Abstract
Coronavirus disease 2019 (COVID-19) pandemic has spread rapidly across the world. The vast majority of patients with COVID-19 manifest mild to moderate symptoms but may progress to severe cases or even mortalities. Young adults of reproductive age are the most affected population by SARS-CoV-2 infection. However, there is no consensus yet if pregnancy contributes to the severity of COVID-19. Initial studies of pregnant women have found that COVID-19 significantly increases the risk of preterm birth, intrauterine growth restriction, and low birth weight, which have been associated with non-communicable diseases in offspring. Besides, maternal viral infections with or without vertical transmission have been allied with neurological and behavioral disorders of the offspring. In this review, obstetrical outcomes of women with COVID-19 and possible risks for their offspring are discussed by reviewing maternal immune responses to COVID-19 based on the current evidence. Structural and systemic follow-up of offspring who are exposed to SARS-CoV-2 in-utero is suggested.
Keywords: COVID-19, SARS-CoV-2, Obstetrical outcomes, Offspring, Behavioral disorders
1. Introduction
Coronavirus disease 2019 (COVID-19) by SARS-CoV-2 has been swiftly spreading around the world, prompting World Health Organization (WHO) to declare a pandemic status on March 11, 2020 (Gheblawi et al., 2020; Wu et al., 2020b). COVID-19 has a wide range of disease manifestations, which have been described as five different patterns: asymptomatic (1.2 %), mild to moderate (80.9 %), severe (13.8 %), critical (4.7 %), and mortality cases (2.3 %). In early September 2020, the total number of COVID-19 cases reached more than 28 million, with more than 911,000 mortality cases worldwide. However, the actual count of reported cases may not reflect the real number of patients since the real COVID-19 screening tests performed in each country are different (Jin et al., 2020, World Health Organization (WHO, 2020).
The clinical manifestations of COVID-19 can be divided into three stages. Stage-I is characterized by the symptoms of mild flu-like syndrome. The majority of patients remain in Stage-I, and a small proportion of cases may progress further. Pulmonary impairment becomes more evident in Stage-II than Stage-I. Patients in Stage-II develop viral pneumonia and present tachypnea, cough, and fever with the absence (Stage-IIA) or presence of hypoxia (Stage-IIB). Stage-III is the most severe phase, which is characterized by systemic manifestations of hyper-inflammation with a cytokine release syndrome (CRS), or also known as cytokine storm syndrome (CSS), leading to lung injury and multi-organ failures (Feng et al., 2020; Siddiqi and Mehra, 2020; Wu et al., 2020a).
COVID-19 is more prevalent among young adults of reproductive age than the older age group, with slightly more cases in men than women. The mortality is higher with advanced age and the presence of comorbidities, such as obesity, diabetes, and hypertension. Interestingly, pregnant women may develop the same comorbidities during pregnancy, including excessive weight gain, gestational diabetes, and hypertensive disorder during pregnancy, namely gestational hypertension, preeclampsia (PE), eclampsia, and hemolysis, elevated liver enzyme, low platelet (HELLP) syndrome (Wu et al., 2020b). The impact of COVID-19 on the health of pregnant women is still poorly understood. Initial studies failed to deliver a clear picture of defining pregnancy as a risk factor for the severity of COVID-19, unlike other pulmonary viral infections, such as H1N1 infection (Malinowski et al., 2011; Knight et al., 2020). Few studies have evaluated the prevalence of COVID-19 in pregnant women. The study from a maternity hospital in New York, NY, US, reported a 15.4 % prevalence of SARS-CoV-2 infection in pregnant women admitted for delivery, including 1.9 % symptomatic and 13.5 % asymptomatic cases (Sutton et al., 2020). Another study reported a 12.2 % prevalence of SARS-CoV-2 infection among pregnant women without specifying each trimester (Fox and Melka, 2020). With the emerging SARS-CoV-2 antibody testing, 6.2 % (80/1,293) of parturient women in Philadelphia, PA, US (from April 4 to June 3, 2020) was reported to have IgG and/or IgM SARS-CoV-2-specific antibodies (Flannery et al., 2020).
Initial studies have shown that COVID-19 increases the risk of preterm birth (PTB), low birth weight (LBW), and the need for neonatal hospitalization in a neonatal intensive care unit (NICU) (Juan et al., 2020; Smith et al., 2020). However, the literature has not yet clearly defined the risks for pregnancy loss (miscarriage and stillbirth), PE, and the vertical transmission of the disease in pregnant women with COVID-19 (Dashraath et al., 2020; Karimi-Zarchi et al., 2020; Mendoza et al., 2020; Smith et al., 2020). Although, in a small series of pregnant women with severe COVID-19, a PE-like syndrome, a variant clinical manifestation of PE, has been reported (Mendoza et al., 2020). During pregnancy, several viral infections have been known to increase the risk of fetal malformations, PTB, and intrauterine growth restriction (IUGR) without a significant long-term impact on the offspring's health (Racicot and Mor, 2017). Recent experimental models in animals and epidemiological studies in humans, however, have shown that offspring exposed to maternal viral infection in-utero or born with LBW are at high risk for chronic non-communicable diseases (NCDs) and psychiatric disorders (Smith et al., 2007; Choi et al., 2016; He et al., 2017; Al Salmi and Hannawi, 2020). Therefore, we aim to review the maternal immune responses and obstetrical outcomes in pregnant women with COVID-19 and discuss the potential impact on the offspring's health, particularly the long-term health concerns, based on the current data in the literature.
2. SARS-CoV-2 infection and systemic immune responses
SARS-CoV-2 infects human beings through angiotensin-converting enzyme 2 (ACE2) receptor, which is highly expressed in the alveolar epithelium and also present in various cells at the maternal-fetal junction and fetal tissues (Eguchi et al., 2018). Briefly, the SARS-CoV-2 spike protein binds to the ACE2 receptor, leading to the down-regulation of these receptors with excessive production of the vasoconstrictor angiotensin II (AngII) and reduction of the vasodilator angiotensin-(1–7) [Ang-(1–7)]. Increased AngII interacts with the angiotensin-1 receptor (AT1R) and activates the downstream nuclear factor kappa B pathway. Consequently, it increases the production of IL-6, TNF-α, IL-1ß and IL-10, and pulmonary vascular permeability. At the same time, the low concentration of Ang-(1–7) contributes to the loss of its modulatory effect via Mas receptor (MasR) that attenuates inflammatory response (Eguchi et al., 2018; Murakami et al., 2019; Jing et al., 2020). Studies have shown that inflammatory cytokines, chemokines, and factors in the peripheral blood, such as interleukin (IL)-2, IL-6, IL-7, granulocyte colony-stimulating factor (G-CSF), macrophage inflammatory protein (MIP) 1-α, tumor necrosis factor-α (TNF-α), C-reactive protein (CRP), ferritin, and D-dimer are significantly elevated in the patients with the severe form of COVID-19 (Feng et al., 2020; Siddiqi and Mehra, 2020; Wu et al., 2020a).
