Abstract
Our study aimed to assess the existing evidence on whether severe coronavirus disease 2019 (COVID-19) is associated with elevated inflammatory markers.
The PubMed, Embase, Web of Science, Scopus, Chinese National Knowledge Infrastructure, WanFang, and China Science and Technology Journal databases were searched to identify studies published between January 1 and April 21, 2020 that assayed inflammatory markers in COVID-19 patients. Three reviewers independently examined the literature, extracted relevant data, and assessed the risk of publication bias before including the meta-analysis studies.
Fifty-six studies involving 8719 COVID-19 patients were identified. Meta-analysis showed that patients with severe disease showed elevated levels of white blood cell count (WMD: 1.15, 95% CI: 0.78–1.52), C-reactive protein (WMD: 38.85, 95% CI: 31.19–46.52), procalcitonin (WMD: 0.08, 95% CI: 0.06–0.11), erythrocyte sedimentation rate (WMD: 10.15, 95% CI: 5.03–15.46), interleukin-6 (WMD: 23.87, 95% CI: 15.95–31.78), and interleukin-10 (WMD: 2.12, 95% CI: 1.97–2.28). Similarly, COVID-19 patients who died during follow-up showed significantly higher levels of white blood cell count (WMD: 4.11, 95% CI: 3.25–4.97), C-reactive protein (WMD: 74.18, 95% CI: 56.63–91.73), procalcitonin (WMD: 0.26, 95% CI: 0.11–0.42), erythrocyte sedimentation rate (WMD: 10.94, 95% CI: 4.79–17.09), and interleukin-6 (WMD: 59.88, 95% CI: 19.46–100.30) than survivors.
Severe COVID-19 is associated with higher levels of inflammatory markers than a mild disease, so tracking these markers may allow early identification or even prediction of disease progression.
Keywords: Coronavirus disease 2019, inflammatory marker, meta-analysis, severe disease
1. Introduction
The outbreak of coronavirus disease 2019 (COVID-19) in December 2019, caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), poses a severe threat to global public health. Data from the World Health Organization indicate that as of April 26, 2020, there were more than 2 million confirmed COVID-19 infections and nearly 200,000 COVID-19 deaths in 208 countries or territories.[1] The number of new cases continues to rise rapidly worldwide, which poses a significant challenge to public health.[1] While the disease is mild or even asymptomatic in most patients, and usually self-resolves without the need for hospitalization, it can rapidly and unpredictably progress to a severe form requiring hospitalization and intensive care.
Single-center studies suggest that numerous inflammation markers are elevated in patients in the intensive care unit[2] or patients with severe disease[3–5] relative to patients with milder conditions. These markers include leukocyte count, procalcitonin level (PCT), C-reactive protein (CRP), interleukin-6 (IL-6), and interleukin-10 (IL-10). A meta-analysis also suggested that patients with increased PCT are nearly 5-fold more likely to have severe infection.[6]
Although several studies have suggested that severe disease may be associated with elevated WBC count, CRP, PCT, and IL-6,[5,7–9] the results across these studies are not entirely consistent. So far, it is unclear whether inflammatory markers are significantly higher in patients with severe COVID-19 than in those with mild disease. Therefore, to gain a clearer picture of the potential association between inflammatory markers and severe COVID-19, we meta-analyzed the relevant literature. The results may provide a basis for detecting or even predicting disease progression quickly enough to improve prognosis.
2. Data and methods
2.1. Search strategy
According to the Preferred Reporting Items for Meta-Analyses of Observational Studies in Epidemiology Statement,[10] our meta-analysis was carried out. We selected relevant studies published between January 1, 2020 and April 21, 2020. The literature was systematically searched using 7 databases: PubMed, Embase, Web of Science, Scopus, Chinese National Knowledge Infrastructure, WanFang, and the China Science and Technology Journal Database. Only literature available online was included, and no language restriction was imposed. The following keywords were used, both separately and in combination, when searching each database: “Coronavirus,““2019-nCoV,” “COVID-19,” “SARS-CoV-2,” “IL-6,” “IL-8,” “IL-10,” “tumor necrosis factor-alpha (TNF-α),” “erythrocyte sedimentation rate (ESR),” “procalcitonin,” “C-reactive protein,” “ESR,” or “Laboratory finding.”
2.2. Study eligibility
A study was included in the meta-analysis if it had a cohort, case-control, or case series design involving more than 20 patients with confirmed COVID-19; if it contained patients with the mild, severe, or critical disease, or it contained survivor and nonsurvivor groups; and if it reported sufficient details about inflammatory markers. The diagnosis and severity classification was based on the New Coronavirus Pneumonia Prevention and Control Program in China or WHO interim guideline, and patients were grouped into different types such as mild, moderate, severe, and critical diseases. The mild and moderate diseases were defined as “mild,” while severe and critical patients were categorized as “severe” in this study. All analyses were based on previously published studies. Thus no ethical approval and patient consent are required.
2.3. Data extraction and quality assessment
Three reviewers independently screened the articles’ titles and abstract to assess whether they were eligible for inclusion. The following data were extracted from included studies into an Excel database: the first author's surname, the publication date of the article, study design, sample size, age, outcome indicators, and assessment of bias risk. A fourth reviewer resolved disagreements. When necessary, authors of the original studies were contacted to obtain further information or clarification.
The quality of included studies was assessed based on the Newcastle-Ottawa Scale guidelines.[11] Three reviewers assessed study quality, and differences were resolved through discussion. Studies scoring more than 6 out of the total possible 9 points were considered high quality.
2.4. Statistical analyses
For studies that reported continuous data as ranges or as medians and interquartile ranges, the means and standard deviation were calculated as described.[12] All meta-analyses were performed using STATA 12 (StataCorp, TX) and a significance definition of 2-tailed P < .05. We calculated the weighted mean difference (WMD) and 95% confidence interval (CI) for differences in continuous variables between patients with severe or mild COVID-19 and between all COVID-19 patients who survived or died follow-up. Heterogeneity between studies was analyzed using the χ2 test with significance set at P < .10 and was quantified using the I2 statistic. The fixed-effect model was utilized when there was no significant heterogeneity in the pooled data. Otherwise, a sensitivity analysis was used to identify the study or studies explaining most of the heterogeneity, then these studies were removed, and the remaining ones were meta-analyzed using a random-effects model. Publication bias was assessed using funnel plots, Egger regression asymmetry test, and Begg test.
3. Results
3.1. Literature screening and assessment
A total of 1417 records were identified from the various databases examined, and 35 additional records were identified from the Chinese Medical Journal Network. After a detailed assessment based on the inclusion criteria, 56 studies[2–5,8,9,13–62] involving 8719 COVID-19 patients were included in the meta-analysis (Fig. 1).
Figure 1.
