Abstract
Backgrounds:
Pulmonary tuberculosis (PTB) is an oldest-known and most formidable disease. The standard microbiology culture is time-wasting. Monokine induced by gamma interferon (MIG) has been reported as a new biomarker to auxiliarily detect PTB. In our study, we used meta-analysis to assess the diagnostic value of MIG for PTB.
Methods:
PubMed, Embase, Web of Science, and Cochrane Library were searched for relative records up to April 2, 2020. The pooled sensitivity, specificity, positive likelihood ratio, negative likelihood ratio, diagnostic odds ratio, area under the curve, and summary receiver operating characteristic curve were estimated.
Results:
Eight studies including 1487 participants were included. The pooled sensitivity, specificity, positive likelihood ratio, and negative likelihood ratio of MIG for detecting PTB were 84%, 84%, 5.19, and 0.19, respectively. The diagnostic odds ratio and area under the curve were 27.88 and 0.90, respectively, indicating a good diagnostic ability of MIG. Meta-regression analysis showed that human immunodeficiency virus status might be a source of heterogeneity (P = .02).
Conclusions:
Our results showed that MIG had a good diagnostic value for PTB.
Keywords: detection, meta-analysis, monokine induced by gamma interferon, pulmonary tuberculosis
1. Introduction
Tuberculosis (TB) is one of the oldest and most formidable diseases in humans, with approximately 10,000,000 newly confirmed cases and 1,500,000 deaths in 2018.[1,2] Pulmonary TB (PTB), accounting for 3-quarters of TB cases, contributes substantially to TB mortality, especially in developing countries and in individuals with human immunodeficiency virus (HIV) coinfection.[3,4] The accurate and rapid detection of PTB is critical for eradicating TB globally by 2035.[5]
Currently, microbiological culture and sputum smear microscopy are utilized for the routine diagnosis of PTB.[6] However, these approaches have various drawbacks, including the time delay for positive culture and poor sensitivity (20%–60%) of microscopy.[7,8] The Xpert MTB/RIF assay is recommended by the World Health Organization for the diagnosis of PTB.[9] However, this method is costly and limited in smear-negative sputum, especially in HIV-coinfected cases.[10] Therefore, additional methods are needed for accurate and practical PTB detection.
Monokine induced by gamma interferon (MIG) is a C-X-C motif chemokine receptor 3 ligand. After TB infection, MIG induces immune effector functions in the host by binding to the C-X-C motif chemokine receptor 3 receptor of monocytes and macrophages.[11] It is highly expressed in patients with pulmonary and extrapulmonary TB.[3] The high MIG level is reversed by anti-TB treatment.[12] Several studies have reported that MIG might be an auxiliary biomarker for PTB detection.[13–20] To address the gap in knowledge regarding the MIG in PTB, we evaluated its diagnostic value. In particular, we performed a meta-analysis to synthesize data related to the detection value of MIG for PTB.
2. Materials and methods
2.1. Data sources and search strategy
This study was conducted based on the preferred reporting items for systematic reviews and meta-analyses.[21] Four reference databases (ie, PubMed, Embase, Web of Science, and Cochrane Library) were searched for relevant articles published up to April 2, 2020. The search terms were “chemokine CXCL9,” “monokine induced by IFN-γ,” “small inducible cytokine B9,” “tuberculosis,” “active tuberculosis,” and “pulmonary tuberculosis.” The search was limited to studies published in English. A detailed search strategy (MeSH and title/abstracts) was used in PubMed: (((“Chemokine CXCL9”[Mesh]) OR (((((((chemokine CXCL9[Title/Abstract]) OR monokine induced by IFN-γ[Title/Abstract]) OR monokine induced by interferon gamma [Title/Abstract]) OR Small Inducible Cytokine B9[Title/Abstract]) OR SCYB9[Title/Abstract]) OR MIG[Title/Abstract]) OR CXCL9[Title/Abstract]))) AND ((“Tuberculosis”[Mesh]) OR ((((((tuberculosis[Title/Abstract]) OR TB[Title/Abstract]) OR active tuberculosis[Title/Abstract]) OR ATB[Title/Abstract]) OR pulmonary tuberculosis[Title/Abstract]) OR PTB[Title/Abstract])). The reference lists of identified articles were manually screened for eligible studies.
2.2. Inclusion and exclusion criteria
The inclusion criteria of studies reporting MIG for detection of PTB were as follows:
-
(1)
studies assessing blood samples of participants with PTB,
-
(2)
studies using MIG as an index test,
-
(3)
studies involving positive microbiological culture as the gold standard, and
-
(4)
studies presenting the sensitivity and specificity of MIG as the primary outcome.
