Abstract
The field of tissue engineering and regenerative medicine has made numerous advances in recent years in the arena of fabricating multifunctional, three-dimensional (3D) tissue constructs. This can be attributed to novel approaches in the bioprinting of stem cells. There are expansive options in bioprinting technology that have become more refined and specialized over the years, and stem cells address many limitations in cell source, expansion, and development of bioengineered tissue constructs. While bioprinted stem cells present an opportunity to replicate physiological microenvironments with precision, the future of this practice relies heavily on the optimization of the cellular microenvironment. In order to fabricate tissue constructs that are useful in replicating physiological conditions in laboratory settings, or in preparation for transplantation to a living host, the microenvironment must mimic conditions that allow bioprinted stem cells to proliferate, differentiate, and migrate. The advances of bioprinting stem cells and directing cell fate have the potential to provide feasible and translatable approach to creating complex tissues and organs. This review will examine the methods through which bioprinted stem cells are differentiated into desired cell lineages through biochemical, biological, and biomechanical techniques.
Graphical Abstract

1. INTRODUCTION
The field of tissue engineering and regenerative medicine has made expeditious advancements in creating multifunctional, three-dimensional (3D) tissue constructs.1,2 This is largely attributed to the progress in numerous bioprinting approaches.1-4 The ability to bioprint a singular construct that has the potential to mature into a functional tissue would facilitate an expansion of in vitro experimental designs, as well as a more rapid translation of a bioprinted tissue or organ to living models.5,6 There are expansive options in bioprinting technologies that have become more refined and specialized over the years. Approaches to cell delivery vary from multicellular, cell aggregate, and droplet-based or single cell bioprinting methodologies. Multicellular approaches include jetting-based, microextrusion-based, laser-assisted, and stereolithography-based techniques.
Notably, the use of stem cells in bioprinting has addressed many limitations in cell source, expansion, and development of bioengineered tissue constructs. To this end, the use of stem cells in bioprinting offers a feasible option. The bioprinting of cells with an ability to mature to differing functional phenotypes presents an abundance of applications in lab-based models and clinical treatments. Stem cells present an opportunity in that they have the ability to replicate rapidly, as well as differentiation to a functional cell type based on various cues in the culture environment. Stem cells present varying potencies and capabilities toward differentiation, which inform their potential uses in tissue constructs.7-9 Potency is an important consideration in selecting the type of stem cells to employ in bioprinted constructs. Cell sources such as embryonic stem cells (ESCs), induced pluripotent stem cells (iPSCs) and adult stem cells have differing differentiation potentials, and thus, can be utilized for different tissue applications or purposes. Multiple bioprinting approaches have been paired with stem cell differentiation techniques to successfully generate target tissue constructs.
One major consideration in the development of constructs comprised of bioprinted stem cells is the future applications or uses of the fabricated tissue construct. While some uses may be for disease modeling or pharmaceutical research in in vitro settings, other uses may be targeted to clinical and therapeutic applications for patients. The desired utilization of the construct may dictate the bioprinting technologies, stem cell type or cell source, and what factors of the microenvironment are manipulated or optimized. One of the most crucial factors in the progress of this field is the optimization of the cellular microenvironment. In order to fabricate constructs that are useful in replicating in vivo conditions in laboratory settings, the selection of the optimal conditions is vital. Fabricating a microenvironment that mimics physiological settings, including incorporating components into the printing process, as well as introducing them into the culture of the construct post-printing determines the success of outcomes. These range from the inclusion of biochemical cues, such as small molecules, growth factors, peptides, exosomes, small RNAs, bioink additives, and other influential factors. Similarly, the development of a scaffold that reflects the natural extracellular matrix (ECM) is vital. Equally important are the mechanical properties of biomaterials that facilitate proliferation, differentiation, and maturation of stem cells. These include, but are not limited to, the mimicry of a functional ECM, the topography of the bioprinted construct or scaffold, and the stiffness and elasticity of bioinks and other materials.
This review will investigate the aforementioned aspects of optimizing a microenvironment for bioprinted stem cells, as well as examine recent literature and studies pertaining to advances in numerous tissue and organ systems within the last five years. Modern research in stem cell bioprinting has produced novel approaches in bone, cartilage, heart, liver, muscular, neural, and skin tissue systems. As each tissue and organ requires distinct conditions to induce the growth, migration, and fate of cells, we will examine how similar techniques and factors have been utilized to create disparate microenvironments to foster the growth of the aforementioned tissue types. The advances of bioprinting stem cells and directing cell fate have the potential to provide feasible and translatable approach to creating complex tissues and organs. This review will examine the methods through which bioprinted stem cells are differentiated into desired cell lineages through biochemical, biological, and biomechanical techniques.
2. 3D BIOPRINTING OF STEM CELLS
2.1. Overview of Bioprinting Methodologies
Many methodologies have been utilized to bioprint stem cells toward various applications. These approaches include tactics to print multiple cells simultaneously using methods such as inkjet-based bioprinters, extrusion bioprinting, laser-assisted technologies, and stereolithography-based methods.5,10,11 Each approach has unique benefits and limitations, which determine what applications would be most suitable for each methodology. 3D multicellular bioprinting allows for replicable printing of cells at adjustable volumes with high levels of precision and resolution of the resulting construct.12 Typically, large quantities of cells can be delivered, and multiple cells are dispersed at once.11
2.1.1. Multicellular Bioprinting.
Multicellular bioprinting can be categorized by the type of technology used to deliver cell-laden bioinks. In early bioprinting efforts, commercially available inkjet printers were modified for the purpose of cell bioprinting, in which cells suspended in bioink were stored in the ink cartridges and computers facilitated the printing process and pattern execution.5,13-19 Presently, inkjet bioprinting exists as a distinct and well-developed technique for cellular bioprinting, in which cells are delivered through drops.11,13-19 Inkjet printing allows for constructs to be printed at a high speed of 1 to 10,000 droplets per second, with a moderate throughput of materials. This facilitates a relatively short fabrication time with sufficient resolution of 10 μm to 1 mm.1,3,5,10,11,14-22 Research utilizing inkjet bioprinting offered delivery of a limited cell density of under 106 cells/mL with a cell viability of approximately 85% post printing.1,3,5,10,11,14-22 However, this method requires a moderate viscosity of bioprinting material, and has a limited three-dimensional building capability.11,14-19 Printed constructs can be crosslinked in numerous ways, including the methods that utilize ionic properties, thermal properties, pH mediation, varying types of polymerization, and enzymatic approaches.1,3,10,20-22 With a low overall cost and propensity for high resolution constructs, inkjet printing is beneficial due to the ability to print without a nozzle which allows for minimal shear stress on cells.1,3,5,10,11,14-22 However, this printing method is known to have a tendency to cause some levels of mechanically-induced abnormality in the printed cells.11,14-19
Microextrusion bioprinting technology involves the dispersal of continuous lines of cell-laden bioink, as opposed to drops or droplets.11,23-31 Microextrusion bioprinting requires slower printing speeds as compared to the inkjet method due to the continuous nature of the approach; however it allows constructs to be printed in relatively short fabrication times with high throughput.1,3,5,10,11,20-22,24-31 While this approach provides printing resolutions of 100 μm to 1 mm, delivery of high cell density is possible with post-printing cell viability ranging from 40% to 95%.1,3,5,10,11,20-22,24-31 The ability to use high viscosity materials allows for building mechanically stable three-dimensional constructs, and crosslinking of constructs can be achieved through ionic, thermal, and pH-mediated methods, as well as polymerization and shear-thinning approaches.1,3,5,10,11,20-22,24-31 Other advantages include the ability to replicate the extracellular matrix and deliver cells at concentrations translatable to densities seen in physiological settings.1,3,10,20-22 However, the cell viability is primarily hindered by the high shear stress, which can inadvertently lead to phenotypical changes in printed cells.1,3,10,20-22 This is a notable limitation in the application of printing stem cells, as differentiation is typically an aspect that is heavily regulated for a desired outcome.
Laser-assisted bioprinting involves the use of lasers to transfer cell-laden bioinks to a desired substrate.1,3,10,20-22,32 This approach allows moderate to fast printing speeds of 200 to 1,000 mm transferred per second, however requires longer fabrication times and involves moderate throughput of materials.1,3,5,10,11,20-22,33-36 Despite longer fabrication times, laser-assisted methods allow for exceptional resolution of equal to or greater than 10 μm.1,3,5,10,11,20-22,33-36 Moderate densities of cells can be printed, approximately less than 108 cells/mL, but at a notably high rate of cell viability of 95%.1,3,5,10,11,20-22,33-36 The approach requires moderate to high material viscosities, and allows for moderate build-up of three-dimensional constructs.1,3,10,20-22 Crosslinking can be achieved through ionic methods, thermal methods, enzyme-based methods, and polymerization.11,33-36 Advantages of laser-assisted bioprinting technologies involve limited shear stress on cells, however limitations include difficulty accommodating viscous materials, achieving high cell printing densities, and the high costs associated with the approach.1,3,5,10,11,20-22,33-36
Stereolithography-based printing utilizes liquid photocurable polymer cured with ultraviolet (UV) light to build a construct in a layer-by-layer fashion.11,17,37-41 This method allows for high speed printing with high throughput, as well as the propensity for high resolution constructs.11,37-41 A moderate cell density can be printed with stereolithography with limited cell viability.11,37-41 Constructs are crosslinked with photopolymerization.11,37-41 While advantages include adequate ability for 3D build-up, major limitations of stereolithography-based printing include limited biomaterials-based resins available.11,37-41
2.1.2. Cell Aggregate Bioprinting.
Cell aggregate bioprinting differs from multicellular printing modalities in that the cell aggregates imitate physiological conditions and structures through the use of organoids, as opposed to printing droplets.11,40,42,43 This method allows for moderate printing speeds and high throughput, with high resolution constructs.11,40,42,43 While cell density capabilities are low, cell viability in aggregate printing is moderate to high.11,40,42,43 Cross-linking methods involve shear-thinning, ionic methods, and thermal methods.11,40,42,43 Notably, this is a high cost approach.11,40,42,43
2.1.3. Droplet-Based Bioprinting.
Droplet-based, or single cell, bioprinting allows for the deposition of single cells at a time, making it ideal for optimal control and study of cell types in bioprinting.11,44-47 Based on the singular nature of the method, throughput and cell densities are understandably low, while printing speed is efficient.11,44-47 Despite the printing of low cell densities, this delicate approach allows for high cell viability post-printing.11,44-47 Ionic or thermal treatments can be used to induce crosslinking, and the cost of this modality is high due to the specificity of the approach.11,44-47
There are many options in selecting which bioprinting approach may be most appropriate for certain applications. In the setting of bioprinting stem cells, many approaches have proven efficacy, and may be appropriate for different target tissues or organ systems. In the setting of fabricating complex tissues or organs, bioprinting is ideal in that it allows for the precise arrangement of multiple cell types and microenvironments during the process of printing a single construct.1 This organization, paired with the use of stem cells, gives limitless potential to what is able to be fabricated using bioprinting technologies. The selection of bioprinting modality must be taken into account not only with the desired construct, but also with the cell lines to be printed. Furthermore, the microenvironment of bioprinted stem cells plays a significant role in the outcomes and applications of the construct. Thus, the selection of a bioprinting methodology that is also able to support the development of a microenvironment ideal for cell proliferation and differentiation is an important consideration.
2.2. Commonly Used Stem Cells in Bioprinting
While many cell types can be utilized in bioprinting 3D tissue constructs, there are certain stem cell types that have been highly preferential in this application. These include, but are not limited to, ESCs, iPSCs and adult stem cells.48 These cell types are frequently utilized due to their pluripotency and their ability to replicate in an undifferentiated fashion. Considerations of which stem cell to use in tissue engineering is heavily based on the potency of the cell line. Stem cells can be totipotent, pluripotent, or multipotent, and this may influence which cell is best suited for a particular tissue or application.49 Totipotent stem cells can give rise to virtually any cell found in an embryo or in the placental mass, which includes over 200 distinct cell types.49 Pluripotent stem cells can give rise to any cell type in the body, but excludes the cells found in the placenta.49 Finally, multipotency involves cells that are able to develop into any cells in a particular lineage, such as hematopoietic cells or cardiac cells.49 Based on the construct that is being fabricated, certain cells have advantages and disadvantages in their potential to differentiate.
ESCs are often obtained from the blastocyst stage of any embryo, and are undifferentiated.48,50 Their pluripotency makes them ideal for stem cell bioprinting, as well as their ability to self-renew in an undifferentiated fashion which can later be guided to direct the cells toward a specific phenotype.48,50 The major limitations of using ESCs in bioprinting efforts is their tendency to form teratomas, possible host immune response leading to injection, and ethical concerns surrounding stem cell harvesting from embryos.48,51-53 As ESCs can differentiate into virtually any cell in the body, they are an attractive option for bioprinted constructs. Depending on which state the research occurs in and their laws and regulations surrounding the use of fetal and embryonic stem cells, this may or may not be a feasible option in studies, especially for therapeutic applications.
With many similarities in differentiation potential to ESCs, iPSCs offer pluripotency and are derived from somatic cells.48,57 This overcomes some of the issues surrounding the ethical use of stem cells derived from embryonic material.57 These cells are more plentiful and readily derived from many areas in the body, however differentiation methods may be difficult, or lead to cellular malformations and teratoma formation.48,57 Due to federal and state regulations on the utilization of embryonic and fetal cells in research, iPSCs are currently a very popular avenue for stem cell research. Furthermore, the ability to induce the phenotype offers the multifaceted use of the cells in various tissue and organ applications.
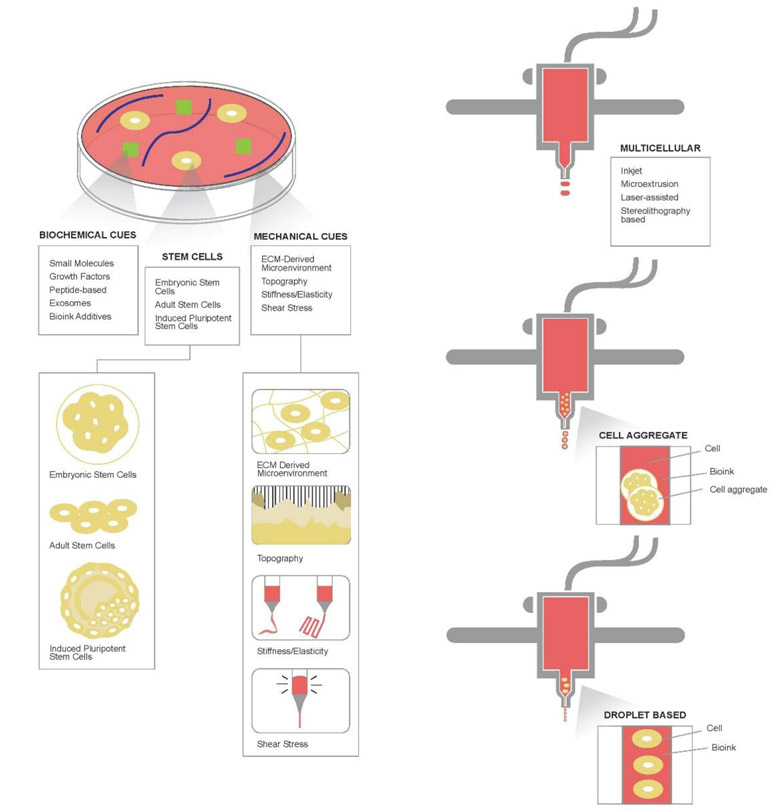
Adult stem cells, for example, bone marrow-derived mesenchymal stem cells (BMSCs), are undifferentiated, self-renewing cells that are multipotent, often having the ability to differentiate to cell types within the tissue or organ from which they originate.48,54 While recent research shows that the potency of these cells is greater than previously expected, limitations in expression have been seen in differentiated adult stem cells, which may hinder function of the final construct.48,54-56 One of the obvious limitations of using adult stem cells is the health of the donor, and the quality of the harvested cells. Depending on the age, health status, or cell availability within a patient, it may be difficult to isolate viable adult stem cells. In cases where an organ is extensively damaged or systemically affected by disease, there may be no way to feasibly obtain autologous cells, and donor cells may induce an immune reaction. While adult stem cells have some inherent limitation in potency and application, they are a promising alternative to ESCs in regards to attainability. Figure 1 shows the overview of bioprinted stem cell-based constructs with various bioprinting methodologies.
Figure 1.
Illustrated overview of bioprinted stem cell constructs, including the biochemical cues, types of stem cells that can be utilized, mechanical cues, and available bioprinting methodologies.
3. BIOCHEMICAL CUES IN MICROENVIRONMENT MAINTENANCE FOR STEM CELL PROLIFERATION AND DIFFERENTIATION
3.1. Small Molecules
Small molecules are a widely used method of cell differentiation due to their specific abilities in influencing cell fate. Small molecules can occur naturally, or can be chemically engineered for a specific use.58-60 They can be used to dictate transcription, and can be targeted to deoxyribonucleic acid (DNA) or ribonucleic acid (RNA).58,59 Additionally, small molecules can be used to target specific steps in signal transduction pathways in order to influence differentiation and cell fate.59 One option to introducing small molecules to cells is through supplementation of the growth medium.59 While the term “small molecules” refers to a plethora of chemical substances, typically this moniker is used to describe molecules of low molecular weight that includes monosaccharides, lipids, components of second messenger systems, natural products, metabolites, and pharmaceutical agents.61 Additionally, this term often speaks to a distinction between small molecules and complex macromolecules or proteins.61 As such, small molecules perform an abundance of physiological functions at the cellular level. This makes them ideal agents in maintaining or directing the renewal or differentiation of stem cells in 3D bioprinted tissue constructs.
As a component of the microenvironment of a bioprinted construct, small molecules may assist printed stem cells in continuing self-renewal and retaining their potency depending on the application of the construct. The self-renewal of cells involves the replication of cells in a way that increases the population while maintaining the same characteristics in regards to multi- or pluripotency.59,62 While this process is a natural tendency of many cells, stem cells have often required some supplementation in culture to continue indefinite growth.63 Furthermore, the biochemical aspects driving self-renewal may change at different stages in the cell life cycle, requiring input from the microenvironment.59,62 Small molecules can be instrumental in supplementing or directing the self-renewal of certain stem cell types, or in certain printing conditions. While some applications may require the preservation of stem cells with universal potency and replication potential, other applications may aim to expedite the differentiation of stem cells within their lineage of origin, or toward a preferred cellular phenotype. As cells mature, they often become more limited in their potency as they are committed to a specific cell type or function.59,64 Small molecules in the microenvironment can assist in the regulation of cell fate, and guide the commitment of cells to a target cell or tissue type.
Numerous small molecules that modulate stem cell fate can be utilized for bioprinting stem cells. Among those, we will discuss well known small molecules that have been used for stem cell differentiation. Dexamethasone is a synthetic adrenal corticosteroid, and is known by the IUPAC name of (8S,9R,10S,11S,13S,14S,16R,17R)-9-fluoro-11,17-dihydroxy-17-(2-hydroxyacetyl)-10,13,16-trimethyl-6,7,8,11,12,14,15,16-octahydrocyclopenta[a]phenanthren-3-one.65,66 Dexamethasone is a fluorinated steroid that is 9-fluoropregna-1,4-diene substituted by hydroxyl groups at positions 11, 17 and 21, a methyl group at position 16 and oxo groups at positions 3 and 20. It is a glucocorticoid and has powerful anti-inflammatory properties, making it useful as an immunosuppressive and anti-inflammatory medication.66 As a corticosteroid, dexamethasone targets intracellular cytoplasmic receptors in target tissues.67 Additionally, dexamethasone also inhibits NF-κB activation and apoptotic pathways.59 In the function of determining cell fates, it has been shown to induce CCAAT/enhancer-binding protein β (C/EBPβ), which is associated with transcription and activation leading to differentiation pancreatic cells to a hepatic lineage.68,69 Dexamethasone is also an integral factor in the differentiation of BMSCs to osteoblastic, adipocytic, and chondrocytic phenotypes.70 This small molecule is known to influence additional pathways, as it inhibits cyclin D2 and promotes cardiomyocyte differentiation and proliferation from progenitor cells.71
Ascorbic acid is also known as Vitamin C and (2R)-2-[(1S)-1,2-dihydroxyethyl]-3,4-dihydroxy-2H-furan-5-one, and exists as the L-enantiomer in humans.72-74 It is a natural water soluble vitamin and has great efficacy as a reducing agent and antioxidant.74 In addition to being integral in the formation of collagen, it also functions as a coenzyme in many reactions.74 Ascorbic acid has been utilized as a conjugate to mesoporous silica nanoparticles to induce differentiation of human ESCs to cardiomyocytes.75
β-glycerophosphate is known as glycerol-2-phosphate, or as 1,3-dihydroxypropan-2-yl phosphate.76 It is a dianion of glycerol 2-phosphate arising from deprotonation of the phosphate OH groups, and is a source for the phosphate in hydroxyapatite and therefore influences intracellular signaling molecules and pathways.77,78 β-glycerophosphate has been utilized to differentiate stem cells to osteogenic phenotypes. Furthermore, it has been used to inhibit the matrix extracellular phophoglycoprotein (MEPE) gene, leading to the determination of cell fate in dental pulp stem cells.78,79
Isobutylmethylxantine (IBMX) is known as 3-isobutyl-1-methylxantine, or 1-methyl-3-(2-methylpropyl)-7H-purine-2,6-dione.80,81 It is derived from 9H-xanthine and is a tautomer of 3-isobutyl-1-methyl-7H-xanthine.81 IBMX has been utilized in neural differentiation due to its role in the elevation of the second messenger, cyclic adenosine monophosphate (cAMP). Increases in the concentration of intracellular cAMP activate the transcription factor CREB through a cascade of protein kinase activation.82 Furthermore, IBMX has been used to differentiate 3T3 cells to adipocytes through transcription factors activated by the C/EBPβ family of proteins, as expression of the CEBPβ gene is induced directly by IBMX.83
Indomethacin, known as 2-[1-(4-chlorobenzoyl)-5-methoxy-2-methylindol-3-yl]acetic acid, is a synthetic nonsteroidal indole derivate introduced in 1963.84-86 As a non-steroidal anti-inflammatory drug (NSAID), it functions as a powerful nonselective inhibitor to the enzyme cyclooxygenase (COX), preventing COX-mediated DNA adduct formation by heterocyclic aromatic amines.85,86 Additionally, indomethacin also activates phosphatases that inhibit migration and proliferation of cancer cells, inhibit phospholipase A and C, reduce neutrophil migration, and decrease T-cell and B-cell proliferation.85,86 Indomethacin has been shown to influence endochondral ossification based on the differentiation stage of chondrocytes through the inhibition of COX.87 As the inhibition is non-specific, indomethacin inhibition of COX-1 or COX-2 during the early stages of chondrocyte differentiation influences prostaglandin expression, and therefore differences in chondrogenic phenotype.87 Indomethacin also has efficacy in differentiation bone marrow-derived MSCs to neural phenotypes when used with IBMX.88
Peroxisome proliferator-activated receptor γ (PPARγ) agonists function as activators to the nuclear ligand-activated PPARγ, which is found in various tissues, including muscle, fat, and hepatic tissues.67,86,89 The receptor in involved with the regulation of transcription of genes that influence β-oxidation of fatty acids, lipid metabolism, glucose metabolism, insulin signal transduction, and the differentiation of adipocytes and other tissues.67,86,89 PPARγ agonists such as KR-62776 and T131 have been utilized in adipocyte differentiation from 3T3 cells due to their effect on adipogenic gene expression.90 Their efficacy is related to their action causing an upregulation of lipin-1, which is a cytosolic phosphatidic acid phosphatase that can generate diacylclycerol (DAG), a precursor involved in a signaling cascade related to adipocyte differentiation.90 Additionally, PPARγ agonists are capable of meibocyte differentiation through the activation of lipogenic gene expression.91
DNA demethylation is a process in which methyl groups are removed from the base pairs cytosine, and can be classified as passive or active.92 Passive demethylation involves the absence of methylation via inhibition of DNA methyltransferase (DNMT), while active demethylation involves the direct removal of the methyl group.92 Agents that induce both passive and active demethylation of DNA have been utilized in the differentiation of progenitor and stem cells. Additionally, DNA demethylating agents have been used to prime MSCs and induce differentiation to pancreatic endoderm.93 Some agents, such as 5-azacytidine function through the inhibition of the enzyme DNA methylase.68 Furthermore, DNA demethylating agents such as decitabine which functions through passive demethylation, have been used to regulate differentiation of cells through the NOTCH1 pathway.94 The NOTCH1 pathway is heavily involved in cell fate determination through ligand-mediated receptors and signaling.95 Structures, targets, pathways, and uses of small molecules are outlines in Table 1.
Table 1.
