Abstract
Diffusion weighted imaging (DWI) is a neuroimaging technique that has allowed us an unprecedented look at the role that white matter microstructure may play in mental illnesses, such as psychosis. Psychosis related illnesses, including schizophrenia, are increasingly viewed as existing along a spectrum; spectrums may be defined based on factors such as stage of illness, symptom severity, or genetic liability. This review first focuses on an overview of some of the recent findings from DWI studies. Then, it examines the ways in which DWI analyses have been extended across the broader psychosis spectrum, or spectrums, and what we have learned from such approaches.
Keywords: schizophrenia, psychosis, neuroimaging, white matter, diffusion tensor imaging, spectrum
Diffusion Weighted Imaging and Schizophrenia
Diffusion weighted imaging (DWI) refers to a set of techniques that use the diffusion patterns of water molecules to characterize tissue properties. DWI is a powerful yet non-invasive tool, and can be performed using a standard magnetic resonance imaging (MRI) machine. It has provided insights into a diverse range of topics including our understanding of overall white matter (WM) architecture and microstructure, brain-structure changes such as myelination during healthy development, and altered WM properties in mental illnesses such as schizophrenia. Schizophrenia is a multifaceted disorder associated with a diverse constellation of symptoms. These include both the commonly described positive (e.g. hallucinations and delusions) and negative symptoms (e.g. flattened affect), as well as a number of associated cognitive and social deficits. Due to their complexity, it is difficult to attribute the symptoms of schizophrenia to impairment in any one brain region or structure — the symptoms often appear to occur as the result of a breakdown in multiple-component functions. Likewise, the cognitive functions which are impaired in patients with schizophrenia are often complex processes relying on widespread brain networks. Thus, in part because of the nature of these deficits, it has been hypothesized that, at its heart, schizophrenia may be a disorder of disrupted connectivity [1, 2]. That is to say, it’s not due to the impairment of any one brain region, but rather to an impairment of multiple regions and of the coordination and connections between them. This growing focus on connectivity is one of the reasons why DWI measures of structural connectivity, both within and between brain regions and networks, is a rapidly growing area of research.
The review first briefly covers the state of the existing body of DWI work in schizophrenia, followed by a discussion of emerging directions of particular interest in diffusion research in psychosis. In particular, neuroimaging work has recently begun to reflect the idea of seeing schizophrenia as part of a psychosis spectrum, with analyses including not only diagnosed patients but also those at clinical high risk, those showing subclinical level symptoms, and those showing variations of genetic risk. In addition, beyond focusing on the spectrum of severity within schizophrenia, there has been more work recently on cross-diagnostic approaches, or spectrums of symptoms that vary between diagnoses, for example, varying degrees of psychosis across schizophrenia and bipolar disorder. Both of these conceptualizations of a spectrum-based illness are consistent with the NIMH Research Domain Criteria (RDoC) approach, which seeks to understand, across disorders and levels of affectedness, the way that variability in function of different domains (e.g. executive function, social function) may scale with variability in underlying biological changes. In addition, the review will cover some of the future directions for the field necessary for building a richer understanding of WM connectivity, including integration with other imaging modalities, predictive utility of imaging measures, and the extension of newly developed diffusion techniques to clinical populations.
White Matter Alterations in Schizophrenia
In the DWI field, the diffusion tensor model (DTI model) is the most frequently employed technique. DTI analysis yields an index known as fractional anisotropy (FA), a measure that reflects a combination of myelination, neural fiber coherence, axon diameter, and organization of the WM tracts, and is thought to serve as a general index of “neuronal integrity” [3]. Furthermore, FA can be broken down into secondary measures of radial (RD) and axial diffusivity (AD) which putatively serve as more specific indices of myelination and axonal organization, respectively [4] (Figure 1). However, recent evidence may support the notion that even these measures are more complex than previously thought and may include other factors such as tract spacing [5].
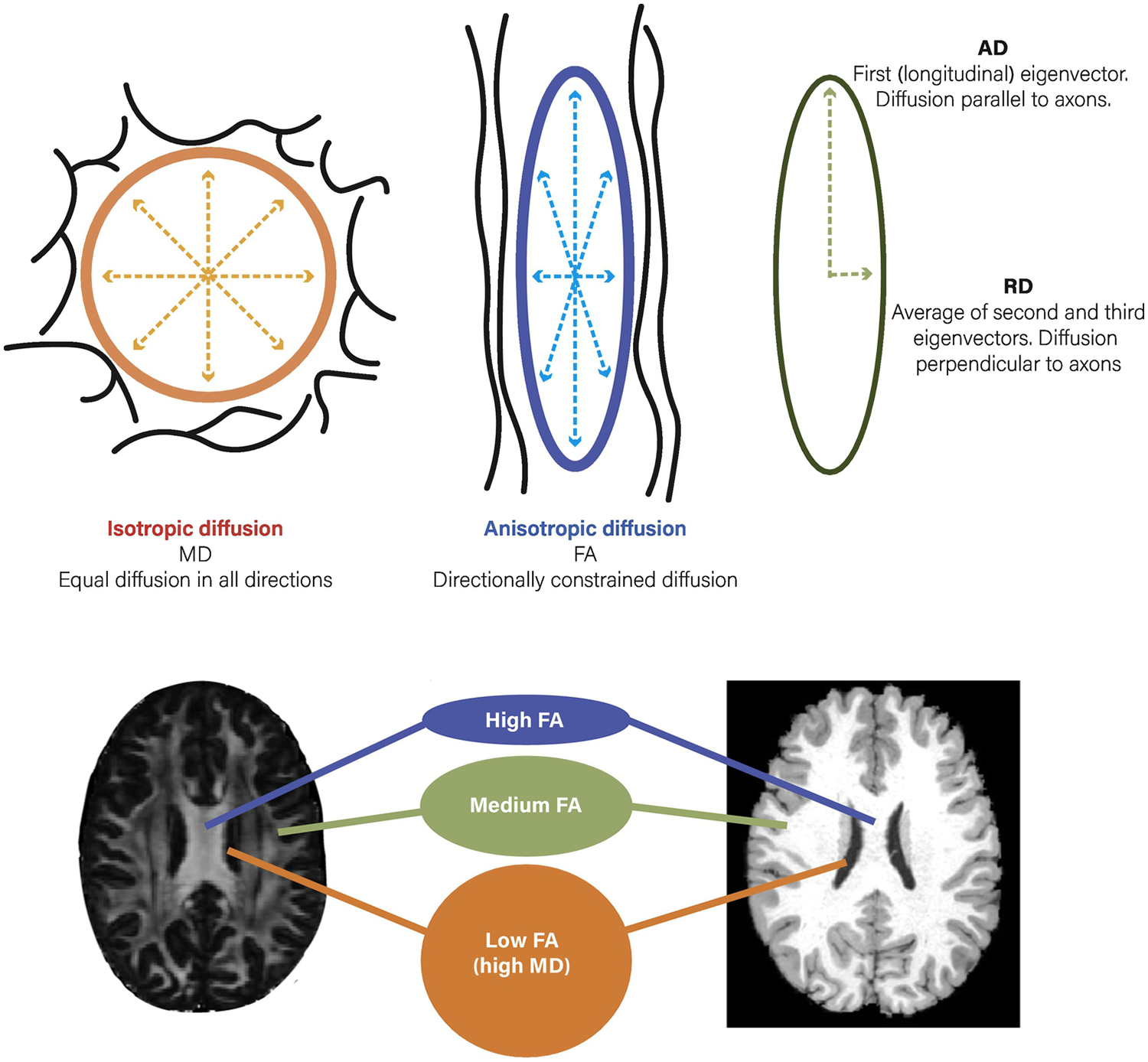
Figure 1.