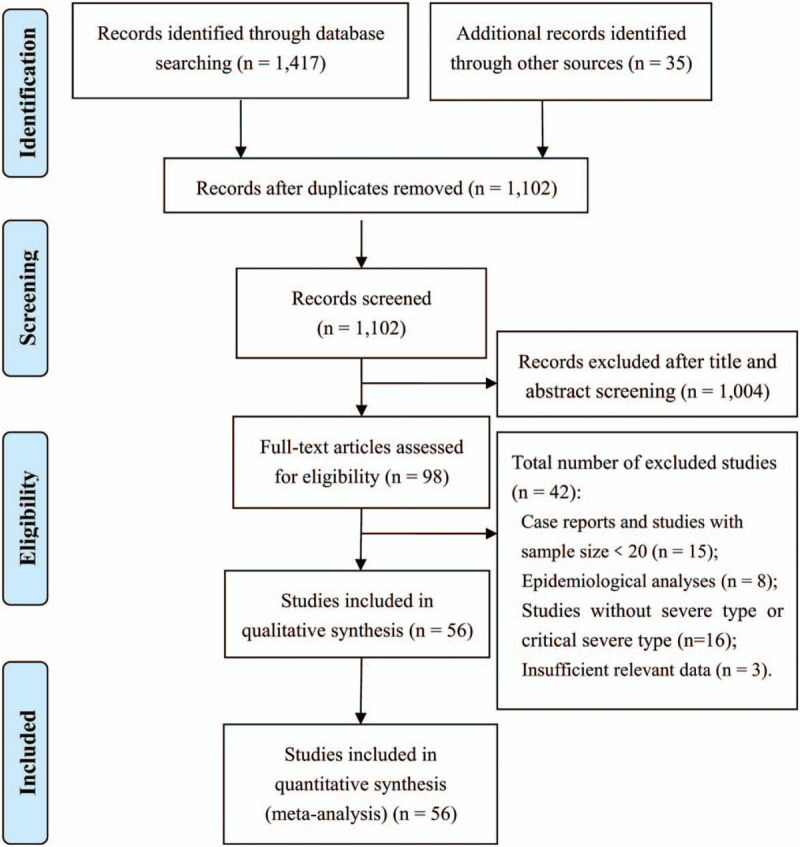
Flowchart of literature screening.
3.2. Characteristics of included studies
All studies included in the meta-analysis were conducted in China and published between February 6, 2020 and April 21, 2020. These retrospective studies examined Chinese patients distributed across 31 provinces. Follow-up data were reported for most patients. All studies received quality scores varying from 6 to 9 points, indicating high quality (Table 1).
Table 1.
Basic characteristics of included studies.
| First author | Publication date in 2020 | n | Single- or multicenter∗ | Patient population | Age†, yr | Follow-up | Diagnosis and severity criteria‡ | Outcomes§ | Quality score|| |
| Huang CL[2] | Feb 15 | 41 | Single | Mild and severe COVID-19 patients in Hubei Province | 49 (41–58) | Dec 2019 to Jan 2, 2020 | WHO interim guideline | ① | 7 |
| Wan SX[3] | Apr 16 | 123 | Single | Mild and severe COVID-19 patients in Chongqing Three Gorges Central Hospital | 43.1 ± 13.1/61.2 ± 15.6 | Jan 26 to Feb 4 | WHO interim guideline | ⑥⑦ | 8 |
| Qin C[4] | Mar 12 | 452 | Single | Mild and severe COVID-19 patients in Tongji Hospital Affiliated to Huazhong University of Science and Technology | 52.1 ± 15.1/60.3 ± 13.4 | Jan 10 to Feb 12 | Trial fifth Edition | ①②③④⑤⑥⑦ | 8 |
| Xiao KH[5] | Feb 27 | 143 | Single | Mild, severe, and critically ill COVID-19 patients in Chongqing Three Gorges Central Hospital | 45.1 ± 1.0 | Jan 23 to Feb 28 | Trial fifth Edition | ⑤ | 9 |
| Yuan J[8] | Mar 06 | 223 | Single | Mild and severe COVID-19 patients in Chongqing Public Medical Center | 46.5 ± 16 | Jan 24 to Feb 23 | Trial sixth Edition | ①③ | 9 |
| Fang XW[9] | Feb 25 | 79 | Single | Mild and severe COVID-19 patients in Anhui Provincial Hospital | 45 ± 16.6 | Jan 22 to Feb 18 | Trial sixth Edition | ①② | 6 |
| Wei WX[13] | Mar 18 | 95 | Single | Mild, severe, and critically ill COVID-19 patients in Chengdu Public Health Clinical Medical Center | 45.5 ± 58.6/56.7 ± 39.8/66.3 ± 28.2 | Jan 16 to Feb 18 | Trial fifth Edition | ①③ | 7 |
| Shi YL[14] | Feb 27 | 164 | Single | Mild, severe, and critically ill COVID-19 patients in Guangzhou Eighth Peoples Hospital | NR | Jan to Feb | Trial fifth Edition | ①③ | 8 |
| Liu et al[15] | Feb 28 | 79 | Multi | Mild and severe COVID-19 patients in 3 tertiary hospitals in Wuhan | 38 (33,57) | Dec 30 to Jan 15 | Trial fourth Edition | ①②③④ | 7 |
| Deng Y[16] | Mar 20 | 225 | Multi | Survival and non-survival COVID-19 patients in 2 tertiary hospitals in Wuhan | 43 ± 18/68 ± 9 | Jan 1 to Feb 21 | Survival and non-survival | ①② | 8 |
| Zhou F[17] | Mar 11 | 191 | Multi | Survival and nonsurvival COVID-19 patients in Wuhan Jinyintan Hospital and Wuhan Pulmonary Hospital | 56 (46–67) | Dec 2019 to Jan 31, 2020 | WHO interim guideline | ①③⑤ | 8 |
| Chen T[18] | Mar 26 | 274 | Single | Survival and non-survival COVID-19 patients in Tongji Hospital Affiliated to Huazhong University of Science and Technology | 62 (44–70) | Dec 2019 to Feb 28, 2020 | Survival and non-survival | ①②③④⑤ | 7 |
| Wang Y[19] | Apr 8 | 344 | Single | Survival and non-survival COVID-19 patients in Tongji Hospital Affiliated to Huazhong University of Science and Technology | 52–72 | Jan 25 to Feb 25 | WHO interim guideline | ①②③⑤ | 7 |
| Cheng KB[20] | Mar 12 | 463 | Single | Mild and severe COVID-19 patients in Wuhan Jinyintan Hospital | 15–90 | Dec 2019 to Feb 06, 2020 | Trial fifth Edition | ①②③④ | 7 |
| Wang D[21] | Feb 08 | 138 | Single | Mild and severe COVID-19 patients in Zhongnan Hospital of Wuhan University | 56 (42–68) | Jan 1 to Jan 28 | WHO interim guideline | ① | 7 |
| Liu M[22] | Feb 17 | 30 | Single | Mild and severe COVID-19 patients in Jianghan University Affiliated Hospital | 35 ± 8 | Jan 10 to Jan 31 | Trial fifth Edition | ① | 6 |
| Zhong SH[23] | Mar 26 | 62 | Single | Mild, severe, and critically ill COVID-19 patients in Hainan General Hospital | 51.8 ± 13.5 | Jan 21 to Feb 10 | Trial third Edition | ① | 6 |
| Guan W[24] | Feb 06 | 1099 | Multi | Mild and severe COVID-19 patients in 552 Hospitals in 31 provinces | 47.0 | NR | WHO interim guideline | ① | 9 |
| Qian GQ[25] | Mar 17 | 88 | Multi | Mild and severe COVID-19 patients in 5 hospitals in Zhejiang province | 50 (36.5–57) | Jan 20 to Feb 11 | WHO interim guideline | ①② | 9 |
| LI KH[26] | Feb 15 | 41 | Single | Mild and severe COVID-19 patients in Hubei Province | 49 (41–58) | Dec 2019 to Jan 2, 2020 | Trial fifth Edition | ①②③ | 7 |
| Wan SX[27] | Feb 29 | 83 | Single | Mild and severe COVID-19 patients in The Second Affiliated Hospital of Chongqing Medical University | 45.5 ± 12.3 | Jan to Feb | WHO interim guideline | ①②③ | 8 |
| Gao Y[28] | Mar 21 | 135 | Single | Mild and severe COVID-19 patients in Chongqing Three Gorges Central Hospital | 47 (36–55) | Jan 23 to Feb 8 | WHO interim guideline | ①②③⑤ | 6 |
| Zhang JJ[29] | Mar 17 | 43 | Single | Mild and severe COVID-19 patients in Fuyang Second People's Hospital | 45 ± 7.7/43 ± 14 | Jan 23 to Feb 2 | trail version 3–5 | ①②③⑧ | 7 |
| Chen W[30] | Mar 17 | 91 | Single | Mild, severe, and critically ill COVID-19 patients in Jingmen First People's Hospital | 41.6 ± 15.6 | Dec 2019 to Feb 21, 2020 | Trial seventh Edition | ① | 7 |
| Li D[31] | Mar 26 | 80 | Single | Mild and severe COVID-19 patients in tertiary hospitals in Zhuzhou | 47.8 ± 19.5 | Jan 20 to Feb 27 | Trial fifth Edition | ①②③④ | 7 |
| Li D[32] | Apr 2 | 62 | Single | Mild, severe, and critically ill COVID-19 patients in Renmin Hospital of Wuhan University | 49 ± 37/59 ± 31 | Jan 31 to Feb 25 | Trial sixth Edition | ①②③ | 6 |
| Xiong J[33] | Mar 03 | 89 | Single | Mild, severe, and critically ill COVID-19 patients in Renmin Hospital of Wuhan University | 53 ± 16.9 | Jan 17 to Feb 20 | Trial sixth Edition | ①②③ | 7 |
