Abstract
There is uncertainty regarding the potential virologic outcome associated with a change in antiretroviral therapy (ARV) among PLHIV who had previous documented virologic failure or who have been exposure to mono/dual nucleoside reverse transcriptase inhibitors (NRTI) therapy. The objective was to measure the potential impact of exposure to previous virologic failure or mono/dual NRTI regimen on virologic outcome of PLHIV following a switch to dolutegravir with 2 NRTIs from a viremia suppressive ARV therapy.
Data from the Quebec HIV Cohort including 10219 PLHIV were collected through routine clinical care at 4 clinical sites in Montreal, Canada. This study includes patients whose ARV therapy was switched to dolutegravir with 2 NRTIs since 2013 with undetectable viral load for ≥6 months before switch. The association between exposure and post-switch virologic outcome was measured by marginal hazard ratio estimated using the Inverse probability weighting Cox model.
Among the 1199 eligible PLHIV, 478 (39.9%) previously experienced at least one virologic failure or were exposed to mono/dual therapy before dolutegravir switch. Post-switch virologic failure after 30 months occurred in 4.1% (95% CI 2.1–7.9) of exposed compared to 4.1% (95% CI 2.3–7.4) in unexposed participants. The adjusted hazard ratio for the association between exposure and post-switch virologic failure was 0.84 (95% CI 0.35–2.01).
Our findings suggest that switch to dolutegravir with 2 NRTIs from a suppressive therapy is a safe option for PLHIV with documented virologic failure and/or previous exposure to mono/dual NRTI therapy.
Keywords: Human immunodeficiency virus, antiretroviral therapy, switch, dolutegravir, previous virologic failure, exposure to mono/dual NRTI therapy, virologic outcome
1. Introduction
Antiretroviral (ARV) regimens are used to reduce plasma Human Immunodeficiency Virus (HIV) RNA levels to below the level of detection in people living with HIV (PLHIV). The majority of ARV regimens include a combination of 2 nucleoside reverse transcriptase inhibitors (NRTIs) and a third agent. Integrase Strand Transfer inhibitors (INSTIs) acting by blocking the integration of HIV DNA into the lymphocyte genome[1,2] such as raltegravir, elvitegravir, bictegravir and dolutegravir, are now recommended as first line 3rd agent of ARV regimens by treatment guidelines[3,4] due to their recognised therapeutic efficacy, safety and immune restoration capacity.[5,6] These newer agents can also be used in experienced patients in context of virologic failures, or in patients with a suppressed viral load (VL) who would derive a benefit from the switch through reduction of pill burden, drug-drug interaction, or adverse effects. However, caution should be used before switching any patients with a suppressed viral load (VL), to a new ARV regimen when the existence of prior resistance mutation is documented or suspected.[4]
Evidence from several clinical trials has demonstrated that patients with previous virologic failures experience greater rates of post-switch virologic failures when switching to new regimens with third agents such as efavirenz, nevirapine,[7,8] or raltegravir as it has been shown in the SWITCHMRK trials.[9] Following these studies, patients with prior virologic failures, documented or suspected resistance mutations or previous exposure to mono/dual NRTI therapies have been excluded from switch studies.[10–13] Moreover, The US Department of Health and Human Services (DHHS)[4] guidelines recommend that if there is uncertainty about prior resistance, it is generally not advisable to replace a viremia suppressive ARV regimen unless the new regimen is likely to be at least as active against a potential resistant virus as the suppressive regimen. The European AIDS Clinical Society (EACS)[3] guidelines recommend that a ritonavir-boosted protease inhibitor (PI/r) regimen may be switched to an INSTI only if full activity of the 2 NRTIs remaining in the regimen can be guaranteed. Due to the higher genetic barrier of dolutegravir it is currently unclear however, if a switch to dolutegravir-based regimens also requires full activity of 2 NRTIs in the combination. Data from the DAWNING[14] and SAILING[15] trials indicate that dolutegravir with 2 NRTIs is superior to a PI/r or a raltegravir based regimen. A few recent studies also suggest the efficacy of dolutegravir in patients with documented resistance mutations.[18–20] Therefore, our objective was to measure the impact of previous virologic failure or exposure to mono/dual NRTI (suboptimal) therapy on virologic outcomes in an observational cohort of virologically suppressed PLHIV switched to dolutegravir with 2 NRTIs.
2. Methods
The Quebec HIV Cohort is an observational cohort including PLHIV followed in 4 sites specialized in HIV care in Montreal: 2 community clinics “Clinique médicale l’Actuel (CMA)” and “Clinique de médecine urbaine du Quartier Latin (CMUQL)” and 2 hospital clinics: “Centre hospitalier de l’Université de Montréal (CHUM)” and “McGill University Health Center (MUHC)”. Clinical HIV cohorts were compiled at each site using data collected prospectively from patient charts during clinical visits, which were generally scheduled every 3 to 4 months. Data collection began in 1985 at CMA, in 1997 at CMUQL and in 1989 at both CHUM and MUHC. In the present study, we use data collected up to August 2017. Databases from each site were then de-identified and merged to form the Quebec HIV Cohort database using an encrypted identifier to link data for patients followed in multiple sites. Data merged into the central database were first analyzed to identify outliers, missing data, and inconsistent information between sites for patients who visited more than 1 site. Completeness and correction of final database was performed after a second round of data collection/verification at each site. The following variables were collected: site, clinical visit date, HIV diagnosis date, risk factors for HIV acquisition, lifetime history of ARV prescriptions and ARV start/end dates, reason for ARV discontinuation, demographic variables (age, sex), resistance mutations and laboratory results including: CD4 count, viral load (VL), hepatitis C antibodies (anti-HCV), and hepatitis B surface antigen (HBsAg).
