Abstract
PURPOSE
BRAFV600 mutations are commonly found in melanoma and thyroid cancers and to a lesser degree in other tumor types. Subprotocol H (EAY131-H) of the NCI-MATCH platform trial sought to investigate the selective BRAF inhibitor dabrafenib and the MEK1/2 inhibitor trametinib in patients with solid tumors, lymphomas, or multiple myeloma whose tumors harbored a BRAFV600 mutation.
PATIENTS AND METHODS
EAY131-H is an open-label, single-arm study. Patients with melanoma, thyroid, or colorectal cancer were excluded; patients with non–small-cell lung cancer were later excluded in an amendment. Patients received dabrafenib 150 mg twice per day and trametinib 2 mg per day continuously until disease progression or intolerable toxicity. The primary end point was centrally assessed objective response rate (ORR); secondary end points included progression-free survival (PFS), 6-month PFS, and overall survival.
RESULTS
Thirty-five patients were enrolled, and 29 were included in the primary efficacy analysis as prespecified in the protocol. Median age was 59 years, and 45% of the patients had received ≥ 3 lines of therapy. The confirmed ORR was 38% (90% CI, 22.9% to 54.9%) with P < .0001 against a null rate of 5%, and PFS was 11.4 months (90% CI, 8.4 to 16.3 months); responses were seen in 7 distinct tumor types. Seven patients had a duration of response of > 12 months, including 4 patients with a duration of response of > 24 months. An additional 8 patients had a PFS > 6 months. The median overall survival was 28.6 months. Reported adverse events were comparable to those noted in previously reported profiles of dabrafenib and trametinib.
CONCLUSION
This study met its primary end point, with an ORR of 38% (P < .0001) in this mixed histology, pretreated cohort. This promising activity warrants additional investigations in BRAFV600-mutated tumors outside of currently approved indications.
INTRODUCTION
Activating mutations in BRAF have garnered a great deal of attention over the past decade. Nearly one half of all melanomas have been shown to harbor a mutation in BRAF at codon 600, resulting in constitutive activation of the MAPK pathway.1 Successful inhibition of this pathway with BRAF/MEK inhibitors results in a clinically meaningful benefit and initially established these agents as a standard-of-care option in patients with BRAFV600-mutated metastatic melanoma.2
Context
Key Objective
BRAF/MEK inhibitor therapy has shown promising clinical activity in certain BRAF-mutated cancers, such as melanoma, non–small-cell lung cancer, and thyroid cancer. Early data suggested that responses to therapy may be histology dependent, because BRAF-mutated colorectal cancer demonstrated a relative resistance to therapy. In many cancers, the incidence of BRAF mutations is low, and limited information exists regarding the sensitivity of other tumor types to BRAF/MEK inhibition. This study sought to evaluate the efficacy of the BRAF/MEK inhibitors dabrafenib and trametinib in patients whose cancers have a BRAFV600 mutation after progression on standard therapy.
Knowledge Generated
Dabrafenib and trametinib therapy resulted in responses in 38% of patients and showed a high rate of disease control. With more than 16 different tumor types represented, many patients seemed to benefit for several months.
Relevance
This study suggests that BRAF/MEK inhibition may be a viable treatment strategy across the majority of BRAFV600-mutated cancers.
In most other tumor types, BRAF has been shown to be altered at lower frequencies; it is estimated that the incidence of BRAF mutations is approximately 1% to 3% for cancers overall.1,3,4 Outside of melanoma, the efficacy of targeting this pathway has been replicated in selected cancers, such as non–small-cell lung cancer (NSCLC), hairy cell leukemia, and thyroid cancer.5-8 However, these results contrast with the low activity of BRAF/MEK–targeted therapy in patients with BRAF-mutant colorectal cancer (CRC), suggesting that responses are histology dependent.9 Early data suggested that epidermal growth factor receptor (EGFR)-mediated reactivation of the MAPK pathway may be a mechanism underlying resistance to therapy.10 This hypothesis has now been confirmed in a randomized phase III study, which demonstrated a survival benefit with the addition of cetuximab to encorafenib and binimetinib in BRAF-mutated CRC.11
Limited information exists about the activity of BRAF/MEK–targeted therapy in other tumor types, and it is largely limited to anecdotal reports or small case series. A phase II basket study of vemurafenib suggested activity in additional cancers that harbor BRAF mutations at a low rate.12 A more recent report from the glioma cohort of this study also demonstrated clinical activity with a response rate of 25%.13 A recent report has also suggested the potential for benefit with dabrafenib and trametinib in BRAF-mutated biliary tract cancers, in which a cohort of 33 patients treated with dabrafenib and trametinib resulted in a response rate of 36% and a median progression-free survival (PFS) of 7.2 months.14 However, given the low overall rate of BRAF mutations in most tumor types, the feasibility of conducting disease-specific studies is limited. The NCI-MATCH trial was designed as a platform precision medicine study in which patients were assigned to receive treatment on the basis of genetic testing results from pretreatment biopsies, irrespective of tumor type. Subprotocol H evaluated the BRAF and MEK1/2 inhibitors dabrafenib and trametinib in patients whose tumors harbored a BRAFV600E/K/R/D mutation. Here, we report the clinical efficacy and safety in this patient population.
PATIENTS AND METHODS
Study Design and Population
The National Cancer Institute Molecular Analysis for Therapy Choice (NCI-MATCH) trial (ClinicalTrials.gov identifier: NCT02465060), developed by the ECOG-ACRIN Cancer Research Group and the National Cancer Institute (NCI), aimed to find signals of efficacy for treatments targeted to actionable molecular alterations found in any tumor type; a more detailed overview of the trial has been published previously.15 The study was designed as a precision medicine platform trial with flexibility to open and close subprotocols, and accrual on additional subprotocols is ongoing. To date, the overall study has comprised 37 subprotocols and is open at nearly 1,100 centers throughout the United States. The study was performed in accordance with provisions of the Declaration of Helsinki and Good Clinical Practice guidelines. The protocol was reviewed by institutional review boards at each participating center. Written informed consent was obtained for all participants. EAY131-H opened to enrollment in August 2015 and completed accrual in February 2018. This subprotocol allowed the enrollment of patients with BRAFV600E/K/R/D mutated solid tumors, lymphoma, or multiple myeloma whose disease had progressed on at least 1 standard therapy (Data Supplement).
Tumor profiling was accomplished as described in Lih et al.16 After the end of central profiling of 5,540 tumor samples in May 2017, patients were accepted if they had eligible molecular alterations identified by molecular profiling performed for clinical reasons at Clinical Laboratory Improvement Amendments (CLIA)-accredited laboratories approved to identify NCI-MATCH–eligible patients. Patients were assigned using a prospectively defined NCI-designed informatics rules algorithm (MATCHBOX).17
For subprotocol H, patients with melanoma, thyroid cancer, or CRC were excluded. In addition, patients with NSCLC were excluded after the US Food and Drug Administration approved dabrafenib and trametinib for this indication. Central pathologic review was performed for all specimens when adequate material was available. All patients had disease progression after standard therapy. Patients were required to have measurable disease according to standard practice for the specified tumor type.18-21 Patients were required to have an Eastern Cooperative Oncology Group performance status of 0-1 and acceptable laboratory parameters. Patients were excluded if they had had prior exposure to a BRAF or MEK1/2 inhibitor, had any history of a RAS mutation–positive cancer, or had a left ventricular ejection fraction below the institutional lower limit of normal.
Study Therapy and Assessments
Patients received dabrafenib 150 mg twice per day and trametinib 2 mg per day in continuous 28-day cycles. Patients continued to receive therapy until disease progression, intolerable toxicity, or study withdrawal. Up to 3 dose reductions were permitted for dabrafenib, and up to 2 dose reductions were permitted for trametinib. Treatment toxicities were evaluated using the National Cancer Institute Common Terminology Criteria, version 4.0.22 Dermatologic examinations were performed at baseline and then every 2 months while patients received study therapy, and every 2-3 months after discontinuation of therapy to monitor for the development of cutaneous squamous cell carcinomas, keratoacanthomas, or other concerning skin lesions. Response assessments were performed every 8 weeks.
