Abstract
The ability to safely and precisely deliver genetic materials to target sites in complex biological environments is vital to the success of gene therapy. Numerous viral and nonviral vectors have been developed and evaluated for their safety and efficacy. This study will feature progress in synthetic polymers as nonviral vectors, which benefit from their chemical versatility, biocompatibility, and ability to carry both therapeutic cargo and targeting moieties. The combination of synthetic gene carrying constructs with advanced delivery techniques promises new therapeutic options for treating and curing genetic disorders.
1 |. INTRODUCTION
Genetic disorders leading to the onset of diseases, such as cystic fibrosis and Parkinsons, represent ongoing threats to human health. For these types of diseases, conventional therapies using small molecule or protein drugs have largely failed to provide lasting treatments or cures. Gene therapy, which seeks to modify malfunctioning genetic expression, has emerged as an important alternative to conventional molecular therapies. Successful gene therapy hinges on transport of therapeutic genes across multiple biological barriers before reaching the target. Although bare nucleic acids can be delivered in vivo by direct introduction of DNA or RNA, rapid clearance and loss of expression limit the effectiveness of this approach (Herweijer & Wolff, 2003). As such, numerous types of delivery vehicles are under investigation for encapsulating genetic material during transportation to the desired location. While both RNA and DNA have been examined extensively in gene therapy research, this review focuses primarily on DNA delivery.
The objective of any gene delivery approach is to select carriers that maximize both patient safety and therapeutic efficacy. Both viral and nonviral vectors have been studied in gene delivery and evaluated for their cytotoxicity, transfection efficiency, and efficacy. Adenoviruses and retroviruses constitute the most widely studied gene delivery vectors, with high transfection efficacy stemming from their intrinsic ability to invade cells (Hardee, Arévalo-Soliz, Hornstein, & Zechiedrich, 2017; Kotterman, Chalberg, & Schaffer, 2015; H. Yin et al., 2014). Despite being the most efficient gene delivery systems, viral vectors have recognized drawbacks, including immunogenicity and limited loading capacity, that drives research towards alternative solutions (Cox, Platt, & Zhang, 2015; Thomas, Ehrhardt, & Kay, 2003). Thus, this review will focus on gene therapy using synthetic reagents, such as polymers, which promise revolutionary advances when used effectively as delivery vectors in medicine.
Figure 1 illustrates examples of current gene delivery approaches being pursued by researchers and clinicians. Chemical methods (Figure 1a) such as those employing lipid assemblies and synthetic polymers, exploit the nanoscale size and controllable surface properties of organic and polymeric molecules. Such constructs are intended to match or exceed the performance of viral vectors (Figure 1b) with fewer immunogenic complications. Indeed, the nanoscale size of polymers, nanoparticles (NPs), and vesicles represent quintessential nanotherapeutics that combines drugs and targeting groups into nanoscale packages. Notably, in addition to liposomal and polymeric systems, virus-like particles—nanostructures composed of self-assembled viral proteins—represent another class of nonviral gene delivery vectors which shown promise in mouse models (Maharaj et al., 2014; Smith et al., 2007). Physical delivery methods, such as electroporation and sonoporation, destabilize membranes as a mechanism for introducing genetic material (Figure 1c). Importantly, none of these methods must necessarily be used in isolation and this study includes examples of combining different methods, especially chemical and physical, to enhance gene delivery in vitro and in vivo.
FIGURE 1.
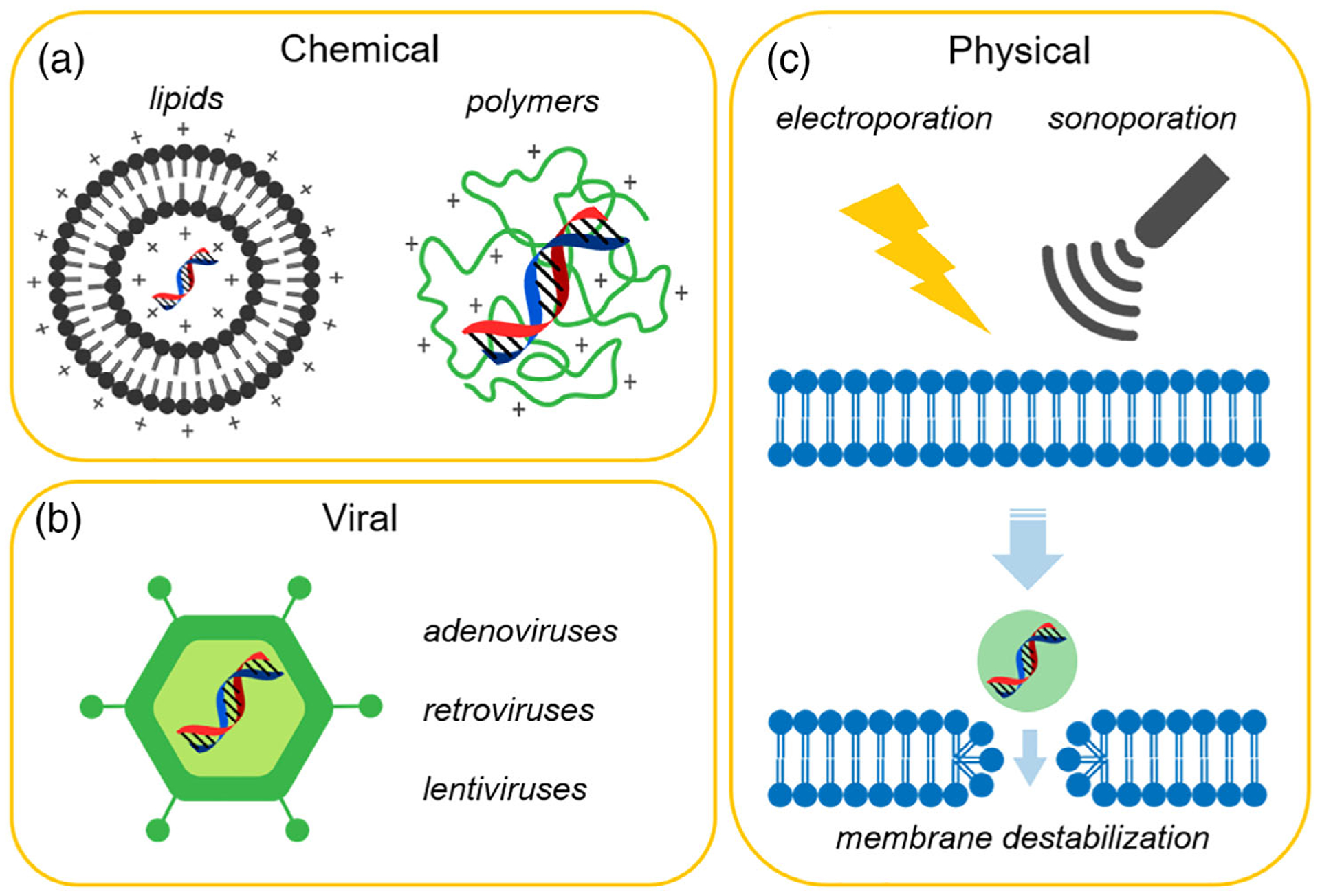
Summary of gene delivery approaches (viral, physical, and chemical): (a) chemical systems involve cationic lipids or polymers which complex negatively charged nucleic acids; (b) biological systems utilize deactivated viral vectors; and (c) physical methods, such as electroporation and sonoporation, create temporary pores in the cell membrane using electronic pulses or ultrasound
2 |. GENE DELIVERY: FROM VIRAL VECTORS TO POLYMER AND NANOSCALE SYSTEMS
2.1 |. Viral vectors
Performing gene delivery with deactivated or nonreplicating viral vectors comprises about two-thirds of clinical trials (Ginn, Amaya, Alexander, Edelstein, & Abedi, 2018), with the selection of any particular viral vector depending on the therapeutic target. For example, the frequently employed adenovirus serotype 5 (Ad5) vectors can target either dividing or nondividing cells. Although Ad5 viruses are stable and easy to manipulate genetically, their immunogenicity hinders clinical translation. Retroviruses and lentiviruses can integrate their genome into host cells, resulting in long-term transgene expression. However, retroviruses can only transfect actively dividing cells, and thus are precluded from targeting nondividing cells (e.g., in brain tissue). Moreover, retroviral and lentiviral vectors are costly to manufacture and less stable as recombinant vectors than Ad5, hindering reproducibility of gene transfer.
Innate and adaptive immune responses induced by viral vectors further limit their efficacy. Thus, many efforts focus on masking immunogenicity by covalent attachment of synthetic polymers, such as poly(ethylene glycol) (PEG) and poly-N-(2-hydroxypropyl) methacrylamide poly(HPMA). For example, when Ad5 was coated with HPMA-oligolysine copolymers, higher transduction and cell viability was observed relative to unmodified virus (C. H. K. Wang et al., 2011). Such polymer-virus hybrids can generate stable, sustained gene expression, and transfect nondividing cells (Ramsey, Vu, & Pack, 2010). Unfortunately, the reduction in side-effects coincides with an undesirably large reduction in transfection efficiency, an unsolved tradeoff that can only be overcome with greater understanding of fundamental structure-property relationships of synthetically modified biological structures.
While this study focuses on soft materials and polymers, we note that metal and semiconductor NPs have been examined extensively in gene delivery, with Au NPs proving especially popular for their amenability to delivery, diagnostics, and tailorable size and surface chemistry. Cationic Au NPs (functionalized with amines or amino acids) readily complex electrostatically to DNA and RNA, while the robust Au-sulfur interactions allow for attachment of thiolated nucleic acids to NP surfaces. In one example, Mirkin and coworkers prepared polyvalent DNA-functionalized AuNPs using thiol-modified DNA, which resulted in significantly lower immune response in RAW 264.7 and HeLa cells relative to lipid vectors (Massich et al., 2009). Lipid- and polymer-coated Au NPs have likewise shown promise as gene delivery vectors (Du et al., 2017; Song, Du, Sun, Zhang, & Wang, 2010). Recent studies on NP-stabilized capsules, involving arginine-functionalized Au NPs and siRNA, enable direct cytosolic delivery, with the hydrophobic fluid core allowing encapsulation of lipophilic drugs in codelivery mechanisms (Hardie et al., 2016; Y. Jiang et al., 2015).
2.2 |. Physical gene delivery methods
The search for reproducibly efficient gene transfer to mammalian cells led to the exciting discovery of electroporation (i.e., electropermeabilization) in the 1980s (Neumann, Schaefer-Ridder, Wang, & Hofschneider, 1982). Electroporation utilizes a pulsed electric field to introduce DNA into cells, exploiting the weak interactions of lipid bilayers to create membrane pores. Electroporation has since been applied extensively in gene transfer, DNA vaccination, and drug delivery, such as in the early work of Titomirov on electroporation-induced gene (electrogene) transfer in mice (Titomirov, Sukharev, & Kistanova, 1991). Subsequent studies expanded the technique to numerous targets, including cartilage, kidney, prostate, testis, and brain tissue. Additional applications have included DNA delivery for treating cancer and arthritis, and for promoting tissue regeneration (Yarmush, Golberg, Sersa, Kotnik, & Miklavcic, 2014). Major ongoing challenges in electrogene transfer include variable transfection efficiency in different tissues and a lack of targeting. In another physical approach, sonoporation generates pores via ultrasound, exploiting the weak interactions of the lipid bilayers to insert pores into the plasma membrane by acoustic streaming or the unidirectional movement of fluid that is mediated by ultrasound. Additionally, sonoporation can facilitate local transport across more complex biological structures, such as skeletal muscle cells, vasculature, and cells residing within solid tumors (Delalande, Kotopoulis, Postema, Midoux, & Pichon, 2013; Mullick Chowdhury, Lee, & Willmann, 2017). Despite the many advantages of the physical approaches described, major unsolved challenges include variable efficiency levels in different tissues, and, by default, a lack of targeting. Complexation of DNA with a variety of targeted carriers, including microbubbles, polymers, or lipids, holds promise for promoting targeting to cells or intracellular locations. In later sections, we will describe the combination of physical methods with polymers and targeting strategies.
2.3 |. “Polyplexes” as designer, macromolecular gene delivery vectors
Nonviral vectors prepared from polymers, liposomes, or other nanoscale structures offer routes to overcome the drawbacks of viral vectors. In liposomes, bilayer lipid assembly exposes cationic head groups to the external aqueous environment (Bhattacharya & Bajaj, 2009). Genetic material may be encapsulated in the inner aqueous environment or complexed electrostatically to the cationic surface; in either case, the lipid vectors are somewhat limited by issues associated with reproducibility, scale up production and in vivo stability (Nayerossadat, Maedeh, & Ali, 2012; Nordling-David & Golomb, 2013; Tros de Ilarduya, Sun, & Düzgüneş, 2010). Thus, polymers present potential advantages, as they exhibit longer circulation times, and their cytotoxicity can be decreased by the inclusion of structures to promote biocompatibility, such as PEG (Yang et al., 2012). Moreover, a major advantage of polymers is their tunable chemical structure and architecture, permitting designer materials preparation tailored to specific diseases.
Synthetic polycations in gene therapy research are typically amine-rich structures with pKa values that produce positive charge under physiological conditions. Especially prominent examples include polyethyleneimine (PEI), polypeptides, and poly(beta-amino esters), each of which complex negatively charged DNA or RNA into nanoscale “polyplexes” suitable for in vivo experiments (Xu, Wiehle, Roth, & Cristiano, 1998). Figure 2 illustrates polymer-based gene delivery, including the sequential steps of cell entry, endosomal escape, and nuclear entry. Despite the promising attributes of polyplex delivery, cytotoxic effects result in permanent cell membrane damage, undesirable mitochondrial interactions, and nuclear membrane permeabilization (Grandinetti, Ingle, & Reineke, 2011; Grandinetti, Smith, & Reineke, 2012; Monnery et al., 2017). As a result, polymer chemists and biologists increasingly collaborate to optimize the capabilities of synthetic polymers; significant advances emerging from these partnerships include novel polymer designs for polyplex formation, targeted systems with enhanced efficacy, and advanced synthetic constructs with improved stability to proteins and erythrocytes in blood.
FIGURE 2.
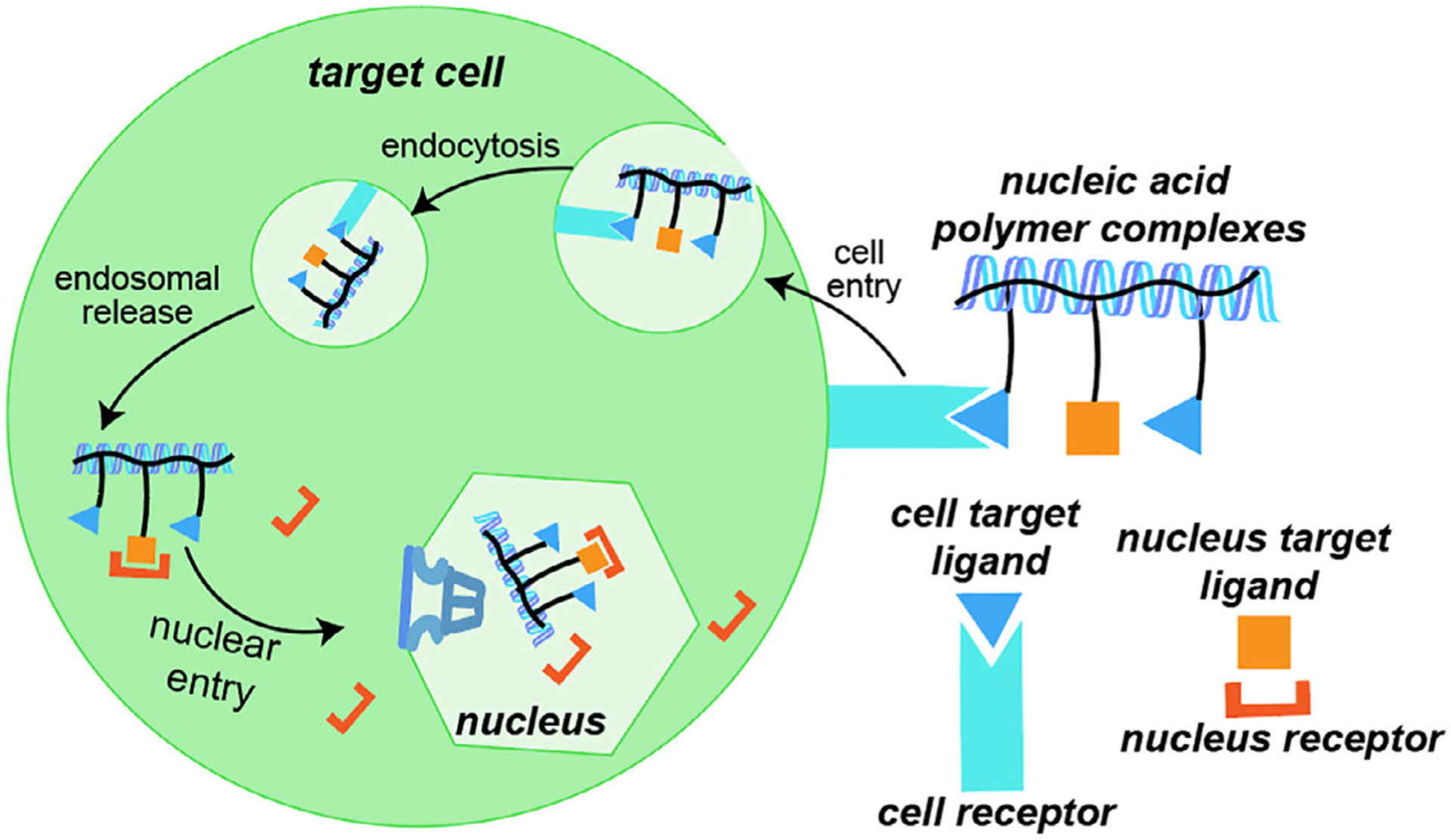
Schematic diagram of gene delivery using polyplexes, with steps including cell entry, lysosomal escape, and nuclear entry
PEI and poly-L-lysine (PLL), shown in Figure 3, represent amine-rich polymers that are appealing for their commercial availability and cationic character under physiological conditions (Boussif et al., 1995; Olins, Olins, & von Hippel, 1967). The relatively high transfection efficiency of PEI-based polyplexes is attributed in part to the so-called “proton sponge effect” (Behr, 1997); following endocytosis, the acidity of the endosomal microenvironment drives PEI to become increasingly cationic, triggering counter ion influx, osmotic pressure increase, endosomal rupture, and DNA release into the cytosol. Notably, the ability to alter the fundamental features of polymers influences gene therapy outcomes. PEI molecular weight and architecture appear to impact transfection efficiency, loading capacity, and cytotoxicity. Reports on polyplexes formed from higher molecular weight PEI show both greater transfection efficiencies and cytotoxicity (Godbey, Wu, & Mikos, 1998; Monnery et al., 2017), an outcome that typifies a long-standing problem in the field. Polyplexes prepared from branched PEI (obtained by ring-opening polymerization of aziridine) (Thomas et al., 2003) showed higher uptake than those from linear PEI (obtained by the hydrolysis of poly(2-oxazoline)); however, after endosomal escape, the polyplexes formed from linear PEI decomplex more easily than the branched PEI structures (Dai, Gjetting, Mattebjerg, Wu, & Andresen, 2011; Itaka et al., 2004). A study in A549 cells showed branched PEI to have higher cytotoxicity because of its higher pKa, which facilitated phospholipid hydrolysis and resultant cell membrane disruption (Grandinetti et al., 2011). Simulations seek to elucidate such differences in polymer-DNA binding modes as a function of polymer molecular weight, architecture, and chain flexibility; specifically, simulations described linear PEI interacting with DNA in a “cord-like” fashion that prevents binding to other DNA molecules and thus promotes release. In contrast, branched PEI-DNA polyplexes were bead-like, permitting the multiple DNA binding and aggregation, which results in more stable polyplexes and favorable cell uptake (Sun, Tang, & Uludağ, 2012).
