Objectives:
The aim of this study was to determine electric-acoustic masking in cochlear implant users with ipsilateral residual hearing and different electrode insertion depths and to investigate the influence on speech reception. The effects of different fitting strategies—meet, overlap, and a newly developed masking adjusted fitting (UNMASKfit)—on speech reception are compared. If electric-acoustic masking has a detrimental effect on speech reception, the individualized UNMASKfit map might be able to reduce masking and thereby enhance speech reception.
Design:
Fifteen experienced MED-EL Flex electrode recipients with ipsilateral residual hearing participated in a crosssover design study using three fitting strategies for 4 weeks each. The following strategies were compared: (1) a meet fitting, dividing the frequency range between electric and acoustic stimulation, (2) an overlap fitting, delivering part of the frequency range both acoustically and electrically, and (3) the UNMASKfit, reducing the electric stimulation according to the individual electric-on-acoustic masking strength. A psychoacoustic masking procedure was used to measure the changes in acoustic thresholds due to the presence of electric maskers. Speech reception was measured in noise with the Oldenburg Matrix Sentence test.
Results:
Behavioral thresholds of acoustic probe tones were significantly elevated in the presence of electric maskers. A maximum of masking was observed when the difference in location between the electric and acoustic stimulation was around one octave in place frequency. Speech reception scores and strength of masking showed a dependency on residual hearing, and speech reception was significantly reduced in the overlap fitting strategy. Electric- acoustic stimulation significantly improved speech reception over electric stimulation alone, with a tendency toward a larger benefit with the UNMASKfit map. In addition, masking was significantly inversely correlated to the speech reception performance difference between the overlap and the meet fitting.
Conclusions:
(1) This study confirmed the interaction between ipsilateral electric and acoustic stimulation in a psychoacoustic masking experiment. (2) The overlap fitting yielded poorer speech reception performance in stationary noise especially in subjects with strong masking. (3) The newly developed UNMASKfit strategy yielded similar speech reception thresholds with an enhanced acoustic benefit, while at the same time reducing the electric stimulation. This could be beneficial in the long-term if applied as a standard fitting, as hair cells are exposed to less possibly adverse electric stimulation. In this study, the UNMASKfit allowed the participants a better use of their natural hearing even after 1 month of adaptation. It might be feasible to transfer these results to the clinic, by fitting patients with the UNMASKfit upon their first fitting appointment, so that longer adaptation times can further improve speech reception.
Keywords: Cochlear implant, Electric-acoustic stimulation, Fitting strategy, Ipsilateral masking, Residual hearing, Speech reception
INTRODUCTION
Users of electric-acoustic stimulation (EAS) combine the stimulation via a cochlear implant (CI) with residual acoustic hearing in the same ear (von Ilberg et al. 1999; Kiefer et al. 2004). Typically a hearing aid (HA), inbuilt into the speech processor, delivers low-frequency acoustic stimulation and complements the CI, which delivers the previously inaudible high frequencies. Overall, adding acoustic stimulation to the electric stimulation via the implant has shown a clear benefit, especially in adverse listening conditions such as speech in background noise (Turner et al. 2004; Gifford et al. 2013), even with marginal residual hearing (Büchner et al. 2009). An increasing portion of the CI population uses the combined EAS, as during the past decade less traumatic surgical techniques (Gantz & Turner 2004; Gstoettner et al. 2004) and softer electrode designs (Lenarz et al. 2009; Suhling et al. 2016) showed successful hearing preservation. This led to an extension of CI criteria toward patients with more residual hearing (Skarzynski et al. 2007). Consequently, the question of how to deliver the best possible stimulation to this increasing number of patients has become a focus of interest of researchers and clinicians.
Still, no clear consensus exists on how to optimally program the speech processor that delivers both acoustic and electric stimulation. The two stimulation modalities complement each other by sharing and dividing the input frequency range, but are otherwise independent from each other. EAS devices include an additional fitting parameter to control the bandwidth assigned to the acoustic and the electric stimulation, the so-called crossover frequency. The division of spectral information and assignment to acoustic or electric stimulation has been investigated in several studies with CI users with residual hearing (for a review, see Incerti et al. 2013) with large variability of fittings reported for optimal speech reception performance. Especially for bimodal stimulation, that is, a CI and a HA in the contralateral, nonimplanted ear, a maximal bandwidth for both acoustic and electric stimulation, which results in an overlap between acoustic and electric frequency range, is generally considered to be the optimal choice for speech reception outcomes (Zhang et al. 2010b; Sheffield & Gifford 2014). However, there are also studies showing better speech reception performance in bimodal stimulation with limited CI bandwidth (Fowler et al. 2016; Gifford et al. 2017). This variability is increased across speech perception outcomes with ipsilateral EAS. Several studies report a subjective preference and maximal speech reception outcomes for a fitting strategy where acoustic and electric stimulation divide the input frequency range at a certain frequency with neither overlap nor a gap between stimulated frequency ranges (Fraysse et al. 2006; Vermeire et al. 2008; Karsten et al. 2013). In these studies, a physiological gap between residual hearing and electrode insertion can be assumed, as insertion depths ranged between 10 and 20 mm. Some interfering effects between electric and acoustic stimulation might limit the possible advantage of delivering information through two modes of stimulation when these two modes are presented ipsilaterally. These effects do not seem to be influenced by a spectral gap between the frequency ranges, as Karsten et al. (2013) did not find better speech reception outcomes for EAS subjects that were programmed with a spectral gap fitting of half an octave. In a study investigating EAS overlap for standard length electrode recipients, there were variable outcomes with some listeners achieving best outcomes with overlap and others with minimal-to-no overlap (Gifford et al. 2017).
In addition, the optimal distance between electric and acoustic stimulation for the best EAS speech reception performance and long-term preservation of residual hearing is still unknown, and several aspects have to be taken into account. Different studies found varying effects of electrode insertion on speech reception for electric stimulation, with some reporting better performance with deeper insertion (Hochmair et al. 2003; Hamzavi & Arnoldner 2006; Buchman et al. 2014; Büchner et al. 2017) but others reporting no effect of insertion depth (van der Jagt et al. 2016). At the same time, deeper insertion has been shown to adversely affect hearing preservation in the implanted ear (O'Connell et al. 2017). It increases the risk of insertion trauma (Adunka & Kiefer 2006) resulting in hair cell apoptosis (Eshraghi & van De Water 2006) as well as the possibility of adverse effects of electric stimulation on the survival of outer hair cells (Dodson et al. 1986).
Recent studies found that combined electric and acoustic stimulation in the same ear shows masking during simultaneous (Lin et al. 2011; Krüger et al. 2017) and nonsimultaneous presentation (Imsiecke et al. 2018). The results indicate that better residual hearing in the EAS population leads to an increase in masking. Krüger et al. (2017) found an asymmetry between electric and acoustic maskers with electric maskers producing a pronounced threshold elevation (TE) of acoustic probes that depended strongly on the electric-acoustic frequency difference (EAFD). The EAFD describes the distance between electric and acoustic stimulation in octaves of the place frequency. The effects of acoustic maskers on electric probes on the contrary showed a very broad range of masking and a less pronounced dependency on EAFD. Forward masking effects show the same asymmetry and decay times of around 100 msec were found for electric and acoustic forward masking (Imsiecke et al. 2018). Animal studies have also shown the reduction of acoustically evoked neural responses with additional electric stimulation in animals with high-frequency hearing loss (Stronks et al. 2011) and more strongly in normal hearing animals (Stronks et al. 2010). Electrophonic interaction, that is, the electric stimulation of hair cells, might be the origin of the observed masking effects (Stronks et al. 2011). Alternatively, electroneural interaction which reduces the response of the neurons to acoustic stimulation following electric stimulation is also possible (Nourski et al. 2005). With even deeper insertion of electrodes and improved preservation of residual hearing at the same time, masking might indeed become clinically relevant. The question whether masking has an influence on the speech reception performance of EAS users has not previously been investigated.
In EAS users, masking between electric and acoustic stimulation may be of special importance, as interaction effects could have a severe impact on the information delivered by the HA or the CI. Temporal fine structure plays an important role in speech recognition especially in temporally fluctuating background noises (e.g., babble noise or reverberation). In CI users, speech reception in quiet is influenced by the ability to identify gaps with different durations (Sagi et al. 2009) and speech reception in noise is severely limited. In EAS, additional acoustic stimulation supplements information mainly in the low frequencies (Zhang et al. 2010a) for early EAS implant recipients and increasingly in medial frequencies in users with better residual hearing (Sheffield & Gifford 2014) and thus potentially restores temporal fine structure (Vaerenberg et al. 2013). This may benefit speech reception in noise (Büchner et al. 2009) as well as music and pitch perception (Dorman et al. 2008). Currently, the gain applied to the HA and the fitting parameters of the CI are adjusted without taking into account possible interactions such as masking effects between acoustic and electric stimulation. Acoustic stimulation is always delivered to the full possible range, limited by the residual hearing of the implanted ear. At the same time, electrodes are inserted deeply into the cochlea and due to the large spread of excitation (Hughes et al. 2013; Padilla & Landsberger 2016), a wide range of neurons is stimulated, possibly even the same neurons that are activated by acoustic stimulation. Thus speech reception outcomes might be optimized by introducing a physiological gap between electric and acoustic stimulation in cases of strong interaction. An investigation of masking effects and their relation to speech reception performance is necessary to expand the knowledge of electric-acoustic interaction. Previous studies investigating the effect of EAS fitting did not investigate the relation between masking and speech reception, which might explain some of the variability observed in the literature. As masking effects are characteristic to a normal hearing system, similarly, electric-acoustic masking might not influence an impaired hearing system of CI and residual hearing. On the other hand, detriments to speech reception might arise from masking between electric and acoustic stimulation by limiting the available information.
