, and re-suspended with 1 ml of HBC. Then, 250 μl of this suspension was mixed with 5 μl of biotin-conjugated DBA or HPA (50 μg/ml final concentration) and incubated for 30 min on ice. After washing with HBC, the bacteria were incubated with 2 μg/ml Alexa Fluor-594-conjugated streptavidin (Thermo Fisher Scientific, Rockford, IL) for 30 min on ice. To visualize the capsule of D39 and SPEC6B, the bacteria were also incubated with Hyp2M2 and Hyp6BM1, respectively, and visualized with Alexa Fluor-488-conjugated goat anti-mouse IgM [23]. Bacteria were washed and re-suspended in PBS with 5 μM DRAQ5 (Thermo Fisher Scientific) and incubated in the dark for 15 min at room temperature (RT). After washing twice with PBS, the bacterial pellet was re-suspended in 10% neutral buffered formalin (Thermo Fisher Scientific) and transferred to a cover slip to dry for 15 min at RT. The coverslip was washed three times with PBS, allowed to dry, and then placed on a slide with 20 μl of Fluromount G (Thermo Fisher Scientific). After a 1-hour incubation at RT, bacteria were examined with either confocal or structured illumination microscopy (Nikon A1R), using a 100X oil objective.
2.5. Genetic manipulation
For all genetic manipulations, genomic DNA was extracted using an Easy-DNA Kit (Invitrogen, Carlsbad, CA), plasmid DNA was extracted using a QIA Prep Spin Miniprep Kit (Qiagen, Germantown, MD), and PCR products were purified using an E.Z.N.A. Gel Extraction Kit (Omega Bio-tek, Norcross, GA). Competent DH5α cells (Zymo Research, Irvine, CA) and a pPEP1 vector (Addgene, Watertown, MA) [24] were used for cloning. Pce gene PCR amplification was performed using primers 5946 and 3780 and was sequenced using primers 5947–5952. DNA sequencing was performed by the Heflin Center for Genomic Science Core Laboratory at the University of Alabama at Birmingham. All primer sequences are listed in Supporting Information Table S1 and all manipulated strains are described in Supporting Information Table S2.
The pce gene was deleted from R36A, D39, TREP11A, and MNZ741 pneumococcal strains by allelic knock-out of pce with the Janus cassette [25,26]. The Janus cassette (JS) allows marker-free allelic replacement or knock-out in pneumococcus via sequential positive and negative selection. The cassette encodes a kanamycin (Km) resistance selection marker and a counter-selectable rpsL+ marker that confers streptomycin (Sm) sensitivity in a Sm-resistant background [25]. In this case, JS was used only for pce knock-out, with the cassette remaining in situ. As such, absent counter-selection, a Sm-resistant background was not required of the recipient strains. Briefly: PCR products for JS and the flanking regions (~500 bp) immediately up- and down-stream of the pce gene were obtained with primer pairs 51015/3865, 5954/3864, and 51016/3819, respectively. Constructs comprising JS and the homologous pce flanking regions were then synthesized using overlap-extension PCR, before being transformed into the four strains, as previously described [26]. Successful △pce transformants were selected by Km resistance and the genetic changes were verified by PCR and sequencing.
To insert the pce allele A into R36A△pce, D39△pce, and SPEC19F, the promoter region of the pce gene and the pce gene itself were obtained by PCR with primer pairs 51011/3861 and 51012/3862 respectively (R36A was the template for R36A△pce and SPEC19F, whilst D39 was the template for D39△pce). The two fragments were then fused by overlap-extension PCR. The fused promoter/pce product was digested with EcoRI-HF and BamHI-HF (New England BioLabs, Ipswich, MA) and ligated with a similarly digested recipient pPEP1 plasmid using a Quick Ligation Kit (New England BioLabs), all according to supplier’s recommendations. Resultant constructs were then transformed into competent DH5α cells and cultured in Luria-Bertani broth supplemented with spectinomycin (Sp). Plasmid DNA was extracted, verified by PCR and sequencing, and used to transform pneumococcal strains R36A△pce and D39△pce. Successful pce knock-in transformants were selected by Sp resistance and the genetic changes were verified by PCR and sequencing. Note that pPEP1 is a chromosomal integration vector for pneumococcus, with a Sp resistance cassette, that integrates at a transcriptionally silent locus near amiF [24]. Similarly, to insert the pce allele B into MNZ741△pce and D39△pce, the pce gene and its promoter region were amplified from TREP11A using primer pairs 51018/3861 and 51012/3862. The same cloning procedure described above was performed afterwards, except that NheI-HF and BamHI-HF (New England BioLabs) were used for the restriction digest.
3. RESULTS
3.1. Flow cytometry shows DBA binds to a majority of pneumococcal strains expressing diverse capsule types
To investigate if capsule type interferes with the binding of DBA to pneumococci, we examined 15 different pneumococcal strains expressing diverse capsule types. All strains were encapsulated, except for R36A. As presented in Figure 1, flow cytometry showed that 12 of the 15 pneumococcal strains exhibited positive DBA binding (11 encapsulated strains and the one non-encapsulated strain). DBA bound best to the non-encapsulated strain (R36A), based on the ratios of geometric mean fluorescence (GMF) for the primary plus secondary antibodies relative to the secondary antibody only (GMF2/GMF1). Thus, the presence of a capsule attenuated DBA binding but it was not an absolute inhibitor of DBA binding. For example, strains BLS141 and BLS157 still showed a high DBA binding signal even though they are encapsulated with serotype 15B and 15C capsules. Conversely, strain TREP19A showed much weaker DBA binding than other strains. DBA did not bind at all for three strains expressing serotypes 3, 6A, and 19F (strains OREP3, TREP6A, and SPEC19F).
FIGURE 1.