A study was included twice when both stimulated and unstimulated MIG were reported. Additionally, 2 researchers independently conducted study selection.
The exclusion criteria were animal experiments, reviews, non-English publications, guidelines, conference abstracts, mechanistic studies, and case reports.
2.3. Data extraction and quality assessment
The data extracted included the author, year, country, where the research was conducted, TB incidence (per 100,000 individuals), study type, samples from patients with PTB and non-TB controls, reference standard, cut-off of index test (MIG), HIV status, type of non-TB control, technology for MIG detection, antigen for MIG, sensitivity (%), specificity (%), area under the curve (AUC), and the true positive, false positive, false negative, and true negative rates. Two researchers independently extracted data from the included articles, and disagreements were resolved by discussion and consensus. Previous publications were included without the requirement for ethical reviews.
The Quality Assessment of Diagnostic Accuracy Studies-2 tool was used to evaluate the risk of bias and applicability of included studies, as implemented in RevMan 5.3.[22] Quality Assessment of Diagnostic Accuracy Studies-2 was composed of patient selection, index test, reference standard, and flow and timing.
2.4. Statistical and meta-analyses
Stata 14.0 was used to evaluate the primary data. The Spearman correlation coefficient was used to investigate the threshold effect. I2 was utilized to evaluate heterogeneity, where values of I2 < 50% and P > .1 indicated low heterogeneity and values of I2 > 50% and P < .1 indicates high heterogeneity.[23,24] An I2 value of 0% indicated no inconsistency. A Galbraith plot analysis was used to identify outlier studies.
A bivariate random effects model was used to determine the pooled sensitivity, specificity, positive likelihood ratio (PLR), negative likelihood ratio (NLR), diagnostic odds ratio (DOR), and AUC to evaluate the diagnostic performance of MIG for PTB.[25] An AUC value exceeding 0.9 indicated that MIG had an excellent diagnostic ability for PTB. A summary receiver operating characteristic curve was also generated to determine the diagnostic accuracy of MIG.[26]
In addition, a meta-regression analysis was applied to explore possible sources of heterogeneity. A subgroup analysis, including HIV status (HIV-coinfected or not), type of non-TB control (healthy controls or other respiratory diseases), technology for MIG detection (Luminex multiplex immunoassay or not), and antigen for MIG (stimulated or unstimulated), was also performed. Deeks’ funnel plot was used to judge whether publication bias existed (P < .05) or not (P > .05).[27]
3. Results
3.1. Research findings
Overall, 462 literature records were identified (Fig. 1). Initially, we removed 197 duplications. We excluded 243 records by screening titles and abstracts: 64 were focused on other diseases; 90 were focused on other cytokines (vitamin D, interleukin-4, and interleukin-8); 21 were reviews, case reports, or guidelines; 45 were animal experiments, including studies of cattle, mice, macaques, and African buffaloes, 14 were not eligible based on primary outcomes, and 9 were other unrelated topics. Ultimately, 22 full studies were assessed for inclusion, and 8 studies were included in the meta-analysis.[13–20]
Figure 1.
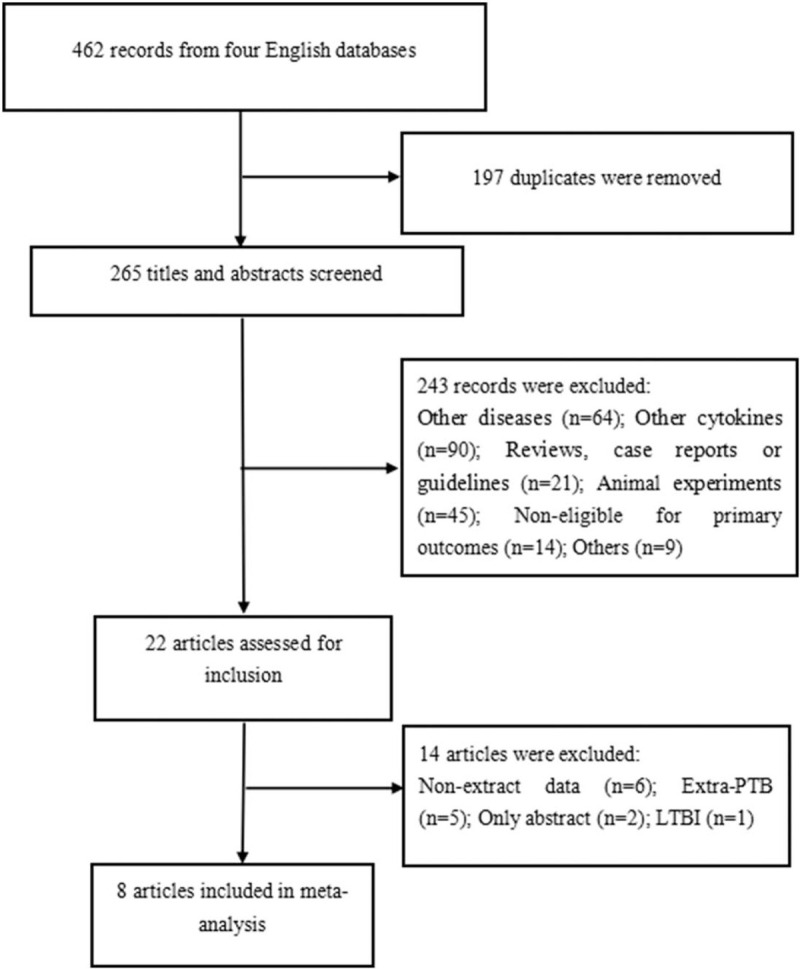
Flow chart of the process of included articles.