The Quebec HIV Cohort included 10 219 PLHIV of whom 5 844 were engaged in care as of August 31, 2017. The median age (interquartile range (IQR)) at cohort entry was 36.4 years (30.1–43.8) and males represented 84.1% of the patients. For our study, we included all PLHIV in the cohort whose regimen has been switched to dolutegravir with 2 NRTIs after January 2013 and who had an undetectable VL (<50 copies/ml) for at least 6 months before switch. Figure 1 depicts the patient selection process from the cohort. The total number of eligible patients was 1199 after the exclusion of 10 patients with missing information on previous ARV). Patients included could have come from any type of virologically suppressive regimen despite any previous documented mutations and were all naïve to dolutegravir.
Figure 1.
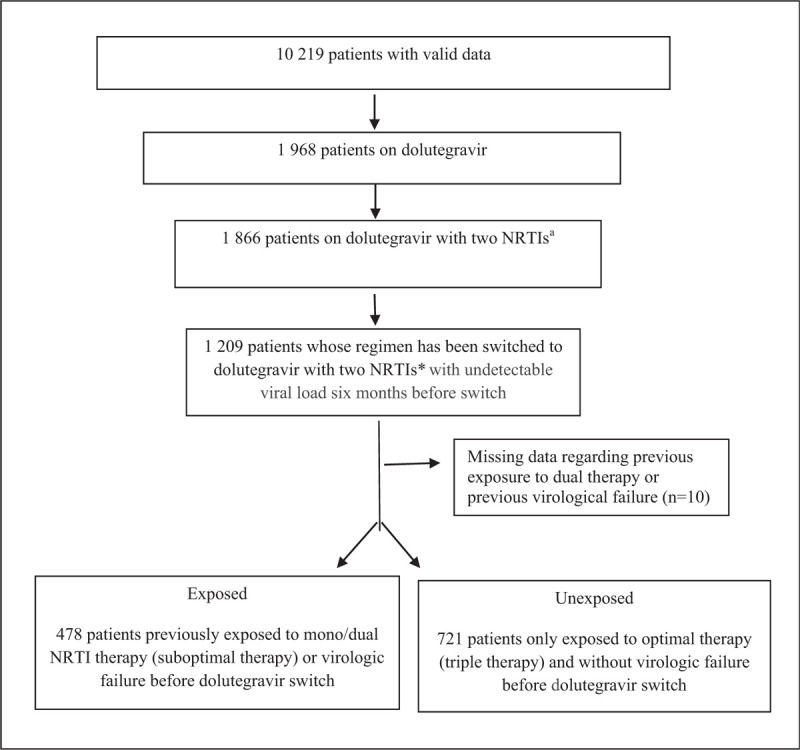
aTwo NRTIs = abacavir + lamivudine or tenofovir disoproxil + emtricitabine or tenofovir disoproxil + lamivudine NRTIs = nucleoside reverse-transcriptase inhibitors.
Ethical approval for the development of The Quebec HIV Cohort and for this study protocol was received from the Research Ethics Boards (REBs) of the MUHC, CHUM and the Sainte Justine University Hospital Center. Patient consent was waived as all data were collected from medical charts.
3. Statistical analysis
Descriptive analyses were performed using proportions (and 95% confidence intervals (CI)) for categorical variables and mean (standard deviation (SD)) and median (interquartile range (IQR)) for continuous variables. We conducted a time-to-event analysis to measure post-switch virologic outcomes. Follow-up time was measured from the dolutegravir switch date (index date), up to the earliest date of virologic failure or censored at the most recent clinical visit with a VL measure. In the event of dolutegravir treatment interruption, follow-up time was censored at the clinical visit date when dolutegravir was discontinued. Kaplan–Meier (KM) method with log-rank test was used to estimate and compare the cumulative incidence of post-switch virologic outcomes among exposed and unexposed groups.
The primary exposure analyzed in this study was a composite of previous virologic failure and/or history of mono/dual NRTI regimens. We defined documented pre-switch virologic failure as[16]:
-
1.
VL > 1000 copies/ml after 16 weeks of therapy or a VL > 400 copies/ml after 24 weeks of therapy or 2 consecutive VL tests > 50 copies/ml in the 48 weeks following first-line ARV initiation (naïve patient), or
-
2.
VL > 50 copies/ml at the time of a prior regimen change, or
-
3.
2 consecutive VL > 50 copies/ml after an undetectable VL.
We defined exposure to mono/dual NRTI therapy as any exposure to monotherapy or dual NRTI therapy for at least 1 month before dolutegravir switch. The primary outcome (post-switch virologic failure) was defined as the presence of 2 consecutive VL > 50 copies/ml during the follow-up or a VL > 50 copies/ml at the last documented visit.
Cox regression modelling was used to estimate hazard ratios (HR) with 95% confidence intervals (CIs) for the association between exposure and virologic outcome. Both exposures (exposure to mono/dual NRTI therapy and previous virologic failure) were considered together in a single variable and a sensitivity analysis was performed in order to analyze both exposures individually. Adjusted Hazard Ratios (aHR) were estimated using 2 methods:
-
1.
using the change-in-estimates method and
-
2.
using inverse probability of treatment weighting (IPTW).[17]
For both adjustment methods, the list of following potential confounders (measured at dolutegravir switch) were considered: ARV treatment duration (continuous), time since HIV diagnosis (continuous), history of hepatitis B (HBsAg positive/negative) or hepatitis C (anti HCV antibodies positive/negative), CD4 count (continuous), number of treatment discontinuations (continuous), reasons for discontinuation of the previous treatment and switch to dolutegravir (side effects/intolerance, simplification or others reasons (insurance coverage, treatment interruption, clinical trial participation)), age (continuous), sex (male/female) and sites (CMA, CMUQL, CHUM, or MUHC). The change-in-estimates method was applied using a 5% cut-off. Inverse probability weights were estimated from a non-parsimonious multivariable logistic regression model in which virologic failure was modelled as the dependent variable, and all previously listed covariates were included. Interactions between continuous variables and higher-order moments displaying standardized differences >10% between exposed and unexposed were also added. Using this approach, an absolute standardized difference <10% was obtained for all covariates in the final weighted sample (Supplemental Digital Content (Figure S1)). Marginal HR (and 95% CI) were then estimated using weights into the Cox model. Stata version 14 software (Stata Corp, College Station, Texas, USA) was used for all statistical analyses.