| Liu J[34] | Mar 27 | 91 | Single | Mild, severe, and critically ill COVID-19 patients in Wuhan Children's Hospital | NR | Jan 25 to Feb 18 | Trial fifth Edition | ①③ | 6 |
| Gao W[35] | Mar 31 | 90 | Single | Mild, severe, and critically ill COVID-19 patients in Beijing Youan Hospital | 51.7 ± 18.6 | Jan to Feb | Trial sixth Edition | ①②③ | 7 |
| Xie HS[36] | Apr 2 | 79 | Single | Mild and severe COVID-19 patients in Wuhan Jinyintan Hospital | 60 (48–66) | Feb 2 to Feb 23 | Trial sixth Edition | ①②④ | 7 |
| Zhang YF[37] | Apr 2 | 121 | Single | Mild and severe COVID-19 patients in Zhongnan Hospital of Wuhan University | 43.9 ± 15/65 ± 1 | Dec 2019 to Feb 22, 2020 | Trial fifth Edition | ① | 7 |
| Chen L[38] | Feb 6 | 29 | Single | Mild, severe, and critically ill COVID-19 patients in Tongji Hospital Affiliated to Huazhong University of Science and Technology | 26–79 | Jan | Trial fifth Edition | ①⑤⑥⑦ | 6 |
| Peng YD[39] | Mar 2 | 112 | Single | Mild and severe COVID-19 patients in western district of Union Hospital in Wuhan | 62 (5567) | Jan 20 to Feb 15 | Trial sixth Edition | ①③ | 7 |
| Chen M[40] | Feb 27 | 54 | Single | Mild, severe, and critically ill COVID-19 patients in Hubei No. 3 People's Hospital | 43.8–69 | Jan 24 to Feb 8 | Trial fifth Edition | ①②③④ | 6 |
| Wan Q[41] | Feb 24 | 153 | Single | Mild and severe COVID-19 patients in Chongqing Public Health Medical Center | 43.4 ± 15/57.7 ± 13 | Jan 26 to Feb 5 | Trial fifth Edition | ① | 8 |
| Li D[42] | Mar 5 | 30 | Single | Mild and severe COVID-19 patients in Shenyang sixth people's hospital | 21–72 | Jan 22 to Feb 8 | Trial fifth Edition | ① | 6 |
| Ling Y[43] | Mar 18 | 292 | Single | Mild and severe COVID-19 patients in Shanghai Public Health Clinical Center | 48.7 ± 16/65.5 ± 16 | Jan 20 to Feb 10 | Trial fifth Edition | ①②③④ | 9 |
| Bin YF[44] | Feb 29 | 55 | Single | Mild and severe COVID-19 patients in Huangpi District Chinese Medicine Hospital of Wuhan | 53.9 ± 17. 1 | Jan 29 to Feb 16 | Trial fifth Edition | ① | 6 |
| Xia XT[45] | Apr 7 | 63 | Single | Mild and severe COVID-19 patients in Hubei Provincial Hospital of Integrated Chinese and Western Medicine | 62.3 ± 15.1/64.6 ± 14.9 | Jan 26 to Feb 20 | Trial fifth Edition | ① | 6 |
| Chen C[46] | Mar 6 | 150 | Single | Mild and severe COVID-19 patients in Tongji Hospital Affiliated to Huazhong University of Science and Technology | 59 ± 16 | Jan to Feb | Trial sixth Edition | ① | 6 |
| Liu SJ[47] | Apr 2 | 342 | Single | Mild, severe, and critically ill COVID-19 patients in Ezhou Central Hospital | NR | Jan 23 to Feb 12 | Trial sixth Edition | ①②③④ | 7 |
| An W[48] | Apr 16 | 110 | Single | Survival and nonsurvival COVID-19 patients in Hubei No.3 People’ s Hospital of Jianghan University | NR | Jan 24 to Feb 19 | Current trail version | ①③④ | 7 |
| Feng Y[49] | Apr 10 | 476 | Multi | Mild, severe, and critically ill COVID-19 patients from 3 hospitals in Wuhan, Shanghai and Anhui | NR | Jan 1 to Feb 15 | Trial fifth Edition | ①②③④ | 8 |
| Cai QX[50] | Apr 2 | 298 | Single | Mild and severe COVID-19 patients in The Third People's Hospital of Shenzhen | 42.7 ± 18.7/61.5 ± 7.6 | Jan 11 to Feb 6 | WHO interim guideline | ①②③④⑤ | 6 |
| Zheng F[51] | Mar | 161 | Single | Mild and severe COVID-19 patients in Changsha First Hospital | 40.7 ± 15.0/56.5 ± 15.2 | Jan 17 to Feb 7 | Trial fifth Edition | ①② | 7 |
| Tang JS[52] | Apr 13 | 40 | Single | Mild, severe, and critically ill COVID-19 patients in The Ninth People's hospital of DongGuan | NR | Jan to Feb | Trial sixth Edition | ①③⑤ | 8 |
| Zheng YL[53] | Apr 10 | 99 | Single | Mild and severe COVID-19 patients in Chengdu Public Health Clinical Medical Center | 42.5 ± 15.1/63.8 ± 16.5 | Jan 16 to Feb 20 | Trial fifth Edition | ①② | 6 |
| Wang L[54] | Mar 30 | 339 | Single | Survival and non-survival COVID-19 patients in Renmin Hospital of Wuhan University | 68.7 ± 7.5/76.3 ± 9.9 | Jan 1 to Feb 6 | Trial sixth Edition | ①③⑤ | 7 |
| Ruan QR[55] | Apr 6 | 150 | Multi | Mild and severe COVID-19 patients in Wuhan Jinyintan Hospital and Tongji Hospital Affiliated to Huazhong University of Science and Technology | 58.3 ± 27.9/54.3 ± 50.0 | NR | Survival and nonsurvival | ①②⑤ | 8 |
| Tu WJ[56] | Apr 6 | 174 | Single | Survival and non-survival COVID-19 patients in Zhongnan Hospital of Wuhan University | 50.0 ± 18.7/71.3 ± 21.6 | Jan 3 to Feb 24 | Survival and non-survival | ①⑤ | 8 |
| Chen XH[57] | Apr 17 | 48 | Single | Mild and severe COVID-19 patients in General Hospital of Central Theater Command | 52.8 ± 14.2/63.7 ± 15.2/79.6 ± 12.6 | Jan 1 to Feb 19 | Trial sixth Edition | ①③⑤ | 9 |
| Ma J[58] | Apr 13 | 37 | Single | Mild and severe COVID-19 patients in Renmin Hospital of Wuhan University | 61.0 ± 4.9/66.5 ± 9.6 | Jan 1 to Mar 30 | Trial seventh Edition | ①⑤ | 7 |
| Wang ZL[59] | Mar 16 | 69 | Single | Mild and severe COVID-19 patients in western district of Union Hospital in Wuhan | 40.0 ± 14.5/69.8 ± 12.4 | Jan 16 to Jan 29 | Trial third Edition | ①②③④⑤⑥⑦ | 7 |
| Zou QX[60] | Apr 21 | 50 | Single | Mild, severe, and critically ill COVID-19 patients in Sino-French New City Campus of Tongji Hospital Affiliated to Huazhong University of Science and Technology | 62.3 ± 10.6/65.8 ± 11.1/72.4 ± 8.5 | Feb to Mar | Trial sixth Edition | ①⑦ | 6 |
| He XW[61] | Apr 21 | 56 | Single | Survival and non-survival COVID-19 patients in Sino-French New City Campus of Tongji Hospital Affiliated to Huazhong University of Science and Technology | 64.8 ± 12.3/69.7 ± 13.0 | Feb 3 to Feb 24 | Trial seventh Edition | ①④ | 8 |
| Zuo FT[62] | Apr 14 | 50 | Single | Mild and severe COVID-19 patients in Nanyang Central Hospital | 46.6 ± 16.3/53.7 ± 10.1 | Jan 19 to Mar 20 | Trial fifth Edition | ①②③ | 8 |
All studies were retrospective.