3.2. Characteristics and quality appraisal of the included studies
The baseline characteristics of the 8 studies are shown in Table 1. From 2012 to 2019, 1487 participants, including 814 patients with PTB and 673 non-TB controls, were included. The TB incidence ranged from 6.1 to 781 per 100,000 residents. Seven studies had cohort designs, and 1 study used a cross-sectional design.[20] All studies used positive culture as the reference standard. Xpert MTB/RIF and positive sputum smears were also used in 3 studies.[13,14,20] The index test was MIG. The cut-off MIG ranged from 111.4 to 2183 pg/mL. Rates of HIV coinfection in 2 studies were 8.65% and 25.5%.[13,15] Five studies selected individuals with other respiratory diseases as non-TB controls,[13–15,17,19] and 3 studies included healthy controls.[16,18,20] With respect to detection technology (MIG), 3 studies used a multiplex immunoassay,[13–15] 3 studies used enzyme-linked immunosorbent assay,[17–19] 1 study used reverse transcription-polymerase chain reaction,[16] and 1 study used microbead-based assays.[20] Half of the studies detected stimulated MIG,[14,16,19,20] while the remaining studies focused on unstimulated MIG.[13,15,17,18] The sensitivity, specificity, AUC, true positive, false positive, false negative, and true negative of MIG for PTB are listed in Table 2.
Table 1.
Characteristics of included studies.
| Samples (N) | |||||||||
| Author | Year | Country/where the research was conducted | TB incidence (/100,000) | Study type | PTB patients | non-TB controls | Reference standard | Index test | Cut-off (MIG) |
| Manngo PM[11] | 2019 | South Africa | 781 (543–1060) | Cohort | 35 | 69 | Positive culture, Xpert MTB/RIF | MIG | 940.3 pg/mL |
| La Manna MP[12] | 2018 | Italy | 6.1 (5.3–7.1) | Cohort | 27 | 20 | Positive culture, Xpert MTB/RIF | MIG | 6.456 relative fluorescence intensity |
| Jacobs R[13] | 2016 | South Africa | 781 (543–1060) | Cohort | 22 | 33 | Positive culture | MIG | 1700 pg/mL |
| Kim S[14] | 2015 | Republic of Korea | 77 (71–82) | Cohort | 28 | 29 | Positive culture | MIG | 9.18 |
| Chung W[15] | 2015 | Republic of Korea | 77 (71–82) | Cohort | 201 | 52 | Positive culture | MIG | 155.1 pg/mL |
| Lee K[16] | 2015 | Republic of Korea | 77 (71–82) | Cohort | 165 | 256 | Positive culture | MIG | 111.4 pg/mL |
| Chung WY[17] | 2014 | Republic of Korea | 77 (71–82) | Cohort | 158 | 58 | Positive culture | MIG | 2183 pg/mL |
| Wang X[18] | 2012 | China | 64 (55–74) | Cross sectional | 178 | 156 | Positive culture, positive sputum smears | MIG | 368.5 pg/mL |
MIG = monokine induced by gamma interferon, PTB = pulmonary tuberculosis, TB = tuberculosis.
Table 2.
