Abstract
Xanthium strumarium is native to North America and now has become one of the invasive alien species (IAS) in China. In order to detect the effects of the invader on biodiversity and evaluate its suitable habitats and ecological distribution, we investigated the abundance, relative abundance, diversity indices, and the number of the invasive and native plants in paired invaded and non-invaded quadrats in four locations in North and Northeast China. We also analyzed the effects of monthly mean maximum and minimum temperatures, relative humidity (%), and precipitations (mm). Strong positive significant (P < 0.01) correlation and maximum interspecific competition (41%) were found in Huailai between invaded and non-invaded quadrats. Shannon’s Diversity Index showed that non-invaded plots had significantly (P < 0.05) more diversified species than invaded ones. The significant (P < 0.05) Margalef’s Richness Index was found in Huailai and Zhangjiakou in non-invaded recorded heterogeneous nature of plant communities. Similarly, significant (P < 0.05) species richness found in Huailai and Zhangjiakou in non-invaded quadrats compared to invaded ones. Maximum evenness of Setaria feberi (0.47, 0.37), Seteria viridis (0.43) found in Fushun and Zhangjiakou recorded more stable in a community compared to other localities. Evenness showed positive relationship of Shannon Entropy within different plant species. The higher dissimilarity in plant communities found in Huailai (87.06%) followed by Yangyuan (44.43%), Zhangjiakou (40.13%) and Fushun (29.02%). The significant (P < 0.01) value of global statistics R (0.943/94.3%) showed high species diversity recorded in Huailai followed by Zhangjiakou recorded by non-metric multidimensional scaling and analysis of similarity between invaded and non-invaded plots. At the end it was concluded that the diversity indices reduced significantly (P < 0.05) in invaded quadrats indicated that native plant species become less diverse due to X. strumarium invasion. The degrees of X. strumarium invasion affected on species richness resulted to reduce diversity indices significantly in invaded quadrats.
Introduction
Invasive plants are a major threat to the biodiversity and functioning of ecological systems [1–3]. Thus, biological invasions have become one of the hotspots in ecological research [4], The invasive plant Xanthium strumarium L. competes with native plant species for soil nutrients, moisture, “shelter”, “light”, and other resources, severely influencing natural vegetations [5]. The invasive plant is monoecious and annual herb of Asteraceae family, with broad leaves and tap-roots [6, 7]. It is native to North América and Argentina [8–10], but now become a noxious invader in China due to its strong colonization potential in new areas [11]. It has strong ability to adapt in diverse soils and climates. This plant has spread in six provinces in China including Hebei and Liaoning.
Invasive alien species has been recognized cause of biodiversity loss in the world [12]. X. strumarium has become a major concern in China, posing severe problems on the rangeland biodiversity, agriculture lands, parks, banks of river, and lakes, dams, roadsides, and even in urban areas, with great economic and ecological consequences. This plant is a severe threat to agriculture field crops such as soybeans, cotton, maize, sunflower and groundnuts in many parts of the world along with China [13]. It can also invade pastures and grazing lands causing reductions in forage production and fur damage through the thorns on the fruits [14]. It competes with, or even out-competes native species, decreasing genetic and species diversity within populations and altering ecosystem [15]. Multivariate analysis for ordination and analysis of similarity showed decreases in biodiversity indices in invaded over control sites, indicating that plant communities become less diverse due to X. strumarium invasion [16]. This plant has imposed negative impacts on ecosystem properties [17, 18].
The competitive patterns between invasive and native plant species may be different at different invasion steps such as transport stage, colonization stage, establishment stage, landscape spread stage (Fig 1) involved in invasion success [19].
Fig 1. Main stages and factors affecting invasion success of introduced plants [20, 21].

X. strumarium usually competes with native plant species, and cause great loss in biodiversity. In order to detect the effects of the invader on biodiversity and evaluate its suitable habitats and ecological distribution, we investigated the abundance, relative abundance, diversity indices, and the number of the invasive and native plants in ten pairs of invaded and non-invaded quadrats in four locations in North and Northeast China (S1 Table in S1 File). In this study, we want to explore the following questions. (1) Does X. strumarium have significant effects on native plant species in paired comparison with diversity indices? (2) What is the paired correlation of different plant species both invaded and non-invaded quadrats in beta diversity? (3) How does X. strumarium invade and compete with native plant species in a paired comparison? (4) What is the relationship of Shannon Entropy with species diversity and evenness?