Statistical Considerations
The primary objective was to evaluate the objective response rate (ORR) for each subprotocol. The accrual goal was 35 patients, to obtain 31 eligible and treated patients. With 31 patients, power is 91.8% to conclude an agent is promising if the true response rate is 25%, and the type 1 error rate (1 sided) is 1.8% under the null response rate of 5%. An observed response rate of 5 of 31 patients (16%) or more was considered a signal of activity. Secondary objectives were PFS at 6 months, PFS, overall survival, toxicity assessment, and evaluation of predictive biomarkers (co-mutations or other factors that potentially predict which patients will respond). Eligible and treated patients who were enrolled on the basis of the MATCH assay or who were enrolled on the basis of outside assays with molecular abnormalities confirmed by the MATCH assay were included in the primary analyses (n = 29). Given that fewer than 31 patients were in the primary analysis population, primary efficacy was assessed using 5% 1-sided exact binomial tests of the null hypothesis that the response rate was ≤ 5%. Secondary analysis results by combining all eligible and treated patients (n = 33) are included in the Appendix (online only).
RESULTS
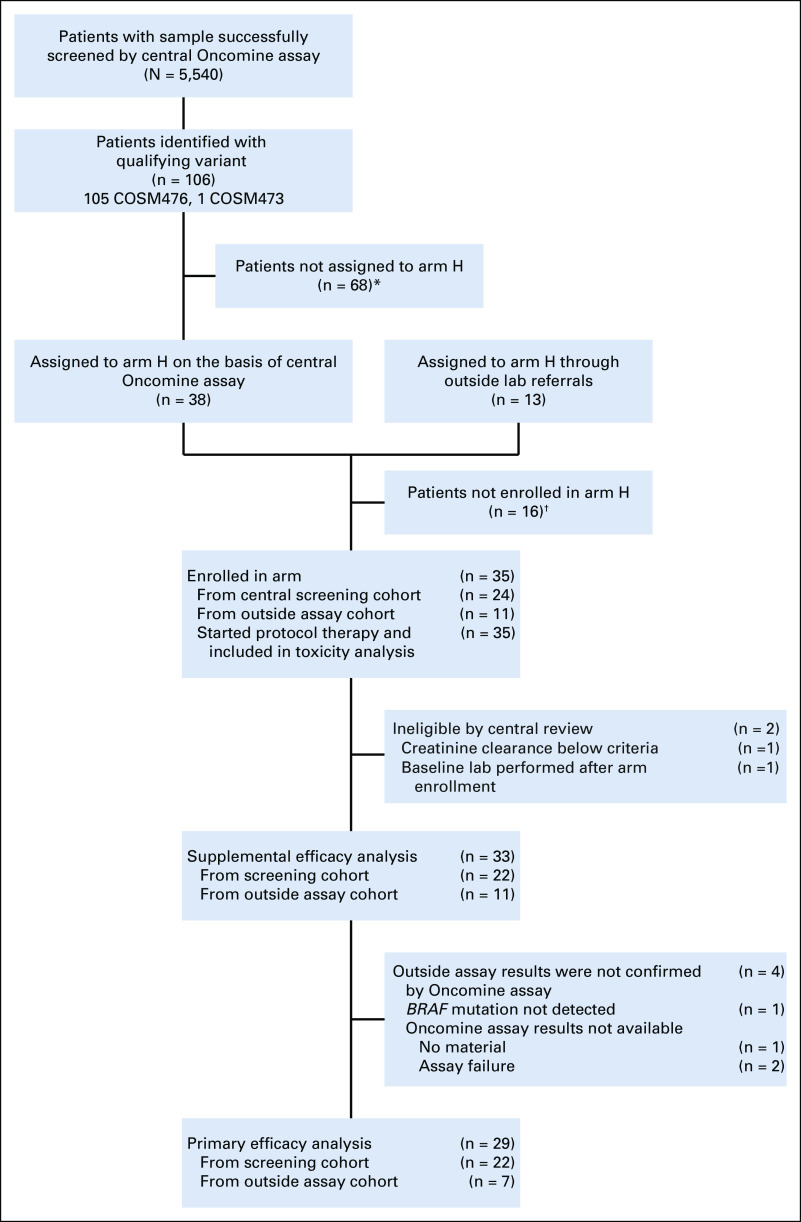
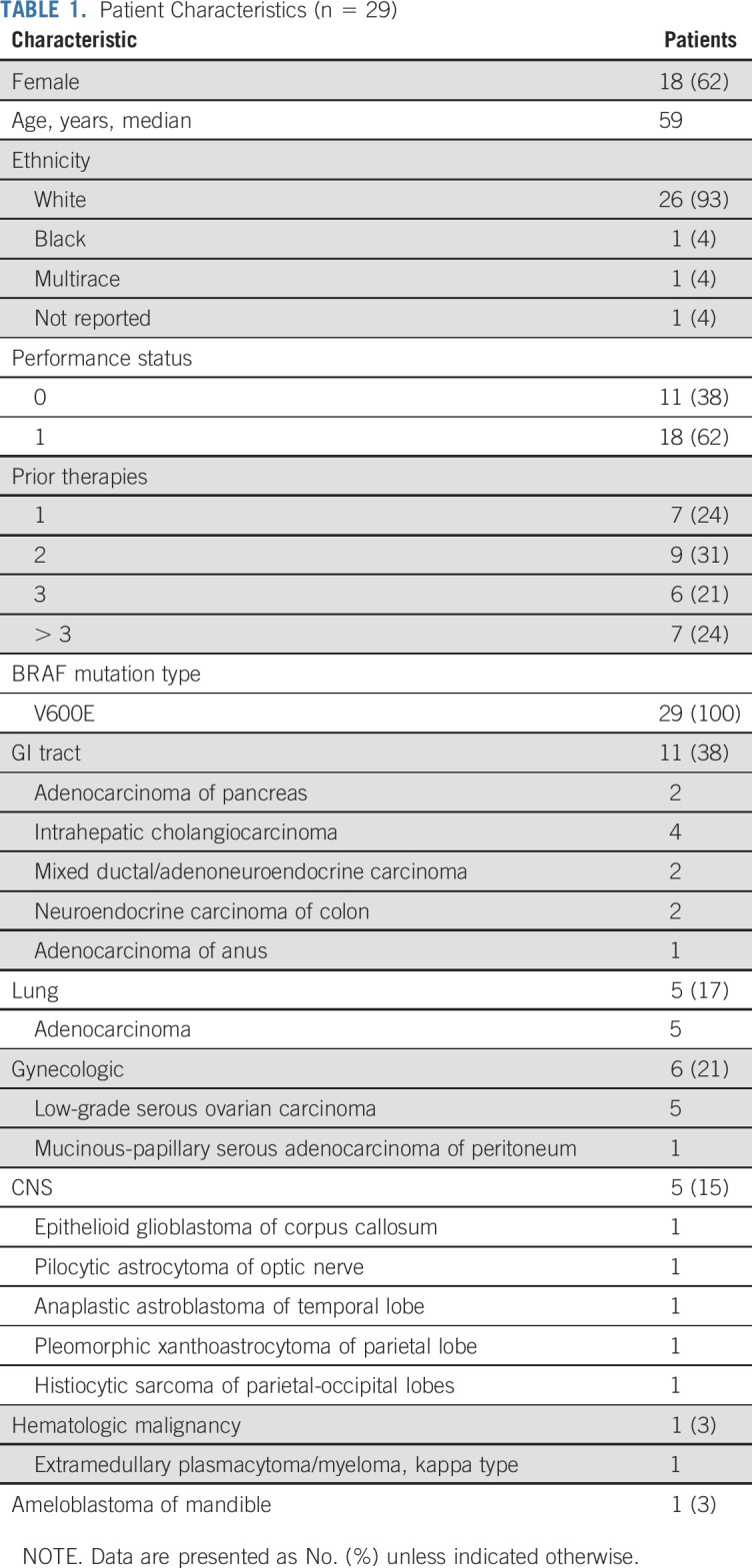
Thirty-five patients with BRAFV600 mutations were enrolled. Among the 5,540 patients screened by the NCI-MATCH central assay, 106 (1.9%) were determined to have a qualifying variant, and 38 were assigned to subprotocol H; 68 patients were not assigned because of histologic exclusions (Fig 1). Of these 38 patients assigned on the basis of central assay, 24 were enrolled subsequently. The remaining 11 were enrolled on the basis of outside next-generation sequencing (NGS) assay results. Two patients who were enrolled through central assay were deemed ineligible on the basis of central review: 1 patient’s creatinine clearance was below the threshold for inclusion, and 1 patient had screening laboratories performed outside of the study-specified window. Per protocol, eligible and treated patients who were enrolled on the basis of the central MATCH assay (n = 22) or who were enrolled on the basis of outside assays with molecular abnormalities confirmed by central assay (7 of the 11) were included in the primary analyses (29 patients, the protocol prespecified primary analysis population); data on the 33-patient cohort (overall eligible and treated population, which included 4 patients with molecular abnormalities unable to be confirmed by the central assay, as reviewed in Fig 1) is presented in Appendix Figures A2-A4 and Tables A2 and A3 (online only). Characteristics of the 29 patients included in the primary analysis are summarized in Table 1. Sixty-two percent of the patients were female, with a median age of 59 years (range, 21-85 years). Ninety-three percent of the patients were White, and 45% of the patients had received at least 3 prior therapies (range, 1-7 therapies). Sixteen distinct tumor types were represented: the most common histologies were low-grade serous ovarian carcinoma (LGSOC; 5 patients), adenocarcinoma of the lung (5 patients), and cholangiocarcinoma (4 patients). All patients had tumors with a BRAFV600E mutation.