The present study therefore examines psychoacoustic masking in EAS users with a simultaneous masking paradigm and relates this measure to speech reception performance under different fitting conditions. Fitting strategies were tested that implement changes in crossover frequency between electric and acoustic stimulation as well as adjustment of electric stimulation levels to minimize interaction effects and thus optimize speech reception in EAS users. As the acoustic stimulation in EAS users conveys both voice pitch (F0) and temporal fine structure and thus a more natural signal, it was considered feasible to convey the full acoustic range. Also, previous studies investigating acoustic masking showed that it is weaker in comparison to electric masking and affects a wider range of electric stimuli (Stronks et al. 2012; Krüger et al. 2017), possibly making it more difficult to effectively reduce the influence of acoustic masking. Thus the present study focuses on electric masking and does not investigate or discuss the phenomenon of acoustic masking. The results are investigated for their implications for the clinical routine of fitting patients with EAS devices.
MATERIALS AND METHODS
Subjects
Of 18 included CI users with a severe to profound high frequency hearing loss but normal to severe low frequency hearing loss, 15 completed the full study. Active users of EAS using the ipsilateral HA in their daily life were included as subjects. Subjects had been implanted with MED-EL electrodes of the FLEX-Series (Flex28, Flex24, Flex20 or the Hanover Custom Made Device Flex16; Timm et al. 2018) at least 10 months before the first appointment. Some subjects did not receive a full insertion, instead, the electrode was only partially inserted (Lenarz et al. 2019), this is indicated in Table 1. All subjects had residual acoustic hearing at middle to low frequencies, with a high variability between subjects. The clinical pure-tone audiograms at the time of the first appointment are shown in Figure 1. Subjects are ordered according to their low frequency pure-tone average (PTA) for the frequencies 125 to 500 Hz. The gray line indicates the typical crossover frequency for EAS users. The demographic data for the study participants are given in Table 1. All subjects gave written informed consent to the experiment as approved by the Hanover Medical School's Institutional Review Board.
TABLE 1.
Subject data with subject ID, gender, side of implantation, age at testing for present study, duration of implant use, electrode type, with PI indicating partial insertion, insertion depth angle, and corresponding place frequency according to the spiral ganglion map of Stakhovskaya et al. (2007) as well as crossover frequency of the acoustic stimulation
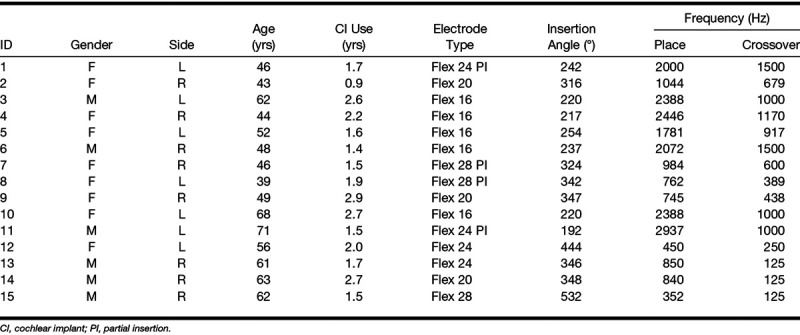
Fig. 1.
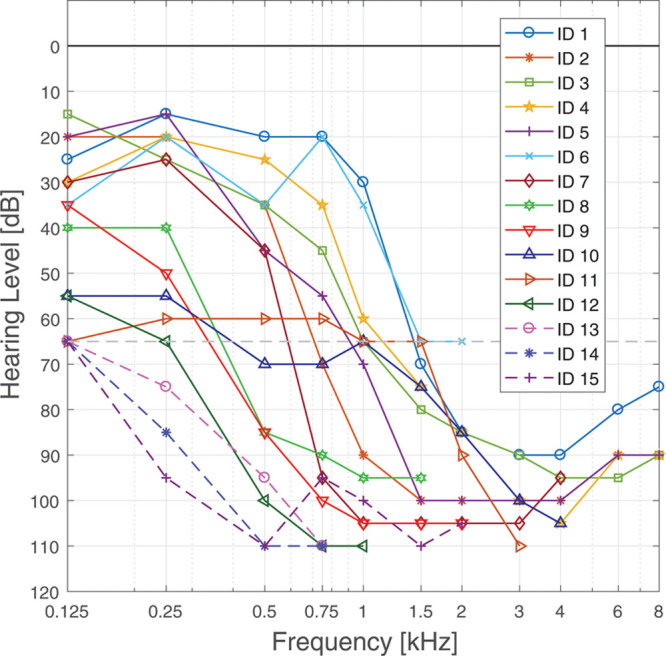
Clinical audiograms of all subjects at time of testing. The gray line indicates 65 dB HL to identify the individual crossover frequency.
Psychoacoustic Masking Experiment
Stimuli •
Electric and acoustic stimuli were presented during this experiment, based on the software described by Krüger et al. (2017). Electric maskers were 500 msec and acoustic probes were 200 msec long and controlled in Matlab (MathWorks Inc., MA). The shorter acoustic probe tone was presented simultaneously to the longer electric masker stimulus, the probe started 150 msec after the start of the masker, which placed it at the center of the masker. Electric masker stimuli were 1000 pps pulse trains presented at maximum comfortable level (100% dynamic range [DR]) at the 5 most apical electrodes. They consisted of biphasic pulses with 33 µsec length, had an interphase gap of 2.1 µsec and were stimulated directly through a research interface (MED-EL RIB2 interface). Acoustic probe stimuli were generated by a NI-DAQ sound card (National Instruments, Austin, TX) and delivered via Sennheiser HDA-200 headphones (Sennheiser electronic GmbH & Co. KG, Wedemark, Germany) connected to a headphone amplifier (Lake People electronic GmbH, Konstanz, Germany). Presented frequencies depended on residual hearing, they are indicated in Figure 4. Acoustic stimulation was calibrated with an artificial ear and levelmeter (Brüel & Kjäer, Naerum, Denmark). Synchrony between electric and acoustic stimulation was obtained by a trigger out signal of the research interface connected to a trigger in channel of the sound card.
Fig. 4.
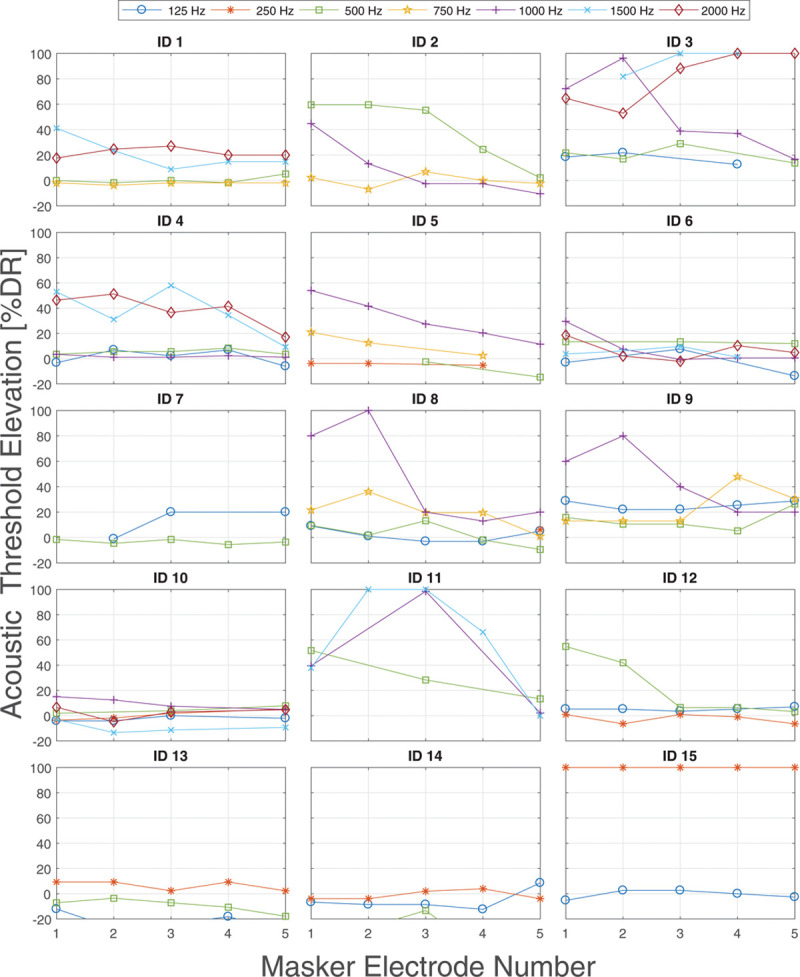
Individual results of threshold elevation of acoustic probes due to electric maskers in % DR for all subjects. The DR is different for each subject and frequency. DR indicates dynamic range.
Procedure
Initially, loudness balancing was conducted across electrodes to ensure the masker stimuli were all presented at equally perceived loudness levels, by adjusting each stimulated electrode to the fixed stimulation of an adjacent electrode twice (for greater detail, see Krüger et al. 2017). Levels of electric stimuli were adjusted in dBel with a reference value of 1 µA. Subsequently, the psychoacoustic masking experiment was started with different combinations of maskers and probes for the simultaneous masking experiment. Threshold estimates were obtained with a 3-interval-2-alternative forced-choice experiment. The last four turning points of the adaptive paradigm were averaged to obtain the threshold value and variance. If the standard deviation exceeded 4 dB, two times the final step size, the corresponding threshold estimate was rejected from analysis. Whenever this became apparent during the measurement (i.e., by strongly fluctuating runs), the threshold estimate was repeated. Unmasked threshold estimations were repeated to obtain more reliable results. The test–retest variability for repeated threshold estimates was 3.1 dB.