Materials and methods
This study was carried out in Hebei and Liaoning Provinces in the summer (August and September) of 2018 using an ecological line transects method [22]. Three locations were selected in Hebei, i.e Yangyuan (40° 12′ 749″ N, 114° 39′ 920″ E), Huailai (40° 22′ 411′′ N, 115° 31′ 581′′ E), and Zhangjiakou (40° 51′ 290′′ N, 114° 51′ 378′′ E) and one location was selected in Liaoning, i.e., Fushun (41° 51′ 279′′ N, 123° 49′ 126′′ E). The precipitation is higher but monthly mean temperatures are lower in Fushun than in Zhangjiakou, Huailai and Yangyuan (S1 Fig in S1 File). In each location, 10 places were selected (10 replicates per location); and at each place, three pairs of invaded and non-invaded quadrats (100 * 100 cm) were setup (3 replicates per place). The 10 spaces in each location were at least 20 km apart from each other [23]. In order to diminish potential confounding effects of habitat heterogeneity on comparisons between invaded and non-invaded quadrats (i.e., the effects of the invader), the quadrats with and without X. strumarium were less than 2 m apart in each pair, and the quadrats in different pair were spaced at least 5 m [23]. The non-invaded quadrats were setup at second vegetations, and the invaded quadrats were dominated by X. strumarium [24]. In total, we had 120 pairs of quadrats with and without X. strumarium (4 locations × 10 places × 3 replicates). All plant species were identified and their individuals were counted in all invaded and non-invaded studied quadrats (S1 Table in S1 File) along with plant species did not found in studied quadrats but also found in four locations (S2 Table in S1 File). The averages of monthly mean maximum and minimum temperatures (Max. T and Mini. T, respectively), monthly mean precipitation (PPT/ppt) and monthly mean relative humidity (RH) was collected from data base of http://data.cma.cn/data/weatherBK.html (S1 Fig in S1 File).
Non-metric multidimensional scaling and analysis of similarity were conducted to determine the resemblance of species composition between the invaded and non-invaded quadrats through Bray-Curtis similarity/dissimilarity following log-transformation of plant species abundance data due to zero/no species count in some plots using one way analysis of variance with Global statistics R through PRIMER 7 programming [25]. The estimations of global measurement (R) values vary between +1 and -1; and the bigger of the R value is (close to 1), the more significant the dissimilarity (P < 0.01 or 1%) between the invaded and non-invaded quadrats is 0 to 100 with 100 expressing the most extreme dissimilarity [10, 26]. Similarity percentage method was used to assess contribution of each species to the dissimilarity between the invaded and non-invaded quadrats [27]. The degree of invasion impact was evaluated using diversity indices including Margalef's Richness Index, Shannon Entropy, Simpson’s Diversity Index, Shannon’s Diversity Index, Species Richness, Species Equitability or Evenness, and Abundance of different plant species between invaded and non-invaded quadrats [25, 28]. The differences in the diversity indices between the invaded and non-invaded quadrats were determined by analysis of variance with invasion status and locations as a factor in SPSS 13.0 (SPSS Inc., Chicago, IL, USA) and using Permutation analysis of variance through PRIMER 7 software. The competitive pattern was recorded between plant species within the selected quadrats calculated on the basis of Relative Abundance [25, 28–30]. The difference in plant species in each location between invaded, non-invaded quadrats and their correlation were calculated individually by paired t-test through SPSS 13.0 (SPSS Inc., Chicago, IL, USA).
Results
Twelve plant species from ten genus and eight families were identified in our study, eight forbs, four grasses. Only two species (Xanthium strumarium and Chenopodium album) occurred in all four locations, with three species (Artemisia annua, Setaria feberi, and Setaria viridis) in three locations, two in two locations, and five in only one location (S1 Table in S1 File).
Some plant species were identified during field survey but not present in the selected quadrats. This data found that only one plant species found in three locations, eleven found in two locations and fifteen found in only one location, seventeen forbs, one sedge, three shrubs and six grass plant species (S2 Table in S1 File).
Shannon Entropy recorded in Malva verticilata (0.36) and Ch. album (0.36) with maximum evenness found in Avena sativa (0.20) and S. viridis (0.20) in its invaded quadrats were more stable in the community followed by other plant species. M. verticilata (0.94) and Ch. album (0.95) were more diversified in a community in its non-invaded quadrats at Yangyuan (S3A Table in S1 File). The calculated values of Shannon Entropy recorded high in S. viridis (0.33), Cynunchum chinensis (0.32); however, similar evenness was found by Ch. album (0.15) and S. viridis (0.15) in its invaded quadrats. X. strumarium was more diversified in the community at Huailai (0.74) compared to all other locations. Similarly, S. feberi (0.32), Ch. album (0.30), A. annua (0.30) found maximum Shannon Entropy with maximum evenness (0.15, 0.14, 0.14) was recorded that gave better stability in the community in its non-invaded quadrats at Huailai (S3B Table in S1 File). More Shannon Entropy was recorded by S. feberi (0.87) found with high evenness (0.37) recorded more diversified and stable in the community in its non-invaded quadrats at Zhangjiakou. Similar results were recorded with high diversity of S. feberi (0.47) and S. viridis (0.43) in its non-invaded quadrats at Fushun (S3C and S3D Table in S1 File).