Commonly Used Small Molecules in Stem Cell Differentiation. Structures created with PubChem Sketcher.
| Molecule | Structure | Targets/ Pathway(s) |
Mechanism of Action |
Uses | References |
|---|---|---|---|---|---|
| Dexamethasone | 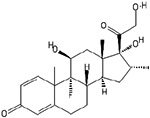 |
CCAAT/enhancer-binding protein β (C/EBPβ) | Inhibition of NFκB, inhibition of cyclin D2 | Promotes differentiation toward adipocyctic, cardiomyogenic, chondrocyctic, and osteoblastic phenotypes, | 59, 67-71 |
| Ascorbic Acid | 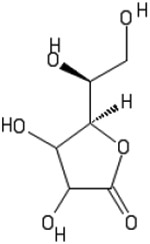 |
Reducing agent, antioxidant | Reprogramming efficiency, differentiation toward cardiomyogenic phenotype | 60, 73, 74 | |
| β-glycerophosphate | 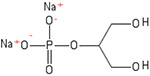 |
MEPE gene | Phosphate donor | Differentiation toward osteogenic phenotype, dental pulp stem cell determination | 77-79 |
| IBMX | 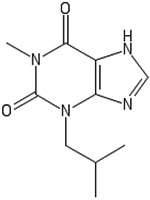 |
CCAAT/enhancer-binding protein β (C/EBPβ) | cAMP activation, CREB factor activator | Differentiation toward adipocytic and neural phenotypes | 82, 83 |
| Indomethacin | 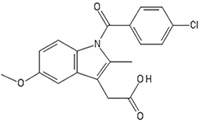 |
COX | COX inhibition, Phosphatase activation | Differentiation toward chondrogenic and neural phenotypes | 85-88 |
| KR-62776 | 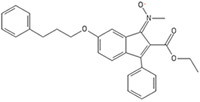 |
PPAR-γ | PPAR-γ agonist, upregulation of lipin-1 | Differentiation of meibocytes, differentiation toward adipogenic phenotype | 90, 91 |
| 5-azacytidine | 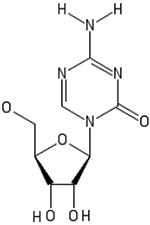 |
DNA-demethylating agent | DNA methylase inhibitor | Promotes differentiation toward adipogenic, cardiomyogenic, epitheial, hepatic, and myogenic phenotypes, reprogramming of iPSCs | 59, 60, 68, 194 |
In the realm of bioprinting stem cells, small molecules are often utilized post-printing to create a microenvironment with the potential to induce differentiation. Factors such as dexamethasone, ascorbic acid, β-glycerophosphate, IBMX, indomethacin, and the PPARγ agonist rosiglitazone have all been added to differentiation media used to culture and differentiate bioprinted constructs.96-99 Other studies have utilized small molecules as a component of differentiation media that can be pre-differentiated several days prior to bioprinting for immediate use.10 Currently, the addition of small molecules to bioink to be printed with cells has not been intensively researched. However, identification of factors that have ability to withstand the mechanical stress of the bioprinting process while maintaining function could be highly applicable in future studies.
3.2. Growth Factors
Growth factors are polypeptides that have the potential to influence and modify the proliferation, differentiation, and migration of cells.100 They are distinct from hormones, in that the site of production and the target of action are not limited or restricted to specific tissues, broadening the scope of their potential affects.100 The use of growth factors and the signaling pathways they utilize can influence the proliferation and differentiation of stem cells, and can be controlled for optimal in vitro manipulation.
The transforming growth factor β (TGF-β) group of growth factors is widely utilized in stem cell differentiation. These growth factors are a family of cytokines and trophic hormones that have a myriad of physiological functions.101 They are integral in growth, development, inflammatory response, repair, and immune response.101 TGF-β factors function through ligand-regulated transmembrane enzymes, and make use of pathways such as tyrosine kinase cascades.86 (Figure 2A) TGF-β mediates differentiation in multiple cell types and lineages. Notably, all immune cells, dendritic cells, and macrophages secrete factors within the family, which influence differentiation, proliferation, and function.102,103 Members of the TGF-β family have also been shown to be a mediator in oligodendrocyte differentiation, with specific activity in oligodendrocyte-type-2 astrocytes.104 Furthermore, members of the family increase chondrogenesis.105
Figure 2.
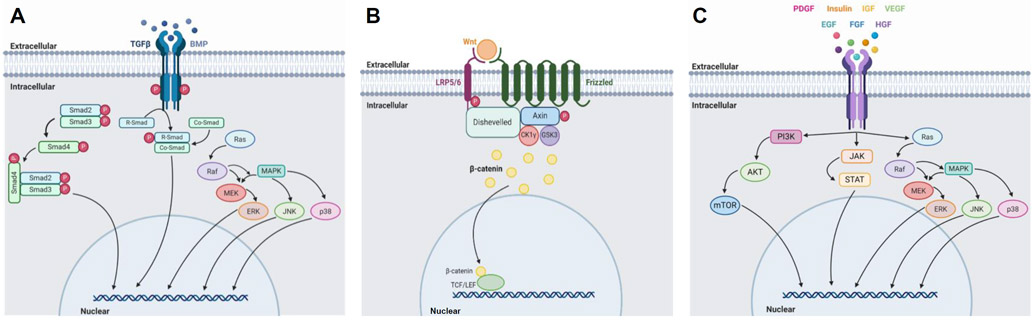
(A) Illustrated overview of (A)TGF-β signaling pathway, utilized by ligands belonging to the TGFβ family and the BMP subfamily101,106, (B) Wnt signaling pathway113, and (C) tyrosine kinase receptor signaling pathways, utilized by PDGF, insulin, IGF, VEGF, EGF, FGF, and HGF.86,116,120,123,127,130,134 Created with Biorender.com.
Bone morphogenic proteins (BMPs) are a subgroup of proteins in the TGF-β family that modulate the differentiation of mesenchymal cells into bone and bone marrow cell phenotypes. BMPs have a major role in temporal, spatial, and biological features of bone development.106,107 They function through signal transduction initiated by the binding of heterodimeric complexes to kinase receptors specific to their structure. Through the receptors, they mediate phosphorylation of transcription factors, leading to functional outcomes in skeletal and bone development (Figure 2A).106 There are several types of BMPs, including BMP-2, BMP-3, and BMP-7. In laboratory settings, the activity of BMPs have been utilized to promote proliferation and control bone and cartilage morphogenesis and differentiation in a controlled fashion.108-112
The Wnt signaling pathway plays an integral role in cell migration, cell polarity, organogenesis during embryonic development, and regulating cell fate and stem cell potency.113,114 The Wnt ligand is a glycoprotein that is secreted by cells and binds to Frizzled receptors. This begins a cascade of activity, in which the Wnt receptor complex begins a signaling pathway that leads to the eventual activation of phosphorylating agents, regulating transcription, and promoting proliferation (Figure 2B).113,115 Mesenchymal cell differentiation is heavily influenced by Wnt signaling activity. Multiple studies have demonstrated the relation between MSC differentiation and Wnt signal transduction pathways at various stages of embryonic development. Notably, Wnt signals are believed to be linked to redox reactions and signals in MSC lineage differentiation.114
Platelet-derived growth factors (PDGFs) are trophic hormones that interact with ligand-regulated transmembrane enzymes (Figure 2C).86 In addition to contributing to cell viability and proliferation, PDGFs have been shown to contribute to the development of the neural crest, the central nervous system, skin, intestines, lungs, reproductive organs, skeletal system, blood vessels, and other aspects of hematopoiesis.116-118
Insulin is a naturally occurring anabolic hormone that functions in glucose storage and energy regulation.86 In the microenvironment, as well as in the body, insulin is a powerful adipogenic agent that functions through the activation of transcription factors (Figure 2C).119 Insulin-like growth factor (IGF) is a naturally occurring endocrine hormone with expansive effects on cell differentiation and determination (Figure 2C).86,120 Two major types, IGF-1 and IGF-2, are involved in the differentiation of the three germ layers of the embryo, as well as the regulation of growth hormone.120,121 Furthermore, IGF plays a role in neural cell growth, proliferation, apoptosis, and differentiation.122
Vascular endothelial growth factor (VEGF) is an angiogenic growth factor and is produced by a plethora of cells, including but not limited to macrophages and platelets.123 In addition to physiological functions pertaining to hematopoiesis, bone formation, and somatic development, VEGF is instrumental in hematopoietic, osteogenic, and chondrogenic proliferation, viability, and differentiation (Figure 2C).123-126
Epidermal growth factor (EGF) is a trophic hormone that interacts with ligand-regulated transmembrane enzymes, such as tyrosine kinase receptors, and is involved in signaling cascades (Figure 2C).86 While research remains to be seen on the effect of EGF on differentiation, it is known to be instrumental in the proliferation, migration, and viability of stem cells.127-129
The fibroblast growth factor (FGF) family consists of over 20 growth factors which interact with tyrosine kinase receptors to stimulate signaling pathways (Figure 2C).130 This family of factors is instrumental in stimulating proliferation and increasing growth rate in stem cells, and demonstrates a preference toward chondrogenic differentiation over time.131-133
Hepatocyte growth factor (HGF) is a cytokinetic growth factor that has the ability to influence the proliferation, migration, viability, and differentiation on a wide array of progenitor and stem cells (Figure 2C).134 Its applicability to many cell types lends promise to use in the microenvironment of a bioprinted construct. A summary of the growth factors and their effects is included in Table 2.
Table 2.
Growth Factors Used in Stem Cell Differentiation
| Growth factor (family) |
Effects | References |
|---|---|---|
| TGF-β Family | Increased proliferation. Differentiation toward chondrogenic and oligodendric phenotype. | 86, 101-105 |
| BMP Subfamily | Increased proliferation. Differenation toward chondrogenic and osteogenic phenotype. | 106-112 |
| Wnt Family | Increase proliferation and differentiation. | 113-115 |
| PDGF | Increased proliferation and viability. Differentiation toward neural, pulmonary, dermal, gastrointestinal, skeletal, vessel, and hematopoietic cell types. | 86, 116-118 |
| Insulin | Differentiation toward adipogenic phenotype. | 86, 119 |
| IGF | Increased proliferation and viability. Influences apoptosis. Differentiation toward neural phenotype. | 86, 120-122 |
| VEGF | Increased proliferation and viability. Differentiation toward chondrogenic, hematopoietic, and osteogenic phenotypes. | 123-126 |
| EGF | Increased proliferation, migration, and viability. | 127-129 |
| FGF | Increased proliferation. Differentiation toward chondrogenic phenotype. | 130-132 |
| HGF | Increased proliferation and viability. | 134 |
There are many approaches that have combined growth factors with bioprinting methodologies. Growth factors, such as PDGF and FGF, have been used as additives in bioprinting.99 Similar to the use of small molecules, some growth factors, including TGF-β and insulin, are added as a supplement to media in order to promote differentiation of printed constructs, and can also be used for pre-differentiation before bioprinting.96 Growth factors can also be incorporated into bioinks prior to bioprinting, and conjugated to biomaterial scaffolds and co-printed with cells to direct cell fate before, during, and after biporinting.135 In a study conducted by Lee et al., neural stem cells were co-printed alongside a fibrin hydrogel conjugated with VEGF.10,136 The cells showed a tendency to migrate toward VEGF and demonstrate more progressive differentiation than the cells printed without VEGF.136 Similarly, Poldervaart et al. utilized BMP-2 in bioprinted alginate for osteogenicity in mice and rats.5,137 The use of growth factors as components of differentiation media and as bioprinting additives offer promising options for bioprinting stem cells, and allow for the guiding of stem cell fate at virtually any point in the printing process.
3.3. Synthetic Peptide Molecules
Peptide-based biomolecules can assist in the mimicry of physiological conditions to aid in the success of a printed biomaterial.68 The use of peptide sequences to facilitate self-assembly, or to imitate other substances such as growth factors, can assist in developing the biochemical and structural components of a bioprinted construct.68 The conjugation or incorporation of synthetic peptide sequences to bioprinted constructs can be instrumental in facilitating the development of a microenvironment that allows for stem cell proliferation and differentiation.68,138 A common approach in developing biomaterials is to create biomimetic peptide sequences to emulate growth factors, modular proteins, or adhesive peptides.68,138-140
Growth factors, such as the BMP subfamily and the TGF-β superfamily, are commonly used to induce differentiation of printed stem cells.68,138 Furthermore, sequences have been fabricated to replicate binding domains in modular proteins such as osteocalcin (OCN) and sialoprotein to influence the microenvironment toward desired applications.138 Other peptides that contribute to the microenvironment have been targeted for replication, such as cytomodulin (CM), due to the ability to stimulate collagen synthesis, and QK, due to its proangiogenic properties.141,142 Other proteins are targeted for their effects on cells, such as N-cadherin and its association with cell-cell adhesion, differentiation, and migration.143 A commonly used amino acid sequence, arginine-glycine-aspartate (RGD), is known to assist with binding of proteins typically seen in the extracellular matrix, as well as the attachment of various cell types.139,140 The incorporation of synthetic peptides to mimic certain conditions can expedite the maturation of a bioprinted construct toward a specified or desired tissue type. A summary of synthetic peptide sequences utilized is included in Table 3.
Table 3.
Synthetic peptides used to induce differentiation.
| Peptide | Amino Acid Sequence(s) | Effects | References |
|---|---|---|---|
| BMP | KIPKASSVPTELSAISTLYL | Differentiation toward osteogenic phenotype. | 68 |
| BMP-2 | KIPKACCVPTELSAISMLYL (AAs: 17-25) | Promotes stem cell differentiation. | 138 |
| BMP derivatives | KIPKASSVPTELSAISTLYL (Cys & Met replaced by Ser & Thr) | Promotes stem cell differentiation. | 138 |
| OCN | γEPRRγEVCγEL (AAs: 17–25) | Differentiation toward chondrogenic and osteoblastic phenotypes. | 138 |
| OCN derivatives | KIPKASSVPTELSAISTLYLAAAAγEPRRγEVAγEL KIPKASSVPTELSAISTLYLAAAAγEPRRAVAγEL KIPKASSVPTELSAISTLYLAAAAγEPRRAVAAL KIPKASSVPTELSAISTLYLAAAAEPRREVAEL |
Differentiation toward chondrogenic and osteoblastic phenotypes. | 138 |
| QK peptide | (Ac-K-[Acryl]-LTWQELYQLK(Ac)YK(Ac)GI-NH2) | Mimics 17-25 helix region of VEGF, accelerates microvascularization. | 151 |
| N-cadherin | Ac-HAVDIGGGC Ac-AGVGDHIGC |
Differentiation toward chondrogenic phenotype. | 152 |
| Arginine-glycine-aspartate | RGD | Binds to fibronectin, fibrinogen, osteopontin, bone sialoprotein, vitronectin. | 139, 140 |
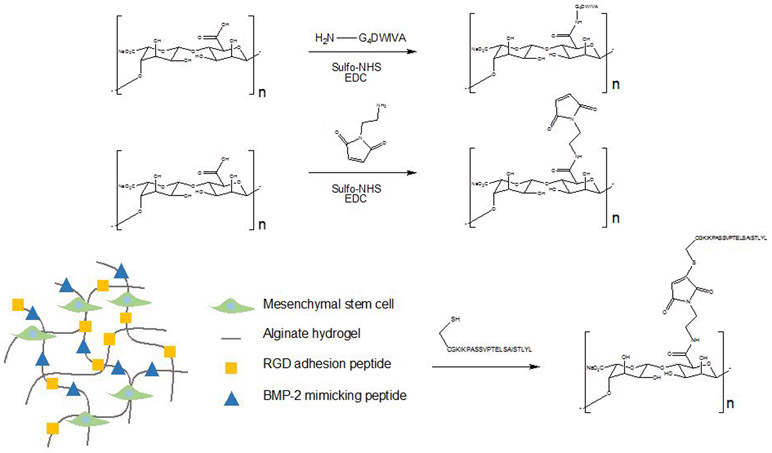
Madl et al. synthesized the peptide mimics of BMP-2 by solid phase Fmoc-peptide synthesis.144 These BMP-2 mimicking peptides were covalently bound to alginate hydrogel by carbodiimide and sulfhydryl-based coupling strategies, which is depicted in Figure 3.144 The BMP-2 mimicking peptide promoted the alkaline phosphatase activity in clonally derived murine osteoblasts.144 In addition, these peptides initiated Smad signaling and upregulated osteopontin production and mineral deposition of clonally derived murine mesenchymal stem cells.144
Figure 3.
Synthetic BMP-mimetics created via solid phase Fmoc-peptide synthesis, in which BMP-2 mimicking peptides were covalently bound to alginate hydrogel through carbodiimide and sulfyldryl-based coupling reactions.144
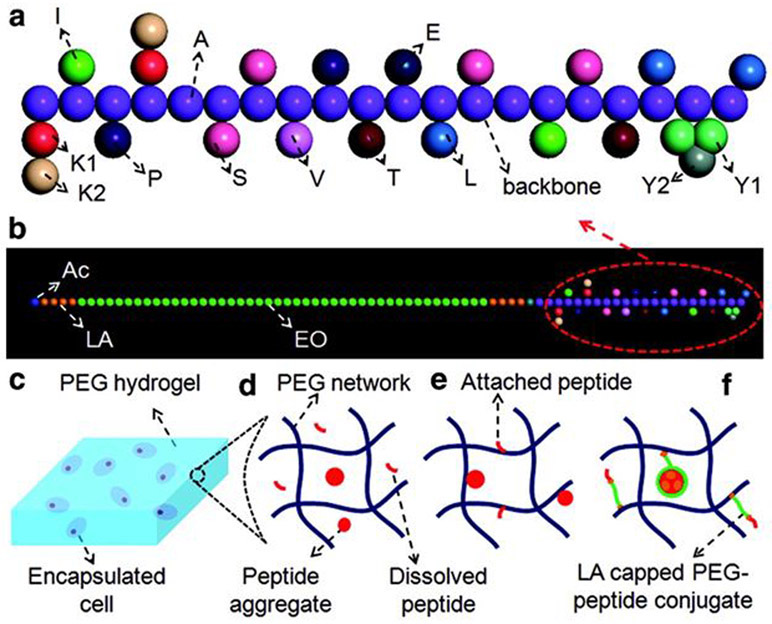
Moeinzadeh et al. investigated the effect of concentration and hydrophobicity of the BMP-2 peptide on peptide aggregation and osteogenic differentiation of human mesenchymal stem cells encapsulated in a PEG hydrogel (Figure 4).145 Acrylamide-terminated BMP-2 peptide was synthesized by the reaction between N-terminal amine of the peptide with acrylic acid on the resin using the amino acid coupling reaction. Lactide segments were capped on ends of PEG-peptide chain by coupling the amino acid sequence cysteine-glycine-glycine to the lysine end of the BMP-2 peptide, which abled control of the peptide hydrophobicity. For conjugation, the Michael addition reaction, which forms bonds between carbons through a mechanism involving carbanion formation, facilitated a bond between the cysteine’s sulfhydryl group on the peptide and the acrylate on the macromer was used. The BMP-2 peptide with a positive index of hydrophobicity formed aggregates, which had a critical micelle concentration. The osteoinductive potential of the BMP-2 peptide was significantly less than that of BMP-2 protein despite the 1000-fold higher peptide concentration. In addition, conjugation of BMP-2 peptide to lactide-capped PEG reduced critical micelle concentration and osteoinductive potential of the peptide.
Figure 4.
Conjugation of bone morphogenic protein-2 (BMP-2) peptide to PEG hydrogel to induce osteogenic differentiation of mesenchymal stem cells. (A) Illustration of BMP-2 peptide. (B) BMP-2 peptide with a lactide-capped PEG conjugate. Lactide is shown in brown, ethylene oxide is shown in green, and acrylate is shown in blue. (C) Illustration showing MSCs encapsulated in PEGDA hydrogel matrix in different experimental groups, including (D) the peptide (shown in red) dissolved in the hydrogel network, (E) covalent attachment of the peptide to the hydrogel network, and (F) the peptide and lactide-capped PEG conjugate attached to the hydrogel network. Reproduced with permission from Moeinzadeh et al.145 Copyright 2015, Mary Ann Liebert, Inc.
He et al. investigated the effects of RGD and BMP peptides that were conjugated to a hydrogel structure on osteogenic differentiation and mineralization of bone marrow stem cells (Figure 5).146 Acrylic acid of RGD peptide was reacted with the N-terminal amine group of the peptide to form the functionalized Ac-GRGD peptide. On the other hand, the PEGylated BMP peptide was reacted with 4-carboxybenzenesulfonazide to produce an azide functionalized Az-mPEG-BMP peptide. Then they crosslinked functionalized RGD and BMP peptide to poly (lactide-co-ethylene oxide-co-fumarate) (PLEOF) macromer. BMSC cells seeded on the RGD+BMP peptide modified hydrogels showed increase in ALPase activity after two weeks and increase in calcium content after three weeks of culture.
Figure 5.
Conjugation of RGD and BMP peptides to hydrogel structure to induce osteogenic differentiation and mineralization of bone marrow stem cells.146
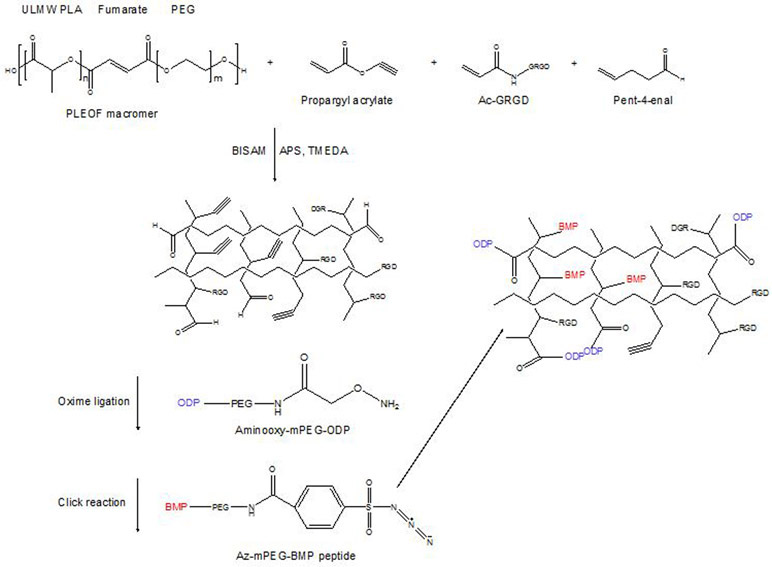
In a subsequent study, He et al. conjugated osteopontin peptide and bone morphogenetic protein-2 (BMP) peptide to an RGD conjugated hydrogel and observed their effect on osteogenic and vasculogenic differentiation of bone marrow stromal cells.147 Propargyl acrylate and 4-pentenal were conjugated to the hydrogel by click chemistry and oxime ligation. The osteopontin peptide was grafted by the reaction between aminooxy moiety of aminooxy-mini-poly(ethylene glycol)-osteopontin and aldehyde moiety in the hydrogel. The synthesis is outlined in Figure 6. The BMP peptide on the other hand, was grafted by the reaction between the azide moiety of Az-mPEG-BMP and the propargyl moiety in the hydrogel. Among the comparison between, RGD, RGD+BMP, RGD+BMP+mOPD, and RGD+BMP+OPD hydrogels, the extent of mineralization was highest in RGD+BMP+mOPD group. In addition, only the bone marrow stromal cells in RGD_BMP_OPD grafted hydrogels showed positive staining for vasculogenic markers alpha-SMA, PECAM-1, and VE-cadherin.
Figure 6.
Conjugation of osteopontin and BMP to an RGD-conjugated hydrogel to induce osteogenic and vasculogenic differentiation of bone marrow stem cells.147
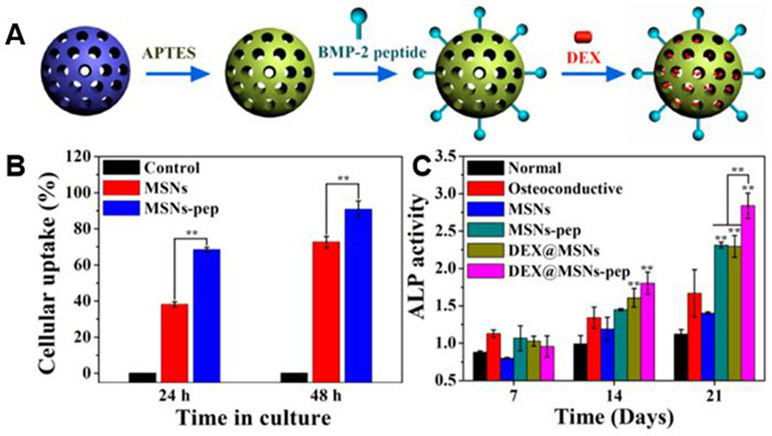
Incorporation of residues 73-92 of BMP-2 (BMP-2 peptide) to mesoporous silica nanoparticles (MSNs-pep) was performed in one study.148 MSNs-pep were synthesized by covalently grafting BMP-2 peptide on the surface of nanoparticle by an aminosilane linker. Then, dexamethasone (DEX) was loaded into the pores of MSNs to form nanoparticulate osteogenic delivery systems (Figure 7A). In vitro results with BMSCs show that the MSNs-pep had batter cytocompatibility and higher cellular uptake efficiency than the bare MSN’s (Figure 7B). In addition, BMSCs cultured with MSN’s-pep showed higher alkaline phosphatase (ALP) activity, calcium deposition, and expression of bone-related protein than in bare MSN’s. When DEX was loaded into the MSNs-pep, the osteogenic differentiation of BMSCs were further promoted (Figure 7C).
Figure 7.
Osteogenic delivery systems fabricated from mesoporous silica nanoparticles (MSNs-pep) conjugated with BMP-2 and loaded with dexamethasone. (A) Illustration of the conjugation of dexamethasone and BMP-2 to nanoparticles. (B) Mean fluorescence uptake of MSNs and BMP-2 conjugated MSNs against BMSCs in vitro. (C) Alkaline phosphatase (ALP) activity of BMSCs after treatment with noted samples for differing culture periods. Reproduced with permission from Zhou et al.148 Copyright 2015, American Chemical Society.
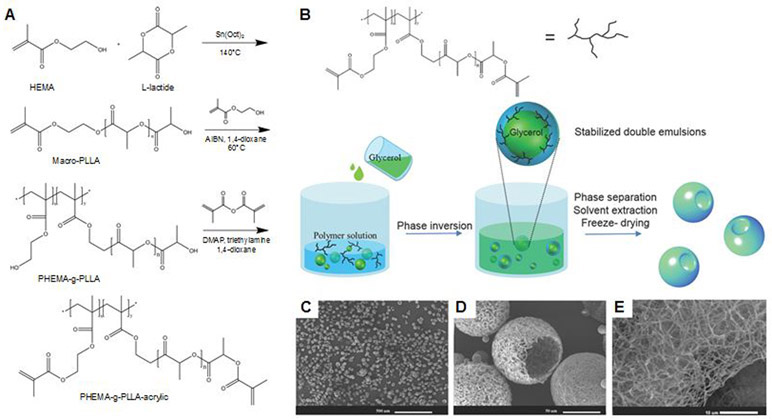
In another study, Zhang et al. synthesized functional nanofibrous hollow microspheres (FNF-HMS) and conjugated with peptides to induce selective differentiation pathways of rabbit BMSCs.149 They conjugated FNF-HMS to two peptides, TGFβ1 mimicking peptide CM and BMP-2 mimicking peptide P24. FNF-HMS were conjugated with thiolated peptides through thiol-ene click reaction (Figure 8). Both in vitro and in vivo studies showed that the FNF-HMS present CM to BMSCs and induce chondrogenesis of BMSCs for cartilage formation. In addition, P24 conjugated to FNF-HMS induced ectopic bone formation in nude mice.