Water diffusion patterns associated with different diffusion tensor imaging metrics. Top Left: Mean Diffusivity (MD), a pattern of isotropic diffusion seen in cerebrospinal fluid and areas without directional impediments such as grey matter. Top Middle: Fractional Anisotropy (FA), anisotropic diffusion that occurs in regions with directional constraints, such as in white matter tracts. Top Right: Radial (RD) and Axial (AD) diffusivity, measures used to provide more detailed information than FA about restricted diffusion perpendicular and parallel to the long axis of the ellipse. Lower panel: Example of regions with higher FA (corpus callosum, with unidirectional white matter), medium FA (smaller white matter tracts or those with crossing fibers), and low FA (ventricles, grey matter). Scans displayed are MP-RAGE and DTI (FA) images from a single participant in the Human Connectome Project (HCP).
DTI studies in schizophrenia and related disorders have primarily focused on cortico-cortico connections, consisting of the WM tracts that serve as the physical links between major cortical regions, including the majority of large-scale brain networks, such as the default mode network (DMN) and executive network. In general, while there has been some variability in findings, the overwhelming consensus of the literature — including from the largest study thus far, performed by the ENIGMA consortium [6] — is that on the whole, FA is decreased in patients with schizophrenia across a number of tracts, including most major WM fasciculi [7]. Decreased FA has been related to cognitive functions such as processing speed [8, 9], inhibitory processes [10], episodic memory [11, 12], social function [13, 14] and executive functions [15, 16] as well as variation in symptom severity [17, 18], indicating that these structural changes have functional relevance. Beyond the initial work on cortical connectivity, there is a growing interest in integrating other brain regions into the picture, such as subcortical structures; this seems particularly relevant given that many of the etiological models of schizophrenia are based on hypothesized alterations in subcortical-cortical loops. One example is the growing body of imaging work, including both structural and functional approaches, focused on thalamocortical connections [19–22]. The thalamus has long been implicated in schizophrenia, and thalamic volume reductions represent some of the earliest neuroimaging findings [23]. The roles of the thalamus, including its integration with the cortex and brainstem have resulted in the hypothesis that disruption in thalamic connectivity might result in a diverse array of sensory and cognitive disruptions, as are seen in schizophrenia [22, 24]. In general, across modalities, connectivity patterns have taken the form of a reduction in thalamo-prefrontal connectivity [19, 25] and an increase in thalamo-somatomotor connectivity [26]. In addition, there has also been a growing focus on striatal connections [27–30], in part because cortico-striatal loops have been implicated in foundational models of disrupted dopamine function in schizophrenia [31] and thus such studies may have the potential to address key questions pertaining to the neurobiological underpinnings of psychosis. Further, with technical and analytic developments allowing more fine-grained analyses, it is also now possible to look at the more local striatal-midbrain connections associated with dopaminergic function [32].
Complicating factors in patient-control analyses are medication status, duration of illness, and developmental stage, each of which has the potential to impact WM [7]. However, reductions in FA have been found even in unmedicated patients [33, 34], as well as unaffected first-degree relatives [35–37], indicating that effects are not likely to be solely attributable to medication confounds. Furthermore, WM deficits, as indexed by FA, have been found even in individuals in the very early stages of the illness [38–41], indicating that this may be a core feature of the disorder. Similarly, how or whether observed deficits might change with age, duration of illness, or across disease progression is an active topic of investigation. In terms of lifespan development, very few studies have looked at age-related trajectories within groups of diagnosed patients, either early in development or in aging populations. Of those that have, multiple studies that have investigated age-related change in younger patients have shown a blunted pattern that is consistent with a hypothesized failure to exhibit the normal adolescent increase in myelination and thus in FA [42, 43]. However, data on mid-adulthood age-related differences in FA are less clear, with some showing no age by group interactions at all [44]. However, recent graph theory-based analyses have revealed changes in more subtle features — such as “small world” connectivity metrics or a reduced integration of brain networks over time — even in adult patients [45], indicating that neural changes may be occurring at a level more refined than that indexed by standard tract based FA approaches. In advanced aging populations, there is some evidence for differential age related change [46] that may point to potential increases in age-related decline [18, 47]. Finally, in a relatively newer area of the literature, there is a growing attention on the potential for an alteration in “brain age” as a key feature of schizophrenia based on a hypothesis of overall accelerated aging [48–50]. Most of this work has been in structural MRI of gray matter. However, one must question the degree to which the pattern of ‘aging’ would expect to be consistent or synchronized across tissue types. Recently, there have been extensions to considering how altered (accelerated or lagged) aging might manifest in WM [18, 47]. An important consideration for this emerging area is the degree to which “brain age” changes reflect consistent delay or acceleration of brain aging across the lifespan, or whether there may be developmentally staged periods associated with either acceleration or lags. Thus, more work is needed to understand progressive change and how it may vary across the lifespan.