FIGURE 3.
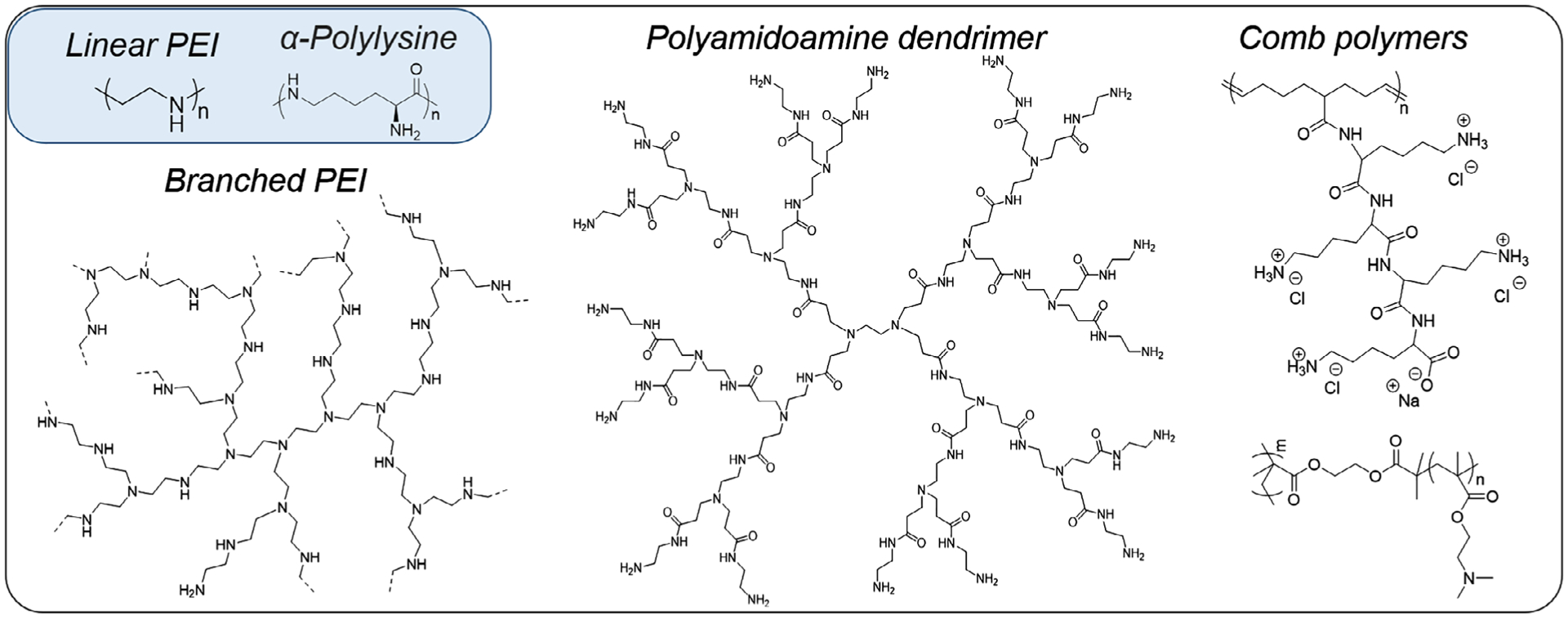
Branched PEI, poly(amidoamine) dendrimers, and comb polymers represent examples of polymer vectors with tunable nucleic acid binding and targeting capacity. Upper-left: chemical structures of linear polyethyleneimine (PEI) and poly-l-lysine, two widely used polymers in gene delivery research
The exclusively primary amines of PLL contrast that of PEI and consequently PLL-based polyplexes are thought to not participate in a proton-sponge effect, resulting in less efficient transfection; nonetheless, the relatively low cytotoxicity of PLL drives interest in improving its transfection performance (Itaka et al., 2004; Yamagata et al., 2007). Such efforts mainly comprise using small molecule additives in conjunction with PLL, and/or covalent modification of PLL. For example, transfection of PLL-based polyplexes is improved in the presence of chloroquine, the buffering capacity of which inhibits degradation of endocytosed PLL/DNA by neutralizing the acidic endosomal compartments (Erbacher, Roche, Monsigny, & Midoux, 1996). In other approaches, conjugating histidine or arginine to PLL promoted endosomal escape and enhanced transfection, likely due to advantageous pH buffering (Fang et al., 2018; Kodama et al., 2015). Covalent attachment of nuclear localization signal (NLS) peptides also improved PLL-based transfection due to recognition by cellular nuclear import machinery and importins (Chan, Senden, & Jans, 2000). Notably, reconfiguration of PLL into a comb polymer structure, composed of a hydrophobic polyolefin backbone and oligolysine pendent groups, exhibited both high cell viability and high transfection efficiency (Breitenkamp & Emrick, 2008). These comb polymers (Figure 3, upper right) were prepared by ring-opening metathesis polymerization of oligolysine-substituted cyclooctene monomers and formed stable polyplexes which, when tested in transfection with multiple cell lines (e.g., C2C12, SKOV3, and HeLa), outperformed many commercial reagents for efficiency and were especially impressive for their low cytotoxicity (Breitenkamp & Emrick, 2008; Parelkar, Chan-eng, & Emrick, 2011).
Stimulations by Jayaraman and coworkers showed the polymers with pendant oligolysines to have a lower binding free energy to DNA relative to linear PLL, due in part to the hydrophobic backbone that constrained those interactions (Elder, Emrick, & Jayaraman, 2011). Current macromolecular targets include incorporating zwitterionic components into polyplexes to further reduce cytotoxicity and enhance colloidal stability (Ghobadi et al., 2016). Additional comb polymer studies by Pun and coworkers (Olden, Cheng, Yu, & Pun, 2018) showed that poly(2-dimethylaminoethyl methacrylate) (pDMAEMA)-grafted structures (Figure 3, lower right) produced high transfection efficiency and cell viability for human T-cell transfection. We will later describe successful in vivo combination of comb polymer structures with physical delivery techniques such as sonoporation.
Other examples of synthetic polymers used in gene therapy include dendritic structures and functional, biomimicking polymers. Commercialized poly(amidoamine) (PAMAM) dendrimers have been especially prominent, as synthesized originally by Tomalia et al. (1985) and studied by numerous groups in gene therapy. The dendritic “generations” synthesized by iterative organic coupling methods allow detailed study of structure-property relationships, leading to implementation of these polymers as components of commercial transfection kits. For example, the “DEP docetaxel“ developed by Starpharma, a PEGylated PLL dendrimer, was noted for its safety and sustained release relative to conventional docetaxel in a Phase 1 trial (Taxotere) (Palmerston Mendes, Pan, & Torchilin, 2017; Starpharma, 2018). Typical of synthetic polymers, PAMAM dendrimers may be modified to reduce cytotoxicity, for example, by PEGylation of terminal amines (Luo, Haverstick, Belcheva, Han, & Saltzman, 2002). Creative examples of functional linear polymers for transfection include trehalose-containing PEI (Figure 4a), prepared by Reineke and coworkers, which successfully delivered pDNA to HeLa cells (Srinivasachari et al., 2006). In another example, poly(glycoamidoamine)s (Figure 4b), consisting of linear chains of sugar units and PEI connected via triazole linkages, was nontoxic in pDNA delivery experiments conducted in multiple mammalian cell lines, including BHK-21, HeLa, and HepG2 (Liu & Reineke, 2005). Hammond (2012) applied electrostatic layer-by-layer (LbL) assembly techniques to encapsulate nucleic acids and achieve controlled release and targeted delivery. Enhanced antitumor efficacy was achieved in mice with nonsmall cell lung cancer by using a combination RNA-chemotherapy built on LbL NPs, in which cisplatin was encapsulated in liposomes and RNA is coated as the electrolyte layer (L. Gu, Deng, Roy, & Hammond, 2017). The outermost layer of LbL NPs is then available for functionalization with multiple targeting ligands to improve the treatment efficacy (Choi et al., 2019).
FIGURE 4.
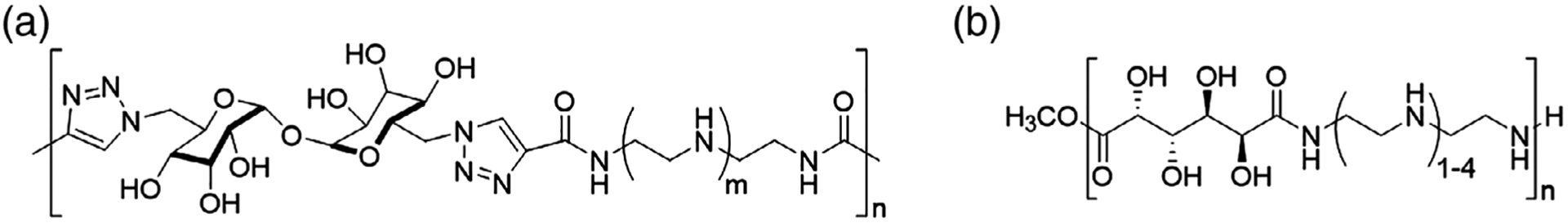
Chemical structures of (a) trehalose-triazole “click” polymer (Srinivasachari, Liu, Zhang, Prevette, & Reineke, 2006) and (b) poly(glycoamidoamine) (Jiang, Lodge, & Reineke, 2018)
From the standpoint of structure-property relationships, rationally designed block copolymers may improve the colloidal stability of polyplexes and their resultant efficacy in transfection. As shown in Figure 5, the amphiphilic poly((2-dimethylamino)ethyl methacrylate)-block-poly(n-butyl methacrylate) formed “beads-on-a-string,” and upon incorporation of PEG blocks these so-called “micelleplexes” became less compact and colloidal stability improved with PEG chain length (Y. Jiang, Lodge, et al., 2018). Poly (2-methacrylamido-2-deoxy glucopyranose) and the zwitterionic poly(2-methacryloyloxyethyl phosphorylcholine) (PMPC) were also tested as the hydrophilic blocks, with the PMPC-containing structures showing comparable colloidal stability, higher cell uptake, and enhanced gene silencing relative to the PEGylated polyplexes (Buckwalter, Sizovs, Ingle, & Reineke, 2012; Jackson et al., 2017). Isothermal titration calorimetry evaluates DNA-polycation binding thermodynamics by measuring the enthalpy change (heat adsorption) associated with complexation, which provides quantitative insight into binding mechanisms and optimal N/P ratios for polymer/DNA pairs. Reineke and coworkers used ITC to probe complexation strength of DNA and poly(2-deoxy-2-methacrylamido glucopyranose)-block-poly(N-(2-aminoethyl) methacrylamide) block copolymers, revealing enthalpy changes of 100–400 cal per mole of amine groups at optimal N/P ratios (Jung, Lodge, & Reineke, 2017).
FIGURE 5.
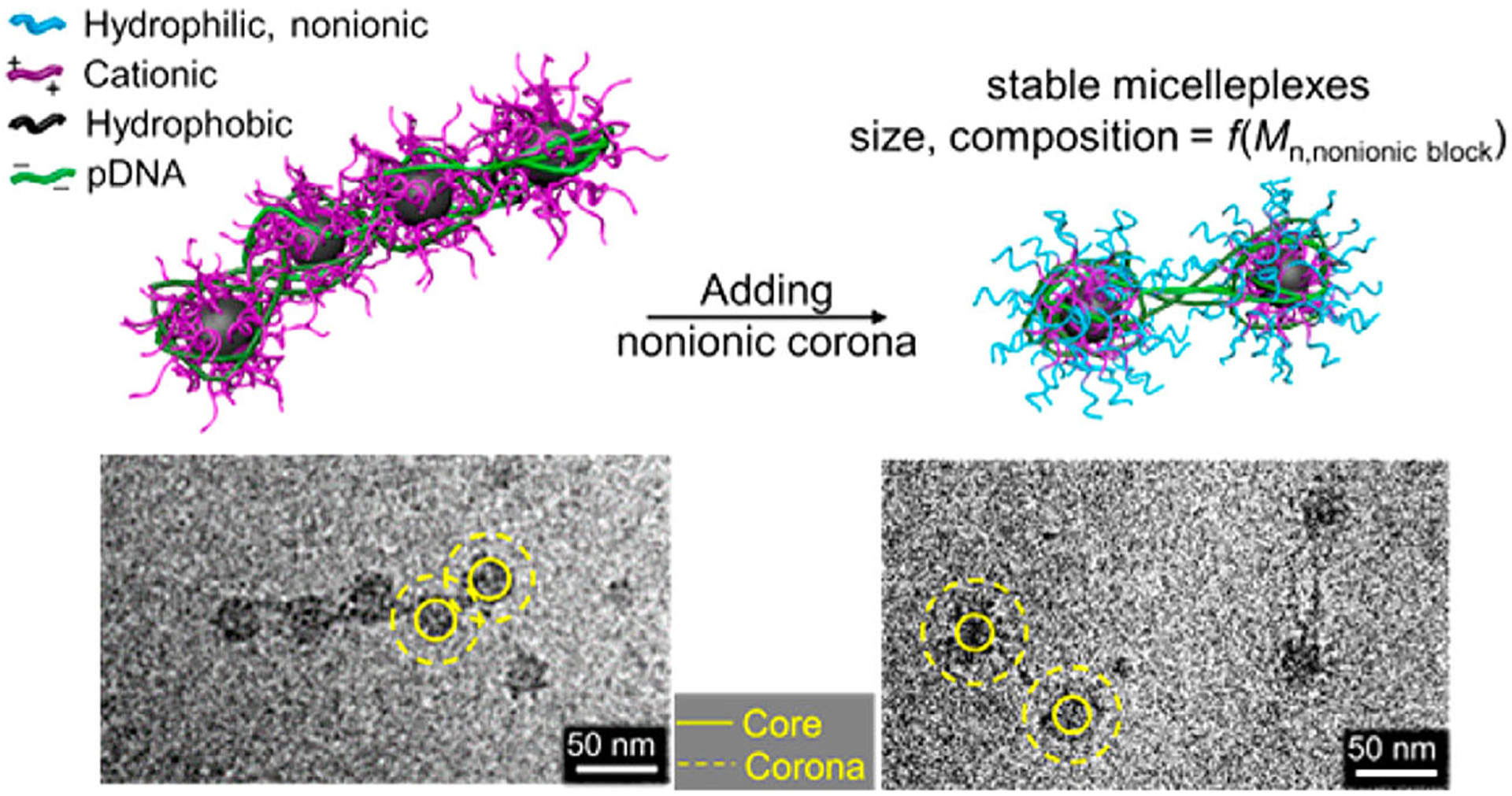
Cryo-TEM images of self-assembled micelleplexes with different poly(ethylene glycol) corona length (Jiang, Lodge, et al., 2018)
Synthetic polymers with conjugated backbones offer additional opportunities in gene delivery, as their intrinsic fluorescence allows for in vitro and in vivo polyplex tracking without the need for additional (and potentially cytotoxic) organic chromophores. Conjugated polymers (CPs) have tunable absorption and emission profiles that enable sensitive measurements at low concentration (Zhu, Liu, Yang, Lv, & Wang, 2012). While CP design typically is intended for optoelectronic devices, water-soluble versions have emerged as versatile, multifunctional polynucleotide carriers. For example, the polyfluorene (PF)-containing structure in Figure 6a, displaying quaternary ammonium groups, was fluorescent in water and capable of binding electrostatically with dsDNA, as reported by Yu et al. (2008). The range of CPs studied in gene delivery now includes poly(phenylene vinylene) (PPV), poly(phenylene ethynylene) (PPE), and a variety of copolymer structures (H. Chen et al., 2018; X. Feng et al., 2012; X. Feng, Tang, Duan, Liu, & Wang, 2010; R. Jiang et al., 2013; Moon, Mendez, Kim, & Kaur, 2011; G. Wang et al., 2013; M. Yin et al., 2008; Yu et al., 2008). In 2013, Jiang et al. prepared NPs from brush-like PFs with flexible cationic pendent moieties (denoted PFNBr) that exhibited high complexation efficiency and stabilization of siRNA (i.e., >30 mol siRNA per mole of polymer). The blue emission of PFNBr allowed for fluorescence-based tracking into human pancreatic cancer cells (PANC-1), while decomplexation of Cy3-labeled siRNA was visualized directly by fluorescence resonance energy transfer experiments (R. Jiang et al., 2013). Additional creative CP designs include a built-in endosomal escape mechanism by light-induced formation of reactive oxygen species (ROS; S. Li et al., 2016; Zhang et al., 2018). For example, Li et al. prepared cationic PPVs for siRNA delivery, finding that in HeLa cells the fluorescent PPV/siRNA complexes produced ROS upon exposure to 400–800 nm light, which then triggered endosome membrane disruption and gene silencing of siRNA (S. Li et al., 2016). While CPs for gene delivery remain relatively unexplored compared to other polymeric carriers, their innate fluorescence and potential for endosomal escape mechanisms make them promising vectors for further research.