Figure 8.
Synthesis of functional nanofibrous hollow microspheres (FNF-HMS) conjugated with CM and P24 to induce differentiation of bone marrow-derived mesenchymal stem cells (BMSCs). (A) Synthesis of PLLA-based block copolymer PHEMA-g-PLLA-acrylic. (B) Illustration of the emulsification and separation techniques to fabricate FNF-HMS and (C-E) scanning electron microscopy (SEM) images of the fabricated microspheres. Reproduced with permission from Zhang et al.149 Copyright 2015, John Wiley & Sons, Inc.
Seo et al. developed in situ-forming click-crosslinked hyaluronic acid hydrogel (Cx-HA-CM) containing chemically conjugated CM-2 (Figure 9).150 The developed hydrogel was tested for chondrogenic differentiation of human periodontal ligament stem cells (hPLSCs). Cx-HA was formed by click-reaction between tetrazine-modified HA and transcyclooctene-modified HA150. CM, which is a chondrogenic differentiation factor, was covalently linked to Cx-HA and it enabled prolonged release profile of CM compared with CM physically loaded onto Cx-HA. In addition, Cx-HA-CM induced better chondrogenic differentiation of hPLSCs.
Figure 9.
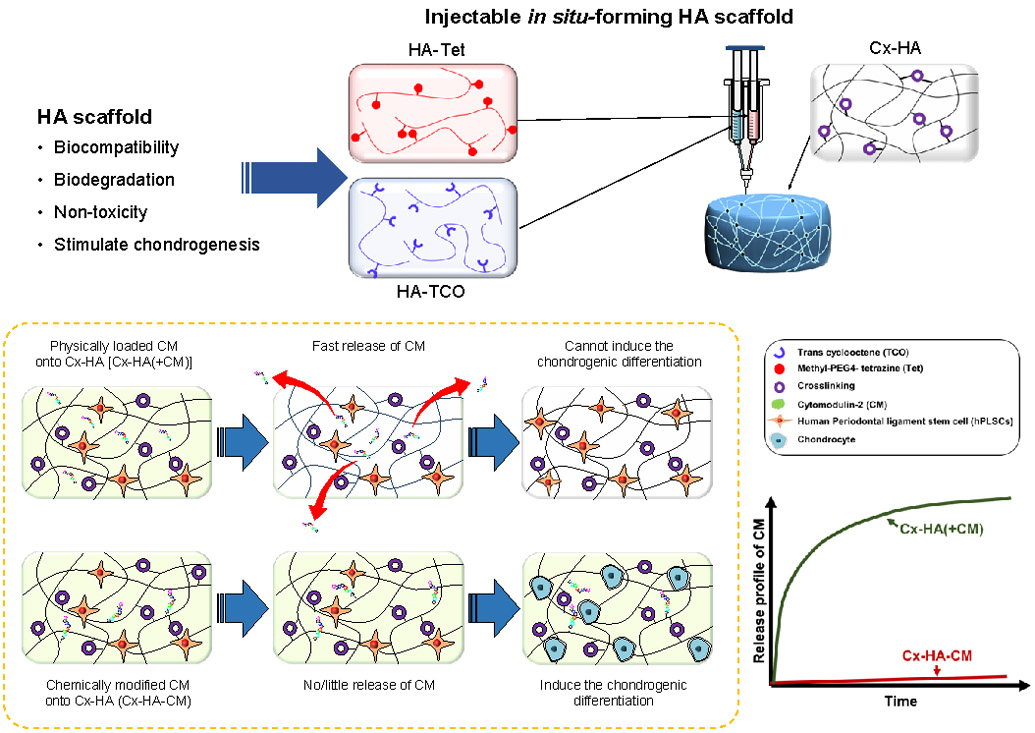
In situ-forming hyaluronic acid hydrogel containing a chemically conjugated cytomodulin-2 mimetic.150
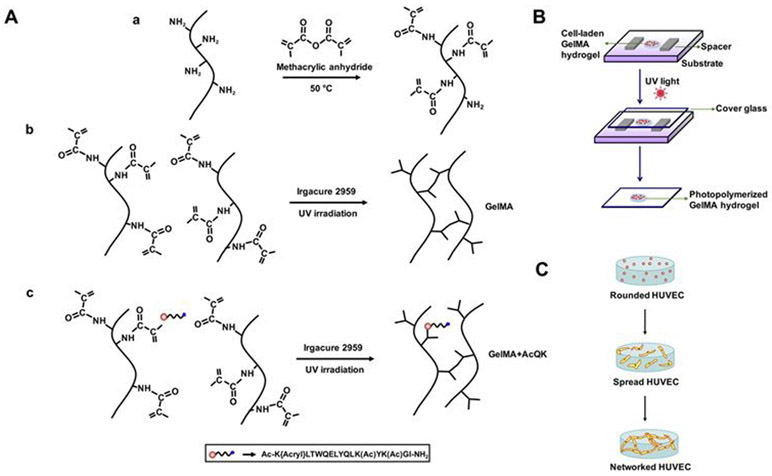
The conditions necessary for microvascularization in a tissue-engineered construct was investigated using an agonist peptide (QK), which mimics the helix region 17-25 of VEGF that has the effect of isoform VEGFA-165 on VEGF receptors.151 In this study, gelatin methacrylate (GelMA) based cell-laden hydrogel was covalently linked with VEFG-mimicking acrylated peptide (Ac-K-[Acryl]-LTWQELYQLK(Ac)YK(Ac)GI-NH2) and inactive form of the peptide (Ac-VK[Acryl]FMDVYQRSYCHPNH2) (Figure 10A). Human umbilical vein endothelial cells (HUVECs) was encapsulated and cultured in three groups of hydrogels; (i) GelMA (control), (ii) the active form of the peptide linked with GelMA (GelMA+AcqK), and (iii) the inactive from of the peptide linked with GelMA (GelMA+InactQk) (Figure 10B-C). Their expression of vascular-specific genes was analyzed, and the results showed that the employment of AcQK peptide accelerated microvascularization.
Figure 10.
Fabrication of construct conjugated with agonist QK peptide to mimic VEGF activity in order to accelerate microvascularization. (A) Chemical formation of active and inactive peptide linked GelMA structures, (B) the preparation of cell-laden GelMA, and (C) formation of HUVEC network in GelMA hydrogels following culture. Reproduced with permission from Parthiban et al.151 Copyright 2017, Elsevier.
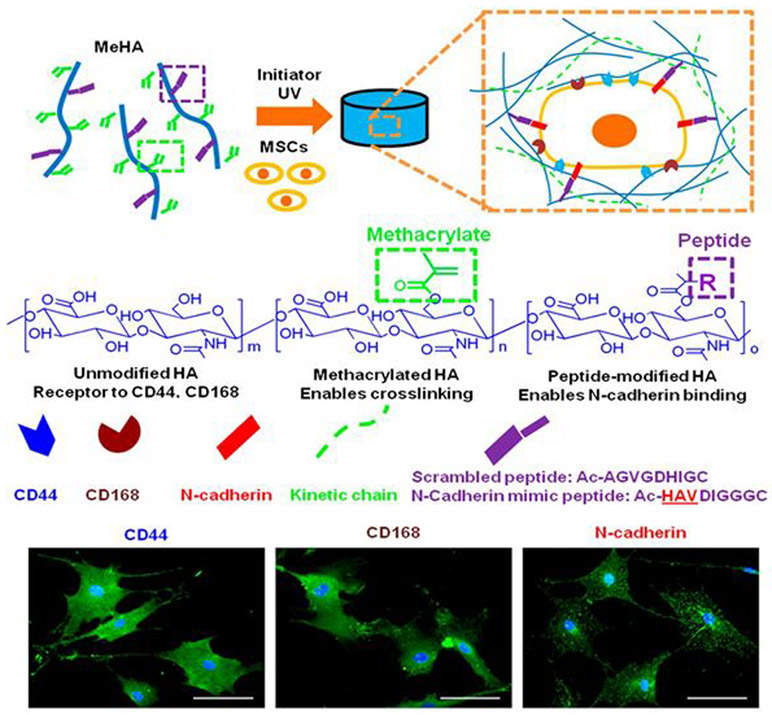
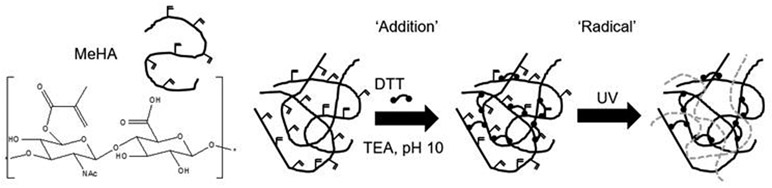
In another study, functionalized methacrylated-HA (MeHA) hydrogels with N-cadherin mimetic peptides (Ac-HAVDIGGGC) were developed and investigated their effect in regulating chondrogenesis and cartilage matrix deposition using encapsulated MSCs.152 N-cadherin peptide conjugation to MeHA hydrogels upregulated initial chondrogenesis of MSCs and cartilage-specific matrix production, compared to non-functionalized control MeHA hydrogel or a scrambled peptide domain (Ac-AGVGDHIGC)-conjugated HA hydrogel. Both Scrambled and N-cadherin mimic peptides with a cysteine residue at the C-terminal end allowed Michael-type addition reaction with MeHA (Figure 11).
Figure 11.
Development of functionalized methacrylated-HA (MEHA) hydrogels conjugated with N-cadherin mimetic peptides to induce chondrogenic differentiation on MSCs. Reproduced with permission from Bian et al.152 Copyright 2013, PNAS.
The process of bioprinting peptides offers many similar options to other biochemical cues. Synthetic peptides, like BMP and RGD, are widely used as additives to differentiation media or as components of bioink to be co-printed with cells.5,10,96,99,135,153 Table 3 lists the most commonly used synthetic peptides. The versatility and specificity of engineering synthetic peptides offer many options in the field of bioprinted stem cell constructs.
3.4. Microvesicles and Exosomes
Exosomes are small, single-membrane vesicles that exists in many cell types, and is notably present and active in MSCs.154,155 Exosomes participate in many cellular functions, such as cell-cell communication through secretion, protein transport, and RNA transport (Figure 12). Their properties as carriers make them ideal for transporting molecules for targeted cell delivery in many applications, including drug delivery and direction of stem cell differentiation. Recent research has explored the use of exosomes derived from MSCs in tissue engineering and regenerative medicine applications. Exosomes have been successfully isolated and utilized as a means of directing differentiation of stem cells toward specific tissue lineages and phenotypes, in vitro and in vivo.156 Efforts in isolating exosomes have led to the testing and development of several techniques and approaches.
Figure 12.
Illustration of exosome leaving cell to deliver contents for extracellular transport.154,155 Created with BioRender.com.
Approaches such as ultracentrifugation, size-based separation, immunoaffinity, precipitation, and microfluidics-based separation have been evaluated for their efficacy in obtaining exosomes.157 Furthermore, size exclusion chromatography has been used in isolated exosomes, which were structurally conserved throughout the process. Field-flow fractionation has also been utilized to separate exosomes through channel-based flow systems based on size and diffusion potential. Hydrostatic filtration dialysis has also used flow-based methods to separate exosomes based on size through the use of pressure and filtration approaches. In the realm of immunoaffinity-based techniques, enzyme-linked immunosorbent assay (ELISA) has been developed to capture and quantify exosomes from various bodily fluids, such as plasma, serum, and urine. Magnetic labeling, mass spectrometric assays, and other immunoassay-based approaches have been specialized to isolate exosomes. Precipitation and microfluidic-based techniques have also been utilized. Precipitation of exosomes has been achieved through the incubation of exosomes with factors that facilitate precipitation, followed by low-speed centrifugation or filtration. This is ideal for large sample sizes and is a cost-efficient scalable approach to isolating exosomes for tissue engineering purposes. Microfluidic chips of varying channel sizes have also been developed to allow exosomes to separate from their surrounding physiological fluids and components.157
The propensity of exosomes to carry and release various biochemical elements make them ideal in delivering factors that can control stem cell fate in a bioprinted microenvironment. While harvesting and isolation of exosomes is ever-advancing, the use of exosomes in tissue engineering and factor delivery is a new area of research that is continually being developed. Exosomes have shown efficacy in assisting with the adhesion of ECM proteins to biomaterials.156 Exosomes have been repurposed and used to induce differentiation toward osteogenic, dental pulp, and stromal phenotypes and lineages.156,158-160
While research regarding the use of exosomes to differentiate stem cells is still in the early stages, several groups have made strides in establishing the efficacy of exosomes in applications. Based on previous research determining that exosomes containing miRNAs and proteins directed MSC differentiation, and that exosomes could be endocytosed by target cells, Narayana et. al hypothesized that exosomes isolated from osteogenic mesenchymal stem cells could be used to promote lineage-specific differentiation of human mesenchymal stem cells.156 The group went on to validate this hypothesis in both in vitro and in vivo settings. In a later study by the same group, Huang et al. utilized exosomes isolated from dental pulp stem cells to demonstrate endocytosis of the exosomes by other dental pulp stem cells and human MSCs to promote differentiation and regeneration of dental pulp tissue.158 The study further demonstrated that endocytosed exosomes were activating the p38 mitogen activated protein kinase (MAPK) pathway, and also that exosomes were capable of adhering to biomaterials through binding to collagen and fibronectin.158 Similarly, Chowdhury et al. utilized exosomes secreted by prostate cancer cells to differentiation of BMSCs.159 In this study, the exosomes were found to deliver TGF-β to induce differentiation, and directed differentiation toward a myofibroblastic phenotype.159 Webber et al. also used cancer-derived exosomes bearing TGF-β to induce myofibroblastic differentiation in stromal cells.160 The ability of exosomes to direct stem cell fate through multiple mechanisms by transporting a range of factors with differing bioactivity offers novel opportunities for use in bioprinted stem cell constructs. Future studies in the field are likely to examine a wider array of exosome sources and target applications.
3.5. Role of Small RNAs in Directing Stem Cell Differentiation
Synthetically developed small RNAs have been used to manipulate gene expression in cellular targets, and can be utilized in tissue engineering to aid in regeneration and repair of damaged or compromised tissues or organs.68 Small double stranded sequences of RNA are often used for interference, in which they induce posttranscriptional gene expression and repress the translation of certain proteins.68 In the setting of stem cells, this can assist in the regulation of self-renewal, proliferation, and differentiation of cells.68 Studies have shown that the use of small RNAs delivered to cellular cultures can assist in the production of iPSCs from a population of ESCs, implying that this methods can be utilized to change or enhance stem cell potency and future phenotypical expressions.68,161,162
Two of the most research classes of small RNAs include small interfering RNA sequences (siRNAs) and micro RNA sequences (miRNAs). While these two types of small RNAs have similar mechanisms of action in tissue engineering, the biological production and native function differ.68 While siRNAs tend to be 21-27 base pair sequences with the function of protecting the host genome from viral or pathogenic nucleic acids, miRNAs are generally approximately 22 base pair sequences of non-coding genetic information that is conserved and passed from plants to eukaryotes.68 Despite difference in origin and intended function, both synthetic siRNAs and miRNAs are utilized to control gene and protein expression through the aforementioned interference mechanism.
Numerous miRNA sequences have been researched and proven to influence stem cell differentiation to specific tissue lineages, including but not limited to adipogenesis, osteogenesis, chondrogenesis, cardiac regeneration, liver regeneration, myogenesis, neurogenesis, angiogenesis, and other applications.68,163 MicroRNA sequences such as miR-23a, miR-30c, miR-34c, miR-133a, miR-135a, miR-137, miR-138, miR-204, miR-205, miR-217, miR-218, miR-338, miR-199a, miR-18a, miR-221, miR-675, miR-29 and miR-29b have been used for bone and cartilage applications.68 Others, like miR-1, miR-27, miR-133, miR-188, and miR-206 have been used to induce differentiation toward skeletal muscle applications.68 Notably, the miR-15 family and miR-7, miR-133b, and miR-206 have been used in cardiovascular and neural settings to promote native tissue repair and regeneration.68 Many of the investigated applications of small RNAs in tissue engineering have focused on tissue repair and regeneration in the host tissue, circumventing the use of these approaches in the bioprinting of stem cells.68 As delivery methods for the therapeutic potential of small RNAs are being developed, it is imperative to consider the role of the small RNAs in a microenvironment on cells that have not yet been introduced into the native physiological environment. The use of these nucleic acid sequences in bioprinted stem cell constructs could be advantageous in determining cellular fate and construct application prior to implantation. The potential use and delivery of genetic factors along with bioprinted stem cell constructs has promising potential for use in tissue engineering and regenerative medicine.
3.6. Bioink Additives and Influence on Cellular Fate
Bioink additives involving stem cells offer the option to include materials in the bioink formulation that assist in the regulation and differentiation of stem cells into the desired cell phenotype or functional profile. Commonly used substances include calcium phosphate-based ceramics, bioactive glass, microcarriers, and other chemicals.10,164-166
3.6.1. Calcium Phosphate-Based Ceramics.
Hydroxyapatite (HA), also known as pentacalcium hydroxide triphosphate, is a calcium phosphate mineral component of bones.167 Due to its hexagonal structure, stability, and chemical properties, it is often utilized as a hydrogel additive in the bioprinting of bone constructs.168,169 HA is widely recognized as a material suitable for tissue engineering due to its bioactivity, biocompatibility, and lack of inflammatory response induction in biological settings.170 As an osteoconductive additive, hydroxyapatite is commonly added to hydrogel, gelatin, alginate and other bioinks to provide a microenvironment analogous to human bone.171-173 HA has been demonstrated to show efficacy in formation of the bone extracellular matrix needed to sustain the microenvironment, and remodeling consistent with physiological bone.174 In addition to being an integral component of bone matrix formation, HA also acts as an agent to increase viscosity and printability in extrusion-based methods.172
Another commonly used calcium phosphate-based bioink additive is β-tricalcium phosphate (β-TCP). The β configuration is one of many crystalline polymorphs of tricalcium phosphate, which is derived from the chemical treatment of hydroxyapatite.175,176 β-TCP can also be synthesized by many methods, including through the sol-gel process, the synthesis or double decomposition of Ca(NO3)2 and (NH4)2HPO4, the neutralization of CaCO3 and H3PO4, or through a water-ethanol synthesis of Ca(NO3)2•4H2O and (NH4)2HPO4.177,178 A notable characteristic of β-TCP is its degradation profile. While other calcium phosphates may inadvertently inhibit the growth of new bone after scaffold implantation, β-TCP creates an equilibrium between scaffold absorption and new bone formation in target tissues.179 Another beneficial property of β-TCP is the ability to pair it with BMPs, which could guide the fate of stem cells in a bioprinted bone microenvironment.180
The benefits of both HA and β-TCP can be realized through their synthesis, in a combination known as biphasic calcium phosphate particles (BCPs).181 BCPs are a popular ceramic in inducing stem cell differentiation and microenvironment replication.182,183 While the ratio of HA to β-TCP can have varying effects on construct degradation, the growth of cells on BCP-containing matrices produces integral elements analogous to the native ECM, including structural proteins, adhesive proteins, and functional proteoglycans.183 The direct association with formation of the microenvironment makes calcium phosphate ceramics a promising bioink additive in stem cell bioprinting.
3.6.2. Bioactive Glass.
Bioactive glasses are biomaterials comprised of glass and ceramics, and are widely used for their bioactivity and biocompatibility in stem cell bioprinting.184 Bioactive glasses and their ions are capable of inducing stem cell differentiation and promoting angiogenesis in bioprinted constructs.185-187 The ability to direct stem cell fate while also giving rise to the vascularization of a printed construct makes bioactive glasses incredibly instrumental in the application of stem cell bioprinting.
3.6.3. Microcarriers.
An additional bioink additive that has shown great potential is the use of microcarriers (MCs), which allow for the attachment of adhesive cell types while in suspension.10,188-191 MCs consist of various sizes and promote cell attachment and growth while remaining chemically inert if desired.10,188-191 MCs can alternatively be further modified to direct the differentiation of stem cells.10,192 MCs have been successfully modified in the areas of size, ranging from 60 to 400 μm, as well as being bound to growth factors or chemicals, such as TGF-β3, BMP-2, and other soluble factors.10,137,188,189,191,193
4. BIOMATERIAL ORGANIZATION AND COMPOSITION
4.1. Extracellular Matrix-Derived Microenvironment
The extracellular matrix (ECM) is one of the most important components in tissue and organ function, and is composed of various fibrous proteins, proteoglycans, and glycoproteins.68,194 The ECM provides structural support, topographical organization, and a biochemical microenvironment, all of which combine to facilitate cell growth, proliferation, migration, differentiation, and biochemical function.68,195-197 Notably, the ECM possesses the ability to influence and direct the fate and differentiation of embedded stem cells through the regulation of growth factors, enzymes, and other biochemical cues, as well as through physical topography and structural cues68. The properties of the ECM utilized in stem cell bioprinting has a profound influence on the structure, function, and applications of the construct, and is one of the major factors of the cellular microenvironment post-printing.
4.1.1. Structural Proteins in the ECM.
Proteins comprise a major component of the ECM, specifically defined by fibrous proteins, which are the most prominent type of protein observed in the human ECM.68,198 Collagen and elastin are two of the most abundant proteins in the ECM, and play an integral role in maintaining the physical structure of the matrix (Figure 13). Collagen is the main structural protein in the ECM, accounting for approximately one-third of protein content in the entire body.68,198 Collagen is vital in providing the ECM with strength and support, but also assists in cellular adhesion, migration, and development.198 There are many members of the collagen family in various tissues throughout the body, some with shared or similar functions. Type I collagen is the most abundant form of collagen in the body, and exists as a fibrillar protein that is the prominent protein found in dermal and osteogenic tissues.68 Type II collagen is found primarily in the cartilage, and Type III is the preeminent fibrillary collagen in elastic tissues, such as the lungs and vasculature.68 Type IV collagen is mainly found in basement membranes and is responsible for forming supportive networks68. One of the more broadly dispersed forms of fibrillar collagen is Type V, which is found in various tissues and organs, and is also instrumental in structural stability.68 While there are many other forms of collagen in the body with different tissue specifications and functions, Types I-V are the most prominent and plentiful.
Figure 13.
(A) Illustration of the extracellular matrix and its components. Created with Biorender.com. (B) Examples of ECM components.68
Elastin is another fibrous protein found in the ECM, and is instrumental in the elastic recoil of the ECM.68,198 Elastin and collagen are closely associated in the ECM, and their combined functions allow for robust structural support and stability, while also allowing flexibility and appropriate conformations for tissue function.
4.1.2. Proteoglycans and Polysaccharides in the ECM.
Proteoglycans, like proteins, are an abundant component of the ECM, and contribute to the hydration of the matrix, the expansion of its volume, and the resistance of compressive forces.68 Proteoglycans are structurally comprised of a diverse array of core proteins associated with glycosaminoglycans (GAGs) (Figure 13).68,198 GAGs are enormously important and widely researched in ECM biochemistry and structure, as well as in their efficacy to contribute to the microenvironment of bioprinted stem cells. GAGs are unbranched polysaccharides consisting of several repeating disaccharide components.68,198 One of the most notable functional characteristics of GAGs is that they hold a significant negative charge, which attracts positively charged ions, electrolytes, and water molecules.68 Through osmosis, GAGs facilitate the formation of gels that have the potential to expand, contributing to the volume of the ECM.68 This allows for the ability to resist compression, and is useful in areas of the body prone to mechanical stress, compression, and in need of lubrication.
There are numerous types of GAGs that may complex with protein cores in the ECM, however certain specific GAGs are more commonly observed and targeted for mimicry to control stem cell fate in bioprinted constructs. The most commonly occurring GAGs include two main groups of sulfated and non-sulfated structures.198 The sulfated GAGs include heparan sulfate, chondroitin sulfate, keratan sulfate, dermatan sulfate, and the most common non-sulfated GAG is hyaluronic acid.68
Heparan sulfate is closely related to heparin in structure and function, and is also known to have anticoagulative properties, as well as efficacy in signaling and cellular development.199 Chondroitin sulfate is similarly instrumental in molecular signaling during development, and has been demonstrated as contributing to the structural integrity of basement membranes in many body systems, including the central nervous system.200 Keratan sulfate, in addition to contributing to the structure of the ECM, also plays a role in hydration and signaling, especially in cartilage and corneal tissues.201 Dermatan sulfate is a stereoisomer of chondroitin sulfate, and commonly complexes with chondroitin sulfate in developmental functions, especially in the nervous system.202 Hyaluronic acid, the main non-sulfated GAG, is commonly used in hydrogels for biomedical and tissue engineering applications.203 This is primarily due to the ability of hyaluronic acid to form various crosslinking associations, as well as the biocompatibility.203 Due to their properties and contributions to the ECM, GAGs are commonly targeted for replicating the effects of the ECM in microenvironments for bioprinted stem cells.