4. Results
In our study, 1199 eligible PLHIV (whose regimen has been switched to dolutegravir with 2 NRTIs after undetectable VL (<50 copies/ml) for at least 6 months) have been included. Among these patients, 478 (39.9%) had previously been exposed to mono/dual NRTI therapy and/or had documented history of virologic failure (171 exposed to mono/dual NRTI therapy and 437 to pre-switch virologic failure) whereas 721 (60.1%) where unexposed (only had triple therapy without any documented virologic failures). In addition to dolutegravir, 947 (79.0%) received abacavir/lamivudine, 246 (20.5%) tenofovir disoproxil/emtricitabine and 6 (0.5%) tenofovir disoproxil/lamivudine as the NRTI backbone regimen. The mean (SD) post-switch follow-up time was 1.5 (0.9) year. Table 1 describes the characteristics of patients in both groups at switch. The mean age at dolutegravir switch was 53.2 (SD 9.3) years in the group exposed to previous virologic failure or mono/dual NRTI therapy and 48.6 (SD 11.2) years in the unexposed group. The mean ARV treatment duration at switch was 15.2 (SD 5.5) years and 8.1 (SD 5.2) years respectively for exposed and non-exposed patients. The reasons for treatment discontinuation and switch to dolutegravir included simplification (49.0 and 49.6% for exposed and unexposed group, respectively) and side effects or intolerance (25.3 and 25.4%, respectively). The most common resistance mutation detected before switch (index date) was M184V/I and was documented in 11.3% (54/478) of PLHIV in the exposed group (one of whom experienced post-switch virologic failure) and in 5.1% (37/721) of non-exposed group (none of whom experienced post-switch virologic failure). Other resistance mutation to NRTIs (apart from M184V/I) were found in 12.3% (59/478) and 7.5% (54/721) of exposed and unexposed patients respectively. PLHIV who had both M184V/I and at least one other INTI mutation(s) accounted for 9.2% (44/478) and 3.5% (25/721) of those exposed and unexposed, respectively.
Table 1.
Characteristics of the 1199 patients according to exposure status at dolutegravir switch.
| Patient characteristics | Exposed group: patients exposed to mono/dual NRTI therapy or virologic failure before dolutegravir switch (n = 478) | Unexposed group: patients only exposed to optimal therapy (triple therapy) without virologic failure before dolutegravir switch (n = 721) | |
| Age at switch | |||
| Mean (SD) | 53.2 (9.3) | 48.6 (11.2) | |
| Median (IQR) | 53.1 (47.4–59.5) | 49.1 (40.5-56.4) | |
| Sex (%) | Male | 411 (86.0%) | 623 (86.4%) |
| Female | 67 (14.0%) | 98 (13.6%) | |
| Risk factor for HIV acquisition | |||
| MSM (%) | Yes | 327 (68.4%) | 525 (72.8%) |
| No | 151 (31.6%) | 196 (27.2%) | |
| Bisexual (%) | Yes | 12 (2.5%) | 14 (1.9%) |
| No | 466 (97.5%) | 707 (98.1%) | |
| Heterosexual (%) | Yes | 100 (20.9%) | 105 (14.6%) |
| No | 378 (79.1%) | 616 (85.4%) | |
| From endemic countries (%) | Yes | 60 (12.6%) | 61 (8.5%) |
| No | 418 (87.4%) | 660 (91.5%) | |
| Vertical transmission (%) | Yes | 2 (0.4%) | 1 (0.1%) |
| No | 476 (99.6%) | 720 (99.9%) | |
| CD4 count at switch (copies/mL) | |||
| Mean (SD) | 675.6 (285.0) | 717.1 (264.2) | |
| Median (IQR) | 630.0 (488.0-840.0) | 679.0 (535.0–869.0) | |
| ARV treatment duration at switch (in years) | |||
| Mean (SD) | 15.2 (5.5) | 8.1 (5.2) | |
| Median (IQR) | 16.9 (11.5–18.9) | 7.1 (3.7–11.5) | |
| Time since HIV diagnosis at switch (in years) | |||
| Mean (SD) | 16.9 (6.5) | 10.6 (6.6) | |
| Median (IQR) | 17.7 (12.6–20.8) | 9.7 (5.3–14.8) | |
| Number of prior treatment changes at switch | |||
| Mean (SD) | 10.1 (5.7) | 4.5 (2.8) | |
| Median (IQR) | 9 (6-13) | 4 (2–6) | |
| Mutations documented at switch | |||
| M184 V/I mutation | Yes | 54 (11.3%) | 37 (5.1%) |
| No | 102 (21.3%) | 280 (38.9%) | |
| Not tested | 322 (67.4%) | 404 (56.0%) | |
| Other NRTI mutation(s) (except M184 V/I)b | Yes | 59 (12.3%) | 54 (7.5%) |
| No | 97 (20.3%) | 263 (36.5%) | |
| Not tested | 322 (67.4%) | 404 (56.0%) | |
| M184 V/I and at least one other NRTI mutation(s) | Yes | 44 (9.2%) | 25 (3.5%) |
| No | 112 (23.4%) | 292 (40.5%) | |
| Not tested | 322 (67.4%) | 404 (56.0%) | |
| History of virologic failure at switch | Yes | 437 (91.4%) | N/A |
| No | 41 (8.6%) | N/A | |
| Exposure to mono/dual NRTI therapy at switchc | Yes | 171 (35.8%) | N/A |
| No | 307 (64.2%) | N/A | |
| Reason for discontinuation of the previous treatment/ switch to dolutegravir | Side effects/intolerance | 121 (25.3%) | 183 (25.4%) |
| Simplification | 234 (49.0%) | 358 (49.6%) | |
| Others | 97 (20.3%) | 116 (16.1%) | |
| Missing variable | 26 (5.4%) | 64 (8.9%) | |
| History of hepatitis B at switchd | Positive for HBsAg | 19 (4.0%) | 16 (2.2%) |
| Negative for HBsAg | 459 (96.0%) | 705 (97.8%) | |
| History of hepatitis C at switchd | Positive for anti-HCV | 48 (10.0%) | 41 (5.7%) |
| Negative for anti-HCV | 430 (90.0%) | 680 (94.3%) | |
SD = standard deviation; MSM = men who have sex with men; ARV = antiretroviral; HIV = human immunodeficiency viruses; HBsAg = Hepatitis B surface antigen; N/A = not applicablea At dolutegravir switch unless reported differently.