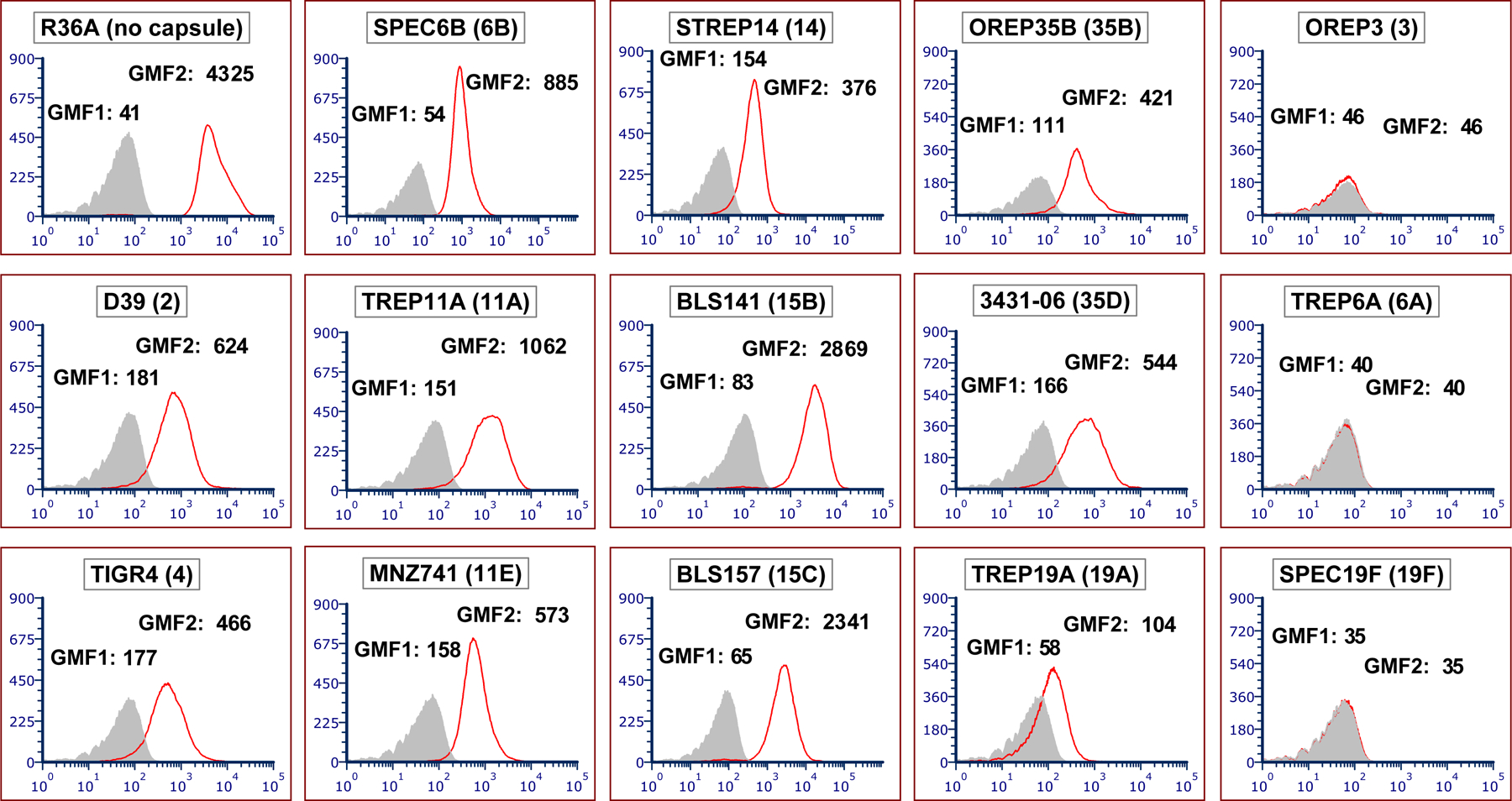
DBA binds to a majority of pneumococcal strains by flow cytometry. Flow cytometric histograms showing binding of DBA to different target strains. Strain names are indicated at the top of each panel, with the associated serotype in parentheses. The y-axis indicates the number of events per channel and the x-axis indicates fluorescence intensity. The grey-filled areas were obtained with bacteria co-incubated with fluorescein-conjugated streptavidin (secondary antibody) only, and this background signal is represented as geometric mean fluorescence 1 (GMF1). The red line represents DBA binding for each strain, with bacteria co-incubated with biotin-conjugated DBA (primary antibody) and fluorescein-conjugated streptavidin, and this signal intensity is represented as GMF2
3.2. Fluorescence microscopy locates DBA binding to the subcapsular area and confirms the results obtained with flow cytometry
When DBA binding to all 15 strains was examined by confocal fluorescence microscopy, these results were consistent with the flow cytometry data (data not shown). We next determined the location of the DBA binding sites by simultaneously staining D39 and SPEC6B with anti-capsule antibodies and DBA, and then examining them with super-resolution structured illumination microscopy. The cross-sectional views showed that DBA (red stain) did not stain cytoplasm but stained only the area underneath the capsule (green stain) (Figure 2). There were tendencies for DBA to often not stain septal areas and for anti-capsule antibodies to not stain homogenously. While additional studies need to be performed to better define the non-homogenous staining of capsule, the microscopy data indicate that DBA does not bind capsule but does bind to the subcapsular area where pneumococcal WTA and LTA are present.
FIGURE 2.
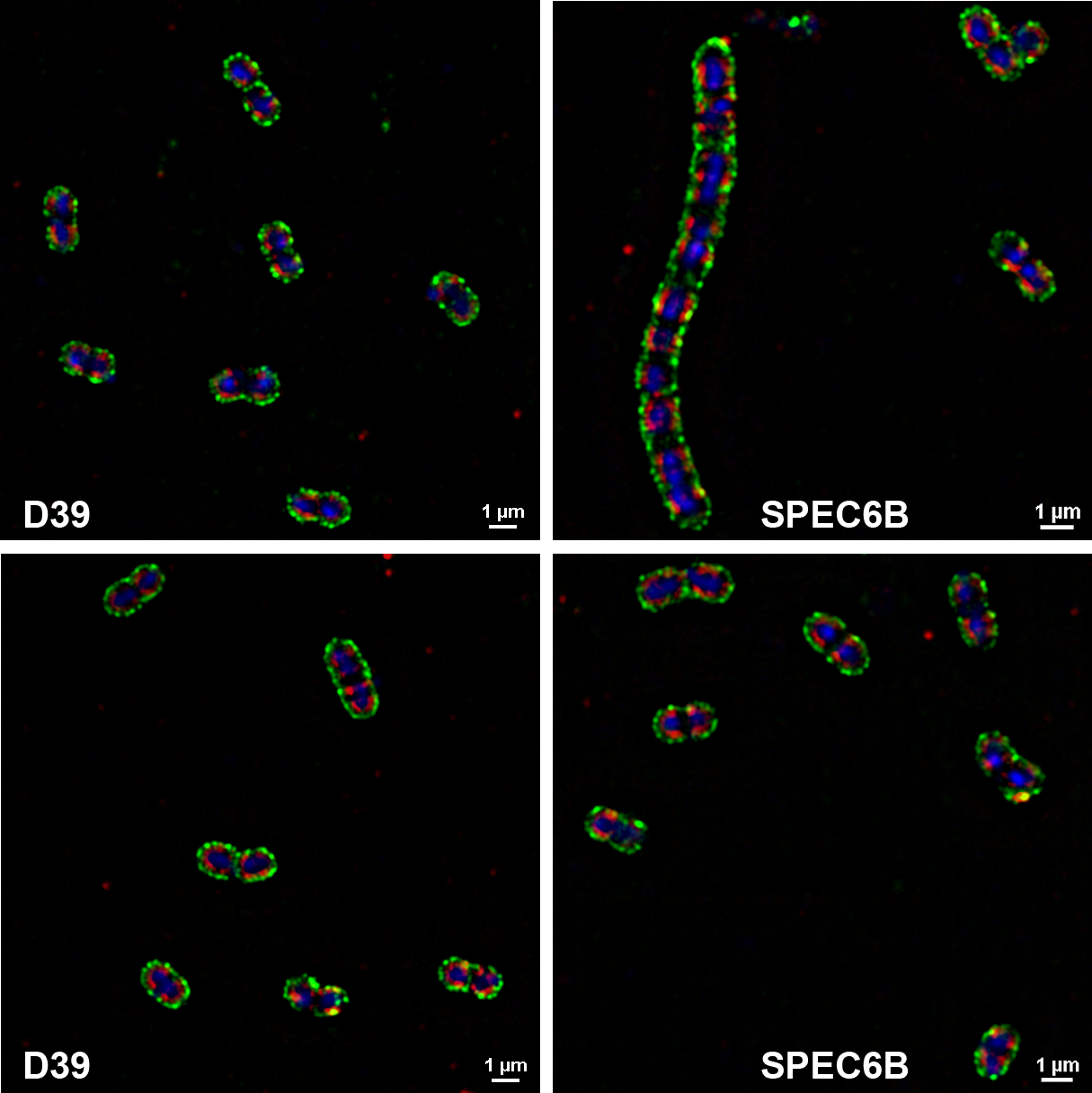