Strong significant (P < 0.01) correlation was recorded among invaded and non-invaded quadrats found in Huailai comparable to other locations showed nonsignificant (P > 0.05) investigations. Species richness recorded highly significant (P < 0.001) results in all locations. Native plant species are more diversified found significant (P < 0.05) individuals per quadrat in its non-invaded compared to invaded by X. strumarium (Table 1 and Fig 2).
Table 1. Analysis of variance of the invasion impact on species richness among invaded and non-invaded quadrats at different locations.
| Locations | Invaded quadrat (Mean+SE) | Non-Invaded quadrat (Mean+SE) | Correlation of I versus NI | Species Richness |
|---|---|---|---|---|
| Yangyuan | 19.60+0.86 | 40.71+0.49 | N | *** |
| Huailai | 12.68+0.64 | 34.29+0.82 | ** | *** |
| Zhangjiakou | 34.20+1.32 | 50.09+1.10 | N | *** |
| Fushun | 37.92+1.17 | 57.96+1.91 | N | *** |
Whereas N = 30 (Number of quadrats 10 x 3 replication), I (Invaded), NI (Non-invaded); Mean + 1 SE
**, P < 0.01
***, P < 0.001, N, P > 0.05.
Fig 2.
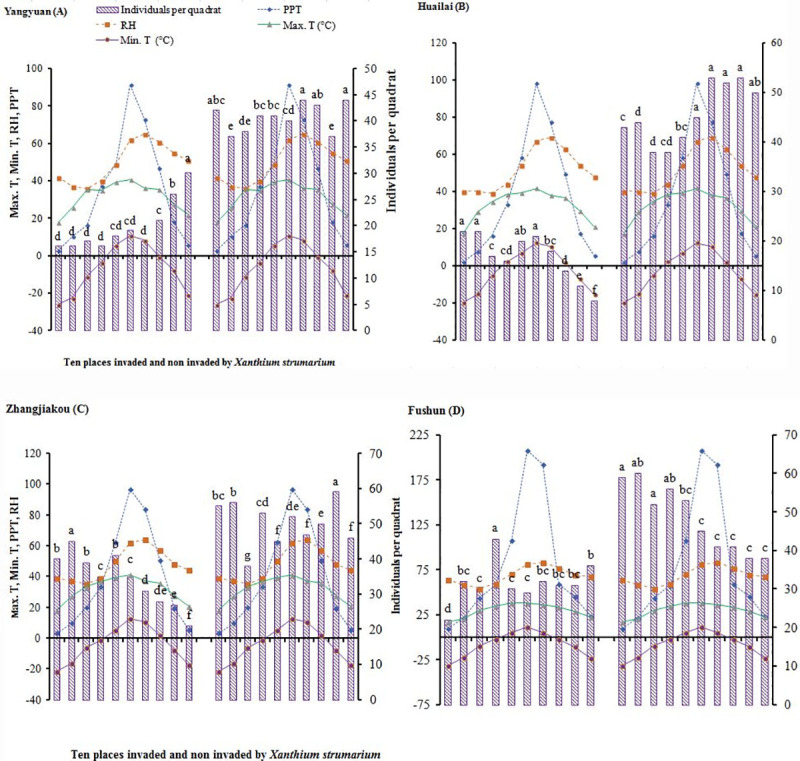
Number of plant individuals per quadrat (N = 10) and environmental factors (from February to November in 2018) found at ten invaded and ten non-invaded quadrats in Yangyuan (A), Huailai (B), Zhangjiakou (C) and Fushun (D), respectively. Max. T., average of monthly mean maximum temperature; Mini. T., average of monthly mean minimum temperature; RH, average of monthly mean relative humidity; PPT, average of monthly mean precipitation. Different letters indicates significant difference (P < 0.05; analysis of variance).
Significantly higher (P < 0.05) species richness was recorded at non-invaded quadrats in Zhangjiakou, Huailai and Yangyuan (Fig 3D). Significantly higher (P < 0.05) paired abundance (Fig 3A) was found in Huailai (t = 18.45, P = 0.003), Zhangjiakou (t = 7.56, P = 0.017) compared to Fushun (t = 2.18, P = 0.161). Strong positive nonsignificant (P = 0.281) paired samples correlations (r = 0.904) were recorded in Huailai. Shannon’s Diversity Index was significantly higher (P < 0.05) in non-invaded quadrats at Yangyuan (t = 13.86, P = 0.005), Huailai (t = 4.84, P = 0.040), Zhangjiakou (t = 30.44, P = 0.001), and Fushun (t = 10.61, P = 0.009) described in Fig 3B. Similarly highly significant (P < 0.01) Margalef's Richness Index found at Zhangjiakou (t = 23.09, P = 0.002), Huailai (t = 22.52, P = 0.002), Yangyuan (t = 5.20, P = 0.035), however nonsignificant (P > 0.05) index found in Fushun in both quadrats (t = 0.00, P = 1). There is a strong positive paired correlation (r = 1) and highly significant (P = 0.000) Margalef’s Richness Index found in Huailai and Zhangjiakou but strong negative and significant paired sampled correlation recorded in Yangyuan compared to moderate positive correlation (r = 0.5) recorded nonsignificant (P > 0.05) relationship at Fushun (Fig 3C).