Investigations Across the Spectrum
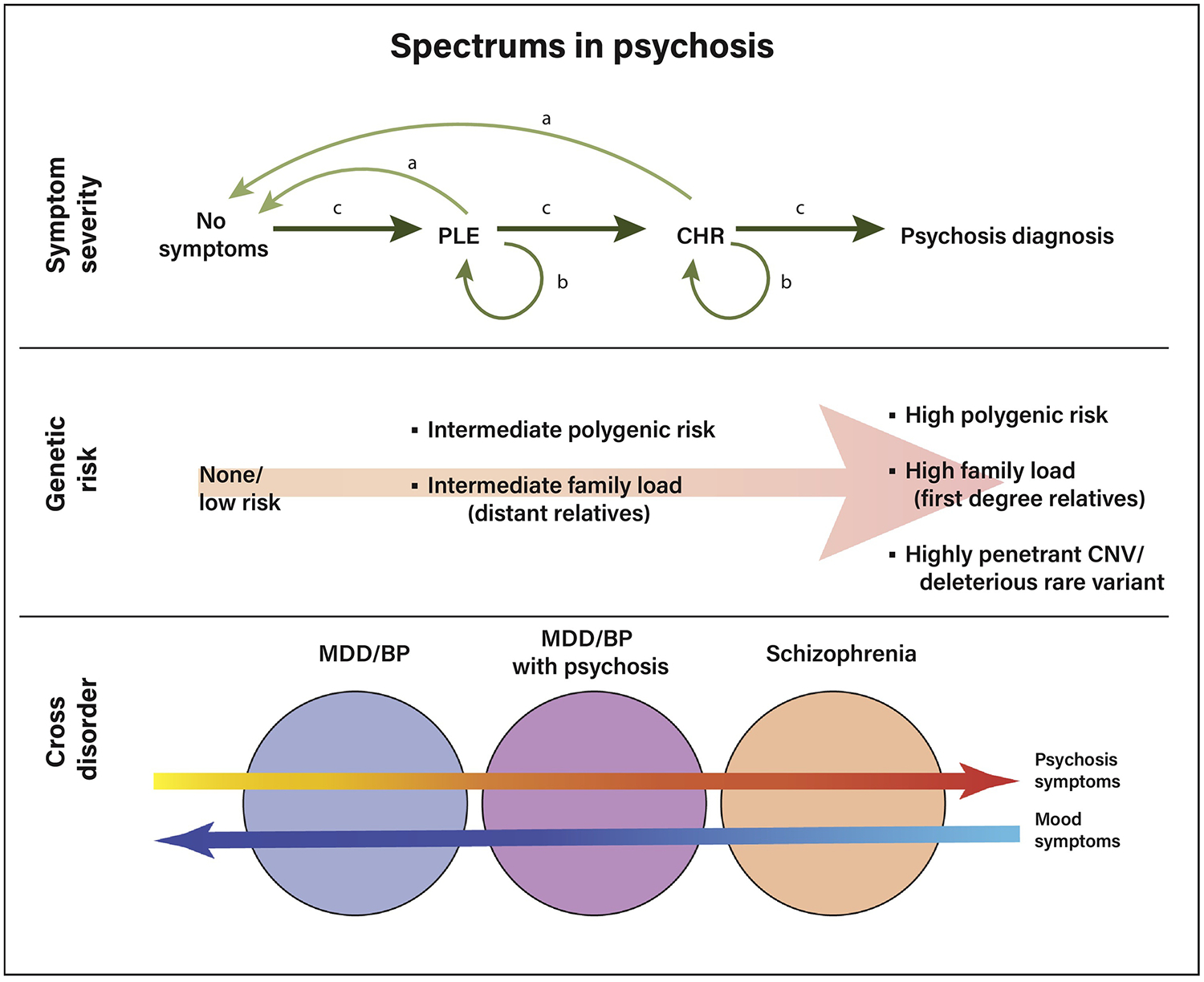
Schizophrenia is increasingly being understood as a spectrum disorder, which is an approach based on similarities in symptom expression and neural changes across related disorders. For example, individuals with schizoaffective disorder, schizophrenia, and schizophreniform disorder all experience psychosis, as do some individuals with major depressive disorder or bipolar disorder. This has led to both a corresponding increase in spectrum-based work, defined as variability either in 1) psychosis symptom severity from very mild to severe, 2) progression across disease stage, or 3) psychosis symptoms across different disorders, such as major depressive disorder with psychosis (Figure 2).
Figure 2.
Different theoretical approaches to the psychosis spectrum. Top panel: symptom severity-based spectrum, ranging from no symptoms, to subclinical psychotic-like experiences (PLEs), to the clinical high-risk state (CHR) to a psychosis spectrum diagnosis. Individuals may progress through the levels of symptoms, or, as indicated by the arrows, those with PLEs or in the CHR state may either improve (pattern a), remain stable (pattern b), or advance to a diagnosis (pattern c). Middle panel: spectrum of genetic liability; genetic liability may be assessed as related to degree of relatedness to an affected individual (proband), number of common low penetrance risk genes, and presence of highly penetrant rare variants, including copy number variations (CNVs) such as 22q deletion syndrome. Bottom panel: severity of specific symptom domains may also vary across disorders, for instance psychosis or mood symptoms may be present in major depressive disorder (MDD) or bipolar disorder (BP) or psychosis at varying degrees of severity.
Clinical High Risk
In particular, there is growing research emphasis on individuals on the low end of the spectrum, due to either low level symptoms or variable levels of genetic risk. The largest body of spectrum-based work has been in individuals identified as being at clinical high risk (CHR) for developing a schizophrenia or psychotic spectrum disorder also referred to as an at-risk mental state (ARMS). Such individuals are typically considered to be showing symptoms consistent with the prodromal stage of the disease. CHR studies have a unique strength, as longitudinal tracking of such individuals has the potential to help gain a clearer understanding of the changes that occur with onset of psychosis. This may help predict which individuals will convert to a psychosis spectrum disorder, as well as develop early interventions. However, there are also challenges with interpretation of data from CHR samples, as only a subset of individuals characterized as being at risk will progress to a diagnosed psychotic disorder, with others either improving or remaining in a subclinical state [51].
CHR individuals are typically identified based on a combination of factors. For example, individuals may show sub-diagnostic psychotic symptoms (symptoms that are the same as those in schizophrenia but that are experienced at a lower level of intensity), brief psychotic symptoms (symptoms the same as, and as intense as, those in schizophrenia but too infrequent to meet diagnostic criteria), or a gradual decline in functioning occurring in the context of genetic risk [51]. To achieve sufficient sample sizes, such studies are often carried out through large scale consortiums such as the North American Longitudinal Prodromal Study (NAPLS) or the European Prediction of Psychosis (EPOS) study. Recent high-risk studies have found WM alterations, primarily decreases in FA, to be present even in these relatively early stages of illness [39, 41, 52, 53]. Moreover, there appear to be differences in the maturational patterns, or patterns of change across time in these populations. This is similar to what has been observed in diagnosed patient groups, where there may be a blunting or deceleration of WM maturation [39, 40, 54, 55]. In addition, as previously discussed, subcortical regions may be particularly relevant for investigations focused on illness onset and etiology. Accordingly, subcortical investigations have started to be carried out in CHR populations as well [20, 56]. These findings are of interest as they may be able to shed unique light on the course of disease onset and early progression of illness. Furthermore, recent work employing more sophisticated network analyses [53] or combinations of structural and functional connectivity assays [57] indicates that it may be possible to differentiate CHR/ARMS patients who more closely resemble patients versus controls in an effort to predict which individuals may ultimately transition to a psychosis spectrum disorder.
Subclinical Psychosis Symptoms
The concept that subclinical (low level) symptoms can exist on a spectrum contiguous with typical experiences [58, 59] has inspired long-standing debate [60]. However, in the last several years there has been a notable increase in the number of neuroimaging studies looking at individuals who experience low levels of subclinical symptoms but who may or may not ever progress to a diagnosed psychosis spectrum disorder [61]. This research trend is likely driven, in part, by the growing availability of large publicly shared data sets such as the Philadelphia Neurodevelopmental Cohort (PNC; [62]) and Human Connectome Project (HCP [63]), in which large numbers of individuals from the community have undergone cognitive assessments, clinical assessments, and neuroimaging procedures. These samples include natural variability in low level symptomatology and typically include individuals who, unlike most CHR/ARMS populations, are not seeking treatment for their symptoms. This has enabled the study of neural correlates of variability in relatively low levels of symptoms [64–66]. Essentially, a focus on such subclinical symptoms, sometimes referred to as psychotic-like experiences or PLEs, is consistent with the idea that the low end of the psychosis spectrum may be conceptualized in a way similar to how we now treat high-functioning autism spectrum individuals, or to how we have come to think of mild cases of depression and anxiety, with the idea that there are individuals who live with and manage persistent low levels of symptoms, perhaps for their entire lives [67], and who do not necessarily progress to a diagnosed disorder. However, while many people with such symptoms do not develop a clinically-diagnosed disorder, there is a higher than typical rate of progression to a psychotic illness among them [60, 68–71]. In addition, such individuals can be at a higher risk for not only psychosis, but a range of other mental illnesses [72], which is consistent with our growing appreciation for the overlap, in terms of both symptoms and neurobehavioral phenotypes, between disorders.