FIGURE 6.
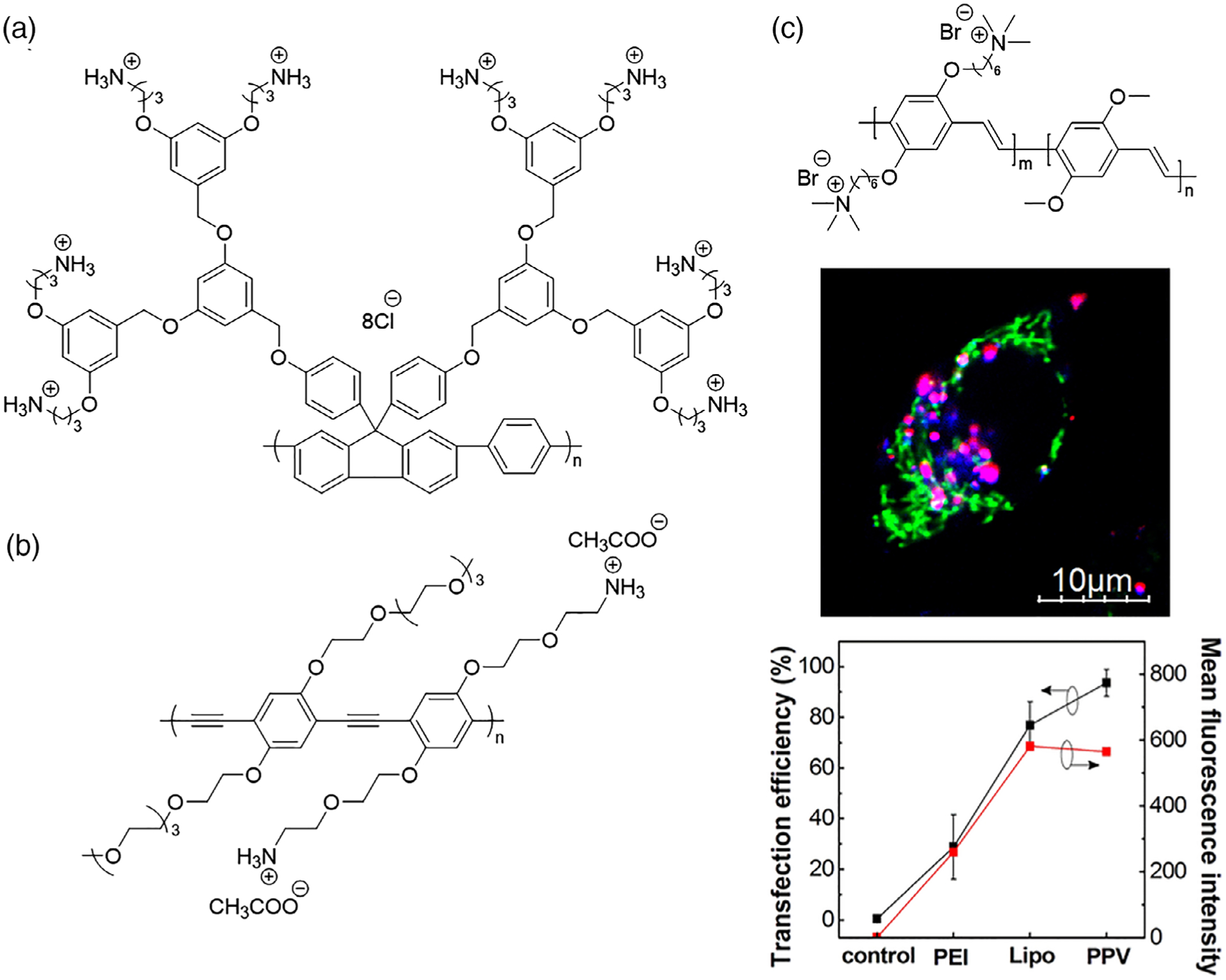
Examples of cationic conjugated polymers used for gene transfection: (a) polyfluorene (Yu, Liu, & Wang, 2008); (b) poly(phenylene ethynylene) (Jiang et al., 2013); and (c) poly(phenylene vinylene) (Li et al., 2016); Fluorescence microscopy shows colocalization of PPV/Cy5-labeled siRNA polyplex (red/green) with endosomes (blue; Lysotracker). Transfection efficiency of the PPV/Cy5-labeled siRNA polyplex, measured by fluorescence-activated cell sorting (FACS), exceeded that of PEI and Lipofectamine (Li et al., 2016)
3 |. TARGETING STRATEGIES
Functional polymers offer opportunities to increase the efficiency and specificity of transfection. The following discussion centers on directing polymers and polyplexes to specific cells and their surface receptors, as well as localization to cellular compartments, including the nucleus. Nonviral vectors, especially cationic polymers, have emerged as attractive alternatives to viral vectors due to their versatile design, low cytotoxicity, and controlled payload release. Despite some advantages of nonviral vectors described so far, limitations in transfection efficiency and off-target accumulation must be overcome; as such, targeting moieties are integrated into therapeutic constructs with increasing frequency.
3.1 |. Functionalization and targeting of cationic polymers
Synthetic delivery systems are especially exciting when utilized in combination therapy or codelivery with antitumor drugs and genes. Key features of polymer-mediated gene and drug delivery include the potential to minimize off-target effects and immune response as well as achieve greater circulation stability and tissue permeability. Early targeting efforts in gene delivery used a peptide-nucleic acid (PNA) motif to tether the targeting ligand transferrin (Tf) to a Luciferase reporter plasmid (pLuc). Because PNA is composed of DNA bases within an uncharged polyamide backbone, it can bind to specific sequences in double stranded DNA to form a stable triplex, and is easily modified with targeting ligands. However, challenges to PNA conjugate systems include poor transfection efficiency, likely due to the formation of charge-neutral complexes that bind weakly to cells. This limitation can be overcome by introducing polycations, such as PEI, to dramatically enhance transfection efficiency in numerous delivery designs.
Surface-shielding modules are extensively used in gene therapy to target therapeutic genes to distant tumors. For example, Kircheis et al. (2001) utilized a ligand-modified polycation module to shield the surface of PEI/pLuc polyplexes and target the Tf receptor, as shown in Figure 7a. Intravenous delivery of the targeted pLuc complexes resulted in 100- to 500-fold greater plasmid accumulation in mice bearing subcutaneous Neuro2a tumors, and lung toxicity was significantly lower when employing low molecular weight PEI. In a follow-up study, this Tf-PEI system achieved targeted delivery of pDNA encoding for tumor necrosis factor-α (TNF-α, a cytokine with potent antitumor activity; Kircheis et al., 2002). The study reported a significant increase in tumor expression of TNF-α and a significant reduction of tumor growth in targeted TNF-α compared to control groups following systemic application via tail vein of A/J mice bearing subcutaneous Neuro2a tumors. Importantly, this delivery system resulted in insignificant off-target expression of the therapeutic gene (Kircheis et al., 2002). These results were successfully replicated in a MethA fibrocarcinoma preclinical model (Kircheis et al., 2002). Intravenous injection of this Tf-shielded PEI/TNF-α system in BALB/c mice subcutaneously bearing MethA fibrocarcinoma resulted in complete tumor regression in 60% of treated mice and protection from tumor recurrence in rechallenge experiments.
FIGURE 7.
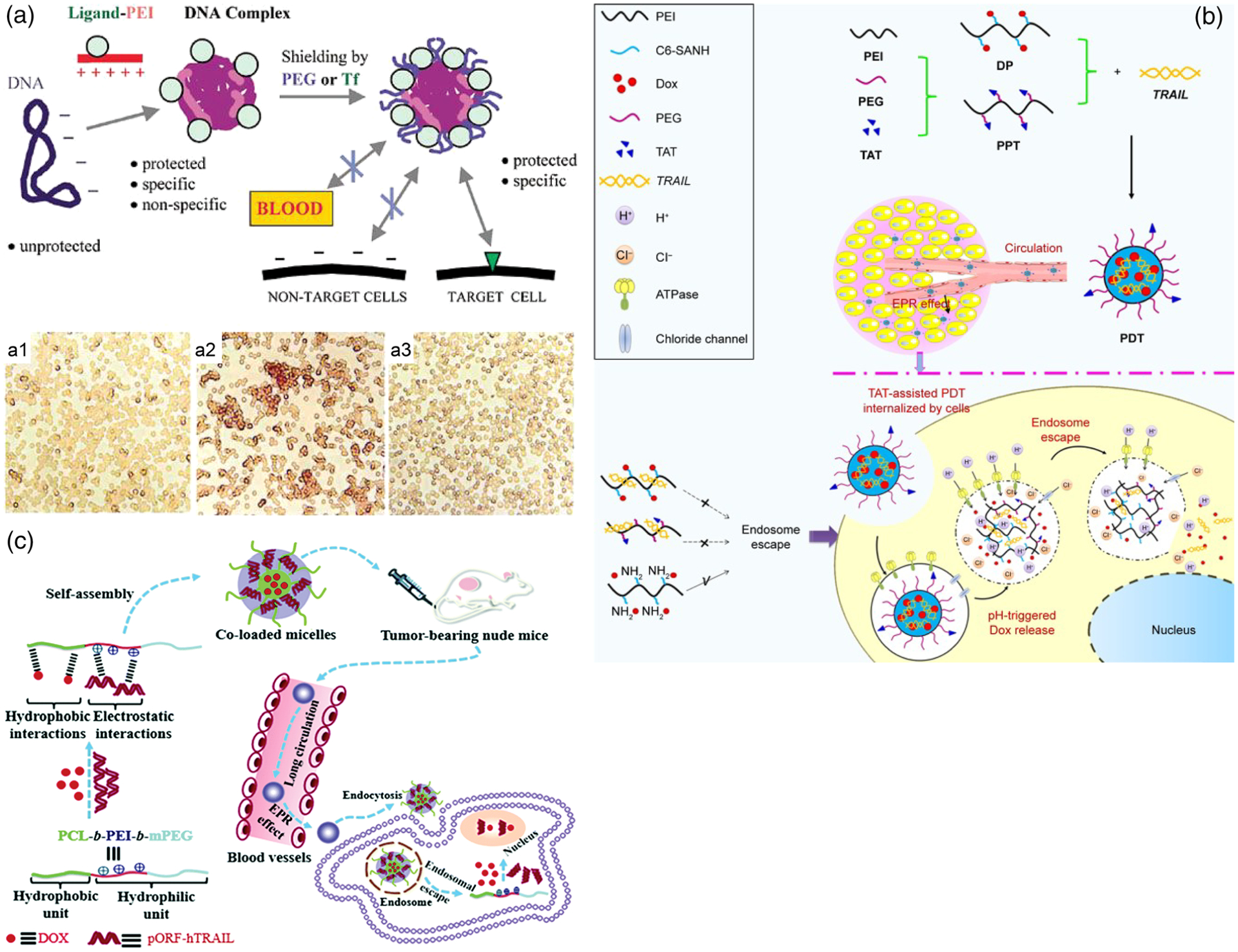
Targeted gene delivery modalities. (a) Shielding with PEG or Tf can enhance targeting specificity of polyplexes. Surface shielding of polyplexes reduces nonspecific interactions (a1), while nonshielded polyplexes induce aggregation of erythrocytes (a2), suggesting increased nonspecific interactions. Nontreated control erythrocytes are shown in (a3) (Kircheis, Wightman, Kursa, Ostermann, & Wagner, 2002). (b) Synthesis of the PEI-PEG-TAT system for delivery of plasmid DNA (pDNA) encoding TRAIL as well as the drug DOX (Jiang et al., 2017). (c) Codelivery of the drug DOX and TRAIL-encoding plasmid DNA via redox sensitive Tf-targeted micelles, and hypothesized route following intravenous injection of drug/pDNA-loaded micelles in a syngeneic tumor model (Feng et al., 2018)
Further improvements to PEI-based polyplexes include self-assembling and redox-sensitive micelle-based systems. For example, D. Jiang et al. (2017) reported effective in vitro and in vivo codelivery of Doxorubicin (DOX) and pDNA encoding TNF-related apoptosis inducing ligand (TRAIL) via a redox-sensitive PEI-PEG system, shown in Figure 7b. Successful improvements to targeted systems included complexes functionalized with the cell-penetrating peptide TAT (Cheng, Tietjen, Saucier-Sawyer, & Saltzman, 2015; Han et al., 2015; Koren, Apte, Jani, & Torchilin, 2012; Peng et al., 2014). The TAT-targeted version of the PEI-PEG complexes (PPT) and pH-sensitive material (DP), when complexed with DOX—TRAIL (PDT), demonstrated synergistic antitumor effects in both HEPG2 and SKOV3 cells, as evaluated by an MTT cell viability assay with varying concentrations of Dox and TRAIL-loaded PPT, PDT, and intermediate products for DP synthesis. This combination treatment also inhibited the growth of subcutaneous H22 hepatoma tumor xenografts in BALB/c mice following intravenous injection and without any significant effect on body weight as compared to controls (D. Jiang et al., 2017). Moreover, Feng and coworkers reported codelivery of DOX and the therapeutic gene encoding human tumor necrosis factor-related apoptosis-inducing ligand (pORF-hTRAIL) via the redox-sensitive disulfide-bridged poly(ethylene glycol-co-ethyleneimine-co-caprolactone)-SS-poly(caprolactone-co-ethyleneimine-co-ethylene glycol) (PEG-PEI-PCL-SS-PCL-PEG) polymer (Figure 7c; L. Feng et al., 2018). DOX/TRAIL codelivery was augmented when Tf receptors (TfR) were targeted by utilizing oligopeptide HAIYAPRH (Bertrand, Wu, Xu, Kamaly, & Farokhzad, 2014; Oh, Kim, Singh, Lai, & Sasaki, 2009). Most notably, intravenous DOX/TRAIL codelivery with this redox-sensitive polymer in BALB/c nude mice bearing subcutaneous MCF-7 breast tumors significantly inhibited tumor growth relative to single modality treatments (L. Feng et al., 2018).
Gene delivery by PEI complexes has also been examined in preclinical studies in which sodium iodide symporter (NIS) is “repurposed” for targeting and treating nonthyroid tumors following gene transfer. The transmembrane protein NIS regulates iodide concentration in the thyroid and was originally utilized in the diagnosis and therapeutic application of radioiodine in treating thyroid cancer, remaining for >70 years as the gold standard in treating this cancer type. While NIS gene therapy pre-dominantly employs viral vectors (Hingorani et al., 2010; Spitzweg et al., 2001), a number of studies appear promising for targeting nonthyroid tumors with PEI/NIS complexes. For example, Klutz et al. utilized GE11, a peptide ligand for the epidermal growth factor receptor (EGFR) discovered by phage display (Z. Li et al., 2005), to target PEG-shielded linear PEI/NIS (LPEI-PEG-GE11/NIS) polyplexes to human hepatocellular carcinoma (HCC) cells. Initial in vitro validation studies showed a 22-fold increase in iodide uptake in HuH7 (a HCC cell line) transfected with LPEI-PEG-GE11/NIS relative to LPEI-PEG-GE11/antisense-NIS control (Klutz et al., 2011). And since NIS serves the dual role of a theranostics agent, that is, as a cancer therapeutic and a noninvasive reporter, 124I-positron emission tomography or 123I-scintigraphy imaging can detect NIS gene delivery to tumors. For instance, experiments examining NIS uptake following intravenous delivery of this EGFR targeting system in mice bearing subcutaneous HuH7 tumors showed high levels of iodide uptake in 80% of mice, with insignificant uptake in off-target organs relative to nontargeted controls (Klutz et al., 2011). Therefore, systemic gene delivery using LPEI-PEG-GE11/NIS polyplexes can promote tumor-specific uptake of iodide, thus serving as a desirable, noninvasive approach for treating metastatic cancers.