FIG 1.
CONSORT diagram. (*) Did not receive an assignment because of ineligible histology: colorectal cancer (n = 44), melanoma (n = 14), papillary thyroid cancer (n = 9), and non–small-cell lung cancer (n = 1); (†) reasons not enrolled after receiving an assignment: central screening cohort: death (n = 2), patient refusal (n = 1), prior treatment (n = 2), disease progression (n = 1), BRAF inhibitor started before assignment (n = 2), inadequate organ/marrow function (n = 2), not able to swallow tablets (n = 1), no longer met master protocol eligibility (n = 1), and unknown (n = 2); outside assay cohort: deteriorating performance status (n = 1) and unknown (n = 1).
TABLE 1.
Patient Characteristics (n = 29)
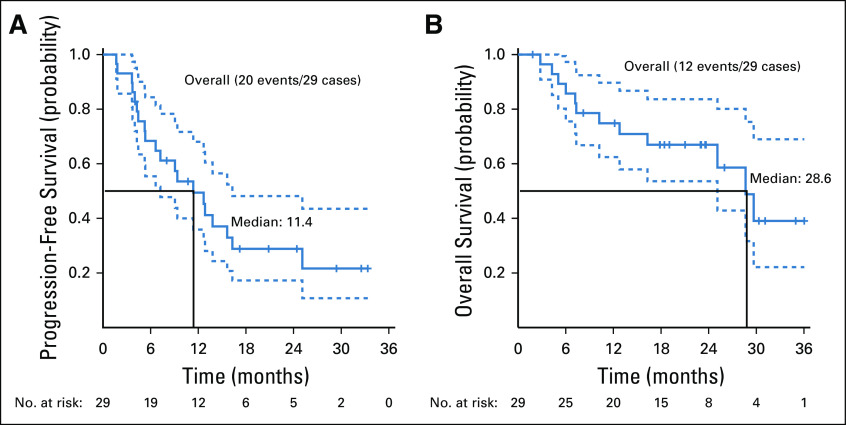
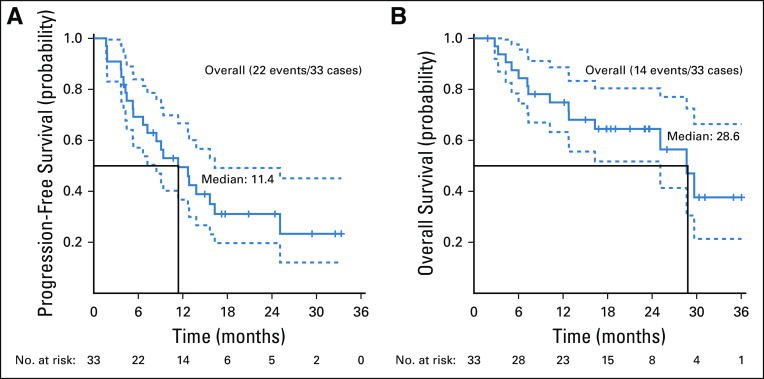
Twenty-nine patients were included in the primary efficacy analysis. Five patients were considered unevaluable for response (4 patients had baseline scans out of window; 1 patient had clinical deterioration before response assessment). The confirmed ORR was 37.9% (90% CI, 22.9% to 54.9%; P < .0001 against a null rate of 5%), with a median duration of response of 25.1 months (90% CI, 12.8 months to NA). For the cohort of 33 patients, the ORR was 33.3% (90% CI, 19.9% to 49.1%) (Table A3, online only). An additional 11 patients had stable disease (SD), resulting in a disease control rate (DCR) of 75.9% (Table 2). Median PFS was 11.4 months (90% CI, 7.2 to 16.3 months; Fig 2A), and the 6-month PFS rate was 68.4% (90% CI, 55.4% to 84.4%). With a median follow-up of 23.0 months, median OS was 28.6 months (Fig 2B). At the time of data cutoff in August 2019, 6 patients continued on treatment.
TABLE 2.
Response Assessment (n = 29)
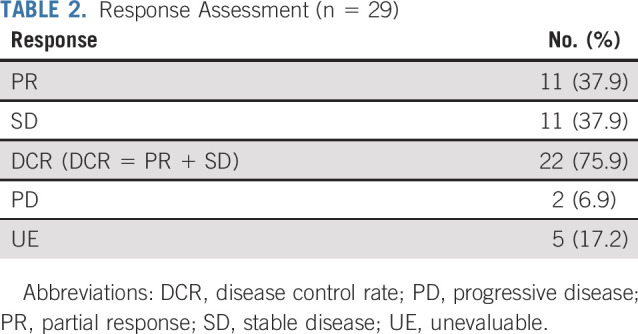
FIG 2.
(A) Progression-free survival. (B) Overall survival.
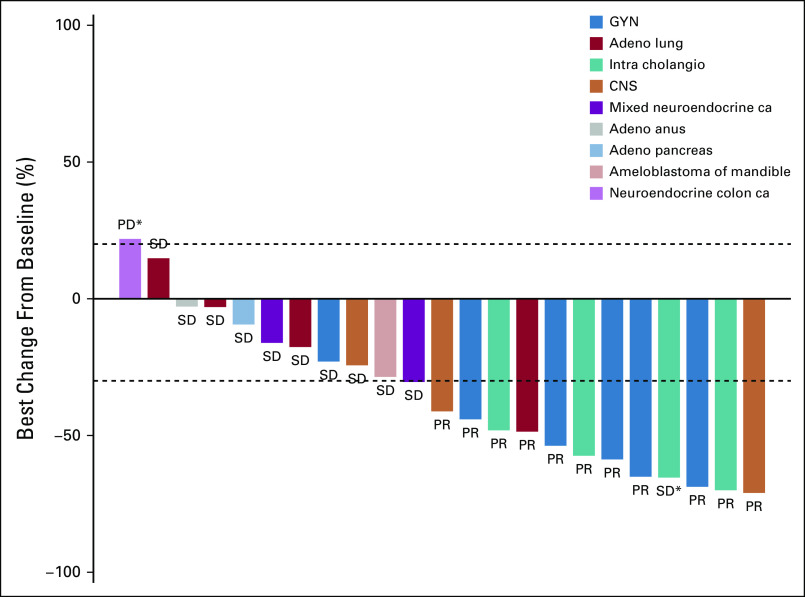
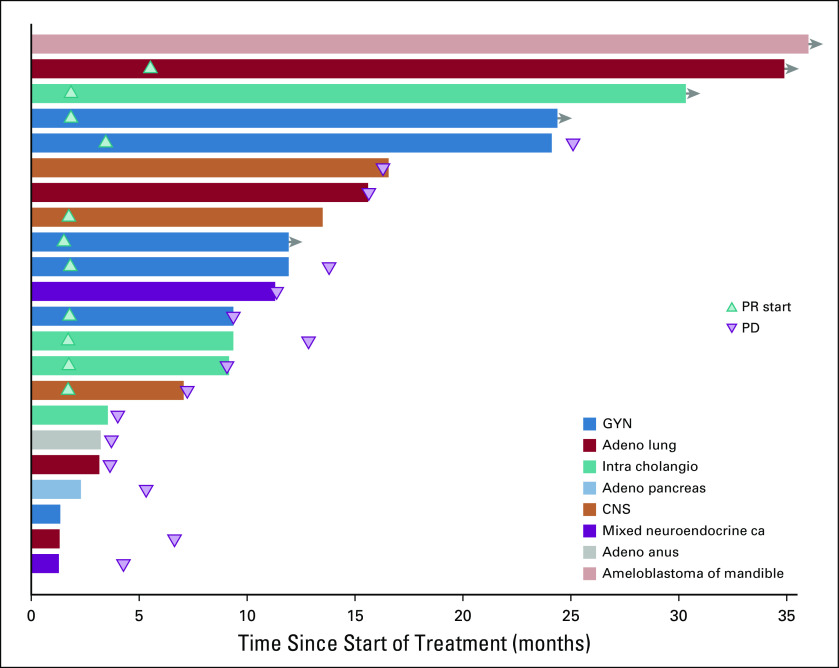
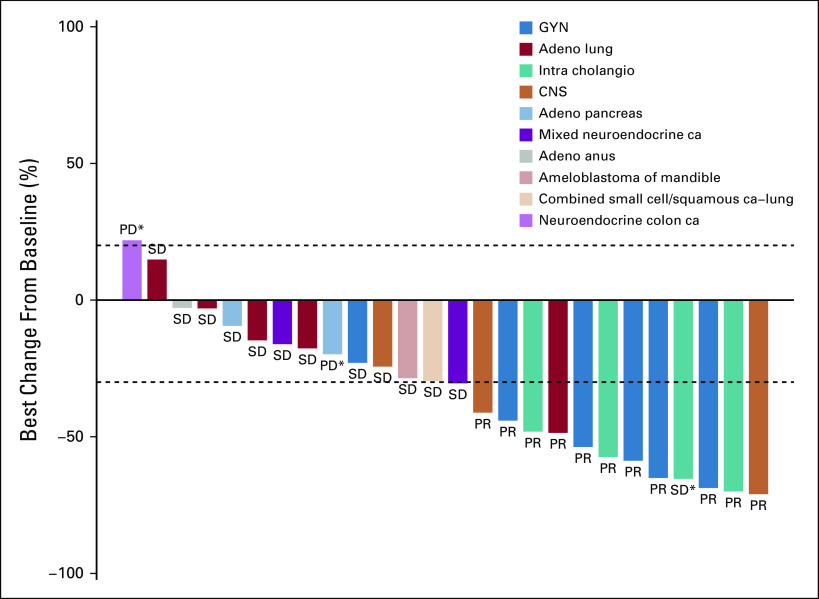
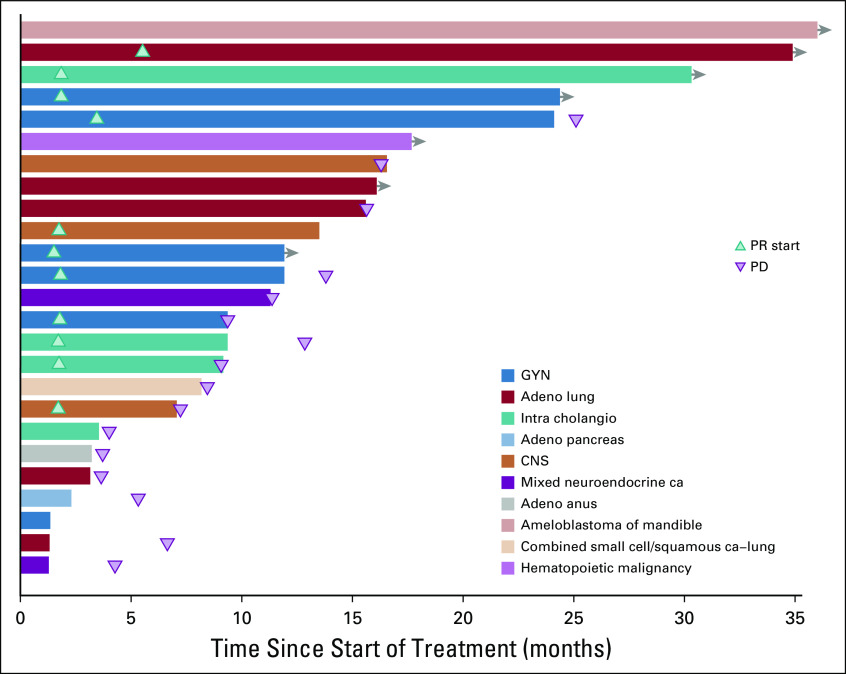
Although no complete responses were observed, durable partial responses (PRs) were seen across a variety of tumor types (Fig 3). These included papillary adenocarcinoma of the lung, LGSOC, mucinous-papillary serous adenocarcinoma of the peritoneum, histiocytic sarcoma of the brain, pleomorphic xanthoastrocytoma (PXA) of the parietal lobe, and cholangiocarcinoma. Of note, among 4 patients who were considered unevaluable for response because of baseline scans being outside of window, 1 patient with lung adenocarcinoma had a maximal decrease in the sum of measured lesions of 81%, and 1 patient with epithelioid glioblastoma had a decrease of 59%. Four of 5 patients with LGSOC had a PR, and 1 patient had SD. Three of the PRs lasted 12 months or longer (24.4 [progression free as of data cutoff], 25.1, and 13.8 months), and the fourth patient was progression free at 10.7 months. Three of the 4 patients with cholangiocarcinoma demonstrated a PR (individual PFS of 12.8, 9.1, and 29.4 months). Of the 5 patients with lung adenocarcinoma, 1 patient