other documented resistance mutations included mutations at position 70, 67, 65, 210, 41, 74, 215, 69 in the group of exposed and 69, 41, 215, 67, 65, 70, 74, 151 in the group of unexposed.
Monotherapy with 1 or 2 NRTIs for at least one month.
Patients who have not been tested for HBsAg or anti-HCV antibodies were considered negative.
Figure 2 shows the cumulative incidence of post-switch virologic failures, stratified by exposure groups. The number of subjects at risk in the survival analysis included only PLHIV who had at least 1 VL measure after dolutegravir switch (453 among those exposed and 695 among those unexposed). No difference was observed in the cumulative incidence of virologic outcomes between exposure groups (log-rank test: P = .31). The 30-months cumulative incidence of virologic failure was 4.1% (95% CI 2.1–7.9) for exposed patients compared to 4.1% (95% CI 2.3–7.4) for unexposed patients. Discontinuation of dolutegravir (censure) after switch was uncommon and not associated with exposure (11.3% and 8.5% for the exposed and unexposed group, respectively).
Figure 2.
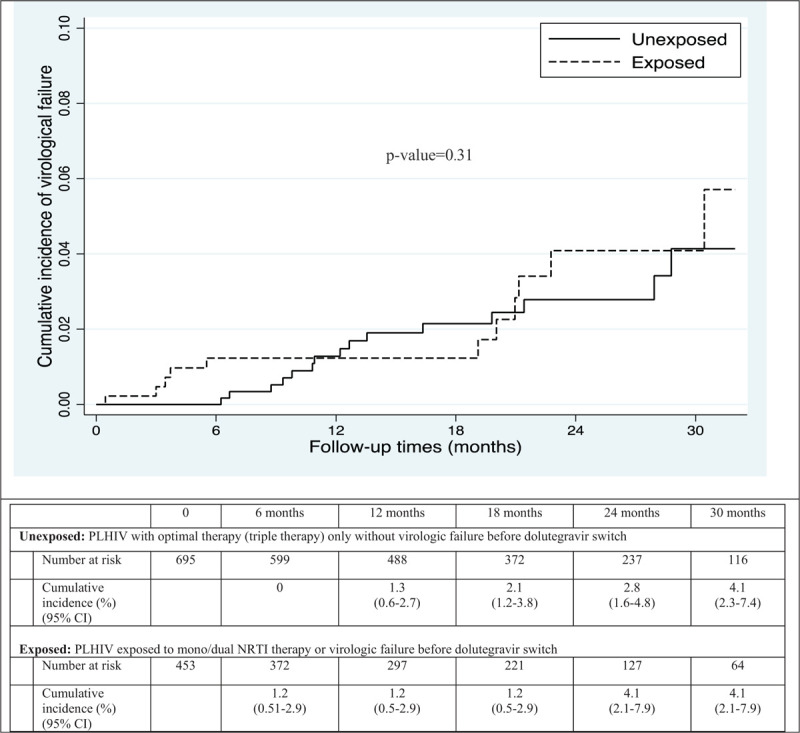
∗Number at risk excluded PLHIV without viral load measures after dolutegravir switch CI = confidence interval.
Table 2 presents the crude and adjusted HR for the association between post-switch virologic failure and exposure. Exposed patients did not experience increased risks of virologic failure post-switch relative to the unexposed group [(crude HR = 1.46, 95% CI 0.69–3.07), aHRs using change-in-estimates method (1.14, 95% CI 0.44–2.93) and aHRs using IPTW (0.84, 95% CI 0.35–2.01)]. Additionally, sensitivity analyses comparing the associations for individual exposure (pre-switch virologic failures, exposure to mono/dual NRTI therapy or mutations) showed similar results.
Table 2.
Crude and adjusted Hazard ratio for the association between post-switch virologic failure and exposure.
| Exposure | Person-years (n = 1148)a | Number of virologic failures | Incidence rate (95% CI) | Crude HR (95% CI) | Multivariate adjusted HR b (95% CI) | IPTW marginal HR† (95% CI) c |
| Unexposed: Optimal therapy only without virologic failure before switch (n = 695) | 1082.62 | 15 | 0.014 (0.008–0.023) | 1 (Reference) | 1 (Reference) | 1 (Reference) |
| Exposed: Mono/dual NRTI therapy and/or virologic failure before switch (n = 453) | 654.01 | 13 | 0.019 (0.011–.034) | 1.46 (0.69–3.07) | 1.14d (0.44–2.93) | 0.84 (0.35–2.01) |
| Individual exposureg | ||||||
| Mono/dual NRTI therapy | 235.29 | 2 | 0.008 (0.002–-.033) | 0.62 (0.11–2.75) | 0.15e (0.02–1.19) | |
| Previous virologic failure | 596.26 | 12 | 0.020 (0.011–0.035) | 1.48 (0.70–3.18) | 1.24f (0.50–3.10) |
CI = confidence interval; HR = hazard Ratio; NA = not applicable.
Analysis included 1148 PLHIV (51 patients excluded because they had no VL measures after switch).
Adjusted using the change in estimates method for the variables among the list described in the method section that changed the HR by +/-5%.
Marginal HR estimated by IPTW with Cox regression model.