4.1.3. Glycoproteins in the ECM.
Members of the glycoprotein family are also highly represented in the ECM, and contribute to the assembly of the ECM, its ability to perform cell signaling, and, notably, its ability to adhere cells.68 Common glycoproteins present in the ECM are fibronectin, laminin, fibrillin, tenascin, vitronectin, and osteonectin (Figure 13).68,198 Fibronectin is one of the most well-studied and understood glycoproteins, and is noted for its extensive adhesive profile.68,204 Fibronectin’s structure gives it the ability to complex with itself, numerous other ECM components, and cellular receptors. This is instrumental in anchoring cells to the ECM in order to carry out biological processes.204 Similarly, the laminin family is integral in cellular adhesion, and can activate cell surface receptors to facilitate intracellular signaling, leading to changes in polarization, migration, viability, and gene expression.205
4.1.4. Approaches in Mimicking the ECM for Bioprinted Stem Cells.
There are numerous approaches to decellularize tissue-derived matrices in order to bioengineer them for various applications. One of the purposes of matrix decellularization is to preserve the structural integrity of the derived matrix while removing all cellular residues that can trigger immune responses or interfere with the regeneration of new tissue.206 Matrices can be harvested from many areas of the body, including but not limited to cardiac matrices, hepatic matrices, dermal matrices, and others.68,207 Protocols involve physical, chemical, and biological approaches, which include methods of freezing, use of cellular detergents, and enzymatic digestion, respectively.68,206-208 After the elimination of cellular components, the matrix can be prepared and used in numerous ways to foster stem cell growth in bioprinted materials to generate a new construct. This can be achieved by the conversion of a decellularized matrix to bioinks or hydrogels.206,207 An alternative to using matrices derived from biological sources is the development of ECM mimetic materials. This is a commonly used approach in 3D bioprinting, as bioinks have been developed to replicate the structure and function of ECM while optimizing printability.209
A common approach in ECM biomimetic engineering is the use of adhesive molecules, such as RGDs or especially GAGs, which are the polysaccharide components of proteoglycans, and major components of the ECM.68,210 In addition to contributing to the ECM structure, GAGs are also instrumental in cellular differentiation as part of the cellular microenvironment.210 There have been various efforts to synthesize GAGs such as heparan sulfate for other applications, namely the use of structurally related heparin, which may have some efficacy in bioprinting applications with further research.199 Some commonly utilized GAG mimetics include readily available oligosaccharides, such as chitosan, polysaccharides, or synthetically engineered polymers.210 GAG mimetics, like other ECM mimetics, have advantages in that they are not encumbered by the limitations of biological GAGs, such as variation and harvesting-related challenges.210 This makes these structures promising in the future of 3D bioprinting microenvironments to accommodate stem cell proliferation and differentiation.
4.2. Influence of Elasticity and Topography of Bioprinted Constructs on Stem Cell Differentiation
The surface profile and mechanical properties of a bioprinted construct holds a great deal of significance as to the fate of the cells being deposited. This is known as the topology or topography of the surface, which characterizes the geometry of the grooves and channels created by the bioprinting process.211-217 Grooves, ridges, pillars, and the alignment of cells in a bioprinted construct can be utilized to promote differentiation based on the cell’s spatial relation to other cells.211,218-221 Generally, parallel bioprinted patterns of grooves and channels are ideal for promoting differentiation and organization of cells or tissues that require an aligned relation, such as ligaments, fibroblasts, neural cells, and cardiac cells.10,211,212,219,220,222-230
There are many approaches in manufacturing micro- or nanosurface topographical profiles in order to guide stem cell fate. Some techniques include photolithography, electron beam lithography, soft lithography, electrospinning, and microstereolithography.68 In photolithography and electron beam lithography, light is used to modulate certain topographies to promote desired cellular growth profiles.68 In soft lithography, polymers are printed into a pattern to facilitate cell growth, migration, or communication in a specific way.68 This is especially feasible in the applications of 3D bioprinting. Electrospinning is a biofabrication technique similar to bioprinting, but focuses on the alignment of polymers to form matrices with desired topographical characteristics.68 This can be used to create a matrix in which stem cells and microenvironment factors are delivered onto an existing surface. Microstereolithography allows the fabrication of constructs with precision, facilitating the execution of complex topography.68 The combination of these approaches with bioprinting modalities can allow for an ideal physical environment for stem cell proliferation and differentiation.
The topographical aspects of bioprinting have the potential to impact the differentiation and fate of stem cells.221 The spatial relationship of cells to each other in a fabricated construct can influence the differentiation, self-renewal, potency, or functional profiles of bioprinted stem cells.231,232 To account for this, methodologies have been developed to control the structures of tissue engineered and bioprinted constructs on a nano and microscale.221,233-236 The small-scale organizational approaches are still being developed, but offer promise to the future of topography as a consideration in the differentiation process in bioprinting.
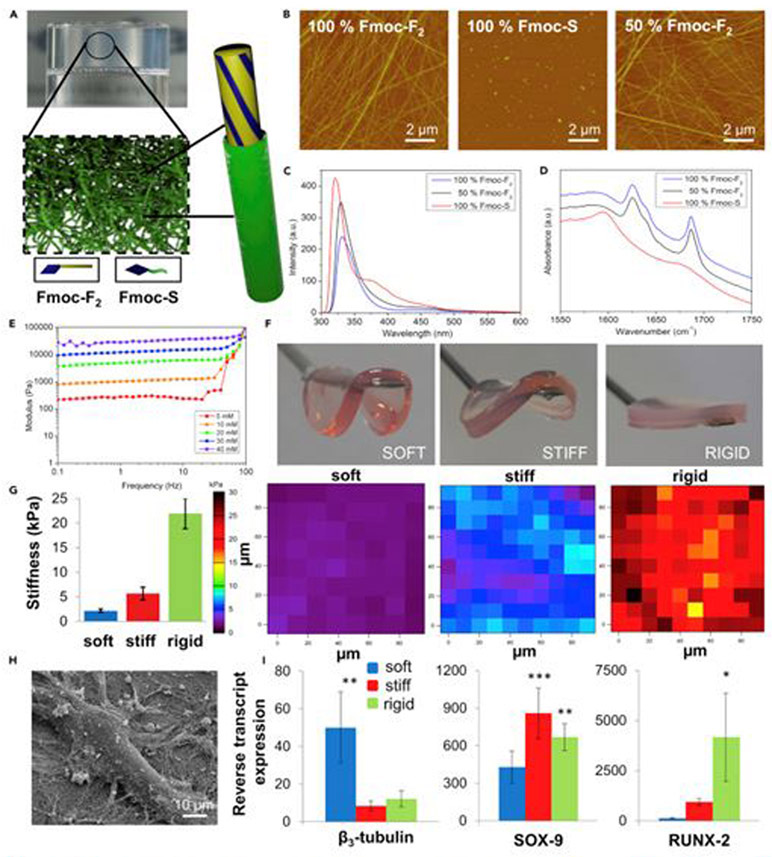
4.3. Stiffness and Elasticity of Bioprinted Materials and Cellular Outcomes
Stem cell differentiation, maturation and morphogenesis are widely recognized to be affected by matrix elasticity. Mechanical forces contribute to cells during early developmental stages by directing patterning and organogenesis.237 Mechanical interactions of cells can also influence intercellular signaling, contributing to the development of tissues or organs.237 The elasticity of the construct matrix contributes to cell migration, motility, and contractility.231
Mechanoregulation of cell fate can be controlled by the stiffness of the printing medium through the adjustment of rheological properties.237,238 Hydrogel stiffness can be adjusted independently of biochemical properties, which allows the analysis of cellular responses to certain mechanical changes. Tissue elasticity varies from organ to organ and increases from ~ 1 kPa for soft tissues such as the brain to ~ 500 kPa for cartilage, and ~ 20 GPa for hard tissues such as cortical bone.239,240 Forces or strains put on the cells throughout the bioprinting process can contribute to the cellular behavior, phenotype, or fate post-printing.241
When cultured in soft, heavy and stiff hydrogels, MSCs show increased expression of neurogenic, myogenic and osteogenic properties. Engler et al. performed a study which used collagen-modified polyacrylamide hydrogels. The preference of naive mesenchymal stem cells (MSC) toward a specific lineage was found to be regulated by the stiffness of the culture matrix.242 It was later found that the stem cell senses the mechanical properties of the environment by adhering and pulling the ECM components of the scaffold.243 Over the past decade, the importance of substrate stiffness for direct stem cell differentiation has been demonstrated for various types of stem cells. Substrate stiffness affects not only stem cell differentiation, but also cell maturation. Cells generally differentiate and mature more effectively when cultured on substrates that are similar to the mechanical properties of native tissue.244
In another study, Marklein et al. fabricated methacrylic hyaluronic acid (MEHA) and allowed it to crosslink through both Michael-type addition and radical polymerization using light (Figure 14).245 A wide range of dynamics (3 kPa to 100 kPa) in both uniform and patterned hydrogels was observed by changing the initial methacrylate consumption via additive crosslinking, limiting UV light to specific areas and modifying UV exposure time. hMSCs showed increased diffusion and proliferation with stiffer gels compared to cells cultured with soft gels. In addition, cells grown on gels with patterned mechanics showed diffusion and proliferation behavior related to local mechanics. This method of spatially controlling matrix dynamics represents a novel hydrogel system for adjusting the microenvironment of stem cells.246
Figure 14.
Methacrylic hyaluronic acid (MEHA) crosslinked via Michael-type addition and the UV light-induced radical polymerization.246
In an effort to examine the properties of hydrogels, Yu and colleagues showed that hydrogels which are most similar in mechanical properties to adults result in the formation of adult-like hepatocytes in human pluripotent stem cell-derived hepatocytes (hpst-Hep).246 Hydrogels with modulus of elasticity of 20, 45 and 140 kPa were made from 20 kPa hydrogels with the stiffness most similar to liver.247,248 The highest value of albumin production, which is a measure closely related to hepatocyte maturation, decreased with increasing scaffold stiffness in cells cultured on the softest hydrogels.247,248 Expression of major enzymes involved in drug metabolism, CYP1A2 and CYP3A4, is also associated with hepatocyte maturation and is higher in adult liver than in fetal.247,248 When expression of these enzymes was examined in hpst-Hep cells, expression levels were found to be highest for cells cultured in the smoothest scaffolds.247,248
In another study, Wang et al. utilized silk hydrogels with tunable mechanical properties that were developed by introducing inert silk fibroin nanofibers (SNF) into a regenerated silk fibroin (RSF) system connected to enzyme silk.249 After RSF cross-linking reaction, inert SNF was buried in RSF hydrogel matrix to improve mechanical properties (Figure 15). In addition, the proliferation of rat bone marrow-derived mesenchymal stem cells cultured on composite hydrogels and differentiated into endothelial cells, myoblasts and osteoblasts was presumably improved to control the stiffness of the hydrogel.249 Bioactive and tunable silk-based hydrogels are manufactured via a composite SNF and cross-linked RSF system, creating a new strategy for designing silk biomaterials with tunable mechanical and biological performance.249
Figure 15.
Silk hydrogel developed by introducing inert silk fibroin nanofibers (SNF) into a silk fibroin (RSF) system connected to enzyme silk.249
In another study, Hanjaya-Putra et al. demonstrated the effect of substrate stiffness on the tubule production of endothelial precursor cells (EPCs) cultured in poly(ethylene glycol)-diacrylate (PEGDA) crosslinked hyaluronic acid (HA)-gelatin hydrogel.250 The number, length and thickness of the formed tubes increased with decreasing scaffold stiffness. EPCs cultured on soft substrates readily assembled into chains and formed the longest tubes with the largest open lumen space. However, tube formation in all scaffolds was possible only at high vascular endothelial growth factor (VEGF) concentrations. VEGF activated the production of matrix metalloproteinases (MMPs), which enabled cell-mediated scaffold remodeling for cell migration.250
Alakpa et al. identified specific bioactive lipids, and they identified different hydrogel stem cell differentiation phenomena with variable stiffness and metabolomics.251 Stem cells around blood vessels were cultured on supramolecular peptide gels of different stiffness, and were depleted of metabolites. Neuronal, chondrogenic, and osteogenic differentiation is observed in soft (1 kPa), moderately rigid (13 kPa) and rigid (32 kPa) gels, respectively, indicating that these stem cells undergo rigid-oriented fate selection. The analysis of changes in metabolite concentrations during differentiation suggested that these metabolites are involved in the differentiation process. In order to clarify that lipid metabolites play an important role in the induction of differentiation, these individual lipids, when fed into standard stem cell cultures, contribute to chondrocyte and osteoblast phenotypes. It has been demonstrated that differentiation can be induced.252 Multiple aspects of the study are outlined and described in Figure 16.
Figure 16.
Neuronal, chondrogenic, and osteogenic differentiation based on rigidity of hydrogel stiffness. (A) Schematic of proposed core-shell nanostructures. (B) AFM images of Fmoc-F2, Fmoc-S, and the 1:1 mixture of the two. (C) Fluorescence emission spectra corresponding to monomeric emission of the fluorenyl moiety (sharp peak at 320 nm), formation of aggregated excimers (broad peak at 460-480 nm), and differential fluorenyl organization upon formation of spherical aggregates (peak at 380 nm). (D) FITR spectra demonstrating that Fmoc-F2 possesses peptide amide I modes consistent with well-ordered β-sheet-like arrangement (band at 1,625 cm−1) and ordered carbamate moiety (band at 1,687 cm−1). For Fmoc-S, carboxylate peak evident at 1,590 cm−1 loses intensity and broadens in the mixture of the two components, indicating co-assembly. (E) Oscillatory rheology of the gels demonstrates elastic moduli of different concentration in gels. Stiffness increases with concentration. (F) Macroscopic images for 10, 30, and 40 mM gels. (G) Young’s modulus and AFM stiffness maps of soft, stiff, and rigid gels. (H) SEM image of single MSC attached to Fmoc-F2/S gel fibers. (I) Assessment of pericytes for the expression of β3-tubulin (neural cells), SOX-9 (chondrocytes), and RUNX2 (osteoblasts) on each hydrogel type. Primary expression of β3-tubulin was observed on the 1 kPa (soft) surface, SOX-9 was observed on the 13 kPa (stiff) surface, and RUNX2 was observed on the 32 kPa (rigid) surface after 1 week of culture. Cells were only cultured in basal media with no differentiation-enhancing factors, and so differentiation is purely substrate driven. Statistics were obtained by ANOVA and Bonferroni post hoc tests; the control was pericytes on glass coverslips with standard media, *p < 0.05, **p < 0.01, and ***p < 0.001. Reproduced with permission from Alakpa et al.252 Copyright 2016, Elsevier.
In a recent experiment, Khetan et al. demonstrated that stem cell responses to the mechanical properties of scaffolds depend on the culture dimension. In general, 3D printed scaffolds are cells encapsulated in a 3D hydrogel.253 Cell proliferation was increased when MSCs were cultured in HA-based hydrogels, but the opposite trend was observed for cells encapsulated within hydrogels. Cells cultured in stiff, highly cross-linked hydrogels did not diffuse and showed a predominantly rounded shape. However, in other studies, Burdick and colleagues showed that by introducing a protein-cleavable crosslink, cells that diffuse within covalently bound hydrogels can be induced.253 In addition, using MSCs, studies showed no correlation with cell morphology during 3D encapsulation, as in previous studies on 2D surfaces.252
These studies speak to the larger potential of mechanical properties and external cues to play an instrumental role in the determination of cell fate and behavior in bioprinted constructs. The mechanical properties and influence of the cellular microenvironment post-printing is one of the most vital elements in controlling stem cell differentiation.
5. Applications in Complex Tissues and Organs
Controlling stem cell fate via the microenvironment and its components is essential for the success of bioprinted constructs fabricated from naïve cells with variable potency. However, mechanisms of cellular differentiation may differ in efficacy between different target tissue and organ types. There are innumerable approaches to fabricating tissue engineered constructs for every conceivable organ, and in the setting of bioprinted stem cells, some approaches have been notably successful in different arenas.
5.1. Adipose Tissue
Surgical procedures that involve the repair of adipose tissue are often in need of a way to compensate for volume loss incurred through trauma, resection of malignant tissue, or congenital abnormalities.254 Constructs for use in soft tissues have demonstrated advantages in stem cell localization, attachment, proliferation, and differentiation in adipose tissues.255 Tissue engineering with stem cell sources has been long studied in meeting this clinical need, and the approach of bioprinted constructs can facilitate in the expedition of these efforts.254
Biochemical cues can be utilized to direct adipogenesis of stem cells in bioprinting. Ninomiya and colleagues demonstrated the differentiation of human MSCs to an adipogenic phenotype through the use of differentiation media containing dexamethasone, insulin, methylisobutylxantine, and the PPAR-γ agonist rosiglitazone.10,256 They shortened the differentiation cultivation period from 2-3 weeks to 7-8 days.256 Other groups have also utilized the PPAR-γ agonist rosiglitazone to facilitate the differentiation of MSCs to an adipogenic phenotype.10,257 Bioprinted adipose tissue has been constructed through the use of decellularized ECM combined with ADSCs and a PCL framework.258,259 This approach demonstrated improved adipogenesis and cell-material interactions.258,259
5.2. Bone and Cartilage Tissues
Clinical applications in which bone constructs are needed include traumatic injuries, surgical procedures where bone is excised or grafted, and repair in the settings of fractures or severe breaks.260 Bone repair is particularly timely and onerous, especially in vulnerable populations like the elderly.260 In these settings, a bone construct could be used in expediting the bone regeneration and healing process.261 Cartilage is also damaged as a result of trauma or degenerative joint diseases due to aging or through genetic predisposition.262 Similarly, cartilage has limited regenerative abilities, and efforts to accelerate cartilage repair are desperately needed in order to facilitate healing and restore joint function.263 The three-dimensional printing of stem cells for bone and cartilage constructs offer advancements toward engineering constructs analogous to the complex multicellular structures of bone and cartilage. Varying approaches have been used toward this goal.
Many groups have employed members of the TGF-β superfamily and BMPs to differentiate stem cells into osteogenic or chondrogenic phenotypes.10,264,265 Diekman and colleagues demonstrated the differentiation of adipose-derived stem cells (ASCs) to chondrogenic cells through the use of BMP-6 and many other molecular signals.10,266 In their study, the ASCs were encapsulated in alginate beads and differentiated in the presence of dexamethasone, EGF, FGF-2, TGF-β1, and various expansion factors.266 In another study, Awad and colleagues observed similar results in differentiation of ASCs to a chondrogenic phenotype with TGF-β1 while examining differences between alginate and agarose hydrogels and gelatin scaffolds.267 In both cases, the use of TGF-β1 supplemented culture media led to increased presence of cartilage matrix components post-differentiation.10,266,267
In another study, Gruene and colleagues utilized laser-induced forward transfer (LIFT) to print MSCs directly into differentiation media, directing differentiation into osteogenic or chondrogenic cell types.10,268 To differentiate to the osteogenic lineage over a period of three weeks, MSCs were cultured in media supplemented with icroglycerophosphate, insulin, transferrin, selenious acid, and ascorbate-2 phosphate.268 Alternatively, to differentiate MSCs to a chondrogenic lineage, the culture media contained TGF-β3, sodium pyruvate, dexamethasone, insulin, transferrin, selenious acid, and ascorbate-2 phosphate.268 Additionally, this method can be employed to differentiate cells several days prior to the bioprinting process.10
Through another approach, Du and colleagues demonstrated that the use of BMP-2 as a bioink additive led to higher cell viability in bone mesenchymal stem cell (BMSC)-laden methacrylamide gelatin scaffolds during bioprinting.10,269 BMP-2 was bound to collagen microfibers with collagen binding domain (CBD), and BMSC-laden scaffolds were produced by a customized 3D bioprinter.269 Furthermore, the BMSCs were differentiated into osteocytes within 14 days in a more efficient manner compared to BMSCs differentiated with osteogenic medium.269
Small RNAs have also been utilized to induce osteogenic and chondrogenic differentiation in the laboratory setting. Micro RNA sequences such as miR-23a, miR-30c, MiR-34c, miR-133a, miR-135a, miR-137, miR-204, miR-205, miR-217, miR-218, and miR-338 have been shown to cause osteogenic differentiation by targeting transcription factor Runx2, while miR-138 achieved the same outcome through inhibition of focal adhesion kinase and its signaling cascade.68,270,271 In chondrogenic differentiation, miR-140 has been continually proven to have a role in targeting IGF binding protein 5, histone deaceylase 4, and Smad3.68,272-274 Other small mRNAs like miR-199a, miR-18a, miR-221, miR-675, miR-29 and miR-29b have been identified as affecting transcription factors, structural proteins, and signaling pathways to aid in chondrogenic differentiation of stem cells.68,275-280
Modifications of the bioink are widely used in bone and cartilage applications. Wang and colleagues differentiated rabbit MSCs to an osteogenic phenotype through the process of bioprinting onto nano-hydroxyapatite/polyamide (n-HA/PA) scaffolds.10,165 Biphasic calcium phosphate particles (BCPs), consisting of HA and β-tricalcium phosphate, were also utilized and showed more evidence of differentiation than controls in an in vivo mouse model.10,165 Similarly, Gao and colleagues were able to achieve osteogenic differentiation of MSCs through the use of poly(ethylene glycol) dimethacrylate (PEGDMA) bioinks supplemented with either HA or bioactive glass.10,281 Again, there was more evidence of differentiation, as well as an increased level of secretion of collagen toward the process of ECM remodeling.10,281
Through the utilization of microcarriers (MCs), Tseng and colleagues demonstrated the ability to differentiate MSCs to osteogenic cell types through the use bioprinting onto collagen supplemented with polysterene MCs.10,192 Similarly, Levato et al. had similar success in differentiating MSCs to an osteogenic phenotype with the coating of poly(lactic acid) (PLA) MCs with collagen.10,188 In a related effort, the coating of MCs with pharmacologically active agents has also shown great potential in cell differentiation of bioprinted constructs. Bouffi et al. and colleagues have successfully differentiated MSCs to chondrogenic phenotypes with the use of poly(lactic-co-glycolic acid) (PLGA) MCs coated with fibronectin, which allowed the release of TGF-β1.193 Similarly, Poldervaart and colleagues utilized MCs engineered to release BMP-2 to differentiate goat MSCs to an osteogenic phenotype.10,137 The use of these MCs in an alginate bioink successfully showed osteogenic differentiation in vitro and in vivo.10,137 In another study, Zhu and colleagues demonstrated the ability to differentiate MSCs to a chondrogenic phenotype through the use of TGF-β1 embedded nanospheres and the supplementation of GelMA with PEGDA.282 Their results showed that cells 3D printed under these conditions exhibited higher levels of viability and proliferation as compared to controls, and demonstrated sustained release of TGF-β1 for up to 3 weeks after printing, facilitating chondrogenic differentiation.282
Additionally, Gao and colleagues demonstrated the crucial role of NR2F2 in chondrogenic differentiation.283 The use of NR2F2 in bioprinted MSCs showed significantly enhanced differentiation to chondrocytes via biochemical and histological analysis.283 Furthermore, Nguyen and colleagues showed that the use of nanofibrillated cellulose with alginate or with HA contributed to the chondrogenic differentiation of 3D printed human iPSCs.284
5.3. Cardiovascular and Cardiomyogenic Tissue
Cardiovascular disease is the leading cause of death globally, and damage to cardiac tissue through infarctions and other mechanisms is a major contributor to this figure.285 Adult cardiac cells have a limited ability to self-renew and proliferate, so healing and regeneration of functional tissue are virtually non-existent in the clinical setting.285 To combat these outcomes, many researchers have pursued developing techniques to repair and regenerative cardiac tissue with the use of stem cells. Like many other organs, the heart is a complex, multicellular network that has the capability to contract and transmit signals initiated by electrical stimuli. Bioprinting offers a reasonable way to replicate the complex cellular network seen in cardiac tissue, and many advances have been made in using bioprinted stem cell constructs in this setting.
Many groups have utilized molecular signals to differentiate stem cells into cardiomyocytes or cardiomyogenic tissue in the bioprinting process. Xu and colleagues utilized molecular signals in order to differentiate human bone marrow MSCs to a cardiomyogenic phenotype through the use of DNA demethylating agent 5-azacytidine in the culture media10,286 In a related approach, Gaetani and colleagues demonstrated the efficacy of printing cardiac-derived cardiomyocyte progenitor cells in a scaffold comprised of alginate hydrogel.259,287 Printing the stem cells along with scaffold material facilitated the conservation of the cardiac lineage and promoted cardiac gene expression.259,287 A later study by the same group exemplified the use of a gelatin/HA scaffold in a similar experimental design.259,288 The outcomes showed that the cells retained the ability to differentiate into cardiomyogenic phenotypes with similarly increased cardiomyogenic gene expression. These models were used in an in vivo mouse MI model.259,288 Similarly, Young and colleagues showed successful differentiation of pre-cardiac cells expanded on thiolated HA-hydrogels coated with collagen.259,289 This resulted in a considerable increase in cardiac-specific marker expression.259,289
Small RNA sequences have been investigated for efficacy in the cardiovascular setting. The well-researched miR-15 family of sequences has demonstrated a role in facilitating resistance of cardiac tissue to ischemia, has aided in the reduction of infarct size, and has enhanced cardiac tissue function following an ischemic injury.68,290,291 This could be applied in bioprinted stem cell constructs intended to replace damaged tissue, as it could prevent tissue damage in the same location in the case of a chronic or recurrent issue.