SARS-CoV-2 infection is known to induce a decrease in CD4+ and CD8+ T and natural killer (NK) cell levels, more evidently in critically ill patients. Despite decreased T cells, the immune response of COVID-19 is characterized by the increased Th17 response, with a concurrent reduction of regulatory T (Treg)/Th17 cell ratios. Studies have suggested that the uncontrolled release of pro-inflammatory cytokines in COVID-19 cases is due to the exaggerated Th17 response (Muyayalo et al., 2020; Xu et al., 2020). Yet, the immune mechanisms of cytokine storm syndrome (CSS) in COVID-19 are not fully understood. Interestingly, it has also been described in several severe acute respiratory syndromes, such as middle east respiratory syndrome (MERS), severe acute respiratory syndrome (SARS), and seasonal influenza (H5N1 and H1N1). The cytokine profiles described in the CSS of each respiratory syndromes were similar, suggesting common pathophysiology; SARS [IFN-γ, IL-1, IL-6, IL-12, TGF-β, monocyte chemoattractant protein (MCP)-1, monokine induced by gamma (MIG), IFN-γ inducible protein (IP)-10, IL-8], MERS [IFN-α, IL-1β, IL-2, IL-6, IL-8, MCP-1, MIP-1a, CCL-5], H1N1 Influenza (IFN-γ, TNF-α, IL- 6, IL-8, IL-9, IL-17, IL-15, IL-12p70), and H1N5 influenza (IFN-γ, IL-6, IL-8, IL-10, MCP-1, MIG, IP-10) (Gao et al., 2020). A recent study of the inflammatory cytokine profiles in three different groups of patients with respiratory infections, including hospitalized-but-stable COVID-19 patients, COVID-19 patients requiring intensive care unit (ICU) admission, and patients with severe community-acquired pneumonia requiring ICU support, revealed that the inflammatory cytokines, including IL-1β, IL-6, IL-8, and soluble TNF receptor 1 (sTNFR1) were significantly elevated in COVID-19 cases when compared to patients who required ICU support without COVID-19 (McElvaney et al., 2020). This finding was consistent with a recent meta-analysis, demonstrating the elevation of several inflammatory markers, such as procalcitonin, CRP, and IL-6, in patients with COVID-19 (Feng et al., 2020), and another study which described changes in inflammatory markers, such as IL-6, IL-1β, IL-10, TNF, GM-CSF, IP-10, IL-17, MCP-3, and IL-1ra in COVID-19 patients (Wang et al., 2020; Wu et al., 2020a).
3. Immune inflammatory responses in pregnant women with COVID-19
The expression of ACE2 in the female reproductive tract and placental tissue suggests that SARS-CoV-2 infection may compromise pregnancy outcomes and vertically transmit to a fetus (Jing et al., 2020). Indeed, electron microscope and molecular biology studies have identified the presence of SARS-CoV-2 in the placental villous stroma and membranes of infected mothers (Algarroba et al., 2020; Penfield et al., 2020). Hence, intrauterine fetuses in mothers with SARS-Cov-2 infection can be exposed to pro-inflammatory milieu either directly induced by fetal or placental tissue or indirectly by maternal immune responses. In COVID-19 cases, shifted Th17 immunity has been reported to induce pro-inflammatory cytokine excess (Muyayalo et al., 2020; Xu et al., 2020). Notably, the balanced Treg/Th17 immune responses are critical for embryonic implantation and healthy pregnancy (Figueiredo and Schumacher, 2016; Jørgensen et al., 2019), while reduced levels of Treg cells and increased levels of Th17 cells are associated with obstetric complications, such as miscarriage, preeclampsia, and PTB (Zenclussen et al., 2005; Sasaki et al., 2007; Lee et al., 2012; Jabalie et al., 2019; Krechetova et al., 2020). Therefore, it is speculated that pro-inflammatory immune responses to SARS-CoV-2 by maternal and fetal immune effectors and trophoblasts may increase maternal risks for obstetrical complications and possibly offspring risk for short- and long-term NCD.
Pro-inflammatory immune responses are dominant during implantation and parturition in normal pregnancy, systemically and locally (Mor et al., 2017; Liu et al., 2020). The pro-inflammatory immune response of pregnant women with COVID-19 appears to be similar to that of non-pregnant women. However, the risk of adverse clinical outcomes in pregnant women with COVID-19 is still unclear. Some authors have observed that pregnant women with COVID-19 had a clinical course of the disease similar to that of non-pregnant women. In contrast, others suggest that the pregnant status raises the morbidity of COVID-19, especially in the presence of risk factors such as black and Hispanic race, obesity, advanced maternal, age, and medical comorbidities (Brandt et al., 2020).
Mortality in pregnant women with COVID-19 appears to be similar to that observed among pregnant women without COVID-19 and the general population. The COVID-19 related maternal mortality ratio (MMR) in 194 obstetric units in the UK was 1% (5/427 pregnant women) and in 33 French maternity units was 0.2 % (1/617 pregnant women) (Kayem et al., 2020; Knight et al., 2020). An epidemiological study of reproductive age women with COVID-19, performed in the period January 22– June 7, 2020, in the US, revealed that pregnant women have the same MR as non-pregnant women [16/8,207 (0.2 %) versus 208/83,205 (0.2 %), adjusted Relative Risk (aRR) 0.9, 95 % confidence interval (CI) 0.5–1.5]. However, pregnant women with COVID-19 were more likely to be admitted to the intensive care unit (ICU) (aRR 1.5, 95 % CI 1.2–1.8) and receive mechanical ventilation (aRR 1.7, 95 % CI 1.2–2.4) (Ellington et al., 2020). Contrarily, Takemoto et al. reported a significantly higher MMR among Brazilian pregnant women with COVID-19 (12.7 %) than that of the general population (overall 5%) (Takemoto et al., 2020).
3.1. Placental pathology induced by SARS-CoV-2
Structurally, placental tissue appears to be a potential site for SARS-CoV-2 infection since the presence of Ang-(1–7) and the ACE2 enzyme was demonstrated in various cells in the placenta, including syncytiotrophoblast, villous cytotrophoblasts, invasive and intravascular trophoblast, decidual cells, vascular smooth muscle cells in primary villi, and vascular endothelial cells in umbilical vessels (Jing et al., 2020). Only a few cells co-express ACE2 and transmembrane protease, serine 2 (TMPRSS2) in any trimester of pregnancy, and approximately 1/10,000 trophoblast cells express the canonical cell-entry-mediators for SARS-CoV-2 (ACE2/TMPRSS2) (Pique-Regi et al., 2020). The presence of alternative entryways for SARS-CoV-2 when ACE2 and TMPRSS2 are not expressed on the cells has been suggested (Gordon et al., 2020). Indeed, the electronic microscopic image of coronavirus virions invading into the syncytiotrophoblasts in the placental villi was reported in a COVID-19 positive pregnant woman over 28 weeks of gestation (Algarroba et al., 2020). Subsequently, other investigators challenged the image as clathrin-coated vesicles, not SARS-CoV-2 particles (Kniss, 2020). However, the presence of SARS-CoV-2 was confirmed by PCR of placental tissue/amniotic membrane, and immunohistochemistry and in-situ hybridization assays of formalin-fixed paraffin-embedded tissue sections later (Best Rocha et al., 2020; Penfield et al., 2020).
Histopathological studies of the placenta in pregnant women with COVID-19 revealed the presence of SARS-CoV-2 in about 27 % of cases (Penfield et al., 2020). The histopathological findings of the placenta affected by COVID-19 were similar to those observed in previous outbreaks by other coronaviruses (SARS and MERS) (Wong et al., 2004; Ng et al., 2006). The underlying immune etiologies for the histopathologic changes in the infected placenta were not clearly understood; however, placental pathologies, such as the chronic villitis in pregnant women with H1N1 influenza infection was speculated to be induced by the burst of inflammatory cytokines accompanying the influenza virus infection (Meijer et al., 2014). The first published study of three third-trimester placentas from pregnant women with COVID-19 reported a high degree of fibrin deposition and syncytial knots accompanied by a chorangioma or massive placental infarction (Hosier et al., 2020). Later on, the major placental pathologies of COVID-19 patients have been described as fetal vascular malperfusion (FVM), fetal vascular thrombosis, maternal vascular malperfusion (MVM), massive infection with generalized inflammation (presence of M2 macrophages, cytotoxic and helper T cells, and activated B-lymphocytes), fibrin deposition and intervillous thrombosis (Baergen and Heller, 2020; Hosier et al., 2020; Shanes et al., 2020). In a study of placental pathology, including 76 third-trimester placentas [SARS-CoV-2 positive (n = 51) versus SARS-CoV-2 negative (n = 25)], the placentas from SARS-CoV-2-positive women were more likely to show evidence of MVM with villous agglutination and subchorionic thrombus than those from SARS-CoV-2 negative women. Interestingly, the frequency of these histopathological findings was not different between symptomatic and asymptomatic COVID-19 cases (Smithgall et al., 2020). Therefore, the placental pathology seems to progress regardless of maternal clinical manifestation, and even asymptomatic pregnant women with COVID-19 may have an increased risk of developing obstetrical complications.