Changes in threshold from unmasked to masked presentation were measured. In some subjects, it was not possible to determine some masked thresholds due to masking effects greater than the possible level of the probe (upper comfortable level). The subjects were instructed to repeat the task of detecting the right interval for at least 20 presentations of that combination and to guess at the right answer. As the paradigm will not easily converge with only chance performance, the run was aborted if after these 20 presentations the subject still reported to not be able to detect the test tone and the performance was below or at chance level. The corresponding threshold was set to maximum level.
Data Analysis
Acoustic thresholds were measured with and without the presence of electric maskers. Acoustic threshold level (THL) and masked acoustic THL in dB were subtracted to obtain TE for each combination of probe frequency and masker electrode according to the equation used by Lin et al. (2011):
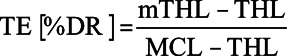 |
For the evaluation of the strength of masking, TE was calculated in percentage DR, where DR is defined as the dB difference between most comfortable level and THL. This was necessary, because the range between just audible and comfortable level of probes differs strongly between subjects but also between frequencies, the individual results of DR of probe frequencies and masker electrodes are shown in Figure 2. DR was used as a scale to compare electric and acoustic stimulation. Experiments in normal hearing animals indicate a monotonic relation between electric masker level and masking strength (Stronks et al. 2010). Previous studies modeling masking in electric or acoustic hearing used a linear relation between masker level and TE (Baumgarte et al. 1997; Nogueira et al. 2005). For the sake of simplicity, we also assumed a linear relationship between electric masker level and TE in the scale of DR.
Fig. 2.
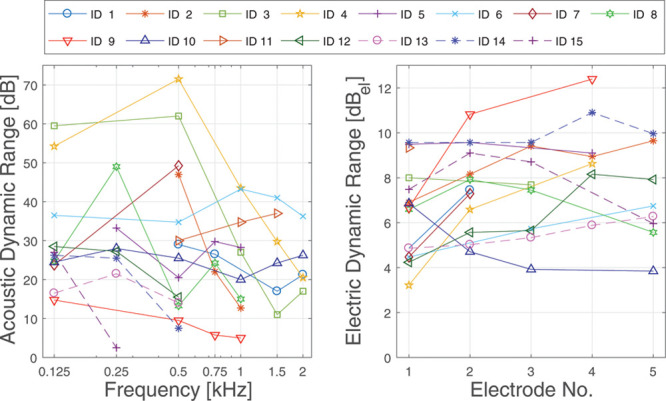
Dynamic range in dB of acoustic (left) and electric (right) stimulation for all subjects.
Krüger et al. (2017) introduced the EAFD measurement which was included to interpret the obtained data with regard to the stimulation site separation of electric and acoustic stimuli. The frequency map of Stakhovskaya et al. (2007) was used to assign place frequencies to insertion angles which were obtained for all electrodes from medical imaging data. Masking is consequently interpreted using the EAFD for all subjects to ensure comparability independent of geometry and electrode length. Individual TE was tested for significance with a bootstrapping method according to Aronoff et al. (2011).
Fitting Strategies
To investigate the influence of masking effects on speech reception in each individual subject, different fitting strategies with FS4 sound coding were programmed. Three strategies were employed, a meet map conforming to the clinical practice of the German Hearing Center Hannover, an overlap map, and a newly designed masking adjusted fitting (UNMASKfit).
For each map, the acoustic amplification delivered by the inbuilt HA component was kept unchanged. It was adjusted to compensate each subject's individual hearing loss. The bandwidth of amplification extended to the frequency at which the subject's hearing loss exceeds 65 dB hearing level (HL), defined as the crossover frequency (Kiefer et al. 2005). The descriptor “overlap” used here refers to the spectral overlap, not a physiological overlap. Physiological overlap was not identified in any of the subjects that participated in the study for the frequencies delivered by the acoustic component. The meet and UNMASKfit map have a continuous spectral distribution, with the physiological gap being increased in the UNMASKfit map.
The three maps differed in their setting of electric upper levels and frequency allocation for apical electrodes, as illustrated in Figure 3. At the first appointment, electric comfortable and THLs were determined with the subjects using a 10-step scale for behavioral loudness scaling and loudness balancing. Balancing was conducted for all electrodes by balancing four adjacent electrodes at a time and shifting two electrodes, until the whole array is covered. Individual settings such as deactivation of electrodes or reduction of level due to unpleasant side effects were preserved in each map.
Fig. 3.
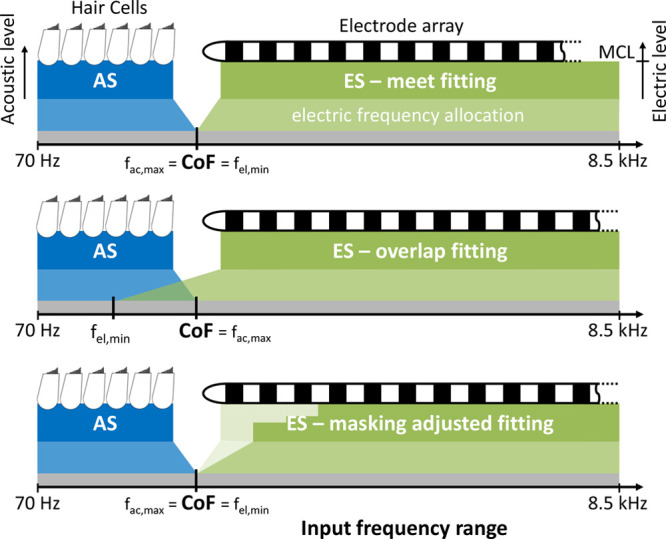
Schematic overview over the three fitting strategies used in the study: meet fitting that divides the ES and AS at the CoF (top), overlap fitting with extended electric frequency range (middle), and UNMASKfit map with reduced electric stimulation levels (bottom). AS indicates acoustic stimulation; CoF, crossover frequency; ES, electric stimulation; MCL, most comfortable level; UNMASKfit, masking adjusted fitting.
Meet Map •
Standard clinical fitting of electric stimulation. Electric frequency bandwidth started at the default crossover frequency and delivered up to 8500 Hz, distributed logarithmically across all active electrode contacts.
Overlap Map •
Extended fitting of electric map to overlap with the acoustic map and to possibly result in informational or electrophonic masking of the acoustic information. Electric lower frequency was decreased to also deliver frequencies up to two octaves below the crossover frequency, based on the upper range of overlap applied by Vermeire et al. (2008). As a complete shift of the frequency, allocation table was assumed to interfere with the adaptation to the new map, a frequency band shift was only allowed for the three most apical electrodes. In 1 subject (ID 2), even this change in frequency allocation was not tolerated upon fitting the map, so overlap was reduced to one octave.
UNMASKfit •
Reduced electric fitting to decrease masking on acoustic stimulation. Electric lower frequency started at the crossover frequency and levels were reduced from most comfortable levels according to each subject's individual strength of masking. The following rules were applied to achieve the reduction while trying to prevent potential loss of information due to the decrease of electric stimulation:
Assessment of the individual electric on acoustic masking effects, given in percentage of DR of each acoustic probe. If subjects did not show masking, a reduction of levels was applied that corresponded to the mean reduction exhibited by the other subjects.
Based on the assumption of linearity of electric masking strength with masker level, a linear reduction of electric DR was applied to achieve masking of less than 20% DR for the acoustic stimulation.
Electrodes were switched off, if the new level lay below THL or if the new level lay below 60% of the original DR. Judging by the loudness growth functions shown by Shannon (1985) and McKay et al. (2003), the upper half of the DR contributes the most to the perceived loudness, and stimulation below this level might become inaudible or too soft to still deliver enough information.
A maximum of one electrode was switched off, its frequency band was then allocated to the neighboring two electrodes to preserve the frequency allocation table for middle and basal electrodes. Reduction of levels for further electrodes was limited to 60% DR.
Study Design
Subjects used each strategy for a period of 4 weeks and at each return visit speech reception measurements were taken with the experienced fitting, before programming another experimental fitting strategy. Subjects were blinded to the specifications of the programmed fitting, but were asked to subjectively report on the differences in sound and speech reception. The test order of fitting conditions was randomized across subjects.
Speech Reception Measurements
Speech reception measurements were obtained for each study participant at each of the appointments. The Oldenburg Matrix Sentence test (OLSA) (Wagener et al. 1999) was employed to measure speech reception thresholds (SRTs) in noise. A procedure was used to reach the signal to noise ratio that yields 50% correctly repeated words by adapting the speech level and fixing the noise level at 65 dB SPL. The step size was changed adaptively according to the number of correct words, and SRTs calculated based on the estimated psychometric function. After initial training, 2 lists of 20 sentences were measured for each listening mode. Tested modes were EAS, electric and acoustic stimulation in stationary speech shaped noise of the OLSA speech material (Wagener & Brand 2005) as well as EAS in the ICRA-5 fluctuating noise with male speaker temporal modulations (ICRA EAS) (Dreschler et al. 2001). Speech tests were conducted in direct coupling, by connecting the speech processor to a headphone amplifier via audio cable. The two conditions electric-only and acoustic-only were achieved by removing either the earmold and processor from the ear or the coil from the implant receiver during the test lists. Hence it was certain that the subjects could not make use of their ipsilateral or contralateral residual hearing during electric only presentation. Previous to measurements with subjects, a Sonnet EAS speech processor and a MED-EL detectorbox, which emulates a receiver-stimulator, were used to calibrate the necessary loudness to achieve stimulation levels for direct coupling equal to the stimulation via free field loudspeakers calibrated to 65 dB SPL with stationary noise. It was adjusted so that the root mean square value of the electric stimulation on several electrodes, measured on an oscilloscope connected to the detectorbox, was identical for direct coupling and free field presentation.