The use of both synthetic and natural ECM has been explored in the application of cardiac tissue bioprinting. Prestwich and colleagues demonstrated the use of a HA-based synthetic ECM, which utilized HA from natural derivatives.259,292 This would ideally allow the expansion and differentiation of stem cells to mature cardiogenic lineages.259,292 Conversely, Jang and colleagues demonstrated the advantageous approach of printing cardiac progenitor cells onto decellularized ECM, which was UVA crosslinked by vitamin B2-induced and subsequently underwent a thermal gelation process.259,293 The resulting bioprinted construct showed increased cell viability, proliferation, and cardiomyogenic differentiation.259,293
Topographical patterns and surface topology have been used in cardiovascular tissue engineering applications. Bauwens et al. demonstrated the differentiation of embryonic stem cells (ESCs) to various tissue fates via the use of embryoid body (EB)-induced differentiation.259,294 This guides EB formation and size, allowing for control over differentiation into cardiogenic phenotypes.259,294 In another approach, Tijore et al. agues developed a microchanneled gelatin hydrogel that facilitated the differentiation of human MSCs to a cardiomyogenic phenotype in the setting of 3D bioprinting.211 The alignment of the cells during the bioprinting process, coupled with the engineered topography of the hydrogel scaffold, allowed a cardiomyogenic commitment of the stem cells.211 Similarly Miao and colleagues were able to differentiate human MSCs to a cardiomyogenic phenotype through the utilization of surface pattern technology in 4D bioprinting.221
5.4. Hepatic Tissue
The liver is the largest solid organ in the body, and plays a crucial role in filtering toxins and regulating other physiological functions.295 While liver disease and damage can be debilitating, hepatocytes have a uniquely regenerative capacity.295 Thus, tissue engineered hepatic tissue is a lucrative alternative to liver transplantation and cellular therapy in the clinical setting. The complex structure of hepatic functional units makes bioprinting a highly useful technique in preparing constructs analogous to the native tissue. Furthermore, stem cells can accelerate the healing and regenerative process that is inherent to hepatic tissue.
Biochemical signals have been utilized to achieve hepatogenic cells and tissues through bioprinting. Faulkner-Jones and colleagues demonstrated the differentiation of human pluripotent stem cells (PSCs) to hepatocyte-like cells through the use of differentiation media supplemented with activin A, Wnt, and knockout-Dulbecco’s modified Eagle’s medium (KO-DMEM) containing 20% serum replacement media, 1% non-essential amino acids, and 1% dimethyl sulfoxide (DMSO).296 The cells were 3D bioprinted using an alginate hydrogel matrix.296 Similarly, Tuleuova and colleagues successfully employed growth factors and patterned printing to differentiate ESCs to hepatic cell lineages.259,297 Through a combination of hepatic growth factor, basic fibroblast growth factor, and BMP4 mixed in solution with fibronectin and collagen, the mouse ESCs were printed into patterned arrays on silane-modified glass.297 The resulting constructs demonstrated a loss of pluripotency and expressed commitment to the hepatic lineage.297
One of the most notable features of hepatic tissue is the characteristic hexagonal subunits that comprise the organ. Ma and colleagues were able to replicate this native geometry, and subsequently demonstrated the ability of human iPSCs bioprinted with ADSCs and HUVECs in gelatin methacrylate and glycidal methacrylate-HA to replicate hepatic structure and function.259,298
5.5. Skeletal Muscle Tissue
Musculoskeletal tissue can be damaged through injury and degenerative disease, and the native tissue has a limited ability for healing and regrowth.299 Stem cells offer potential therapies through tissue engineering and regeneration, and many groups have examined the efficacy of microenvironment manipulation on bioprinted stem cells in skeletal muscle tissues. Many research groups have demonstrated the ability to differentiate primary muscle-derived stem cells (MDSCs) into myogenic lineages through the use of molecular signals in bioprinting.259,300,301 As described earlier, Phillippi and colleagues showed that the use of BMP-2 bioprinted in a pattern on glass slides coated with fibrin facilitates myogenic differentiation.153,259
The formation of a matrix is also important in the construction of skeletal muscle tissue. Ker and colleagues replicated an ECM-like patterned structure bound with growth factors to facilitate differentiation of C2C12 cells into myogenic tissues.259,302 The growth factors utilized included FGF-2 and BMP-2.259,302
Small RNAs have been extensively researched in the setting of skeletal muscle applications. Sequences such as miR-1, miR-27, miR-133, miR-188, and miR-206 are notable mediators associated with the regulation of myogenic expression.68,303-306 These miRNAs are found in myogenic tissues, such as skeletal, cardiac, and smooth muscles.68 They have been shown to regulate transcription factors that contribute to myogenic differentiation, including MyoD and myocyte enhancer factor 2.68,303,307 The inclusion of these agents in the microenvironment can aid in the direction of differentiation toward a muscular phenotype.
The topography and patterns play an important role in the bioprinting of skeletal muscle tissue. Li and colleagues were able to differentiate human MSCs to a myogenic phenotype through the use of topographical alignment of microchannels.10,229 The scaffolds were printed via laser machining of the cells on a poly(L-lactide-co-ε-caprolactone) (PLCL) scaffold.10,229 In another study, Gao and colleague have shown that C2C12 myoblasts can be patterned into precise designs to allow for guiding differentiation into myotubes.259,281 Cui and colleagues demonstrated the rapid functional maturation of C2C12 cells printed in alignment, compared with cells printed in a random pattern.259,308 In comparison to controls, the aligned printed cells demonstrated myogenic phenotypes 10 days earlier.259,308 Furthermore, Yu and colleagues capitalized off of the mimicry of the texture of native muscle to induce focal adhesions, and thus differentiation of bioprinted patterns of MDSCs to myogenic phenotypes demonstrating myotubule formation.259,309
5.6. Neural Tissue
Similar to many of the aforementioned tissues, neural tissue has limited regenerative ability, and can be damaged through infarct, degeneration, or injury.310 Furthermore, innervation of engineered tissues is also a goal of regenerative medicine, and the ability to produce neural networks in fabricated constructs is integral to success and integration of the construct.259,311 Bioprinted neural models with stem cells have been utilized for in vitro modeling, but are also needed for clinical and therapeutic applications.310 Bioprinting stem cells with a supportive microenvironment could address the need for neural regeneration and tissue engineering.
The differentiation of neural stem cells (NSCs) can be achieved through the use of molecular signals, such as macromolecules or transcription factors.259,312 Hsieh and colleagues demonstrated the capability of thermoresponsive polyurethane hydrogel as a bioink for neural stem cells printing and differentiation.313 NSCs printed in a dispersion of water-based biodegradable polyurethane exhibited repair potential in a neural injury in a zebrafish model.313 Similarly, Gu and colleagues showed that a polysaccharide-based bioink was effective in direct-write printing and differentiation of human NSCs.314 The bioink contained alginate, carboxymethyl-chitosan and agarose, and their results showed that differentiated neurons formed synaptic networks, were spontaneously active, and had physiological behavior characteristic of neural cells.314 In a related effort, Lee and colleagues utilized molecular signals to influence the migration and behavior of stem cells in an engineered hydrogel.136 Murine neural stem cells were bioprinted in close proximity with collagen hydrogel loaded with VEGF.10,136 In addition to increased cell viability, the bioprinted construct demonstrated a higher degree of differentiation than the control construct, migration of the cells toward fibrin gel, as well as sustained release of VEGF from the construct.10,136 Additionally, Tay and colleagues demonstrated the differentiation of human MSCs into neurogenic cells through the use of fibronectin-coated PLGA.217,259 Gene expression was consistent with differentiation into the desired target tissue type.217,259 Furthermore, Xu and colleagues successfully employed FGF-2 and ciliary neurotrophic factor (CNTF) as part of a hydrogel to bioprint NSCs into a construct in which the cells differentiated into astrocytes.5,259,315,316 The differentiated cells demonstrated appropriate gene expression and phenotypical presentation.5,259,315,316
Small RNAs have been instrumental in facilitating neural tissue repair and regrowth in many studies. Sequences such as miR-7, miR-133b, and miR-206 have been used to enhance self-renewal of neural stem cells, promote regeneration of damaged neural cells, and inhibit the progression of neural degeneration, respectively.68,317-319
Characteristics of the matrix can be instrumental in stem cell differentiation to a desired phenotype. For example, MSCs show preferential differentiation to neurogenic lineages when bioprinted onto soft matrices, as compared to stiffer matrices.312,320
The patterns in which neurological precursor cells are arranged are of great importance to the fate of stem cells. Jiang and colleagues successfully differentiated human MSCs to neural phenotypes through the combined efforts of topographical considerations and drug release.10,230 Retinoic acid was loaded onto PCL nanofibers and sustained release of the drug, along with attention to topographical nanofiber conformation, facilitated the differentiation of the cells to a neurogenic lineage.10,230 Additionally, Mattotti and colleagues demonstrated the differentiation of astrocytes to radial glia-like cells through the bioprinting of the astrocytes on a micropatterned polymethylmethacrylate (PMMA) grooved scaffold.259,321 The organization and alignment of the cells onto the scaffold allowed the differentiation of the cells without added factors.259,321
5.7. Dermal Tissue
Skin has an innate ability to regenerate and heal, however injuries to large surface areas, such as burns and extensive trauma, complicate the healing process.322 Bioprinted stem cells are ideal for skin tissue engineering, and the necessity of a strong microenvironment to direct the growth, proliferation, and development of functional structures can revolutionize the ways in which skin wounds are regenerated.
Skardal and colleagues demonstrated the use of molecular signals in skin bioprinting by utilizing AFSCs and MSCs printed in a fibrin-collagen gel to promote skin regeneration for wound healing applications36,259 The formation of a matrix on which cells are deposited is especially vital in skin constructs. Rutz and colleagues successfully printed MSCs onto a gelatin and fibrin scaffold matrix, which allowed the tissue to maintain a matrix conformation after the degradation of the original substrate.259,323 In an attempt to create an ECM, Huang et al. created a 3D ECM with hydrogels comprised of gelatin and sodium alginate to support the regeneration of sweat glands.259,324 This construct demonstrated the differentiation of mouse epidermal progenitors into sweat glands that displayed the appropriate markers and functionality.259,324
Koch et al. examined the use of topographical approaches in skin bioprinting with the use of LIFT bioprinting to create a skin construct made up of layers of MSCs, keratinocytes, and fibroblasts.5,259,325,326 The cells were printed in an alginate hydrogel with blood plasma and exemplified the ability of computer-controlled patterning techniques that could direct cellular interaction and tissue formation.5,259,325,326
6. LIMITATIONS OF 3D BIOPRINTING IN STEM CELL APPLICATIONS AND FUTURE DIRECTIONS
6.1. Effect of Printing Methodology on Cell Viability and Construct Quality
As discussed previously, the selection of printing methodology comes with many advantages and disadvantages in the realms of printing speed, fabrication time, cell viability, cost, and other categories. Some of these limitations are specific to certain bioprinting modalities. Inkjet bioprinting presents with limited cell viability due to some of the high temperatures and pressures cells are subjected to as part of the process.327 Similarly, extrusion-based printing may have issues with cell viability as a result of shear stress, as well as being hindered by limited printing resolution. Laser-assisted bioprinting also has issues with cell viability and is prohibitively expensive.327 In addition to the aforementioned limitations, stereolithography-based methods accommodate few biomaterials that are able to be polymerized, and constructs exhibit limited desirability in mechanical properties.327
In general, bioprinting methods require more efficient printing speeds and fabrication times, as well as optimal resolution and scalability in the printed constructs. Another major limitation in the field is the development of 3D bioprinters that can effectively produce translatable biological products. This may be achieved in the future by combining aspects of the various bioprinting methods.3
6.2. Cell Source, Proliferation, and Differentiation
One of the continued challenges in tissue engineering is obtaining a reliable and expandable cell source that is virtually unlimited in potency. Stem cells have overcome many of the obstacles related to cell sources in bioprinting, however more research is needed to fully understand and optimize the signals that direct stem cell growth, migration, and differentiation.311 Strides have been made in the harvesting and derivation of stem cells, as well as in the use of biochemical cues that facilitate targeted proliferation and differentiation.3 While research is rapidly advancing in this endeavor, continued investigation is needed on the varying potencies of cells within the same populations, and the optimal methods to utilize heterogeneous stem cell sources.3 Moving forward, the ability to direct self-renewal and differentiation of stem cells in an in vivo setting is a necessity if constructs are intended for use in physiological settings.328 The ability to replicate cell microenvironments reliably in vitro prior to translation would be a major advancement in the use of stem cells in bioprinting 3D constructs.328 Lastly, the controversies surrounding the use of stem cells in laboratory research could present a hindrance to the progress of research toward clinical studies.329 The ethical debate surrounding the harvesting of stem cells remains divisive, though cell populations such as iPSCs provide potential alternatives.
6.3. Integration of Biochemical Cues and Biomaterials into the Fabrication Process
Despite promising research in various biochemical cues to direct stem cell self-renewal, proliferation, migration, and targeted differentiation, there are still areas for improvement.328 The physiological microenvironment, unlike in vitro experiments, utilizes a combination of many small molecules, growth factors, peptides, cytokines, and other biochemical agents.328 While specific research on one or two of these components can offer valuable insight to specific functions in the microenvironment, mimicry of physiological conditions will need to involve the eventual combination of many of these molecules and their interactions.328
Another approach to moving forward in the biochemical replication of human physiology could involve the combination of naturally derived, complex components with synthetic factors or polymers.3 For example, a naturally decellularized ECM could be combined with growth factors, synthetic peptides, and other biomolecules to achieve biocompatibility while optimizing function.3 Additionally, future research should consider the ability to deliver biochemical molecules via bioprinting techniques in a way that maintains their ability to direct differentiation of the cell source.330 This could present an intensive process for ensuring that these components are compatible with bioinks and other biomaterials to be utilized in the fabrication of the construct.330
6.4. Vascularization, Innervation, and Maturation of Complex Tissues
Among of the major challenges across all approaches in regenerative medicine are the tasks of providing tissue-engineered constructs with innervation, vasculature, and the capability of translation into a clinical model. The challenge of vascularizing a construct involves ensuring the entirety of the construct is perfused and receiving adequate nutrients and oxygen for long-term survival.3,311 Additionally, a well-developed vascular network is necessary for larger tissues, including vessels of varying sizes, like capillaries and microvessels. It is imperative that developed vessels are able to withstand physiological pressure and mechanical stress.3 In addition to vascularization, the ability to innervate bioprinted tissue constructs is integral to the function of the tissues in both in vitro and clinical settings.3
Some methods that have been utilized to address the issue of vascularization, innervation, and maturation include the fabrication of scaffolds that secrete angiogenic factors over time, and direct incorporation of endothelial cells or vasculature in the scaffolds.311 Additionally, increasing the pore size in constructs allows the penetration and flow of nutrients and facilitates vascular growth327. However, the incorporation of angiogenic factors and use of pores has been mainly demonstrated as small-scale in vitro studies, and may not be rapid enough to support the growth of complex, large tissue constructs.327 Functional arteries have been fabricated by seeding ECs and SMCs onto a vascular scaffold, and maturing the vessel in a bioreactor.311 Like vessels, neural networks could be introduced through the use of bioreactors to mature incorporated cells, or after fabrication during growth via pharmacological or biochemical agents.3
The use of bioreactors to foster the growth of vasculature and nerve networks is a promising approach, as bioreactors can mimic physiological microenvironments with controlled temperature, pH, and delivery of nutrients through circulating medium.3 Furthermore, bioreactors offer sufficient time to precondition and mature a construct and foster the development of a microenvironment prior to clinical translation.3,327
Another possibility is the incorporation of 3D printers into surgical technology in order to deliver bioprinted stem cell-laden constructs directly into the patient to facilitate proliferation and differentiation into target tissue.3 This approach has been used in skin tissue engineering, and offers advantages in the efficient and clinically relevant delivery of tissues to a physiological environment.3
6.5. Specific Limitations Based on Target Tissue Type
Bioprinted adipose constructs have shown difficulty in maintaining viability in the full thickness of the construct, as a result of poor vascularization throughout. The aforementioned efforts toward developing vasculature may be relevant in this application.259
In developing bioprinted bone with stem cells, one of the challenges is replicating the structure of native bone, with a calcific outer layer and a rich capillary network in the center.331 3D bioprinting can be instrumental in the organization of this construct during fabrication, but a great deal of structural integrity is needed for bone constructs to be physiologically appropriate.331
Cartilage constructs are a field of extensive research, however the ability to regenerate hyaline cartilage is still being developed.332 One of the main limitations is the ability to translate laboratory research to meaningful options for clinical problems.327 The use of 3D printing incorporated into surgical tools is a possible solution in this setting.332
The heart is an incredibly complex organ with various functional regions and tissue types. In addition to complexity in muscle fiber orientation, the layers are extremely thick requiring adequate supply of oxygen and nutrients.259 The combination of these features makes replicating the structure with bioprinting, as well vascularizing the construct, incredibly difficult.259 The use of stem cells is being actively studied for cardiac applications, as mature myocardium displays limited self-renewal abilities.259 Future studies will need to address these issues, as well as develop constructs that are capable of synchronized contraction in response to electrical stimulation to ensure functionality in clinical settings.259
The liver is a metabolically active organ, and developing a microenvironment for fabricated constructs that mimics this physiological setting is challenging.259 Like the heart, the liver structure involves many cell types of varying functions in a complex structure that is not easily replicated.259 Like all tissues, vascularization is a major limitation in hepatic engineering, and is necessary to examine the function of bioprinted hepatic constructs.259 Furthermore, biomaterials used in this application need to withstand the unique microenvironment of the liver, including the aforementioned metabolic activity, as well as high albumin levels.259
Skeletal muscle constructs need to be designed in a way that allows contraction in response to electrical stimulation.259 Vascularization and innervation are important for constructs that mimic physiological thickness.259 Additionally, in vitro studies must be able to replicate and allow for synaptic communication at neuromuscular junctions to provide function of musculoskeletal tissue.259
A specific requirement of engineering neural tissues is the optimization of bioinks and biomaterials that can support the growth and differentiation of neural cell precursors, as well as the development of complex organizations of functional neural networks.259
Promising advances have been made in the area of bioprinted skin, however future research should focus on the complexity of dermal and epidermal structures, such as sweat glands, hair follicles, and other functional constituents.259 The use of multipotent precursors, as well as the printing of multiple cell types together, could be instrumental in moving forward toward optimal structures.259
7. CONCLUSIONS AND OUTLOOK
The development of an optimal microenvironment for bioprinted stem cells requires the concurrent consideration of multiple aspects described above. In optimizing and combining certain approaches, progress can be made toward developing viable, functional, complex bioprinted tissue constructs for multiple applications.
Bioprinting methodologies developed specifically for biological materials have undergone extensive advances in recent years. Moving forward, the use of multicellular bioprinting approaches, in combination with highly specific low volume methods, may be advantageous in fabricating constructs consisting of multiple cell types and bioinks to increase functionality and complexity. These methodologies would benefit from addressing the aspects of printing speed, fabrication time, resolution, and throughput of materials. Additionally, optimizing cell viability while subjecting the cells to minimal mechanical stress, shear stress, pressure, and temperature can aid in producing high quality functional constructs.
The use of stem cells remains to be a promising option in the field of cellular bioprinting, despite ethical concerns with certain stem cell types, such as embryonic stem cells. To overcome this issue, the use of iPSCs has been increasingly popular, and offers a bevy of benefits in applicability, availability, and ease of collection, isolation, and expansion. While more research is needed on the control and guiding of self-renewal, proliferation, migration, and differentiation of stem cells, this field remains one of the most promising in modern scientific discovery.
The use of biochemical cues in directing the proliferation and differentiation of stem cells is especially exciting in the applications of bioprinting. Ideally, as research further defines the roles of small molecules, growth factors, peptides, and ECM components in the role of the microenvironment as individual elements, efforts will transition to exploring the interaction of multiple factors and their interactions. Additional research into replicating biological function through synthetic components, such as through synthetic peptide sequences, can also assist in the large-scale production and replication of clinically relevant experiments. The developing research into exosomes and other fairly recent approaches toward biochemical agent delivery in bioprinted constructs also offers numerous potential applications for future studies. Collaboration between researchers specializing in individual biochemical factors would be ideal in creating novel approaches to mimicking the physiological microenvironment. Furthermore, the limitless possible combinations between the biochemical cues may present research that differs from, but is functionally similar to, the native microenvironment. This could lead to the optimization of in vitro efforts for pharmaceutical testing or disease modeling.
Similarly, more research surrounding bioink additives that are capable of delivering components that direct stem cell fate would be beneficial to the development of methodologies. The delivery of cell-laden bioink containing biochemical factors to a scaffold with optimal topography may also further influence targeted cell maturation in creating tissue constructs. These components could contribute to ideal mechanical properties in bioprinted tissues and organs.
The expedition of translating constructs to clinical models is an ambitious, but ever-approaching goal. Through the use of bioreactors or 3D printing incorporated into surgical robotics, the delivery of stem cell constructs with the ability to develop extensive vascular and nerve networks is plausible. These modalities may also enhance the maturation of constructs to a level that is physiologically functional and promotes in vivo viability.
A concerted effort between all of the aforementioned aspects can assist in creating constructs for both in vitro clinical modeling, and for in vivo transplantation. The perpetual discovery of new approaches and advancement of techniques makes 3D printing for tissue engineering a field with limitless potential. As technology develops to make this research a reality, the efforts toward composing an archetypal microenvironment that embodies the harmonious function of physiological components will be instrumental in new discoveries.
Table 4.
Summary of Applications in Complex Tissues and Organs
| Tissue | Efforts Toward Optimizing Stem Cell Microenvironment in Bioprinted Constructs | References |
|---|---|---|
| Adipose |
|
10, 256 10, 257 258, 259 |
| Bone |
|
10, 264, 265 268 269 68, 270 68, 271 10, 165 10, 281 10, 189, 192 10, 137 |
| Cartilage |
|
10, 266 10, 267 268 68, 272-274 68, 275-280 193 282 283 284 |
| Cardiovascular & Cardiomyogenic |
|
10, 286 259, 287, 288 259, 289 68, 290, 291 259, 292 259, 293 259, 294 211 221 |
| Hepatic |
|
296 259, 297 259, 298 |
| Skeletal Muscle |
|
259, 153 259, 302 68, 303-306 10, 229 259, 281 259, 308 259, 309 |
| Neural |
|
313 314 10, 136 217, 259 316 68, 317-319 312, 320 10, 279 259, 321 |
| Dermal |
|
36, 259 259, 323 259, 324 5, 259, 325, 326 |
Table 5.
Summary of Limitations in Complex Tissues and Organs
| Tissue | Major Limitations in Bioprinted Stem Cell Constructs | References |
|---|---|---|
| Adipose | Difficulties maintaining viability in relation to thickness of construct, issues with vascularization | 259 |
| Bone | Replication of native bone structure | 331 |
| Cartilage | Difficulties regenerating hyaline cartilage, issues in translatability to clinical setting | 332 |
| Cardiovascular & Cardiomyogenic | Replicating heart structure, issues with vascularization | |
| Hepatic | Constructs that can withstand metabolic activity of liver microenvironment | 259 |
| Skeletal Muscle | Issues with vascularization in relation to thickness of construct, development of sufficient innervation and response to electrical stimulation | 259 |
| Neural | Need for bioinks that support neural cell proliferation and differentiation, difficulty fabricating complex neural networks | 259 |
| Dermal | Development and optimization of functional components of skin, such as sweat glands and hair follicles | 259 |
Acknowledgements
We would like to thank Ms. Emily Gregg for illustration. This review was supported, in part, by National Institutes of Health (1P41EB023833-346 01).
Footnotes
The authors declare no competing financial interest.