The placental abnormalities, such as MVM, observed in the cases of pregnant women with COVID-19 have been associated with IUGR (Wong et al., 2004; Ng et al., 2006; Levytska et al., 2017). Previously, the presence of intense infiltration of macrophages in the placenta and elevated levels of placental inflammatory markers have been reported to be associated with a reduction in fetal growth rate (Sharps et al., 2020). Additionally, 83.3 % of pregnant women diagnosed with IUGR and abnormal fetal Doppler flow measurements have placental pathologies compatible with MVM (Rotshenker-Olshinka et al., 2019). Hence, SARS-CoV-2 related placental pathology may be induced by the local activation of both innate and acquired immune effectors at the maternal-fetal junction and pro-inflammatory cytokine milieu. Careful follow up of pregnant women with COVID-19, including asymptomatic cases, should be considered.
3.2. Obstetrical outcomes of mothers with COVID-19
The impact of COVID-19 on pregnancy outcomes is still poorly understood. Studies in pregnant women during previous outbreaks of other coronaviruses (SARS and MERS) have observed an increased risk for miscarriage, PTB, and IUGR (Wong et al., 2004; Dashraath et al., 2020). The occurrence of other flu-like syndromes during pregnancy, such as H1N1 influenza, is also associated with an elevated risk of PTB (RR 3.9, 95 % CI: 2.7–5.6) and LBW (RR 4.6, 95 % CI: 2.9–7.5) (Meijer et al., 2015; Newsome et al., 2019). The initial pregnancy outcomes reports of pregnant women infected with COVID-19 suggest an increase in the risk of PTB and LBW (Huntley et al., 2020; Smith et al., 2020), particularly in the latter half of the pregnancy (≥ 20 weeks gestation) (Badr et al., 2020).
3.2.1. Preterm birth
Recently, in a systemic review evaluating the pregnancy outcomes of nine Chinese studies with pregnant women with COVID-19 (n = 92), increased prevalence of PTB [63.8 % (30/47)], LBW [42.8 % (9/21)], C-section [80 % (40/50)], fetal distress [61.1 % (11/18)], and neonatal ICU admission [76.9 % (11/13)] has been reported when compared to those of overall Chinese population (Smith et al., 2020). The systematic review (13 studies) by Capobianco et al. revealed a 23 % PTB rate in Chinese pregnant women (Capobianco et al., 2020), while another systematic review by Huntley et al., including ten studies from China, two from the US, and one from Italy, observed a PTB rate of 20.1 % (57/284) (Huntley et al., 2020). In a prospective cohort of 427 pregnant women hospitalized with confirmed COVID-19 in the UK, 27 % delivered prematurely (Knight et al., 2020). Trocado et al. reported a 35 % (18/51) PTB rate and a 20 % (10/51) LBW rate among newborns by reviewing 8 Chinese studies (Trocado et al., 2020). Other studies have not found a higher rate of PTB among pregnant women with COVID-19 (Zhang et al., 2020). Prematurity could be spontaneous or induced in pregnant women with COVID-19, and the severity of COVID-19 may be a factor inducing PTB. Unfortunately, the majority of the studies did not report the nature of PTB precisely. It should also be noted that there are undoubtedly differences in practice between countries that influence the results. In general, these data suggest a trend that maternal COVID-19 increases the risk for PTB and LBW.
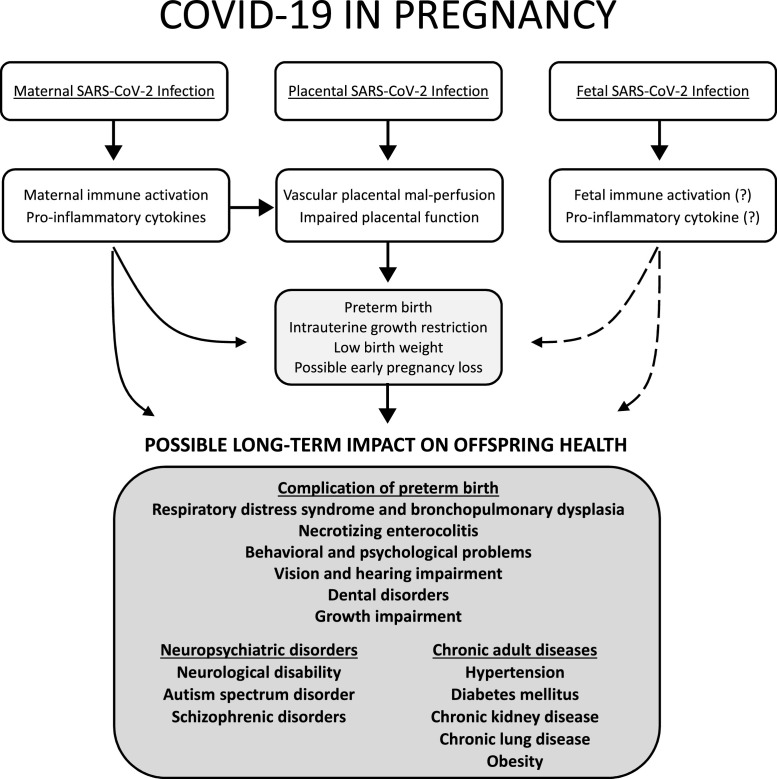
The PTB has been a global concern for maternal health, neonatal mortality, and morbidity. Recently, particular attention has been given to repercussions throughout the lives of preterm babies. The global prevalence of PTB was estimated to be 10.6 % (Chawanpaiboon et al., 2019). The PTB rates in China and the US are 7.3 % and 10 %, respectively (Chen et al., 2019; Martin et al., 2019). When compared to the overall prevalence of PTB in different countries, the first report of the obstetrical outcomes of pregnant women with COVID-19 revealed a significant increase in the PTB (Capobianco et al., 2020; Huntley et al., 2020; Knight et al., 2020; Smith et al., 2020). Consequently, these newborns have short-, medium-, and long-term health risks. In the neonatal period and the first months of life, the main complications of premature babies are respiratory distress, retinopathy of prematurity, patent ductus arteriosus, bronchopulmonary dysplasia, metabolism disorders, late-onset sepsis, necrotizing enterocolitis, intraventricular hemorrhage, and periventricular leukomalacia (Stoll et al., 2010). Premature birth may lead to the following long-term complications, including cerebral palsy, impairment of lung function, impaired learning, vision and hearing abnormalities, dental disorders, behavioral and psychological problems, growth impairment, and chronic adult diseases, namely insulin resistance, chronic kidney disease (CKD), and hypertension (Holsti et al., 2017; Kuint et al., 2017). Fig. 1 narrates the possible COVID-19-related long-term offspring risks.
Fig. 1.
Possible lofng-term impact on offspring health exposed to SARS-CoV-2 infection during pregnancy. Maternal SARS-CoV-2 infection may directly and indirectly via placental pathology, induce preterm birth, intrauterine growth restriction, low birth weight, and possible early pregnancy loss. Placenta, with and without the presence of SARS-CoV-2, may develop vascular placental mal-perfusion and functional impairment, consequently developing obstetrical complications. Fetal SARS-CoV-2 infection has been reported. However, the evidence of fetal immune activation or pro-inflammatory cytokine production by fetal immune effectors has not been reported. If any, fetal functional immune responses to SARS-CoV-2 may induce pregnancy complications as well (dotted lines). Considering maternal immune responses to SARS-CoV-2, and obstetrical complications associated with COVID-19, possible long-term impacts on offspring health are presented.