Visualization of capsule and DBA staining with super-resolution structured illumination microscopy. D39 and SPEC6B were stained with DBA (red) and anti-capsule antibodies (green; Hyp2M2 for D39 and Hyp6BM1 for SPEC6B). DNA was stained with DRAQ5 (blue)
3.3. DBA binding is associated with Pce activity
Since Pce removes the two PC groups from the terminal repeating unit and creates FA, we hypothesized that DBA non-binding would be associated with Pce activity. To test this hypothesis, we measured the Pce activity of the 15 bacterial strains with a colorimetric assay using p-nitrophenylphosphorylcholine (p-NPPC) as the artificial substrate. The 15 different strains could be naturally divided into two starkly different groups, five strains with no Pce activity and 10 strains with abundant activity (Figure 3); for the purpose of this study, strains were defined as having no Pce activity if the enzyme activity was less than 0.05 units. The three DBA non-binders (OREP3, TREP6A, and SPEC19F) did not have measurable Pce activity, whereas the 10 DBA binders were positive for Pce activity. Thus, except for the two DBA binders with no Pce activity (TREP11A and MNZ741), DBA binding correlated with Pce activity (Table 1).
FIGURE 3.
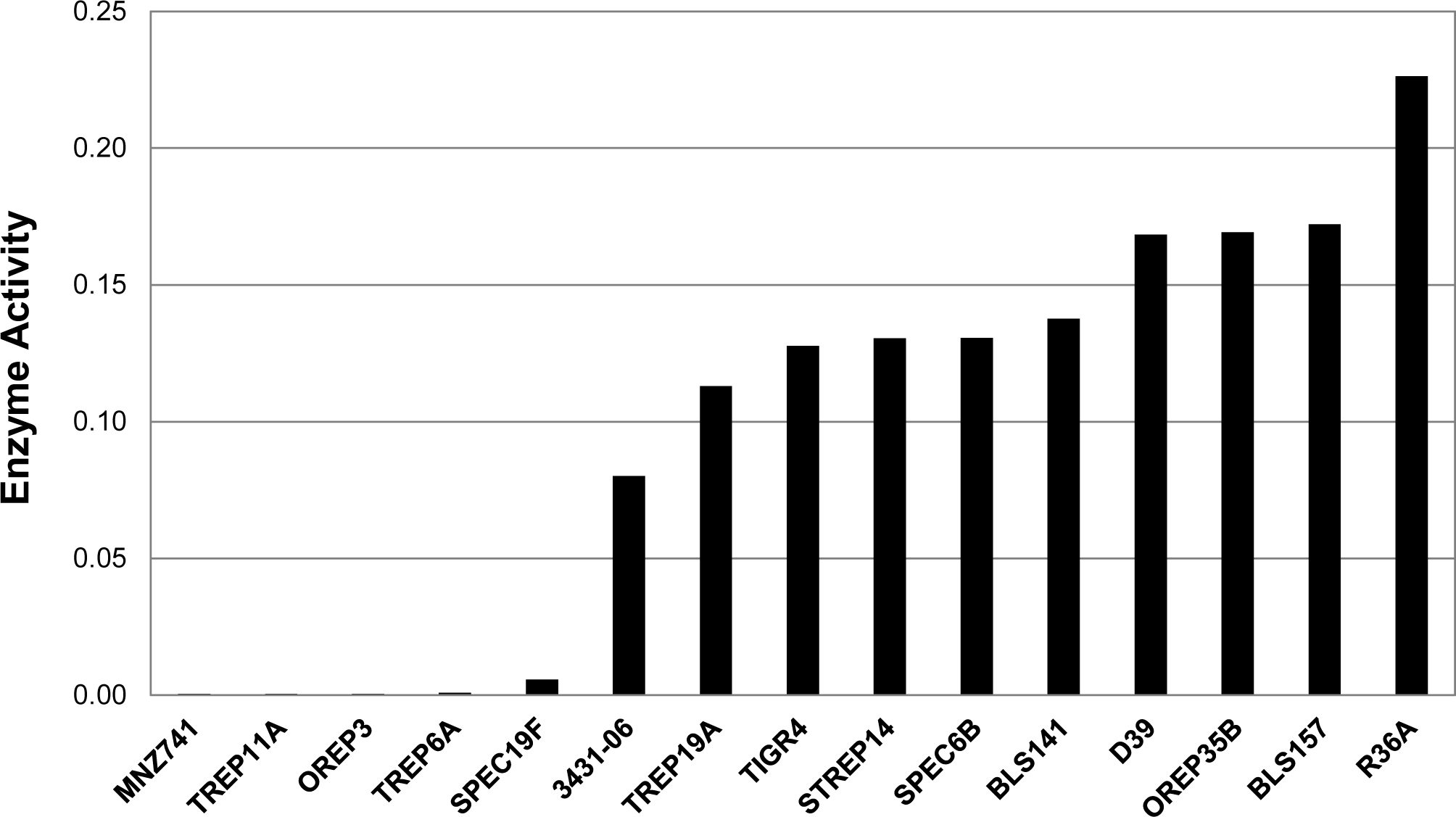
In vitro Pce enzyme activity of various pneumococcal strains using p-NPPC as the substrate. The in vitro Pce enzyme activity was defined as the average slope of OD405 over the first 8 h, normalized to the OD600 value of the strain’s working stock. Strains are arranged by increasing Pce activity. Strains with enzyme activity of less than 0.05 units were defined as having no Pce activity for the purpose of this study
TABLE 1.
Association of DBA binding* with Pce enzyme activity
| DBA (+) | DBA (−) | Total | |
|---|---|---|---|
| Pce (+) | 10 | 0 | 10 |
| Pce (−) | 2 | 3 | 5 |
| Total | 12 | 3 | 15 |
DBA binding was determined by flow cytometry and microscopy.