Baseline data of included studies.
| Author | Year | HIV status | Type of non-TB controls | Technology (MIG) | Antigen (MIG) | Sensitivity (%) | Specificity (%) | AUC | TP | FP | FN | TN |
| Manngo PM[11] | 2019 | 9 (8.65%) | Other respiratory diseases | Multiplex immunoassay | Unstimulated | 70 | 57 | 0.73 | 25 | 30 | 10 | 39 |
| La Manna MP[12] | 2018 | None | Other respiratory diseases | Multiplex immunoassay | Stimulated | 94.44 | 90 | 0.8944 | 25 | 2 | 2 | 18 |
| Jacobs R[13] | 2016 | 14 (25.5%) | Other respiratory diseases | Multiplex immunoassay | Unstimulated | 68 | 88 | 0.81 | 15 | 4 | 7 | 29 |
| Kim S[14] | 2015 | None | Healthy controls | RT-PCR | Stimulated | 85.71 | 86.21 | - | 24 | 4 | 4 | 25 |
| Chung W[15] | 2015 | None | Other respiratory diseases | ELISA | Unstimulated | 81.1 | 88.5 | 0.89 | 163 | 6 | 38 | 46 |
| Lee K[16] | 2015 | None | Healthy controls | ELISA | Unstimulated | 89.3 | 89.1 | 0.935 | 147 | 28 | 18 | 228 |
| Chung WY[17] | 2014 | None | Other respiratory diseases | ELISA | Stimulated | 88.6 | 87.9 | 0.941 | 140 | 7 | 18 | 51 |
| Wang X[18] | 2012 | None | Healthy controls | Microbead-based assay | Stimulated | 85.4 | 80.8 | 0.896 | 152 | 30 | 26 | 126 |
AUC = area under curve, ELISA = enzyme-linked immunosorbent assay, FN = false negative, FP = false positive, HIV = human immunodeficiency virus, RT-PCR = reverse transcription-polymerase chain reaction, TB = tuberculosis, TN = true negative, TP = true positive.
The quality of eligible studies is summarized in Figure 2. Patient selection bias was unclear for 1 study because the time of participant enrolment was unknown.[20] Half of the studies had unclear bias in index tests because we could not determine whether the MIG detection was blinded.[13,14,20] Three studies had unclear bias in the reference standard because other methods (Xpert MTB/RIF and positive sputum smears) were additionally used.[13,14,20] Flow and timing bias were unclear for 3 studies because data for a few participants were lost without explanation.[13,14,19] Applicability concerns were generally low.
Figure 2.
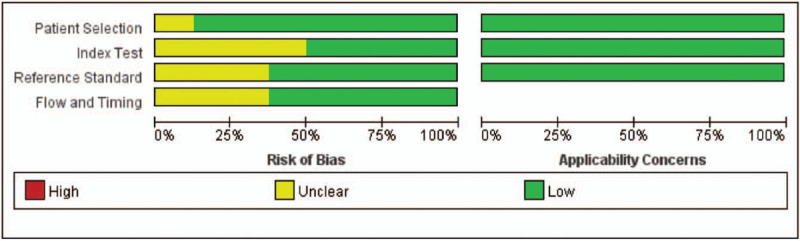
Quality of included studies.
3.3. Galbraith plot and pooled analysis
In the meta-analysis, no threshold effect was detected (P = 1.00). Heterogeneity was low (I2 = 0%, P = .234). In addition, based on the Galbraith plot, there were no outlier studies (Fig. 3).
Figure 3.
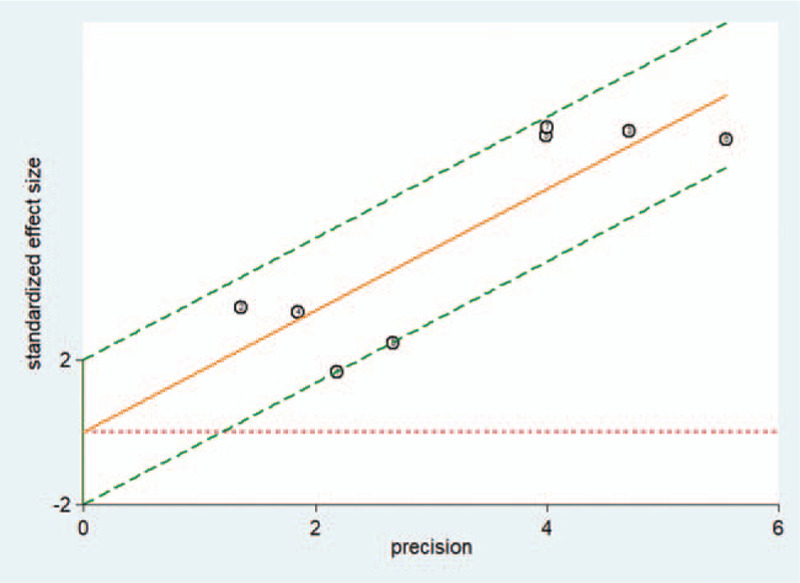
The Galbraith plot of MIG to detect PTB. MIG = monokine induced by gamma interferon, PTB = pulmonary tuberculosis.