3.2.2. Low birth weight
WHO defines LBW as a birth weight less than 2500 g (up to and including 2499 g), regardless of gestational age (World Health Organization (WHO, 2004). LBW is an indicator of PTB or IUGR (World Health Organization (WHO, 2004), and these two obstetric conditions appear to be more prevalent among pregnant women with COVID-19 (Silverwood et al., 2013; Cutland et al., 2017; Al Salmi and Hannawi, 2020; Kanda et al., 2020). The prevalence of LBW was reported to be higher in suspected COVID-19 cases (17.6 %), and COVID-19 positive cases (10.5 %), as compared to controls (2.5 %) (Li et al., 2020).
The overall prevalence of LBW is estimated to be between 15–20 %, ranging from 6% in East Asia and the Pacific, 13 % in Sub-Saharan Africa, and up to 28 % in South Asia (Cutland et al., 2017; Kanda et al., 2020). LBW is associated with high neonatal mortality (>20 times greater than neonates with a birth weight of >2.5Kg), long-term neurologic disability, impaired language development, impaired academic achievement, and increased risk of chronic NCDs in offspring, such as obesity, CKD, cardiovascular disease (CVD) and diabetes mellitus (DM) (Silverwood et al., 2013; Cutland et al., 2017; Al Salmi and Hannawi, 2020; Kanda et al., 2020). Since the Baker’s initial study on the fetal origin of diseases in adulthood, fetal exposure to adverse conditions during pregnancy is considered as a risk factor for NCDs (The fetal origin of the adult disease - Barker’s Hypothesis). There are likely two main mechanisms involved. The first one is that immature organ development may be induced by adverse conditions during pregnancy, such as infections and placental pathologies, which leads to structural changes and impaired functioning of the affected organ, for example, reduced islet cell mass and β cell numbers, reduction in the number of functional nephrons. The second mechanism is epigenetic modification (Barker et al., 2009). To date, no study assesses the long-term effects on the offspring of pregnant women infected by previous outbreaks of other coronaviruses (SARS and MERS). However, some studies assess the long-term impact of viral infections, such as H1N1 influenza, during pregnancy on the health of offspring. Song et al. observed that H1N1 influenza infection during pregnancy was associated with PTB (RR 1.4, 95 % CI 1.3–1.4) and LBW (RR 1.2, 95 % CI 1.1–1.2) in newborns, which might paradoxically increase the risk of obesity in the long-term (Song et al., 2020).
A recent meta-analysis reviewing more than 7 million patients (135 publications) confirmed the association between birth weight and chronic diseases in adulthood. LBW participants (birth weight <2.5 kg) experienced a 45 % (OR 1.45, 95 % CI 1.33–1.59) higher risk to develop type 2 DM than those with birth weight ≥2.5 kg. The relationship was stronger for females (OR 1.45, 95 % CI 1.34–1.57) than males (OR 1.34, 95 % CI 1.05–1.62). Regarding the risk of CVD, individuals with LBW had a 30 % higher risk (OR 1.30, 95 % CI 1.01–1.67) compared with those with birth weight ≥2.5 kg. LBW was also associated with the increased risk of hypertension by 30 % (OR 1.30, 95 % CI 1.16–1.46) compared with those with birth weight ≥2.5 kg (Knop et al., 2018).
Babies born prematurely from mothers with inflammatory conditions have an increased risk of chronic NCDs in adulthood (Rogers and Velten, 2011). In a recent meta-analysis, a higher prevalence of type 1 DM (T1DM) was reported in the children of women with a history of maternal infection during pregnancy (OR 1.31, 95 % CI 1.07–1.62). The etiologic agent of viral infections was not identified in 12 of the 18 studies. However, in the remaining studies, enterovirus was responsible for maternal infections. Since the studies did not define the gestational ages at the occurrence of viral infection (Yue et al., 2018), it is unclear to determine the timing of viral insult. In another systematic review, Allen et al. also reported a significant association between viral infection during pregnancy and T1DM in offspring (OR 2.16, 95 % CI 1.22–3.80), but not with islet cell autoimmunity (OR 1.45, 95 % CI 0.63–3.31). It was speculated that the fetal immune response to vertically-transmitted enterovirus might be associated with the increased risk for T1DM in offspring (Allen et al., 2018). Therefore, obstetrical complications, particularly related to immune-inflammatory conditions, may have a link to long-term NCD in affected children. At this point, there is no data to support the increased risk of NCD later in the life of COVID-19 affected children, and the vertical transmission of SARS-CoV-2 seems to happen rarely. Hence, it remains as a hypothetical question.
3.2.3. Pregnancy loss
The association between viral infection and pregnancy loss has been debated for decades. Some viral infections increase the risk of miscarriage and stillbirth. The mechanisms that induce pregnancy loss are still poorly understood (Racicot and Mor, 2017). Giakoumelou et al. suggested that the direct viral action or maternal immunological changes, which induce trophoblastic and placental lesions, are involved in the pathophysiology of pregnancy loss (Giakoumelou et al., 2016). The ideal maternal immune response for a successful pregnancy depends on a balance between T-helper 1 (Th1) and T-helper-2 (Th2) immunity. Therefore, from an immunological point of view, hypothetically, the severe form of COVID-19 may increase the risk of pregnancy losses (Liu et al., 2020).
Segars et al. compared pregnancy outcomes between the three recent coronavirus outbreaks, COVID-19, SARS, and MERS, highlighting miscarriage rates of 2%, 25 %, and 18 %, respectively (Segars et al., 2020). Recently, Cosma et al. showed no significant difference in the cumulative incidence of COVID-19 in women who experienced a miscarriage during the first-trimester than those with ongoing pregnancies (11 % versus 9.6 %, p = 0.73) (Cosma et al., 2020). Mascio et al., analyzing a series of 388 pregnant women infected by COVID-19, observed rates of spontaneous first-trimester abortion (n = 6, 19.4 % of the 31 women with the first-trimester infection) and stillbirth (6/266, 2.3 %) similar to pregnant women not infected by COVID-19 (Di Mascio et al., 2020). Again, there was no difference in the risk of pregnancy loss in symptomatic pregnant women infected by SARS-CoV-2 compare with asymptomatic pregnant women (Di Mascio et al., 2020).
Contrarily, Khalil et al. described an increase in the stillbirth rate at St George’s University Hospital, London, during the COVID-19 pandemic period (from February 1, 2020, to June 14, 2020) when compared to the pre-pandemic period (from October 1, 2019, to January 31, 2020); 9.31 per 1000 births (16/1718 births) versus 2.38 per 1000 births (4/1681 births), a difference of 6.93 (95 % CI 1.83–12.0) per 1000 births (p = 0.01) (Khalil et al., 2020). The authors suggested a possible relationship with SARS-CoV-2 infection or a worsening of the quality of care for pregnant women during the COVID-19 pandemic. Therefore, it is necessary to carry out studies that assess the impact of COVID-19 on the risk of pregnancy loss.
4. Vertical transmission and possible long-term offspring risks
Since the beginning of the COVID-19 pandemic, researchers have investigated the possibility of SARS-CoV-2 vertical transmission and the consequential impact on fetuses. Although several studies have already described placental infection with SARS-CoV-2, vertical transmission appears to be a rare event. The first study described an absence of vertical transmission (Karimi-Zarchi et al., 2020). Subsequently, Vivanti et al. described a case of vertical transmission of SARS-CoV-2 in an infected pregnant woman at 35 weeks gestation. In addition to the vertical transmission, the authors described an intense placental inflammation and neonatal viremia, with the newborn showing neurological symptoms from cerebral vasculitis (Vivanti et al. 2020).