Reported as range, mean ± SD, or median (interquartile range).
Version of New Coronavirus Pneumonia Prevention and Control Program in China, or WHO interim guideline.
NR = not reported. ① WBC = white blood cell count, ② CRP = C-reactive protein, ③ PCT = procalcitonin, ④ ESR = erythrocyte sedimentation rate, ⑤ IL-6 = interleukin-6, ⑥ IL-10 = interleukin-10, ⑦ TNF-α = tumor necrosis factor-α.
Score based on the Newcastle-Ottawa Scale guidelines.[11]
3.3. Meta-analysis
3.3.1. Inflammatory markers
Pooled results revealed that patients with severe disease showed significantly higher (WMD: 1.15, 95% CI: 0.78–1.52), CRP (WMD: 38.85, 95% CI: 31.19–46.52), PCT (WMD: 0.08, 95% CI: 0.06–0.11), erythrocyte sedimentation rate (WMD: 10.15, 95% CI: 5.03–15.46), IL-6 (WMD: 23.87, 95% CI: 15.95–31.78), and IL-10 (WMD: 2.12, 95% CI: 1.97–2.28) (Figs. 2–5, Table 2). In contrast, the 2 groups showed similar TNF-α levels.
Figure 2.
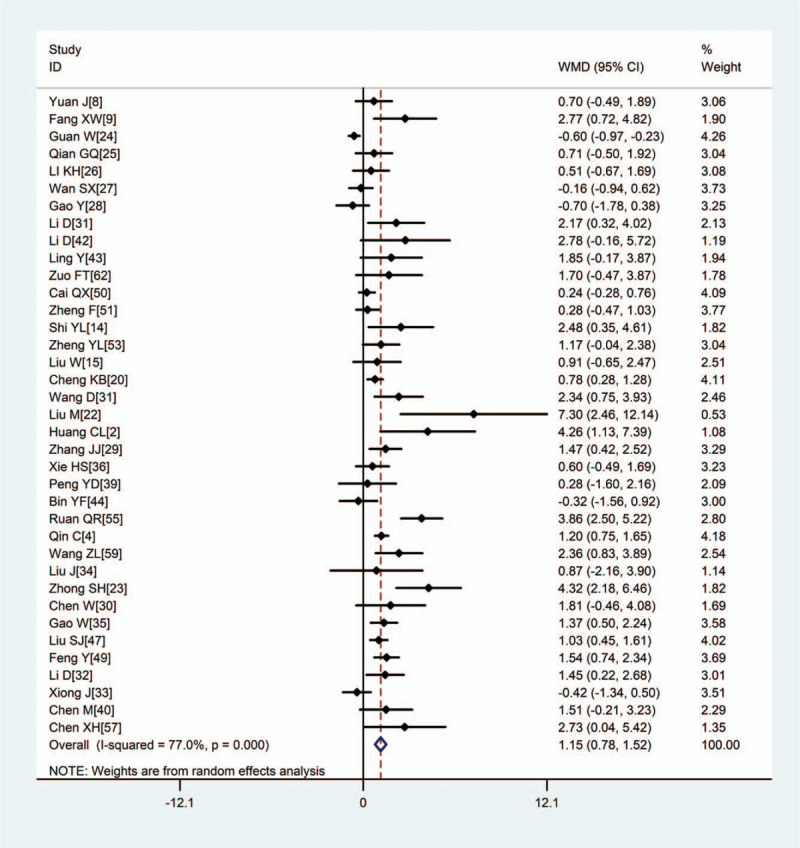
Meta-analysis of the difference in white blood cell count (×1012/L) between patients with mild or severe COVID-19. WMD = weighted mean difference, COVID-19 = coronavirus disease 2019.
Figure 5.
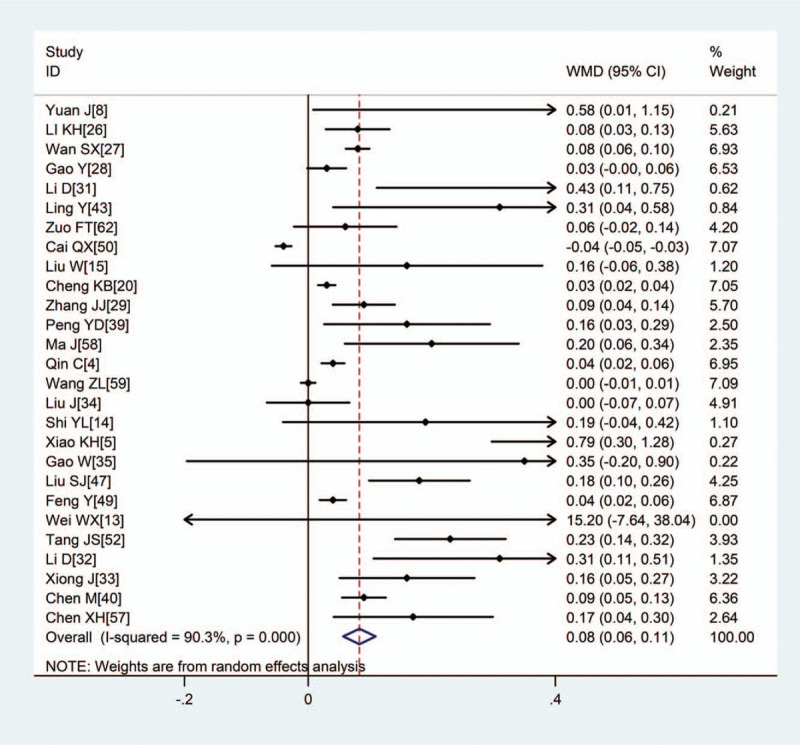
Meta-analysis of the difference in PCT (ng/mL) between COVID-19 patients with mild or severe disease. WMD = weighted mean difference. COVID-19 = coronavirus disease 2019, PCT = procalcitonin.