Kim, Lee, Kim, Park, and Kim (2016) reported a variation on this PEG/PEI vector motif by introducing phenylboronic acid (PBA) groups into the polymers. PBA binds to the cis-diols of sugar units in a pH-dependent manner (weaker binding at lower pH) (Springsteen & Wang, 2002; J. Yan, Springsteen, Deeter, & Wang, 2004) and exchanges for the more thermodynamically stable binding product of 1,2-diols of ribose units in adenosine triphosphate (ATP), which is abundant intracellularly. This exchange represents an intracellular controlled release mechanism of the gene payload. PBA can be repurposed as a targeting moiety due to its selective binding to sialylated proteins, which are typically overexpressed at the surface of cancer cells. This system was used to systemically deliver pDNA-encoding soluble vascular growth factor receptor (sVEGFR or sFlt-1) into a CT-26 colon cancer xenograft model (Kim et al., 2016). In animal studies, although this PBA-PEG-functionalized PEI nanoplex accumulated in off-target organs, no significant weight loss was observed and tumor size was reduced significantly relative to control groups (Kim et al., 2016). This demonstrates the effectiveness of angiogenesis inhibition via transfection with soluble VEGFR delivered by PBA-functionalized PEI/PEG polyplexes.
Other variations of PEI-based gene delivery systems include biodegradable PEI systems functionalized with phage display peptides (Lee, Lee, Kim, Park, & Kim, 2015; Markoishvili, Tsitlanadze, Katsarava, Glenn, & Sulakvelidze, 2002). For example, CPIEDRPMC (RPM) was used to target integrin α5β1 in invasive colorectal cancer (Lee et al., 2015). This study utilized PEGylated, branched PEI polymers containing disulfide bonds (IR820-SS-bPEI-PEG-RPM) to enhance transfection efficiency while maintaining low toxicity (Figure 8; Lee et al., 2015). The transfection efficiency of this integrin-targeting system was evaluated in vitro using luciferase as the reporter gene (pLuc) in human colon cancer cells. Luciferase activity was measured following plasmid delivery using either the integrin-targeted polyplex or a nontargeted polyplex (IR820-SS-bPEI-PEG) as a control. The presence of disulfides imparted biodegradability and enabled pDNA unpacking at the targeted site. Furthermore, in vivo imaging of fluorescently labeled RPM-conjugated and nonconjugated polyplexes revealed high levels of fluorescence throughout the whole animal at 30 min postinjection. After 1 day, the RPM-conjugated polyplexes showed preferential accumulation in subcutaneously growing tumors, in contrast to low accumulation at untargeted tissues. When the pLuc was delivered intravenously using this system, tumor transfection was considerably higher compared to the nontargeted vector (Figure 8b). These results suggest that therapeutic gene delivery to target cancer cells or other pathological conditions where integrins are upregulated, such as in rheumatoid arthritis (Lowin & Straub, 2011), could augment transfection levels while maintaining biocompatibility.
FIGURE 8.
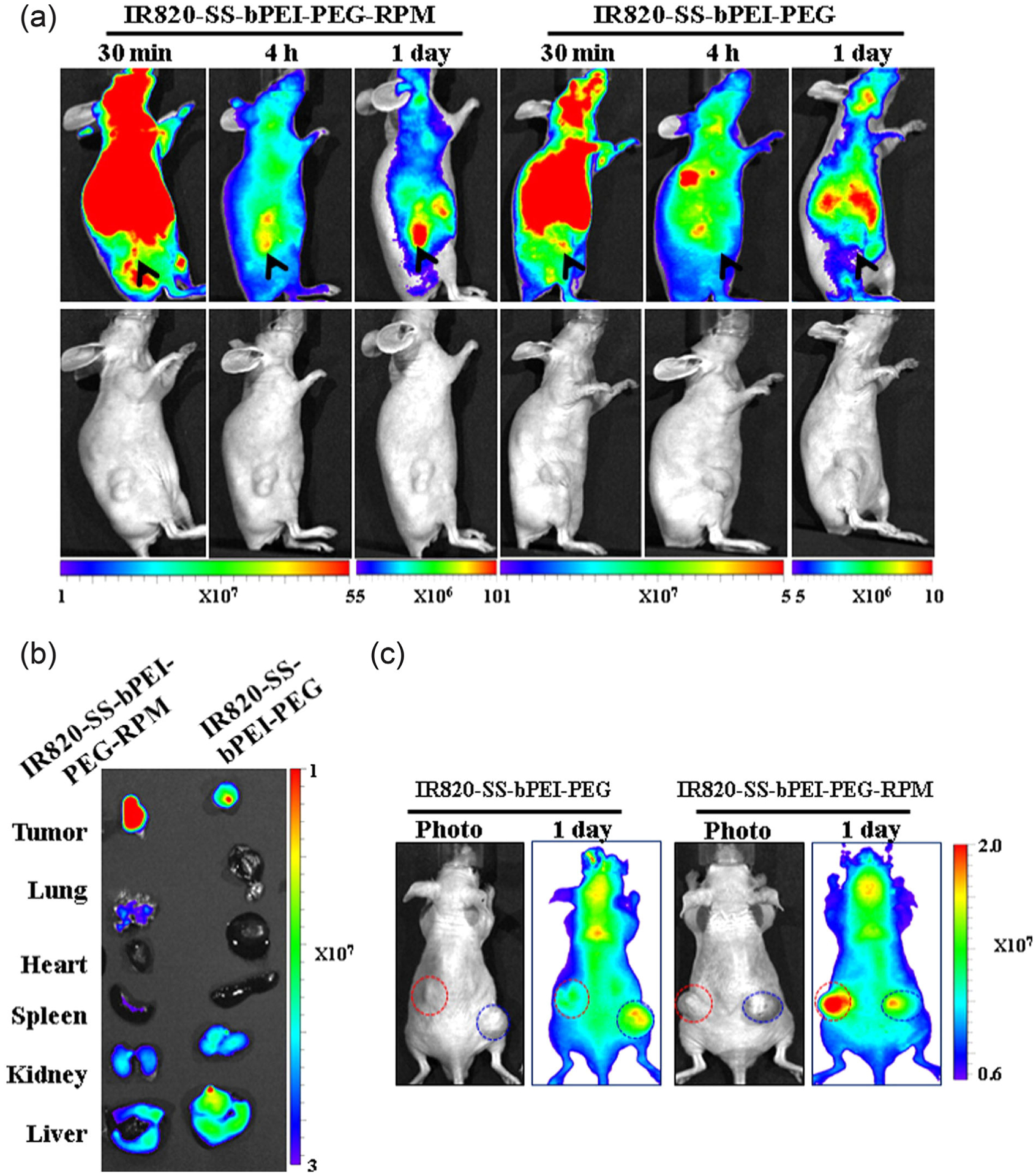
In vivo and ex vivo imaging of polyplex biodistribution. (a) in vivo time-dependent near infrared (NIR) imaging following injection with RPM-conjugated (IR820-bPEI-PEG-RPM) or nontargeted (IR820-SS-bPEI-PEG) polyplexes. (b) ex vivo images of organs collected 4 hr following intravenous injection with RPM-conjugated or nontargeted polyplexes. (c) in vivo imaging of HT-29 (RPM-positive cells, left) and HCT-116 (RPM-negative cells, right) in dual tumor bearing mice following injection with RPM-conjugated polyplexes (Lee et al., 2015)
Another important recent development uses peptides to both target polyplexes to cells and facilitate cellular entry. For instance, PEG-PEI copolymers were synthesized with targeting and cell-penetrating peptides to facilitate gene delivery to the central nervous system (CNS) (J. Wang et al., 2013). Wang et al. functionalized PEG-PEI copolymers with a C-end rule (C-endR) peptide moiety (RGERPPR) for gene delivery to glioblastoma multiforme (GBM) tumors (J. Wang et al., 2013). CendR peptides exhibit high binding affinities to neuropilin-1 (NRP-1) receptors, which are over-expressed in numerous tumors, including GBM (X. Li et al., 2011; Nasarre et al., 2010; Osada et al., 2004). C-endR peptides also serve as a tumor-penetrating motif that enhances the localized transfection efficiency of PEG-PEI (J. Wang et al., 2013). The transfection efficiency of this system was evaluated in vitro and in vivo. The reporter genes enhanced green fluorescent protein (pEGFP-N2) and luciferase (pGL4.2) were individually used to evaluate transfection efficiency in U87 glioma cells. Both in vitro systems demonstrated enhanced expression in conjunction with RGERPPR-PEG-PEI relative to a nontargeted control polymer. Transfection was enhanced approximately twofold, as determined by flow cytometry for EGFP detection and luciferase assay. In vivo validation utilized a red fluorescent protein (pDsRED) as a reporter gene in mice bearing intracranial U87 glioblastoma, and demonstrated greater than sixfold increase in fluorescence intensity compared to nontargeted control. J. Wang et al. (2013) also used an intracranial U87 glioblastoma xenograft model to demonstrate the transfection efficiency of the PEG-PEI-RGERPPR complexed with pDNA encoding for red fluorescent protein and delivered intravenously. The NRP-1 targeted polyplexes exhibited an approximately sixfold increase in brain-specific fluorescence intensity relative to nontargeted polyplexes, while naked pDNA exhibited negligible fluorescence in the brain. While this study provided evidence for the potential of RGERPPR-PEG-PEI as an effective system for the delivery of cancer therapeutics, further studies examining the toxicity and off-target accumulation of this delivery vehicle are lacking.
Collaborative work by Figueiredo and Emrick has sought to improve upon the capabilities of PLL and PEI with new synthetic polymer designs. For gene delivery to tumors or skeletal muscle, we employed comb polymer architectures composed of a polyolefin backbone and NLS oligopeptides (Figure 9) as pendent groups for pDNA complexation and cell transfection. For example, polymers with PKKKRKV heptapeptide pendent groups were prepared with two orientations, one with the proline residue connected to the polymer backbone (NLS) and the other with the valine connected to the backbone (reverse NLS, rNLS). These novel NLS-polycations promoted superior gene delivery in vitro relative to commercial reagents, including PEI and Lipofectamine 2000 (Parelkar et al., 2011, 2014). Notably, the NLS orientation influenced transfection performance, with the rNLS structures proving superior. Importantly, this advantageous pDNA complexation and nuclear localization found in cell culture translated to in vivo experiments in combination with intramuscular ultrasound-mediated delivery (Figueiredo Neto, Letteri, Chan-Seng, Emrick, & Figueiredo, 2015; Parelkar et al., 2014), producing a high level of reporter gene expression in mice and demonstrating therapeutic promise in vivo against bone-metastatic prostate tumors (Zolochevska et al., 2013).
FIGURE 9.
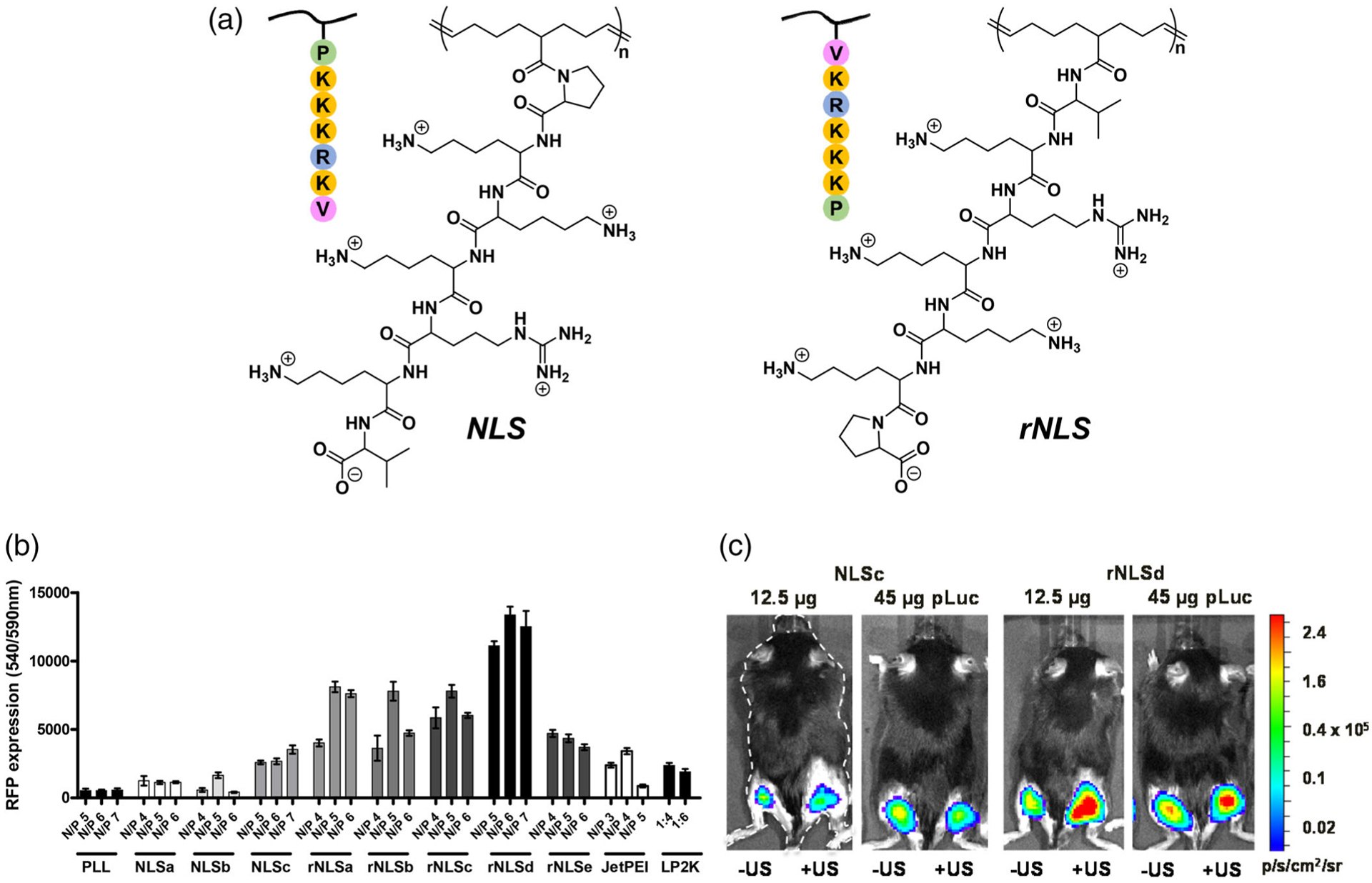
(a) Structures of NLS and rNLS-comb polymers. (b) in vitro transfection performance of polyplexes in SKOV3 cells. (c) in vivo intramuscular gene delivery (with or without ultrasound mediation) in mice by NLS- and rNLS-based polyplexes (Parelkar et al., 2014)
3.2 |. Functional polyamidoamine “dendriplexes”
The well-defined architecture and large number of functional chain ends of dendritic macromolecules makes them useful scaffolds for targeted gene therapy. In one example, epidermal growth factor (EGF) ligands were embedded into polyamidoamine (PAMAM) dendrimers to afford self-assembled PAMAM/DNA/EGF polyplexes (J. Li et al., 2016). In vitro cytotoxicity assays demonstrated that incorporation of EGF-PAMAM complexes reduced cytotoxicity with greater incorporation of EGF relative to plain PAMAM dendriplexes. Similarly, Li et al. observed enhanced targeting to EGFP+ MDA-MB-231 breast tumors in vivo when transfected with a luciferase-containing plasmid via EGF-containing polyplexes (J. Li et al., 2016). In vitro cellular uptake and in vivo biodistribution of PAMAM dendrimers were examined by labeling EGF-containing and EGF-free polyplexes with the near-infrared (NIR) dye LSS670, and observing their distribution by NIR fluorescence imaging (Figure 10). Both EGF-targeted and untargeted dendriplexes accumulated in the abdomen, while significant accumulation could also be detected at the tumor implantation site of mice receiving EGF dendriplexes via tail vein injection. Tumor implantation sites were confirmed by tumor bioluminescence, but organ-specific distribution or off-target toxicity was not addressed. These findings indicate that introducing EGF to novel nanoscale vectors may be useful for in vivo targeting to cells that overexpress EGFR. As this study examined the incorporation of EGF into complexes through electrostatic interactions rather than its complexation via covalent binding to the dendrimers, it would be interesting in future studies to examine EGF covalently conjugated to dendritic structures to potentially enhance its targeting potential.
FIGURE 10.