Multivariate model adjusted for the following variables measured at dolutegravir switch: age, treatment duration, number of treatment discontinuation, reasons for discontinuation of the previous treatment and sex.
Multivariate model adjusted for the following variables measured at dolutegravir switch: age, treatment duration, time since HIV diagnosis, number of treatment discontinuation, reasons for discontinuation of the previous treatment, history of hepatitis C at switch and sex.
Multivariate model adjusted for the following variables measured at dolutegravir switch: age, time since HIV diagnosis, number of treatment discontinuation and sex.
Non mutually exclusive (total failure in individual category did not sum up total virologic failure in the combined group because virologic failure occurred in patients exposed to both individual group).
5. Discussion
Many PLHIV have been exposed to mono/dual NRTI therapy, virologic failure or harbor resistance mutations and uncertainty remains about the safety of simplifying or changing their regimen. We aimed to assess whether the presence of documented virologic failure or exposure to mono/dual NRTI therapy had an impact on the virologic outcome of patients with a suppressive therapy being replaced by dolutegravir with 2 NRTIs. The results of this study have showed similar virologic outcomes whether or not subjects have been exposed to mono/dual NRTI therapy or previous virologic failure suggesting that the switch to dolutegravir with 2 NRTIs is a safe option in these patients.
Previous studies showed that switches can be risky for PLHIV with a history of virologic failures, resistance mutations or who have been exposed to mono/dual NRTI therapy.[7–9,18] Early switch studies in patients with a suppressed VL on 2 NRTIs with a boosted protease inhibitor (PI) have shown that replacing the boosted PI by either abacavir, efavirenz or nevirapine could lead to more virologic failures, relative to maintaining the boosted PI.[7,8,18] The SWITCHMRK studies,[10] 2 parallel randomized control trials including a total of 702 subjects also showed that replacing lopinavir/ritonavir (r) by raltegravir led to more virologic failures among participants exposed to prior treatment failures. Among PLHIV with documented virologic failures, after a follow-up of 24 weeks, 91.9% of participants in the lopinavir/r group had a VL < 50 copies/ml vs 76.6% in the raltegravir group (treatment difference -15.3%, 95% CI −24.9–−6.2) while no difference was found between the 2 treatment arms in participants without previous virologic failure. However, our results such as some others suggest that dolutegravir might be an effective alternative for patients with a history of virologic failure or previous exposure to mono/dual NRTI therapy. Some studies in naïve patients suggested that dolutegravir has a higher barrier to resistance than other first generation INSTIs like raltegravir or elvitegravir.[19–24] Spring-2 study,[25] a randomized non-inferiority control trial comparing dolutegravir and raltegravir in 411 naïve patients in each arm showed a lower risk of resistance mutations in the dolutegravir group as no NRTI or INSTI resistance mutations occurred at failure among patients on dolutegravir compared to 4 cases of NRTI mutations and 1 case of INSTI mutation in the raltegravir arm. In experienced patients, the SAILING study[15] compared dolutegravir and raltegravir in INSTI-naïve patients failing their current regimen with documented resistance mutations to at least 1 member of 2 of the other ARV classes. Dolutegravir was shown to be superior to raltegravir when given with an optimized background regimen suggesting a higher antiviral potency of dolutegravir in patients with viruses harboring mutations. Among subjects who received NRTIs only as optimized background with either raltegravir or dolutegravir, 0/32 failed in the dolutegravir arm compared to 7/32 in the raltegravir arm and almost half had none or only one fully active NRTI (13 in the dolutegravir arm with no failure reported). The DAWNING study[14] also showed a better efficacy of dolutegravir with 2 NRTIs compared to PI/r in patients with current virologic failure to a first line non-nucleoside reverse transcriptase inhibitor (NNRTI) based regimen. After 48 weeks of follow-up, 84% and 70% of patients on dolutegravir and those on PI/r respectively had a VL < 50 copies/ml (adjusted difference 13.8, 95% CI 7.3–20.3). A prospective study,[26] including 5 European HIV cohorts also showed no statistically significant difference for the presence M184V/I mutations among patients on suppressive therapy whose regimen was switched to abacavir/lamivudine/dolutegravir. In this study, the cumulative incidence of virologic failures in patients without and with M184V/I mutations was 1.1 and 3.0%, respectively (P = .09) over a median of follow-up time of 288.5 days. Our observational study showed similar outcomes among patients in whom a suppressive ARV regimen has been switched to dolutegravir with 2 NRTIs, regardless of previous exposure to documented virologic failure or mono/dual NRTI therapy. This suggests that a regimen switch to dolutegravir is a safe option for patients with documented virologic failure or exposure to suboptimal therapy. The replacement of a PI/r by dolutegravir in the ARV regimen may also offer other advantages such as reducing the risk of lipid disorders,[10,27] and potentially cardiovascular disease.[28]
Our study presents strengths and limitations. Our study provides real world data in patients with documented virologic failures and/or exposure to mono/dual NRTI therapy before the replacement of their suppressive ARV therapy to dolutegravir. The Quebec HIV Cohort includes a significant number of PLHIV providing good statistical power, external validity and real-life effectiveness results. Inherent limitation to all observational studies, however, is the potential for confounding bias although we used conservative approaches in order to control for confounders. This study was also subject to information bias, especially regarding whether or not the prescription were actually taken by patients and with which adherence, and regarding the detection of mutations (although this was not the primary exposure). The genomic mutation assay was performed in 39.4% (473/1199) of the patients which is representative of many clinical situations in which previous genotypes are not available or were not performed. A sensitivity analysis for the associations between the presence/absence of the M184V/I mutation and post-switch virological failure undertaken among patients who have been tested for mutations provides similar results with an adjusted HR (and 95%CI) of 0.47 (0.05–4.15) for the presence of M184V/I. Indeed, the DHHS guidelines recommends to assume presence of drug-specific resistance when patients are failing on a ARV with a low barrier to resistance such as a NNRTI, elvitegravir, raltegravir, lamivudine or emtricitabine.[4] The possible long duration of virological suppression before dolutegravir switch may also have resulted in lower relevance of past mutations. We also pooled all PLHIV having either exposition to mono/dual NRTI therapy or virologic failure before switch in our study. Viral loads were not available in the era of mono/dual NRTI therapy and virologic failure has been presumed for all of these patients which may not be always the case. In sensitive analysis, we considered both exposures individually and no statistically significant difference were found. Moreover, in order to have a similar definition of virological failure used in randomized control studies, our definition of post-switching virological failure was considered with a single VL > 50 copies/ml at the last visit as a marker for virological failure. This definition could have considered blips in each group leading to non-differential bias. The number of patients with post-switching virological failure defined with a single VL > 50 copies/ml at the last visit was only 9/13 in the exposed group and 5/15 in those unexposed. Finally, the follow-up time was also limited because dolutegravir remains a more recently available ARV, but this is a longer follow-up than any previous trials which are 48 to 96 weeks in duration.