A total of 1487 participants were evaluated. Sensitivity ranged from 68% to 94.44% (pooled sensitivity: 0.84, 95% confidence interval [CI]: 0.80–0.88). The specificity ranged from 57% to 90% (pooled specificity: 0.84, 95% CI: 0.76–0.89). The pooled PLR and NLR were 5.19 (95% CI: 3.37.7–97) and 0.19 (95% CI: 0.13–0.26), respectively. The pooled DOR and AUC were 27.88 (95% CI: 13.43–57.89) and 0.90 (95% CI: 0.88–0.93), respectively, indicating that MIG had a good diagnostic value for PTB. The summary receiver operating characteristic curves are shown in Figure 4.
Figure 4.
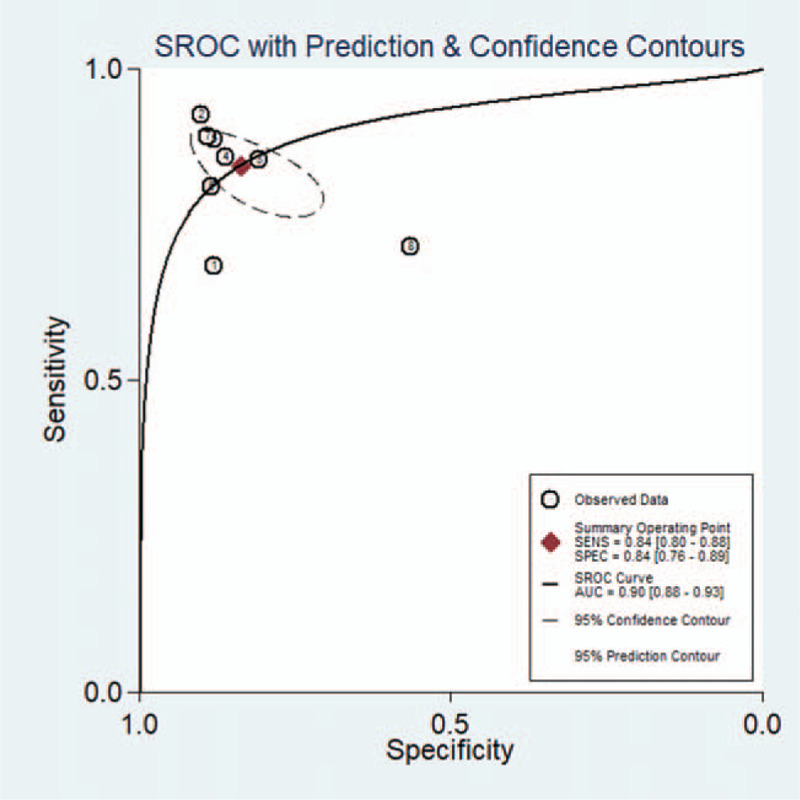
The SROC curve for assessment of MIG to detect PTB. AUC = area under curve, MIG = monokine induced by gamma interferon, PTB = pulmonary tuberculosis, SROC = summary receiver operating characteristic.
3.4. Meta-regression and subgroup analyses
In a meta-regression analysis, HIV status was a potential source of heterogeneity (P = .02). The type of non-TB control, technology, and antigen for MIG were not sources of heterogeneity (P = .36, .23, and .17, respectively).
Concerning HIV status, 23/159 (14.47%) participants were co-infected with HIV, and 1328 participants were not co-infected with HIV. The sensitivity and specificity for participants with PTB/HIV co-infection were much lower than those for patients with PTB alone (0.70 vs 0.86 and 0.70 vs 0.87, respectively). The overall performance was slightly higher for studies using healthy controls than for studies using patients with other respiratory diseases (sensitivity: 0.87 and 0.82; specificity: 0.86 and 0.83). With respect to the MIG detection technology, the sensitivity and specificity of the luminex multiplex immunoassay/microbead-based assay were lower than those of enzyme-linked immunosorbent assay/reverse transcription-polymerase chain reaction (0.82 vs 0.86, 0.77 vs 0.88, respectively). With respect to the antigen for MIG, the diagnostic performance was slightly higher for stimulated MIG than unstimulated MIG (sensitivity: 0.88 and 0.81; specificity: 0.86 and 0.81).