with a papillary variant had a PR and is progression free at 32.5 months, and 1 patient who was considered unevaluable, with an 81% reduction in the sum of measured lesions, had a PFS of 12.7 months. Three patients had SD for 15.6, 6.6, and 3.6 months. One patient with a PXA had a PR lasting 7.2 months, and 1 patient with histiocytic sarcoma of the brain had a PR and is progression free at 20.9 months. In addition to the 11 PRs, a total of 8 patients demonstrated a PFS of > 6 months. Treatment duration data are presented in Fig 4.
FIG 3.
Best percentage change from baseline in 23 patients with evaluable measurements. This plot excludes 5 unevaluable patients and 1 patient with pancreatic adenocarcinoma who was classified as having progressive disease but for whom complete data for target lesions were not available. (*) New lesions. Adeno, adenocarcinoma; ca, carcinoma; GYN, gynecologic; intra cholangia, intrahepatic cholangiocarcinoma; PD, progressive disease; PR, partial response; SD, stable disease.
FIG 4.
Duration of treatment in patients who achieved partial response (PR) or stable disease. Adeno, adenocarcinoma; ca, carcinoma; GYN, gynecologic; intra cholangio, intrahepatic cholangiocarcinoma; PD, progressive disease.
Adverse event (AE) analysis included all treated patients (n = 35). AEs were comparable to previously reported profiles of dabrafenib and trametinib, and no new AEs were identified. The most frequent AEs felt to be at least possibly related to treatment were fatigue in 26 of 35 patients (74%), nausea in 20 of 35 patients (57%), and fever and chills in 18 and 19 of 35 patients (51% and 54%), respectively. Headache was reported in 10 of 35 patients (29%), alkaline phosphatase elevation in 11 of 35 patients (31%), and aspartate aminotransferase elevation in 10 of 35 patients (29%). The most common grade 3 AEs felt to be possibly related to treatment were fatigue, neutropenia, hyponatremia, and hypophosphatemia; there was 1 grade 4 sepsis, (Appendix Table A1, online only). There were no grade 5 AEs.
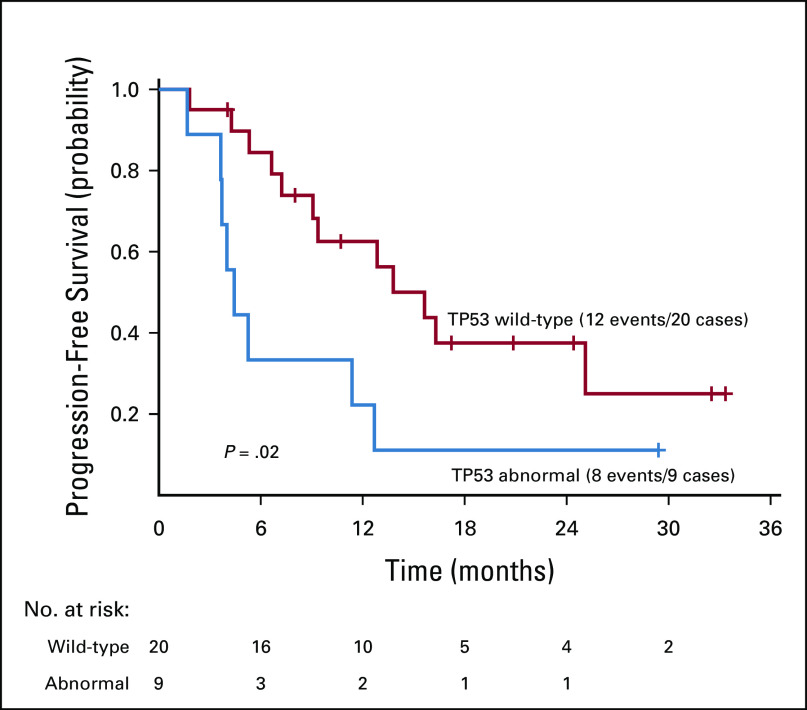
The NCI-MATCH NGS assay was designed to detect a number of predefined genomic alterations (Appendix Table A4, online only).16 Co-occurring mutation data were available for the 29 patients included in the primary analysis. In this cohort, 11 patients were identified as having an additional genetic alteration (Fig 5).The most common co-occurring mutation was a missense mutation in TP53, which was detected in 8 patients. One additional patient had an insertion-deletion in TP53. Overall, there was a high degree of heterogeneity, with no additional overlap in co-occurring mutations across patients. In an exploratory analysis, patients with TP53 alterations did seem to have a shorter PFS than that of patients with wild-type TP53 (nominal P = .02, HR = 2.8; Appendix Fig A1, online only). The response rate in the TP53-altered group was 11%, and it was 50% in the TP53 wild-type group, but this did not reach statistical significance (nominal P = .1).