6. Conclusion
In conclusion, our study found that the effectiveness of the switch to dolutegravir with 2 NRTIs is similar regardless of a history of virologic failures or exposure to mono/dual NRTI therapy. This study suggests that therapeutic switches to dolutegravir with 2 NRTIs provide a safe option for patients on suppressive ARV regimens in the presence of documented virologic failure and previous exposure to mono/dual NRTI therapy.
Acknowledgment
The authors are grateful to Anne-Fanny Vassal and Mariève Beauchemin (CMA), to Joseph Niyibizi (Sainte-Justine Hospital), to Tudor Luncean (CHUM Hospital), to Jessica Lumia and Leo Wong (MUHC) and to all other contributing research staff for their help in collecting and managing data in all sites.
Author contributions
All authors of this research paper have directly contributed to the conception and design (HT, MNS, JGB, AdP, RT, MK, CT, MD, MR), or acquisition of data (MNS, JGB, RT, MK, CT, LL, ZG, CP, NM, MD, MR, IH, HT), or data management, analysis and interpretation (MNS, HT, JGB, AdP, CL, NM, MD, CD) of the study. MNS, HT, JGB and AdP wrote the first draft of the manuscript. All authors have subsequently read, revised, and approved the version that is being submitted.
Conceptualization: Mohamed N’dongo Sangaré, Jean-Guy Baril, Alexandra de Pokomandy, Marina Klein, Réjean Thomas, Cécile Tremblay, Helen Trottier.
Data curation: Mohamed N’dongo Sangaré, Jean-Guy Baril, Alexandra de Pokomandy, Claudie Laprise, Marina Klein, Réjean Thomas, Cécile Tremblay, Michel Roger, Costa Pexos, Zoe Greenwald, Nima Machouf, Madeleine Durand, Isabelle Hardy, Mamadou Dakouo, Louise Laporte, Helen Trottier.
Formal analysis: Mohamed N’dongo Sangaré, Jean-Guy Baril, Alexandra de Pokomandy, Claudie Laprise, Catherine Deshaies, Costa Pexos, Nima Machouf, Madeleine Durand, Helen Trottier.
Funding acquisition: Mohamed N’dongo Sangaré, Jean-Guy Baril, Alexandra de Pokomandy, Marina Klein, Réjean Thomas, Cécile Tremblay, Helen Trottier.
Investigation: Jean-Guy Baril, Alexandra de Pokomandy, Marina Klein, Réjean Thomas, Cécile Tremblay, Michel Roger, Helen Trottier.
Methodology: Mohamed N’dongo Sangaré, Jean-Guy Baril, Alexandra de Pokomandy, Claudie Laprise, Catherine Deshaies, Réjean Thomas, Cécile Tremblay, Michel Roger, Costa Pexos, Zoe Greenwald, Nima Machouf, Madeleine Durand, Helen Trottier.
Project administration: Jean-Guy Baril, Alexandra de Pokomandy, Costa Pexos, Louise Laporte, Helen Trottier.
Resources: Jean-Guy Baril, Alexandra de Pokomandy, Michel Roger, Louise Laporte, Helen Trottier.
Software: Alexandra de Pokomandy, Helen Trottier.
Supervision: Jean-Guy Baril, Alexandra de Pokomandy, Helen Trottier.
Validation: Jean-Guy Baril, Alexandra de Pokomandy, Helen Trottier.
Visualization: Jean-Guy Baril, Alexandra de Pokomandy, Helen Trottier.
Writing – original draft: Mohamed N’dongo Sangaré, Jean-Guy Baril, Alexandra de Pokomandy, Helen Trottier.
Writing – review & editing: Mohamed N’dongo Sangaré, Jean-Guy Baril, Alexandra de Pokomandy, Claudie Laprise, Catherine Deshaies, Marina Klein, Réjean Thomas, Cécile Tremblay, Michel Roger, Costa Pexos, Zoe Greenwald, Nima Machouf, Madeleine Durand, Isabelle Hardy, Mamadou Dakouo, Louise Laporte, Helen Trottier.
Supplementary Material
Footnotes
Abbreviations: aHR = Adjusted Hazard Ratios, anti-HCV = hepatitis C antibodies, ARV = antiretroviral therapy, CHUM = Centre hospitalier de l’Université de Montréal, CMA = Clinique médicale l’Actuel, CMUQL = Clinique de médecine urbaine du Quartier Latin, CI = confidence interval, DHHS = department of health and human services, EACS = European AIDS Clinical Society, HBsAg = hepatitis B surface antigen, HIV = Human Immunodeficiency Virus, HR = hazard ratios, INSTIs = Integrase Strand Transfer inhibitors, IPTW = inverse probability of treatment weighting, IQR = interquartile range, KM = Kaplan–Meier, MUHC = McGill University Health Center, NNRTI = non-nucleoside reverse transcriptase inhibitor, NRTI = nucleoside reverse transcriptase inhibitors, PLHIV = people living with HIV, PI/r = ritonavir-boosted protease inhibitor, REBs = Research Ethics Boards, SD = standard deviation, VL = viral load.