The presentation conditions were tested randomly, and each condition was tested twice, with the requirement that test–retest variability stayed below 1 dB. If not, a third test list was measured and the deviating list omitted, which always resulted in the requirement being met. Values given for SRTs are mean values of the two lists, due to the small variability the SD is not shown.
RESULTS
Masking
Masked TE of acoustic probes was measured under the presence of electric masker electrodes. Across all subjects, electrodes, and frequencies, a mean TE of 3.2 ± 6.5 dB (bootstrapped P < 0.001) was observed, which corresponded to 14.9% DR mean TE. The individual masking results for the different combinations of frequencies and electrodes are shown in % DR in Figure 4. Masking was observed in several subjects for some of the tested probe frequencies, with higher frequencies and more apical electrodes often resulting in higher masking than lower frequencies. At the same time, some subjects did not show masking across all tested frequencies and electrodes. For these subjects, TE seemed to vary around zero, within the test–retest variability of 3.1 dB. A special case is subject ID 15, where masking for the tested frequency of 250 Hz is at 100% DR for all masker electrodes. This is deemed insignificant, as the absolute elevation was only 3 dB.
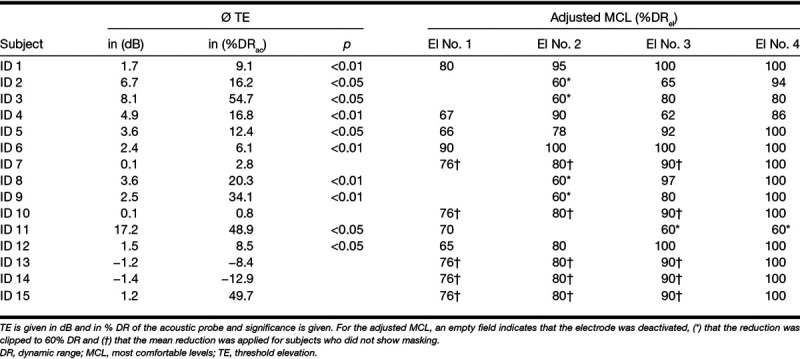
Collapsed across all combinations of probe frequency and masker electrode, 10 subjects showed a significant (bootstrapped p < 0.05) and of these 5 subjects showed a highly significant (p<0.01) mean TE, as shown in Table 2.
TABLE 2.
Mean TE of acoustic probes due to electric masking and resulting adjusted MCL of electric stimulation in the masking adjusted fitting
To allow a group analysis of masking the relation between electric and acoustic stimulation was taken into account, to cover the differences in electrode locations and amounts of residual hearing in the variable subject group. This relation is given in EAFD as the distance in octaves between acoustic stimulus frequency and electric place frequency according to Stakhovskaya et al. (2007). Figure 5 shows the results of absolute and % DR TE for acoustic probes under the influence of simultaneous electric maskers. The color indicates the maskers' comfortable levels in dBel. Stronger interaction effects are seen at smaller EAFDs, especially in the range of 1 to 2 octaves, with masking of up to 37 dB TE in 1 subject. At the same time, some subjects did not show masking, even at zero EAFD. The difference in masker level which are shown by the color scale could only explain a small portion of the variability, as determined by using a multiple linear model (R2 = 0.03, p < 0.01). Taking into account other factors such as probe frequency and threshold, insertion angle and EAFD did not result in R2 > 0.15, for any combination.
Fig. 5.
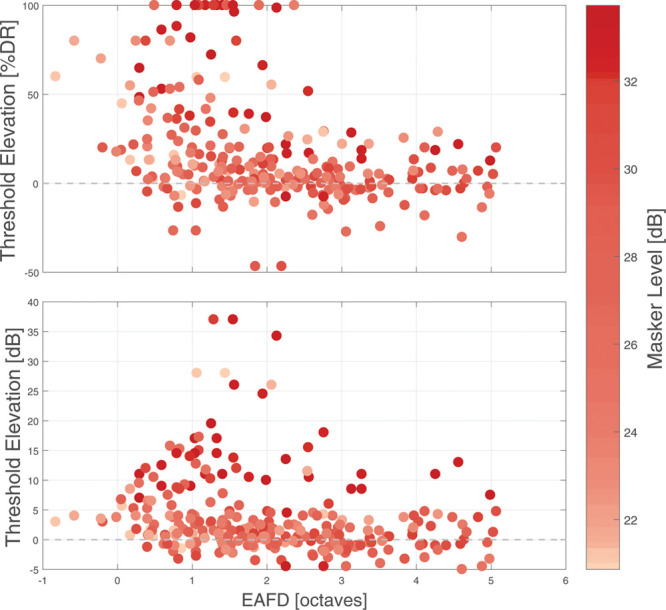
Threshold elevation of acoustic probes due to electric maskers in % DR (top) and in dB (bottom) for all subjects as a function of EAFD. The electric masker level is indicated as a color bar, with darker colors corresponding to lower thresholds. DR indicates dynamic range; EAFD, electric-acoustic frequency difference.
TE of 20% DR or below lay within the average test–retest variability as determined by pilot data as well as previous studies (Krüger et al. 2017; Imsiecke et al. 2018). To reduce the possibility of random variance affecting the reduction of electric stimulation, only TE of more than 20% DR was considered relevant. Consequently, the electric levels of the UNMASKfit map were reduced to reduce masking of the acoustically transmitted frequencies. The new levels applied for the different subjects are listed in Table 2. The subjects in whom no significant masking was observed did not receive an individual UNMASKfit map, but a mean UNMASKfit map, which is indicated in the table.
Speech Reception
Individual SRTs for each subject are shown in Figure 6 for the three fitting strategies (meet, overlap, UNMASKfit) at the four listening modes (EAS, acoustic stimulation, electric stimulation, ICRA EAS). Better speech reception scores are represented by negative values, which are oriented toward the top of the figures. SRTs with the acoustic listening mode could not be obtained for four subjects (IDs 12 to 15) as performance stayed below 50% words correct, even when additionally tested in quiet.
Fig. 6.
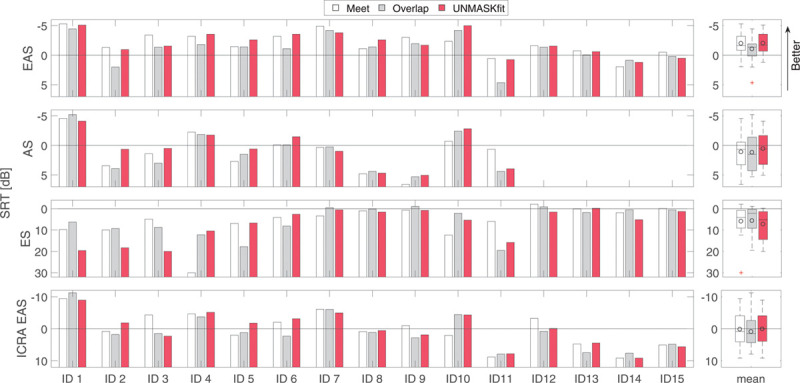
Individual SRTs, tested in listening modes EAS, ES, AS, and temporally modulated noise (ICRA EAS) with different fitting conditions meet (white), overlap (gray), and UNMASKfit. More negative SRTs indicate better performance. Box plots on the right show group statistics for the different listening modes. The circles denote mean values. AS indicates acoustic only; EAS, electric-acoustic stimulation; ES, electric only stimulation; ICRA EAS, ICRA-5 fluctuating noise with male speaker temporal modulations; SRT, speech reception thresholds; UNMASKfit, masking adjusted fitting.
To test the hypothesis whether fitting has an influence on SRT in the EAS listening mode, which corresponds to the everyday usage setting, a one-factor repeated-measures analysis of variance was calculated. Fitting strategy (meet, overlap, or UNMASKfit) was the independent variable and UNMASKfit fitting group (“individualized” or “mean”) the intersubject factor. Fitting showed a significant effect (F = 3.323; p = 0.05) on SRTs. Posthoc tests revealed a significant decrease of the overlap map (mean SRT −1.0 dB) to the meet (mean SRT −1.9 dB, F = 4.79; p = 0.047) and the UNMASKfit (mean SRT −2.0, F = 5.38; p = 0.037) map. Mean values improved 1 dB, which is the test–retest variability of the OLSA in negative SRT (Hey et al. 2014), but individual differences were higher, indicating the relevance of the difference in mean results. The meet and UNMASKfit map were not significantly different.
In addition, the hypothesis whether the listening mode has an influence on the changes in SRT elicited by the different fittings was analyzed by a two-factor analysis of variance by adding the factor listening mode (EAS, electric, ICRA EAS) as an independent variable. The acoustic listening mode had to be omitted due to missing data in four subjects. A highly significant effect of listening mode was found (F = 9.08; p < 0.001) and a significant interaction between UNMASKfit fitting group and listening mode (F = 3.76; p = 0.037), but no effect of fitting strategy (F = 1.226; p = 0.328) or interaction of fitting strategy with listening mode (F = 0.432; p = 0.783) was found. Intersubjects contrasts revealed that SRTs were significantly worse for ICRA EAS (F = 8.21; p = 0.01) and electric in stationary noise (F = 17.73; p < 0.001) listening mode than for EAS in stationary noise mode.