However, in a recent systematic review, which included 936 newborns from COVID-19 infected mothers, 27 neonates had SARS-CoV-2 viral RNA positive by nasopharyngeal swab, indicating a pooled proportion of 3.2 % (95 % CI 2.2–4.3 %) for vertical transmission. The authors also investigated the presence of SARS-CoV-2 viral RNA in different sites; in neonatal cord blood, SARS-CoV-2 was positive in 2.9 % (1/34) of samples, 7.7 % (2/26) of placenta tissues, and 9.7 % (3/31) of fecal/rectal swabs. No case had the presence of SARS-CoV-2 in amniotic fluid and newborn urine [0% (0/51) and 0% (0/17), respectively]. Neonatal serology was positive in 3/82 (3.7 %) based on IgM SARS-CoV-2 antibodies (Kotlyar et al., 2020).
To date, there is no observational study to assess the impact of maternal infection by other coronaviruses on the behavioral and neurological health of offspring. However, there is strong evidence that different viral infections, such as severe acute respiratory syndrome by influenza H1N1 virus in pregnant women, can compromise the offspring’s health even when the infection is limited to the mother or placental bed without fetal infection (Brown et al., 2004; Canetta et al., 2014b; He et al., 2020; Song et al., 2020). Animal and epidemiological studies suggested that viral infections in pregnant women, which did not cross the placenta, were also associated with neurological and behavioral diseases in offspring (Knuesel et al., 2014; Al-Haddad et al., 2019). Maternal immune responses to SARS-CoV-2 have similarities to those reported in women with flu-like syndrome, hence, raising concern for possible neurological and behavioral disorders in the offspring of pregnant women with COVID-19. In a recent meta-analysis including 7865 COVID-19 patients, a significant decrease in lymphocytes, including CD4+ T cells, CD8+ T cells, CD3+ cells, CD19+ B cells, NK cells, and monocytes, and an increase in the white blood cell, neutrophils, neutrophil to lymphocyte ratio, CRP, erythrocyte sedimentation rate (ESR), ferritin, procalcitonin (PCT), serum amyloid A (SAA), IL-2, IL-2R, IL-4, IL-6, IL-8, IL-10, TNF-α, and IFN-γ were present in the severe cases compared to those of non-severe cases. However, there are no significant differences in IL-1β, IL-17, and CD4/CD8 T cell ratio between these groups (Akbari et al., 2020). These changes were similar to those of H1N1 influenza.
The mechanisms responsible for the long-term disorders in offspring are still poorly understood (Knuesel et al., 2014; Racicot and Mor, 2017). Neurological and behavioral diseases in children of pregnant women with a history of viral infection during pregnancy may result from tissue damage caused by the direct action of the virus on the fetal brain or by the injury of the central nervous system indirectly thru potentiating the fetal inflammatory response, resulting in activation of astrocytes and microglia and causing cytokine release, apoptosis, attenuation of growth, and direct cellular damage (Al-Haddad et al., 2019). Viral infection limited to the placenta is also associated with fetal brain injury. Briefly, inflammatory mediators or cells in the placenta can be transferred to the fetus, which can ultimately damage the fetal brain through the release of cytokines, neurotransmitters, or excitotoxic metabolites (Knuesel et al., 2014; Al-Haddad et al., 2019). The recent outbreak caused by the Zika virus (ZIKV) is an example of the viral impact on the neurological and mental health of offspring thru the direct action of the virus on the fetal brain and the probable indirect effect of infection during pregnancy. Neurodevelopmental abnormalities have been reported in children with in-utero ZIKV exposure, even without congenital Zika syndrome (Mulkey et al., 2020).
4.1. Psychological disorders
Observational studies demonstrate the correlation between viral infections in pregnancy and a higher prevalence of schizophrenia and other psychotic disorders in offspring. The higher maternal levels of IgG and IgM before the delivery of affected offspring with schizophrenia and other psychotic disorders were reported compared with those of controls (Buka et al., 2001). Besides, for the first time, the occurrence of influenza during the first half of pregnancy was reported to increases the risk of schizophrenia spectrum disorders (SSD) (OR 3.00, 95 % CI 0.9–10.1, p = 0.05) (Brown et al., 2004). Additionally, influenza infection during pregnancy seems to increase the risk for offspring to develop bipolar disorder with psychotic features (OR 5.03, 95 % CI 1.38–10.1, p = 0.05) (Canetta et al., 2014b). Maternal exposure to herpes simplex virus 2 is also associated with an increased risk for psychoses among adult offspring (OR 2.6, 95 % CI 1.4–4.6) (Buka et al., 2008). Recent studies suggest that the placental inflammatory process induces changes in local gene expression, increasing the risk of behavioral diseases, such as schizophrenia (Knuesel et al., 2014; Ursini et al., 2018; Al-Haddad et al., 2019). Changes in the secretion of placental neurotransmitters such as serotonin, changes in dopaminergic and GABAergic activity in the fetal brain, and the diverged development of cholinergic neurons in the fetal basal forebrain induced by maternal inflammation are other possible mechanisms that increase the risk of neurological and behavioral disorders in offspring of pregnant women with viral infections (Ozawa et al., 2006; Knuesel et al., 2014; Goeden et al., 2016). Indeed, increased maternal CRP levels were significantly associated with schizophrenia in offspring (OR 1.12, 95 % CI 1.02–1.24, p = 0.019) (Canetta et al., 2014a), and elevated maternal TNF-α and IL-8 levels during pregnancy have also been associated with an increased risk of schizophrenia and other psychotic disorders in offspring (Canetta et al., 2014a). Considering changes in pro-inflammatory cytokines and factors in pregnant women with COVID-19, schizophrenic and psychotic disorders can be a long-term offspring risk of pregnant women with COVID-19.
4.2. Autism spectrum disorders
Over the past few decades, studies have suggested the association between viral infections in pregnancy and autism spectrum disorders (ASD) in offspring. A large population study in Denmark, evaluating 1,612,342 children born between 1980 and 2005, found an increased risk of ASD in children of mothers with a history of viral infection in the first trimester of pregnancy [adjusted hazard ratio (aHR) 2.98 (CI: 1.29–7.15)] and bacterial infection in the second trimester of pregnancy (aHR 1.42, CI 1.08–1.87). A total of 283 children were exposed to viral infection in the first trimester, and the frequent viral infections exposed to these children were influenza (25 %), viral gastroenteritis (20 %), and unspecified viral infection (12 %) (Atladóttir et al., 2010). A meta-analysis of 15 studies with more than 40,000 ASD cases also demonstrated an increased risk for ASD after prenatal exposure to infectious diseases (OR 1.13, 95 % CI 1.03–1.23) (Jiang et al., 2016). In animal models, the role of maternal immune activation (MIA) with an intense inflammatory response has been reported in conditions of behavioral changes in the offspring. In rodent models of MIA, maternal IL-17a promoted abnormal cortical development in offspring and ASD-like behavioral abnormalities in offspring. Treatment with anti-IL-17a blocking antibody in pregnant dams ameliorated MIA-associated behavioral abnormalities (Choi et al., 2016). Using the same animal model, the elevated IL-6 level was associated with changes in offspring behavior. Treatment with anti-IL6 antibodies prevented the exploratory and social deficits caused by the MIA (Smith et al., 2007). In women with severe COVID-19, serum IL-6 levels were significantly higher than that of mild COVID-19 cases, while IL-17 levels were the same. Although it is premature to link COVID-19 exposure in-utero and ASD or psychotic disorders, a systematic and careful follow up of offspring from mothers with severe COVID-19 should be explored.
5. Conclusion
Currently, the COVID-19 pandemic has already reached alarming numbers of cases and deaths, unprecedented in recent medical history. Given the pandemic status and the history of sequelae in children of pregnant women affected by other recent viral outbreaks, it is necessary to assess the impact of COVID-19 on obstetrical outcomes and short and long-term NCD in offspring who had in-utero exposure to SARS-CoV-2 infection. Initial studies demonstrate a possible association between COVID-19 and PTB, IUGR, and LBW, which have been reported to increase risks for long-term NCD in adulthood. Besides, observational studies on outbreaks of other SARS warn the possible risk of behavioral and neurological diseases in children of infected women during pregnancy. However, the association between COVID-19 and long-term sequelae is uncertain and hypothetical at this point. Therefore, the establishment of a long-term follow-up plan for the offspring of pregnant women affected by COVID-19 should be carefully established.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of Competing Interest
None of the authors have a conflict of interest in this work.