Table 2.
Meta-analysis of inflammatory marker levels in Chinese COVID-19 patients.
| Heterogeneity | Meta-analysis | ||||||
| Marker | No. studies | No. patients | P | I2 | Model∗ | WMD (95%CI) | P |
| Mild versus severe disease | |||||||
| WBC, × 10 9/L | 37 | 8973 | <.001 | 77.0% | R | 1.15 (0.78,1.52) | <.001 |
| CRP, mg/L | 34 | 4910 | <.001 | 93.0% | R | 38.85 (31.19,46.52) | <.001 |
| PCT, ng/mL | 27 | 4250 | <.001 | 90.3% | R | 0.08 (0.06,0.11) | <.001 |
| ESR, mm/h | 11 | 2684 | <.001 | 75.7% | R | 10.25 (5.03,15.46) | <.001 |
| IL-6, pg/mL | 11 | 1359 | <.001 | 93.1% | R | 23.87 (15.95,31.78) | <.001 |
| IL-10, pg/mL | 4 | 673 | .791 | 0.0% | F | 2.12 (1.97,2.28) | <.001 |
| TNF-α, pg/mL | 5 | 723 | <.001 | 91.1% | R | 0.20 (−0.60,1.01) | .622 |
| Nonsurvivors versus survivors | |||||||
| WBC, × 1012/L | 4 | 1034 | .057 | 60.1% | R | 4.11 (3.25,4.97) | <.001 |
| CRP, mg/L | 7 | 1522 | <.001 | 76.4% | R | 74.18 (56.63,91.73) | <.001 |
| PCT, ng/mL | 4 | 1067 | <.001 | 93.2% | R | 0.26 (0.11,0.42) | .001 |
| ESR, mm/h | 3 | 440 | .902 | 0.0% | F | 10.94 (4.79,17.09) | <.001 |
| IL-6, pg/mL | 5 | 1322 | <.001 | 98.0% | R | 59.88 (19.46,100.30) | .004 |
CI = confidence interval, CRP = C-reactive protein, ESR = erythrocyte sedimentation rate, IL-10 = interleukin-10, IL-6 = interleukin-6, PCT = procalcitonin, TNF-α = tumor necrosis factor-α, WBC = white blood cell count, WMD = weighted mean difference.
R means random model; F means fixed model.
Figure 3.
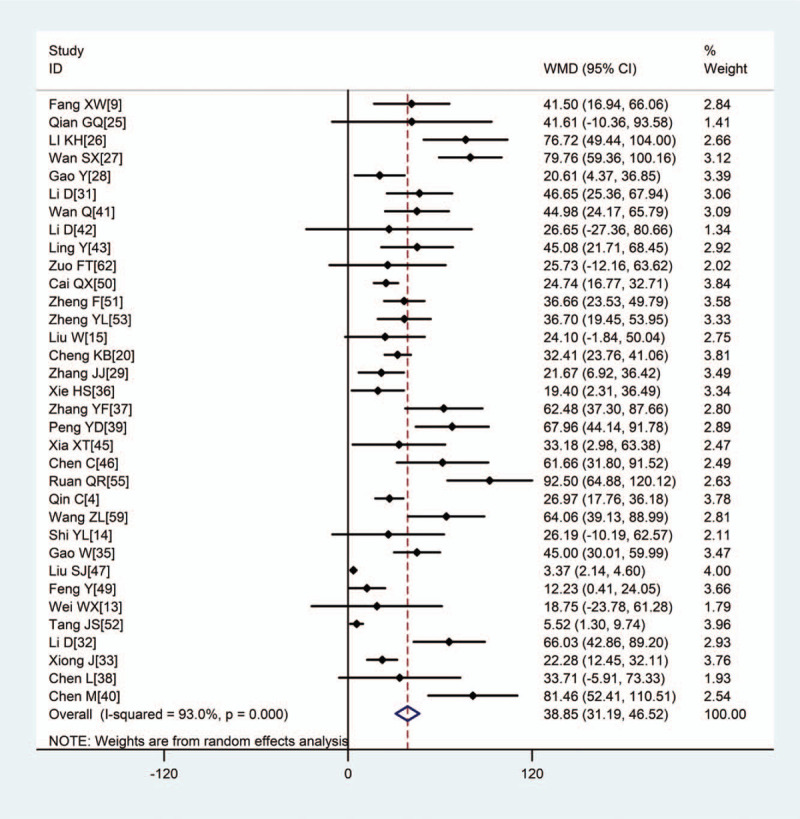
Meta-analysis of the difference in CRP (mg/L) between COVID-19 patients with mild or severe disease. WMD = weighted mean difference, COVID-19 = coronavirus disease 2019, CRP = C-reactive protein.
Figure 4.
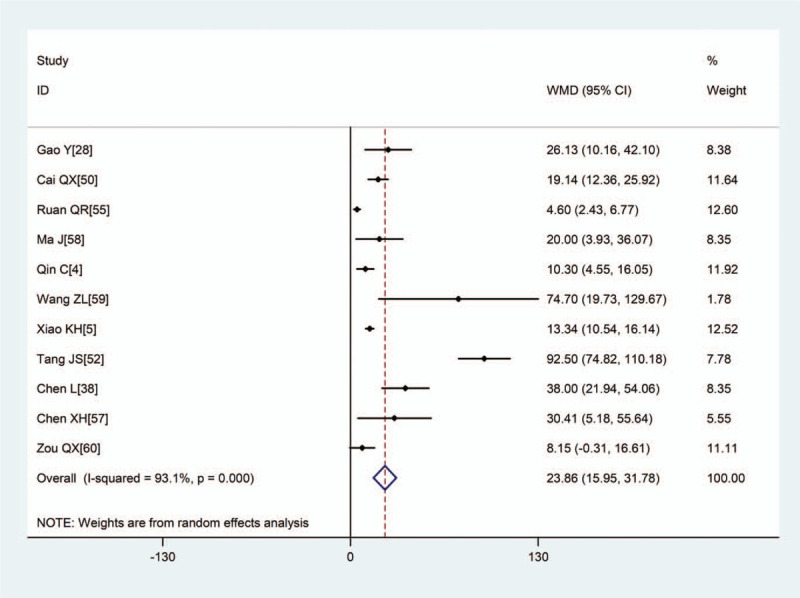
Meta-analysis of the difference in IL-6 (pg/mL) between COVID-19 patients with mild or severe disease. WMD = weighted mean difference, COVID-19 = coronavirus disease 2019, L-6 = interleukin-6.
Eight studies[16–19,48,54,56,61] comparing 543 COVID-19 patients who died during follow-up with 1713 who remained alive during the same period found that on admission, patients who subsequently died showed significantly higher white blood cell count (WMD: 4.11, 95% CI: 3.25–4.97), CRP (WMD: 74.18, 95% CI: 56.63–91.73), PCT (WMD: 0.26, 95% CI: 0.11–0.42), erythrocyte sedimentation rate (WMD: 10.94, 95% CI: 4.79–17.09), and IL-6 (WMD: 59.88, 95% CI: 19.46–100.30) (Table 2).
3.3.2. Sensitivity analysis
For most of the outcomes described in Section 3.3.1, there was heterogeneity in the pooled data, with I2 ranging from 60.1% to 98.5%. Therefore we repeated each meta-analysis after excluding 1 study at a time. We found that the results did not change substantially (Fig. 6), suggesting our original meta-analyses’ reliability.