As the two discrepant strains coincidentally expressed the two related serotypes 11A and 11E, we first investigated the role of the serotype 11A/11E capsule by examining another serotype 11A strain (JC03) and its isogenic capsule variants, JC04 and AMB03. JC04 was created by deleting wcjE (an O-acetyl transferase gene) and phenotypically expresses serotype 11E [27]. AMB03 was derived from JC03 by deleting the cps locus and expresses no capsule (JC03△cps) [27]. All three strains (JC03, JC04, and AMB03) were discrepant (i.e., they bound DBA when there was no Pce activity) (Figure 4). Specifically, the absence of the capsule in AMB03 did not change Pce activity. Thus, the discrepancy is not associated with the serotype 11A/E capsule and capsule-producing genes.
FIGURE 4.
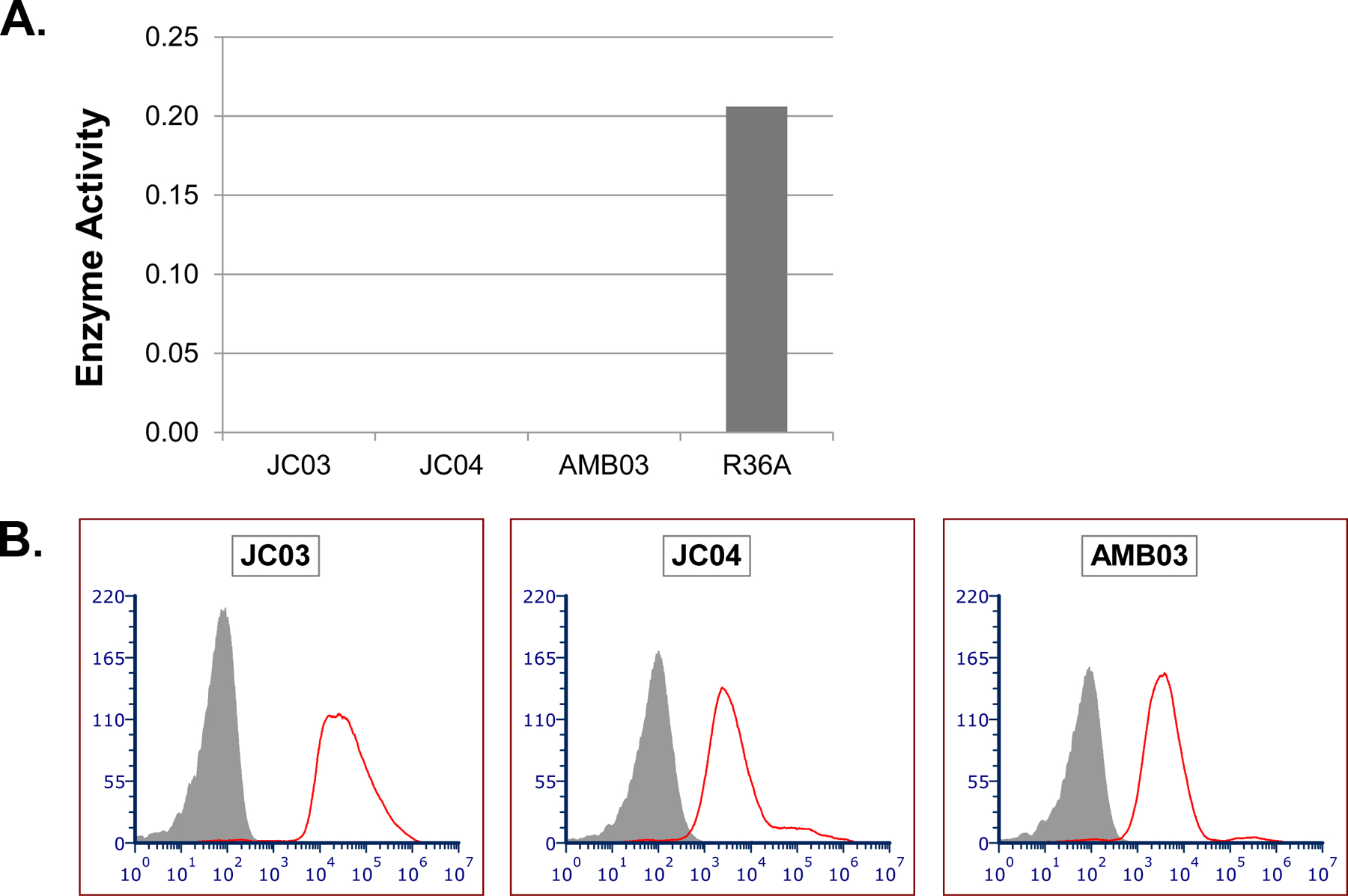
Pce activity and DBA binding results for JC03 and its isogenic capsule variants. (A) In vitro Pce enzyme activity for DBA-binders JC03 and its isogenic capsule variants, JC04 and AMB03, along with R36A for comparison. Enzyme activity was detected using p-NPPC as the substrate and defined as the average slope of OD405 over the first 8 hours. Strains with enzyme activity of less than 0.05 units were defined as having no Pce activity for the purpose of this study. (B) Flow cytometric histograms showing binding of DBA to JC03 and its isogenic capsule variants, JC04 and AMB03 (strain names are indicated at the top of each panel). The y-axis indicates the number of events per channel and the x-axis indicates fluorescence intensity. The grey-shaded areas were obtained with bacteria co-incubated with fluorescein-conjugated streptavidin (secondary antibody) only, and the red line represents DBA binding for each strain
3.4. Pce mutations and alleles explain Pce activity
To further investigate the relationship between Pce activity and DBA binding, we determined the DNA sequences of the pce gene from selected strains, five with normal Pce activity (D39, OREP3, R36A, SPEC19F, and TREP6A) and five with discrepant Pce activity (AMB03, JC03, JC04, MNZ741, and TREP11A). The pce nucleotide sequences for these 10 strains were deposited in GenBank with accession numbers MN380299–MN380308. The pce nucleotide sequences were translated into putative protein sequences, and both DNA and protein sequences were compared with each other, and with the prototypical sequence of R6 from the literature [28]. The amino acid sequences of three representative strains are summarized in Figure 5, following the amino acid numbering system used by Garau et al. [28].
FIGURE 5.