Fig 3. Difference in each ecological index between the invaded and non-invaded quadrats at Yangyuan, Huailai, Zhangjiakou and Fushun.

(A), Abundance; (B), Shannon’s Diversity Index; (C), Margalef’s Richness Index; (D), Species Richness. Mean ± 1 SE (N = 10). *, P < 0.05, **, P < 0.01, NS, P > 0.05.
In all four locations, interspecific competition intensity was significantly stronger (P < 0.05) at invaded relative to non-invaded quadrats. Species richness was significantly higher in non-invaded compared to invaded quadrat in Zhangjiakou (83.49 vs. 57.00), Huailai (57.15 vs. 21.14), Yangyuan (67.83 vs. 32.67), and Fushun (96.60 vs. 63.20) described in Fig 4. Maximum interspecific competition was found in invaded quadrats at Huailai (41%; Fig 4B), which indicates that X. strumarium competes with native plant species greatly in studied ecosystems. However, 14% and 12% interspecific competitions were found at invaded quadrats in Zhangjiakou and Yangyuan, respectively (Fig 4A and 4C). In Zhangjiakou S. feberi (34%), in Fushun S. feberi and S. viridis recorded maximum relative abundance in its non-invaded quadrats (Fig 4C and 4D).
Fig 4. Web plot showed the interspecific competition between different plant species in invaded and non-invaded quadrats based on relative abundance.
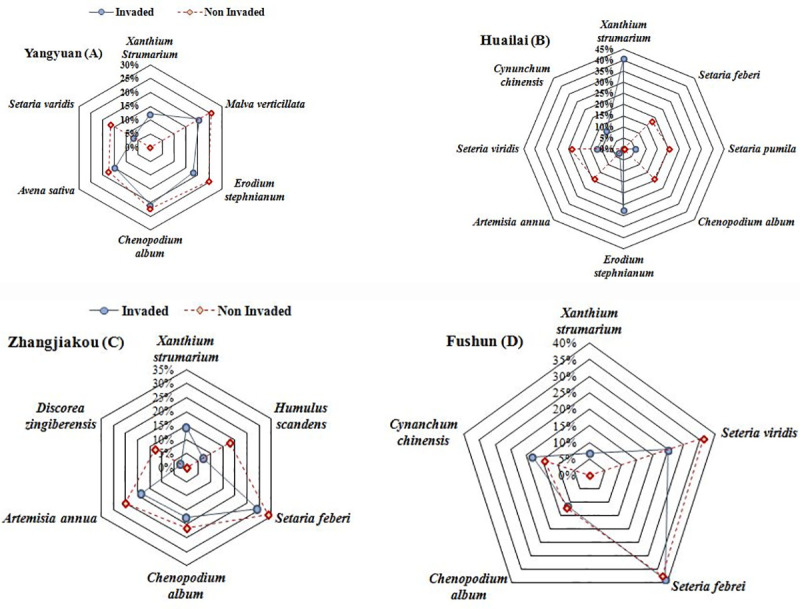
The central point is 0%, each ring gives the indication of the magnitude (%) of interspecific competition among plants in invaded and non-invaded quadrats.
Similarity percentage analysis suggested that abundance of plant species was high in non-invaded relative to invaded quadrats at four locations and their dissimilarities between the non-invaded and invaded quadrats were recorded significant investigations (Table 2).
Table 2. Abundances of the plant species present in each location, their dissimilarities (based on abundance) between the invaded and non-invaded quadrats, and their contributions (%) to the total dissimilarities.