FIG 5.
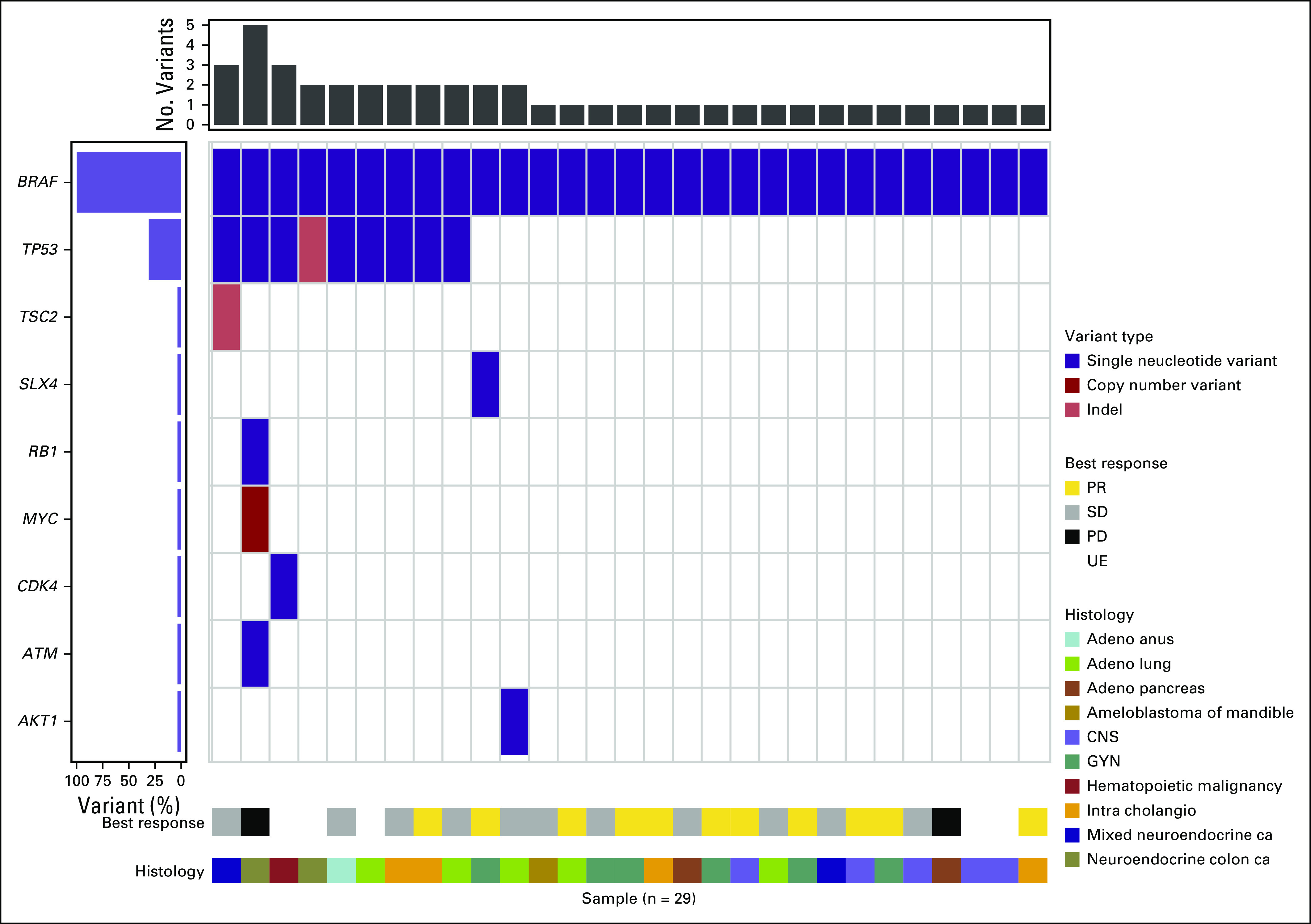
Co-occurring genomic alterations with BRAFV600E using the central NCI-MATCH assay for 29 patients. Single nucleotide variant (purple), copy number variant (dark red), insertion-deletion (light red). Response data and histology for individual patients are listed at the bottom. Adeno, adenocarcinoma; ca, carcinoma; GYN, gynecologic; intra cholangio, intrahepatic cholangiocarcinoma; PD, progressive disease; PR, partial response; SD, stable disease; UE, unevaluable.
DISCUSSION
This study met its primary end point, with an ORR of 37.9% (90% CI, 22.9% to 54.9%; P < .0001); an additional 2 unevaluable patients had clinically meaningful decreases in tumor volume. These data suggest that dabrafenib and trametinib have promising activity outside of their currently approved indications. Although the response rate reported here is lower than that in the first-line setting for diseases such as melanoma and NSCLC, the overall DCR was 75.9% in this heavily pretreated population.5,23 Importantly, disease control seemed to be durable in many patients, with a median duration of response of 25.1 months, a median PFS of 11.4 months, and a median OS of 28.6 months, suggesting prolonged benefit in a patient population with limited therapeutic options Fig 2A,2B
In selected cancers and other neoplastic processes, the frequency of BRAF mutations allows for the design of disease-specific trials.12 However, the overall rate of BRAF mutations in cancer is low, presenting a challenge for the design of histology-dependent studies.1 The NCI-MATCH trial was designed as a precision medicine platform trial, allowing patients whose tumors harbored prespecified molecular alterations to be assigned to a treatment arm on the basis of molecular assay results, independent of tumor histology. As expected, our study enrolled a heterogeneous patient population. This tumor heterogeneity has important therapeutic implications, given that BRAF/MEK–targeted approaches have demonstrated various degrees of efficacy across histologic subtypes. BRAF/MEK inhibition represents a standard-of-care option in BRAFV600-mutated melanoma, NSCLC, and thyroid cancer.2,5,6,8 However, successfully targeting the MAPK pathway has been more challenging in BRAF-mutated CRC, in which dabrafenib and trametinib resulted in response rates of 12% in 1 study, despite evidence of MAPK inhibition in all participants.9 EGFR-mediated reactivation of MAPK signaling seems to drive this resistance at least in part, and triplet therapy with EGFR antibodies combined with BRAF and MEK inhibitors has recently shown promising efficacy in this patient population.11,24 Recent reports in BRAFV600-mutant gliomas also suggest that there may be a histology-dependent variation in response to BRAF inhibition.13
Certain tumor types were more common in our study, but most tumor types were represented only as single cases. All 6 patients with gynecologic primaries seemed to derive clinical benefit from therapy, including 5 of 6 patients with a PR, 3 of which lasted more than 12 months; 1 patient has had SD for 8 months. LGSOC is a rare subtype of ovarian cancer that tends to be indolent and relatively refractory to chemotherapy; only isolated case reports have suggested a benefit with BRAF/MEK inhibition in BRAF-mutated LGSOCs.25,26 To our knowledge, the 5 patients in this study represent the largest report to date regarding the efficacy of dabrafenib and trametinib in LGSOC. A recent report from the biliary tract cancer cohort of a basket trial for BRAFV600E-mutated tumors treated with dabrafenib and trametinib reported PRs in 41% of patients.14 In this study, PRs were seen in 3 of the 4 patients with cholangiocarcinoma, with 1 ongoing at 29 months. These data lend additional support to the approach of BRAF/MEK inhibition in this disease with limited treatment options. As might be expected, the majority of patients with NSCLC benefitted from therapy, with 1 patient having a PR, a second patient with a substantial reduction in tumor volume, and an additional 2 patients with a PFS that exceeded 6 months. In contrast, many of the other malignancies with reported benefit in this study, such as histiocytic sarcoma of the brain and ameloblastoma, are exceedingly rare, with no defined standard therapy.