Some recent studies in this domain have used network based statistics to show that there is a decrease in whole brain structural connectivity that correlates with scores on the Prodromal Questionnaire (PQ) [38] in addition to changes in temporal-lobe-based tracks [6, 73, 74]. Other investigators have found differences in network topology [75] as well as decreased FA and decreased centrality of parietal hubs [76]. Furthermore, effects of developmental risk factors for schizophrenia (birthweight, childhood IQ) on the presence of subclinical symptoms was found to be mediated by WM [77], indicating that PLEs may have a shared etiology with schizophrenia and that WM may play a key role. In further support of this notion, a collection of risk factors, such as cannabis use, subclinical symptoms, and childhood trauma were shown to be collectively associated with WM deficits [78]. In addition, as with youth with schizophrenia and CHR youth, cross-sectional developmental trajectories show a deviation such that in youth with subclinical symptoms there is a failure to show the normal age-related increase in FA [64]. Such changes may have functional implications, as differences in FA at baseline have been shown to be predictive of social competence in youth with PLEs at 12-month follow-up [79].
Taken together, the evidence indicates that, even in individuals with very subtle levels of symptom expression, there may be functionally relevant alterations in WM integrity. This further supports the notion of WM as an intermediate endophenotype, and also supports the use of such large-scale community data as a mechanism for gaining traction on the neural changes associated with broadly defined symptom spectrums.
Along the Genetic Spectrum
In addition to spectrums based on symptom levels or illness stage, it is also possible to conceptualize a spectrum based on genetic liability. The liability threshold model of psychosis [80, 81] would predict that the degree of subclinical affectedness, as well as the severity of intermediate endophenotypes such as WM changes, would be expected to vary with the level of genetic and environmental load. Family and twin studies have long supported the idea that neural changes may scale with genetic risk—for example, finding WM alterations that are greater in those more closely related to (share more genes with) the proband [82]. Concordantly, studies in individuals with first degree relatives with schizophrenia show connectivity differences [83–86]. Such neural differences may contribute to cognitive deficits often observed in unaffected relatives [87]. Moreover, there is evidence that genetic liability may affect developmental trajectories of WM change as well [37].
Familial high-risk studies, as just discussed, can show the differences between individuals known to be at overall different levels of risk without focusing on individual genes. Some recent work has built upon this knowledge base, using more sophisticated techniques to delve into the underlying genetic architecture of the risk. One aspect of genetic risk studies has focused on understanding neural changes associated with individual genes that are rare, but highly penetrant, and known to independently impart high levels of risk [88]. As one example, 22q11.2 deletion syndrome is a copy number variation (CNV) that has been associated with neural changes including WM deficits [89–93], and these have been associated with functional changes such as cognitive decline and symptom levels [89, 92]. Further, individuals with 22q11.2 deletion syndrome vary in their level of psychosis proneness, and recent studies have found that there may be subgroups of these individuals with distinct patterns of WM anomalies, which may contribute to some of this variability in susceptibility to psychosis [90, 91].
In addition to work on individual rare risk loci, genetic research on psychosis in recent years has also focused on understanding the cumulative effects of common genetic risk variants each with relatively small effect [94]. This work has led to a growing emphasis on investigation into how neural changes may scale with polygenetic risk scores (PGRS), which are summary scores that can be calculated based on an individual’s relative load of common schizophrenia risk alleles [94]. Recent studies have investigated the degree to which PGRS correlate with imaging measures [95], in many cases leveraging large scale public datasets. For example, in the UK Biobank study using a massive community sample, no effect of PGRS on global WM integrity was found [96]. Two different studies have looked at the Generation R birth cohort, one found no association between PGRS and whole brain FA [97], and another finding null results for both global and tract-based FA in [98]. However, a study in healthy older adults did find alterations in longitudinal change over time, both in standard DTI measures and graph theory metrics, indicating that the PGRS might impact age-related decline in patients and those at genetic risk [99]. This also may indicate that perhaps more subtle metrics than global WM integrity must be investigated, or that the impact of risk genes may emerge across lifespan development. Finally, these studies assess PGRS in population samples, but while work focused on PGRS specifically in patients is limited, it has been shown to correlate with clinical outcome and symptom severity [100, 101]. It is therefore possible that PGRS may also scale with neural alterations within patient groups, explaining some of the observed heterogeneity.
Thus, with multiple avenues through which to experimentally approach the concept of a genetic risk spectrum, there is now evidence from multiple types of studies that WM, as measured with diffusion weighted imaging, may be one of the neural metrics that scales with genetic differences and putatively contributes to subtle variation in illness presentation.
Cross Disorder Spectrums
Symptom severity and genetic risk, discussed above, are indices of degrees of severity within the schizophrenia spectrum. However, there is also a growing appreciation for the degree to which symptoms (and genetic and environmental risk factors) may be shared between different mental illnesses. Thus, there are increasing efforts to understand the similarities and differences between neural changes in highly overlapping disorders, such as schizophrenia, bipolar disorder, and major depressive disorder. In particular, based on the NIMH RDoC approach, there is interest in the degree to which neural phenotypes may scale with factors such as specific symptom or cognitive domains across multiple illnesses [102]. The most substantial body of literature in this area is on the commonalities and dissociations between schizophrenia and bipolar disorder given the shared genetic factors, similar ages of onset, and overlapping phenotypes (for instance bipolar disorder with psychosis, or schizophrenia with mood symptoms). Findings have been somewhat mixed (for a recent review see [103]), both in terms of impacted regions as well as direction of effects. However, it is to be expected that not all deficits will be identical between the disorders, and perhaps the most interesting aspect of such studies is in the degree to which overlap is related to shared symptomatology [104], particularly psychosis.
In a related direction, emerging largely in this age of big datasets and publicly shared data, there is an effort to capitalize on what differences may exist between disorders to use data driven classification-based approaches to analysis of neuroimaging data, including diffusion data [105, 106]. One such study found that combined evaluation of structural and functional connectivity across three core large-scale brain networks, the salience, executive, and default mode networks, was able to distinguish between patients with schizophrenia and major depressive disorder, with the schizophrenia-specific structural findings being largely located in frontal and, to a lesser extent, parietal regions [107]. As datasets including large samples of individuals with multiple major psychiatric disorders are relatively less common, some hurdles in such studies include the ability to merge very different datasets and to create algorithms that can perform well not just within a given dataset, but also when applied to other data sets. However, despite current limitations, such methods have the potential to be a powerful tool in personalized or precision medicine. Other approaches, such as in work by the Bipolar-Schizophrenia Network for Intermediate Phenotypes (B-SNIP), have been trying to look at neural changes, regardless of disorder, that characterize subgroups of phenotypically similar patients [108], who might then also share risk factors or benefit from the same treatments. Similarly, in large-scale community datasets that include individuals with subclinical symptoms, there have been efforts to explore the neural basis of dimensions of psychopathology that may span across disorders [109]. However, such approaches have more often included structural MRI or functional connectivity and less frequently diffusion-based analyses. Extending such work to include more diffusion imaging may help understand the degree to which WM variability contributes to cross-disorder symptom expression, and whether there may be differences in WM integrity between disorders. Further, one challenge in the use of the kind of large, multi-site datasets that might allow dimensional or subgroup analyses is the frequent inclusion of data from multiple scanners. This is the case, for example, in the work from consortia such as the HCP, or the Adolescent Brain and Cognitive Development Study (ABCD) [110]. To address potential issues based on scanner differences or incompatibilities, there have been recent efforts to establish harmonization techniques [111] as well as pre-processing pipelines, or to standardize processing strategies as is done with the ENIGMA consortium [112].These are important steps towards improving big data style analyses of diffusion data.