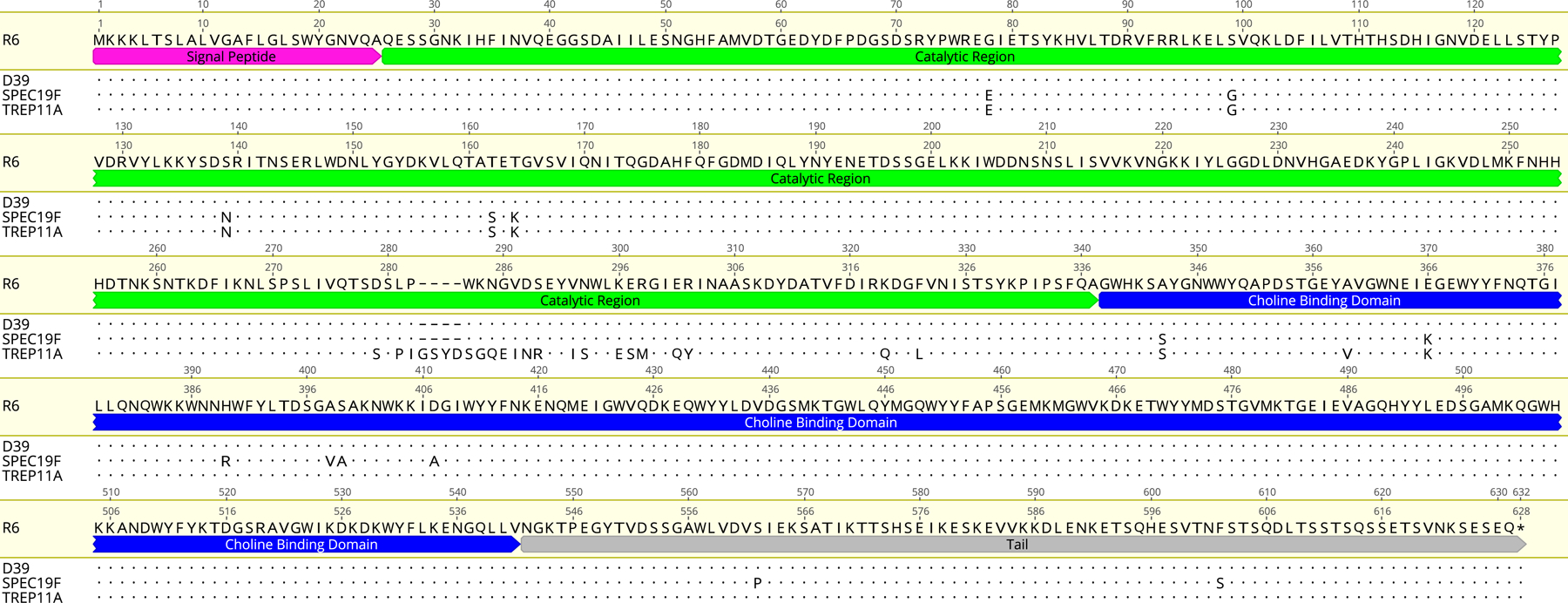
Amino acid sequences of Pce in various pneumococcal strains. The amino acid position numbering shown above the amino acid sequence is that used by Garau et al. [28]. The pce gene encodes a putative protein of 627 amino acids, including a 25-residue typical signal peptide (pink), followed by a 312-residue catalytic region (green), a 204-residue choline-binding domain (blue), and a carboxy-terminal tail of 85 amino acids (grey)
When DBA binders with Pce activity were studied (R36A and D39), their pce gene sequences were identical to the published R6 pce sequence [28]. We named this canonical pce gene sequence “allele A”. When the three DBA non-binders with no Pce activity were studied, two strains showed premature stop codons at amino acid positions 65 (for TREP6A) and 406 (for OREP3). As a result of the premature stop codons, most of the TREP6A Pce is lost and OREP3 Pce lost the C-terminal half of the choline-binding domain. The remaining non-binder, SPEC19F, had no premature stop codons but did have 34 nucleotide polymorphisms, resulting in 13 amino acid changes dispersed over the entire Pce sequence (Figure 5). These changes may have reduced the Pce activity of SPEC19F.
When the pce gene sequences of the five DBA binders without Pce activity (per the colorimetric assay) were studied, they were found to have a variant pce sequence with 12 nucleotide insertions, resulting in four amino acid insertions (GenBank accession numbers MN380304–MN380308). Because the variant pce sequence was found in multiple unrelated strains, we named the variant “allele B”; one of these variant sequences (TREP11A) is shown in Figure 5.
3.5. Pce of either allele is necessary and sufficient for binding DBA
To directly demonstrate that Pce is essential for DBA binding, we first removed pce genes from R36A and D39 (that express pce allele A), resulting in both strains becoming DBA non-binders. The non-binders became DBA binders again when the pce allele A was re-introduced (Figure 6). Thus, pce allele A is necessary, and sufficient, for DBA binding for these strains. We then deleted the pce allele B from TREP11A and MNZ741. Both knock-out strains (TREP11A△pce and MNZ741△pce) became DBA non-binders (Figure 6). When the pce allele B was introduced into D39△pce and MNZ741△pce, both strains became DBA binders (Figure 6). Thus, pce allele B is functional in vivo, even though it is not functional in an in vitro assay using p-NPPC as the substrate. Lastly, we investigated whether SPEC19F has a non-functional Pce due to its numerous amino acid changes. When a functional pce allele A was inserted into SPEC19F it became a DBA binder (Figure 6). Thus, SPEC19F has a non-functional Pce. Taken together, we conclude that the presence of a functional Pce of either allele is necessary, and sufficient, for DBA binding.
FIGURE 6.