A recently published update of the VE-BASKET study of vemurafenib in nonmelanoma BRAFV600 cancers demonstrated a response rate of 33%, a median PFS of 5.8 months, and a median OS of 17.6 months.27 Responses were seen across 13 distinct histologies, lending additional support to the feasibility of BRAF inhibition across numerous cancers. In melanoma, combined BRAF/MEK inhibition has been shown to result in both superior PFS and OS when compared with BRAF-inhibitor monotherapy.23 The favorable median PFS of 11.4 months and median OS of 28.6 months reported in this study suggest that BRAF/MEK inhibition may also be preferred for the majority of BRAFV600-mutated cancers, and that the relative resistance seen in BRAF-driven CRC may be relatively isolated.
Co-occurring mutation data were available for 29 patients included in the primary analysis. TP53 was the most commonly altered gene, a finding seen in other data sets of prospectively sequenced metastatic cancers.28 There was some suggestion that patients in this study with co-occurring TP53 mutations derived less clinical benefit from dabrafenib and trametinib, which could possibly be attributed to previously reported associations with altered TP53 and more clinically aggressive disease.29 An analysis of BRAFV600-mutated nonmelanoma cancers treated with BRAF-inhibitor monotherapy demonstrated that co-occurring alterations in the phosphatidylinositol-3-kinase/mammalian target of rapamycin pathway were associated with a reduced PFS and OS.30 Only 1 patient in this study had a co-occurring AKT1-E17K mutation. Given the small overall numbers and the fact that complete sequencing data were available for most, but not all, patients, it is difficult to draw definitive conclusions regarding the impact of other pathways on this population.
Despite the limitations of a platform trial design, the response rate of 37.9% and high rate of disease control suggest that BRAF/MEK inhibition is likely a viable treatment approach across a wide variety of BRAFV600-mutated cancers. Consistent with data in diseases such as melanoma and NSCLC, de novo resistance to dabrafenib and trametinib was uncommon in this study, with only 2 patients having PD as best response. This study is an informative step in selecting patients for molecularly targeted therapy in BRAFV600-driven cancers, and it stands to serve as a foundation for future work focused on BRAFV600-mutated cancers that currently lack effective standard-of-care therapies. An expansion of this cohort is planned to better characterize the potential benefit of dabrafenib and trametinib in this patient population.
APPENDIX
FIG A1.
Progression-free survival by TP53 status.
FIG A2.
(A) Progression-free survival for patient cohort. (B) Overall survival for patient cohort.
FIG A3.
Best % change from baseline in 26 patients with evaluable measurements (n = 33 patient cohort). This plot excludes 5 unevaluable patients, 1 patient with multiple myeloma who had a minimal response, and 1 patient with pancreatic adenocarcinoma who was classified as having progressive disease but for whom complete data for target lesions were not available. (*) New lesions. Adeno, adenocarcinoma; ca, carcinoma; GYN, gynecologic; intra cholangio, intrahepatic cholangiocarcinoma; PD, progressive disease; PR, partial response; SD, stable disease.
FIG A4.
Duration of treatment in patients who achieved a partial response (PR) or stable disease for n = 33 patient cohort. Adeno, adenocarcinoma; ca, carcinoma; GYN, gynecologic; intra cholangio, intrahepatic cholangiocarcinoma; PD, progressive disease.
TABLE A1.
Treatment-Related Adverse Events
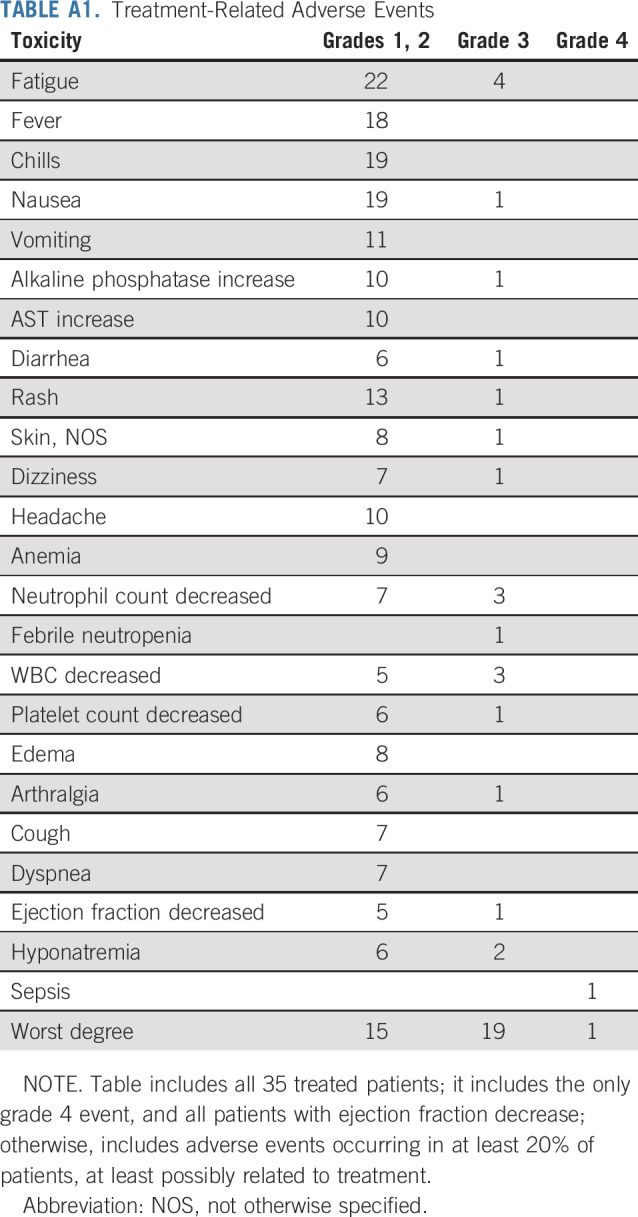
TABLE A2.
Patient Characteristics in Patient Cohort (n = 33)
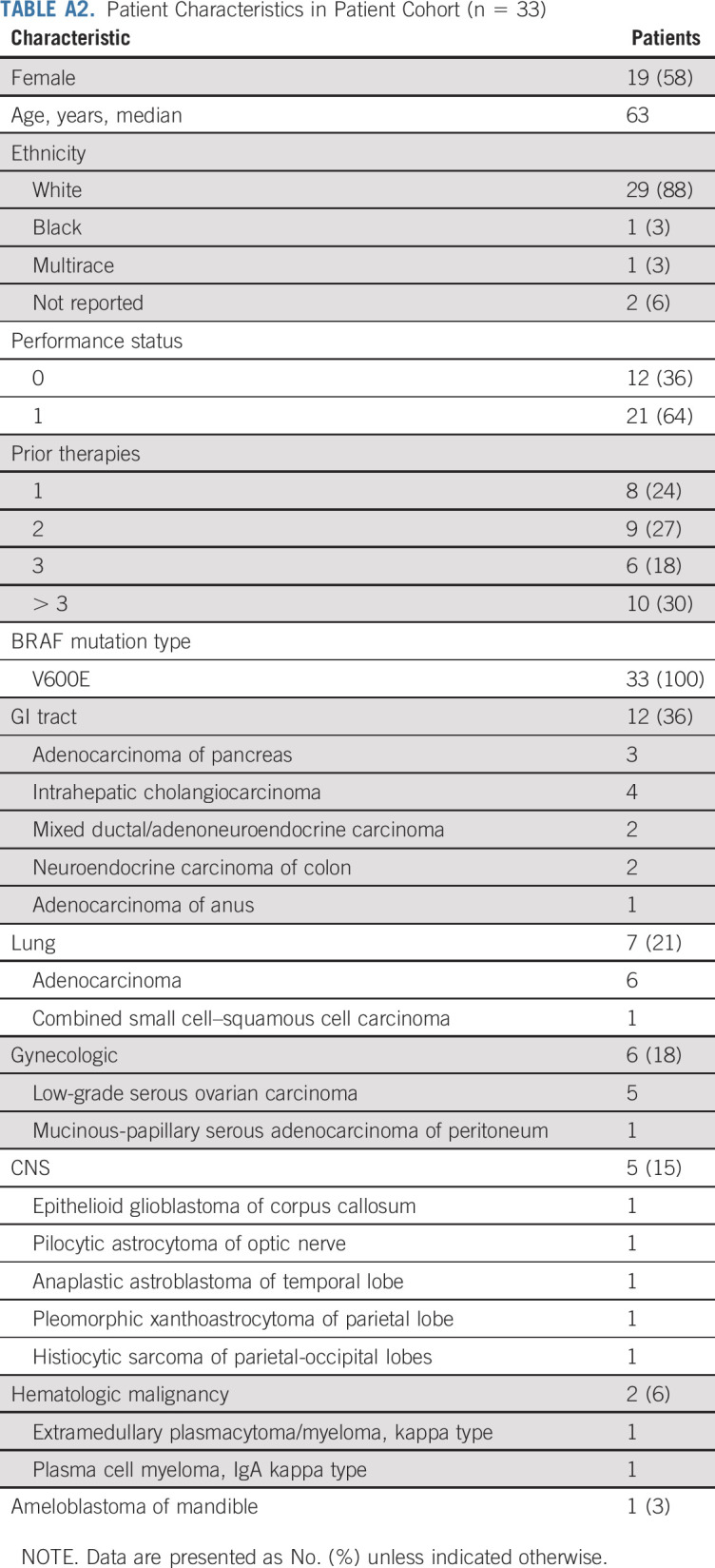
TABLE A3.