Figure 6.
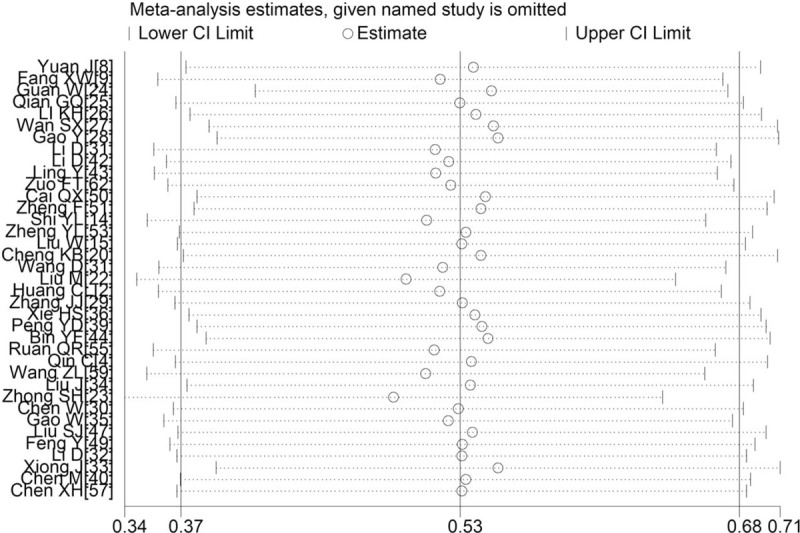
Sensitivity analysis of white blood cell count between patients with mild or severe COVID-19. COVID-19 = coronavirus disease 2019.
4. Discussion
Inflammation markers can appear elevated in infected individuals, including those infected with SARS-CoV-2.[54] Previous work suggested that the magnitude of the elevation WBC count, CRP, PCT, and IL-6 may relate to the severity of the resulting COVID-19.[17,59] The National Health Commission of the People's Republic of China included elevated inflammatory factors such as IL-6 and CRP as potential early warning indicators of severe disease in its widely used “COVID-19 diagnosis and treatment plan (for version 7).”[63] While these considerations imply that monitoring levels of inflammatory markers may help identify progression to severe disease, the literature has not been entirely consistent on which markers may be useful in this regard. For example, at least 2 studies found white blood cell count is similar or even lower in severe disease than in mild disease[23,44] in severe disease, yet other studies found the same marker to be higher in severe disease.[32,35] To help clarify the inflammatory markers whose elevation may signal severe COVID-19, we meta-analyzed the relevant literature from January 1, 2020, at the beginning of what would quickly become a global pandemic. Our analysis of 56 studies[2–5,8,9,13–62] involving 8719 patients with confirmed COVID-19 suggests that WBC, CRP, PCT, ESR, and IL-6 are significantly higher with mild disease, and higher in those who die during follow-up than in those who survive. It is also noteworthy that our results are also in keeping with those of previous studies,[6,64,65] but our study included larger sample size and the analyzed inflammation markers that are more comprehensive. However, there is no insufficient evidence that shows a ranking for inflammatory markers in terms of correlation with the severity of COVID-19. These findings justify the monitoring of inflammatory markers to detect COVID-19 progression as early as possible for timely intervention.
Our results are consistent with the idea that IL-6 levels positively correlate with COVID-19 severity,[38] with levels in critically ill patients exceeding those with the milder disease by up to 10 times.[38] Our results are also consistent with a positive correlation between IL-6 levels and the risk of mortality.[57] IL-6 has strong pro-inflammatory effects.[66–68] Increases in IL-6 also trigger increases in PCT, which may explain why both are significantly higher in severe COVID-19.[69] The reason for there is no significant difference in TNF-α levels between mild and severe groups is unclear, but it may be related to the inhibition of Th2 cells involved in humoral immunity in the early stage of infection[3] or the sample size is small, and the results are not representative.
The increase in inflammatory markers seen with severe COVID-19 is reminiscent of increases in similar markers during infection. For example, upon bacterial infection, PCT is released into the circulation, and elevated levels in peripheral blood correlate with infection severity.[5] Tan et al[70] found that CRP increased significantly in the initial stage of severe COVID-19 infection, while there was no significant difference in CT imaging between the severe group and the mild group. Research is needed to clarify to what extent the increases in inflammatory markers are caused directly by SARS-CoV-2 or reflect comorbidities such as hypertension, diabetes mellitus, and other chronic diseases that, like infectious diseases, trigger a chronic proinflammatory state. Patients with such comorbidities are more likely to develop severe COVID-19 than patients who are otherwise healthy,[71] at least partly because such conditions weaken the innate immune response, increasing the risk of SARS-CoV-2 infection.[70]
Our results suggest that monitoring inflammatory markers may serve as an early warning system for progression to severe COVID-19. Simultaneously, monitoring levels of IL-6, CRP, and PCT can allow early detection of bacterial infections, which may reduce overprescription of antibiotics for patients who do not need them and trigger early antibiotic therapy to prevent sepsis and other severe conditions.[72]
Although our meta-analysis rigorously analyzed data from a large sample of COVID-19 patients, our results are limited by the heterogeneity observed across studies, such as in the disease course and severity, reflecting the difficulties of standardizing methods during an emerging epidemic. We could not control for these and other potential confounders because all studies in our meta-analysis were retrospective. Due to the nature of reporting in the emerging outbreak, we did not perform a risk of bias assessment and presume it to be high across studies, which should be considered when interpreting results.
5. Conclusion
In summary, current evidence showed that higher levels of inflammatory markers such as WBC, CRP, PCT, ESR, IL-6, and IL-10 are associated with the severity of COVID-19 and thus could be used as significant prognostic factors of the disease.
Author contributions
Zhimei Zhong, Hongyuan Li, Jielong Pang, and Bocheng Li collected and analyzed the data. Jianfeng Zhang acquired the funding. Pan Ji and Jieyun Zhu designed the study and wrote the first draft of the manuscript. Jianfeng Zhang designed and supervised the study, and finalized the manuscript, which all authors read and approved.
Conceptualization: Pan Ji, Jielong Pang, Bocheng Li.
Data curation: Zhimei Zhong, Hongyuan Li, Jielong Pang.
Formal analysis: Pan Ji, Zhimei Zhong.
Funding acquisition: Jianfeng Zhang.
Investigation: Zhimei Zhong.
Methodology: Pan Ji, Jielong Pang, Bocheng Li, Jianfeng Zhang.
Resources: Jianfeng Zhang.
Software: Jieyun Zhu, Hongyuan Li, Bocheng Li.
Supervision: Jianfeng Zhang.
Validation: Jianfeng Zhang.
Writing – original draft: Pan Ji, Jieyun Zhu.
Writing – review & editing: Jianfeng Zhang.
Footnotes
Abbreviations: CI = confidence interval, COVID-19 = coronavirus disease 2019, CRP = C-reactive protein, ESR = erythrocyte sedimentation rate, IL-10 = interleukin-10, IL-6 = interleukin-6, = not reported, PCT = procalcitonin, TNF-α = tumor necrosis factor-α, WBC count = white blood cell count, WMD = weighted mean difference.
How to cite this article: Ji P, Zhu J, Zhong Z, Li H, Pang J, Li B, Zhang J. Association of elevated inflammatory markers and severe COVID-19: a meta-analysis. Medicine. 2020;99:47(e23315).
PJ and JZ contributed equally to this work.