| Locations/Plant species | Average abundance in invaded quadrats* | Average abundance in non-invaded quadrats* | Average dissimilarity between invaded and non-invaded quadrats | Standard deviation | Contribution to dissimilarity (%) | |
|---|---|---|---|---|---|---|
| Yangyuan | ||||||
| Malva verticilata | 4.00 | 10.40 | 10.64 | 2.54 | 23.94 | |
| Seteria viridis | 1.40 | 6.80 | 9.24 | 1.95 | 20.80 | |
| Chenopodium album | 4.10 | 9.00 | 8.27 | 2.27 | 18.62 | |
| Avena sativa | 2.90 | 7.10 | 7.38 | 1.48 | 16.62 | |
| Huailai | ||||||
| Seteria viridis | 2.10 | 7.60 | 12.19 | 1.29 | 14.00 | |
| Chenopodium album | 0.00 | 6.10 | 12.05 | 2.14 | 13.84 | |
| Artemisia annua | 0.50 | 6.10 | 11.53 | 1.69 | 13.24 | |
| Setaria pumila | 0.90 | 6.50 | 11.46 | 1.78 | 13.16 | |
| Zhangjiakou | ||||||
| Seteria febrei | 10.00 | 16.80 | 9.79 | 1.30 | 24.40 | |
| Discorea zingiberensis | 0.80 | 6.50 | 7.28 | 1.44 | 18.13 | |
| Artemisia annua | 6.30 | 12.50 | 7.27 | 1.81 | 18.12 | |
| Fushun | ||||||
| Seteria viridis | 7.90 | 17.50 | 11.84 | 2.41 | 40.82 | |
| Seteria febrei | 12.30 | 8.10 | 7.86 | 1.47 | 27.10 | |
| Chenopodium album | 3.60 | 5.80 | 4.31 | 1.58 | 14.86 | |
The plant species collectively explained 79.98%, 54.24%, 60.65% and 82.78% of the total dissimilarities in Yangyuan, Huailai, Zhangjiakou and Fushun, respectively. The total dissimilarities were 44.43, 87.06, 40.13 and 29.02 in Yangyuan, Huailai, Zhangjiakou and Fushun, respectively.
Whereas
*: 1-rare, 2-common, 3-very common, >4-dominant.
The total dissimilarities between non-invaded and invaded quadrats were 44.43, 87.06, 40.13, and 29.02 in Yangyuan, Huailai, Zhangjiakou, and Fushun, respectively. The most commonly occurring species collectively explained 79.98%, 54.24%, 60.65%, and 82.78% of the total dissimilarities found in Yangyuan, Huailai, Zhangjiakou, and Fushun, respectively. According to non-metric multidimensional scaling ordination and similarity analysis, the differences in species compositions between invaded and non-invaded quadrats were also significant, with global statistics R values found 0.62 (P < 0.01), 0.943 (P < 0.01), 0.779 (P < 0.01), and 0.553 (P < 0.01) in Yangyuan, Huailai, Zhangjiakou, and Fushun, respectively (S2 Fig in S1 File).
Discussion
We examined mechanism of Xanthium strumarium invasion on native plant species found interspecific competition. X. strumarium affected directly with native plant species in its invaded quadrats. In Huailai, there was significant (P < 0.05) result among native species found in non-invaded quadrats, however maximum interspecific competition found in invaded quadrats at Huailai (41%). The decreased value of Shannon’s Diversity Index in invaded plot over non-invaded provided a clear indication that native plant species were less diversified due to X. strumarium invasion. In the non-invaded plots, the competition mechanism among native plants was significantly higher than invaded plots. The high Margalef's Richness Index in non-invaded found heterogeneous nature of plant communities. The native plant abundance reduced significantly (P < 0.05) in invaded plots due to X. strumarium invasion. These results are consistent with scientists who reported negative impact of X. strumarium on native plant species [16]. In our study 24 vs. 19 plant individuals present in all non-invaded vs. invaded quadrats are in line with the researchers who reported 70 vs. 31 plant species [25]. Margalef’s Richness Index and Shannon’s Diversity Index significantly (P < 0.05) decreased in the invaded plots up to 70.33% and 69.39% due to X. strumarium invasion [25]. A significant (P<0.05) Shannon’s diversity index recorded between non-invaded and invaded plots at Yangyuan, Huailai and Zhangjiakou. X. strumarium invasion mechanism found diversified in the community at Huailai recorded Simpson’s Diversity index (0.74) compared to all other locations. More Shannon Entropy was recorded by Setaria feberi (0.87) found with high evenness (0.37) recorded more diversified and stable in the community in its non-invaded quadrats at Zhangjiakou. Similar results were recorded with high diversity of Seteria feberi (0.47) and Seteria viridis (0.43) in its non-invaded quadrats at Fushun. Total number of individuals recorded in ten Invaded quadrats (169) compared to non-invaded (457) found strong positive significant correlation (P < 0.01) indicated X. strumarium invasion mechanism in Huailai. There is a positive relationship of Shannon Entropy with species diversity and evenness, however, species evenness increased with the increase of species diversity and Shannon Entropy. Shannon Entropy showed that different plant species in a community had a relative abundance and diverse in a community. These results are in line with the researchers who gave similar recommendations in their experiments [31–33]. The degrees of X. strumarium invasion affected on species richness in invaded quadrat resulted to reduce Shannon’s diversity index significantly (P < 0.05) are in line with the researchers who gave similar recommendations [34]. The native plant species abundance decreased significantly (P<0.05) up to 55.71% due to X. strumarium invasion. The researchers reported that twenty four families were recorded in non-invaded plots compared to fifteen in X. strumarium invaded areas. However, X. strumarium categorized as one of the dominant invasive alien plant that reduced native species