3.5. Publication bias
Deeks’ funnel plot indicated no striking publication bias (P = .49) (Fig. 5).
Figure 5.
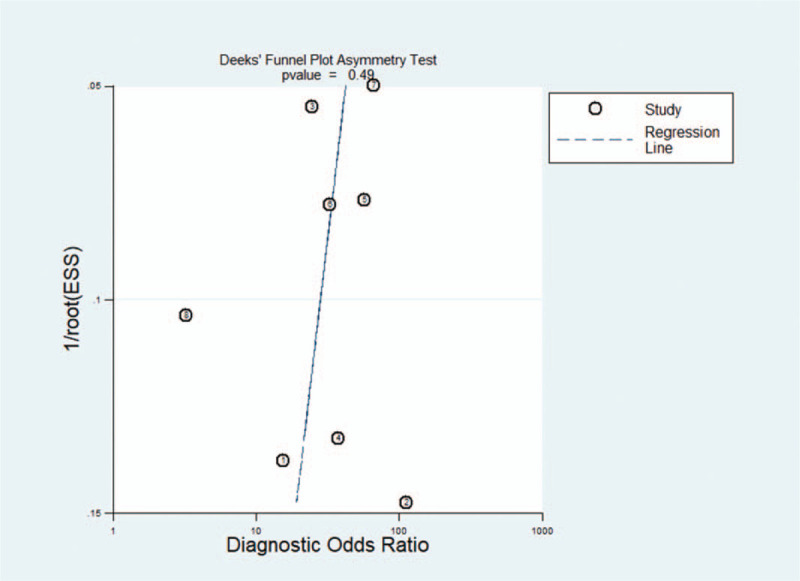
The Deek funnel plot.
4. Discussion
PTB remains a leading cause of death worldwide, especially for patients with HIV-coinfection.[28] The accurate discrimination of PTB is a key element of the World Health Organization “End TB Strategy.”[29] Conventional methods for PTB detection are limited by the need for sputum samples, time, expense, and BCG-vaccination status. In recent years, researchers have explored some new biomarkers (eg, interferon gamma-induced protein 10 and C-reactive protein) for auxiliary discrimination of PTB. Several studies have shown that MIG is a promising marker for PTB.[13–20] However, the overall diagnostic accuracy of MIG is unclear.
We firstly performed a meta-analysis to estimate the overall diagnostic performance of MIG for PTB. MIG has a moderate possibility of missed diagnoses (16%, sensitivity: 84%) and misdiagnoses (16%, specificity: 84%). The DOR and AUC were 27.88 and 0.90, respectively, indicating a good overall performance for PTB detection. A PLR of 5.19 and NLR of 0.19 further suggested that MIG had a good diagnostic value. Although the cut-off for MIG varied substantially (from 111.4 to 2183 pg/mL), the heterogeneity was relatively low (I2 = 0%, P = .234) and no outlier studies were identified by the Galbraith plot, indicating the high stability and reliability of the results. No striking publication bias (P = .49) also improved the objectivity of the results. Besides, MIG is more rapid (requiring no more than 6 hours) than microbiological culture (4–6 weeks to obtain results). MIG also had a higher sensitivity (84%) than that of sputum smear microscopy (20%–60%).
In our meta-regression analysis, HIV-coinfection was identified as a potential source of heterogeneity (P = .02). The sensitivity and specificity for patients with PTB/HIV co-infection were much lower than those for patients with only TB (0.70 vs 0.86, 0.70 vs 0.87). PTB is difficult to diagnose in patients with HIV because sputum samples are paucibacillary and unreliable.[30,31] Furthermore, patients with PTB/HIV coinfection are often in critical condition and have a high rate of death, thereby requiring rapid laboratory confirmation.[32] Only 2 studies (158 participants) reported HIV coinfection; although we agree with the results, further studies of patients with PTB/HIV co-infection are needed.
The precise diagnostic value of MIG for PTB might be lower than that previously reported for several reasons. First, heterogeneity is a concern. HIV coinfection could increase heterogeneity. Second, some of the included studies were from the Republic of Korea, and 2 studies from the same group were conducted at the same hospital; 1 focused on stimulated MIG and enrolled patients from August 2012 to July 2014,[17] and the other concentrated with unstimulated MIG and enrolled patients from January 2010 to April 2012.[19] Selection bias cannot be excluded. Furthermore, MIG is usually evaluated in combination with other biomarkers; however, we did not address the reliability of marker combinations including MIG. Third, publication bias should not be ignored; owing to limited linguistic abilities, only English studies were included.