This study was supported by grants from the National Natural Science Foundation of China (81960343), the Emergency Science and Technology Brainstorm Project for the Prevention and Control of COVID-19, which is part of the Guangxi Key Research and Development Plan (GuikeAB20058002), and the High-level Medical Expert Training Program of Guangxi “139” Plan Funding (G201903027).
The authors have no conflicts of interest to disclose.
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
References
- [1].WHO. Coronavirus disease 2019 (COVID-19) Situation Dashboard[Internet]. Available at: https://covid19.who.int/ [Accessed April 27, 2020]. [Google Scholar]
- [2].Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020;395:497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Wan S, Yi Q, Fan S, et al. Relationships among lymphocyte subsets, cytokines, and the pulmonary inflammation index in coronavirus (COVID-19) infected patients. Br J Haematol 2020;189:428–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Qin C, Zhou L, Hu Z, et al. Dysregulation of immune response in patients with COVID-19 in Wuhan, China. Clin Infect Dis 2020;ciaa248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Xiao KH, Shui LL, Pang XH, et al. Clinical features of coronavirus disease 2019 in Northeast area of Chongqing: analysis of 143 cases. J Third Mil Med Univ 2020;42:549–54. [Google Scholar]
- [6].Lippi G, Plebani M. Procalcitonin in patients with severe coronavirus disease 2019 (COVID-19): a meta-analysis. Clin Chim Acta 2020;505:190–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Ulhaq ZS, Soraya GV. Interleukin-6 as a potential biomarker of COVID-19 progression. Med Mal Infect 2020;50:382–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Yuan J, Sun YL, Zuo YJ, et al. Clinical characteristics of 223 novel coronavirus pneumonia cases in Chongqing. J Southwest Univ (Natural Science Ed) 2020;42:17–24. [Google Scholar]
- [9].Fang XW, Mei Q, Yang TJ, et al. Clinical characteristics and treatment analysis of 79 cases of COVID-19. Chin Pharmacol Bull 2020;36:453–9. [Google Scholar]
- [10].Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. JAMA 2000;283:2008–12. [DOI] [PubMed] [Google Scholar]
- [11].Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010;25:603–5. [DOI] [PubMed] [Google Scholar]
- [12].Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol 2005;5:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Wei WX, He XP, Ying BW, et al. The correlation between acute phase protein combined biochemical indicators and clinical classification of COVID-19. Int J Lab Med 2020;41:1602–7. [Google Scholar]
- [14].Shi YL, Qu JY, Chen X, et al. Expressions of multiple inflammation markers in the patients with 2019 novel coronavirus pneumonia and their clinical values. Chin J Lab Med 2020;43:E013–113. [Google Scholar]
- [15].Liu W, Tao ZW, Wang L, et al. Analysis of factors associated with disease outcomes in hospitalized patients with 2019 novel coronavirus disease. Chin Med J (Engl) 2020;133:1032–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Deng Y, Liu W, Liu K, et al. Clinical characteristics of fatal and recovered cases of coronavirus disease 2019 (COVID-19) in Wuhan, China: a retrospective study. Chin Med J (Engl) 2020;133:1261–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan China: a retrospective cohort study. Lancet 2020;395:1054–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Chen T, Wu D, Chen H, et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ 2020;368:m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Wang Y, Lu X, Chen H, et al. Clinical course and outcomes of 344 intensive care patients with COVID-19. Am J Respir Crit Care Med 2020;201:1430–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Cheng KB, Wei M, Shen H, et al. Clinical characteristics of 463 patients with common and severe type coronavirus disease 2019. Shanghai Med J 2020;1–5. [Google Scholar]
- [21].Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 Novel Coronavirus-infected pneumonia in Wuhan, China. JAMA 2020;323:1061–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Liu M, He P, Liu HG, et al. Clinical characteristics of 30 medical workers infected with new coronavirus pneumonia. Chin J Tuberculosis Respir Diseases 2020;209–14. [DOI] [PubMed] [Google Scholar]
- [23].Zhong SH, Lin F, Shi L. The clinical characteristics and outcome of 62 patients with COVID-19. Medical J Chin People's Liberation Army 2020;1–9. [Google Scholar]
- [24].Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of 2019 novel corona virus infection in China. N Engl J Med 2020;382:1708–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Qian GQ, Yang NB, Ding F, et al. Epidemiologic and clinical characteristics of 91 Hospitalized Patients with COVID-19 in Zhejiang, China: a retrospective, multi-centre case series. QJM 2020;hcaa089.doi:10.1093/qjmed/hcaa089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Li K, Wu J, Wu F, et al. The clinical and chest CT features associated with severe and critical COVID-19 pneumonia. Invest Radiol 2020;1–29. doi:10.1097/RLI.0000000000000672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Wan S, Xiang Y, Fang W, et al. Clinical features and treatment of COVID-19 patients in northeast Chongqing. J Med Virol 2020;92:797–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Gao Y, Li T, Han M, et al. Diagnostic utility of clinical laboratory data determinations for patients with the severe COVID-19. J Med Virol 2020;92:791–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Zhang JJ, Dong X, Cao YY, et al. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy 2020;75:730–41. [DOI] [PubMed] [Google Scholar]
- [30].Chen W, Xu L, Zhang Q, et al. Clinical characteristics of 91 novel coronavirus pneumonia patients in Jingmen First People’ s Hospital. J Inner Mongolia Medical Univ 2020;1–5. 10.16343/j.cnki.issn.2095-512x.20200317.001 [DOI] [Google Scholar]
- [31].Li D, Wang ML, He B, et al. Laboratory test analysis of sixty-two COVID-19 patients. Med J Wuhan Univ 2020;1–5. 10.14188/j.1671-8852.2020.0220 [DOI] [Google Scholar]
- [32].Li D, Long YZ, Huang P, et al. Clinical characteristics of 80 patients with COVID-19 in Zhuzhou City. Chinese J Infect Control 2020;19:227–33. [Google Scholar]
- [33].Xiong J, Jiang WL, Zhou Q, et al. Clinical characteristics, treatment, and prognosis in 89 cases of COVID-2019. Medical J Wuhan Univ (Health Sciences) 2020;1–5. 10.14188/j.1671-8852.2020.0103 [DOI] [Google Scholar]
- [34].Liu J, Luo WJ, Deng ZH, et al. Clinical and epidemiological characteristics of 91 children conformed with COVID-19. Chinese J Nosocomiol 2020;1–5. http://kns.cnki.net/kcms/detail/11.3456.R.20200326.1721.007.html [Google Scholar]
- [35].Gao W, Yang X, Zheng XQ, et al. Clinical characteristic of 90 patients with COVID-19 hospitalized in a Grade-A hospital in Beijing. Academic J Chin PLA Med School 2020;1–4. http://kns.cnki.net/kcms/detail/10.1117.R.20200330.1000.008.html [Google Scholar]
- [36].Xie H, Zhao J, Lian N, et al. Clinical characteristics of non-ICU hospitalized patients with coronavirus disease 2019 and liver injury: a retrospective study. Liver Int 2020;40:1321–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Zhang Y, Zheng L, Liu L, et al. Liver impairment in COVID-19 patients: a retrospective analysis of 115 cases from a single centre in Wuhan city, China. Liver Int 2020;40:2095–103. [DOI] [PubMed] [Google Scholar]