and composition of various plant species in invaded communities [35]. The findings of our analysis are also consistent with the researchers who investigated strong impact of invaded species on native populations [36]. The diversity indices reduced in invaded plots compared to non-invaded supported our hypotheses that X. strumarium competed with native plant species because of their high phenotypic plasticity in the environment. These results are consistent with researchers who reported that X. strumarium showed its toxic effect on native plant populations in ecosystem [17]. A large canopy and tap root system of X. strumarium showed substantial impact on native plant communities, but its population abundance threatens native plant communities seriously. Invaded quadrats were found maximum uniformity and good competitor in ecosystem process. The plant invasions recorded serious influence on vegetation size and composition by interfering with biotic interactions in abiotic networks [37]. Maximum families (Asteraceae, Poaceae and Apocynaceae) found in our study during survey at different locations; however these three families have maximum tendency to flourish in an ecosystem (S2 Table in S1 File). Invasive species are characterized by their large, persistent, elastic, rapid growth, their ability to travel through vast areas and their rich reproduction allowed them to compete with indigenous plant species [38, 39]. The scientists recorded that the X. strumarium was the most aggressive alien-invasive plant in Ethiopia [40]. In invaded and non-invaded squares, researchers previously conducted paired comparative study which indicated that invaded plant species were competed and affected on native plant species [41]. The hypotheses are true that invasive alien plant can compete in a population with native plant species. These findings were consistent with researchers in their experiments who identified heterogeneous plant populations [42]. Maximum dissimilarity or beta diversity was recorded in Huailai (87.06%) followed by Yangyuan (44.43%), Zhangjiakou (40.13%), and Fushun (29.02%). X. strumarium influenced on species abundance in this study which was consistent with the researchers who identified Jaccard's similarity indices between invaded and non-invaded ranges that suggested loss of approximately 38.40% of plant species due to the X. strumarium invasions resulted in a 61.60% dissimilarity index [35]. Significantly (P<0.01) maximum global test for sample statistics R values were reported in Huailai (0.943/94.3%) followed by Zhangjiakou, Yangyuan and Fushun, where similarities were analyzed by one-way analysis of variance taking 999 number of permutations. The higher value of global statistics R suggested that there was a greater impact of species diversity on the variables studied. Yangyuan, Huailai, and Zhangjiakou areas were affected significantly by X. strumarium invasions. The lowest invasion was found in Fushun due to the high dissimilarity or beta diversity in invaded and non-invaded plots. Strong negative and significant (P<0.05) relationship (R = 0.741/74.1%, P<0.05) found between X. strumarium and species richness. These results are consistent with the scientists who stated that the regression equation and the Pearson correlation (-0.861) suggested the presence of negative linear relationship between X. strumarium and native species however, richness decreased significantly with the increased impact of X. strumarium [35].
Conclusion
Xanthium strumarium was a good competitor against natural flora in the studied network. The elevated Shannon’s Diversity Index showed that plant networks in non-invaded areas were increased heterogeneously. X. strumarium invasion mechanism was diversified in the community at Huailai. Shannon Entropy recorded by Setaria feberi found high evenness were found more diversified and stable in the community in its non-invaded quadrats at Zhangjiakou. Similar findings were recorded and found high diversity of Seteria feberi and Seteria viridis at Fushun. Higher estimates of global statistics R showed the increased influence of decent species on the factors studied. The most affected location identified by X. strumarium invasion was Huailai followed by Zhangjiakou and Yangyuan. The mechanism of X. strumarium in our study recorded interspecific competition which suggested that this plant influenced negatively on native plant species. The degrees of X. strumarium invasion can affect species richness in invaded quadrat, resulting to reduce diversity indices significantly. There is dire need to develop integrated invasive plant management strategies to overcome X. strumarium invasion. This invasive plant species are spreading in cropped area of China, and may become future risk for growers. The study also encouraged researchers to explore the mechanism of protection, nutrient cycling, the role of pseudomonas bacteria, mechanism of resistance of invasive and native plants in future.
Supporting information
(DOCX)
Acknowledgments
We are grateful to Dr. Maqsood Ahmed for help during the field survey and also Wei-Wei Feng for her assistance in data analysis. The authors are also thankful to three anonymous reviewers for their helpful comments and suggestion on an early version of this manuscript.
Data Availability
All relevant data have been added within the paper and uploaded minimal data set as a Supporting Information file.