Concluding Remarks
While there is support for the notion that changes in WM are associated with psychotic illness and may scale with symptom severity and risk, additional research is needed. First, although there is a growing body of work in individuals at the low end of the spectrum, as defined by either level of symptoms or genetic risk, research in this area is at relatively early stages. There is also need for deeper understanding of the specific nature (regionally or in terms of affected microstructural measures) and replicability of observed deficits. Moreover, more work on the long-term clinical and neural trajectories of those at the low end of the spectrum is important. With the availability of large population datasets that include DWI data as well as clinical indices, this is likely to be a growing area of investigation. Next, it would be informative to have more cross-modality analyses, for instance DWI combined with functional connectivity as measured by fMRI. While there is work on this area in healthy and developmental samples [113], and some studies in schizophrenia patients, more is needed across the broader psychosis spectrum. Relatedly, there have been technical advances in DWI, such as free-water imaging, diffusion kurtosis imaging (DKI), and neurite orientation dispersion density imaging (NODDI), but they are slow to translate into clinical samples [7]. Many of these techniques require advanced imaging sequences, but with the growing adoption of HCP style sequences, these new and potentially more sensitive techniques will hopefully become more integrated into new studies. Finally, when studying WM in a disease context, it is important to consider that WM varies dynamically across the lifespan [48]. Many studies on schizophrenia or the psychosis spectrum do not adequately consider the effects of neurodevelopmental stage on acquired measures. However, there is a growing interest in developmental factors and how they may impact the onset and course of mental illness, which will hopefully yield an increase in work focused on development in major mental illness, for instance in adolescent psychosis.
In sum, over the last decade we have gained a much deeper understanding of how WM is disrupted in schizophrenia. Moreover, indices of WM disruption scale across (widely construed) spectrum-based approaches, including degrees of symptom severity, levels of genetic risk, and across disorders. These findings support the promise of WM differences as endophenotypes, potential predictors of risk, and future treatment targets. With this strong base of existing work, it is now important to continue to push the field forward, with the hope of ultimately being able to contribute to illness prediction and development of treatment targets.
Outstanding Questions.
Based on a spectrum model of psychosis, white matter changes in those at the low end on the spectrum should be milder than those in individuals with diagnosed schizophrenia. Across the literature, do effect sizes scale with severity of psychosis symptomatology or degree of risk?
What is the long-term developmental trajectory of white matter microstructure in those at the lower end of the spectrum? Is it different in those with transient vs. chronic PLEs?
Would more recently developed advanced diffusion imaging techniques, such as free water imaging (FWI) or NODDI be more sensitive to sub-syndromal white matter difference than diffusion tensor imaging?
Can cross-modality analyses (e.g. structural and functional connectivity) help gain better understanding of variability in subclinical psychosis?
What best practices need to be employed to maximally leverage emerging large multisite datasets? Examples of possible practices include harmonization of data pre- or post-acquisition, standardization of analytic pipelines, and replicability studies.
Highlights.
In recent years, there has been a growing emphasis on approaching psychosis-related illnesses, including schizophrenia, as spectrum-based disorders.
Spectrum-based approaches to psychosis have included investigating spectrums of symptom severity from mild to severe, spectrums of clinical and genetic risk levels, and spectrums of symptoms that manifest to varying degrees across different disorders such as schizophrenia and mood disorders.
There is evidence that microstructural changes in the white matter as indexed by diffusion weighted imaging scale across these spectrums, and are present at even low levels of symptom expression or genetic loading.
Taken together, these findings may indicate that white matter-based structural connectivity changes may be a core feature underlying the expression of psychotic symptoms.
Acknowledgements
This work was funded by NIMH MH115433 (KHK). Brain images for Figure 1 were provided [in part] by the Human Connectome Project, WU-Minn Consortium (Principal Investigators: David Van Essen and Kamil Ugurbil; 1U54MH091657) funded by the 16 NIH Institutes and Centers that support the NIH Blueprint for Neuroscience Research; and by the McDonnell Center for Systems Neuroscience at Washington University.
Glossary
- Diffusion tensor model
Diffusion tensor imaging (DTI) is a version of diffusion weighted imaging. In the diffusion tensor model, water diffusion is characterized as an ellipse, yielding a measure known as fractional anisotropy (FA) that represents the ratio of the longest and shortest axes of the ellipse.
- Diffusion weighted imaging (DWI)
a neuroimaging technique that can be performed on a standard magnetic resonance imaging (MRI) scanner. DWI uses the pattern of movement of water molecules, and the contours where they are restricted by tissue, to characterize the microstructure of brain structures such as white matter.
- Fractional Anisotropy
also known as FA, fractional anisotropy is the main measure yielded from DTI models. Water diffusion can be described as an ellipse, and FA indicates the eccentricity of that ellipse. FA ranges from 0 to 1, with 0 indicating water flow that is unrestricted in any direction (a sphere, as in CSF) and 1 representing the most directionally restricted flow (as in a highly myelinated region).
- Polygenic risk score
is a number that summarizes, across a large number of genetic loci, the aggregate risk an individual has for a disorder or trait
- Prodromal
the prodrome to an illness is a period in which early, but often non-specific, symptoms are present prior to the full onset of acute illness. In schizophrenia, the prodromal period may include manifestations such as a decline in social or role function, as well as symptoms that are either infrequent or low in severity such as increased suspiciousness, social withdrawal, or changes in visual perception.