References
- (1).Mandrycky C; Wang Z; Kim K; Kim DH 3D bioprinting for engineering complex tissues. Biotechnol Adv 2016, 34 (4), 422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Mikos AG; Herring SW; Ochareon P; Elisseeff J; Lu HH; Kandel R; Schoen FJ; Toner M; Mooney D; Atala A et al. Engineering complex tissues. Tissue Eng 2006, 12 (12), 3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Murphy SV; Atala A 3D bioprinting of tissues and organs. Nat Biotechnol 2014, 32 (8), 773. [DOI] [PubMed] [Google Scholar]
- (4).Kang HW; Lee SJ; Ko IK; Kengla C; Yoo JJ; Atala A A 3D bioprinting system to produce human-scale tissue constructs with structural integrity. Nat Biotechnol 2016, 34 (3), 312. [DOI] [PubMed] [Google Scholar]
- (5).Tasoglu S; Demirci U Bioprinting for stem cell research. Trends Biotechnol 2013, 31 (1), 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Lutolf MP; Gilbert PM; Blau HM Designing materials to direct stem-cell fate. Nature 2009, 462 (7272), 433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Spector M; Goldman RD; Leinwand LA Cells. A Laboratory Manual 1998, 1. [Google Scholar]
- (8).Jaenisch R; Young R Stem cells, the molecular circuitry of pluripotency and nuclear reprogramming. Cell 2008, 132 (4), 567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Ooi J; Liu P Pluripotency and its layers of complexity. Cell Regen (Lond) 2012, 1 (1), 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Irvine SA; Venkatraman SS Bioprinting and Differentiation of Stem Cells. Molecules 2016, 21 (9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Ding S; Feng L; Wu J; Zhu F; Tan Z. e.; Yao R Bioprinting of stem cells: Interplay of bioprinting process, bioinks, and stem cell properties. ACS Biomaterials Science & Engineering 2018, 4 (9), 3108. [DOI] [PubMed] [Google Scholar]
- (12).Maiullari F; Costantini M; Milan M; Pace V; Chirivì M; Maiullari S; Rainer A; Baci D; Marei HE; Seliktar D et al. A multi-cellular 3D bioprinting approach for vascularized heart tissue engineering based on HUVECs and iPSC-derived cardiomyocytes. Sci Rep 2018, 8 (1), 13532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Angelopoulos I; Allenby MC; Lim M; Zamorano M Engineering inkjet bioprinting processes toward translational therapies. Biotechnol Bioeng 2019, DOI: 10.1002/bit.2717610.1002/bit.27176. [DOI] [PubMed] [Google Scholar]
- (14).Obregon F; Vaquette C; Ivanovski S; Hutmacher DW; Bertassoni LE Three-Dimensional Bioprinting for Regenerative Dentistry and Craniofacial Tissue Engineering. J Dent Res 2015, 94 (9 Suppl), 143S. [DOI] [PubMed] [Google Scholar]
- (15).Malda J; Visser J; Melchels FP; Jüngst T; Hennink WE; Dhert WJ; Groll J; Hutmacher DW 25th anniversary article: Engineering hydrogels for biofabrication. Adv Mater 2013, 25 (36), 5011. [DOI] [PubMed] [Google Scholar]
- (16).Cui X; Gao G; Yonezawa T; Dai G Human cartilage tissue fabrication using three-dimensional inkjet printing technology. J Vis Exp 2014, DOI: 10.3791/5129410.3791/51294(88). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Lorber B; Hsiao WK; Hutchings IM; Martin KR Adult rat retinal ganglion cells and glia can be printed by piezoelectric inkjet printing. Biofabrication 2014, 6 (1), 015001. [DOI] [PubMed] [Google Scholar]
- (18).Cui X; Dean D; Ruggeri ZM; Boland T Cell damage evaluation of thermal inkjet printed Chinese hamster ovary cells. Biotechnol Bioeng 2010, 106 (6), 963. [DOI] [PubMed] [Google Scholar]
- (19).Benning L; Gutzweiler L; Trondle K; Riba J; Zengerle R; Koltay P; Zimmermann S; Stark GB; Finkenzeller G Cytocompatibility testing of hydrogels toward bioprinting of mesenchymal stem cells. Journal of biomedical materials research. Part A 2017, 105 (12), 3231. [DOI] [PubMed] [Google Scholar]
- (20).Lee VK; Dias A; Ozturk MS; Chen K; Tricomi B; Corr DT; Intes X; Dai G In Bioprinting in regenerative medicine; Springer, 2015. [Google Scholar]
- (21).Knowlton S; Onal S; Yu CH; Zhao JJ; Tasoglu S Bioprinting for cancer research. Trends Biotechnol 2015, 33 (9), 504. [DOI] [PubMed] [Google Scholar]
- (22).Chang CC; Boland ED; Williams SK; Hoying JB Direct-write bioprinting three-dimensional biohybrid systems for future regenerative therapies. J Biomed Mater Res B Appl Biomater 2011, 98 (1), 160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Ouyang L Study on Microextrusion-based 3D Bioprinting and Bioink Crosslinking Mechanisms; Springer, 2019. [Google Scholar]
- (24).Ouyang L; Yao R; Zhao Y; Sun W Effect of bioink properties on printability and cell viability for 3D bioplotting of embryonic stem cells. Biofabrication 2016, 8 (3), 035020. [DOI] [PubMed] [Google Scholar]
- (25).Jones N Science in three dimensions: the print revolution. Nature 2012, 487 (7405), 22. [DOI] [PubMed] [Google Scholar]
- (26).Wilson SA; Cross LM; Peak CW; Gaharwar AK Shear-Thinning and Thermo-Reversible Nanoengineered Inks for 3D Bioprinting. ACS Appl Mater Interfaces 2017, 9 (50), 43449. [DOI] [PubMed] [Google Scholar]
- (27).Derby B Printing and prototyping of tissues and scaffolds. Science 2012, 338 (6109), 921. [DOI] [PubMed] [Google Scholar]
- (28).Ferris CJ; Gilmore KG; Wallace GG; In het Panhuis M Biofabrication: an overview of the approaches used for printing of living cells. Appl Microbiol Biotechnol 2013, 97 (10), 4243. [DOI] [PubMed] [Google Scholar]
- (29).Smith CM; Stone AL; Parkhill RL; Stewart RL; Simpkins MW; Kachurin AM; Warren WL; Williams SK Three-dimensional bioassembly tool for generating viable tissue-engineered constructs. Tissue Eng 2004, 10 (9-10), 1566. [DOI] [PubMed] [Google Scholar]
- (30).Chang R; Nam J; Sun W Effects of dispensing pressure and nozzle diameter on cell survival from solid freeform fabrication-based direct cell writing. Tissue engineering. Part A 2008, 14 (1), 41. [DOI] [PubMed] [Google Scholar]
- (31).Guvendiren M; Lu HD; Burdick JA Shear-thinning hydrogels for biomedical applications. Soft matter 2012, 8 (2), 260. [Google Scholar]
- (32).Antoshin A; Churbanov S; Minaev N; Deying Z; Yuanyuan Z; Shpichka A; Timashev P LIFT-bioprinting, is it worth it? Bioprinting 2019, e00052. [Google Scholar]
- (33).Guillotin B; Guillemot F Cell patterning technologies for organotypic tissue fabrication. Trends Biotechnol 2011, 29 (4), 183. [DOI] [PubMed] [Google Scholar]
- (34).Guillotin B; Souquet A; Catros S; Duocastella M; Pippenger B; Bellance S; Bareille R; Rémy M; Bordenave L; Amédée J et al. Laser assisted bioprinting of engineered tissue with high cell density and microscale organization. Biomaterials 2010, 31 (28), 7250. [DOI] [PubMed] [Google Scholar]
- (35).Ali M; Pages E; Ducom A; Fontaine A; Guillemot F Controlling laser-induced jet formation for bioprinting mesenchymal stem cells with high viability and high resolution. Biofabrication 2014, 6 (4), 045001. [DOI] [PubMed] [Google Scholar]
- (36).Skardal A; Mack D; Kapetanovic E; Atala A; Jackson JD; Yoo J; Soker S Bioprinted amniotic fluid-derived stem cells accelerate healing of large skin wounds. Stem Cells Transl Med 2012, 1 (11), 792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Mulani B; Chabukswar A; Bhalake S; Ramnani S Review On Low Cost 3D Printing. 2019. [Google Scholar]
- (38).Skoog SA; Goering PL; Narayan RJ Stereolithography in tissue engineering. J Mater Sci Mater Med 2014, 25 (3), 845. [DOI] [PubMed] [Google Scholar]
- (39).Wang Z; Abdulla R; Parker B; Samanipour R; Ghosh S; Kim K A simple and high-resolution stereolithography-based 3D bioprinting system using visible light crosslinkable bioinks. Biofabrication 2015, 7 (4), 045009. [DOI] [PubMed] [Google Scholar]
- (40).Tan Y; Richards DJ; Trusk TC; Visconti RP; Yost MJ; Kindy MS; Drake CJ; Argraves WS; Markwald RR; Mei Y 3D printing facilitated scaffold-free tissue unit fabrication. Biofabrication 2014, 6 (2), 024111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Yang X; Sun Y; Wang Q A phase field approach for multicellular aggregate fusion in biofabrication. J Biomech Eng 2013, 135 (7), 71005. [DOI] [PubMed] [Google Scholar]
- (42).Hong S; Lee JY; Hwang C; Shin JH; Park Y Inhibition of Rho-Associated Protein Kinase Increases the Angiogenic Potential of Mesenchymal Stem Cell Aggregates via Paracrine Effects. Tissue engineering. Part A 2016, 22 (3-4), 233. [DOI] [PubMed] [Google Scholar]
- (43).Lei J; Trevino E; Temenoff J Cell number and chondrogenesis in human mesenchymal stem cell aggregates is affected by the sulfation level of heparin used as a cell coating. Journal of biomedical materials research. Part A 2016, 104 (7), 1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Roth EA; Xu T; Das M; Gregory C; Hickman JJ; Boland T Inkjet printing for high-throughput cell patterning. Biomaterials 2004, 25 (17), 3707. [DOI] [PubMed] [Google Scholar]
- (45).Zhang K; Chou CK; Xia X; Hung MC; Qin L Block-Cell-Printing for live single-cell printing. Proc Natl Acad Sci U S A 2014, 111 (8), 2948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (46).Lin L; Chu YS; Thiery JP; Lim CT; Rodriguez I Microfluidic cell trap array for controlled positioning of single cells on adhesive micropatterns. Lab Chip 2013, 13 (4), 714. [DOI] [PubMed] [Google Scholar]
- (47).Zychowicz M; Mehn D; Ruiz A; Frontczak-Baniewicz M; Rossi F; Buzanska L Patterning of human cord blood-derived stem cells on single cell posts and lines: Implications for neural commitment. Acta Neurobiol Exp (Wars) 2012, 72 (4), 325. [DOI] [PubMed] [Google Scholar]
- (48).Osborne J; Hellein J; Singla R; Singal PK; Singla DK Stem cells in three-dimensional bioprinting: Future perspectives. Current Research: Cardiology 2015, 2 (4). [Google Scholar]
- (49).MacDonald A In Cell Science; Technology Networks, 2018. [Google Scholar]
- (50).Solter D From teratocarcinomas to embryonic stem cells and beyond: a history of embryonic stem cell research. Nat Rev Genet 2006, 7 (4), 319. [DOI] [PubMed] [Google Scholar]
- (51).Mountford JC Human embryonic stem cells: origins, characteristics and potential for regenerative therapy. Transfus Med 2008, 18 (1), 1. [DOI] [PubMed] [Google Scholar]
- (52).Singla DK Embryonic stem cells in cardiac repair and regeneration. Antioxid Redox Signal 2009, 11 (8), 1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (53).de Lazaro I; Yilmazer A; Kostarelos K Induced pluripotent stem (iPS) cells: a new source for cell-based therapeutics? Journal of controlled release : official journal of the Controlled Release Society 2014, 185, 37. [DOI] [PubMed] [Google Scholar]
- (54).Sousa BR; Parreira RC; Fonseca EA; Amaya MJ; Tonelli FM; Lacerda SM; Lalwani P; Santos AK; Gomes KN; Ulrich H et al. Human adult stem cells from diverse origins: an overview from multiparametric immunophenotyping to clinical applications. Cytometry A 2014, 85 (1), 43. [DOI] [PubMed] [Google Scholar]
- (55).Tang Y; He H; Cheng N; Song Y; Ding W; Zhang Y; Zhang W; Zhang J; Peng H; Jiang H PDGF, NT-3 and IGF-2 in combination induced transdifferentiation of muscle-derived stem cells into Schwann cell-like cells. PLoS One 2014, 9 (1), e73402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (56).Lue J; Lin G; Ning H; Xiong A; Lin CS; Glenn JS Transdifferentiation of adipose-derived stem cells into hepatocytes: a new approach. Liver Int 2010, 30 (6), 913. [DOI] [PubMed] [Google Scholar]
- (57).Stadtfeld M; Hochedlinger K Induced pluripotency: history, mechanisms, and applications. Genes Dev 2010, 24 (20), 2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (58).Schreiber SL Small molecules: the missing link in the central dogma. Nat Chem Biol 2005, 1 (2), 64. [DOI] [PubMed] [Google Scholar]
- (59).Schugar RC; Robbins PD; Deasy BM Small molecules in stem cell self-renewal and differentiation. Gene Ther 2008, 15 (2), 126. [DOI] [PubMed] [Google Scholar]
- (60).Schmole AC; Hubner R; Beller M; Rolfs A; Frech MJ Small molecules in stem cell research. Curr Pharm Biotechnol 2013, 14 (1), 36. [PubMed] [Google Scholar]
- (61).Nature, 2019.
- (62).Shenghui H; Nakada D; Morrison SJ Mechanisms of stem cell self-renewal. Annual Review of Cell and Developmental 2009, 25, 377. [DOI] [PubMed] [Google Scholar]
- (63).Lukaszewicz AI; McMillan MK; Kahn M Small molecules and stem cells. Potency and lineage commitment: the new quest for the fountain of youth. J Med Chem 2010, 53 (9), 3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (64).Sanchez Alvarado A; Yamanaka S Rethinking differentiation: stem cells, regeneration, and plasticity. Cell 2014, 157 (1), 110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (65).In PubChem; Naitonal Center for Biotechnology Information: United States National Library of Medicine, 2019.
- (66).Chemical Entities of Biological Interest, 2019.
- (67).Whalen K Pharmacology: Lippincott illustrated reviews; Lippincott Williams & Wilkins, 2015. [Google Scholar]
- (68).Lee SJ; Atala A; Yoo JJ In Situ Tissue Regeneration: Host Cell Recruitment and Biomaterial Design; Academic Press, 2016. [Google Scholar]
- (69).Shen CN; Slack JM; Tosh D Molecular basis of transdifferentiation of pancreas to liver. Nat Cell Biol 2000, 2 (12), 879. [DOI] [PubMed] [Google Scholar]
- (70).Ghali O; Broux O; Falgayrac G; Haren N; van Leeuwen JP; Penel G; Hardouin P; Chauveau C Dexamethasone in osteogenic medium strongly induces adipocyte differentiation of mouse bone marrow stromal cells and increases osteoblast differentiation. BMC Cell Biol 2015, 16, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (71).Gay MS; Dasgupta C; Li Y; Kanna A; Zhang L Dexamethasone Induces Cardiomyocyte Terminal Differentiation via Epigenetic Repression of Cyclin D2 Gene. J Pharmacol Exp Ther 2016, 358 (2), 190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (72).In PubChem; National Center for Biotechnology Information: United States National Library of Medicine, 2019.
- (73).Chemical Entities of Biological Interest, 2019.
- (74).National Cancer Institute, 2019.
- (75).Ren M; Han Z; Li J; Feng G; Ouyang S Ascorbic acid delivered by mesoporous silica nanoparticles induces the differentiation of human embryonic stem cells into cardiomyocytes. Mater Sci Eng C Mater Biol Appl 2015, 56, 348. [DOI] [PubMed] [Google Scholar]
- (76).In PubChem; National Center for Biotechnology Information: United States National Library of Medicine, 2019.
- (77).Chemical Entities of Biological Interest, 2019.
- (78).Langenbach F; Handschel J Effects of dexamethasone, ascorbic acid and β-glycerophosphate on the osteogenic differentiation of stem cells in vitro. Stem Cell Res Ther 2013, 4 (5), 117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (79).Liu M; Sun Y; Liu Y; Yuan M; Zhang Z; Hu W Modulation of the differentiation of dental pulp stem cells by different concentrations of β-glycerophosphate. Molecules 2012, 17 (2), 1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (80).In PubChem; National Center for Biotechnology Information: United Staets National Library of Medicine, 2019.
- (81).Chemical Entities of Biological Interest, 2019.
- (82).Thompson R; Casali C; Chan C Forskolin and IBMX Induce Neural Transdifferentiation of MSCs Through Downregulation of the NRSF. Sci Rep 2019, 9 (1), 2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (83).Hua Y; Ke S; Wang Y; Irwin DM; Zhang S; Wang Z Prolonged treatment with 3-isobutyl-1-methylxanthine improves the efficiency of differentiating 3T3-L1 cells into adipocytes. Anal Biochem 2016, 507, 18. [DOI] [PubMed] [Google Scholar]
- (84).In PubChem; National Center for Biotechnology Information: United States National Library of Medicine, 2019.
- (85).National Cancer Institute, 2019.
- (86).Katzung BG; Revor AJ Basic & Clinical Pharmacology 13 ed., 2013. [Google Scholar]
- (87).Caron MMJ; Emans PJ; Cremers A; Surtel DAM; van Rhijn LW; Welting TJM Indomethacin induces differential effects on in vitro endochondral ossification depending on the chondrocyte's differentiation stage. J Orthop Res 2017, 35 (4), 847. [DOI] [PubMed] [Google Scholar]
- (88).Mu MW; Zhao ZY; Li CG Comparative study of neural differentiation of bone marrow mesenchymal stem cells by different induction methods. Genet Mol Res 2015, 14 (4), 14169. [DOI] [PubMed] [Google Scholar]
- (89).UniProt, 2019.
- (90).Kim J; Lee YJ; Kim JM; Lee SY; Bae MA; Ahn JH; Han DC; Kwon BM PPARγ agonists induce adipocyte differentiation by modulating the expression of Lipin-1, which acts as a PPARγ phosphatase. Int J Biochem Cell Biol 2016, 81 (Pt A), 57. [DOI] [PubMed] [Google Scholar]
- (91).Kim SW; Xie Y; Nguyen PQ; Bui VT; Huynh K; Kang JS; Brown DJ; Jester JV PPARγ regulates meibocyte differentiation and lipid synthesis of cultured human meibomian gland epithelial cells (hMGEC). Ocul Surf 2018, 16 (4), 463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (92).Neidhart M DNA Methylation and Complex Human Disease; Academic Press, 2015. [Google Scholar]
- (93).Sivanathan K; Manaph NPA; Nitschke J; Drogemuller C; Zhou X-F; Coates PT DNA Memethylation Agents Promote Pancreatic Endodermal Differentiation of Mesenchymal Stem Cells. Transplantation 2018, 102, S729. [Google Scholar]
- (94).Ramakrishnan S; Hu Q; Krishnan N; Wang D; Smit E; Granger V; Rak M; Attwood K; Johnson C; Morrison C et al. Decitabine, a DNA-demethylating agent, promotes differentiation via NOTCH1 signaling and alters immune-related pathways in muscle-invasive bladder cancer. Cell Death Dis 2017, 8 (12), 3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (95).Cell Signaling Technology, 2019. [Google Scholar]
- (96).Armstrong JP; Burke M; Carter BM; Davis SA; Perriman AW 3D Bioprinting Using a Templated Porous Bioink. Adv Healthc Mater 2016, 5 (14), 1724. [DOI] [PubMed] [Google Scholar]
- (97).Wang X; Ao Q; Tian X; Fan J; Tong H; Hou W; Bai S Gelatin-Based Hydrogels for Organ 3D Bioprinting. Polymers (Basel) 2017, 9 (9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (98).Cofiño C; Perez-Amodio S; Semino CE; Engel E; Mateos-Timoneda MA Development of a Self-Assembled Peptide/Methylcellulose-Based Bioink for 3D Bioprinting. Macromolecular Materials and Engineering 2019. [Google Scholar]
- (99).Bishop ES; Mostafa S; Pakvasa M; Luu HH; Lee MJ; Wolf JM; Ameer GA; He TC; Reid RR 3-D bioprinting technologies in tissue engineering and regenerative medicine: Current and future trends. Genes Dis 2017, 4 (4), 185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (100).Millipore Sigma, 2019.
- (101).Clark DA; Coker R Transforming growth factor-beta (TGF-beta). Int J Biochem Cell Biol 1998, 30 (3), 293. [DOI] [PubMed] [Google Scholar]
- (102).Moustakas A; Pardali K; Gaal A; Heldin CH Mechanisms of TGF-beta signaling in regulation of cell growth and differentiation. Immunol Lett 2002, 82 (1-2), 85. [DOI] [PubMed] [Google Scholar]
- (103).Longobardi L; O'Rear L; Aakula S; Johnstone B; Shimer K; Chytil A; Horton WA; Moses HL; Spagnoli A Effect of IGF-I in the chondrogenesis of bone marrow mesenchymal stem cells in the presence or absence of TGF-beta signaling. J Bone Miner Res 2006, 21 (4), 626. [DOI] [PubMed] [Google Scholar]
- (104).McKinnon RD; Piras G; Ida JA Jr.; Dubois-Dalcq M A role for TGF-beta in oligodendrocyte differentiation. J Cell Biol 1993, 121 (6), 1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (105).Weiss S; Hennig T; Bock R; Steck E; Richter W Impact of growth factors and PTHrP on early and late chondrogenic differentiation of human mesenchymal stem cells. J Cell Physiol 2010, 223 (1), 84. [DOI] [PubMed] [Google Scholar]
- (106).Cheng H; Jiang W; Phillips FM; Haydon RC; Peng Y; Zhou L; Luu HH; An N; Breyer B; Vanichakarn P et al. Osteogenic activity of the fourteen types of human bone morphogenetic proteins (BMPs). J Bone Joint Surg Am 2003, 85 (8), 1544. [DOI] [PubMed] [Google Scholar]
- (107).Urist MR; Strates BS Bone morphogenetic protein. J Dent Res 1971, 50 (6), 1392. [DOI] [PubMed] [Google Scholar]
- (108).Reddi AH; Cunningham NS Initiation and promotion of bone differentiation by bone morphogenetic proteins. J Bone Miner Res 1993, 8 Suppl 2, S499. [DOI] [PubMed] [Google Scholar]
- (109).Luu HH; Song WX; Luo X; Manning D; Luo J; Deng ZL; Sharff KA; Montag AG; Haydon RC; He TC Distinct roles of bone morphogenetic proteins in osteogenic differentiation of mesenchymal stem cells. J Orthop Res 2007, 25 (5), 665. [DOI] [PubMed] [Google Scholar]
- (110).Lou J; Xu F; Merkel K; Manske P Gene therapy: adenovirus-mediated human bone morphogenetic protein-2 gene transfer induces mesenchymal progenitor cell proliferation and differentiation in vitro and bone formation in vivo. J Orthop Res 1999, 17 (1), 43. [DOI] [PubMed] [Google Scholar]
- (111).Chang SC; Chung HY; Tai CL; Chen PK; Lin TM; Jeng LB Repair of large cranial defects by hBMP-2 expressing bone marrow stromal cells: comparison between alginate and collagen type I systems. Journal of biomedical materials research. Part A 2010, 94 (2), 433. [DOI] [PubMed] [Google Scholar]
- (112).Stewart A; Guan H; Yang K BMP-3 promotes mesenchymal stem cell proliferation through the TGF-beta/activin signaling pathway. J Cell Physiol 2010, 223 (3), 658. [DOI] [PubMed] [Google Scholar]
- (113).Komiya Y; Habas R Wnt signal transduction pathways. Organogenesis 2008, 4 (2), 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (114).Visweswaran M; Pohl S; Arfuso F; Newsholme P; Dilley R; Pervaiz S; Dharmarajan A Multi-lineage differentiation of mesenchymal stem cells - To Wnt, or not Wnt. Int J Biochem Cell Biol 2015, 68, 139. [DOI] [PubMed] [Google Scholar]
- (115).Baksh D; Boland GM; Tuan RS Cross-talk between Wnt signaling pathways in human mesenchymal stem cells leads to functional antagonism during osteogenic differentiation. J Cell Biochem 2007, 101 (5), 1109. [DOI] [PubMed] [Google Scholar]
- (116).Andrae J; Gallini R; Betsholtz C Role of platelet-derived growth factors in physiology and medicine. Genes Dev 2008, 22 (10), 1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (117).Krausgrill B; Vantler M; Burst V; Raths M; Halbach M; Frank K; Schynkowski S; Schenk K; Hescheler J; Rosenkranz S et al. Influence of cell treatment with PDGF-BB and reperfusion on cardiac persistence of mononuclear and mesenchymal bone marrow cells after transplantation into acute myocardial infarction in rats. Cell Transplant 2009, 18 (8), 847. [DOI] [PubMed] [Google Scholar]
- (118).Chase LG; Lakshmipathy U; Solchaga LA; Rao MS; Vemuri MC A novel serum-free medium for the expansion of human mesenchymal stem cells. Stem Cell Res Ther 2010, 1 (1), 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (119).Klemm DJ; Leitner JW; Watson P; Nesterova A; Reusch JE; Goalstone ML; Draznin B Insulin-induced adipocyte differentiation. Activation of CREB rescues adipogenesis from the arrest caused by inhibition of prenylation. J Biol Chem 2001, 276 (30), 28430. [DOI] [PubMed] [Google Scholar]
- (120).Laron Z Insulin-like growth factor 1 (IGF-1): a growth hormone. Mol Pathol 2001, 54 (5), 311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (121).Youssef A; Aboalola D; Han VK The Roles of Insulin-Like Growth Factors in Mesenchymal Stem Cell Niche. Stem Cells Int 2017, 2017, 9453108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (122).Hellström M; Kalén M; Lindahl P; Abramsson A; Betsholtz C Role of PDGF-B and PDGFR-beta in recruitment of vascular smooth muscle cells and pericytes during embryonic blood vessel formation in the mouse. Development 1999, 126 (14), 3047. [DOI] [PubMed] [Google Scholar]
- (123).Duffy AM; Bouchier-Hayes DJ; Harmey JH In Madame Curie Bioscience Database [Internet]; Landes Bioscience, 2013. [Google Scholar]
- (124).Hu K; Olsen BR Osteoblast-derived VEGF regulates osteoblast differentiation and bone formation during bone repair. J Clin Invest 2016, 126 (2), 509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (125).Pons J; Huang Y; Arakawa-Hoyt J; Washko D; Takagawa J; Ye J; Grossman W; Su H VEGF improves survival of mesenchymal stem cells in infarcted hearts. Biochem Biophys Res Commun 2008, 376 (2), 419. [DOI] [PubMed] [Google Scholar]