5. Conclusion
This meta-analysis showed that MIG has good diagnostic value for PTB. Further multi-center, large, and prospective studies are required to support this finding.
Author contributions
Conceptualization: Yang Li.
Data curation: Yang Li, Dengqi He.
Formal analysis: Yang Li, Dengqi He, Yinfu Che, Xinchen Zhao.
Funding acquisition: Yang Li.
Investigation: Yang Li, Dengqi He, Yinfu Che, Xinchen Zhao.
Methodology: Yang Li, Dengqi He, Yinfu Che.
Software: Yang Li, Dengqi He, Yinfu Che, Xinchen Zhao.
Writing – original draft: Yang Li.
Writing – review & editing: Yang Li.
Footnotes
Abbreviations: 95% CI = 95% confidence interval, AUC = area under the curve, DOR = diagnostic odds ratio, HIV = human immunodeficiency virus, MIG = monokine induced by gamma interferon, NLR = negative likelihood ratio, PLR = positive likelihood ratio, PTB = pulmonary tuberculosis.
How to cite this article: Li Y, He D, Che Y, Zhao X. Monokine induced by gamma interferon for detecting pulmonary tuberculosis: a diagnostic meta-analysis. Medicine. 2020;99:47(e23302).
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
This work was supported by the Scientific Research Management Project Grants from Health Industry of Gansu Province (GWGL2014-06).
The authors have no conflicts of interest to disclose.
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
References
- [1].Boardman NJ, Moore T, Freiman J, et al. Pulmonary tuberculosis disease among immigrant detainees: rapid disease detection, high prevalence of asymptomatic disease and implications for tuberculosis prevention. Clin Infect Dis 2020;ii:ciaa 434.[Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- [2].The World Health Organizations. Global tuberculosis report 2019. Available at: www.aidsdatahub.org/global-tuberculosis-report-2019-who-2019. Accessed 17 October, 2019. [Google Scholar]
- [3].Araujo Z, Macias-Segura N, Lopez-Ramos JE, et al. Diagnostic accuracy of combinations of serological biomarkers for identifying clinical tuberculosis. J Infect Dev Ctries 2018;12:429–41. [DOI] [PubMed] [Google Scholar]
- [4].Denkinger CM, Schumacher SG, Boehme CC, et al. Xpert MTB/RIF assay for the diagnosis of extrapulmonary tuberculosis: a systematic review and meta-analysis. Eur Respir J 2014;44:435–46. [DOI] [PubMed] [Google Scholar]
- [5].Lönnroth K, Raviglione M. The WHO's new end TB strategy in the post-2015 era of the sustainable development goals. Trans R Soc Trop Med Hyg 2016;110:148–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Turner CT, Gupta RK, Tsaliki E, et al. Blood transcriptional biomarkers for active pulmonary tuberculosis in a high-burden setting: a prospective, observational, diagnostic accuracy study. Lancet Respir Med 2020;8:407–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Hu X, Liao S, Bai H, et al. LncRNA and predictive model to improve the diagnosis of clinically diagnosed pulmonary tuberculosis. J Clin Microbiol 2020;58:e01973-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Walusimbi S, Bwanga F, De Costa A, et al. Meta-analysis to compare the accuracy of GeneXpert, MODS and the WHO 2007 algorithm for diagnosis of smear-negative pulmonary tuberculosis. BMC Infect Dis 2013;13:507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Esmail A, Tomasicchio M, Meldau R, et al. Comparison of Xpert MTB/RIF (G4) and Xpert Ultra, including trace readouts, for the diagnosis of pulmonary tuberculosis in a TB and HIV endemic setting. Int J Infect Dis 2020;95:246–52. [DOI] [PubMed] [Google Scholar]
- [10].Theron G, Peter J, van Zyl-Smit R, et al. Evaluation of the Xpert MTB/RIF assay for the diagnosis of pulmonary tuberculosis in a high HIV prevalence setting. Am J Respir Crit Care Med 2011;184:132–40. [DOI] [PubMed] [Google Scholar]
- [11].Chegou NN, Heyckendorf J, Walzl G, et al. Beyond the IFN-γ horizon: biomarkers for immunodiagnosis of infection with Mycobacterium tuberculosis. Eur Respir J 2014;43:1472–86. [DOI] [PubMed] [Google Scholar]
- [12].Almeida Cde S, Abramo C, Alves CC, et al. Anti-mycobacterial treatment reduces high plasma levels of CXC-chemokines detected in active tuberculosis by cytometric bead array. Mem Inst Oswaldo Cruz 2009;104:1039–41. [DOI] [PubMed] [Google Scholar]