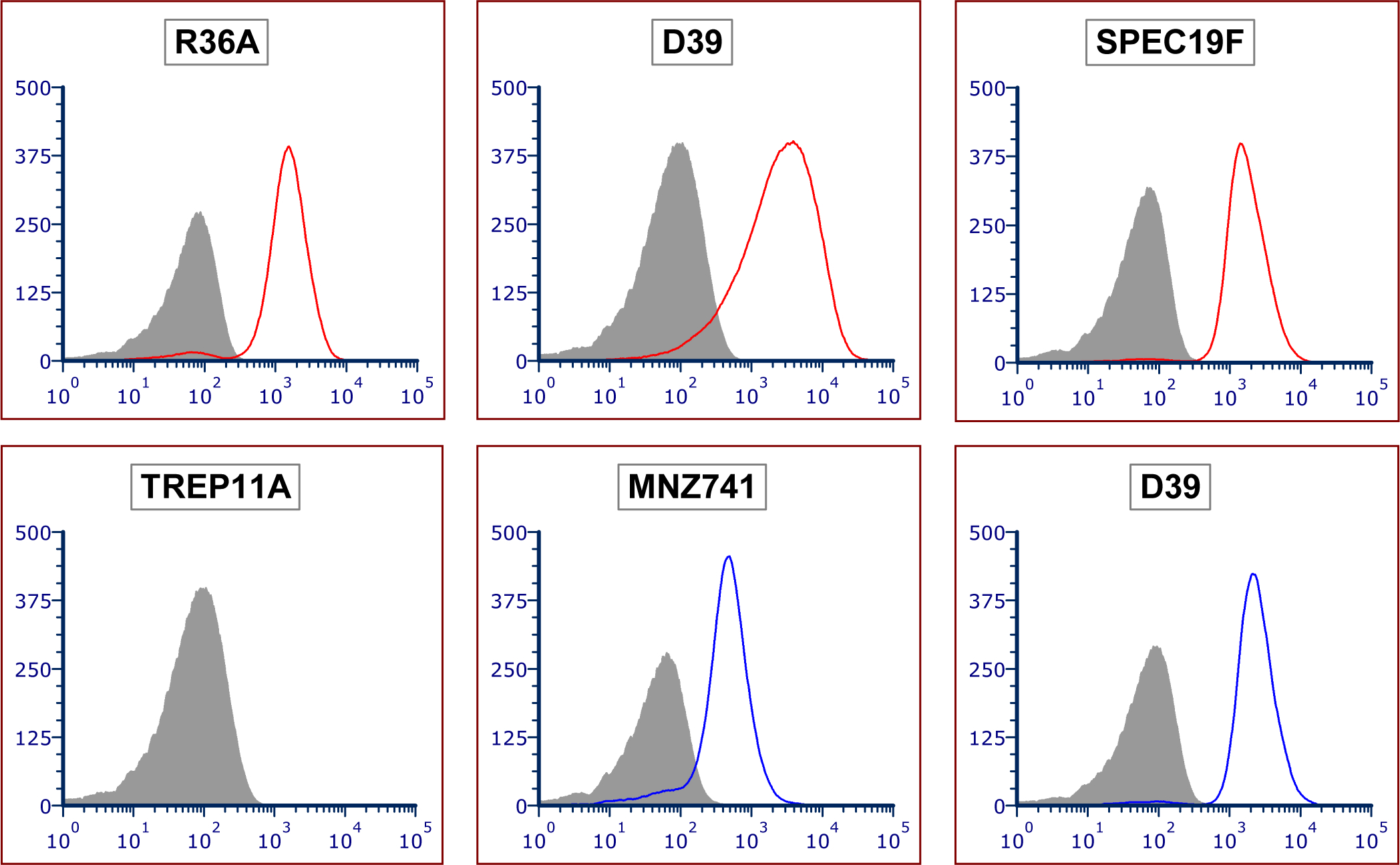
DBA binding to various genetic mutant pneumococcal strains. The names of the background strains are indicated at the top of each panel. The grey-filled areas indicate DBA binding to mutant strains with the pce gene deleted (or the background strain in the case of SPEC19F). Red lines indicate △pce mutants with pce allele A knocked-in. Blue lines indicate △pce mutants with pce allele B knocked-in. TREP11A has no pce allele knock-in
3.6. Binding of HPA to pneumococcus
HPA is another lectin known to target the FA. To study its target at the molecular level, we investigated HPA binding to various pneumococcal strains that were used for the DBA binding studies. Similar to what was found with DBA, HPA bound to the two DBA binding-positive strains (D39 and TREP11A) but not the two DBA non-binders (OREP3 and SPEC19F) (Figure 7). The two HPA binders became HPA non-binders after pce allele A or allele B was removed from strains D39 or TREP11A, respectively (Figure 7). The confocal fluorescence microscopy observations were confirmed with flow cytometric studies (data not shown). Thus, the HPA binding pattern is identical to the DBA binding pattern, and HPA binding requires Pce, which removes PC from the terminal RU of WTA/LTA.
FIGURE 7.
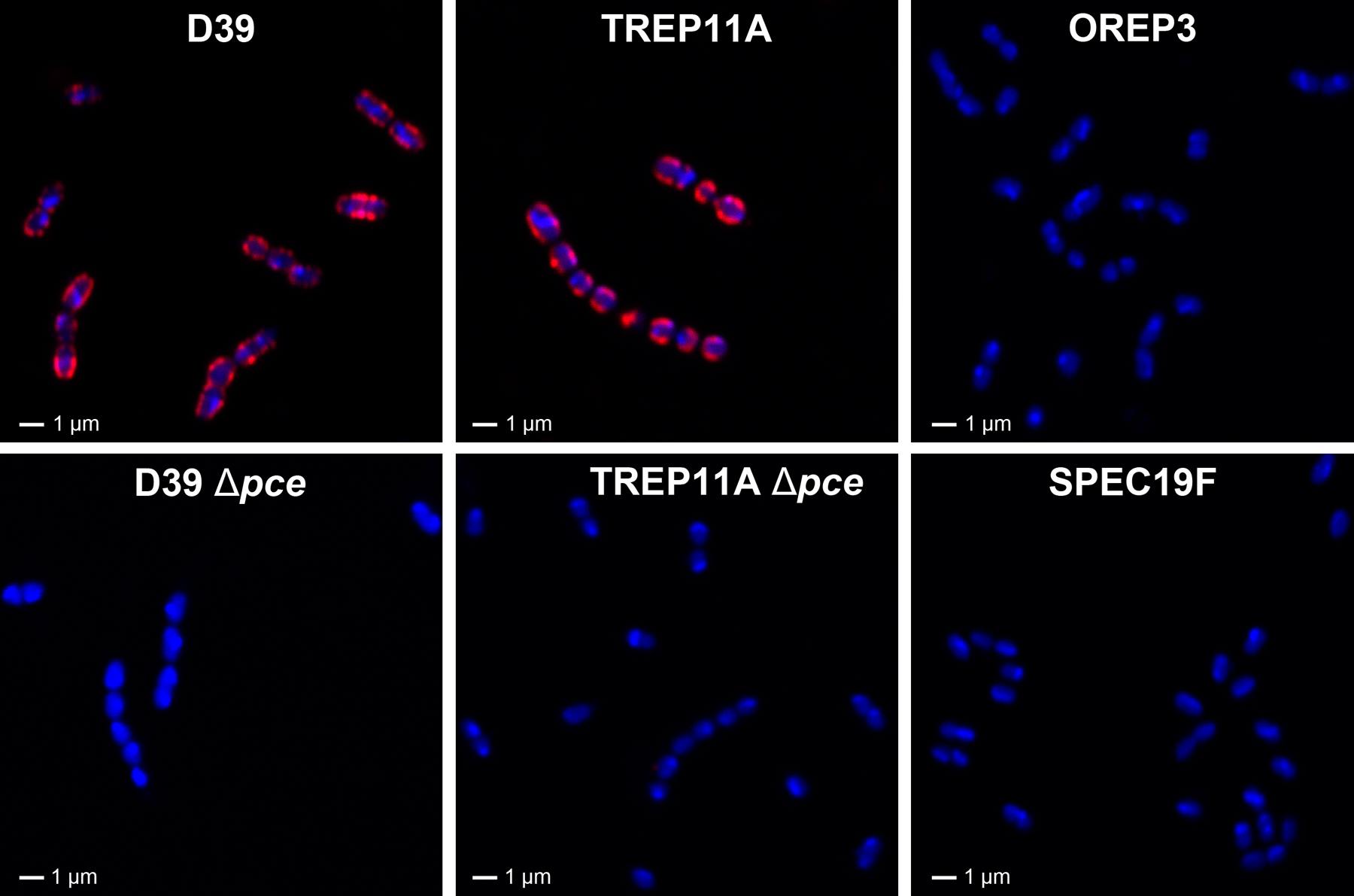
Visualization of HPA binding to various pneumococcus strains using confocal fluorescence microscopy. Six representative strains, including D39 (pce allele A), TREP11A (pce allele B), their corresponding pce knock-out mutants, and two strains with no Pce activity (OREP3 and SPEC19F) are shown for HPA staining. Red represents HPA staining and blue represents DNA counter-staining using DRAQ5
4. DISCUSSION
Although classical lectins DBA and HPA have been used as markers in many cell differentiation studies [29–32], their use in microbiology has been somewhat limited. While there have been a few previous attempts to use them to identify various streptococcal species [17,18,20,21], the molecular targets of the two lectins have remained unclear.