Funding Statement
We are thankful to the support of National Key R&D Program of China (2017YFC1200101), the National Natural Science Foundation of China (31670545, and 31971557). The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Parker IM, Simberloff D, Lonsdale WM, Goodell K, Wonham M, Kareiva PM, et al. : Impact: toward a framework for understanding the ecological effects of invaders. Biological invasions 1999, 1(1):3–19. [Google Scholar]
- 2.Ehrenfeld JG: Ecosystem consequences of biological invasions. Annual review of ecology, evolution, and systematics 2010, 41:59–80. [Google Scholar]
- 3.Simberloff D: How common are invasion-induced ecosystem impacts? Biological invasions 2011, 13(5):1255–1268. [Google Scholar]
- 4.Khan MA, Qureshi RA, Gillani SA, Ghufran MA, Batool A, Sultana KN: Invasive species of federal capital area Islamabad, Pakistan. Pak J Bot 2010, 42(3):1529–1534. [Google Scholar]
- 5.Nigussie ST, Amare SA, Manaye MM, Edget MB, Ashenafi AH, Girum FB, et al. : Invasion and impacts of Xanthium strumarium in Borena Zone of Oromia Region, Ethiopia. Journal of Coastal Life Medicine 2017, 5(8):350–355. [Google Scholar]
- 6.Dekker J: The EEW 2nd Edition Narrative Book. 2018. [Google Scholar]
- 7.Dekker J: Evolutionary ecology of weeds Agronomy Department, Iowa State University, Ames, IA: 2011. [Google Scholar]
- 8.Stešević D, Petrović D: Preliminary list of plant invaders in Montenegro. Biologica Nyssana 2010, 1(1–2). [Google Scholar]
- 9.Nel JL, Richardson DM, Rouget M, Mgidi TN, Mdzeke N, Le Maitre DC, et al. : A proposed classification of invasive alien plant species in South Africa: towards prioritizing species and areas for management action: working for water. South African Journal of Science 2004, 100(1–2):53–64. [Google Scholar]
- 10.Osunkoya OO, Akinsanmi OA, Lim LSA, Perrett C, Callander J, Dhileepan K: Parthenium hysterophorus L. (Asteraceae) invasion had limited impact on major soil nutrients and enzyme activity: Is the null effect real or reflects data insensitivity? Plant and soil 2017, 420(1–2):177–194. [Google Scholar]
- 11.Harrison SK, Regnier EE, Schmoll JT: Postdispersal predation of giant ragweed (Ambrosia trifida) seed in no-tillage corn. Weed Science 2003, 51(6):955–964. [Google Scholar]
- 12.Genovesi P, Shine C: European strategy on invasive alien species: Convention on the Conservation of European Wildlife and Habitats (Bern Convention): Council of Europe; 2004. [Google Scholar]
- 13.Hussain Z, Marwat KB, Cardina J, Khan IA: Xanthium strumarium L. impact on corn yield and yield components. Turkish journal of agriculture and forestry 2014, 38(1):39–46. [Google Scholar]
- 14.Sartorato I, Berti A, Zanin G: Estimation of economic thresholds for weed control in soybean (Glycine max (L.) Merr.). Crop Protection 1996, 15(1):63–68. [Google Scholar]
- 15.Alpert P: The advantages and disadvantages of being introduced. Biological Invasions 2006, 8(7):1523–1534. [Google Scholar]
- 16.Qureshi H, Anwar T, Arshad M, Osunkoya OO, Adkins SW: Impacts of Xanthium strumarium L. Invasion on vascular plant diversity in Pothwar region (Pakistan). Annali di Botanica 2019, 9:73–82. [Google Scholar]
- 17.Lemma B, Tessema T, Fessehaie R: Distribution, abundance and socio-economic impacts of invasive plant species (IPS) in Borana and Guji Zones of Oromia National Regional State, Ethiopia. Ethiopia Journal of Agricultural Science and Review 2015, 4(9):271–279. [Google Scholar]
- 18.Tadesse NS, Assefa AS, Motbaynor MM, Betsiha EM, Hailu AA, Beyene GF, et al. : Invasion and impacts of Xanthium strumarium in Borena Zone of Oromia Region, Ethiopia. Journal of Coastal Life Medicine 2017, 5(8):350–355. [Google Scholar]
- 19.Lockwood JL, Cassey P, Blackburn T: The role of propagule pressure in explaining species invasions. Trends in ecology & evolution 2005, 20(5):223–228. 10.1016/j.tree.2005.02.004 [DOI] [PubMed] [Google Scholar]
- 20.Rai PK: Plant invasion ecology: Impacts and Sustainable management: Nova Science Publishers, Incorporated; 2013. [Google Scholar]
- 21.Rai PK: What makes the plant invasion possible? Paradigm of invasion mechanisms, theories and attributes. Environmental Skeptics and Critics 2015, 4(2):36. [Google Scholar]
- 22.Iqbal MF, Feng YL, Liu MC, Lu XR, Nasir M, Sikandar A: Parasitic activity of powdery mildew (Pathogen strain HMLAC 226) on prostrate knotweed (Polygonum aviculare L.) at various locations of Shenyang, Northeast China. Applied Ecology and Environmental Research 2019, 17(6):13383–13394. [Google Scholar]