Acknowledgments
N/A.
References
- Akbari H., et al. The role of cytokine profile and lymphocyte subsets in the severity of coronavirus disease 2019 (covid-19): a systematic review and meta-analysis. Life Sci. 2020:118167. doi: 10.1016/j.lfs.2020.118167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al Salmi I., Hannawi S. Birth weight is inversely correlated with blood pressure: population-based study. J. Hypertens. 2020 doi: 10.1097/HJH.0000000000002545. July 6. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- Algarroba G.N., et al. Visualization of sars-cov-2 virus invading the human placenta using electron microscopy. Am. J. Obstet. Gynecol. 2020;223(2):275–278. doi: 10.1016/j.ajog.2020.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Haddad B.J.S., et al. The fetal origins of mental illness. Am. J. Obstet. Gynecol. 2019;221:549–562. doi: 10.1016/j.ajog.2019.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen D.W., et al. Maternal virus infections in pregnancy and type 1 diabetes in their offspring: systematic review and meta-analysis of observational studies. Rev. Med. Virol. 2018;28:e1974. doi: 10.1002/rmv.1974. [DOI] [PubMed] [Google Scholar]
- Atladóttir H.O., et al. Maternal infection requiring hospitalization during pregnancy and autism spectrum disorders. J. Autism Dev. Disord. 2010;40:1423–1430. doi: 10.1007/s10803-010-1006-y. [DOI] [PubMed] [Google Scholar]
- Badr D.A., et al. Are clinical outcomes worse for pregnant women at ≥20 weeks’ gestation infected with coronavirus disease 2019? A multicenter case-control study with propensity score matching. Am. J. Obstet. Gynecol. 2020;27(20):S0002–9378. doi: 10.1016/j.ajog.2020.07.045. 30776-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baergen R.N., Heller D.S. Placental pathology in covid-19 positive mothers: preliminary findings. Pediatr. Dev. Pathol. 2020;23:177–180. doi: 10.1177/1093526620925569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker D.J., et al. Growth and chronic disease: findings in the Helsinki birth cohort. Ann. Hum. Biol. 2009;36:445–458. doi: 10.1080/03014460902980295. [DOI] [PubMed] [Google Scholar]
- Best Rocha A., et al. Detection of sars-cov-2 in formalin-fixed paraffin-embedded tissue sections using commercially available reagents. Lab. Invest. 2020:1–5. doi: 10.1038/s41374-020-0464-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt J.S., et al. Epidemiology of coronavirus disease 2019 in pregnancy: risk factors and associations with adverse maternal and neonatal outcomes. Am. J. Obstet. Gynecol. 2020;25(20):S0002–9378. doi: 10.1016/j.ajog.2020.09.043. 31134-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A.S., et al. Serologic evidence of prenatal influenza in the etiology of schizophrenia. Arch. Gen. Psychiatry. 2004;61:774–780. doi: 10.1001/archpsyc.61.8.774. [DOI] [PubMed] [Google Scholar]
- Buka S.L., et al. Maternal infections and subsequent psychosis among offspring. Arch. Gen. Psychiatry. 2001;58:1032–1037. doi: 10.1001/archpsyc.58.11.1032. [DOI] [PubMed] [Google Scholar]
- Buka S.L., et al. Maternal exposure to herpes simplex virus and risk of psychosis among adult offspring. Biol. Psychiatry. 2008;63:809–815. doi: 10.1016/j.biopsych.2007.09.022. [DOI] [PubMed] [Google Scholar]
- Canetta S., et al. Elevated maternal C-reactive protein and increased risk of schizophrenia in a national birth cohort. Am. J. Psychiatry. 2014;171:960–968. doi: 10.1176/appi.ajp.2014.13121579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canetta S.E., et al. Serological documentation of maternal influenza exposure and bipolar disorder in adult offspring. Am. J. Psychiatry. 2014;171:557–563. doi: 10.1176/appi.ajp.2013.13070943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capobianco G., et al. Covid-19 in pregnant women: a systematic review and meta-analysis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020;252:490–501. doi: 10.1016/j.ejogrb.2020.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chawanpaiboon S., et al. Global, regional, and national estimates of levels of preterm birth in 2014: a systematic review and modelling analysis. Lancet Glob. Health. 2019;7:e37–e46. doi: 10.1016/S2214-109X(18)30451-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C., et al. Preterm birth in china between 2015 and 2016. Am. J. Public Health. 2019;109:1597–1604. doi: 10.2105/AJPH.2019.305287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi G.B., et al. The maternal interleukin-17a pathway in mice promotes autism-like phenotypes in offspring. Science (New York, N.Y.) 2016;351:933–939. doi: 10.1126/science.aad0314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosma S., et al. Covid-19 and first trimester spontaneous abortion: a case-control study of 225 pregnant patients. Am. J. Obstet. Gynecol. 2020 doi: 10.1016/j.ajog.2020.10.005. S0002-9378(20)31177-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutland C.L., et al. Low birth weight: case definition & guidelines for data collection, analysis, and presentation of maternal immunization safety data. Vaccine. 2017;35:6492–6500. doi: 10.1016/j.vaccine.2017.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dashraath P., et al. Coronavirus disease 2019 (covid-19) pandemic and pregnancy. Am. J. Obstet. Gynecol. 2020;222:521–531. doi: 10.1016/j.ajog.2020.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Mascio D., et al. Risk factors associated with adverse fetal outcomes in pregnancies affected by coronavirus disease 2019 (COVID-19): a secondary analysis of the wapm study on covid-19. J. Perinat. Med. 2020 doi: 10.1515/jpm-2020-0355. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Eguchi S., et al. Understanding angiotensin ii type 1 receptor signaling in vascular pathophysiology. Hypertension (Dallas, Tex. 1979) 2018;71:804–810. doi: 10.1161/HYPERTENSIONAHA.118.10266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellington S., et al. Characteristics of women of reproductive age with laboratory-confirmed sars-cov-2 infection by pregnancy status - united states, January 22-June 7, 2020. MMWR Morb. Mortal. Wkly. Rep. 2020;69:769–775. doi: 10.15585/mmwr.mm6925a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng X., et al. Immune-inflammatory parameters in covid-19 cases: a systematic review and meta-analysis. Front. Med. (Lausanne) 2020;7:301. doi: 10.3389/fmed.2020.00301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueiredo A.S., Schumacher A. The T helper type 17/regulatory T cell paradigm in pregnancy. Immunology. 2016;148:13–21. doi: 10.1111/imm.12595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flannery D.D., et al. Sars-cov-2 seroprevalence among parturient women in Philadelphia. Sci. Immunol. 2020;5 doi: 10.1126/sciimmunol.abd5709. eabd5709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox N.S., Melka S. Covid-19 in pregnant women: case series from one large New York city obstetrical practice. Am. J. Perinatol. 2020;37:1002–1004. doi: 10.1055/s-0040-1712529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y.M., et al. Cytokine storm syndrome in coronavirus disease 2019: a narrative review. J. Intern. Med. 2020 doi: 10.1111/joim.13144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gheblawi M., et al. Angiotensin-converting enzyme 2: Sars-cov-2 receptor and regulator of the renin-angiotensin system: celebrating the 20th anniversary of the discovery of ace2. Circ. Res. 2020;126:1456–1474. doi: 10.1161/CIRCRESAHA.120.317015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giakoumelou S., et al. The role of infection in miscarriage. Hum. Reprod. Update. 2016;22:116–133. doi: 10.1093/humupd/dmv041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goeden N., et al. Maternal inflammation disrupts fetal neurodevelopment via increased placental output of serotonin to the fetal brain. J. Neurosci. 2016;36:6041–6049. doi: 10.1523/JNEUROSCI.2534-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon D.E., et al. A sars-cov-2-human protein-protein interaction map reveals drug targets and potential drug-repurposing. Nature. 2020;583:459–468. doi: 10.1038/s41586-020-2286-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J., et al. A systematic review and meta-analysis of influenza a virus infection during pregnancy associated with an increased risk for stillbirth and low birth weight. Kidney Blood Press. Res. 2017;42:232–243. doi: 10.1159/000477221. [DOI] [PubMed] [Google Scholar]