Herein we clearly show, by multiple approaches, that DBA and HPA bind to the non-reducing termini of WTA/LTA. First, we used super-resolution microscopy to show that the lectins do not bind capsule or the cytoplasmic area, but bind only to the subcapsular layer where WTA/LTA are located. Second, we showed that the lectin binding is critically dependent on the Pce enzyme, which removes the two PC groups from the terminal repeating unit of WTA/LTA [7,33]. Taken together, we conclude that DBA binds only to the PC-free terminal GalNAcα1→3GalNAcβ1 of pneumococcal WTA or LTA that is created by Pce.
Recent development of various forms of high-resolution microscopy has significantly improved our ability to identify subcellular sites of synthesis and organization of bacterial surface molecules [34,35]. Our clarification of the target for these two lectins will greatly facilitate studies of WTA/LTA synthesis in pneumococci. Pneumococci are emerging as a preferred model organism for these surface structure studies because of its acknowledged clinical importance as a human pathogen and because of its inherent genetic malleability. For instance, the expression of pce can be regulated by inducible promoters [24,36] and DBA can be used to identify the subcellular location where Pce functions. Thus, these lectins will help us study the synthesis and attachment of WTA/LTA to PG.
The lectins should be also useful in studying Pce structure and function because the lectin binding is so critically dependent on Pce activity in vivo. For example, the SPEC19F Pce was found to have 13 individual mutations and was essentially non-functional (it did show some slight activity with the p-NPPC assay but no DBA binding). When the 13 mutations were analyzed for their effect on enzyme function using the Protein Variation Effect Analyzer (PROVEAN) website (http://provean.jcvi.org), one mutation (G78E) was considered significant, with a neutral-to-negative charge change. Moreover, a crystallography study of the Pce enzyme found that positions G78–T81 in the Pce structure delimit the substrate binding pocket at the end of the elongated loop, which might contribute to modulating the shape of the PC binding site and/or accessibility to the teichoic acid substrate [28]. Thus, the G78E mutation may underlie the significantly reduced activity of SPEC19F Pce with regard to its ability to hydrolyze p-NPPC in vitro but completely eliminated its ability to remove PC residues from the terminal repeating unit of WTA/LTA in vivo, and thereby may possibly explain the inactive form of Pce in SPEC19F.
An unexpected finding from this study is our discovery of a Pce “allele B”. Relative to the canonical “allele A”, the major difference found in allele B is a 12-nucleotide insertion, contributing to four amino acids inserted between residues 282 and 283, resulting in a very distinct 23 amino acid stretch covering the 6th alpha helix region (residues 279–326), located at the C-terminal end of the catalytic domain [28] (Figure 5). This change in the catalytic domain may compensate for the predicted negative effect of the G78E mutation because allele B has the G78E mutation and is functional in vivo. The change in the catalytic domain may also explain why Pce allele B is inactive when assayed in vitro with the artificial substrate p-NPPC but is actually functional in vivo.
Pce is an important virulence factor and its mechanism of action has been extensively studied at the molecular level using crystallography and the p-NPPC in vitro assay [11,12,22]. We show that the in vitro assay does not accurately reflect the actual in vivo situation. Thus, previous studies of Pce structure and function are limited by this assay. Now, DBA binding to pneumococci can serve as a more representative assay for Pce function in vivo and one can now critically examine the molecular requirements for Pce function.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by grants from the China Scholarship Council Fund (No. 201806210441, MLZ) and the NIH (AG050607, MHN). We would like to acknowledge assistance from Jigui Yu, Patsy Oliver, Feroze Ganaie, Aubree Perkins, and Lei Zhang.
((Funded by
China Scholarship Council Fund; grant number: 201806210441
U.S. Department of Health and Human Services, National Institutes of Health, National Institute on Aging; grant number: AG050607
Footnotes
Additional Supporting Information may be found online in the supporting information tab for this article.
CONFLICT OF INTERESTS
All authors declare no financial/commercial conflict of interest.