Group results can be compared using the box plots on the right in Figure 6. For the EAS listening mode in stationary noise, average SRTs for all three fitting strategies were negative (i.e., speech was softer than the noise), with small variability across subjects. The significant decrease in performance can be seen in the overlap fitting strategy. A poorer mean SRT can also be observed in the ICRA EAS listening mode for the overlap fitting, but it is not significant (p = 0.2) due to larger variability of the SRTs in this more demanding condition. The acoustic listening mode yields mostly positive SRTs and shows a higher variability, which is accounted for by the difference in residual hearing of the different subjects. This listening mode could not be completed by all subjects (N - 4), due to poor residual hearing. The electric listening mode shows very poor SRTs, as the crossover frequency severely limits the amount of low frequency information transmitted via the implant alone in many subjects. Better median SRTs for the overlap map can be seen in the electric listening mode.
The acoustic benefit, meaning the improvement of SRT from electric to EAS listening mode due to the residual natural hearing of the subjects is highly significant (F = 17.728; p < 0.001). As the EAS performance is similar in the meet and UNMASKfit map, but a lower mean performance in the electric listening mode can be observed, the acoustic benefit as the difference in mean performance is larger in the UNMASKfit map. This difference is bigger in the individual UNMASKfit map group (see Fig. 7, left). In contrast, the results for the mean UNMASKfit map group (Fig. 7, right) are not different across the different fitting conditions in EAS and ICRA EAS listening mode. However, this increase in acoustic benefit for the individual fitting group is not statistically significant (F = 2.08, p = 0.17 for UNMASKfit versus overlap and F = 0.87, p = 0.37 for meet versus overlap for listening modes EAS versus electric). Some subjects (e.g., ID 3, 11) showed a decrement in performance in the electric listening mode for both newly adjusted maps, and also did not show an increase in the UNMASKfit map in EAS mode, as opposed to subjects with similar performance across fittings in the electric stimulation mode (e.g., ID 5, 8).
Fig. 7.
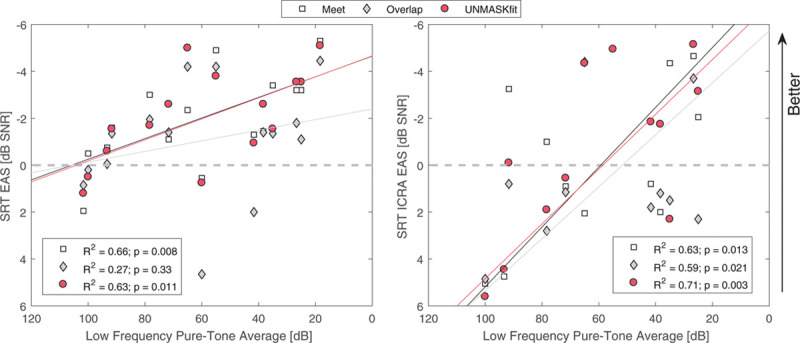
Speech reception thresholds, tested for conditions EAS, ES, AS, and temporally modulated noise with EAS (ICRA EAS) for grouped subjects with different fitting strategies meet (white), overlap (gray), and UNMASKfit (red). Subjects are grouped according to their fitting, that is, individualized (left) or mean UNMASKfit map (right). Box plots show the group statistics and the circles denote mean values. The text inset N − x for acoustic listening mode indicates the number of subjects that could not be tested. AS indicates acoustic only; EAS, electric-acoustic stimulation; ES, electric only stimulation; ICRA EAS, ICRA-5 fluctuating noise with male speaker temporal modulations; UNMASKfit, masking adjusted fitting.
Overall, speech performance in all subjects is significantly better with the combined mode of electric and acoustic stimulation, with some subjects benefiting more and others less from the additional acoustic component. There is a division between different subjects, some with good performance in EAS mode (EAS SRT < 0) and significant acoustic benefit, and the other group with poor or nonmeasurable SRTs in acoustic listening mode and better performance with the implant alone. The latter subjects showed crossover frequencies below 300 Hz and also a PTA above 65 dB HL. Figure 8 shows on the left that SRTs correlate significantly with better residual hearing for the meet and UNMASKfit (R2 = 0.63; p = 0.011) shown in red circles and white boxes. This is not the case for the overlap fitting gray diamonds, where the residual hearing does not improve SRTs significantly. The UNMASKfit does not improve SRTs with respect to the meet map, while the overlap map results in less improvement in SRT with respect to the meet map. On the right in Figure 8, SRTs for the ICRA EAS listening mode are shown for the different fitting strategies. All fitting conditions correlate significantly with PTA, with very similar correlation values for the meet (R2 = 0.63; p = 0.013) and overlap (R2 = 0.59; p = 0.021) maps and the highest correlation for the UNMASKfit map (R2 = 0.71; p = 0.003). There is no benefit or decrement of UNMASKfit or the overlap map with respect to the meet map.
Fig. 8.
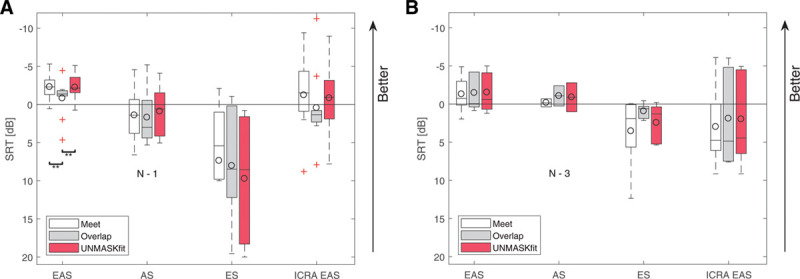
SRT for different fitting strategies meet, overlap, and UNMASKfit fitting for the EAS listening mode in stationary noise (left) and the ICRA fluctuating noise (right) as a function of the low frequency pure-tone average (125–500 Hz). AS indicates acoustic only; EAS, electric-acoustic stimulation; ES, electric only stimulation; SRT, speech reception threshold; UNMASKfit, masking adjusted fitting.
Figure 9 shows the improvement of SRT of the two fitting conditions overlap and UNMASKfit over the meet fitting for the listening condition EAS (left) and ICRA EAS (right) as a function of the masking described by the integral of TE. For this, the integral was calculated with a numerical rectangular approximation of the acoustic TE in dB across EAFD:
Fig. 9.
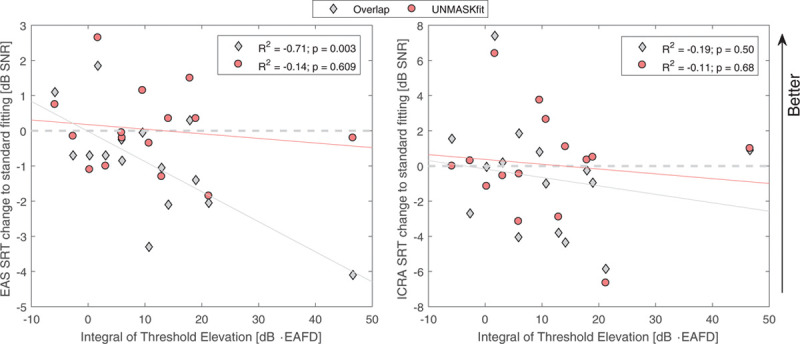
SRT benefit of overlap and UNMASKfit strategies in comparison to the meet fitting as a function of the integral over threshold elevation for the EAS (left) and the ICRA EAS (right) listening modes. EAFD, electric-acoustic frequency difference; EAS, electric-acoustic stimulation; SNR, signal to noise ratio; SRT, speech reception threshold; UNMASKfit, masking adjusted fitting.
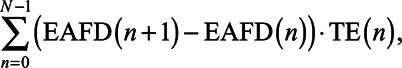 |
where N is the number of measured TE for each subject. For the EAS listening mode, no improvement of UNMASKfit is found over the meet fitting condition, and there is no relation to the strength of TE. However, the overlap fitting condition results in a significant decline of SRT with increasing electric masking (R2 = −0.71; p = 0.003). This relation is not present in the ICRA EAS listening mode, with the average SRT change below zero (mean change −0.69 dB) for the overlap fitting compared with the mean change of close to zero for the UNMASKfit strategy (mean change of 0.08 dB).
DISCUSSION
This study investigated electric-acoustic interaction by measuring masking for different electrodes and frequencies in subjects with different insertion depths and residual hearing. Strong masking was confirmed for small EAFDs. The results of this study show individual masking in 10 of 15 subjects. Masking was correlated to speech reception scores with the overlap fitting. It was hypothesized that masking influences speech reception which was confirmed for the overlap fitting in stationary noise. The overlap fitting resulted in significant worsening in SRT in subjects with strong masking. A masking adjusted map termed UNMASKfit was developed to reduce the masking strength and possibly restore previously lost information. The UNMASKfit map significantly improves speech performance with respect to the overlap map in subjects with masking. No significant difference was observed between the UNMASKfit and meet maps.
Electric Masking
In the electric masking experiment, the masking of acoustic probes under the influence of the stimulation from different masker electrodes was confirmed. Ten of the 15 subjects showed significant elevation of acoustic probe thresholds. For these subjects, a dependency on EAFD was observed with a peak in masking between one and two octaves. Some subjects exhibited masking greater than the audible range of the acoustic probe, resulting in a clipping effect for the results in %DR. The TE in dB decreases rapidly below one and above 2 octaves EAFD. The decrease in TE in dB below one octave is not visible in the scale of % DR. This is partially due to the fact that higher frequencies with more hearing loss were tested for the combinations with lower EAFD. This results in a decrease in DR, which exaggerates the masking effect in the scale of % DR.