- He J.R., et al. Maternal infection in pregnancy and childhood leukemia: a systematic review and meta-analysis. J. Pediatr. 2020;217:98–109. doi: 10.1016/j.jpeds.2019.10.046. e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holsti A., et al. Chronic conditions and health care needs of adolescents born at 23 to 25 weeks’ gestation. Pediatrics. 2017;139 doi: 10.1542/peds.2016-2215. [DOI] [PubMed] [Google Scholar]
- Hosier H., et al. Sars-cov-2 infection of the placenta. MedRxiv. 2020 doi: 10.1172/JCI139569. 2020.04.30.20083907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huntley B.J.F., et al. Rates of maternal and perinatal mortality and vertical transmission in pregnancies complicated by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection: a systematic review. Obstet. Gynecol. 2020;136:303–312. doi: 10.1097/AOG.0000000000004010. [DOI] [PubMed] [Google Scholar]
- Jabalie G., et al. Metabolic syndrome mediates pro-inflammatory responses of inflammatory cells in preeclampsia. Am J Reprod Immunol (New York, N.Y. 1989) 2019;81 doi: 10.1111/aji.13086. [DOI] [PubMed] [Google Scholar]
- Jiang H.-Y., et al. Maternal infection during pregnancy and risk of autism spectrum disorders: a systematic review and meta-analysis. Brain Behav. Immun. 2016;58:165–172. doi: 10.1016/j.bbi.2016.06.005. [DOI] [PubMed] [Google Scholar]
- Jin Y., et al. Virology, epidemiology, pathogenesis, and control of covid-19. Viruses. 2020;12:372. doi: 10.3390/v12040372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing Y., et al. Potential influence of covid-19/ace2 on the female reproductive system. Mol. Hum. Reprod. 2020;26:367–373. doi: 10.1093/molehr/gaaa030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jørgensen N., et al. The tolerogenic function of regulatory t cells in pregnancy and cancer. Front. Immunol. 2019;10:911. doi: 10.3389/fimmu.2019.00911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juan J., et al. Effects of coronavirus disease 2019 (COVID-19) on maternal, perinatal and neonatal outcomes: a systematic review. Ultrasound Obstet. Gynecol. 2020;56:15–27. doi: 10.1002/uog.22088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanda T., et al. Low birth weight trends: possible impacts on the prevalences of hypertension and chronic kidney disease. Hypertens. Res. 2020;43:859–868. doi: 10.1038/s41440-020-0451-z. [DOI] [PubMed] [Google Scholar]
- Karimi-Zarchi M., et al. Vertical transmission of coronavirus disease 19 (COVID-19) from infected pregnant mothers to neonates: a review. Fetal Pediatr. Pathol. 2020;39:246–250. doi: 10.1080/15513815.2020.1747120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayem G., et al. A snapshot of the covid-19 pandemic among pregnant women in France. J. Gynecol. Obstet. Hum. Reprod. 2020;49 doi: 10.1016/j.jogoh.2020.101826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalil A., et al. Change in the incidence of stillbirth and preterm delivery during the covid-19 pandemic. JAMA. 2020;324:705–706. doi: 10.1001/jama.2020.12746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight M., et al. Characteristics and outcomes of pregnant women admitted to hospital with confirmed sars-cov-2 infection in uk: national population based cohort study. BMJ. 2020;369:m2107. doi: 10.1136/bmj.m2107. Clinical research ed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kniss D.A. Alternative interpretation to the findings reported in visualization of severe acute respiratory syndrome coronavirus 2 invading the human placenta using electron microscopy. Am. J. Obstet. Gynecol. 2020 doi: 10.1016/j.ajog.2020.06.016. S0002-9378(20)30632-30633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knop M.R., et al. Birth weight and risk of type 2 diabetes mellitus, cardiovascular disease, and hypertension in adults: A meta-analysis of 7,646,267 participants from 135 studies. J. Am. Heart Assoc. 2018;7 doi: 10.1161/JAHA.118.008870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knuesel I., et al. Maternal immune activation and abnormal brain development across CNS disorders. Nat. Rev. Neurol. 2014;10:643–660. doi: 10.1038/nrneurol.2014.187. [DOI] [PubMed] [Google Scholar]
- Kotlyar A., et al. Vertical transmission of COVID-19: a systematic review and meta-analysis. Am. J. Obstet. Gynecol. 2020 doi: 10.1016/j.ajog.2021.09.029. S0002-9378(20)30823-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krechetova L.V., et al. Lymphocyte activation in the development of immune tolerance in women with recurrent pregnancy loss. Biochemistry Mosc. 2020;85:583–593. doi: 10.1134/S0006297920050077. [DOI] [PubMed] [Google Scholar]
- Kuint J., et al. Rehospitalization through childhood and adolescence: association with neonatal morbidities in infants of very low birth weight. J. Pediatr. 2017;188:135–141. doi: 10.1016/j.jpeds.2017.05.078. e2. [DOI] [PubMed] [Google Scholar]
- Lee S.K., et al. Th17 and regulatory t cells in women with recurrent pregnancy loss. Am. J. Reprod. Immunol. (New York, N.Y. 1989) 2012;67:311–318. doi: 10.1111/j.1600-0897.2012.01116.x. [DOI] [PubMed] [Google Scholar]
- Levytska K., et al. Placental pathology in relation to uterine artery doppler findings in pregnancies with severe intrauterine growth restriction and abnormal umbilical artery doppler changes. Am. J. Perinatol. 2017;34:451–457. doi: 10.1055/s-0036-1592347. [DOI] [PubMed] [Google Scholar]
- Li N., et al. Maternal and neonatal outcomes of pregnant women with covid-19 pneumonia: a case-control study. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa352. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H., et al. Why are pregnant women susceptible to COVID-19? An immunological viewpoint. J. Reprod. Immunol. 2020;139 doi: 10.1016/j.jri.2020.103122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malinowski A.K., et al. H1N1 in pregnancy: a tertiary care centre experience. J. Obstet. Gynaecol. Can. 2011;33:698–704. doi: 10.1016/S1701-2163(16)34954-4. [DOI] [PubMed] [Google Scholar]
- Martin J.A., et al. Births: final data for 2018. Natl. Vital Stat. Rep. 2019;68:1–47. [PubMed] [Google Scholar]
- Mcelvaney O.J., et al. Characterization of the inflammatory response to severe covid-19 illness. Am. J. Respir. Crit. Care Med. 2020 doi: 10.1164/rccm.202005-1583OC. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijer W.J., et al. High rate of chronic villitis in placentas of pregnancies complicated by influenza A/H1N1 infection. Infect. Dis. Obstet. Gynecol. 2014;2014 doi: 10.1155/2014/768380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijer W.J., et al. Influenza virus infection in pregnancy: a review. Acta Obstet. Gynecol. Scand. 2015;94:797–819. doi: 10.1111/aogs.12680. [DOI] [PubMed] [Google Scholar]
- Mendoza M., et al. Pre-eclampsia-like syndrome induced by severe covid-19: a prospective observational study. BJOG. 2020 doi: 10.1111/1471-0528.16339. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mor G., et al. The unique immunological and microbial aspects of pregnancy. Nat. Rev. Immunol. 2017;17:469–482. doi: 10.1038/nri.2017.64. [DOI] [PubMed] [Google Scholar]