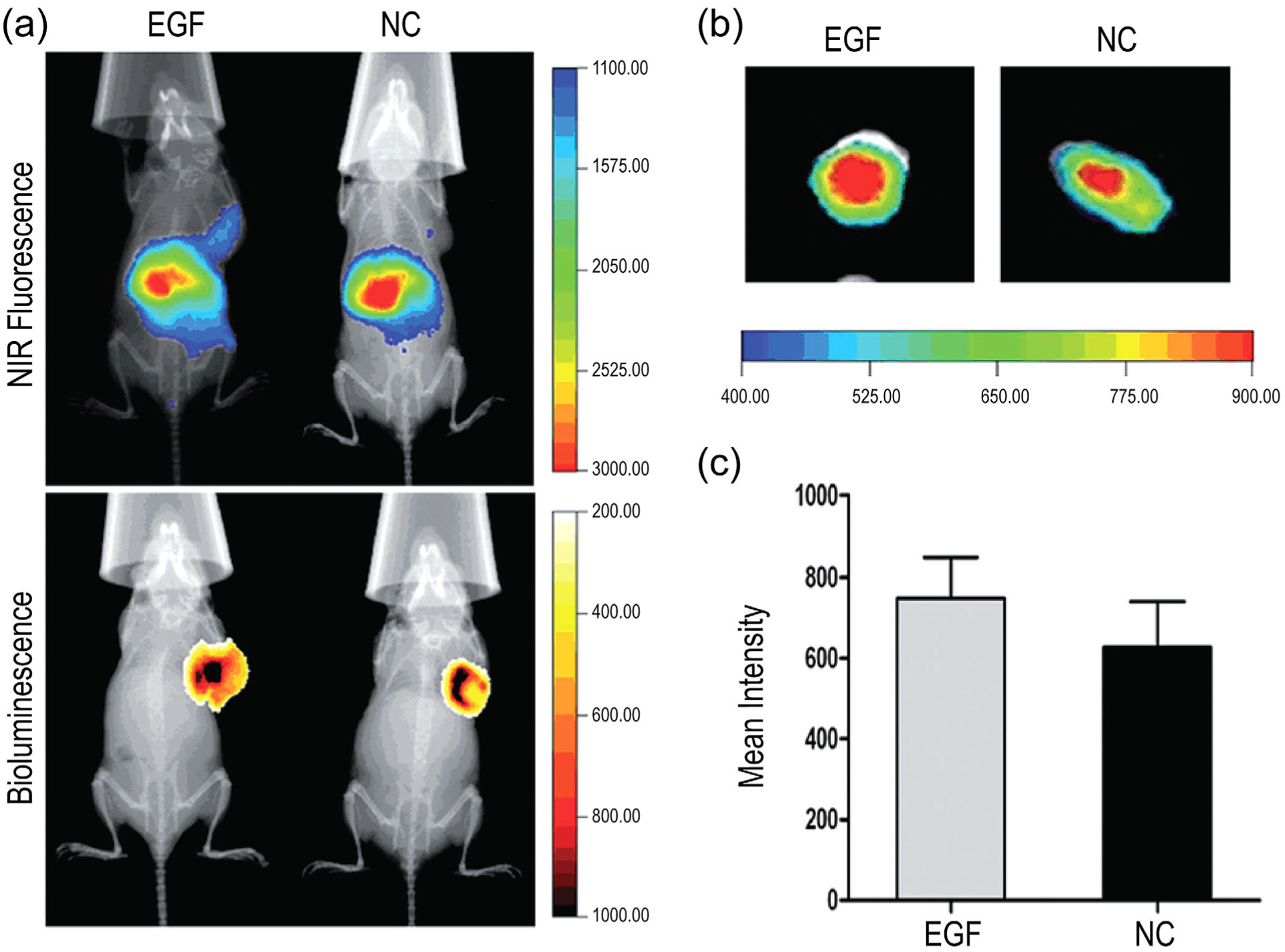
In vivo and ex vivo imaging of tumor-bearing mice treated with nonconjugated (NC) or EGF-conjugated dendriplexes (EGF). (a) Upper panel: localization of EGFR-targeted and untargeted dendrimers via near infrared (NIR) fluorescence at 2 hr posttreatment. Lower panel: luminescence imaging of the localization of breast cancer tumor cells labeled with luciferase (MDA-MB-231-Luc), implanted subcutaneously, and following administration of 150 mg/kg D-luciferin. (b) Overlay of X-ray and ex vivo NIR fluorescence images of tumors treated with NC or EGF-conjugated dendriplexes. (c) Mean ± SD ex vivo fluorescence intensity of tumors 2 hr posttreatment with NC or EGF-conjugated dendriplexes (J. Li et al., 2016)
3.3 |. Targeting with aspartamide-containing polymers
Gene delivery research continues to focus on developing stable carriers capable of condensing DNA into nanoscale structures, shielding the surface of DNA-nanoplexes to ensure retention in circulation, effective off-loading of genetic material at target sites, and efficient cellular uptake. Towards these objectives, Kataoka and coworkers developed a polyplex micelle from a self-assembling multifunctional block catiomer PEG20C, or PEG20kDa-poly [N′-[N-(2-aminoethyl)-2-aminoethyl] aspartamide]-cholesterol (PEG-PAsp(DET)-cholesteryl; Q. Chen et al., 2017; Ge et al., 2014). Hydrophobic core stabilization by the cholesteryl residues, combined with PEG-shielding, provided superior retention in the bloodstream relative to traditional PEG-PAsp(DET) complexes. Interestingly, specific formulation steps were included to ensure the elimination of minute quantities of free cationic structures that would elicit undesired toxicity. Cell viability was evaluated using the Cell Counting Kit-8 (CCK-8) in HeLa, HuH-7, or HUVEC cells following incubation with polyplex micelles (PEG20C) or control polyplexes (PEG20 and PEI) at varying ratios (Q. Chen et al., 2017). Transfection efficiencies were evaluated in vitro in HeLa or HUVEC cells using luciferase as the reporter gene, by measuring its activity in lysed cells 48 hr post-transfection (Q. Chen et al., 2017; Ge et al., 2014). Addition of a cyclic oligopeptide (cRGD) allowed targeting of ανβ3 and ανβ5 integrins (Oba et al., 2007), facilitated accumulation of micelles at the tumor site (Q. Chen et al., 2017; Ge et al., 2014), and correlated with effective antitumor activity relative to untargeted control micelles (Q. Chen et al., 2017; Ge et al., 2014). This copolymer assembly also enabled delivery of pDNA encoding the sFlt-1 gene in a BxPC3 pancreatic adenocarcinoma xenograft tumor model following intravenous injection (Q. Chen et al., 2017). Tumors treated with PEG20C/sFlt-1 exhibited approximately threefold decrease in size, while tumor vascular densities were significantly lower than control groups. This approach indicates that integrin targeting, coupled with optimized vehicle designs with free polymer exclusion, enhances the delivery of therapeutic genes while minimizing potential toxic effects.
A redox-sensitive, detachable PEG gene delivery system, also described by Kataoka, utilized a P-[Asp(DET)] polymer that promoted significantly higher in vivo gene expression of human TNF-α and antitumor effects in a pancreatic cancer mouse model following administration into the peritoneal cavity of mice (Kumagai et al., 2012; Takae et al., 2008). While this delivery system promised elimination of pancreatic tumors, it lacked targeting modalities to ensure accumulation at target tumors and minimization of any cytotoxic effects. Ping, Hu, Tang, and Li (2013) expanded upon this PEG-detachable concept in studies of fibroblast growth factor receptor-targeted β-cyclodextrin-crosslinked PEI polyplexes (MCP/Ad-SS-PEG). In vivo transfection of reporter gene pLuc showed that although all groups exhibited liver and lung accumulation, MCP/Ad-SS-PEG +pDNA polyplexes accumulated more significantly in tumor tissue, suggesting that such redox-sensitive PEG-based polyplexes could potentially maximize target specificity while minimizing toxicity (Ping et al., 2013).
3.4 |. Functionalization and targeting with poly(propyleneimine) and methacrylate polymers
Multidrug resistance (MDR) in cancer is characterized by overexpression of ATP-binding cassette (ABC) transmembrane transporter protein P-glycoprotein (P-gp/MDR1) (Leighton & Goldstein, 1995), which promotes a reduction in cytosolic drug concentration and cytotoxicity by pumping drugs out of cells. Thus, P-gp inhibition is of interest for its role in retaining drugs in the cytosol (Ganguly et al., 2011; Mei et al., 2009; Milane, Ganesh, Shah, Duan, & Amiji, 2011; Pan, Liu, He, Wang, & Shi, 2013; Stege, Krühn, & Lage, 2010; Yadav, van Vlerken, Little, & Amiji, 2009). A study examining enhanced delivery of nucleic acids to reverse MDR utilized polypropylenimine (PPI) dendrimers conjugated to Pluronic P123. The Pluronic block not only stabilized the nanocomplexes to promote cell uptake, but also served as an inhibitor of P-gp. This approach was reported by J. Gu, Fang, Hao, and Sha (2015) to reduce cellular internalization and transfection efficiency. Additionally, in this polyplex, an anti-CD44 antibody was incorporated as the targeting moiety. pDNA encoding a short hairpin RNA (shRNA) against the expression of P-gp was complexed with PPI-P123 and delivered to the MDR CD44+ MCF-7 breast cancer mouse model. A 2.5-fold increase in transfection efficiency was observed in mice treated with targeted (anti-CD44-P123-PPI/pLuc) relative to nontargeted pLuc nanoplexes (J. Gu et al., 2015). Additionally, tumor growth was inhibited in groups treated with DOX alone or DOX with targeted anti-P-gp nanoplexes (anti-CD44-P123-PPI/pDNA-P-gp/MDR1-shRNA). At the end of the 24-day study, the combination of DOX with targeted anti-P-gp nanoplexes reduced tumor volume by greater than sixfold relative to free DOX and nontargeted nanoplex (J. Gu et al., 2015). These results illustrated that shielding modifications can enable nanoplex targeting with moieties significantly larger than oligopeptides, such as antibodies.
The delivery of therapeutics to the CNS is severely restricted by the blood brain barrier (BBB). Early clinical trial efforts to circumvent the BBB employed intracerebral injection of deactivated viral vectors (Lehrman, 1999). More recent work by Qian et al. described diblock copolymers composed of methoxy-PEG-PDMAEMA and maleimide-PEG-PDMAEMA, the latter appended with a 12 amino acid oligopeptide (TNG) known to target the BBB (J. Li et al., 2011; Qian et al., 2013). The transfection efficiency of TNG-PEG-PDMAEMA/pDNA exceeded that of nontargeted polyplexes by threefold. Unfortunately, both untargeted and targeted polyplexes displayed off-target organ accumulation in the heart and lung, suggesting that clinical translation of these polyplexes for gene delivery would remain challenging in this particular formulation.
3.5 |. Future directions in preclinical and clinical translation of polymers for pDNA delivery
Our groups have been interested in translating polymer-based gene delivery for treating disease states associated with uncontrolled growth (tumor models), uncontrolled inflammation (arthritis), and musculoskeletal tissue repair (cartilage and bone repair). To our knowledge, very limited clinical trial data is available on these specific therapeutic applications. The existing state-of-the-art includes adenoviral or adenoviral-associated vectors, or cell-based therapies. Interestingly, the majority of translated gene delivery strategies have included physical methods, of which electroporation is prominent. Promising future directions in translating the polymer systems described in this review include adapting targeted polymers to achieve gene delivery in vivo. A major challenge will be the charged nature of cationic polymers, and for this purpose, shielding with neutral, biocompatible polymers will likely be needed to facilitate future translation to the clinic. For example, Moffatt, Papasakelariou, Wiehle, and Cristiano (2006) reported promising results for a branched PEI25kDa-PEG copolymer functionalized with an anti-PSMA (prostate-specific membrane antigen) antibody (J591). PSMA is a transmembrane protein known to be overexpressed in prostate cancer tissue. Expression of a reporter gene (pCMV-β galactosidase) through this delivery system showed a 10-fold increase in reporter activity in tumor tissue relative to off-target organs (Moffatt et al., 2006). This study provided preliminary evidence for polymeric delivery systems to target cancer therapy, which is lacking in prostate cancer research. It remains to be seen what extent of transfection efficiency will be required for effectiveness of gene products in a clinical setting; this will be critical for determining whether targeted polymer gene delivery is best utilized alone or in combination therapies. It is most likely that gene carriers will continue to be utilized in the context of combination therapies. For example, a recent preclinical prostate cancer approach reported by Dufes and coworkers included in vivo experiments demonstrating the potential of Tf-conjugated generation 3-aminobutyric polypropyleneimine (DAB) dendrimers as effective gene carriers (Al Robaian, Chiam, Blatchford, & Dufès, 2014; Altwaijry et al., 2018). Al Robaian et al. (2014) complexed Tf-DAB with either pTNFα, pTRAIL, or pIL-12 to observe therapeutic effects following intravenous injection in PC3 and DU145 xenograft mouse models. Treatment with targeted pTNFα (Tf-DAB-pTNFα) resulted in tumor growth reduction in 50–60% of the animals, whereas pTRAIL or pIL-12 only led to suppression in 10–20% of the tumors (Figure 11; Al Robaian et al., 2014). Remarkably, alteration of the targeting motif to lactoferrin (Lf) dramatically enhanced the therapeutic efficacy, inducing complete tumor suppression in 50–70% of the animals; Lf targeting also improved the efficacy of pTRAIL and pIL-12 (Altwaijry et al., 2018). Importantly, treatment with Tf- or Lf-conjugated DAB complexes did not induce toxicity or weight loss in the animals, suggesting that DAB-pDNA polyplexes were well tolerated (Al Robaian et al., 2014; Altwaijry et al., 2018). These efficiencies could be very promising for eventual clinical translation of pTRAIL/pIL12 targeted polymer gene delivery modalities.
FIGURE 11.
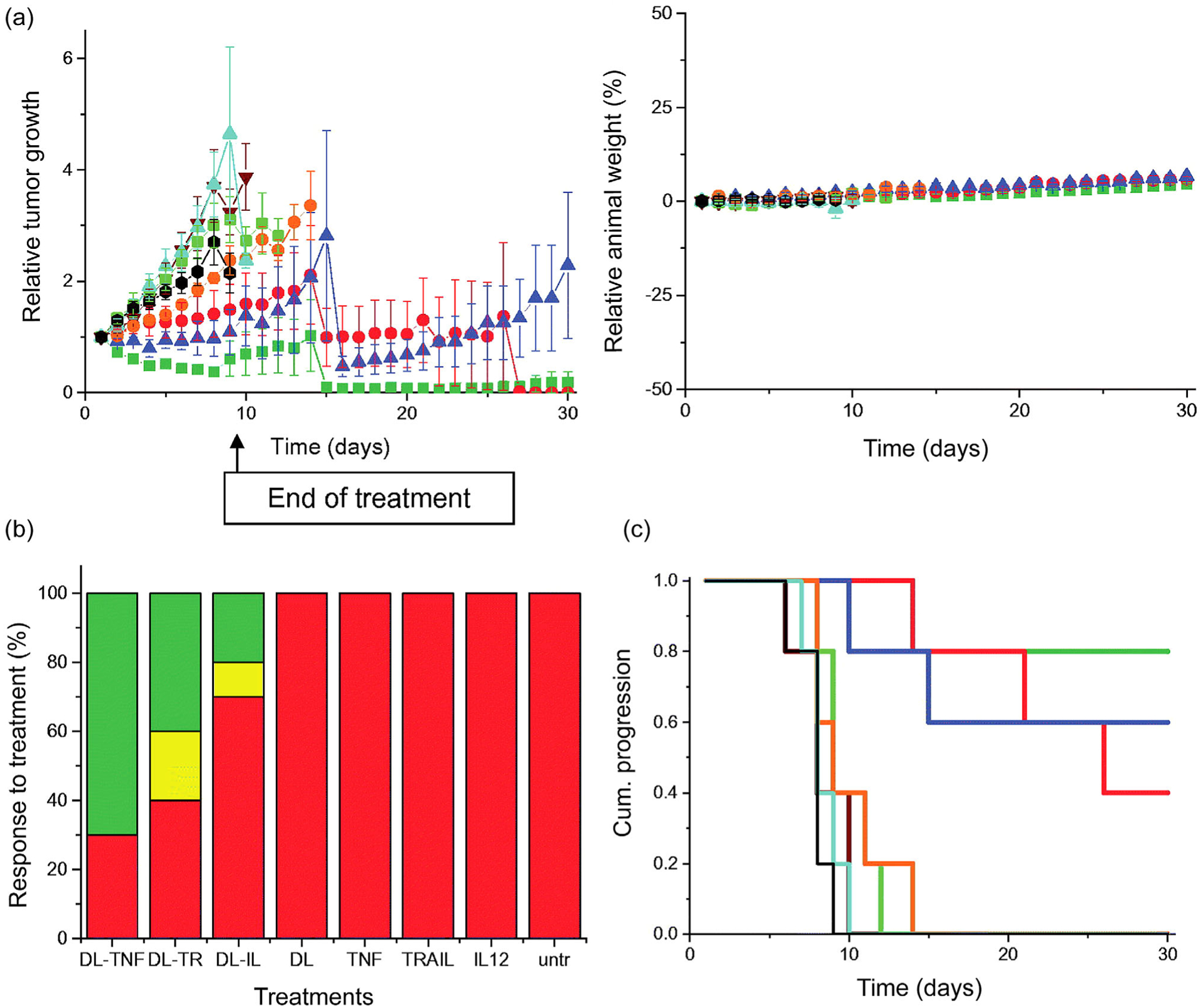
Targeted gene therapy in a PC3 tumor xenograft mouse model. (a) Tumor growth studies following intravenous injection with DAB-Lf dendriplex encoding TNF-α (■, green), TRAIL (●, red), IL-12 (▲, blue), all at 50 mg pDNA/injection. DAB-Lf (▼, brown), naked pTNFα (■, pale green), naked pTRAIL (●, orange), naked pIL-27 (▲, cyan), and nontreated tumors (●, black). (b) Relative mouse weight throughout the study. (c) Overall tumor response to treatment evaluated according to the Response Evaluation Criteria in Solid Tumors (RECIST). Red, progressive response; orange, stable response; yellow, partial response; green, complete response. (d) Time to disease progression. Y-axis indicates the percent of surviving animals. Animals were removed from the study when the tumor reaches 10 mm in diameter. Color coding in b and d are similar to a (Al Robaian et al., 2014)
For inflammatory diseases, current gene delivery approaches primarily rely on viral vectors. In arthritis, since inflammation of single or multiple joints is a hallmark of disease, effective antagonism of inflammatory receptors, such as interleukin-1 receptor (IL-1R), has been the focus of numerous studies. A randomized, double-blind, placebo-controlled clinical trial on osteoarthritis patients, for instance, demonstrated that direct intra-articular injection of the biologic drug anakinra could alleviate pain at an early time point in the study, but pain relief was not sustained 4 weeks after the injection (Chevalier et al., 2009). Therefore, if polyplexes are to control inflammatory diseases, it will be critical to introduce complexes that enhance bioavailability of therapies at the target tissue, using targeting moieties or other methods to enable intra-articular retention or accumulation. In a very recent study, Nixon et al. (2018), utilized a helper-dependent adenovirus (HDAd) for intra-articular gene delivery of the endogenous IL-1R antagonist (HDAd-IL-1Rα), which proved effective in mice and a horse model. Treatment with HDAd-IL-1Rα significantly improved clinical parameters (lameness, range of motion, flexion pain, and joint swelling) compared to controls without any pathological changes or off-target vector accumulation in the liver, spleen, heart, or ovaries of the horses tested. This exciting report demonstrates that therapeutic efficacy can be improved with nonintegrative delivery vehicles (episomally maintained, such as HDAd); however, a gap still exists in the availability of targeted, nonviral joint gene delivery modalities that are biocompatible and effective in reducing cytotoxic effects. Some drugs are already being delivered to osteoarthritic joints in mouse models via targeted nanoscale systems using WYRGRL peptides and PLGA NPs (T. Jiang et al., 2018); however, to our knowledge, gene delivery has not been pursued yet for arthritis treatment with polymer-peptide combinations.