- Schizophrenia
schizophrenia is a major mental illness primarily associated with a combination of ‘positive’ symptoms (such as delusions, hallucinations, paranoia, disorganization) and ‘negative’ symptoms (such as flat affect, social withdrawal, and lack of motivation). In addition, schizophrenia has been associated with cognitive deficits and neural changes.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bleuler E (1950) Dementia Praecox or The Group of Schizophrenias, International Universities Press. [Google Scholar]
- 2.Friston KJ and Frith CD (1995) Schizophrenia: a disconnection syndrome? Clin Neurosci 3 (2), 89–97. [PubMed] [Google Scholar]
- 3.Beaulieu C (2002) The basis of anisotropic water diffusion in the nervous system - a technical review. NMR Biomed 15 (7–8), 435–55. [DOI] [PubMed] [Google Scholar]
- 4.Wozniak JR and Lim KO (2006) Advances in white matter imaging: a review of in vivo magnetic resonance methodologies and their applicability to the study of development and aging. Neuroscience and Biobehavioral Reviews 30 (6), 762–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang EH et al. (2017) The role of myelination in measures of white matter integrity: Combination of diffusion tensor imaging and two-photon microscopy of CLARITY intact brains. Neuroimage 147, 253–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Erp TGM et al. (2018) Cortical Brain Abnormalities in 4474 Individuals With Schizophrenia and 5098 Control Subjects via the Enhancing Neuro Imaging Genetics Through Meta Analysis (ENIGMA) Consortium. Biol Psychiatry 84 (9), 644–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Karlsgodt KH (2016) Diffusion Imaging of White Matter In Schizophrenia: Progress and Future Directions. Biol Psychiatry Cogn Neurosci Neuroimaging 1 (3), 209–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kochunov P et al. (2017) Association of White Matter With Core Cognitive Deficits in Patients With Schizophrenia. JAMA Psychiatry 74 (9), 958–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kochunov P et al. (2016) Diffusion-weighted imaging uncovers likely sources of processing-speed deficits in schizophrenia. Proc Natl Acad Sci U S A 113 (47), 13504–13509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Du X et al. (2017) The role of white matter microstructure in inhibitory deficits in patients with schizophrenia. Brain Stimul 10 (2), 283–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Green AE et al. (2016) White matter correlates of episodic memory encoding and retrieval in schizophrenia. Psychiatry Res Neuroimaging 254, 188–98. [DOI] [PubMed] [Google Scholar]
- 12.Hidese S et al. (2019) The relationship between the Wechsler Memory Scale-Revised scores and whole-brain structure in patients with schizophrenia and healthy individuals. Cogn Neuropsychiatry 24 (1), 80–91. [DOI] [PubMed] [Google Scholar]
- 13.Koshiyama D et al. (2018) Role of frontal white matter and corpus callosum on social function in schizophrenia. Schizophr Res 202, 180–187. [DOI] [PubMed] [Google Scholar]
- 14.Saito Y et al. (2018) Impaired white matter connectivity between regions containing mirror neurons, and relationship to negative symptoms and social cognition, in patients with first-episode schizophrenia. Brain Imaging Behav 12 (1), 229–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schaeffer DJ et al. (2015) White matter structural integrity differs between people with schizophrenia and healthy groups as a function of cognitive control. Schizophr Res 169 (1–3), 62–68. [DOI] [PubMed] [Google Scholar]
- 16.Subramaniam K et al. (2018) White matter microstructure predicts cognitive training-induced improvements in attention and executive functioning in schizophrenia. Schizophr Res 193, 276–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Parnanzone S et al. (2017) Alterations of cerebral white matter structure in psychosis and their clinical correlations: a systematic review of Diffusion Tensor Imaging studies. Riv Psichiatr 52 (2), 49–66. [DOI] [PubMed] [Google Scholar]
- 18.Peters BD and Karlsgodt KH (2015) White matter development in the early stages of psychosis. Schizophr Res 161 (1), 61–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Giraldo-Chica M et al. (2018) Prefrontal-Thalamic Anatomical Connectivity and Executive Cognitive Function in Schizophrenia. Biol Psychiatry 83 (6), 509–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cho KI et al. (2016) Altered Thalamo-Cortical White Matter Connectivity: Probabilistic Tractography Study in Clinical-High Risk for Psychosis and First-Episode Psychosis. Schizophr Bull 42 (3), 723–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lyall AE et al. (2019) Diffusion Magnetic Resonance Imaging Advances the Study of Nuclei-Specific Thalamocortical Connectivity in Early Stage Psychosis. Biol Psychiatry 85 (1), 10–12. [DOI] [PubMed] [Google Scholar]
- 22.Anticevic A (2017) Understanding the role of thalamic circuits in schizophrenia neuropathology. Schizophr Res 180, 1–3. [DOI] [PubMed] [Google Scholar]
- 23.Andreasen NC et al. (1994) Regional brain abnormalities in schizophrenia measured with magnetic resonance imaging. Jama 272 (22), 1763–9. [PubMed] [Google Scholar]
- 24.Andreasen NC et al. (1994) Thalamic abnormalities in schizophrenia visualized through magnetic resonance image averaging. Science 266 (5183), 294–8. [DOI] [PubMed] [Google Scholar]
- 25.Hamoda HM et al. (2018) Abnormalities in thalamo-cortical connections in patients with first-episode schizophrenia: a two-tensor tractography study. Brain Imaging Behav. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cho KIK et al. (2019) Disturbed thalamocortical connectivity in unaffected relatives of schizophrenia patients with a high genetic loading. Aust N Z J Psychiatry, 4867418824020. [DOI] [PubMed] [Google Scholar]
- 27.Lee KH et al. (2018) Functional and Structural Connectivity of the Cerebellar Nuclei With the Striatum and Cerebral Cortex in First-Episode Psychosis. J Neuropsychiatry Clin Neurosci, appineuropsych17110276. [DOI] [PubMed] [Google Scholar]
- 28.Levitt JJ et al. (2017) Reduced Structural Connectivity in Frontostriatal White Matter Tracts in the Associative Loop in Schizophrenia. Am J Psychiatry 174 (11), 1102–1111. [DOI] [PubMed] [Google Scholar]
- 29.Delvecchio G et al. (2018) A diffusion weighted imaging study of basal ganglia in schizophrenia. Int J Psychiatry Clin Pract 22 (1), 6–12. [DOI] [PubMed] [Google Scholar]
- 30.James A et al. (2016) Abnormal frontostriatal connectivity in adolescent-onset schizophrenia and its relationship to cognitive functioning. Eur Psychiatry 35, 32–8. [DOI] [PubMed] [Google Scholar]
- 31.McCutcheon RA et al. (2019) Schizophrenia, Dopamine and the Striatum: From Biology to Symptoms. Trends Neurosci 42 (3), 205–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rivas-Grajales AM et al. (2018) Striato-nigro-striatal tract dispersion abnormalities in patients with chronic schizophrenia. Brain Imaging Behav. [DOI] [PubMed] [Google Scholar]
- 33.Li F et al. (2018) Altered White Matter Connectivity Within and Between Networks in Antipsychotic-Naive First-Episode Schizophrenia. Schizophr Bull 44 (2), 409–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang XY et al. (2016) Extensive white matter abnormalities and clinical symptoms in drug-naive patients with first-episode schizophrenia: a voxel-based diffusion tensor imaging study. J Clin Psychiatry 77 (2), 205–11. [DOI] [PubMed] [Google Scholar]
- 35.Cho KIK et al. (2019) Microstructural Changes in Higher-Order Nuclei of the Thalamus in Patients With First-Episode Psychosis. Biol Psychiatry 85 (1), 70–78. [DOI] [PubMed] [Google Scholar]