- 23.Zhao Y-Z, Liu M-C, Feng Y-L, Wang D, Feng W-W, Clay K, et al. : Release from below-and aboveground natural enemies contributes to invasion success of a temperate invader. Plant and Soil 2020:1–10. [Google Scholar]
- 24.Arifa Z, Ahmad SS, Sardar AA, Zaheer-ud-Din K: Evaluation of ecological aspects of natural vegetation of Pakpattan District using multivariate techniques. Journal of Biodiversity and Environmental Sciences (JBES) 2014, 5(4):230–238. [Google Scholar]
- 25.Qureshi H, Arshad M, Bibi Y, Osunkoya OO, Adkins SW: Multivariate impact analysis of Parthenium hysterophorus invasion on above-ground plant diversity of Pothwar region of Pakistan. Applied Ecology and Environmental Research 2018, 16(5):5799–5813. [Google Scholar]
- 26.Clarke KR, Gorley RN, Somerfield PJ, Warwick RM: Change in marine communities: an approach to statistical analysis and interpretation: Primer-E Ltd; 2014. [Google Scholar]
- 27.Clarke KR, Warwick RM: Change in marine communities. An approach to statistical analysis and interpretation 2001. [Google Scholar]
- 28.Iqbal MF, Feng Y-L: Species diversity of different insect families trapped under beer-based volatile fermentation. BMC Chemistry 2020, 14(1):48 10.1186/s13065-020-00699-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chao A, Chazdon RL, Colwell RK, Shen TJ: A new statistical approach for assessing similarity of species composition with incidence and abundance data. Ecology letters 2005, 8(2):148–159. [Google Scholar]
- 30.Maszura CM, Karim SMR, Norhafizah MZ, Kayat F, Arifullah M: Distribution, Density, and Abundance of Parthenium Weed (Parthenium hysterophorus L.) at Kuala Muda, Malaysia. International Journal of Agronomy 2018, 2018. [Google Scholar]
- 31.Murugesan M, Chinnappu J, Manoharan P, Matheswaran P, Raja L, Gani SB: Assessment of diversity and relative richness of aquatic entomofauna in Jedarpalayam Dam, Namakkal District, Tamil Nadu. International Journal of Entomology Research 2020, 5(2):103–110. [Google Scholar]
- 32.Reshi Z, Khuroo AA, Dar GH: Plant species diversity in the Kashmir Himalayan grasslands along an elevational gradient. International Jour Ecology and Environmental Sciences 2009, 35(1):91–100. [Google Scholar]
- 33.Kara B: Assessment of the distribution and diversity of street tree species in Aydin, Turkey. Journal of Food, Agriculture & Environment 2012, 10(3&4):919–928. [Google Scholar]
- 34.Si C, Liu X, Wang C, Wang L, Dai Z, Qi S, et al. : Different degrees of plant invasion significantly affect the richness of the soil fungal community. PLoS One 2013, 8(12):e85490 10.1371/journal.pone.0085490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Seifu A, Seboka N, Misganaw M, Bekele T, Merawi E: Impact of Invasive Alien Plant, Xanthium Strumarium, On Species Diversity and Composition of Invaded Plant Communities in Borena Zone, Ethiopia. Biodiversity Int J 1 (1): 00004 10.15406/bij 00004 Xanthium strumarium is one of the invasive alien species those posing negative impacts on country’s biodiversity. Xanthium strumarium 2017. [DOI] [Google Scholar]
- 36.Vila M, Weiner J: Are invasive plant species better competitors than native plant species?–evidence from pair‐wise experiments. Oikos 2004, 105(2):229–238. [Google Scholar]
- 37.Levine JM, Hille Ris Lambers J: The importance of niches for the maintenance of species diversity. Nature 2009, 461(7261):254 10.1038/nature08251 [DOI] [PubMed] [Google Scholar]
- 38.Valéry L, Fritz H, Lefeuvre J-C, Simberloff D: In search of a real definition of the biological invasion phenomenon itself. Biological invasions 2008, 10(8):1345–1351. [Google Scholar]
- 39.Sujay YH, Sattagi HN, Patil RK: Invasive alien insects and their impact on agroecosystem. Karnataka Journal of Agricultural Sciences 2010, 23(1):26–34. [Google Scholar]
- 40.Berhanu L, Taye T, Rezene F: Distribution, abundance and socio-economic impacts of invasive plant species in Borana and Guji Zones of Oromia National Regional State, Ethiopia. Basic Res J Agric Sci Rev 2015, 4(9):271–279. [Google Scholar]
- 41.Powell KI, Chase JM, Knight TM: Invasive plants have scale-dependent effects on diversity by altering species-area relationships. science 2013, 339(6117):316–318. 10.1126/science.1226817 [DOI] [PubMed] [Google Scholar]
- 42.Mahajan M, Fatima S: Frequency, abundance, and density of plant species by list count quadrat method. International Journal of Multidisciplinary Research 2017, 3(7):1–8. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
Data Availability Statement
All relevant data have been added within the paper and uploaded minimal data set as a Supporting Information file.