Other promising targeted polymer-pDNA complexes for bone-metastatic cancers remain to be developed; currently, polymers (some lacking targeting moieties) typically only delivered nucleic acids or drugs (Elazar et al., 2010; Miller et al., 2011). However, some targeted polymer-pDNA systems appear in the literature. For example, the rise of CRISPR/Cas9 as a genomic editing tool has opened new venues for polymer-mediated gene delivery. Recently, Liang et al. (2017) functionalized PEG-PEI-cholesterol (PPC) lipopolymers with an osteosarcoma (OS) cell-specific aptamer (LC09) to encapsulate and deliver CRISPR/Cas9 plasmids encoding VEGFA gRNA and Cas9 in mice bearing OS K7M2 tumors. In this study, the targeting and therapeutic capacity of this PPC-CRISPR/Cas9 system was examined for treating both localized and metastatic tumor cells. This system inhibited orthotopic OS growth, bone destruction, and lung metastasis through the inhibition of VEGFA, a known angiogenic and metastasis-promoting factor (Daft, Yang, Napierala, & Zayzafoon, 2015; Tanaka et al., 2013; Zhao, Zhang, Zhao, Ma, & Fan, 2015). In vivo results demonstrated tumor-specific expression, and antitumor/anti-metastasis activity of PPC-CRISPR/Cas9 plasmids, with insignificant accumulation in off-target organs such as the liver and the heart relative to controls following systemic administration. Thus, this study demonstrated the effectiveness of aptamers for targeting of CRISPR/Cas9 and possibly other therapeutic genes to both localized and metastatic tumor cells.
Clinical approaches using polymers for gene delivery include PPC in a Phase I clinical trial, where pIL-12 was delivered to ovarian cancer patients (NCT00137865) (Safety Study of phIL-12–005/PPC to Treat Recurrent Ovarian Cancer, 2013). In this study, phIL12/PPC gene therapy combined with carboplatin/docetaxel chemotherapy doses were well tolerated in patients with recurrent ovarian cancer. Also, IFN-γ and TNF-α concentrations in patient intraperitoneal fluid samples exhibited dose-dependent increases in response to treatments, demonstrating that phIL-12/PPC elicited IL-12 biological activity (Anwer et al., 2013). Although phIL-12/PPC did not appear to reduce the efficacy of chemotherapy treatment, one disadvantage of gene delivery and carboplatin/docetaxel was the attenuation of TNF-α and IFN-γ increases mediated by the phIL-12/PPC monotherapy, likely due to the use of dexamethasone to prevent hypersensitivity to docetaxel. This work demonstrated the feasibility of gene delivery as a strategy to treat aggressive cancers and opened new avenues for exploring other combination therapies, such as concurrent delivery of genes and immunomodulating agents, a rapidly growing and promising field in cancer therapeutics.
Irrespective of the presence or type of targeting groups on polymer delivery systems, numerous challenges must be overcome to apply these delivery systems in clinical translation. Biomolecule dissociation kinetics and polyplex stability (or lack thereof) in circulation, and detailed characterization of biodistribution and cytotoxicity associated with any off-target organ accumulation, are key considerations. Difficult-to-deliver charged polyplexes will likely require additional strategies such as shielding by covalent modification with either PEG or by incorporating high densities of targeting agents (e.g., transferrin) without PEGylation. One supporting example for such strategies is the use of PPC lipopolymer-encapsulated IL-12 pDNA (EGEN-001) along with PEGylated Doxorubicin for treating ovarian cancer patients, as reported in a Phase I clinical trial that spanned from 2012 to 2018 (NCT01489371) (EGEN-001 and Pegylated Liposomal Doxorubicin Hydrochloride in Treating Patients With Recurrent or Persistent Ovarian Epithelial Cancer, Fallopian Tube Cancer, or Primary Peritoneal Cancer, 2019; Thaker, Brady, Bradley, Anwer, & Alvarez, 2015). A Phase II clinical trial is currently employing intratumoral gene transfer of plasmids encoding genes that sensitize cancer cells to gemcitabine complexed with linear PEI (CYL-02) for pancreatic adenocarcinoma guided by endoscopy (NCT02806687) (Effect of Intratumoral Injection of Gene Therapy for Locally Advanced Pancreatic Cancer (THERGAP-02), 2019). CYL-02 encodes for SSTR2 (somatostatin receptor subtype 2, downregulated in ~95% of pancreatic ductal adenocarcinoma) and DCK::UMK, a gene fusion that encodes deoxycytidine kinase and uridylate monophosphate kinase. In a relatively complex study protocol, the trial is examining intratumoral injection of CYL-02 within the primary tumor under endoscopic ultrasound guidance and followed by three IV infusions of Gemcitabine at 48 hr and then 3 weeks/month for 6 months (or until progression). The study will measure progression-free survival as the primary outcome, and overall survival, tumor response, biological tumor markers (CA19–9 levels), biodistribution, quality of life change, adverse events, and peak plasma concentration for the drug as well as quantification of the transgenes by qPCR in blood and tumor biopsies of samples before and after gene transfer. These trials will be instrumental in paving the way for translation of a wider variety of targeted or untargeted polymer:pDNA therapeutic complexes.
3.6 |. Properties of gene delivery systems in clinical trials
The delivery system used in the first clinical trial discussed above (NCT00137865) was a lipopolymer-based system containing PPC, composed of branched, low molecular weight PEI linked covalently to methoxypolyethyleneglycol (PEG) and cholesterol (CHOL). This delivery system was utilized due to its ability to form stable nanoplexes with pDNA, protect pDNA from degradation by DNAses, and facilitate efficient gene transfer in both in vitro and in preclinical solid tumor models (Fewell et al., 2005). PEG-PEI-CHOL and nitrogen-to-phosphate (N/P) ratios were optimized for high pDNA transfection efficiency. In vivo transfection efficiency of this delivery system was evaluated in BALB/c mice bearing subcutaneous 4T-1 syngeneic breast tumors following intratumoral injection of PPC/pLuc. Similarly, the therapeutic efficacy of this gene delivery system was evaluated using the same tumor model in combination with an investigational chemotherapeutic called Genexol-PM (Fewell et al., 2005), a formulation of paclitaxel and a biodegradable polymeric solubilizer, HySolv (Janát-Amsbury et al., 2004). Significant reduction in tumor volumes was observed in response to Genexol-PM+ pmIL-12/PPC relative to controls (Fewell et al., 2005). It is important to note that this delivery approach is nontargeted and relies on direct intratumoral injection, which may not be readily applicable to distant tumor metastases.
4 |. CONCLUSION
Within the extensive array of polymeric materials being examined in as gene delivery vectors, common objectives and overarching principles will continue to include synthetic designs that effectively modulate charge density, mask toxicity, and optimize structure to include targeting ligands. The diverse array of opportunities with polymers is enhanced by the availability of pi-conjugated structures that allow facile fluorescent tracking in addition to DNA/RNA complexation and delivery. New, emerging polymer designs, when combined with advanced delivery techniques, will drive discovery of improved therapeutic treatments. This is expected to include the development of biocompatible polymers with degradable backbones that facilitate fast/triggered cargo release, while the degradability and stability of the polyplexes should be well balanced (Benner et al., 2019; Y. Yan, Xiong, Zhang, Cheng, & Siegwart, 2017). Combining physical methods such as electroporation and sonoporation to assist polyplex transfection enhances targeting effects and reduces off-target toxicity. Recently translated gene delivery protocols employing polymers and pDNA have been used in the context of chemotherapy for different cancer types, promising low toxicity and biological activity of the gene products. As such, the future is promising for the continued application of polymer gene delivery for challenging-to-treat conditions, including uncontrolled growth (tumor models), uncontrolled inflammation (arthritis), and musculoskeletal tissue repair (cartilage and bone repair).
Funding information
National Institutes of Health, Grant/Award Numbers: R01AR069079, R01CA196947, R01CA196947-S1
Footnotes
CONFLICT OF INTEREST
The authors have declared no conflicts of interest for this article.
RELATED WIRES ARTICLE
Plant viral and bacteriophage delivery of nucleic acid therapeutics
REFERENCES
- Al Robaian M, Chiam KY, Blatchford DR, & Dufès C (2014). Therapeutic efficacy of intravenously administered transferrin-conjugated dendriplexes on prostate carcinomas. Nanomedicine, 9(4), 421–434. 10.2217/nnm.13.25 [DOI] [PubMed] [Google Scholar]
- Altwaijry N, Somani S, Parkinson JA, Tate RJ, Keating P, Warzecha M, … Dufès C (2018). Regression of prostate tumors after intravenous administration of lactoferrin-bearing polypropylenimine dendriplexes encoding TNF-α, TRAIL, and interleukin-12. Drug Delivery, 25(1), 679–689. 10.1080/10717544.2018.1440666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anwer K, Kelly FJ, Chu C, Fewell JG, Lewis D, & Alvarez RD (2013). Phase I trial of a formulated IL-12 plasmid in combination with carboplatin and docetaxel chemotherapy in the treatment of platinum-sensitive recurrent ovarian cancer. Gynecologic Oncology, 131(1), 169–173. 10.1016/j.ygyno.2013.07.081 [DOI] [PubMed] [Google Scholar]
- Behr J (1997). The proton sponge: A trick to enter cells the viruses did not exploit. International Journal for Chemistry, 2(1), 34–36. [Google Scholar]
- Benner NL, McClellan RL, Turlington CR, Haabeth OAW, Waymouth RM, & Wender PA (2019). Oligo(serine ester) charge-altering releasable transporters: Organocatalytic ring-opening polymerization and their use for in vitro and in vivo mRNA delivery. Journal of the American Chemical Society, 141(21), 8416–8421. 10.1021/jacs.9b03154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand N, Wu J, Xu X, Kamaly N, & Farokhzad OC (2014). Cancer nanotechnology: The impact of passive and active targeting in the era of modern cancer biology. Advanced Drug Delivery Reviews, 66, 2–25. 10.1016/j.addr.2013.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya S, & Bajaj A (2009). Advances in gene delivery through molecular design of cationic lipids. Chemical Communications, (31), 4632–4656. 10.1039/b900666b [DOI] [PubMed] [Google Scholar]
- Boussif O, Lezoualc’h F, Zanta MA, Mergny MD, Scherman D, Demeneix B, & Behr JP (1995). A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: Polyethylenimine. Proceedings of the National Academy of Sciences, 92(16), 7297–7301. 10.1073/pnas.92.16.7297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitenkamp BR, & Emrick T (2008). Pentalysine-grafted ROMP polymers for DNA complexation and delivery. Biomacromolecules, 9, 2495–2500. [DOI] [PubMed] [Google Scholar]
- Buckwalter DJ, Sizovs A, Ingle NP, & Reineke TM (2012). MAG versus PEG: Incorporating a poly(MAG) layer to promote colloidal stability of nucleic acid/”click cluster” complexes. ACS Macro Letters, 1(5), 609–613. 10.1021/mz300081d [DOI] [PubMed] [Google Scholar]
- Chan CK, Senden T, & Jans DA (2000). Supramolecular structure and nuclear targeting efficiency determine the enhancement of transfection by modified polylysines. Gene Therapy, 7(19), 1690–1697. 10.1038/sj.gt.3301275 [DOI] [PubMed] [Google Scholar]
- Chen H, Fang X, Jin Y, Hu X, Yin M, Men X, … Wu C (2018). Semiconducting polymer nanocavities: Porogenic synthesis, tunable host-guest interactions, and enhanced drug/siRNA delivery. Small, 14(21), 1800239 10.1002/smll.201800239 [DOI] [PubMed] [Google Scholar]
- Chen Q, Osada K, Ge Z, Uchida S, Tockary TA, Dirisala A, … Kataoka K (2017). Polyplex micelle installing intracellular self-processing functionalities without free catiomers for safe and efficient systemic gene therapy through tumor vasculature targeting. Biomaterials, 113, 253–265. 10.1016/j.biomaterials.2016.10.042 [DOI] [PubMed] [Google Scholar]
- Cheng CJ, Tietjen GT, Saucier-Sawyer JK, & Saltzman WM (2015). A holistic approach to targeting disease with polymeric nanoparticles. Nature Reviews. Drug Discovery, 14(4), 239–247. 10.1038/nrd4503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevalier X, Goupille P, Beaulieu AD, Burch FX, Bensen WG, Conrozier T, … Appleton BE (2009). Intraarticular injection of anakinra in osteoarthritis of the knee: A multicenter, randomized, double-blind, placebo-controlled study. Arthritis Care & Research, 61(3), 344–352. 10.1002/art.24096 [DOI] [PubMed] [Google Scholar]
- Choi KY, Correa S, Min J, Li J, Roy S, Laccetti KH, … Hammond PT (2019). Binary targeting of siRNA to hematologic cancer cells in vivo using layer-by-layer nanoparticles. Advanced Functional Materials, 29(20), 1900018 10.1002/adfm.201900018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox DBT, Platt RJ, & Zhang F (2015). Therapeutic genome editing: Prospects and challenges. Nature Medicine, 21(2), 121–131. 10.1038/nm.3793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daft PG, Yang Y, Napierala D, & Zayzafoon M (2015). The growth and aggressive behavior of human osteosarcoma is regulated by a CaMKII-controlled autocrine VEGF signaling mechanism. PLoS One, 10(4), e0121568 10.1371/journal.pone.0121568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai Z, Gjetting T, Mattebjerg MA, Wu C, & Andresen TL (2011). Elucidating the interplay between DNA-condensing and free polycations in gene transfection through a mechanistic study of linear and branched PEI. Biomaterials, 32(33), 8626–8634. 10.1016/j.biomaterials.2011.07.044 [DOI] [PubMed] [Google Scholar]
- Delalande A, Kotopoulis S, Postema M, Midoux P, & Pichon C (2013). Sonoporation: Mechanistic insights and ongoing challenges for gene transfer. Gene, 525(2), 191–199. 10.1016/j.gene.2013.03.095 [DOI] [PubMed] [Google Scholar]
- Du B, Gu X, Han X, Ding G, Wang Y, Li D, … Wang J (2017). Lipid-coated gold nanoparticles functionalized by folic acid as gene vectors for targeted gene delivery in vitro and in vivo. ChemMedChem, 12(21), 1768–1775. 10.1002/cmdc.201700391 [DOI] [PubMed] [Google Scholar]
- Effect of Intratumoral Injection of Gene Therapy for Locally Advanced Pancreatic Cancer (THERGAP-02). (2019).
- EGEN-001 and Pegylated Liposomal Doxorubicin Hydrochloride in Treating Patients With Recurrent or Persistent Ovarian Epithelial Cancer, Fallopian Tube Cancer, or Primary Peritoneal Cancer. (2019). Retrieved from https://ClinicalTrials.gov/show/NCT01489371.