- 36.Zhou Y et al. (2017) White Matter Integrity in Genetic High-Risk Individuals and First-Episode Schizophrenia Patients: Similarities and Disassociations. Biomed Res Int 2017, 3107845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Domen P et al. (2017) Differential Time Course of Microstructural White Matter in Patients With Psychotic Disorder and Individuals at Risk: A 3-Year Follow-up Study. Schizophr Bull 43 (1), 160–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oestreich LKL et al. (2019) White matter connectivity reductions in the pre-clinical continuum of psychosis: A connectome study. Hum Brain Mapp 40 (2), 529–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Krakauer K et al. (2018) White matter maturation during 12 months in individuals at ultra-high-risk for psychosis. Acta Psychiatr Scand 137 (1), 65–78. [DOI] [PubMed] [Google Scholar]
- 40.Karlsgodt KH et al. (2009) White matter integrity and prediction of social and role functioning in subjects at ultra-high risk for psychosis. Biol Psychiatry 66 (6), 562–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vijayakumar N et al. (2016) White matter integrity in individuals at ultra-high risk for psychosis: a systematic review and discussion of the role of polyunsaturated fatty acids. BMC Psychiatry 16 (1), 287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Douaud G et al. (2009) Schizophrenia delays and alters maturation of the brain in adolescence. Brain 132 (Pt 9), 2437–48. [DOI] [PubMed] [Google Scholar]
- 43.Epstein KA and Kumra S (2015) White matter fractional anisotropy over two time points in early onset schizophrenia and adolescent cannabis use disorder: A naturalistic diffusion tensor imaging study. Psychiatry Res 232 (1), 34–41. [DOI] [PubMed] [Google Scholar]
- 44.Tonnesen S et al. (2018) White matter aberrations and age-related trajectories in patients with schizophrenia and bipolar disorder revealed by diffusion tensor imaging. Sci Rep 8 (1), 14129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sun Y et al. (2016) Disruption of brain anatomical networks in schizophrenia: A longitudinal, diffusion tensor imaging based study. Schizophr Res 171 (1–3), 149–57. [DOI] [PubMed] [Google Scholar]
- 46.Ota M et al. (2009) Progressive brain changes in schizophrenia: a 1-year follow-up study of diffusion tensor imaging. Acta Neuropsychiatr 21 (6), 301–7. [DOI] [PubMed] [Google Scholar]
- 47.Kochunov P et al. (2016) Heterochronicity of white matter development and aging explains regional patient control differences in schizophrenia. Hum Brain Mapp 37 (12), 4673–4688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kochunov P and Hong LE (2014) Neurodevelopmental and neurodegenerative models of schizophrenia: white matter at the center stage. Schizophr Bull 40 (4), 721–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shahab S et al. (2019) Brain structure, cognition, and brain age in schizophrenia, bipolar disorder, and healthy controls. Neuropsychopharmacology 44 (5), 898–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schnack HG et al. (2016) Accelerated Brain Aging in Schizophrenia: A Longitudinal Pattern Recognition Study. Am J Psychiatry 173 (6), 607–16. [DOI] [PubMed] [Google Scholar]
- 51.Addington J et al. (2019) Clinical and functional characteristics of youth at clinical high-risk for psychosis who do not transition to psychosis. Psychol Med 49 (10), 1670–1677. [DOI] [PubMed] [Google Scholar]
- 52.Krakauer K et al. (2017) Patterns of white matter microstructure in individuals at ultra-high-risk for psychosis: associations to level of functioning and clinical symptoms. Psychol Med 47 (15), 2689–2707. [DOI] [PubMed] [Google Scholar]
- 53.Choi SH et al. (2017) Brain network characteristics separating individuals at clinical high risk for psychosis into normality or psychosis. Schizophr Res 190, 107–114. [DOI] [PubMed] [Google Scholar]
- 54.Saito J et al. (2017) Longitudinal study examining abnormal white matter integrity using a tract-specific analysis in individuals with a high risk for psychosis. Psychiatry Clin Neurosci 71 (8), 530–541. [DOI] [PubMed] [Google Scholar]
- 55.Mittal VA et al. (2014) Neurological soft signs predict abnormal cerebellar-thalamic tract development and negative symptoms in adolescents at high risk for psychosis: a longitudinal perspective. Schizophr Bull 40 (6), 1204–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bernard JA et al. (2017) Cerebello-thalamo-cortical networks predict positive symptom progression in individuals at ultra-high risk for psychosis. Neuroimage Clin 14, 622–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang C et al. (2016) Disrupted salience network functional connectivity and white-matter microstructure in persons at risk for psychosis: findings from the LYRIKS study. Psychol Med 46 (13), 2771–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Beer MD (1996) The dichotomies: psychosis/neurosis and functional/organic: a historical perspective. Hist Psychiatry 7 (26 Pt 2), 231–55. [DOI] [PubMed] [Google Scholar]
- 59.Strauss JS (1969) Hallucinations and delusions as points on continua function. Rating scale evidence. Arch Gen Psychiatry 21 (5), 581–6. [DOI] [PubMed] [Google Scholar]
- 60.DeRosse P and Karlsgodt KH (2015) Examining the Psychosis Continuum. Curr Behav Neurosci Rep 2 (2), 80–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.McGorry PD et al. (2018) Beyond the “at risk mental state” concept: transitioning to transdiagnostic psychiatry. World Psychiatry 17 (2), 133–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Satterthwaite TD et al. (2014) Neuroimaging of the Philadelphia neurodevelopmental cohort. Neuroimage 86, 544–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Somerville LH et al. (2018) The Lifespan Human Connectome Project in Development: A large-scale study of brain connectivity development in 5–21 year olds. Neuroimage 183, 456–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hegarty CE et al. (2018) Disruptions in White Matter Maturation and Mediation of Cognitive Development in Youths on the Psychosis Spectrum. Biol Psychiatry Cogn Neurosci Neuroimaging. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Barber R.a.C. E (2016) A knockoff filter for high-dimensional selective inference. arXiv 1602.03574.
- 66.Sheffield JM et al. (2016) Cingulo-opercular network efficiency mediates the association between psychotic-like experiences and cognitive ability in the general population. Biol Psychiatry Cogn Neurosci Neuroimaging 1 (6), 498–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Guloksuz S and van Os J (2018) The slow death of the concept of schizophrenia and the painful birth of the psychosis spectrum. Psychol Med 48 (2), 229–244. [DOI] [PubMed] [Google Scholar]
- 68.Chapman LJ et al. (1994) Putatively psychosis-prone subjects 10 years later. J Abnorm Psychol 103 (2), 171–83. [DOI] [PubMed] [Google Scholar]
- 69.Poulton R et al. (2000) Children’s self-reported psychotic symptoms and adult schizophreniform disorder: a 15-year longitudinal study. Archives of General Psychiatry 57 (11), 1053–8. [DOI] [PubMed] [Google Scholar]
- 70.Cannon M et al. (2002) Evidence for early-childhood, pan-developmental impairment specific to schizophreniform disorder: results from a longitudinal birth cohort. Arch Gen Psychiatry 59 (5), 449–56. [DOI] [PubMed] [Google Scholar]
- 71.Hanssen M et al. (2005) The incidence and outcome of subclinical psychotic experiences in the general population. Br J Clin Psychol 44 (Pt 2), 181–91. [DOI] [PubMed] [Google Scholar]
- 72.Mennigen E and Bearden CE (in press) Psychosis Risk and Development: What do we know from population based studies? Biological Psychiatry [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.O’Hanlon E et al. (2015) White Matter Differences Among Adolescents Reporting Psychotic Experiences: A Population-Based Diffusion Magnetic Resonance Imaging Study. JAMA Psychiatry 72 (7), 668–77. [DOI] [PubMed] [Google Scholar]