Response Assessment in Patient Cohort (n = 33)
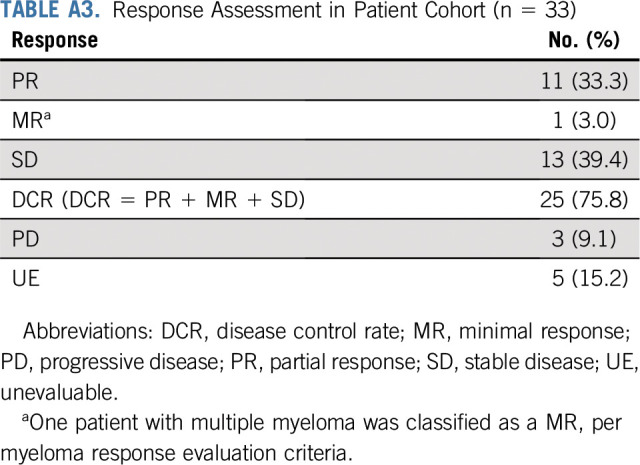
TABLE A4.
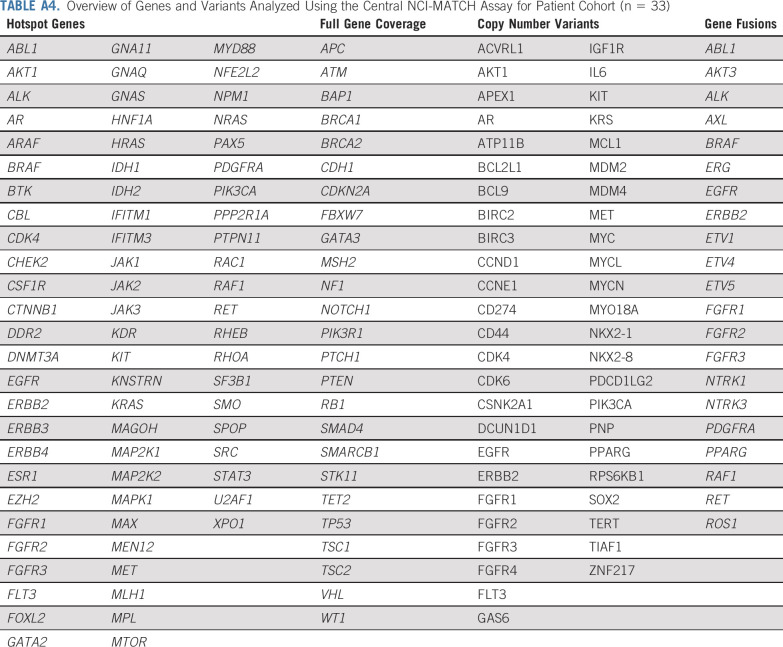
Overview of Genes and Variants Analyzed Using the Central NCI-MATCH Assay for Patient Cohort (n = 33)
PRIOR PRESENTATION
Presented in part at the 2019 ASCO Annual Meeting, Chicago IL, May 31-June 4, 2019.
SUPPORT
DISCLAIMER
The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, nor does mention of trade names, commercial products, or organizations imply endorsement by the US government.
AUTHOR CONTRIBUTIONS
Conception and design: April K. S. Salama, Shuli Li, Erin R. Macrae, Edith P. Mitchell, James A, Zweibel, Helen X. Chen, Robert J. Gray, Lisa M. McShane, Larry V. Rubenstein, David Patton, P. Mickey Williams, Stanley R. Hamilton, Barbara A. Conley, Carlos L Arteaga, Peter J. O’Dwyer, Alice P. Chen, Keith T. Flaherty
Financial support: Stanley R. Hamilton
Administrative support: Helen X. Chen, P. Mickey Williams, Stanley R. Hamilton, Barbara A. Conley, Lyndsay N. Harris
Provision of study material or patients: Edith P. Mitchell, Stanley R. Hamilton, Alice P. Chen
Collection and assembly of data: April K. S. Salama, Erin R. Macrae, Edith P. Mitchell, David Patton, P. Mickey Williams, Stanley R. Hamilton, Deborah K. Armstrong, Alice P. Chen
Data analysis and interpretation: April K. S. Salama, Shuli Li, Erin R. Macrae, Jong-In Park, Edith P. Mitchell, Robert J. Gray, Lisa M. McShane, David Patton, P. Mickey Williams, Stanley R. Hamilton, Deborah K. Armstrong, Barbara A. Conley, Lyndsay N. Harris, Peter J. O’Dwyer, Alice P. Chen, Keith T. Flaherty
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Dabrafenib and Trametinib in Patients With Tumors with BRAFV600E Mutations: Results of the NCI-MATCH Trial Subprotocol H
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
April K. S. Salama
Consulting or Advisory Role: Merck, Array BioPharma, Novartis, Regeneron
Research Funding: Bristol Myers Squibb (Inst), Merck (Inst), Dynavax (Inst), Immunocore
Edith P. Mitchell
Honoraria: Sanofi
Consulting or Advisory Role: Genentech, Novartis, Merck, Bristol Myers Squib
Speakers' Bureau: Ipsen
Research Funding: Genentech (Inst), Sanofi (Inst)
James A. Zwiebel
Consulting or Advisory Role: Boston Pharmaceuticals, Scandion Oncology
Robert J. Gray
Research Funding: Agios, Amgen, AstraZeneca, Bristol Myers Squibb, Boehringer Ingelheim, Celgene, Genentech/Roche, Genomic Health, Genzyme, GlaxoSmithKline, Janssen-Ortho, Onyx, Pfizer, Sequenta, Syndax, Novartis, Takeda, AbbVie, Sanofi, Merck Sharp & Dohme
P. Mickey Williams
Research Funding: Illumina (Inst)
Patents, Royalties, Other Intellectual Property: I was a co-inventor of the diffuse large B-cell lymphoma cell of origin patent recently filed by the National Institutes of Health
Stanley R. Hamilton
Stock and Other Ownership Interests: The Johns Hopkins University School of Medicine
Consulting or Advisory Role: HalioDx, Thermo Fisher Scientific, Bristol Myers Squibb, Loxo, Merck, Guardant Health, Cell Medica Limited
Deborah K. Armstrong
Consulting or Advisory Role: CUE Biopharma, AbbVie, Eisai
Research Funding: Clovis Oncology (Inst), AstraZeneca (Inst), Advaxis (Inst), Syndax (Inst), Pfizer (Inst), Tesaro (Inst), Eisai (Inst)
Other Relationship: AstraZeneca
Carlos L. Arteaga
Leadership: American Association for Cancer Research
Stock and Other Ownership Interests: Provista Diagnostics, Y-Trap
Consulting or Advisory Role: Novartis, Lilly, Sanofi, Radius Health, Taiho Pharmaceutical, Puma Biotechnology, Merck, H3 Biomedicine, Symphogen, Origimed, Petra Pharma, Third Rock Ventures, Immunomedics, Daiichi Sankyo, Athenex, G1 Therapeutics, Clovis Oncology
Research Funding: Puma Biotechnology, Pfizer, Lilly, Radius Health, Takeda, Bayer
Other Relationship: Susan G. Komen for the Cure
Lyndsay N. Harris
Patents, Royalties, Other Intellectual Property: Philips Healthcare
Peter J. O’Dwyer
Consulting or Advisory Role: Genentech, Array
Research Funding: Bristol Myers Squibb, Pfizer, Novartis, Genentech, Mirati Therapeutics, Celgene, GlaxoSmithKline, BBI Healthcare, Merck, Pharmacyclics, Bayer, Five Prime Therapeutics, Forty Seven, Amgen, H3 Biomedicine (Inst), Taiho (Inst), Array BioPharma (Inst), Lilly/ImClone (Inst)
Expert Testimony: Bayer, Lilly
Keith T. Flaherty
Stock and Other Ownership Interests: Clovis Oncology, Loxo, X4 Pharma, Strata Oncology, PIC Therapeutics, Fount Therapeutics, Shattuck Labs, Apricity Health, Oncoceutics, Fog Pharma, Tvardi, Checkmate Pharmaceuticals, Kinnate
Consulting or Advisory Role: Novartis, Genentech, Merck, Lilly, Amgen, Sanofi, Oncoceutics, Bristol Myers Squibb, Adaptimmune, Aeglea Biotherapeutics, Loxo, Roche, Asana Biosciences, Incyte, Shattuck Labs, Tolero Pharmaceuticals, Array BioPharma, FOG Pharma, Neon Therapeutics, Tvardi, Takeda, Verastem, Boston Biomedical, Pierre Fabre, Cell Medica, Debiopharm Group
Research Funding: Novartis, Sanofi
Travel, Accommodations, Expenses: Pierre Fabre, Debiopharm Group
No other potential conflicts of interest were reported.