- Elazar V, Adwan H, Bäuerle T, Rohekar K, Golomb G, & Berger MR (2010). Sustained delivery and efficacy of polymeric nanoparticles containing osteopontin and bone sialoprotein antisenses in rats with breast cancer bone metastasis. International Journal of Cancer, 126(7), 1749–1760. 10.1002/ijc.24890 [DOI] [PubMed] [Google Scholar]
- Elder RM, Emrick T, & Jayaraman A (2011). Understanding the effect of polylysine architecture on DNA binding using molecular dynamics simulations. Biomacromolecules, 12(11), 3870–3879. 10.1021/bm201113y [DOI] [PubMed] [Google Scholar]
- Erbacher P, Roche AC, Monsigny M, & Midoux P (1996). Putative role of chloroquine in gene transfer into a human hepatoma cell line by DNA/lactosylated polylysine complexes. Experimental Cell Research, 225(1), 186–194. 10.1006/excr.1996.0169 [DOI] [PubMed] [Google Scholar]
- Fang H, Guo Z, Lin L, Chen J, Sun P, Wu J, … Chen X (2018). Molecular strings significantly improved the gene transfection efficiency of polycations. Journal of the American Chemical Society, 140, 11992–12000. 10.1021/jacs.8b05341 [DOI] [PubMed] [Google Scholar]
- Feng L, Yan S, Zhu Q, Chen J, Deng L, Zheng Y, … Guo R (2018). Targeted multifunctional redox-sensitive micelle co-delivery of DNA and doxorubicin for the treatment of breast cancer. Journal of Materials Chemistry B, 6(20), 3372–3386. 10.1039/c8tb00748a [DOI] [PubMed] [Google Scholar]
- Feng X, Lv F, Liu L, Yang Q, Wang S, & Bazan GC (2012). A highly emissive conjugated polyelectrolyte vector for gene delivery and transfection. Advanced Materials, 24(40), 5428–5432. 10.1002/adma.201202145 [DOI] [PubMed] [Google Scholar]
- Feng X, Tang Y, Duan X, Liu L, & Wang S (2010). Lipid-modified conjugated polymer nanoparticles for cell imaging and transfection. Journal of Materials Chemistry, 20(7), 1312–1316. 10.1039/b915112e [DOI] [Google Scholar]
- Fewell JG, Matar M, Slobodkin G, Han S-O, Rice J, Hovanes B, … Anwer K (2005). Synthesis and application of a non-viral gene delivery system for immunogene therapy of cancer. Journal of Controlled Release, 109(1), 288–298. 10.1016/j.jconrel.2005.09.024 [DOI] [PubMed] [Google Scholar]
- Figueiredo Neto M, Letteri R, Chan-Seng D, Emrick T, & Figueiredo ML (2015). Sonodelivery facilitates sustained luciferase expression from an episomal vector in skeletal muscle. Materials, 8(7), 4608–4617. 10.3390/ma8074608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganguly A, Banerjee K, Chakraborty P, Das S, Sarkar A, Hazra A, … Choudhuri SK (2011). Overcoming multidrug resistance (MDR) in cancer in vitro and in vivo by a quinoline derivative. Biomedicine & Pharmacotherapy, 65(6), 387–394. 10.1016/j.biopha.2011.04.024 [DOI] [PubMed] [Google Scholar]
- Ge Z, Chen Q, Osada K, Liu X, Tockary TA, Uchida S, … Kataoka K (2014). Targeted gene delivery by polyplex micelles with crowded PEG palisade and cRGD moiety for systemic treatment of pancreatic tumors. Biomaterials, 35(10), 3416–3426. 10.1016/j.biomaterials.2013.12.086 [DOI] [PubMed] [Google Scholar]
- Ghobadi AF, Letteri R, Parelkar SS, Zhao Y, Chan-Seng D, Emrick T, & Jayaraman A (2016). Dispersing zwitterions into comb polymers for nonviral transfection: Experiments and molecular simulation. Biomacromolecules, 17(2), 546–557. 10.1021/acs.biomac.5b01462 [DOI] [PubMed] [Google Scholar]
- Ginn SL, Amaya AK, Alexander IE, Edelstein M, & Abedi MR (2018). Gene therapy clinical trials worldwide to 2017: An update. Journal of Gene Medicine, 20(5), e3015 10.1002/jgm.3015 [DOI] [PubMed] [Google Scholar]
- Godbey WT, Wu KK, & Mikos AG (1998). Size matters: Molecular weight affects the efficiency of poly(ethylenimine) as a gene delivery vehicle. Journal of Biomedical Materials Research, 45(3), 268–275. [DOI] [PubMed] [Google Scholar]
- Grandinetti G, Ingle NP, & Reineke TM (2011). Interaction of poly(ethylenimine)-DNA polyplexes with mitochondria: Implications for a mechanism of cytotoxicity. Molecular Pharmaceutics, 8(5), 1709–1719. 10.1021/mp200078n [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandinetti G, Smith AE, & Reineke TM (2012). Membrane and nuclear permeabilization by polymeric pDNA vehicles: Efficient method for gene delivery or mechanism of cytotoxicity? Molecular Pharmaceutics, 9(3), 523–538. 10.1021/mp200368p [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu J, Fang X, Hao J, & Sha X (2015). Reversal of P-glycoprotein-mediated multidrug resistance by CD44 antibody-targeted nanocomplexes for short hairpin RNA-encoding plasmid DNA delivery. Biomaterials, 45, 99–114. 10.1016/j.biomaterials.2014.12.030 [DOI] [PubMed] [Google Scholar]
- Gu L, Deng ZJ, Roy S, & Hammond PT (2017). A combination RNAi-chemotherapy layer-by-layer nanoparticle for systemic targeting of KRAS/P53 with cisplatin to treat non-small cell lung cancer. Clinical Cancer Research, 23(23), 7312–7323. 10.1158/1078-0432.ccr-16-2186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond PT (2012). Building biomedical materials layer-by-layer. Materials Today, 15(5), 196–206. 10.1016/S1369-7021(12)70090-1 [DOI] [Google Scholar]
- Han S-S, Li Z-Y, Zhu J-Y, Han K, Zeng Z-Y, Hong W, … Zhang X-Z (2015). Dual-pH sensitive charge-reversal polypeptide micelles for tumor-triggered targeting uptake and nuclear drug delivery. Small, 11(21), 2543–2554. 10.1002/smll.201402865 [DOI] [PubMed] [Google Scholar]
- Hardee CL, Arévalo-Soliz LM, Hornstein BD, & Zechiedrich L (2017). Advances in non-viral DNA vectors for gene therapy. Genes, 8(2), 1–22. 10.3390/genes8020065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardie J, Jiang Y, Tetrault ER, Ghazi PC, Tonga GY, Farkas ME, & Rotello VM (2016). Simultaneous cytosolic delivery of a chemotherapeutic and siRNA using nanoparticle-stabilized nanocapsules. Nanotechnology, 27(37), 374001 10.1088/0957-4484/27/37/374001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herweijer H, & Wolff JA (2003). Progress and prospects: Naked DNA gene transfer and therapy. Gene Therapy, 10(6), 453–458. 10.1038/sj.gt.3301983 [DOI] [PubMed] [Google Scholar]
- Hingorani M, Spitzweg C, Vassaux G, Newbold K, Melcher A, Pandha H, … Harrington K (2010). The biology of the sodium iodide symporter and its potential for targeted gene delivery. Current Cancer Drug Targets, 10(2), 242–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itaka K, Harada A, Yamasaki Y, Nakamura K, Kawaguchi H, & Kataoka K (2004). In situ single cell observation by fluorescence resonance energy transfer reveals fast intra-cytoplasmic delivery and easy release of plasmid DNA complexed with linear polyethylenimine. Journal of Gene Medicine, 6(1), 76–84. 10.1002/jgm.470 [DOI] [PubMed] [Google Scholar]
- Jackson MA, Werfel TA, Curvino EJ, Yu F, Kavanaugh TE, Sarett SM, … Duvall CL (2017). Zwitterionic nanocarrier surface chemistry improves siRNA tumor delivery and silencing activity relative to polyethylene glycol. ACS Nano, 11(6), 5680–5696. 10.1021/acsnano.7b01110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janát-Amsbury MM, Yockman JW, Lee M, Kern S, Furgeson DY, Bikram M, & Kim SW (2004). Combination of local, nonviral IL12 gene therapy and systemic paclitaxel treatment in a metastatic breast cancer model. Molecular Therapy, 9(6), 829–836. 10.1016/j.ymthe.2004.03.015 [DOI] [PubMed] [Google Scholar]
- Jiang D, Wang M, Wang T, Zhang B, Liu C, & Zhang N (2017). Multifunctionalized polyethyleneimine-based nanocarriers for gene and chemotherapeutic drug combination therapy through one-step assembly strategy. International Journal of Nanomedicine, 12, 8681–8698. 10.2147/ijn.s142966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang R, Lu X, Yang M, Deng W, Fan Q, & Huang W (2013). Monodispersed brush-like conjugated polyelectrolyte nanoparticles with efficient and visualized SiRNA delivery for gene silencing. Biomacromolecules, 14(10), 3643–3652. 10.1021/bm401000x [DOI] [PubMed] [Google Scholar]
- Jiang T, Kan HM, Rajpura K, Carbone EJ, Li Y, & Lo KW (2018). Development of targeted nanoscale drug delivery system for osteoarthritic cartilage tissue. Journal of Nanoscience and Nanotechnology, 18(4), 2310–2317. 10.1166/jnn.2018.14311 [DOI] [PubMed] [Google Scholar]
- Jiang Y, Lodge TP, & Reineke TM (2018). Packaging pDNA by polymeric ABC micelles simultaneously achieves colloidal stability and structural control. Journal of the American Chemical Society, 140(35), 11101–11111. 10.1021/jacs.8b06309 [DOI] [PubMed] [Google Scholar]
- Jiang Y, Tang R, Duncan B, Jiang Z, Yan B, Mout R, & Rotello VM (2015). Direct cytosolic delivery of siRNA using nanoparticle-stabilized nanocapsules. Angewandte Chemie International Edition, 54(2), 506–510. 10.1002/anie.201409161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung S, Lodge TP, & Reineke TM (2017). Complexation between DNA and hydrophilic-cationic diblock copolymers. Journal of Physical Chemistry B, 121(10), 2230–2243. 10.1021/acs.jpcb.6b11408 [DOI] [PubMed] [Google Scholar]
- Kim J, Lee YM, Kim H, Park D, & Kim WJ (2016). Phenylboronic acid-sugar grafted polymer architecture as a dual stimuli-responsive gene carrier for targeted anti-angiogenic tumor therapy. Biomaterials, 75, 102–111. 10.1016/j.biomaterials.2015.10.022 [DOI] [PubMed] [Google Scholar]
- Kircheis R, Wightman L, Kursa M, Ostermann E, & Wagner E (2002). Tumor-targeted gene delivery: An attractive strategy to use highly active effector molecules in cancer treatment. Gene Therapy, 9, 731–735. 10.1038/sj/gt/3301748 [DOI] [PubMed] [Google Scholar]
- Kircheis R, Wightman L, Schreiber A, Robitza B, Rossler V, Kursa M, & Wagner E (2001). Polyethylenimine/DNA complexes shielded by transferrin target gene expression to tumors after systemic application. Gene Therapy, 8, 28–40. [DOI] [PubMed] [Google Scholar]
- Klutz K, Schaffert D, Willhauck MJ, Grunwald GK, Haase R, Wunderlich N, … Spitzweg C (2011). Epidermal growth factor receptor-targeted (131)I-therapy of liver cancer following systemic delivery of the sodium iodide symporter gene. Molecular Therapy, 19(4), 676–685. 10.1038/mt.2010.296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodama Y, Yatsugi Y, Kitahara T, Kurosaki T, Egashira K, Nakashima M, … Sasaki H (2015). Quaternary complexes modified from pDNA and poly-L-lysine complexes to enhance pH-buffering effect and suppress cytotoxicity. Journal of Pharmaceutical Sciences, 104(4), 1470–1477. 10.1002/jps.24364 [DOI] [PubMed] [Google Scholar]
- Koren E, Apte A, Jani A, & Torchilin VP (2012). Multifunctional PEGylated 2C5-immunoliposomes containing pH-sensitive bonds and TAT peptide for enhanced tumor cell internalization and cytotoxicity. Journal of Controlled Release, 160(2), 264–273. 10.1016/j.jconrel.2011.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotterman MA, Chalberg TW, & Schaffer DV (2015). Viral vectors for gene therapy: Translational and clinical outlook. Annual Review of Biomedical Engineering, 17, 63–89. 10.1146/annurev-bioeng-071813-104938 [DOI] [PubMed] [Google Scholar]
- Kumagai M, Shimoda S, Wakabayashi R, Kunisawa Y, Ishii T, Osada K, … Nakano K (2012). Effective transgene expression without toxicity by intraperitoneal administration of PEG-detachable polyplex micelles in mice with peritoneal dissemination. Journal of Controlled Release, 160(3), 542–551. 10.1016/j.jconrel.2012.03.021 [DOI] [PubMed] [Google Scholar]
- Lee YM, Lee D, Kim J, Park H, & Kim WJ (2015). RPM peptide conjugated bioreducible polyethylenimine targeting invasive colon cancer. Journal of Controlled Release, 205, 172–180. 10.1016/j.jconrel.2015.01.020 [DOI] [PubMed] [Google Scholar]
- Lehrman S (1999). Virus treatment questioned after gene therapy death. Nature, 401(6753), 517–518. 10.1038/43977 [DOI] [PubMed] [Google Scholar]
- Leighton JC, & Goldstein LJ (1995). P-glycoprotein in adult solid tumors: Expression and prognostic significance. Hematology/Oncology Clinics of North America, 9(2), 251–274. 10.1016/S0889-8588(18)30095-9 [DOI] [PubMed] [Google Scholar]
- Li J, Chen L, Liu N, Li S, Hao Y, & Zhang X (2016). EGF-coated nano-dendriplexes for tumor-targeted nucleic acid delivery in vivo. Drug Delivery, 23(5), 1718–1725. 10.3109/10717544.2015.1004381 [DOI] [PubMed] [Google Scholar]
- Li J, Feng L, Fan L, Zha Y, Guo L, Zhang Q, … Wen L (2011). Targeting the brain with PEG-PLGA nanoparticles modified with phage-displayed peptides. Biomaterials, 32(21), 4943–4950. 10.1016/j.biomaterials.2011.03.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Yuan H, Chen H, Wang X, Zhang P, Lv F, … Wang S (2016). Cationic poly(p-phenylene vinylene) materials as a multifunctional platform for light-enhanced siRNA delivery. Chemistry—An Asian Journal, 11(19), 2686–2689. 10.1002/asia.201600447 [DOI] [PubMed] [Google Scholar]
- Li X, Tang T, Lu X, Lu X, Zhou H, Zhou H, … Huang Y (2011). RNA interference targeting NRP-1 inhibits human glioma cell proliferation and enhances cell apoptosis. Molecular Medicine Reports, 4, 1261–1266. 10.3892/mmr.2011.550 [DOI] [PubMed] [Google Scholar]
- Li Z, Zhao R, Wu X, Sun Y, Yao M, Li J, … Gu J (2005). Identification and characterization of a novel peptide ligand of epidermal growth factor receptor for targeted delivery of therapeutics. The FASEB Journal, 19(14), 1978–1985. 10.1096/fj.05-4058com [DOI] [PubMed] [Google Scholar]
- Liang C, Li F, Wang L, Zhang Z-K, Wang C, He B, … Zhang G (2017). Tumor cell-targeted delivery of CRISPR/Cas9 by aptamer-functionalized lipopolymer for therapeutic genome editing of VEGFA in osteosarcoma. Biomaterials, 147, 68–85. 10.1016/j.biomaterials.2017.09.015 [DOI] [PubMed] [Google Scholar]
- Liu Y, & Reineke TM (2005). Hydroxyl stereochemistry and amine number within poly(glycoamidoamine)s affect intracellular DNA delivery. Journal of the American Chemical Society, 127(9), 3004–3015. 10.1021/ja0436446 [DOI] [PubMed] [Google Scholar]
- Lowin T, & Straub RH (2011). Integrins and their ligands in rheumatoid arthritis. Arthritis Research & Therapy, 13(5), 244 10.1186/ar3464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo D, Haverstick K, Belcheva N, Han E, & Saltzman WM (2002). Poly(ethylene glycol)-conjugated PAMAM dendrimer for biocompatible, high-efficiency DNA delivery. Macromolecules, 35(9), 3456–3462. 10.1021/ma0106346 [DOI] [Google Scholar]
- Maharaj DP, Mallajosyula KJ, Lee G, Thi P, Zhou Y, Kearney MC, & McCormick AA (2014). Nanoparticle encapsidation of flock house virus by auto assembly of tobacco mosaic virus coat protein. International Journal of Molecular Sciences, 15(10), 18540–18556. 10.3390/ijms151018540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markoishvili K, Tsitlanadze G, Katsarava R, Glenn J, & Sulakvelidze A (2002). A novel sustained-release matrix based on biodegradable poly(ester amide)s and impregnated with bacteriophages and an antibiotic shows promise in management of infected venous stasis ulcers and other poorly healing wounds. International Journal of Dermatology, 41(7), 453–458. 10.1046/j.1365-4362.2002.01451.x [DOI] [PubMed] [Google Scholar]