- [13].Manngo PM, Gutschmidt A, Snyders CI, et al. Prospective evaluation of host biomarkers other than interferon gamma in QuantiFERON Plus supernatants as candidates for the diagnosis of tuberculosis in symptomatic individuals. J Infect 2019;79:228–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].La Manna MP, Orlando V, Li Donni P, et al. Identification of plasma biomarkers for discrimination between tuberculosis infection/disease and pulmonary non tuberculosis disease. PLoS One 2018;13:e0192664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Jacobs R, Malherbe S, Loxton AG, et al. Identification of novel host biomarkers in plasma as candidates for the immunodiagnosis of tuberculosis disease and monitoring of tuberculosis treatment response. Oncotarget 2016;7:57581–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Kim S, Lee H, Kim H, et al. Diagnostic performance of a cytokine and IFN-γ-induced chemokine mRNA assay after Mycobacterium tuberculosis specific antigen stimulation in whole blood from infected individuals. J Mol Diagn 2015;17:90–9. [DOI] [PubMed] [Google Scholar]
- [17].Chung W, Lee K, Jung Y, et al. Serum CXCR3 ligands as biomarkers for the diagnosis and treatment monitoring of tuberculosis. Int J Tuberc Lung Dis 2015;19:1476–84. [DOI] [PubMed] [Google Scholar]
- [18].Lee K, Chung W, Jung Y, et al. CXCR3 ligands as clinical markers for pulmonary tuberculosis. Int J Tuberc Lung Dis 2015;19:191–9. [DOI] [PubMed] [Google Scholar]
- [19].Chung WY, Lee KS, Jung YJ, et al. A TB antigen-stimulated CXCR3 ligand assay for the diagnosis of active pulmonary TB. Chest 2014;146:283–91. [DOI] [PubMed] [Google Scholar]
- [20].Wang X, Jiang J, Cao Z, et al. Diagnostic performance of multiplex cytokine and chemokine assay for tuberculosis. Tuberculosis (Edinb) 2012;92:513–20. [DOI] [PubMed] [Google Scholar]
- [21].Moher D, Liberati A, Tetzlaff J, et al. The PRISMA group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Whiting PF, Rutjes AW, Westwood ME, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med 2011;155:529–36. [DOI] [PubMed] [Google Scholar]
- [23].Zhou Y, Dendukuri N. Statistics for quantifying heterogeneity in univariate and bivariate meta-analyses of binary data: the case of meta-analyses of diagnostic accuracy. Stat Med 2014;33:2701–17. [DOI] [PubMed] [Google Scholar]
- [24].Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Devillé WL, Buntinx F, Bouter LM, et al. Conducting systematic reviews of diagnostic studies: didactic guidelines. BMC Med Res Methodol 2002;2:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Chappell FM, Raab GM, Wardlaw JM. When are summary ROC curves appropriate for diagnostic meta-analyses? Stat Med 2009;28:2653–68. [DOI] [PubMed] [Google Scholar]
- [27].Deeks JJ, Macaskill P, Irwig L. The performance of tests of publication bias and other sample size effects in systematic reviews of diagnostic test accuracy was assessed. J Clin Epidemiol 2005;58:882–93. [DOI] [PubMed] [Google Scholar]
- [28].Hamada Y, Lujan J, Schenkel K, et al. Sensitivity and specificity of WHO's recommended four-symptom screening rule for tuberculosis in people living with HIV: a systematic review and meta-analysis. Lancet HIV 2018;5:e515–23. [DOI] [PubMed] [Google Scholar]
- [29].Qiu X, Xiong T, Su X, et al. Accumulate evidence for IP-10 in diagnosing pulmonary tuberculosis. BMC Infect Dis 2019;19:924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Boyles TH, Griesel R, Stewart A, et al. Incremental yield and cost of urine Determine TB-LAM and sputum induction in seriously ill adults with HIV. Int J Infect Dis 2018;75:67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Broger T, Nicol MP, Székely R, et al. Diagnostic accuracy of a novel tuberculosis point-of-care urine lipoarabinomannan assay for people living with HIV: a meta-analysis of individual in- and outpatient data. PLoS Med 2020;17:e1003113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Garrido-Cardenas JA, de Lamo-Sevilla C, Cabezas-Fernández MT, et al. Global tuberculosis research and its future prospects. Tuberculosis (Edinb) 2020;121:101917. [DOI] [PubMed] [Google Scholar]