REFERENCES
- [1].Weidenmaier C, Peschel A. Teichoic acids and related cell-wall glycopolymers in Gram-positive physiology and host interactions. Nat Rev Microbiol. 2008;6:276–87. [DOI] [PubMed] [Google Scholar]
- [2].Vollmer W, Blanot D, de Pedro MA. Peptidoglycan structure and architecture. FEMS Microbiol Rev. 2008;32:149–67. [DOI] [PubMed] [Google Scholar]
- [3].Barreteau H, Kovač A, Boniface A, Sova M, Gobec S, Blanot D. Cytoplasmic steps of peptidoglycan biosynthesis. FEMS Microbiol Rev. 2008;32:168–207. [DOI] [PubMed] [Google Scholar]
- [4].Wheeler R, Mesnage S, Boneca IG, Hobbs JK, Foster SJ. Super-resolution microscopy reveals cell wall dynamics and peptidoglycan architecture in ovococcal bacteria. Mol Microbiol. 2011;82:1096–109. [DOI] [PubMed] [Google Scholar]
- [5].Neuhaus FC, Baddiley J. A continuum of anionic charge: structures and functions of d-alanyl-teichoic acids in gram-positive bacteria. Microbiol Mol Biol Rev. 2003;67:686–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Fischer W Pneumococcal lipoteichoic and teichoic acid. Microb Drug Resist. 1997;3:309–25. [DOI] [PubMed] [Google Scholar]
- [7].Waldow F, Kohler TP, Hess N, Schwudke D, Hammerschmidt S, Gisch N. Attachment of phosphorylcholine residues to pneumococcal teichoic acids and modification of substitution patterns by the phosphorylcholine esterase. J Biol Chem. 2018;293:10620–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Zhang JR, Idanpaan-Heikkila I, Fischer W, Tuomanen EI. Pneumococcal licD2 gene is involved in phosphorylcholine metabolism. Mol Microbiol. 1999;31:1477–88. [DOI] [PubMed] [Google Scholar]
- [9].Fischer W Physiology of lipoteichoic acids in bacteria. Adv Microb Physiol. 1988;29:233–302. [DOI] [PubMed] [Google Scholar]
- [10].Denapaite D, Brückner R, Hakenbeck R, Vollmer W. Biosynthesis of teichoic acids in Streptococcus pneumoniae and closely related species: Lessons from genomes. Microb Drug Resist. 2012;18:344–58. [DOI] [PubMed] [Google Scholar]
- [11].de las Rivas B, Garcia JL, Lopez R, Garcia P. Molecular characterization of the pneumococcal teichoic acid phosphorylcholine esterase. Microb Drug Resist. 2001;7:213–22. [DOI] [PubMed] [Google Scholar]
- [12].Vollmer W, Tomasz A. Identification of the teichoic acid phosphorylcholine esterase in Streptococcus pneumoniae. Mol Microbiol. 2001;39:1610–22. [DOI] [PubMed] [Google Scholar]
- [13].Seo HS, Cartee RT, Pritchard DG, Nahm MH. A new model of pneumococcal lipoteichoic acid structure resolves biochemical, biosynthetic, and serologic inconsistencies of the current model. J Bacteriol. 2008;190:2379–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Gisch N, Kohler T, Ulmer AJ, Muthing J, Pribyl T, Fischer K, et al. Structural reevaluation of Streptococcus pneumoniae lipoteichoic acid and new insights into its immunostimulatory potency. J Biol Chem. 2013;288:15654–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Siddiqui B, Hakomori S. A revised structure for the Forssman glycolipid hapten. J Biol Chem. 1971;246:5766–9. [PubMed] [Google Scholar]
- [16].Wu AM, Song SC, Sugii S, Herp A. Differential binding properties of Gal/GalNAc specific lectins available for characterization of glycoreceptors. Indian J Biochem Biophys. 1997;34:61–71. [PubMed] [Google Scholar]
- [17].Kellens JT, Jacobs JA, Peumans WJ, Stobberingh EE. The agglutination of β-haemolytic streptococci by lectins. J Med Microbiol. 1993;39:440–5. [DOI] [PubMed] [Google Scholar]
- [18].Slifkin M, Gil GM. Rapid biochemical tests for the identification of groups A, B, C, F, and G streptococci from throat cultures. J Clin Microbiol. 1983;18:29–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Slifkin M, Gil GM. Identification of group C streptococcal antigen extracts with lectin-bound polystyrene particles. J Clin Microbiol. 1984;19:83–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Avni I, Arffa RC, Robin JB, Rao NA. Lectins for the identification of ocular bacterial pathogens. Metab Pediatr Syst Ophthalmol. 1987;10:45–7. [PubMed] [Google Scholar]
- [21].Domenech M, Garcia E. Fluorescence imaging of Streptococcus pneumoniae with the helix pomatia agglutinin (HPA) as a potential, rapid diagnostic tool. Front Microbiol. 2017;8:1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Fliegera A, Gong S, Faigle M, Neumeister B. Critical evaluation of p-nitrophenylphosphorylcholine (p-NPPC) as artificial substrate for the detection of phospholipase C. Enzyme Microb Technol. 2000;26:451–8. [DOI] [PubMed] [Google Scholar]
- [23].Yu J, Lin J, Kim KH, Benjamin WH Jr., Nahm MH. Development of an automated and multiplexed serotyping assay for Streptococcus pneumoniae. Clin Vaccine Immunol. 2011;18:1900–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Sorg RA, Kuipers OP, Veening JW. Gene expression platform for synthetic biology in the human pathogen Streptococcus pneumoniae. ACS Synth Biol. 2015;4:228–39. [DOI] [PubMed] [Google Scholar]
- [25].Sung CK, Li H, Claverys JP, Morrison DA. An rpsL cassette, Janus, for gene replacement through negative selection in Streptococcus pneumoniae. Appl Environ Microbiol. 2001;67:5190–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Spencer BL, Saad JS, Shenoy AT, Orihuela CJ, Nahm MH. Position of O-acetylation within the capsular repeat unit impacts the biological properties of pneumococcal serotypes 33A and 33F. Infect Immun. 2017;85:e00132–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Calix JJ, Brady AM, Du VY, Saad JS, Nahm MH. Spectrum of pneumococcal serotype 11A variants results from incomplete loss of capsule O-acetylation. J Clin Microbiol. 2014;52:758–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Garau G, Lemaire D, Vernet T, Dideberg O, Di Guilmi AM. Crystal structure of phosphorylcholine esterase domain of the virulence factor choline-binding protein e from Streptococcus pneumoniae: new structural features among the metallo-beta-lactamase superfamily. J Biol Chem. 2005;280:28591–600. [DOI] [PubMed] [Google Scholar]
- [29].Etzler ME, Kabat EA. Purification and characterization of a lectin (plant hemagglutinin) with blood group A specificity from Dolichos biflorus. Biochemistry. 1970;9:869–77. [DOI] [PubMed] [Google Scholar]
- [30].Muramatsu T, Muramatsu H, Kasai M, Habu S, Okumura K. Receptors for Dolichos biflorus agglutinin: new cell surface markers on a spontaneous leukemia. Biochem Biophys Res Commun. 1980;96:1547–53. [DOI] [PubMed] [Google Scholar]
- [31].Nash R, Neves L, Faast R, Pierce M, Dalton S. The lectin Dolichos biflorus agglutinin recognizes glycan epitopes on the surface of murine embryonic stem cells: a new tool for characterizing pluripotent cells and early differentiation. Stem Cells. 2007;25:974–82. [DOI] [PubMed] [Google Scholar]
- [32].Sourial S, Searchfield L, Schuppe-Koistinen I, Betton GR, Riccardi D, Price SA. Application of Dolichos biflorus in immunoassay detection of kidney collecting duct biomarkers. Biomarkers. 2010;15:424–35. [DOI] [PubMed] [Google Scholar]
- [33].Hermoso JA, Lagartera L, Gonzalez A, Stelter M, Garcia P, Martinez-Ripoll M, et al. Insights into pneumococcal pathogenesis from the crystal structure of the modular teichoic acid phosphorylcholine esterase Pce. Nat Struct Mol Biol. 2005;12:533–8. [DOI] [PubMed] [Google Scholar]
- [34].Schlafer S, Meyer RL. Confocal microscopy imaging of the biofilm matrix. J Microbiol Methods. 2017;138:50–9. [DOI] [PubMed] [Google Scholar]
- [35].Bottomley AL, Turnbull L, Whitchurch CB, Harry EJ. Immobilization techniques of bacteria for live super-resolution imaging using structured illumination microscopy. Methods Mol Biol. 2017;1535:197–209. [DOI] [PubMed] [Google Scholar]
- [36].Keller LE, Rueff AS, Kurushima J, Veening JW. Three new integration vectors and fluorescent proteins for use in the opportunistic human pathogen Streptococcus pneumoniae. Genes. 2019;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