In previous studies, Krüger et al. (2017) found electric on acoustic masking in 5 subjects with short 16 mm electrode arrays, with a maximum elevation below one octave and an exponential decay of TE. Lin et al. (2011) showed ipsilateral masking in a single subject implanted with a longer 24 mm electrode array. It is not possible to determine the EAFD of the subject that participated in the latter study, but Krüger et al. (2017) argued toward a similar exponentially decaying electric masking effect. Even though the TE varies more strongly in the present study due to some subjects not showing TE, the existence of electric masking can be confirmed in an EAS population with different electrode types, deeper insertion angles and less residual hearing. Subjects who did not show significant TE in this study had a tendency toward worse PTA than those subjects who did show masking and also than those tested by Krüger et al. (2017). Neither PTA, probe thresholds, rate of electric stimulation, insertion depth of electrodes nor masker comfortable levels predicted TE, indicating toward effects that cannot be quantified at this stage. Subjects who did not show masking were used as a control group for the UNMASKfit map, by applying a mean reduction to the electric stimulation.
The relation between TE and EAFD differs slightly from the results shown by Krüger et al. (2017), who reported a maximum TE for EAFDs below 1 octave. Two quantitative differences become clear. First, in this study, masking does not behave monotonically. Second, the peak in TE is shifted. Only 3 subjects with EAFDs between 0.5 and 1 octave were tested by Krüger et al. (2017), so that it cannot be determined whether masking might decrease for lower EAFD. The shift in maximum TE might be due to differences in electrode arrays. Different electrode array characteristics, that is, insertion depth, electrode location with respect to hair cells and auditory nerve, contact orientation and form as well as electric field spread, could result in different masking profiles. Alternatively, describing masking with the EAFD based on a place frequency estimate of the insertion angle according to the spiral ganglion map of Stakhovskaya et al. (2007) might be suboptimal for highly variable insertion depths, resulting in a misrepresentation of some subjects in the present study.
Speech Reception Results
Speech reception in the tested EAS users was measured for three fitting strategies, a meet programming strategy dividing the frequency range between electric and acoustic stimulation, an overlap strategy that expanded the electric frequency range, and the newly developed, individualized UNMASKfit, that reduced the electric stimulation in apical electrodes which elicited masking in the psychoacoustic experiment. Speech reception in the enrolled subjects had a negative mean SRT for all tested fitting strategies in the EAS listening mode with background stationary noise, meaning the speech was softer than the noise for the 50% correctly repeated words estimate of most subjects. The performance was significantly better than the performance with electric listening mode and also better in comparison than the acoustic listening mode across fitting strategies. The analysis of the relation between residual hearing, as expressed by low frequency PTA, and SRT confirmed the advantageous effect of ipsilateral residual hearing on speech reception performance reported by several previous studies (Turner et al. 2004; Gantz et al. 2005; Kiefer et al. 2005; Gstoettner et al. 2008). The data show a steep increase in SRT with low-frequency hearing which reaches a plateau with expanded residual hearing comparable to the results reported by Büchner et al. (2009) and Zhang et al. (2010b). In the ICRA listening mode, however, an increase in residual hearing continuously improves the SRT, indicating the additional benefit of residual hearing in the medium frequency range for fluctuating noise.
Results under the temporally fluctuating ICRA noise were significantly worse than under the stationary noise in EAS listening mode. This effect is contrary to the effect in normal hearing listeners, who benefit from the temporal gaps in the ICRA-5–modulated noise and show improved SRTs in the OLSA (Wagener & Brand 2005). This effect which is at least partly due to the poor spectral representation and the lack of temporal fine structure transmitted through the CI has been shown previously (Nelson et al. 2003; Zirn et al. 2016) and was the reason to include fluctuating background noise into the analysis. If speech reception performance was influenced by masking, a release from masking with the UNMASKfit strategy might have been more strongly visible in the ICRA EAS listening mode due to the improved exploitation of temporal fine structure delivered by the residual hearing. However, no significant difference between meet and UNMASKfit was observed. No interaction was found between fitting and listening mode. The different amounts of residual hearing in the present subjects of this study as well as their different performance in electric stimulation and corresponding acoustic benefit result in a high variability between subjects in the ICRA EAS listening mode. Thus subjects are not quite comparable and the limited number of subjects might confound a possible effect of a benefit of the fitting strategy on speech reception performance in the ICRA listening mode.
Previous clinical studies with EAS patients showed both advantageous and detrimental effects of an overlap between electric and acoustic frequency ranges (Fraysse et al. 2006; Simpson et al. 2009), with simulations in normal hearing listeners indicating toward better speech recognition with no overlap and minimal gap between electric and acoustic frequency representation maps (Dorman et al. 2005). These contrasting findings were analyzed in the present subjects and correlated to the individual masking results. The SRTs in the EAS listening mode show equivalent speech reception performance with the meet and the UNMASKfit map, whereas the overlap fitting resulted in a significant decrease in SRT. Gifford et al. (2017) reported that a lower electric frequency boundary at the acoustic frequency with 70 dB HL yielded significantly better results (F = 3.3; p = 0.019) in a listening condition with EAS and a contralateral HA, which is consistent to the findings of the meet fitting in this study. As they referenced, this result to a fitting recommendation delivering acoustic amplification to frequencies with less than 90 dB HL, they defined the corresponding map as overlap. In contrast, the overlap fitting defined in this study resulted in a spectral overlap of two octaves within the range of beneficial residual hearing, that is, hearing loss of less than 65 dB HL.
The UNMASKfit map increased the physiological gap between the electric and acoustic stimulation, but did not result in an overall decrease of SRT. At the same time, it reduced the strength of electric stimulation in the apical electrodes for most subjects, causing a lower mean speech reception performance for the electric listening mode. The mean SRT in electric listening mode was lowest for the overlap fitting, but not significantly, as this transmitted more information due to the extended range of electric bandwidth. The mean SRT for the UNMASKfit map was slightly higher in the electric listening mode, but it was not significant, similar to the findings by Arnoldner et al. (2007a) who deactivated two apical electrode contacts. However, the improvement from electric to EAS listening mode due to the residual hearing was lowest in the overlap and largest in the UNMASKfit map. Although the difference in this acoustic benefit was not significant, the results indicate that the residual hearing was able to restore information in the UNMASKfit strategy that was lost in the electric stimulation alone. Consequently, even after only 1 month of adapting to the UNMASKfit strategy, subjects were making better use of their residual acoustic hearing. This relation was stronger in subjects that received the individualized masking map due to significant electric masking. This effect might result in better overall performance after longer adjusting times and could help to preserve residual hearing by better training effects and less possible adverse electric stimulation (Dodson et al. 1986).
Speech Reception and Masking
The results show a negative correlation between masking and speech reception in the overlap fitting. Especially subjects with strong masking suffered significant detriments in SRT due to the overlap fitting (maximum decrease of 4 dB with respect to meet fitting). As the physiological proximity between electric and acoustic stimulation is not changed by this fitting, this might indicate toward more central, informational electric-acoustic masking affecting the speech intelligibility by masking acoustic information. In contrast, electric masking might be more frequent when the analysis bands of electric and acoustic processing overlap and both modalities are stimulated simultaneously. At the same time, electrophonic responses of the organ of Corti elicited by electric stimulation could also result in the observed loss of speech information. Due to the electric signal fine structure processing strategy of the MED-EL speech processor employed in this study, electric pulses are stimulated at zero crossings of the input signal in the low frequency bands and the temporal fine structure is implemented in the sound coding strategy (Arnoldner et al. 2007b). Thus electrophonic masking could be more frequently elicited in the overlap fitting. Masking has not previously been analyzed and correlated to the speech reception outcomes in EAS users. Several studies reported a detrimental effect of the overlap fitting especially in subjects with better residual hearing (Fraysse et al. 2006; Vermeire et al. 2008; Simpson et al. 2009; Karsten et al. 2013). Electric on acoustic masking might be the cause of these findings. Currently, the origin of masking is unclear (Krüger et al. 2017), but several mechanisms have been characterized in animal models, such as electrophonic and electroneural interaction (Stronks et al. 2011; Sato et al. 2017).
The observed masking did not influence the overall performance in EAS listening mode for the meet and UNMASKfit maps. A significant correlation between SRTs and residual hearing was observed, and subjects with better residual hearing were observed to show significant masking. Masking seems to have no detrimental effect in the meet fitting strategy, as the UNMASKfit map does not show a release from masking, which had been expected. It is possible that the psychoacoustically observed masking does not affect the best aided speech reception as it behaves similar to masking observed in a normal hearing system.
Clinical Application and Outlook
A recommendation toward the optimal electric frequency range can be concluded from the presented data. For subjects with poor residual hearing and no masking, an overlap map yielded similar results as the meet map. Because it often sounded unfamiliar (lower, more bass) most users chose to keep the meet fitting when the study ended, which corresponded to their standard clinical fitting. This corresponds to the tendency to prefer the long-term acclimated fittings, observed by many other studies that varied signal processing algorithms or frequency allocation in CI users (Fu et al. 2002; Buechner et al. 2011; Magnusson 2011; Nogueira et al. 2015).
Judging from the results obtained in this study, an overlap map is probably suboptimal in subjects with good residual hearing, especially subjects with strong electric masking should rather not be programmed with an overlap map. Some subjects indicated their difficulty in adjusting to the change in electric frequency range upon the programming of this study map. In 1 subject, the overlap was reduced to one octave to ensure compliance. The other subjects were encouraged to try to adapt to the new sound. Two subjects, who are not included in the presented data, dropped out of the study after trying to adjust to the overlap fitting and reportedly failing to do so. However, neither their degree of masking nor their acoustic benefit differed from the range of the other subjects, so that the remaining sample is not biased toward more or less masking. In fact, these subjects showed medium strength TE of maximum 35% DR for 5 to 6 dB acoustic TE. They did not want to continue with the study at that stage, but choose to use their clinical fitting again. As the masking measurements exceed the typical clinical appointment schedules, a clear recommendation is given against fitting EAS subjects with good residual hearing with an overlap map.