How to cite this article: Sangaré MN, Baril JG, de Pokomandy A, Laprise C, Deshaies C, Klein M, Thomas R, Tremblay C, Roger M, Pexos C, Greenwald Z, Machouf N, Durand M, Hardy I, Dakouo M, Laporte L, Trottier H. Impact of previous HIV resistance and virologic failures on virologic outcome following a switch to dolutegravir with 2 NRTIs among people living with HIV. Medicine. 2020;99:47(e23335).
This study was supported by an unrestricted grant from ViiV Healthcare. Funding for the Quebec HIV Cohort was also provided in part by the Réseau FRQS SIDA-MI. MNS was supported by a doctoral award from Islamic Development Bank, Merit scholarship Program for High Technology (2013-2016), and a doctoral award from Université de Montréal. HT holds a salary award (chercheur-boursier) from the Fonds de la recherche du Québec en santé (FRQ-S) and a New investigator salary award from Canadian Institutes of Health Research (CIHR). AdP holds a salary award (chercheur-boursier) from the Fonds de la recherche du Québec en santé (FRQ-S).
Parts of this manuscript were presented as a poster at the HIV Glasgow 2018 conference (Sangaré M. et al, poster number P106, Virological outcome after switching a suppressive HAART to dolutegravir (DTG) with 2 NRTIs among HIV-1-infected patients: potential effects of previous mono/dual NRTI therapies or previous virological failures) and published as a supplement to the Journal of the international AIDS Society, volume 21, Issue S8.
Parts of this manuscript were also presented at the Quebec HIV Days from April 11 to 12, 2019 in Montreal, Canada.
HT has received unrestricted grants from ViiV Healthcare, Merck and Gilead Sciences. JGB has received honoraria for consulting for ViiV Healthcare, Merck, and Gilead Sciences and for participation as a speaker at conferences from Merck and Gilead Sciences. His institution (CMUQL) has received research grants from GlaxoSmithKline, Merck, and Gilead Sciences. AdP's institution participates in several pharmaceutical clinical trials for HIV antiretrovirals and HCV treatments in which she is the site principal investigator (ViiV Healthcare, Janssen, Merck, Gilead Sciences), and has received honoraria for consulting in advisory board meetings for ViiV Healthcare. RT has received honoraria for consulting for ViiV Healthcare, Merck, and Gilead Sciences, and for participation as a speaker at conferences from Merck and Gilead Sciences. His institution (CMA) has received research grants from GlaxoSmithKline, Gilead Sciences, Merck, and Janssen.
All other co-authors have no conflicts of interest to declare.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Supplemental digital content is available for this article.
References
- [1].Kandel CE, Walmsley SL. Dolutegravir - a review of the pharmacology, efficacy, and safety in the treatment of HIV. Drug Design Development and Therapy 2015;9:3547–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].viiv, healthcare. Highlights of prescribing information for tivicay. 2015. Available at: https://www.natap.org/2015/HIV/us_tivicay2.pdf. Accessed May 2019. [Google Scholar]
- [3].European AIDS Clinical Society (EACS). Guidelines version 10.0. November 2019. Available at: https://www.eacsociety.org/files/guidelines-10.0_final_2_2.pdf. Accessed april 2020. [Google Scholar]
- [4].Department of Health and Human Services. Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the Use of Antiretroviral Agents in Adults and Adolescents with HIV. December 2019. Available at: https://clinicalinfo.hiv.gov/sites/default/files/guidelines/documents/AdultandAdolescentGL.pdf. Accessed April 2020. [Google Scholar]
- [5].Lennox JL, Dejesus E, Lazzarin A, et al. Safety and efficacy of raltegravir-based versus efavirenz-based combination therapy in treatment-naive patients with HIV-1 infection: a multicentre, double-blind randomised controlled trial. Lancet 2009;374:796–806. [DOI] [PubMed] [Google Scholar]
- [6].Cooper DA, Steigbigel RT, Gatell JM, et al. Subgroup and resistance analyses of raltegravir for resistant HIV-1 infection. N Engl J Med 2008;359:355–65. [DOI] [PubMed] [Google Scholar]
- [7].Martinez E, Arnaiz JA, Podzamczer D, et al. Substitution of nevirapine, efavirenz, or abacavir for protease inhibitors in patients with human immunodeficiency virus infection. N Engl J Med 2003;349:1036–46. [DOI] [PubMed] [Google Scholar]
- [8].Maggiolo F, Ripamonti D, Ravasio L, et al. Outcome of 2 simplification strategies for the treatment of human immunodeficiency virus type 1 infection. Clin Infect Dis 2003;37:41–9. [DOI] [PubMed] [Google Scholar]
- [9].Eron JJ, Young B, Cooper DA, et al. Switch to a raltegravir-based regimen versus continuation of a lopinavir-ritonavir-based regimen in stable HIV-infected patients with suppressed viraemia (SWITCHMRK 1 and 2): two multicentre, double-blind, randomised controlled trials. Lancet 2010;375:396–407. [DOI] [PubMed] [Google Scholar]
- [10].Gatell JM, Assoumou L, Moyle G, et al. Immediate versus deferred switching from a boosted protease inhibitor-based regimen to a dolutegravir-based regimen in virologically suppressed patients with high cardiovascular risk or age >/ = 50 years: final 96-week results of the neat022 study. Clin infect Dis 2019;68:597–606. [DOI] [PubMed] [Google Scholar]