- Mulkey S.B., et al. Neurodevelopmental abnormalities in children with in utero zika virus exposure without congenital zika syndrome. JAMA Pediatr. 2020;174:269–276. doi: 10.1001/jamapediatrics.2019.5204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami M., et al. Pleiotropy and specificity: insights from the interleukin 6 family of cytokines. Immunity. 2019;50:812–831. doi: 10.1016/j.immuni.2019.03.027. [DOI] [PubMed] [Google Scholar]
- Muyayalo K.P., et al. COVID-19 and Treg/Th17 imbalance: potential relationship to pregnancy outcomes. Am. J. Reprod. Immunol. (New York, N.Y. 1989) 2020 doi: 10.1111/aji.13304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newsome K., et al. Outcomes of infants born to women with influenza A(H1N1)pdm09. Birth Defects Res. 2019;111:88–95. doi: 10.1002/bdr2.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng W.F., et al. The placentas of patients with severe acute respiratory syndrome: a pathophysiological evaluation. Pathology. 2006;38:210–218. doi: 10.1080/00313020600696280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozawa K., et al. Immune activation during pregnancy in mice leads to dopaminergic hyperfunction and cognitive impairment in the offspring: a neurodevelopmental animal model of schizophrenia. Biol. Psychiatry. 2006;59:546–554. doi: 10.1016/j.biopsych.2005.07.031. [DOI] [PubMed] [Google Scholar]
- Penfield C.A., et al. Detection of severe acute respiratory syndrome coronavirus 2 in placental and fetal membrane samples. Am. J. Obstet. Gynecol. MFM. 2020;2 doi: 10.1016/j.ajogmf.2020.100133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pique-Regi R., et al. Does the human placenta express the canonical cell entry mediators for SARS-CoV-2? ELife. 2020;9 doi: 10.7554/eLife.58716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racicot K., Mor G. Risks associated with viral infections during pregnancy. J. Clin. Invest. 2017;127:1591–1599. doi: 10.1172/JCI87490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers L.K., Velten M. Maternal inflammation, growth retardation, and preterm birth: insights into adult cardiovascular disease. Life Sci. 2011;89:417–421. doi: 10.1016/j.lfs.2011.07.017. [DOI] [PubMed] [Google Scholar]
- Rotshenker-Olshinka K., et al. Recurrent intrauterine growth restriction: characteristic placental histopathological features and association with prenatal vascular doppler. Arch. Gynecol. Obstet. 2019;300:1583–1589. doi: 10.1007/s00404-019-05339-x. [DOI] [PubMed] [Google Scholar]
- Sasaki Y., et al. Proportion of peripheral blood and decidual CD4(+) CD25(bright) regulatory T cells in preeclampsia. Clin. Exp. Immunol. 2007;149:139–145. doi: 10.1111/j.1365-2249.2007.03397.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segars J., et al. Prior and novel coronaviruses, COVID-19, and human reproduction: What is known? Fertil. Steril. 2020;113:1140–1149. doi: 10.1016/j.fertnstert.2020.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanes E.D., et al. Placental pathology in COVID-19. Am. J. Clin. Pathol. 2020;154:23–32. doi: 10.1093/ajcp/aqaa089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharps M.C., et al. Increased placental macrophages and a pro-inflammatory profile in placentas and maternal serum in infants with a decreased growth rate in the third trimester of pregnancy. Am. J. Reprod. Immunol. (New York, N.Y. 1989) 2020;84 doi: 10.1111/aji.13267. [DOI] [PubMed] [Google Scholar]
- Siddiqi H.K., Mehra M.R. COVID-19 illness in native and immunosuppressed states: a clinical-therapeutic staging proposal. J. Heart Lung Transplant. 2020;39:405–407. doi: 10.1016/j.healun.2020.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverwood R.J., et al. Low birth weight, later renal function, and the roles of adulthood blood pressure, diabetes, and obesity in a British birth cohort. Kidney Int. 2013;84:1262–1270. doi: 10.1038/ki.2013.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S.E., et al. Maternal immune activation alters fetal brain development through interleukin-6. J. Neurosci. 2007;27:10695–10702. doi: 10.1523/JNEUROSCI.2178-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith V., et al. Maternal and neonatal outcomes associated with covid-19 infection: a systematic review. PLoS One. 2020;15 doi: 10.1371/journal.pone.0234187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smithgall M.C., et al. Third trimester placentas of sars-cov-2-positive women: histomorphology, including viral immunohistochemistry and in situ hybridization. Histopathology. 2020 doi: 10.1111/his.14215. doi: 10.1111/his.14215. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J.Y., et al. Paradoxical long-term impact of maternal influenza infection on neonates and infants. BMC Infect. Dis. 2020;20:502. doi: 10.1186/s12879-020-05236-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoll B.J., et al. Neonatal outcomes of extremely preterm infants from the NICHD neonatal research network. Pediatrics. 2010;126:443–456. doi: 10.1542/peds.2009-2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton D., et al. Universal screening for SARS-CoV-2 in women admitted for delivery. NEJM. 2020;382:2163–2164. doi: 10.1056/NEJMc2009316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemoto M.L.S., et al. The tragedy of COVID-19 in brazil: 124 maternal deaths and counting. Int. J. Gynaecol. Obstet. 2020 doi: 10.1002/ijgo.13300. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trocado V., et al. Pregnancy and COVID-19: a systematic review of maternal, obstetric and neonatal outcomes. J. Matern. Fetal. Neonatal. Med. 2020:1–13. doi: 10.1080/14767058.2020.1781809. [DOI] [PubMed] [Google Scholar]
- Ursini G., et al. Convergence of placenta biology and genetic risk for schizophrenia. Nat. Med. 2018;24:792–801. doi: 10.1038/s41591-018-0021-y. [DOI] [PubMed] [Google Scholar]
- Wang J., et al. Cytokine storm and leukocyte changes in mild versus severe sars-cov-2 infection: review of 3939 COVID-19 patients in china and emerging pathogenesis and therapy concepts. J. Leukoc. Biol. 2020;108:17–41. doi: 10.1002/JLB.3COVR0520-272R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong S.F., et al. Pregnancy and perinatal outcomes of women with severe acute respiratory syndrome. Am. J. Obstet. Gynecol. 2004;191:292–297. doi: 10.1016/j.ajog.2003.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization (WHO) 2nd ed. World Health Organization; Geneva: 2004. ICD-10: International Statistical Classification of Diseases and Related Health Problems: Tenth Revision. [Google Scholar]
- World Health Organization (WHO) 2020. Who Coronavirus Disease (COVID-19) Dashboard.https://covid19.who.int/ [Google Scholar]
- Wu R., et al. An update on current therapeutic drugs treating covid-19. Curr. Pharmacol. Rep. 2020:1–15. doi: 10.1007/s40495-020-00216-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y.C., et al. The outbreak of covid-19: an overview. J. Chin. Med. Assoc. 2020;83:217–220. doi: 10.1097/JCMA.0000000000000270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z., et al. Pathological findings of covid-19 associated with acute respiratory distress syndrome. Lancet Respir. Med. 2020;8:420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue Y., et al. Maternal infection during pregnancy and type 1 diabetes mellitus in offspring: a systematic review and meta-analysis. Epidemiol. Infect. 2018;146:2131–2138. doi: 10.1017/S0950268818002455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zenclussen A.C., et al. Abnormal T-cell reactivity against paternal antigens in spontaneous abortion: adoptive transfer of pregnancy-induced CD4+CD25+ T regulatory cells prevents fetal rejection in a murine abortion model. Am. J. Pathol. 2005;166:811–822. doi: 10.1016/S0002-9440(10)62302-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., et al. [Analysis of the pregnancy outcomes in pregnant women with COVID-19 in Hubei province] Zhonghua Fu Chan Ke Za Zhi. 2020;55:166–171. doi: 10.3760/cma.j.cn112141-20200218-00111. [DOI] [PubMed] [Google Scholar]