The UNMASKfit map did not result in a decrease of speech reception in the EAS mode on average, indicating that no information was lost due to the decrease of electric stimulation. Some subjects however showed a decrement in electric stimulation SRTs and they did not benefit as much from the UNMASKfit map as subjects with steady electric performance. This suggests that more time may be necessary to adapt to the new electric fitting. The possibly confounding factor of insufficient adaptation time could be eliminated in a further study that instead of changing the electric stimulation reduces the acoustic stimulation to obtain a physiological gap. The results might indicate that a fitting strategy with less electric stimulation in the apical region is feasible for the clinical practice, as it reduces the amount of electric current the surviving hair cells are exposed to. As Dodson et al. (1986) showed damage to the outer hair cells and the efferent functionality of the cochlear nerve due to loud electric stimulation, implementing a reduction of stimulation of apical electrodes in a standard fitting practice could help to protect outer hair cells in the long-term. Coco et al. (2007) did not find an effect of chronic suprathreshold electric stimulation on the survival of inner and outer hair cells, however, they did not address the status of the hair cells. Similarly, in a case report, Quesnel et al. (2016) did not find a difference in hair cell or spiral ganglion cell count between the implanted and unimplanted ear. The UNMASKfit map developed in the present study may be more appropriate for deeply inserted electrodes and good residual hearing. In contrast, deep electrode insertion might be beneficial in the long-term. If residual hearing is then lost at some later point, electrodes can be reactivated or electrodes added virtually up to the range of one electrode contact by shaping the electrical field (Saoji & Litvak 2010; Macherey et al. 2011; Klawitter et al. 2018). To quickly assess the individual masking effects, the implementation of a fast objective measurement of masking, as proposed by Koka and Litvak (2017) is feasible, but needs to be investigated further. Alternatively, a new surgical procedure in which the electrode is inserted only up to the unmasked frequency is proposed. This surgical procedure may be helpful in protecting the hair cells from inflammatory processes or trauma caused by the electrode array, which should be investigated in a prospective study.
ACKNOWLEDGMENTS
The authors thank the subjects who dedicated their time and effort to this study.
Footnotes
W.N. and M.I. received funding for this research from the DFG (German Research Foundation) Cluster of Excellence EXC 1077/1 ‘Hearing4all,' the DFG project Number 396932747 and MED-EL Medical Electronics.
M.I. designed and performed experiments, co-designed the fitting rule, analyzed data and wrote the article. W.N. designed the experiments, co-designed the fitting rule, provided analysis, and contributed to the writing of the article. B.K. designed the experiments, provided analysis, and critical revision of the manuscript. T.L. and A.B. provided critical revision for experimental design and manuscript.
The remaining authors have no conflicts of interest to disclose.
REFERENCES
- Adunka O., Kiefer J. Impact of electrode insertion depth on intracochlear trauma. Otolaryngol Head Neck Surg, (2006). 135, 374–382. [DOI] [PubMed] [Google Scholar]
- Arnoldner C., Riss D., Baumgartner W. D., et al. Cochlear implant channel separation and its influence on speech perception–implications for a new electrode design. Audiol Neurootol, 2007a). 12, 313–324. [DOI] [PubMed] [Google Scholar]
- Arnoldner C., Riss D., Brunner M., et al. Speech and music perception with the new fine structure speech coding strategy: Preliminary results. Acta Otolaryngol, 2007b). 127, 1298–1303. [DOI] [PubMed] [Google Scholar]
- Aronoff J. M., Freed D. J., Fisher L. M., et al. The effect of different cochlear implant microphones on acoustic hearing individuals' binaural benefits for speech perception in noise. Ear Hear, (2011). 32, 468–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgarte F. A Physiological Ear Model for Auditory Masking Applicable to Perceptual Coding. Preprint 4511, 103rd AES Convention, (1997). September 1997New York, NY, [Google Scholar]
- Buchman C. A., Dillon M. T., King E. R., et al. Influence of cochlear implant insertion depth on performance: A prospective randomized trial. Otol Neurotol, (2014). 35, 1773–1779. [DOI] [PubMed] [Google Scholar]
- Büchner A., Schüssler M., Battmer R., et al. Impact of low-frequency hearing. Audiol Neurotol, (2009). 14: 8–13. [DOI] [PubMed] [Google Scholar]
- Büchner A., Illg A., Majdani O., et al. Investigation of the effect of cochlear implant electrode length on speech comprehension in quiet and noise compared with the results with users of electro-acoustic-stimulation, a retrospective analysis. PLoS One, (2017). 12, e0174900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buechner A., Beynon A., Szyfter W., et al. Clinical evaluation of cochlear implant sound coding taking into account conjectural masking functions, MP3000™. Cochlear Implants Int, (2011). 12, 194–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coco A., Epp S. B., Fallon J. B., et al. Does cochlear implantation and electrical stimulation affect residual hair cells and spiral ganglion neurons? Hear Res, (2007). 225, 60–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodson H. C., Walliker J. R., Frampton S., et al. Structural alteration of hair cells in the contralateral ear resulting from extracochlear electrical stimulation. Nature, (1986). 320, 65–67. [DOI] [PubMed] [Google Scholar]
- Dorman M. F., Spahr A. J., Loizou P. C., et al. Acoustic simulations of combined electric and acoustic hearing (EAS). Ear Hear, (2005). 26, 371–380. [DOI] [PubMed] [Google Scholar]
- Dorman M. F., Gifford R. H., Spahr A. J., et al. The benefits of combining acoustic and electric stimulation for the recognition of speech, voice and melodies. Audiol Neurootol, (2008). 13, 105–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreschler W. A., Verschuure H., Ludvigsen C., et al. ICRA noises: Artificial noise signals with speech-like spectral and temporal properties for hearing instrument assessment. International Collegium for Rehabilitative Audiology. Audiology, (2001). 40, 148–157. [PubMed] [Google Scholar]
- Eshraghi A. A., Van de Water T. R. Cochlear implantation trauma and noise-induced hearing loss: Apoptosis and therapeutic strategies. Anat Rec A Discov Mol Cell Evol Biol, (2006). 288, 473–481. [DOI] [PubMed] [Google Scholar]
- Fowler J. R., Eggleston J. L., Reavis K. M., et al. Effects of removing low-frequency electric information on speech perception with bimodal hearing. J Speech Lang Hear Res, (2016). 59, 99–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraysse B., Macías A. R., Sterkers O., et al. Residual hearing conservation and electroacoustic stimulation with the nucleus 24 contour advance cochlear implant. Otol Neurotol, (2006). 27, 624–633. [DOI] [PubMed] [Google Scholar]
- Fu Q. J., Shannon R. V., Galvin J. J., 3rd. Perceptual learning following changes in the frequency-to-electrode assignment with the Nucleus-22 cochlear implant. J Acoust Soc Am, (2002). 112, 1664–1674. [DOI] [PubMed] [Google Scholar]
- Gantz B. J., Turner C. Combining acoustic and electrical speech processing: Iowa/Nucleus hybrid implant. Acta Otolaryngol, (2004). 124, 344–347. [DOI] [PubMed] [Google Scholar]
- Gantz B. J., Turner C., Gfeller K.E., et al. Preservation of hearing in cochlear implant surgery: Advantages of combined electrical and acoustical speech processing. Laryngoscope, (2005). 115: 796–802. [DOI] [PubMed] [Google Scholar]
- Gifford R. H., Dorman M. F., Skarzynski H., et al. Cochlear implantation with hearing preservation yields significant benefit for speech recognition in complex listening environments. Ear Hear, (2013). 34, 413–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gifford R. H., Davis T. J., Sunderhaus L. W., et al. Combined electric and acoustic stimulation with hearing preservation: Effect of cochlear implant low-frequency cutoff on speech understanding and perceived listening difficulty. Ear Hear, (2017). 38, 539–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gstoettner W., Kiefer J., Baumgartner W. D., et al. Hearing preservation in cochlear implantation for electric acoustic stimulation. Acta Otolaryngol, (2004). 124, 348–352. [DOI] [PubMed] [Google Scholar]
- Gstoettner W. K., van de Heyning P., O'Connor A. F., et al. Electric acoustic stimulation of the auditory system: Results of a multi-centre investigation. Acta Otolaryngol, (2008). 128, 968–975. [DOI] [PubMed] [Google Scholar]
- Hamzavi J., Arnoldner C. Effect of deep insertion of the cochlear implant electrode array on pitch estimation and speech perception. Acta Otolaryngol, (2006). 126, 1182–1187. [DOI] [PubMed] [Google Scholar]
- Hey M., Hocke T., Hedderich J., et al. Investigation of a matrix sentence test in noise: Reproducibility and discrimination function in cochlear implant patients. Int J Audiol, (2014). 53, 895–902. [DOI] [PubMed] [Google Scholar]
- Hochmair I., Arnold W., Nopp P., et al. Deep electrode insertion in cochlear implants: Apical morphology, electrodes and speech perception results. Acta Otolaryngol, (2003). 123, 612–617. [PubMed] [Google Scholar]
- Hughes M. L., Stille L. J., Baudhuin J. L., et al. ECAP spread of excitation with virtual channels and physical electrodes. Hear Res, (2013). 306, 93–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imsiecke M., Krüger B., Büchner A., et al. Electric-acoustic forward masking in cochlear implant users with ipsilateral residual hearing. Hear Res, (2018). 364, 25–37. [DOI] [PubMed] [Google Scholar]