- (126).Wang X; Hu Q; Mansoor A; Lee J; Wang Z; Lee T; From AH; Zhang J Bioenergetic and functional consequences of stem cell-based VEGF delivery in pressure-overloaded swine hearts. Am J Physiol Heart Circ Physiol 2006, 290 (4), H1393. [DOI] [PubMed] [Google Scholar]
- (127).Fan VH; Tamama K; Au A; Littrell R; Richardson LB; Wright JW; Wells A; Griffith LG Tethered epidermal growth factor provides a survival advantage to mesenchymal stem cells. Stem Cells 2007, 25 (5), 1241. [DOI] [PubMed] [Google Scholar]
- (128).Tamama K; Fan VH; Griffith LG; Blair HC; Wells A Epidermal growth factor as a candidate for ex vivo expansion of bone marrow-derived mesenchymal stem cells. Stem Cells 2006, 24 (3), 686. [DOI] [PubMed] [Google Scholar]
- (129).Krampera M; Pasini A; Rigo A; Scupoli MT; Tecchio C; Malpeli G; Scarpa A; Dazzi F; Pizzolo G; Vinante F HB-EGF/HER-1 signaling in bone marrow mesenchymal stem cells: inducing cell expansion and reversibly preventing multilineage differentiation. Blood 2005, 106 (1), 59. [DOI] [PubMed] [Google Scholar]
- (130).Eswarakumar VP; Lax I; Schlessinger J Cellular signaling by fibroblast growth factor receptors. Cytokine Growth Factor Rev 2005, 16 (2), 139. [DOI] [PubMed] [Google Scholar]
- (131).Tsutsumi S; Shimazu A; Miyazaki K; Pan H; Koike C; Yoshida E; Takagishi K; Kato Y Retention of multilineage differentiation potential of mesenchymal cells during proliferation in response to FGF. Biochem Biophys Res Commun 2001, 288 (2), 413. [DOI] [PubMed] [Google Scholar]
- (132).Stewart AA; Byron CR; Pondenis H; Stewart MC Effect of fibroblast growth factor-2 on equine mesenchymal stem cell monolayer expansion and chondrogenesis. Am J Vet Res 2007, 68 (9), 941. [DOI] [PubMed] [Google Scholar]
- (133).Farre J; Roura S; Prat-Vidal C; Soler-Botija C; Llach A; Molina CE; Hove-Madsen L; Cairo JJ; Godia F; Bragos R et al. FGF-4 increases in vitro expansion rate of human adult bone marrow-derived mesenchymal stem cells. Growth Factors 2007, 25 (2), 71. [DOI] [PubMed] [Google Scholar]
- (134).Forte G; Minieri M; Cossa P; Antenucci D; Sala M; Gnocchi V; Fiaccavento R; Carotenuto F; De Vito P; Baldini PM et al. Hepatocyte growth factor effects on mesenchymal stem cells: proliferation, migration, and differentiation. Stem Cells 2006, 24 (1), 23. [DOI] [PubMed] [Google Scholar]
- (135).Huang Y; Zhang X-F; Gao G; Yonezawa T; Cui X 3D bioprinting and the current applications in tissue engineering. Biotechnology Journal 2017, 12 (8), 1600734. [DOI] [PubMed] [Google Scholar]
- (136).Lee YB; Polio S; Lee W; Dai G; Menon L; Carroll RS; Yoo SS Bio-printing of collagen and VEGF-releasing fibrin gel scaffolds for neural stem cell culture. Exp Neurol 2010, 223 (2), 645. [DOI] [PubMed] [Google Scholar]
- (137).Poldervaart MT; Wang H; van der Stok J; Weinans H; Leeuwenburgh SC; Öner FC; Dhert WJ; Alblas J Sustained release of BMP-2 in bioprinted alginate for osteogenicity in mice and rats. PLoS One 2013, 8 (8), e72610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (138).Lee JS; Lee JS; Wagoner-Johnson A; Murphy WL Modular peptide growth factors for substrate-mediated stem cell differentiation. Angew Chem Int Ed Engl 2009, 48 (34), 6266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (139).Bellis SL Advantages of RGD peptides for directing cell association with biomaterials. Biomaterials 2011, 32 (18), 4205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (140).Naghdi P; Tiraihi T; Ganji F; Darabi S; Taheri T; Kazemi H Survival, proliferation and differentiation enhancement of neural stem cells cultured in three-dimensional polyethylene glycol-RGD hydrogel with tenascin. J Tissue Eng Regen Med 2016, 10 (3), 199. [DOI] [PubMed] [Google Scholar]
- (141).Lam HJ; Li S; Lou N; Chu J; Bhatnagar RS Synthetic peptides cytomodulin-1 (CM-1) and cytomodulin-2 (CM-2) promote collagen synthesis and wound healing in vitro. Conf Proc IEEE Eng Med Biol Soc 2004, 7, 5028. [DOI] [PubMed] [Google Scholar]
- (142).Santulli G; Ciccarelli M; Palumbo G; Campanile A; Galasso G; Ziaco B; Altobelli GG; Cimini V; Piscione F; D'Andrea LD et al. In vivo properties of the proangiogenic peptide QK. J Transl Med 2009, 7, 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (143).Derycke LD; Bracke ME N-cadherin in the spotlight of cell-cell adhesion, differentiation, embryogenesis, invasion and signalling. Int J Dev Biol 2004, 48 (5-6), 463. [DOI] [PubMed] [Google Scholar]
- (144).Madl CM; Mehta M; Duda GN; Heilshorn SC; Mooney DJ Presentation of BMP-2 mimicking peptides in 3D hydrogels directs cell fate commitment in osteoblasts and mesenchymal stem cells. Biomacromolecules 2014, 15 (2), 445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (145).Moeinzadeh S; Barati D; Sarvestani SK; Karimi T; Jabbari E Experimental and computational investigation of the effect of hydrophobicity on aggregation and osteoinductive potential of BMP-2-derived peptide in a hydrogel matrix. Tissue Eng Part A 2015, 21 (1-2), 134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (146).He X; Ma J; Jabbari E Effect of grafting RGD and BMP-2 protein-derived peptides to a hydrogel substrate on osteogenic differentiation of marrow stromal cells. Langmuir 2008, 24 (21), 12508. [DOI] [PubMed] [Google Scholar]
- (147).He X; Yang X; Jabbari E Combined effect of osteopontin and BMP-2 derived peptides grafted to an adhesive hydrogel on osteogenic and vasculogenic differentiation of marrow stromal cells. Langmuir 2012, 28 (12), 5387. [DOI] [PubMed] [Google Scholar]
- (148).Zhou X; Feng W; Qiu K; Chen L; Wang W; Nie W; Mo X; He C BMP-2 Derived Peptide and Dexamethasone Incorporated Mesoporous Silica Nanoparticles for Enhanced Osteogenic Differentiation of Bone Mesenchymal Stem Cells. ACS Appl Mater Interfaces 2015, 7 (29), 15777. [DOI] [PubMed] [Google Scholar]
- (149).Zhang Z; Gupte MJ; Jin X; Ma PX Injectable Peptide Decorated Functional Nanofibrous Hollow Microspheres to Direct Stem Cell Differentiation and Tissue Regeneration. Adv Funct Mater 2015, 25 (3), 350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (150).Seo J; Park SH; Kim MJ; Ju HJ; Yin XY; Min BH; Kim MS Injectable Click-Crosslinked Hyaluronic Acid Depot To Prolong Therapeutic Activity in Articular Joints Affected by Rheumatoid Arthritis. ACS Appl Mater Interfaces 2019, 11 (28), 24984. [DOI] [PubMed] [Google Scholar]
- (151).Prakash Parthiban S; Rana D; Jabbari E; Benkirane-Jessel N; Ramalingam M Covalently immobilized VEGF-mimicking peptide with gelatin methacrylate enhances microvascularization of endothelial cells. Acta biomaterialia 2017, 51, 330. [DOI] [PubMed] [Google Scholar]
- (152).Bian L; Guvendiren M; Mauck RL; Burdick JA Hydrogels that mimic developmentally relevant matrix and N-cadherin interactions enhance MSC chondrogenesis. Proc Natl Acad Sci U S A 2013, 110 (25), 10117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (153).Phillippi JA; Miller E; Weiss L; Huard J; Waggoner A; Campbell P Microenvironments engineered by inkjet bioprinting spatially direct adult stem cells toward muscle- and bone-like subpopulations. Stem Cells 2008, 26 (1), 127. [DOI] [PubMed] [Google Scholar]
- (154).Mendt M; Rezvani K; Shpall E Mesenchymal stem cell-derived exosomes for clinical use. Bone Marrow Transplant 2019, 54 (Suppl 2), 789. [DOI] [PubMed] [Google Scholar]
- (155).Pegtel DM; Gould SJ Exosomes. Annu Rev Biochem 2019, 88, 487. [DOI] [PubMed] [Google Scholar]
- (156).Narayanan R; Huang CC; Ravindran S Hijacking the Cellular Mail: Exosome Mediated Differentiation of Mesenchymal Stem Cells. Stem Cells Int 2016, 2016, 3808674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (157).Li P; Kaslan M; Lee SH; Yao J; Gao Z Progress in Exosome Isolation Techniques. Theranostics 2017, 7 (3), 789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (158).Huang CC; Narayanan R; Alapati S; Ravindran S Exosomes as biomimetic tools for stem cell differentiation: Applications in dental pulp tissue regeneration. Biomaterials 2016, 111, 103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (159).Chowdhury R; Webber JP; Gurney M; Mason MD; Tabi Z; Clayton A Cancer exosomes trigger mesenchymal stem cell differentiation into pro-angiogenic and pro-invasive myofibroblasts. Oncotarget 2015, 6 (2), 715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (160).Webber JP; Spary LK; Sanders AJ; Chowdhury R; Jiang WG; Steadman R; Wymant J; Jones AT; Kynaston H; Mason MD et al. Differentiation of tumour-promoting stromal myofibroblasts by cancer exosomes. Oncogene 2015, 34 (3), 290. [DOI] [PubMed] [Google Scholar]
- (161).Judson RL; Babiarz JE; Venere M; Blelloch R Embryonic stem cell-specific microRNAs promote induced pluripotency. Nat Biotechnol 2009, 27 (5), 459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (162).Frith JE; Porrello ER; Cooper-White JJ Concise review: new frontiers in microRNA-based tissue regeneration. Stem Cells Transl Med 2014, 3 (8), 969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (163).Houbaviy HB; Murray MF; Sharp PA Embryonic stem cell-specific MicroRNAs. Dev Cell 2003, 5 (2), 351. [DOI] [PubMed] [Google Scholar]
- (164).Wüst S; Godla ME; Müller R; Hofmann S Tunable hydrogel composite with two-step processing in combination with innovative hardware upgrade for cell-based three-dimensional bioprinting. Acta biomaterialia 2014, 10 (2), 630. [DOI] [PubMed] [Google Scholar]
- (165).Wang H; Li Y; Zuo Y; Li J; Ma S; Cheng L Biocompatibility and osteogenesis of biomimetic nano-hydroxyapatite/polyamide composite scaffolds for bone tissue engineering. Biomaterials 2007, 28 (22), 3338. [DOI] [PubMed] [Google Scholar]
- (166).Fedorovich NE; Leeuwenburgh SC; van der Helm YJ; Alblas J; Dhert WJ The osteoinductive potential of printable, cell-laden hydrogel-ceramic composites. Journal of biomedical materials research. Part A 2012, 100 (9), 2412. [DOI] [PubMed] [Google Scholar]
- (167).In PubChem; National Center for Biotechnology Information: United States National Library of Medicine 2019.
- (168).Kalita SJ; Bhardwaj A; Bhatt HA Nanocrystalline calcium phosphate ceramics in biomedical engineering. Materials Science and Engineering: C 2007, 27 (3), 441. [Google Scholar]
- (169).Mostafa NY; Brown PW Computer simulation of stoichiometric hydroxyapatite: structure and substitutions. Journal of Physics and Chemistry of Solids 2007, 68 (3), 431. [Google Scholar]
- (170).Kantharia N; Naik S; Apte S; Kheur M; Kheur S; Kale B Nano-hydroxyapatite and its contemporary applications. Bone 2014, 34 (15.2), 1.71. [Google Scholar]
- (171).Cox SC; Thornby JA; Gibbons GJ; Williams MA; Mallick KK 3D printing of porous hydroxyapatite scaffolds intended for use in bone tissue engineering applications. Mater Sci Eng C Mater Biol Appl 2015, 47, 237. [DOI] [PubMed] [Google Scholar]
- (172).Wenz A; Janke K; Hoch E; Tovar GE; Borchers K; Kluger PJ Hydroxyapatite-modified gelatin bioinks for bone bioprinting. BioNanoMaterials 2016, 17 (3-4), 179. [Google Scholar]
- (173).Axpe E; Oyen ML Applications of Alginate-Based Bioinks in 3D Bioprinting. Int J Mol Sci 2016, 17 (12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (174).Wenz A; Borchers K; Tovar GEM; Kluger PJ Bone matrix production in hydroxyapatite-modified hydrogels suitable for bone bioprinting. Biofabrication 2017, 9 (4), 044103. [DOI] [PubMed] [Google Scholar]
- (175).Schrödter K; Bettermann G; Staffel T; Wahl F; Klein T; Hofmann T Phosphoric acid and phosphates. Ullmann's Encyclopedia of Industrial Chemistry 2000. [Google Scholar]
- (176).Yashima M; Sakai A; Kamiyama T; Hoshikawa A Crystal structure analysis of β-tricalcium phosphate Ca3 (PO4) 2 by neutron powder diffraction. Journal of Solid State Chemistry 2003, 175 (2), 272. [Google Scholar]
- (177).Chaair H; Labjar H; Britel O Synthesis of beta-tricalcium phosphate. Morphologie 2017, 101 (334), 120. [DOI] [PubMed] [Google Scholar]
- (178).Liu L; Wu Y; Xu C; Yu S; Wu X; Dai H Synthesis, characterization of nano-β-tricalcium phosphate and the inhibition on hepatocellular carcinoma cells. Journal of Nanomaterials 2018, 2018. [Google Scholar]
- (179).Liu B; Lun DX Current application of beta-tricalcium phosphate composites in orthopaedics. Orthop Surg 2012, 4 (3), 139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (180).Urist MR; Lietze A; Dawson E Beta-tricalcium phosphate delivery system for bone morphogenetic protein. Clin Orthop Relat Res 1984, (187), 277. [PubMed] [Google Scholar]
- (181).Habibovic P; Yuan H; van der Valk CM; Meijer G; van Blitterswijk CA; de Groot K 3D microenvironment as essential element for osteoinduction by biomaterials. Biomaterials 2005, 26 (17), 3565. [DOI] [PubMed] [Google Scholar]
- (182).Bouler JM; Pilet P; Gauthier O; Verron E Biphasic calcium phosphate ceramics for bone reconstruction: A review of biological response. Acta biomaterialia 2017, 53, 1. [DOI] [PubMed] [Google Scholar]
- (183).Aslankoohi N; Mondal D; Rizkalla AS; Mequanint K Bone Repair and Regenerative Biomaterials: Towards Recapitulating the Microenvironment. Polymers (Basel) 2019, 11 (9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (184).Boccaccini AR; Brauer DS; Hupa L Bioactive glasses: Fundamentals, technology and applications; Royal Society of Chemistry, 2016. [Google Scholar]
- (185).Ojansivu M; Vanhatupa S; Bjorkvik L; Hakkanen H; Kellomaki M; Autio R; Ihalainen JA; Hupa L; Miettinen S Bioactive glass ions as strong enhancers of osteogenic differentiation in human adipose stem cells. Acta biomaterialia 2015, 21, 190. [DOI] [PubMed] [Google Scholar]
- (186).Naruphontjirakul P; Tsigkou O; Li S; Porter AE; Jones JR Human mesenchymal stem cells differentiate into an osteogenic lineage in presence of strontium containing bioactive glass nanoparticles. Acta biomaterialia 2019, 90, 373. [DOI] [PubMed] [Google Scholar]
- (187).Murphy C; Kolan K; Li W; Semon JA; Day D; Leu M-C 3D bioprinting of stem cells and polymer/bioactive glass composite scaffolds for bone tissue engineering. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (188).Levato R; Visser J; Planell JA; Engel E; Malda J; Mateos-Timoneda MA Biofabrication of tissue constructs by 3D bioprinting of cell-laden microcarriers. Biofabrication 2014, 6 (3), 035020. [DOI] [PubMed] [Google Scholar]
- (189).Levato R; Mateos-Timoneda MA; Planell JA Preparation of biodegradable polylactide microparticles via a biocompatible procedure. Macromol Biosci 2012, 12 (4), 557. [DOI] [PubMed] [Google Scholar]
- (190).Sart S; Agathos SN; Li Y Engineering stem cell fate with biochemical and biomechanical properties of microcarriers. Biotechnol Prog 2013, 29 (6), 1354. [DOI] [PubMed] [Google Scholar]
- (191).Ayyildiz-Tamis D; Avc1 K; Deliloglu-Gurhan SI Comparative investigation of the use of various commercial microcarriers as a substrate for culturing mammalian cells. In Vitro Cell Dev Biol Anim 2014, 50 (3), 221. [DOI] [PubMed] [Google Scholar]
- (192).Tseng PC; Young TH; Wang TM; Peng HW; Hou SM; Yen ML Spontaneous osteogenesis of MSCs cultured on 3D microcarriers through alteration of cytoskeletal tension. Biomaterials 2012, 33 (2), 556. [DOI] [PubMed] [Google Scholar]
- (193).Bouffi C; Thomas O; Bony C; Giteau A; Venier-Julienne MC; Jorgensen C; Montero-Menei C; Noël D The role of pharmacologically active microcarriers releasing TGF-beta3 in cartilage formation in vivo by mesenchymal stem cells. Biomaterials 2010, 31 (25), 6485. [DOI] [PubMed] [Google Scholar]
- (194).Zhang Y; Li W; Laurent T; Ding S Small molecules, big roles -- the chemical manipulation of stem cell fate and somatic cell reprogramming. J Cell Sci 2012, 125 (Pt 23), 5609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (195).Martinez E; Engel E; Planell JA; Samitier J Effects of artificial micro- and nano-structured surfaces on cell behaviour. Ann Anat 2009, 191 (1), 126. [DOI] [PubMed] [Google Scholar]
- (196).Naba A; Clauser KR; Hoersch S; Liu H; Carr SA; Hynes RO The matrisome: in silico definition and in vivo characterization by proteomics of normal and tumor extracellular matrices. Mol Cell Proteomics 2012, 11 (4), M111 014647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (197).Hynes RO The Extracellular Matrix: Not Just Pretty Fibrils. Science 2009, 326 (5957), 1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (198).Frantz C; Stewart KM; Weaver VM The extracellular matrix at a glance. J Cell Sci 2010, 123 (Pt 24), 4195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (199).Fu L; Suflita M; Linhardt RJ Bioengineered heparins and heparan sulfates. Adv Drug Deliv Rev 2016, 97, 237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (200).Keough MB; Rogers JA; Zhang P; Jensen SK; Stephenson EL; Chen T; Hurlbert MG; Lau LW; Rawji KS; Plemel JR et al. An inhibitor of chondroitin sulfate proteoglycan synthesis promotes central nervous system remyelination. Nat Commun 2016, 7, 11312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (201).Pomin VH Keratan sulfate: an up-to-date review. Int J Biol Macromol 2015, 72, 282. [DOI] [PubMed] [Google Scholar]
- (202).Mizumoto S; Yamada S; Sugahara K Molecular interactions between chondroitin-dermatan sulfate and growth factors/receptors/matrix proteins. Curr Opin Struct Biol 2015, 34, 35. [DOI] [PubMed] [Google Scholar]
- (203).Highley CB; Prestwich GD; Burdick JA Recent advances in hyaluronic acid hydrogels for biomedical applications. Curr Opin Biotechnol 2016, 40, 35. [DOI] [PubMed] [Google Scholar]
- (204).Zollinger AJ; Smith ML Fibronectin, the extracellular glue. Matrix Biol 2017, 60-61, 27. [DOI] [PubMed] [Google Scholar]
- (205).Ramovs V; Te Molder L; Sonnenberg A The opposing roles of laminin-binding integrins in cancer. Matrix Biol 2017, 57-58, 213. [DOI] [PubMed] [Google Scholar]
- (206).Agmon G; Christman KL Controlling stem cell behavior with decellularized extracellular matrix scaffolds. Curr Opin Solid State Mater Sci 2016, 20 (4), 193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (207).Han W; Singh NK; Kim JJ; Kim H; Kim BS; Park JY; Jang J; Cho DW Directed differential behaviors of multipotent adult stem cells from decellularized tissue/organ extracellular matrix bioinks. Biomaterials 2019, 224, 119496. [DOI] [PubMed] [Google Scholar]
- (208).Gilbert TW; Sellaro TL; Badylak SF Decellularization of tissues and organs. Biomaterials 2006, 27 (19), 3675. [DOI] [PubMed] [Google Scholar]
- (209).Nam SY; Park SH ECM Based Bioink for Tissue Mimetic 3D Bioprinting. Adv Exp Med Biol 2018, 1064, 335. [DOI] [PubMed] [Google Scholar]
- (210).Rowlands D; Sugahara K; Kwok JC Glycosaminoglycans and glycomimetics in the central nervous system. Molecules 2015, 20 (3), 3527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (211).Tijore A; Irvine SA; Sarig U; Mhaisalkar P; Baisane V; Venkatraman S Contact guidance for cardiac tissue engineering using 3D bioprinted gelatin patterned hydrogel. Biofabrication 2018, 10 (2), 025003. [DOI] [PubMed] [Google Scholar]
- (212).Curtis A; Wilkinson C Topographical control of cells. Biomaterials 1997, 18 (24), 1573. [DOI] [PubMed] [Google Scholar]
- (213).Bourget JM; Laterreur V; Gauvin R; Guillemette MD; Miville-Godin C; Mounier M; Tondreau MY; Tremblay C; Labbé R; Ruel J et al. Microstructured human fibroblast-derived extracellular matrix scaffold for vascular media fabrication. J Tissue Eng Regen Med 2017, 11 (9), 2479. [DOI] [PubMed] [Google Scholar]
- (214).Clark P; Connolly P; Curtis AS; Dow JA; Wilkinson CD Topographical control of cell behaviour: II. Multiple grooved substrata. Development 1990, 108 (4), 635. [DOI] [PubMed] [Google Scholar]
- (215).Kolind K; Leong KW; Besenbacher F; Foss M Guidance of stem cell fate on 2D patterned surfaces. Biomaterials 2012, 33 (28), 6626. [DOI] [PubMed] [Google Scholar]
- (216).Teo BK; Wong ST; Lim CK; Kung TY; Yap CH; Ramagopal Y; Romer LH; Yim EK Nanotopography modulates mechanotransduction of stem cells and induces differentiation through focal adhesion kinase. ACS Nano 2013, 7 (6), 4785. [DOI] [PubMed] [Google Scholar]
- (217).Tay CY; Yu H; Pal M; Leong WS; Tan NS; Ng KW; Leong DT; Tan LP Micropatterned matrix directs differentiation of human mesenchymal stem cells towards myocardial lineage. Exp Cell Res 2010, 316 (7), 1159. [DOI] [PubMed] [Google Scholar]
- (218).Zhou J; Ye X; Wang Z; Liu J; Zhang B; Qiu J; Sun Y; Li H; Zhao Q Development of decellularized aortic valvular conduit coated by heparin-SDF-1α multilayer. Ann Thorac Surg 2015, 99 (2), 612. [DOI] [PubMed] [Google Scholar]
- (219).Metavarayuth K; Sitasuwan P; Luckanagul JA; Feng S; Wang Q Virus Nanoparticles Mediated Osteogenic Differentiation of Bone Derived Mesenchymal Stem Cells. Adv Sci (Weinh) 2015, 2 (10), 1500026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (220).Dalby MJ; McCloy D; Robertson M; Wilkinson CD; Oreffo RO Osteoprogenitor response to defined topographies with nanoscale depths. Biomaterials 2006, 27 (8), 1306. [DOI] [PubMed] [Google Scholar]
- (221).Miao S; Cui H; Nowicki M; Lee SJ; Almeida J; Zhou X; Zhu W; Yao X; Masood F; Plesniak MW et al. Photolithographic-stereolithographic-tandem fabrication of 4D smart scaffolds for improved stem cell cardiomyogenic differentiation. Biofabrication 2018, 10 (3), 035007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (222).Wang PY; Li WT; Yu J; Tsai WB Modulation of osteogenic, adipogenic and myogenic differentiation of mesenchymal stem cells by submicron grooved topography. J Mater Sci Mater Med 2012, 23 (12), 3015. [DOI] [PubMed] [Google Scholar]
- (223).Yim EK; Pang SW; Leong KW Synthetic nanostructures inducing differentiation of human mesenchymal stem cells into neuronal lineage. Exp Cell Res 2007, 313 (9), 1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (224).Annabi N; Tsang K; Mithieux SM; Nikkhah M; Ameri A; Khademhosseini A; Weiss AS Highly Elastic Micropatterned Hydrogel for Engineering Functional Cardiac Tissue. Adv Funct Mater 2013, 23 (39). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (225).Tsang KM; Annabi N; Ercole F; Zhou K; Karst D; Li F; Haynes JM; Evans RA; Thissen H; Khademhosseini A et al. Facile One-step Micropatterning Using Photodegradable Methacrylated Gelatin Hydrogels for Improved Cardiomyocyte Organization and Alignment. Adv Funct Mater 2015, 25 (6), 977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (226).Kai D; Prabhakaran MP; Jin G; Ramakrishna S Guided orientation of cardiomyocytes on electrospun aligned nanofibers for cardiac tissue engineering. J Biomed Mater Res B Appl Biomater 2011, 98 (2), 379. [DOI] [PubMed] [Google Scholar]
- (227).Bian W; Jackman CP; Bursac N Controlling the structural and functional anisotropy of engineered cardiac tissues. Biofabrication 2014, 6 (2), 024109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (228).Bhuthalingam R; Lim PQ; Irvine SA; Agrawal A; Mhaisalkar PS; An J; Chua CK; Venkatraman S A novel 3D printing method for cell alignment and differentiation. International Journal of Bioprinting 2015, 1. [Google Scholar]
- (229).Li H; Wen F; Wong YS; Boey FY; Subbu VS; Leong DT; Ng KW; Ng GK; Tan LP Direct laser machining-induced topographic pattern promotes up-regulation of myogenic markers in human mesenchymal stem cells. Acta biomaterialia 2012, 8 (2), 531. [DOI] [PubMed] [Google Scholar]
- (230).Jiang X; Cao HQ; Shi LY; Ng SY; Stanton LW; Chew SY Nanofiber topography and sustained biochemical signaling enhance human mesenchymal stem cell neural commitment. Acta biomaterialia 2012, 8 (3), 1290. [DOI] [PubMed] [Google Scholar]
- (231).Irawan V; Higuchi A; Ikoma T Physical cues of biomaterials guide stem cell fate of differentiation: The effect of elasticity of cell culture biomaterials. Open Physics 2018, 16 (1), 943. [Google Scholar]
- (232).Lee JH; Park HK; Kim KS Intrinsic and extrinsic mechanical properties related to the differentiation of mesenchymal stem cells. Biochem Biophys Res Commun 2016, 473 (3), 752. [DOI] [PubMed] [Google Scholar]