- Massich MD, Giljohann DA, Seferos DS, Ludlow LE, Horvath CM, & Mirkin CA (2009). Regulating immune response using polyvalent nucleic acid–gold nanoparticle conjugates. Molecular Pharmaceutics, 6(6), 1934–1940. 10.1021/mp900172m [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei L, Zhang Y, Zheng Y, Tian G, Song C, Yang D, … Huang L (2009). A novel docetaxel-loaded poly(ε-caprolactone)/pluronic F68 nanoparticle overcoming multidrug resistance for breast cancer treatment. Nanoscale Research Letters, 4(12), 1530–1539. 10.1007/s11671-009-9431-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milane L, Ganesh S, Shah S, Duan Z. f., & Amiji M (2011). Multi-modal strategies for overcoming tumor drug resistance: Hypoxia, the Warburg effect, stem cells, and multifunctional nanotechnology. Journal of Controlled Release, 155(2), 237–247. 10.1016/j.jconrel.2011.03.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller K, Eldar-Boock A, Polyak D, Segal E, Benayoun L, Shaked Y, & Satchi-Fainaro R (2011). Antiangiogenic antitumor activity of HPMA copolymer-paclitaxel-alendronate conjugate on breast cancer bone metastasis mouse model. Molecular Pharmaceutics, 8(4), 1052–1062. 10.1021/mp200083n [DOI] [PubMed] [Google Scholar]
- Moffatt S, Papasakelariou C, Wiehle S, & Cristiano R (2006). Successful in vivo tumor targeting of prostate-specific membrane antigen with a highly efficient J591/PEI/DNA molecular conjugate. Gene Therapy, 13, 761–772. 10.1038/sj.gt.3302721 [DOI] [PubMed] [Google Scholar]
- Monnery BD, Wright M, Cavill R, Hoogenboom R, Shaunak S, Steinke JHG, & Thanou M (2017). Cytotoxicity of polycations: Relationship of molecular weight and the hydrolytic theory of the mechanism of toxicity. International Journal of Pharmaceutics, 521(1–2), 249–258. 10.1016/j.ijpharm.2017.02.048 [DOI] [PubMed] [Google Scholar]
- Moon JH, Mendez E, Kim Y, & Kaur A (2011). Conjugated polymer nanoparticles for small interfering RNA delivery. Chemical Communications, 47(29), 8370–8372. 10.1039/c1cc10991j [DOI] [PubMed] [Google Scholar]
- Mullick Chowdhury S, Lee T, & Willmann JK (2017). Ultrasound-guided drug delivery in cancer. Ultrasonography, 36(3), 171–184. 10.14366/usg.17021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasarre C, Roth M, Jacob L, Roth L, Koncina E, Thien A, … Bagnard D (2010). Peptide-based interference of the transmembrane domain of neuropilin-1 inhibits glioma growth in vivo. Oncogene, 29, 2381–2392. 10.1038/onc.2010.9 [DOI] [PubMed] [Google Scholar]
- Nayerossadat N, Maedeh T, & Ali PA (2012). Viral and nonviral delivery systems for gene delivery. Advanced Biomedical Research, 1, 27–27. 10.4103/2277-9175.98152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann E, Schaefer-Ridder M, Wang Y, & Hofschneider PH (1982). Gene transfer into mouse lyoma cells by electroporation in high electric fields. The EMBO Journal, 1(7), 841–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixon AJ, Grol MW, Lang HM, Ruan MZC, Stone A, Begum L, … Guse K (2018). Disease-modifying osteoarthritis treatment with Interleukin-1 receptor antagonist gene therapy in small and large animal models. Arthritis & Rheumatology, 70(11), 1757–1768. 10.1002/art.40668 [DOI] [PubMed] [Google Scholar]
- Nordling-David MM, & Golomb G (2013). Gene delivery by liposomes. Israel Journal of Chemistry, 53(9–10), 737–747. 10.1002/ijch.201300055 [DOI] [Google Scholar]
- Oba M, Fukushima S, Kanayama N, Aoyagi K, Nishiyama N, Koyama H, & Kataoka K (2007). Cyclic RGD peptide-conjugated polyplex micelles as a targetable gene delivery system directed to cells possessing αvβ3 and αvβ5 integrins. Bioconjugate Chemistry, 18(5), 1415–1423. 10.1021/bc0700133 [DOI] [PubMed] [Google Scholar]
- Oh S, Kim BJ, Singh NP, Lai H, & Sasaki T (2009). Synthesis and anti-cancer activity of covalent conjugates of artemisinin and a transferrin-receptor targeting peptide. Cancer Letters, 274(1), 33–39. 10.1016/j.canlet.2008.08.031 [DOI] [PubMed] [Google Scholar]
- Olden BR, Cheng Y, Yu JL, & Pun SH (2018). Cationic polymers for non-viral gene delivery to human T cells. Journal of Controlled Release, 282, 140–147. 10.1016/j.jconrel.2018.02.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olins DE, Olins AL, & von Hippel PH (1967). Model nucleoprotein complexes: Studies on the interaction of cationic homopolypeptides with DNA. Journal of Molecular Biology, 24(2), 157–176. 10.1016/0022-2836(67)90324-5 [DOI] [PubMed] [Google Scholar]
- Osada H, Tokunaga T, Nishi M, Nishi M, Hatanaka H, Abe Y, … Nakamura M (2004). Overexpression of the neuropilin 1 (NRP1) gene correlated with poor prognosis in human glioma. Anticancer Research, 24, 547–552. [PubMed] [Google Scholar]
- Palmerston Mendes L, Pan J, & Torchilin VP (2017). Dendrimers as nanocarriers for nucleic acid and drug delivery in cancer therapy. Molecules (Basel, Switzerland), 22(9), 1401 10.3390/molecules22091401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan L, Liu J, He Q, Wang L, & Shi J (2013). Overcoming multidrug resistance of cancer cells by direct intranuclear drug delivery using TAT-conjugated mesoporous silica nanoparticles. Biomaterials, 34(11), 2719–2730. 10.1016/j.biomaterials.2012.12.040 [DOI] [PubMed] [Google Scholar]
- Parelkar SS, Chan-seng D, & Emrick T (2011). Reconfiguring polylysine architectures for controlling polyplex binding and non-viral transfection. Biomaterials, 32(9), 2432–2444. 10.1016/j.biomaterials.2010.12.004 [DOI] [PubMed] [Google Scholar]
- Parelkar SS, Letteri R, Chan-Seng D, Zolochevska O, Ellis J, Figueiredo M, & Emrick T (2014). Polymer-peptide delivery platforms: Effect of oligopeptide orientation on polymer-based DNA delivery. Biomacromolecules, 15(4), 1328–1336. 10.1021/bm401878p [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng L-H, Niu J, Zhang C-Z, Yu W, Wu J-H, Shan Y-H, … Gao J-Q (2014). TAT conjugated cationic noble metal nanoparticles for gene delivery to epidermal stem cells. Biomaterials, 35(21), 5605–5618. 10.1016/j.biomaterials.2014.03.062 [DOI] [PubMed] [Google Scholar]
- Ping Y, Hu Q, Tang G, & Li J (2013). FGFR-targeted gene delivery mediated by supramolecular assembly between beta-cyclodextrin-crosslinked PEI and redox-sensitive PEG. Biomaterials, 34(27), 6482–6494. 10.1016/j.biomaterials.2013.03.071 [DOI] [PubMed] [Google Scholar]
- Qian Y, Zha Y, Feng B, Pang Z, Zhang B, Sun X, … Jiang X (2013). PEGylated poly(2-(dimethylamino) ethyl methacrylate)/DNA polyplex micelles decorated with phage-displayed TGN peptide for brain-targeted gene delivery. Biomaterials, 34(8), 2117–2129. 10.1016/j.biomaterials.2012.11.050 [DOI] [PubMed] [Google Scholar]
- Ramsey JD, Vu HN, & Pack DW (2010). A top-down approach for construction of hybrid polymer-virus gene delivery vectors. Journal of Controlled Release, 144(1), 39–45. 10.1016/j.jconrel.2010.01.031 [DOI] [PubMed] [Google Scholar]
- Safety Study of phIL-12–005/PPC to Treat Recurrent Ovarian Cancer. (2013). Retrieved from https://ClinicalTrials.gov/show/NCT00137865
- Smith ML, Corbo T, Bernales J, Lindbo JA, Pogue GP, Palmer KE, & McCormick AA (2007). Assembly of trans-encapsidated recombinant viral vectors engineered from tobacco mosaic virus and Semliki Forest virus and their evaluation as immunogens. Virology, 358(2), 321–333. 10.1016/j.virol.2006.08.040 [DOI] [PubMed] [Google Scholar]
- Song W-J, Du J-Z, Sun T-M, Zhang P-Z, & Wang J (2010). Gold nanoparticles capped with polyethyleneimine for enhanced siRNA delivery. Small, 6(2), 239–246. 10.1002/smll.200901513 [DOI] [PubMed] [Google Scholar]
- Spitzweg C, Dietz AB, O’Connor MK, Bergert ER, Tindall DJ, Young CYF, & Morris JC (2001). In vivo sodium iodide symporter gene therapy of prostate cancer. Gene Therapy, 8, 1524–1531. 10.1038/sj.gt.3301558 [DOI] [PubMed] [Google Scholar]
- Springsteen G, & Wang B (2002). A detailed examination of boronic acid-diol complexation. Tetrahedron, 58, 5291–5300. [Google Scholar]
- Srinivasachari S, Liu Y, Zhang G, Prevette L, & Reineke TM (2006). Trehalose click polymers inhibit nanoparticle aggregation and promote pDNA delivery in serum. Journal of the American Chemical Society, 128(25), 8176–8184. 10.1021/ja0585580 [DOI] [PubMed] [Google Scholar]
- Starpharma. (2018). DEP™ docetaxel summary and commercial opportunity. Retrieved from https://starpharma.com/drug_delivery/dep_docetaxel
- Stege A, Krühn A, & Lage H (2010). Overcoming multidrug resistance by RNA interference In Zhou J (Ed.), Multi-drug resistance in cancer (pp. 447–465). Totowa, NJ: Humana Press. [DOI] [PubMed] [Google Scholar]
- Sun C, Tang T, & Uludağ H (2012). Molecular dynamics simulations for complexation of DNA with 2 kDa PEI reveal profound effect of PEI architecture on complexation. Journal of Physical Chemistry B, 116(8), 2405–2413. 10.1021/jp211716v [DOI] [PubMed] [Google Scholar]
- Takae S, Miyata K, Oba M, Ishii T, Nishiyama N, Itaka K, … Kataoka K (2008). PEG-detachable polyplex micelles based on disulfide-linked block catiomers as bioresponsive nonviral gene vectors. JACS, 130, 6001–6009. [DOI] [PubMed] [Google Scholar]
- Tanaka T, Yui Y, Naka N, Wakamatsu T, Yoshioka K, Araki N, … Itoh K (2013). Dynamic analysis of lung metastasis by mouse osteosarcoma LM8: VEGF is a candidate for anti-metastasis therapy. Clinical & Experimental Metastasis, 30(4), 369–379. 10.1007/s10585-012-9543-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thaker PH, Brady WE, Bradley WH, Anwer K, & Alvarez RD (2015). Phase I study of intraperitoneal IL-12 plasmid formulated with PEG-PEI-cholesterol lipopolymer administered in combination with pegylated liposomal doxorubicin in recurrent or persistent epithelial ovarian, fallopian tube, or primary peritoneal cancer patients: An NRG/GOG study. Journal of Clinical Oncology, 33(Suppl. 15), 5541 10.1200/jco.2015.33.15_suppl.5541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas CE, Ehrhardt A, & Kay MA (2003). Progress and problems with the use of viral vectors for gene therapy. Nature Reviews Genetics, 4(5), 346–358. 10.1038/nrg1066 [DOI] [PubMed] [Google Scholar]
- Titomirov AV, Sukharev S, & Kistanova E (1991). In vivo electroporation and stable transformation of skin cells of newborn mice by plasmid DNA. Biochimica et Biophysica Acta, 1088, 131–134. [DOI] [PubMed] [Google Scholar]
- Tomalia D, Baker H, Dewald J, Hall M, Kallos G, Martin S, … Smith P (1985). A new class of polymers: Starburst-dendritic macromolecules. Polymer Journal, 17(1), 117–132. [Google Scholar]
- Tros de Ilarduya C, Sun Y, & Düzgüneş N (2010). Gene delivery by lipoplexes and polyplexes. European Journal of Pharmaceutical Sciences, 40(3), 159–170. 10.1016/j.ejps.2010.03.019 [DOI] [PubMed] [Google Scholar]
- Wang CHK, Chan LW, Johnson RN, Chu DSH, Shi J, Schellinger JG, … Pun SH (2011). The transduction of coxsackie and adenovirus receptor-negative cells and protection against neutralizing antibodies by HPMA-co-oligolysine copolymer-coated adenovirus. Biomaterials, 32(35), 9536–9545. 10.1016/j.biomaterials.2011.08.069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Yin H, Yin Ng JC, Cai L, Li J, Tang BZ, & Liu B (2013). Polyethyleneimine-grafted hyperbranched conjugated polyelectrolytes: Synthesis and imaging of gene delivery. Polymer Chemistry, 4(20), 5297–5304. 10.1039/c3py00020f [DOI] [Google Scholar]
- Wang J, Lei Y, Xie C, Lu W, Yan Z, Gao J, … Liu M (2013). Targeted gene delivery to glioblastoma using a C-end rule RGERPPR peptide-functionalised polyethylenimine complex. International Journal of Pharmaceutics, 458(1), 48–56. 10.1016/j.ijpharm.2013.10.017 [DOI] [PubMed] [Google Scholar]
- Xu B, Wiehle S, Roth JA, & Cristiano RJ (1998). The contribution of poly-L-lysine, epidermal growth factor and streptavidin to EGF/PLL/DNA polyplex formation. Gene Therapy, 5(9), 1235–1243. 10.1038/sj.gt.3300719 [DOI] [PubMed] [Google Scholar]
- Yadav S, van Vlerken LE, Little SR, & Amiji MM (2009). Evaluations of combination MDR-1 gene silencing and paclitaxel administration in biodegradable polymeric nanoparticle formulations to overcome multidrug resistance in cancer cells. Cancer Chemotherapy and Pharmacology, 63(4), 711–722. 10.1007/s00280-008-0790-y [DOI] [PubMed] [Google Scholar]
- Yamagata M, Kawano T, Shiba K, Mori T, Katayama Y, & Niidome T (2007). Structural advantage of dendritic poly(L-lysine) for gene delivery into cells. Bioorganic and Medicinal Chemistry, 15(1), 526–532. 10.1016/j.bmc.2006.09.033 [DOI] [PubMed] [Google Scholar]
- Yan J, Springsteen G, Deeter S, & Wang B (2004). The relationship among pKa, pH, and binding constants in the interactions between boronic acids and diols—It is not as simple as it appears. Tetrahedron, 60(49), 11205–11209. 10.1016/j.tet.2004.08.051 [DOI] [Google Scholar]
- Yan Y, Xiong H, Zhang X, Cheng Q, & Siegwart DJ (2017). Systemic mRNA delivery to the lungs by functional polyester-based carriers. Biomacromolecules, 18(12), 4307–4315. 10.1021/acs.biomac.7b01356 [DOI] [PubMed] [Google Scholar]
- Yang XZ, Dou S, Wang YC, Long HY, Xiong MH, Mao CQ, … Wang J (2012). Single-step assembly of cationic lipid-polymer hybrid nanoparticles for systemic delivery of siRNA. ACS Nano, 6(6), 4955–4965. 10.1021/nn300500u [DOI] [PubMed] [Google Scholar]
- Yarmush ML, Golberg A, Sersa G, Kotnik T, & Miklavcic D (2014). Electroporation-based technologies for medicine: Principles, applications, and challenges. Annual Review of Biomedical Engineering, 16, 295–320. 10.1146/annurev-bioeng-071813-104622 [DOI] [PubMed] [Google Scholar]
- Yin H, Kanasty RL, Eltoukhy AA, Vegas AJ, Dorkin JR, & Anderson DG (2014). Non-viral vectors for gene-based therapy. Nature Reviews Genetics, 15(8), 541–555. 10.1038/nrg3763 [DOI] [PubMed] [Google Scholar]
- Yin M, Ding K, Gropeanu RA, Shen J, Berger R, Weil T, & Müllen K (2008). Dendritic star polymers for efficient DNA binding and stimulus-dependent DNA release. Biomacromolecules, 9(11), 3231–3238. 10.1021/bm800797j [DOI] [PubMed] [Google Scholar]
- Yu M, Liu L, & Wang S (2008). Water-soluble dendritic-conjugated polyfluorenes: Synthesis, characterization, and interactions with DNA. Journal of Polymer Science Part A: Polymer Chemistry, 46(22), 7462–7472. 10.1002/pola.23051 [DOI] [Google Scholar]
- Zhang C, Ji J, Shi X, Zheng X, Wang X, & Feng F (2018). Synthesis of structurally defined cationic polythiophenes for DNA binding and gene delivery. ACS Applied Materials & Interfaces, 10(5), 4519–4529. 10.1021/acsami.7b17948 [DOI] [PubMed] [Google Scholar]
- Zhao J, Zhang Z-R, Zhao N, Ma B-A, & Fan Q-Y (2015). VEGF silencing inhibits human osteosarcoma angiogenesis and promotes cell apoptosis via PI3K/AKT signaling pathway. International Journal of Clinical and Experimental Medicine, 8(8), 12411–12417. [PMC free article] [PubMed] [Google Scholar]
- Zhu C, Liu L, Yang Q, Lv F, & Wang S (2012). Water-soluble conjugated polymers for imaging, diagnosis, and therapy. Chemical Reviews, 112(8), 4687–4735. 10.1021/cr200263w [DOI] [PubMed] [Google Scholar]
- Zolochevska O, Ellis J, Parelkar S, Chan-Seng D, Emrick T, Wei J, … Figueiredo ML (2013). Interleukin-27 gene delivery for modifying malignant interactions between prostate tumor and bone. Human Gene Therapy, 24(12), 970–981. 10.1089/hum.2013.091 [DOI] [PMC free article] [PubMed] [Google Scholar]