- Incerti P. V., Ching T. Y., Cowan R. A systematic review of electric-acoustic stimulation: Device fitting ranges, outcomes, and clinical fitting practices. Trends Amplif, (2013). 17, 3–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karsten S. A., Turner C. W., Brown C. J., et al. Optimizing the combination of acoustic and electric hearing in the implanted ear. Ear Hear, (2013). 34, 142–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiefer J., Gstoettner W., Baumgartner W., et al. Conservation of low-frequency hearing in cochlear implantation. Acta Otolaryngol, (2004). 124, 272–280. [DOI] [PubMed] [Google Scholar]
- Kiefer J., Pok M., Adunka O., et al. Combined electric and acoustic stimulation of the auditory system: Results of a clinical study. Audiol Neurootol, (2005). 10, 134–144. [DOI] [PubMed] [Google Scholar]
- Klawitter S., Landsberger D. M., Büchner A., et al. Perceptual changes with monopolar and phantom electrode stimulation. Hear Res, (2018). 359, 64–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koka K., Litvak L. M. Feasibility of using electrocochleography for objective estimation of electro-acoustic interactions in cochlear implant recipients with residual hearing. Front Neurosci, (2017). 11, 337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krüger B., Büchner A., Nogueira W. Simultaneous masking between electric and acoustic stimulation in cochlear implant users with residual low-frequency hearing. Hear Res, (2017). 353, 185–196. [DOI] [PubMed] [Google Scholar]
- Krüger B., Büchner A., Lenarz T., et al. (under review). Electric acoustic interaction measurements in cochlear implant users with ipsilateral residual hearing using electrocochleography. J Acoust Soc Am. [DOI] [PubMed] [Google Scholar]
- Lenarz T., Stöver T., Buechner A., et al. Hearing conservation surgery using the Hybrid-L electrode. Results from the first clinical trial at the Medical University of Hannover. Audiol Neurootol, (2009). 14(Suppl 1), 22–31. [DOI] [PubMed] [Google Scholar]
- Lenarz T., Timm M. E., Salcher R., et al. Individual hearing preservation cochlear implantation using the concept of partial insertion. Otol Neurotol, (2019). 40, e326–e335. [DOI] [PubMed] [Google Scholar]
- Lin P., Turner C. W., Gantz B. J., et al. Ipsilateral masking between acoustic and electric stimulations. J Acoust Soc Am, (2011). 130, 858–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macherey O., Deeks J. M., Carlyon R. P. Extending the limits of place and temporal pitch perception in cochlear implant users. J Assoc Res Otolaryngol, (2011). 12, 233–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnusson L. Comparison of the fine structure processing (FSP) strategy and the CIS strategy used in the MED-EL cochlear implant system: Speech intelligibility and music sound quality. Int J Audiol, (2011). 50, 279–287. [DOI] [PubMed] [Google Scholar]
- McKay C. M., Henshall K. R., Farrell R. J., et al. A practical method of predicting the loudness of complex electrical stimuli. J Acoust Soc Am, (2003). 113(4 Pt 1), 2054–2063. [DOI] [PubMed] [Google Scholar]
- Nelson P. B., Jin S. H., Carney A. E., et al. Understanding speech in modulated interference: Cochlear implant users and normal-hearing listeners. J Acoust Soc Am, (2003). 113, 961–968. [DOI] [PubMed] [Google Scholar]
- Nogueira W., Büchner A., Lenarz T., et al. A psychoacoustic NofM-type speech coding strategy for cochlear implants. EURASIP J Appl Signal Process, (2005). 18, 3044–3059. [Google Scholar]
- Nogueira W., Litvak L. M., Saoji A. A., et al. Design and evaluation of a cochlear implant strategy based on a “Phantom” channel. PLoS One, (2015). 10, e0120148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nourski K. V., Abbas P. J., Miller C. A., et al. Effects of acoustic noise on the auditory nerve compound action potentials evoked by electric pulse trains. Hear Res, (2005). 202, 141–153. [DOI] [PubMed] [Google Scholar]
- O'Connell B. P., Hunter J. B., Haynes D. S., et al. Insertion depth impacts speech perception and hearing preservation for lateral wall electrodes. Laryngoscope, (2017). 127, 2352–2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padilla M., Landsberger D. M. Reduction in spread of excitation from current focusing at multiple cochlear locations in cochlear implant users. Hear Res, (2016). 333, 98–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quesnel A. M., Nakajima H. H., Rosowski J. J., et al. Delayed loss of hearing after hearing preservation cochlear implantation: Human temporal bone pathology and implications for etiology. Hear Res, (2016). 333, 225–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagi E., Kaiser A. R., Meyer T. A., et al. The effect of temporal gap identification on speech perception by users of cochlear implants. J Speech Lang Hear Res, (2009). 52, 385–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saoji A. A., Litvak L. M. Use of “phantom electrode” technique to extend the range of pitches available through a cochlear implant. Ear Hear, (2010). 31, 693–701. [DOI] [PubMed] [Google Scholar]
- Sato M., Baumhoff P., Tillein J., et al. Physiological mechanisms in combined electric-acoustic stimulation. Otol Neurotol, (2017). 38, e215–e223. [DOI] [PubMed] [Google Scholar]
- Shannon R. V. Threshold and loudness functions for pulsatile stimulation of cochlear implants. Hear Res, (1985). 18, 135–143. [DOI] [PubMed] [Google Scholar]
- Sheffield S. W., Gifford R. H. The benefits of bimodal hearing: Effect of frequency region and acoustic bandwidth. Audiol Neurootol, (2014). 19, 151–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson A., McDermott H. J., Dowell R. C., et al. Comparison of two frequency-to-electrode maps for acoustic-electric stimulation. Int J Audiol, (2009). 48, 63–73. [DOI] [PubMed] [Google Scholar]
- Skarzynski H., Lorens A., Piotrowska A., et al. Preservation of low frequency hearing in partial deafness cochlear implantation (PDCI) using the round window surgical approach. Acta Otolaryngol, (2007). 127, 41–48. [DOI] [PubMed] [Google Scholar]
- Stakhovskaya O., Sridhar D., Bonham B. H., et al. Frequency map for the human cochlear spiral ganglion: Implications for cochlear implants. J Assoc Res Otolaryngol, (2007). 8, 220–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stronks H. C., Versnel H., Prijs V. F., et al. Suppression of the acoustically evoked auditory-nerve response by electrical stimulation in the cochlea of the guinea pig. Hear Res, (2010). 259, 64–74. [DOI] [PubMed] [Google Scholar]
- Stronks H. C., Versnel H., Prijs V. F., et al. Effects of electrical stimulation on the acoustically evoked auditory-nerve response in guinea pigs with a high-frequency hearing loss. Hear Res, (2011). 272, 95–107. [DOI] [PubMed] [Google Scholar]
- Stronks H. C., Prijs V. F., Chimona T. S., et al. Spatial overlap of combined electroacoustic stimulation determines the electrically evoked response in the guinea pig cochlea. Otol Neurotol, (2012). 33, 1535–1542. [DOI] [PubMed] [Google Scholar]
- Suhling M. C., Majdani O., Salcher R., et al. The impact of electrode array length on hearing preservation in cochlear implantation. Otol Neurotol, (2016). 37, 1006–1015. [DOI] [PubMed] [Google Scholar]
- Timm M. E., Majdani O., Weller T., et al. Patient specific selection of lateral wall cochlear implant electrodes based on anatomical indication ranges. PLoS One, (2018). 13, e0206435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner C. W., Gantz B. J., Vidal C., et al. Speech recognition in noise for cochlear implant listeners: Benefits of residual acoustic hearing. J Acoust Soc Am, (2004). 115, 1729–1735. [DOI] [PubMed] [Google Scholar]
- Vaerenberg B., Péan V., Lesbros G., et al. Combined electric and acoustic hearing performance with Zebra® speech processor: Speech reception, place, and temporal coding evaluation. Cochlear Implants Int, (2013). 14, 150–157. [DOI] [PubMed] [Google Scholar]
- van der Jagt M. A., Briaire J. J., Verbist B. M., et al. Comparison of the HiFocus Mid-Scala and HiFocus 1J Electrode Array: Angular insertion depths and speech perception outcomes. Audiol Neurootol, (2016). 21, 316–325. [DOI] [PubMed] [Google Scholar]
- Vermeire K., Anderson I., Flynn M., et al. The influence of different speech processor and hearing aid settings on speech perception outcomes in electric acoustic stimulation patients. Ear Hear, (2008). 29, 76–86. [DOI] [PubMed] [Google Scholar]
- von Ilberg C., Kiefer J., Tillein J., et al. Electric-acoustic stimulation of the auditory system. New technology for severe hearing loss. ORL J Otorhinolaryngol Relat Spec, (1999). 61, 334–340. [DOI] [PubMed] [Google Scholar]
- Wagener K. C., Brand T. Sentence intelligibility in noise for listeners with normal hearing and hearing impairment: Influence of measurement procedure and masking parameters. Int J Audiol, (2005). 44, 144–156. [DOI] [PubMed] [Google Scholar]
- Wagener K., Kühnel V., Kollmeier B. Development and evaluation of a german sentence test I: Design of the Oldenburg Sentence test. Zeitschrift Für Audiologie, (1999). 38, 4–15. [Google Scholar]
- Zhang T., Dorman M. F., Spahr A. J. Information from the voice fundamental frequency (F0) region accounts for the majority of the benefit when acoustic stimulation is added to electric stimulation. Ear Hear, 2010a). 31, 63–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T., Spahr A. J., Dorman M. F. Frequency overlap between electric and acoustic stimulation and speech-perception benefit in patients with combined electric and acoustic stimulation. Ear Hear, 2010b). 31, 195–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zirn S., Polterauer D., Keller S., et al. The effect of fluctuating maskers on speech understanding of high-performing cochlear implant users. Int J Audiol, (2016). 55, 295–304. [DOI] [PubMed] [Google Scholar]