- (233).Dang JM; Leong KW Myogenic Induction of Aligned Mesenchymal Stem Cell Sheets by Culture on Thermally Responsive Electrospun Nanofibers. Adv Mater 2007, 19 (19), 2775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (234).Jahani H; Jalilian FA; Wu CY; Kaviani S; Soleimani M; Abassi N; Ou KL; Hosseinkhani H Controlled surface morphology and hydrophilicity of polycaprolactone toward selective differentiation of mesenchymal stem cells to neural like cells. Journal of biomedical materials research. Part A 2015, 103 (5), 1875. [DOI] [PubMed] [Google Scholar]
- (235).Yang F; Murugan R; Wang S; Ramakrishna S Electrospinning of nano/micro scale poly(L-lactic acid) aligned fibers and their potential in neural tissue engineering. Biomaterials 2005, 26 (15), 2603. [DOI] [PubMed] [Google Scholar]
- (236).Lim SH; Liu XY; Song H; Yarema KJ; Mao HQ The effect of nanofiber-guided cell alignment on the preferential differentiation of neural stem cells. Biomaterials 2010, 31 (34), 9031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (237).Vining KH; Mooney DJ Mechanical forces direct stem cell behaviour in development and regeneration. Nat Rev Mol Cell Biol 2017, 18 (12), 728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (238).Ivanovska IL; Shin JW; Swift J; Discher DE Stem cell mechanobiology: diverse lessons from bone marrow. Trends Cell Biol 2015, 25 (9), 523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (239).Budday S; Sommer G; Birkl C; Langkammer C; Haybaeck J; Kohnert J; Bauer M; Paulsen F; Steinmann P; Kuhl E et al. Mechanical characterization of human brain tissue. Acta biomaterialia 2017, 48, 319. [DOI] [PubMed] [Google Scholar]
- (240).Hoenig E; Leicht U; Winkler T; Mielke G; Beck K; Peters F; Schilling AF; Morlock MM Mechanical properties of native and tissue-engineered cartilage depend on carrier permeability: a bioreactor study. Tissue engineering. Part A 2013, 19 (13-14), 1534. [DOI] [PubMed] [Google Scholar]
- (241).Chaudhuri O; Gu L; Klumpers D; Darnell M; Bencherif SA; Weaver JC; Huebsch N; Lee HP; Lippens E; Duda GN et al. Hydrogels with tunable stress relaxation regulate stem cell fate and activity. Nat Mater 2016, 15 (3), 326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (242).Engler AJ; Sen S; Sweeney HL; Discher DE Matrix elasticity directs stem cell lineage specification. Cell 2006, 126 (4), 677. [DOI] [PubMed] [Google Scholar]
- (243).Trappmann B; Gautrot JE; Connelly JT; Strange DG; Li Y; Oyen ML; Cohen Stuart MA; Boehm H; Li B; Vogel V et al. Extracellular-matrix tethering regulates stem-cell fate. Nat Mater 2012, 11 (7), 642. [DOI] [PubMed] [Google Scholar]
- (244).You J; Park SA; Shin DS; Patel D; Raghunathan VK; Kim M; Murphy CJ; Tae G; Revzin A Characterizing the effects of heparin gel stiffness on function of primary hepatocytes. Tissue engineering. Part A 2013, 19 (23-24), 2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (245).Marklein RA; Burdick JA Spatially controlled hydrogel mechanics to modulate stem cell interactions. Soft Matter 2010, 6 (1), 136. [Google Scholar]
- (246).Mittal N; Tasnim F; Yue C; Qu Y; Phan D; Choudhury Y; Tan M-H; Yu H Substrate stiffness modulates the maturation of human pluripotent stem-cell-derived hepatocytes. ACS Biomaterials Science & Engineering 2016, 2 (9), 1649. [DOI] [PubMed] [Google Scholar]
- (247).Nava A; Mazza E; Furrer M; Villiger P; Reinhart WH In vivo mechanical characterization of human liver. Med Image Anal 2008, 12 (2), 203. [DOI] [PubMed] [Google Scholar]
- (248).Huwart L; Peeters F; Sinkus R; Annet L; Salameh N; ter Beek LC; Horsmans Y; Van Beers BE Liver fibrosis: non-invasive assessment with MR elastography. NMR Biomed 2006, 19 (2), 173. [DOI] [PubMed] [Google Scholar]
- (249).Wang X; Ding Z; Wang C; Chen X; Xu H; Lu Q; Kaplan DL Bioactive Silk Hydrogels with Tunable Mechanical Properties. J Mater Chem B 2018, 6 (18), 2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (250).Hanjaya-Putra D; Yee J; Ceci D; Truitt R; Yee D; Gerecht S Vascular endothelial growth factor and substrate mechanics regulate in vitro tubulogenesis of endothelial progenitor cells. J Cell Mol Med 2010, 14 (10), 2436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (251).Alakpa EV; Jayawarna V; Lampel A; Burgess KV; West CC; Bakker SC; Roy S; Javid N; Fleming S; Lamprou DA Tunable supramolecular hydrogels for selection of lineage-guiding metabolites in stem cell cultures. Chem 2016, 1 (2), 298. [Google Scholar]
- (252).Huebsch N; Arany PR; Mao AS; Shvartsman D; Ali OA; Bencherif SA; Rivera-Feliciano J; Mooney DJ Harnessing traction-mediated manipulation of the cell/matrix interface to control stem-cell fate. Nat Mater 2010, 9 (6), 518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (253).Khetan S; Guvendiren M; Legant WR; Cohen DM; Chen CS; Burdick JA Degradation-mediated cellular traction directs stem cell fate in covalently crosslinked three-dimensional hydrogels. Nat Mater 2013, 12 (5), 458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (254).Gomillion CT; Burg KJ Stem cells and adipose tissue engineering. Biomaterials 2006, 27 (36), 6052. [DOI] [PubMed] [Google Scholar]
- (255).Dai R; Wang Z; Samanipour R; Koo KI; Kim K Adipose-Derived Stem Cells for Tissue Engineering and Regenerative Medicine Applications. Stem Cells Int 2016, 2016, 6737345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (256).Ninomiya Y; Sugahara-Yamashita Y; Nakachi Y; Tokuzawa Y; Okazaki Y; Nishiyama M Development of a rapid culture method to induce adipocyte differentiation of human bone marrow-derived mesenchymal stem cells. Biochem Biophys Res Commun 2010, 394 (2), 303. [DOI] [PubMed] [Google Scholar]
- (257).Scott MA; Nguyen VT; Levi B; James AW Current methods of adipogenic differentiation of mesenchymal stem cells. Stem Cells Dev 2011, 20 (10), 1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (258).Pati F; Jang J; Ha DH; Won Kim S; Rhie JW; Shim JH; Kim DH; Cho DW Printing three-dimensional tissue analogues with decellularized extracellular matrix bioink. Nat Commun 2014, 5, 3935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (259).Ong CS; Yesantharao P; Huang CY; Mattson G; Boktor J; Fukunishi T; Zhang H; Hibino N 3D bioprinting using stem cells. Pediatr Res 2018, 83 (1-2), 223. [DOI] [PubMed] [Google Scholar]
- (260).Yousefi AM; James PF; Akbarzadeh R; Subramanian A; Flavin C; Oudadesse H Prospect of Stem Cells in Bone Tissue Engineering: A Review. Stem Cells Int 2016, 2016, 6180487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (261).Black CR; Goriainov V; Gibbs D; Kanczler J; Tare RS; Oreffo RO Bone Tissue Engineering. Curr Mol Biol Rep 2015, 1 (3), 132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (262).Vinatier C; Guicheux J Cartilage tissue engineering: From biomaterials and stem cells to osteoarthritis treatments. Ann Phys Rehabil Med 2016, 59 (3), 139. [DOI] [PubMed] [Google Scholar]
- (263).Makris EA; Gomoll AH; Malizos KN; Hu JC; Athanasiou KA Repair and tissue engineering techniques for articular cartilage. Nat Rev Rheumatol 2015, 11 (1), 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (264).Chen G; Deng C; Li YP TGF-β and BMP signaling in osteoblast differentiation and bone formation. Int J Biol Sci 2012, 8 (2), 272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (265).Sekiya I; Colter DC; Prockop DJ BMP-6 enhances chondrogenesis in a subpopulation of human marrow stromal cells. Biochem Biophys Res Commun 2001, 284 (2), 411. [DOI] [PubMed] [Google Scholar]
- (266).Diekman BO; Estes BT; Guilak F The effects of BMP6 overexpression on adipose stem cell chondrogenesis: Interactions with dexamethasone and exogenous growth factors. Journal of biomedical materials research. Part A 2010, 93 (3), 994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (267).Awad HA; Wickham MQ; Leddy HA; Gimble JM; Guilak F Chondrogenic differentiation of adipose-derived adult stem cells in agarose, alginate, and gelatin scaffolds. Biomaterials 2004, 25 (16), 3211. [DOI] [PubMed] [Google Scholar]
- (268).Gruene M; Deiwick A; Koch L; Schlie S; Unger C; Hofmann N; Bernemann I; Glasmacher B; Chichkov B Laser printing of stem cells for biofabrication of scaffold-free autologous grafts. Tissue engineering. Part C, Methods 2011, 17 (1), 79. [DOI] [PubMed] [Google Scholar]
- (269).Du M; Chen B; Meng Q; Liu S; Zheng X; Zhang C; Wang H; Li H; Wang N; Dai J 3D bioprinting of BMSC-laden methacrylamide gelatin scaffolds with CBD-BMP2-collagen microfibers. Biofabrication 2015, 7 (4), 044104. [DOI] [PubMed] [Google Scholar]
- (270).Zhang Y; Xie RL; Croce CM; Stein JL; Lian JB; van Wijnen AJ; Stein GS A program of microRNAs controls osteogenic lineage progression by targeting transcription factor Runx2. Proc Natl Acad Sci U S A 2011, 108 (24), 9863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (271).Eskildsen T; Taipaleenmaki H; Stenvang J; Abdallah BM; Ditzel N; Nossent AY; Bak M; Kauppinen S; Kassem M MicroRNA-138 regulates osteogenic differentiation of human stromal (mesenchymal) stem cells in vivo. Proc Natl Acad Sci U S A 2011, 108 (15), 6139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (272).Tardif G; Hum D; Pelletier JP; Duval N; Martel-Pelletier J Regulation of the IGFBP-5 and MMP-13 genes by the microRNAs miR-140 and miR-27a in human osteoarthritic chondrocytes. BMC Musculoskelet Disord 2009, 10, 148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (273).Tuddenham L; Wheeler G; Ntounia-Fousara S; Waters J; Hajihosseini MK; Clark I; Dalmay T The cartilage specific microRNA-140 targets histone deacetylase 4 in mouse cells. FEBS Lett 2006, 580 (17), 4214. [DOI] [PubMed] [Google Scholar]
- (274).Pais H; Nicolas FE; Soond SM; Swingler TE; Clark IM; Chantry A; Moulton V; Dalmay T Analyzing mRNA expression identifies Smad3 as a microRNA-140 target regulated only at protein level. RNA 2010, 16 (3), 489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (275).Lin EA; Kong L; Bai XH; Luan Y; Liu CJ miR-199a, a bone morphogenic protein 2-responsive MicroRNA, regulates chondrogenesis via direct targeting to Smad1. J Biol Chem 2009, 284 (17), 11326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (276).Akhtar N; Rasheed Z; Ramamurthy S; Anbazhagan AN; Voss FR; Haqqi TM MicroRNA-27b regulates the expression of matrix metalloproteinase 13 in human osteoarthritis chondrocytes. Arthritis Rheum 2010, 62 (5), 1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (277).Ohgawara T; Kubota S; Kawaki H; Kondo S; Eguchi T; Kurio N; Aoyama E; Sasaki A; Takigawa M Regulation of chondrocytic phenotype by micro RNA 18a: involvement of Ccn2/Ctgf as a major target gene. FEBS Lett 2009, 583 (6), 1006. [DOI] [PubMed] [Google Scholar]
- (278).Kim D; Song J; Jin EJ MicroRNA-221 regulates chondrogenic differentiation through promoting proteosomal degradation of slug by targeting Mdm2. J Biol Chem 2010, 285 (35), 26900. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- (279).Dudek KA; Lafont JE; Martinez-Sanchez A; Murphy CL Type II collagen expression is regulated by tissue-specific miR-675 in human articular chondrocytes. J Biol Chem 2010, 285 (32), 24381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (280).Yan C; Wang Y; Shen XY; Yang G; Jian J; Wang HS; Chen GQ; Wu Q MicroRNA regulation associated chondrogenesis of mouse MSCs grown on polyhydroxyalkanoates. Biomaterials 2011, 32 (27), 6435. [DOI] [PubMed] [Google Scholar]
- (281).Gao G; Cui X Three-dimensional bioprinting in tissue engineering and regenerative medicine. Biotechnol Lett 2016, 38 (2), 203. [DOI] [PubMed] [Google Scholar]
- (282).Zhu W; Cui H; Boualam B; Masood F; Flynn E; Rao RD; Zhang ZY; Zhang LG 3D bioprinting mesenchymal stem cell-laden construct with core-shell nanospheres for cartilage tissue engineering. Nanotechnology 2018, 29 (18), 185101. [DOI] [PubMed] [Google Scholar]
- (283).Gao G; Zhang XF; Hubbell K; Cui X NR2F2 regulates chondrogenesis of human mesenchymal stem cells in bioprinted cartilage. Biotechnol Bioeng 2017, 114 (1), 208. [DOI] [PubMed] [Google Scholar]
- (284).Nguyen D; Hägg DA; Forsman A; Ekholm J; Nimkingratana P; Brantsing C; Kalogeropoulos T; Zaunz S; Concaro S; Brittberg M et al. Cartilage Tissue Engineering by the 3D Bioprinting of iPS Cells in a Nanocellulose/Alginate Bioink. Sci Rep 2017, 7 (1), 658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (285).Hasan A; Waters R; Roula B; Dana R; Yara S; Alexandre T; Paul A Engineered Biomaterials to Enhance Stem Cell-Based Cardiac Tissue Engineering and Therapy. Macromol Biosci 2016, 16 (7), 958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (286).Xu W; Zhang X; Qian H; Zhu W; Sun X; Hu J; Zhou H; Chen Y Mesenchymal stem cells from adult human bone marrow differentiate into a cardiomyocyte phenotype in vitro. Exp Biol Med (Maywood) 2004, 229 (7), 623. [DOI] [PubMed] [Google Scholar]
- (287).Gaetani R; Doevendans PA; Metz CH; Alblas J; Messina E; Giacomello A; Sluijter JP Cardiac tissue engineering using tissue printing technology and human cardiac progenitor cells. Biomaterials 2012, 33 (6), 1782. [DOI] [PubMed] [Google Scholar]
- (288).Gaetani R; Feyen DA; Verhage V; Slaats R; Messina E; Christman KL; Giacomello A; Doevendans PA; Sluijter JP Epicardial application of cardiac progenitor cells in a 3D-printed gelatin/hyaluronic acid patch preserves cardiac function after myocardial infarction. Biomaterials 2015, 61, 339. [DOI] [PubMed] [Google Scholar]
- (289).Young JL; Engler AJ Hydrogels with time-dependent material properties enhance cardiomyocyte differentiation in vitro. Biomaterials 2011, 32 (4), 1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (290).Cannon RO 3rd. Mechanisms, management and future directions for reperfusion injury after acute myocardial infarction. Nat Clin Pract Cardiovasc Med 2005, 2 (2), 88. [DOI] [PubMed] [Google Scholar]
- (291).Hullinger TG; Montgomery RL; Seto AG; Dickinson BA; Semus HM; Lynch JM; Dalby CM; Robinson K; Stack C; Latimer PA et al. Inhibition of miR-15 protects against cardiac ischemic injury. Circ Res 2012, 110 (1), 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (292).Prestwich GD Hyaluronic acid-based clinical biomaterials derived for cell and molecule delivery in regenerative medicine. Journal of controlled release : official journal of the Controlled Release Society 2011, 155 (2), 193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (293).Jang J; Kim TG; Kim BS; Kim SW; Kwon SM; Cho DW Tailoring mechanical properties of decellularized extracellular matrix bioink by vitamin B2-induced photo-crosslinking. Acta biomaterialia 2016, 33, 88. [DOI] [PubMed] [Google Scholar]
- (294).Bauwens CL; Peerani R; Niebruegge S; Woodhouse KA; Kumacheva E; Husain M; Zandstra PW Control of human embryonic stem cell colony and aggregate size heterogeneity influences differentiation trajectories. Stem Cells 2008, 26 (9), 2300. [DOI] [PubMed] [Google Scholar]
- (295).Lee SY; Kim HJ; Choi D Cell sources, liver support systems and liver tissue engineering: alternatives to liver transplantation. Int J Stem Cells 2015, 8 (1), 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (296).Faulkner-Jones A; Fyfe C; Cornelissen DJ; Gardner J; King J; Courtney A; Shu W Bioprinting of human pluripotent stem cells and their directed differentiation into hepatocyte-like cells for the generation of mini-livers in 3D. Biofabrication 2015, 7 (4), 044102. [DOI] [PubMed] [Google Scholar]
- (297).Tuleuova N; Lee JY; Lee J; Ramanculov E; Zern MA; Revzin A Using growth factor arrays and micropatterned co-cultures to induce hepatic differentiation of embryonic stem cells. Biomaterials 2010, 31 (35), 9221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (298).Ma X; Qu X; Zhu W; Li YS; Yuan S; Zhang H; Liu J; Wang P; Lai CS; Zanella F et al. Deterministically patterned biomimetic human iPSC-derived hepatic model via rapid 3D bioprinting. Proc Natl Acad Sci U S A 2016, 113 (8), 2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (299).Loebel C; Burdick JA Engineering Stem and Stromal Cell Therapies for Musculoskeletal Tissue Repair. Cell Stem Cell 2018, 22 (3), 325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (300).Deasy BM; Gharaibeh BM; Pollett JB; Jones MM; Lucas MA; Kanda Y; Huard J Long-term self-renewal of postnatal muscle-derived stem cells. Mol Biol Cell 2005, 16 (7), 3323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (301).Qu-Petersen Z; Deasy B; Jankowski R; Ikezawa M; Cummins J; Pruchnic R; Mytinger J; Cao B; Gates C; Wernig A et al. Identification of a novel population of muscle stem cells in mice: potential for muscle regeneration. J Cell Biol 2002, 157 (5), 851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (302).Ker ED; Nain AS; Weiss LE; Wang J; Suhan J; Amon CH; Campbell PG Bioprinting of growth factors onto aligned sub-micron fibrous scaffolds for simultaneous control of cell differentiation and alignment. Biomaterials 2011, 32 (32), 8097. [DOI] [PubMed] [Google Scholar]
- (303).Rao PK; Kumar RM; Farkhondeh M; Baskerville S; Lodish HF Myogenic factors that regulate expression of muscle-specific microRNAs. Proc Natl Acad Sci U S A 2006, 103 (23), 8721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (304).Rosenberg MI; Georges SA; Asawachaicharn A; Analau E; Tapscott SJ MyoD inhibits Fstl1 and Utrn expression by inducing transcription of miR-206. J Cell Biol 2006, 175 (1), 77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (305).Sokol NS; Ambros V Mesodermally expressed Drosophila microRNA-1 is regulated by Twist and is required in muscles during larval growth. Genes Dev 2005, 19 (19), 2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (306).Zhao Y; Samal E; Srivastava D Serum response factor regulates a muscle-specific microRNA that targets Hand2 during cardiogenesis. Nature 2005, 436 (7048), 214. [DOI] [PubMed] [Google Scholar]
- (307).Chen CZ; Li L; Lodish HF; Bartel DP MicroRNAs modulate hematopoietic lineage differentiation. Science 2004, 303 (5654), 83. [DOI] [PubMed] [Google Scholar]
- (308).Cui X; Gao G; Qiu Y Accelerated myotube formation using bioprinting technology for biosensor applications. Biotechnol Lett 2013, 35 (3), 315. [DOI] [PubMed] [Google Scholar]
- (309).Yu H; Tay CY; Pal M; Leong WS; Li H; Wen F; Leong DT; Tan LP A bio-inspired platform to modulate myogenic differentiation of human mesenchymal stem cells through focal adhesion regulation. Adv Healthc Mater 2013, 2 (3), 442. [DOI] [PubMed] [Google Scholar]
- (310).Knowlton S; Cho Y; Li XJ; Khademhosseini A; Tasoglu S Utilizing stem cells for three-dimensional neural tissue engineering. Biomater Sci 2016, 4 (5), 768. [DOI] [PubMed] [Google Scholar]
- (311).Langer HF; von der Ruhr JW; Daub K; Schoenberger T; Stellos K; May AE; Schnell H; Gauß A; Hafner R; Lang P et al. Capture of endothelial progenitor cells by a bispecific protein/monoclonal antibody molecule induces reendothelialization of vascular lesions. Journal of Molecular Medicine 2010, 88 (7), 687. [DOI] [PubMed] [Google Scholar]
- (312).Lin X; Shi Y; Cao Y; Liu W Recent progress in stem cell differentiation directed by material and mechanical cues. Biomed Mater 2016, 11 (1), 014109. [DOI] [PubMed] [Google Scholar]
- (313).Hsieh FY; Lin HH; Hsu SH 3D bioprinting of neural stem cell-laden thermoresponsive biodegradable polyurethane hydrogel and potential in central nervous system repair. Biomaterials 2015, 71, 48. [DOI] [PubMed] [Google Scholar]
- (314).Gu Q; Tomaskovic-Crook E; Wallace GG; Crook JM 3D Bioprinting Human Induced Pluripotent Stem Cell Constructs for In Situ Cell Proliferation and Successive Multilineage Differentiation. Adv Healthc Mater 2017, 6 (17). [DOI] [PubMed] [Google Scholar]
- (315).Xu T; Jin J; Gregory C; Hickman JJ; Boland T Inkjet printing of viable mammalian cells. Biomaterials 2005, 26 (1), 93. [DOI] [PubMed] [Google Scholar]
- (316).Xu T; Gregory CA; Molnar P; Cui X; Jalota S; Bhaduri SB; Boland T Viability and electrophysiology of neural cell structures generated by the inkjet printing method. Biomaterials 2006, 27 (19), 3580. [DOI] [PubMed] [Google Scholar]
- (317).Cui Y; Xiao Z; Chen T; Wei J; Chen L; Liu L; Chen B; Wang X; Li X; Dai J The miR-7 identified from collagen biomaterial-based three-dimensional cultured cells regulates neural stem cell differentiation. Stem Cells Dev 2014, 23 (4), 393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (318).Yu YM; Gibbs KM; Davila J; Campbell N; Sung S; Todorova TI; Otsuka S; Sabaawy HE; Hart RP; Schachner M MicroRNA miR-133b is essential for functional recovery after spinal cord injury in adult zebrafish. Eur J Neurosci 2011, 33 (9), 1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (319).Jeng SF; Rau CS; Liliang PC; Wu CJ; Lu TH; Chen YC; Lin CJ; Hsieh CH Profiling muscle-specific microRNA expression after peripheral denervation and reinnervation in a rat model. J Neurotrauma 2009, 26 (12), 2345. [DOI] [PubMed] [Google Scholar]
- (320).Jakab K; Norotte C; Marga F; Murphy K; Vunjak-Novakovic G; Forgacs G Tissue engineering by self-assembly and bio-printing of living cells. Biofabrication 2010, 2 (2), 022001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (321).Mattotti M; Alvarez Z; Ortega JA; Planell JA; Engel E; Alcántara S Inducing functional radial glia-like progenitors from cortical astrocyte cultures using micropatterned PMMA. Biomaterials 2012, 33 (6), 1759. [DOI] [PubMed] [Google Scholar]
- (322).Chua AW; Khoo YC; Tan BK; Tan KC; Foo CL; Chong SJ Skin tissue engineering advances in severe burns: review and therapeutic applications. Burns Trauma 2016, 4, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (323).Rutz AL; Hyland KE; Jakus AE; Burghardt WR; Shah RN A multimaterial bioink method for 3D printing tunable, cell-compatible hydrogels. Adv Mater 2015, 27 (9), 1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (324).Huang S; Yao B; Xie J; Fu X 3D bioprinted extracellular matrix mimics facilitate directed differentiation of epithelial progenitors for sweat gland regeneration. Acta biomaterialia 2016, 32, 170. [DOI] [PubMed] [Google Scholar]
- (325).Tricomi BJ; Dias AD; Corr DT Stem cell bioprinting for applications in regenerative medicine. Ann N Y Acad Sci 2016, 1383 (1), 115. [DOI] [PubMed] [Google Scholar]
- (326).Koch L; Kuhn S; Sorg H; Gruene M; Schlie S; Gaebel R; Polchow B; Reimers K; Stoelting S; Ma N et al. Laser printing of skin cells and human stem cells. Tissue engineering. Part C, Methods 2010, 16 (5), 847. [DOI] [PubMed] [Google Scholar]
- (327).Jessop ZM; Al-Sabah A; Gardiner MD; Combellack E; Hawkins K; Whitaker IS 3D bioprinting for reconstructive surgery: Principles, applications and challenges. J Plast Reconstr Aesthet Surg 2017, 70 (9), 1155. [DOI] [PubMed] [Google Scholar]
- (328).Hwang NS; Varghese S; Elisseeff J Controlled differentiation of stem cells. Adv Drug Deliv Rev 2008, 60 (2), 199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (329).Singh S; Choudhury D; Yu F; Mironov V; Naing MW In situ bioprinting - Bioprinting from benchside to bedside? Acta biomaterialia 2019, DOI: 10.1016/j.actbio.2019.08.045 10.1016/j.actbio.2019.08.045. [DOI] [PubMed] [Google Scholar]
- (330).Gaharwar AK; Arpanaei A; Andresen TL; Dolatshahi-Pirouz A 3D Biomaterial Microarrays for Regenerative Medicine: Current State-of-the-Art, Emerging Directions and Future Trends. Adv Mater 2016, 28 (4), 771. [DOI] [PubMed] [Google Scholar]
- (331).Adepu S; Dhiman N; Laha A; Sharma CS; Ramakrishna S; Khandelwal M Three-dimensional bioprinting for bone tissue regeneration. Current Opinion in Biomedical Engineering 2017, 2, 22. [Google Scholar]
- (332).Di Bella C; Fosang A; Donati DM; Wallace GG; Choong PF 3D Bioprinting of Cartilage for Orthopedic Surgeons: Reading between the Lines. Front Surg 2015, 2, 39. [DOI] [PMC free article] [PubMed] [Google Scholar]