- [38].Chen L, Liu HG, Liu W, et al. Analysis of clinical features of 29 patients with 2019 novel coronavirus pneumonia. Chin J Tuberc Respir Dis 2020;43:203–8. [DOI] [PubMed] [Google Scholar]
- [39].Peng YD, Meng K, Guan HQ, et al. Clinical characteristics and outcomes of 112cardiovascular disease patients infected by 2019-nCoV. Chin J Cardiol 2020;48:E0004–14. [DOI] [PubMed] [Google Scholar]
- [40].Chen M, An W, Xia F, et al. Retrospective analysis of COVID-19 patients with different clinical subtypes. Herald Med 2020;39:459–64. [Google Scholar]
- [41].Wan Q, Shi AQ, He T, et al. Analysis of clinical features of 153 patients with novel coronavirus pneumonia in Chongqing. Chin J Clin Infect Dis 2020;16–20. [Google Scholar]
- [42].Li D, Liu HY, Wang Y, et al. Clinical features of 30 cases with novel coronavirus pneumonia. Chin J Infect Dis 2020;38:E018–118. [Google Scholar]
- [43].Ling Y, Lin YX, Qian ZP, et al. Clinical analysis of risk factors for severe patients with novel coronavirus pneumonia. Chinese J Infect Dis 2020;1–0. http://rs.yiigle.com/yufabiao/1185115.htm [Google Scholar]
- [44].Bin YF, Ji P, Liang XD, et al. Clinical characteristics of 55 hospitalized patients with COVID-19 in Wuhan. China. J Guangxi Medical Univ 2020;37:338–42. [Google Scholar]
- [45].Xia XT, Wen MY, Zhan SF, et al. An increased neutrophil/lymphocyte ratio is an early warning signal of severe COVID-19. J South Med Univ 2020;40:333–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Chen C, Chen T, Yan JT, et al. Analysis of myocardial injury in patients with COVID-19 and association between concomitant cardiovascular diseases and severity of COVID-19. Chinese J Cardiol 2020;48:567–71. [DOI] [PubMed] [Google Scholar]
- [47].Liu SJ, Cheng F, Yang XY, et al. A study of laboratory confirmed cases between laboratory indexes and clinical classification of 342 cases with Corona Virus Disease 2019 in Ezhou. Lab Med 2020;1–2. [Google Scholar]
- [48].An W, Xia F, Chen M, et al. Analysis of clinical features of 11 death cases caused by COVID-19. J Pract Med 2020;1–6. [Google Scholar]
- [49].Feng Y, Ling Y, Bai T, et al. COVID-19 with different severity: a multi-center study of clinical features. Am J Respir Crit Care Med 2020;201:1380–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Cai Q, Huang D, Ou P, et al. COVID-19 in a designated infectious diseases hospital outside Hubei Province, China. Allergy 2020;75:1742–52. [DOI] [PubMed] [Google Scholar]
- [51].Zheng F, Tang W, Li H, et al. Clinical characteristics of 161 cases of corona virus disease 2019 (COVID-19) in Changsha. Eur Rev Med Pharmacol Sci 2020;24:3404–10. [DOI] [PubMed] [Google Scholar]
- [52].Tang JS, Xuan C, Lin JT, et al. Clinical significance of detecting c-reactive protein, interleukin-6 and procalcitonin in COVID-19. J Pract Med 2020;36:839–41. [Google Scholar]
- [53].Zheng Y, Xu H, Yang M, et al. Epidemiological characteristics and clinical features of 32 critical and 67 noncritical cases of COVID-19 in Chengdu. J Clin Virol 2020;127:104366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Wang L, He W, Yu X, et al. Coronavirus disease 2019 in elderly patients: characteristics and prognostic factors based on 4-week follow-up. J Infect 2020;80:639–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Ruan Q, Yang K, Wang W, et al. Correction to: clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med 2020;1–4. doi:10.1007/s00134-020-06028-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Tu WJ, Cao J, Yu L, et al. Clinicolaboratory study of 25 fatal cases of COVID-19 in Wuhan. Intensive Care Med 2020;46:1117–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Chen X, Zhao B, Qu Y, et al. Detectable serum SARS-CoV-2 viral load (RNAaemia) is closely correlated with drastically elevated interleukin 6 (IL-6) level in critically ill COVID-19 patients. Clin Infect Dis 2020;ciaa449.doi:10.1093/cid/ciaa449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Ma J, Yin J, Qian Y, et al. Clinical characteristics and prognosis in cancer patients with COVID-19: a single center's retrospective study. J Infect 2020;81:318–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Wang Z, Yang B, Li Q, et al. Clinical features of 69 cases with Coronavirus Disease 2019 in Wuhan, China. Clin Infect Dis 2020;ciaa272.doi:10.1093/cid/ciaa272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Zou QX, Lin FW, Wang Y, et al. Changes and clinical significance of serum IL-2R, IL-6 and TNF-α in elderly patients with COVID-19. J Changchun Univ Chinese Med 2020;1–3. http://kns.cnki.net/kcms/detail/22.1375.R.20200420.1333.002.html [Google Scholar]
- [61].He XW, Lai JS, Cheng J, et al. Impact of complicated myocardial injury on the clinical outcome of severe or critically ill COVID-19 patients. Chin J Cardiol 2020;1–9. http://rs.yiigle.com/yufabiao/1184840.htm [DOI] [PubMed] [Google Scholar]
- [62].Zuo FT, Li CL, Dong ZG, et al. Analysis of thecorrelation between clinical characteristics and disease severity in patients with novel coronavirus pneumonia. Tianjin Med J 2020;5:455–60. [Google Scholar]
- [63].National Health and Health Commission of the people's Republic of China. Diagnosis and Treatment of Pneumonia of New Coronavirus Infection (Trial Version 7). Available at: http://www.nhc.gov.cn/yzygj/s7653p/202003/46c9294a7dfe4cef80dc7f5912eb1989.shtml. Accessed March 3, 2020. [Google Scholar]
- [64].Aziz M, Fatima R, Assaly R. Elevated interleukin-6 and severe COVID-19: a meta-analysis. J Med Virol 2020;Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Zeng F, Huang Y, Guo Y, et al. Association of inflammatory markers with the severity of COVID-19: a meta-analysis. Int J Infect Dis 2020;96:467–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Baran P, Hansen S, Waetzig GH, et al. The balance of interleukin (IL)-6, IL-6 soluble IL-6 receptor (sIL-6R), and IL-6 sIL-6R sgp130 complexes allows simultaneous classic and trans-signaling. J Biol Chem 2018;293:6762–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Taniguchi K, Karin M. IL-6 and related cytokines as the critical lynchpins between inflammation and cancer. Semin Immunol 2014;26:54–74. [DOI] [PubMed] [Google Scholar]
- [68].Lai HS, Lin WH, Lai SL, et al. Interleukin-6 mediates angiotensinogen gene expression during liver regeneration. PLoS One 2013;8:e67868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Fraunberger P, Wang Y, Holler E, et al. Prognostic value of interleukin 6, procalcitonin, and C-reactive protein levels in intensive care unit patients during first increase of fever. Shock 2006;26:10–2. [DOI] [PubMed] [Google Scholar]
- [70].Tan C, Huang Y, Shi F, et al. C-reactive protein correlates with computed tomographic findings and predicts severe COVID-19 early. J Med Virol 2020;92:856–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Yang J, Zheng Y, Gou X, et al. Prevalence of comorbidities and its effects in coronavirus disease 2019 patients: a systematic review and meta-analysis. Int J Infect Dis 2020;94:91–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Zhang C, Wu Z, Li JW, et al. The cytokine release syndrome (CRS) of severe COVID-19 and Interleukin-6 receptor (IL-6R) antagonist Tocilizumab may be the key to reduce the mortality. Int J Antimicrob Agents 2020;105954. [DOI] [PMC free article] [PubMed] [Google Scholar]