REFERENCES
- 1.Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–954. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- 2.Robert C, Karaszewska B, Schachter J, et al. Improved overall survival in melanoma with combined dabrafenib and trametinib. N Engl J Med. 2015;372:30–39. doi: 10.1056/NEJMoa1412690. [DOI] [PubMed] [Google Scholar]
- 3.Paik PK, Arcila ME, Fara M, et al. Clinical characteristics of patients with lung adenocarcinomas harboring BRAF mutations. J Clin Oncol. 2011;29:2046–2051. doi: 10.1200/JCO.2010.33.1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cohn AL, Day BM, Abhyankar S, et al. BRAFV600 mutations in solid tumors, other than metastatic melanoma and papillary thyroid cancer, or multiple myeloma: A screening study. OncoTargets Ther. 2017;10:965–971. doi: 10.2147/OTT.S120440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Planchard D, Smit EF, Groen HJM, et al. Dabrafenib plus trametinib in patients with previously untreated BRAFV600E-mutant metastatic non-small-cell lung cancer: An open-label, phase 2 trial. Lancet Oncol. 2017;18:1307–1316. doi: 10.1016/S1470-2045(17)30679-4. [DOI] [PubMed] [Google Scholar]
- 6.Subbiah V, Kreitman RJ, Wainberg ZA, et al. Dabrafenib and trametinib treatment in patients with locally advanced or metastatic BRAF V600-mutant anaplastic thyroid cancer. J Clin Oncol. 2018;36:7–13. doi: 10.1200/JCO.2017.73.6785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tiacci E, Trifonov V, Schiavoni G, et al. BRAF mutations in hairy-cell leukemia. N Engl J Med. 2011;364:2305–2315. doi: 10.1056/NEJMoa1014209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fukushima T, Suzuki S, Mashiko M, et al. BRAF mutations in papillary carcinomas of the thyroid. Oncogene. 2003;22:6455–6457. doi: 10.1038/sj.onc.1206739. [DOI] [PubMed] [Google Scholar]
- 9.Corcoran RB, Atreya CE, Falchook GS, et al. Combined BRAF and MEK inhibition with dabrafenib and trametinib in BRAF V600-mutant colorectal cancer. J Clin Oncol. 2015;33:4023–4031. doi: 10.1200/JCO.2015.63.2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Corcoran RB, Ebi H, Turke AB, et al. EGFR-mediated re-activation of MAPK signaling contributes to insensitivity of BRAF mutant colorectal cancers to RAF inhibition with vemurafenib. Cancer Discov. 2012;2:227–235. doi: 10.1158/2159-8290.CD-11-0341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kopetz S, Grothey A, Yaeger R, et al. Encorafenib, binimetinib, and cetuximab in BRAF V600E-mutated colorectal cancer. N Engl J Med. 2019;381:1632–1643. doi: 10.1056/NEJMoa1908075. [DOI] [PubMed] [Google Scholar]
- 12.Hyman DM, Puzanov I, Subbiah V, et al. Vemurafenib in multiple nonmelanoma cancers with BRAF V600 mutations N Engl J Med 373726–736.2015[Erratum: N Engl J Med 379:1585, 2018] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaley T, Touat M, Subbiah V, et al. BRAF inhibition in BRAFV600-mutant gliomas: Results from the VE-BASKET study. J Clin Oncol. 2018;36:3477–3484. doi: 10.1200/JCO.2018.78.9990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wainberg ZA, Lassen UN, Elez E, et al: Efficacy and safety of dabrafenib (D) and trametinib (T) in patients (pts) with BRAF V600E–mutated biliary tract cancer (BTC): A cohort of the ROAR basket trial. J Clin Oncol 37:187-187, 2019 .
- 15.Conley BA, Doroshow JH. Molecular analysis for therapy choice: NCI MATCH. Semin Oncol. 2014;41:297–299. doi: 10.1053/j.seminoncol.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 16.Lih CJ, Harrington RD, Sims DJ, et al. Analytical validation of the next-generation sequencing assay for a nationwide signal-finding clinical trial: Molecular analysis for therapy choice clinical trial. J Mol Diagn. 2017;19:313–327. doi: 10.1016/j.jmoldx.2016.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Flaherty KT, Gray R, Chen A, et al. The Molecular Analysis for Therapy Choice (NCI-MATCH) trial: Lessons for genomic trial design J Natl Cancer Inst 10.1093/jnci/djz245 [epub ahead of print on January 10, 2020] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 19.Cheson BD, Fisher RI, Barrington SF, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: The Lugano classification. J Clin Oncol. 2014;32:3059–3068. doi: 10.1200/JCO.2013.54.8800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wen PY, Macdonald DR, Reardon DA, et al. Updated response assessment criteria for high-grade gliomas: Response assessment in neuro-oncology working group. J Clin Oncol. 2010;28:1963–1972. doi: 10.1200/JCO.2009.26.3541. [DOI] [PubMed] [Google Scholar]
- 21.Kumar S, Paiva B, Anderson KC, et al. International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet Oncol. 2016;17:e328–e346. doi: 10.1016/S1470-2045(16)30206-6. [DOI] [PubMed] [Google Scholar]
- 22. https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf US National Institutes of Health: Common Terminology Criteria for Adverse Events (CTCAE), version 4.0.
- 23.Long GV, Stroyakovskiy D, Gogas H, et al. Combined BRAF and MEK inhibition versus BRAF inhibition alone in melanoma. N Engl J Med. 2014;371:1877–1888. doi: 10.1056/NEJMoa1406037. [DOI] [PubMed] [Google Scholar]
- 24.Corcoran RB, André T, Atreya CE, et al. Combined BRAF, EGFR, and MEK inhibition in patients with BRAFV600E-mutant colorectal cancer. Cancer Discov. 2018;8:428–443. doi: 10.1158/2159-8290.CD-17-1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mendivil AA, Tung PK, Bohart R, et al. Dramatic clinical response following dabrafenib and trametinib therapy in a heavily pretreated low grade serous ovarian carcinoma patient with a BRAF V600E mutation. Gynecol Oncol Rep. 2018;26:41–44. doi: 10.1016/j.gore.2018.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sieben NL, Macropoulos P, Roemen GM, et al. In ovarian neoplasms, BRAF, but not KRAS, mutations are restricted to low-grade serous tumours. J Pathol. 2004;202:336–340. doi: 10.1002/path.1521. [DOI] [PubMed] [Google Scholar]
- 27.Subbiah V, Puzanov I, Blay JY, et al. Pan-cancer efficacy of vemurafenib in BRAFV600-mutant non-melanoma cancers. Cancer Discov. 2020;10:657–663. doi: 10.1158/2159-8290.CD-19-1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zehir A, Benayed R, Shah RH, et al. Mutational landscape of metastatic cancer revealed from prospective clinical sequencing of 10,000 patients Nat Med 23703–713.2017[Erratum: Nat Med 23:1004, 2017] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Petitjean A, Achatz MI, Borresen-Dale AL, et al. TP53 mutations in human cancers: Functional selection and impact on cancer prognosis and outcomes. Oncogene. 2007;26:2157–2165. doi: 10.1038/sj.onc.1210302. [DOI] [PubMed] [Google Scholar]
- 30.Sen S, Meric-Bernstam F, Hong DS, et al. Co-occurring genomic alterations and association with progression-free survival in BRAFV600-mutated nonmelanoma tumors. J Natl Cancer Inst. 2017;109:109. doi: 10.1093/jnci/djx094. [DOI] [PMC free article] [PubMed] [Google Scholar]