- [11].Arribas JR, Pialoux G, Gathe J, et al. Simplification to coformulated elvitegravir, cobicistat, emtricitabine, and tenofovir versus continuation of ritonavir-boosted protease inhibitor with emtricitabine and tenofovir in adults with virologically suppressed HIV (STRATEGY-PI): 48 week results of a randomised, open-label, phase 3b, non-inferiority trial. Lancet Infect Dis 2014;14:581–9. [DOI] [PubMed] [Google Scholar]
- [12].Trottier B, Lake J, Logue K, et al. Switching to abacavir/dolutegravir/lamivudine combination (ABC/DTG/3TC FDC) from a PI, INI or NNRTI based regimen maintains HIV suppression. Presented at: Interscience Conference on Antimicrobial Agents and Chemotherapy; September 17-21, 2015; San Diego, CA. [Google Scholar]
- [13].Pozniak A, Markowitz M, Mills A, et al. Switching to coformulated elvitegravir, cobicistat, emtricitabine, and tenofovir versus continuation of non-nucleoside reverse transcriptase inhibitor with emtricitabine and tenofovir in virologically suppressed adults with HIV (STRATEGY-NNRTI): 48 week results of a randomised, open-label, phase 3b non-inferiority trial. Lancet Infect Dis 2014;14:590–9. [DOI] [PubMed] [Google Scholar]
- [14].Aboud M, Kaplan R, Lombaard J, et al. Dolutegravir versus ritonavir-boosted lopinavir both with dual nucleoside reverse transcriptase inhibitor therapy in adults with HIV-1 infection in whom first-line therapy has failed (DAWNING): an open-label, non-inferiority, phase 3b trial. Lancet Infect Dis 2019;19:253–64. [DOI] [PubMed] [Google Scholar]
- [15].Cahn P, Pozniak AL, Mingrone H, et al. Dolutegravir versus raltegravir in antiretroviral-experienced, integrase-inhibitor-naive adults with HIV: week 48 results from the randomised, double-blind, non-inferiority SAILING study. Lancet 2013;382:700–8. [DOI] [PubMed] [Google Scholar]
- [16].Gouvernement du Québec. Ministère de la Santé et des Services Sociaux (MSSS). La thérapie antirétrovirale pour les adultes infectés par le VIH. Guide pour les professionnels de la santé du Québec. In: Québec Gd, ed. ISBN: 978-2-550-83215- Last updated January 2019. Available at: https://publications.msss.gouv.qc.ca/msss/fichiers/2018/18-337-01W.pdf. Accessed April 2020. [Google Scholar]
- [17].Hernán MARJ. Causal Inference. Boca Raton: Chapman & Hall/CRC, forthcoming. In; 2019. [Google Scholar]
- [18].Opravil M, Hirschel B, Lazzarin A, et al. A randomized trial of simplified maintenance therapy with abacavir, lamivudine, and zidovudine in human immunodeficiency virus infection. J Infect Dis 2002;185:1251–60. [DOI] [PubMed] [Google Scholar]
- [19].Sax PE, DeJesus E, Mills A, et al. Co-formulated elvitegravir, cobicistat, emtricitabine, and tenofovir versus co-formulated efavirenz, emtricitabine, and tenofovir for initial treatment of HIV-1 infection: a randomised, double-blind, phase 3 trial, analysis of results after 48 weeks. Lancet 2012;379:2439–48. [DOI] [PubMed] [Google Scholar]
- [20].Rockstroh JK, DeJesus E, Lennox JL, et al. Durable efficacy and safety of raltegravir versus efavirenz when combined with tenofovir/emtricitabine in treatment-naive HIV-1-infected patients: final 5-year results from STARTMRK. J Acquir Immune Defic Syndr 2013;63:77–85. [DOI] [PubMed] [Google Scholar]
- [21].Clotet B, Feinberg J, van Lunzen J, et al. Once-daily dolutegravir versus darunavir plus ritonavir in antiretroviral-naive adults with HIV-1 infection (FLAMINGO): 48 week results from the randomised open-label phase 3b study. Lancet 2014;383:2222–31. [DOI] [PubMed] [Google Scholar]
- [22].Kulkarni R, Abram ME, McColl DJ, et al. Week 144 resistance analysis of elvitegravir/cobicistat/emtricitabine/tenofovir DF versus atazanavir + ritonavir + emtricitabine/tenofovir DF in antiretroviral-naive patients. HIV Clin Trials 2014;15:218–30. [DOI] [PubMed] [Google Scholar]
- [23].Sax PE, Pozniak A, Montes ML, et al. Coformulated bictegravir, emtricitabine, and tenofovir alafenamide versus dolutegravir with emtricitabine and tenofovir alafenamide, for initial treatment of HIV-1 infection (GS-US-380-1490): a randomised, double-blind, multicentre, phase 3, non-inferiority trial. Lancet 2017;390:2073–82. [DOI] [PubMed] [Google Scholar]
- [24].Gallant J, Lazzarin A, Mills A, et al. Bictegravir, emtricitabine, and tenofovir alafenamide versus dolutegravir, abacavir, and lamivudine for initial treatment of HIV-1 infection (GS-US-380-1489): a double-blind, multicentre, phase 3, randomised controlled non-inferiority trial. Lancet 2017;390:2063–72. [DOI] [PubMed] [Google Scholar]
- [25].Raffi F, Jaeger H, Quiros-Roldan E, et al. Once-daily dolutegravir versus twice-daily raltegravir in antiretroviral-naive adults with HIV-1 infection (SPRING-2 study): 96 week results from a randomised, double-blind, non-inferiority trial. Lancet Infect Dis 2013;13:927–35. [DOI] [PubMed] [Google Scholar]
- [26].Olearo F, Nguyen H, Bonnet F, et al. Impact of the M184V/I mutation on the efficacy of abacavir/lamivudine/dolutegravir therapy in HIV treatment-experienced patients. Open Forum Infect Dis 2019;6:ofz330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Chen GJ, Sun HY, Chang SY, et al. Effectiveness of switching from protease inhibitors to dolutegravir in combination with nucleoside reverse transcriptase inhibitors as maintenance antiretroviral therapy among HIV-positive patients. Int J Antimicrob Agents 2019;54:35–42. [DOI] [PubMed] [Google Scholar]
- [28].Friis-Moller N, Sabin CA, Weber R, et al. Combination antiretroviral therapy and the risk of myocardial infarction. N Engl J Med 2003;349:1993–2003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