- 74.Cooper S et al. (2018) White matter alterations in individuals experiencing attenuated positive psychotic symptoms. Early Interv Psychiatry 12 (3), 372–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Drakesmith M et al. (2015) Schizophrenia-like topological changes in the structural connectome of individuals with subclinical psychotic experiences. Hum Brain Mapp 36 (7), 2629–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.van Dellen E et al. (2016) Structural Brain Network Disturbances in the Psychosis Spectrum. Schizophr Bull 42 (3), 782–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Drakesmith M et al. (2016) Mediation of Developmental Risk Factors for Psychosis by White Matter Microstructure in Young Adults With Psychotic Experiences. JAMA Psychiatry 73 (4), 396–406. [DOI] [PubMed] [Google Scholar]
- 78.DeRosse P et al. (2014) Adding insult to injury: childhood and adolescent risk factors for psychosis predict lower fractional anisotropy in the superior longitudinal fasciculus in healthy adults. Psychiatry Res 224 (3), 296–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.DeRosse P et al. (2017) White Matter Abnormalities Associated With Subsyndromal Psychotic-Like Symptoms Predict Later Social Competence in Children and Adolescents. Schizophr Bull 43 (1), 152–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gottesman II and Shields J (1967) A polygenic theory of schizophrenia. Proc Natl Acad Sci U S A 58 (1), 199–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cannon TD and Keller MC (2006) Endophenotypes in the genetic analyses of mental disorders. Annu Rev Clin Psychol 2, 267–90. [DOI] [PubMed] [Google Scholar]
- 82.Bohlken MM et al. (2016) Structural Brain Connectivity as a Genetic Marker for Schizophrenia. JAMA Psychiatry 73 (1), 11–9. [DOI] [PubMed] [Google Scholar]
- 83.Cho KIK et al. (2019) Disturbed thalamocortical connectivity in unaffected relatives of schizophrenia patients with a high genetic loading. Aust N Z J Psychiatry 53 (9), 889–895. [DOI] [PubMed] [Google Scholar]
- 84.Ou J et al. (2018) Decreased white matter FA values in the left inferior frontal gyrus is a possible intermediate phenotype of schizophrenia: evidences from a novel group strategy. Eur Arch Psychiatry Clin Neurosci 268 (1), 89–98. [DOI] [PubMed] [Google Scholar]
- 85.de Leeuw M et al. (2017) Changes in White Matter Organization in Adolescent Offspring of Schizophrenia Patients. Neuropsychopharmacology 42 (2), 495–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Arat HE et al. (2015) Diffusion tensor imaging in first degree relatives of schizophrenia and bipolar disorder patients. Schizophr Res 161 (2–3), 329–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kochunov P et al. (2016) The common genetic influence over processing speed and white matter microstructure: Evidence from the Old Order Amish and Human Connectome Projects. Neuroimage 125, 189–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bearden CE and Forsyth JK (2018) The many roads to psychosis: recent advances in understanding risk and mechanisms. F1000Res 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Nuninga JO et al. (2018) White matter abnormalities in 22q11.2 deletion syndrome patients showing cognitive decline. Psychol Med 48 (10), 1655–1663. [DOI] [PubMed] [Google Scholar]
- 90.Zhan L et al. (2018) Baseline connectome modular abnormalities in the childhood phase of a longitudinal study on individuals with chromosome 22q11.2 deletion syndrome. Hum Brain Mapp 39 (1), 232–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Roalf DR et al. (2017) White matter microstructural deficits in 22q11.2 deletion syndrome. Psychiatry Res Neuroimaging 268, 35–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Olszewski AK et al. (2017) The social brain network in 22q11.2 deletion syndrome: a diffusion tensor imaging study. Behav Brain Funct 13 (1), 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kikinis Z et al. (2017) Abnormalities in brain white matter in adolescents with 22q11.2 deletion syndrome and psychotic symptoms. Brain Imaging Behav 11 (5), 1353–1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.International Schizophrenia, C. et al. (2009) Common polygenic variation contributes to risk of schizophrenia and bipolar disorder. Nature 460 (7256), 748–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Dima D and Breen G (2015) Polygenic risk scores in imaging genetics: Usefulness and applications. J Psychopharmacol 29 (8), 867–71. [DOI] [PubMed] [Google Scholar]
- 96.Reus LM et al. (2017) Association of polygenic risk for major psychiatric illness with subcortical volumes and white matter integrity in UK Biobank. Sci Rep 7, 42140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bolhuis K et al. (2019) Interaction of schizophrenia polygenic risk and cortisol level on pre-adolescent brain structure. Psychoneuroendocrinology 101, 295–303. [DOI] [PubMed] [Google Scholar]
- 98.Jansen PR et al. (2019) Polygenic Scores for Neuropsychiatric Traits and White Matter Microstructure in the Pediatric Population. Biol Psychiatry Cogn Neurosci Neuroimaging 4 (3), 243–250. [DOI] [PubMed] [Google Scholar]
- 99.Alloza C et al. (2018) Polygenic risk score for schizophrenia and structural brain connectivity in older age: A longitudinal connectome and tractography study. Neuroimage 183, 884–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zhang JP et al. (2019) Schizophrenia Polygenic Risk Score as a Predictor of Antipsychotic Efficacy in First-Episode Psychosis. Am J Psychiatry 176 (1), 21–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sengupta SM et al. (2017) Polygenic Risk Score associated with specific symptom dimensions in first-episode psychosis. Schizophr Res 184, 116–121. [DOI] [PubMed] [Google Scholar]
- 102.Insel T et al. (2010) Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. Am J Psychiatry 167 (7), 748–51. [DOI] [PubMed] [Google Scholar]
- 103.Mitelman SA (2019) Transdiagnostic neuroimaging in psychiatry: A review. Psychiatry Res. [DOI] [PubMed] [Google Scholar]
- 104.Anticevic A et al. (2015) Ventral anterior cingulate connectivity distinguished nonpsychotic bipolar illness from psychotic bipolar disorder and schizophrenia. Schizophr Bull 41 (1), 133–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Deng Y et al. (2019) Tractography-based classification in distinguishing patients with first-episode schizophrenia from healthy individuals. Prog Neuropsychopharmacol Biol Psychiatry 88, 66–73. [DOI] [PubMed] [Google Scholar]
- 106.Mikolas P et al. (2018) Machine learning classification of first-episode schizophrenia spectrum disorders and controls using whole brain white matter fractional anisotropy. BMC Psychiatry 18 (1), 97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Han W et al. (2019) Low-rank network signatures in the triple network separate schizophrenia and major depressive disorder. Neuroimage Clin 22, 101725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ivleva EI et al. (2017) Brain Structure Biomarkers in the Psychosis Biotypes: Findings From the Bipolar-Schizophrenia Network for Intermediate Phenotypes. Biol Psychiatry 82 (1), 26–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Xia CH et al. (2018) Linked dimensions of psychopathology and connectivity in functional brain networks. Nat Commun 9 (1), 3003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Casey BJ et al. (2018) The Adolescent Brain Cognitive Development (ABCD) study: Imaging acquisition across 21 sites. Dev Cogn Neurosci 32, 43–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Cetin-Karayumak S et al. (2019) White matter abnormalities across the lifespan of schizophrenia: a harmonized multi-site diffusion MRI study. Mol Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Thompson PM et al. (2014) The ENIGMA Consortium: large-scale collaborative analyses of neuroimaging and genetic data. Brain Imaging Behav 8 (2), 153–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Baum GL et al. (2020) Development of structure-function coupling in human brain networks during youth. Proc Natl Acad Sci U S A 117 (1), 771–778. [DOI] [PMC free article] [PubMed] [Google Scholar]


