PURPOSE
We conducted the phase III double-blind European Organisation for Research and Treatment of Cancer (EORTC) 1325/KEYNOTE-054 trial to evaluate pembrolizumab versus placebo in patients with resected high-risk stage III melanoma. On the basis of 351 recurrence-free survival (RFS) events at a 1.25-year median follow-up, pembrolizumab prolonged RFS (hazard ratio [HR], 0.57; P < .0001) compared with placebo. This led to the approval of pembrolizumab adjuvant treatment by the European Medicines Agency and US Food and Drug Administration. Here, we report an updated RFS analysis at the 3.05-year median follow-up.
PATIENTS AND METHODS
A total of 1,019 patients with complete lymph node dissection of American Joint Committee on Cancer Staging Manual (seventh edition; AJCC-7), stage IIIA (at least one lymph node metastasis > 1 mm), IIIB, or IIIC (without in-transit metastasis) cutaneous melanoma were randomly assigned to receive pembrolizumab at a flat dose of 200 mg (n = 514) or placebo (n = 505) every 3 weeks for 1 year or until disease recurrence or unacceptable toxicity. The two coprimary end points were RFS in the overall population and in those with programmed death-ligand 1 (PD-L1)–positive tumors.
RESULTS
Pembrolizumab (190 RFS events) compared with placebo (283 RFS events) resulted in prolonged RFS in the overall population (3-year RFS rate, 63.7% v 44.1% for pembrolizumab v placebo, respectively; HR, 0.56; 95% CI, 0.47 to 0.68) and in the PD-L1–positive tumor subgroup (HR, 0.57; 99% CI, 0.43 to 0.74). The impact of pembrolizumab on RFS was similar in subgroups, in particular according to AJCC-7 and AJCC-8 staging, and BRAF mutation status (HR, 0.51 [99% CI, 0.36 to 0.73] v 0.66 [99% CI, 0.46 to 0.95] for V600E/K v wild type).
CONCLUSION
In resected high-risk stage III melanoma, pembrolizumab adjuvant therapy provided a sustained and clinically meaningful improvement in RFS at 3-year median follow-up. This improvement was consistent across subgroups.
INTRODUCTION
In concordance with results obtained with immune checkpoint inhibitors and BRAF plus MEK inhibitors in advanced melanoma,1,2 adjuvant therapies with ipilimumab,3-5 nivolumab,6 and pembrolizumab7 in patients with melanoma at high risk for relapse regardless of BRAF mutation status and with dabrafenib plus trametinib8,9 in patients with BRAF mutation demonstrated significant benefits that resulted in US Food and Drug Administration (FDA) approvals for all of these drugs. The ipilimumab,3-5 pembrolizumab,7 and dabrafenib plus trametinib8,9 trials were conducted in patients with stage III disease with the restriction that patients with American Joint Committee on Cancer (AJCC) stage IIIA disease had to be at higher risk of recurrence on the basis of tumor load in the sentinel node (diameter > 1 mm, according to the Rotterdam criteria).10-12 The CheckMate-238 (ClinicalTrials.gov identifier: NCT02388906) nivolumab trial was conducted in patients with stage IIIB-C and completely resected stage IV melanoma.6
CONTEXT
Key Objective
Does pembrolizumab treatment administration for 1 year lead to a sustained improvement of recurrence-free survival (RFS) in resected high-risk stage III melanoma?
Knowledge Generated
Pembrolizumab as adjuvant therapy for patients with resected high-risk stage III melanoma provided a statistically significant and clinically relevant 20% improvement of the RFS rate at 3 years compared with placebo and had a safety profile consistent with the toxicity spectrum that already had been defined. Such RFS improvement was consistent across subgroups, in particular according to programmed cell death-ligand 1 status, American Joint Committee on Cancer Cancer Staging Manual (seventh edition; AJCC-7) and (eighth edition; AJCC-8), and BRAF mutation status.
Relevance
More than 1 year ago, pembrolizumab was already approved by the US Food and Drug Administration and European Medicines Agency. These results confirm the clinical utility of pembrolizumab in the adjuvant setting in resected high-risk stage III melanoma. We expect that these RFS improvements will also translate in terms of distant metastasis–free survival and overall survival when long-term follow-up results are available.
We conducted the phase III, randomized, double-blind European Organisation for Research and Treatment of Cancer (EORTC) 1325/KEYNOTE-054 trial (ClinicalTrials.gov identifier: NCT02362594) to evaluate pembrolizumab versus placebo in patients with resected high-risk stage III melanoma. At the 1.25-year median follow-up, pembrolizumab adjuvant treatment prolonged RFS (hazard ratio [HR], 0.57, P < .0001) compared with placebo.7 This led to the approval of pembrolizumab adjuvant treatment by the European Medicines Agency (EMA) and FDA.
We report an updated analysis at 3-year median follow-up with regard to RFS outcome of the EORTC 1325/KEYNOTE-054 trial to investigate whether the benefit is sustained and whether patient characteristics, particularly programmed cell death-ligand 1 (PD-L1) status; baseline stage according to AJCC Cancer Staging Manual (seventh edition; AJCC-7), and AJCC-8 classifications13,14; and BRAF-V600E/K mutation status are of predictive importance for the treatment difference. Such analyses are important to confirm the initial findings with a shorter follow-up7,15 and to compare them with those provided by the COMBI-AD trial (ClinicalTrials.gov identifier: NCT01682083) in BRAF-V600E/K–mutated melanoma at 44 months median follow-up.9
PATIENTS AND METHODS
Patients
Patients (age ≥ 18 years) with histologically confirmed cutaneous melanoma with metastasis to regional lymph nodes were eligible to enter the study provided that a complete regional lymphadenectomy could be performed within 13 weeks before the start of treatment. Patients had either stage IIIA melanoma (patients with N1a or N2a had to have at least one micrometastasis measuring > 1 mm in greatest diameter) or stage IIIB or IIIC disease with no in-transit metastases according to the AJCC-7 classification.13 Exclusion criteria included Eastern Cooperative Oncology Group performance status 2-4, presence of autoimmune disease, uncontrolled infections, use of systemic corticosteroids, and prior systemic therapy for melanoma. A tumor sample from melanoma-positive lymph nodes was required to be sent for central pathology evaluation of PD-L1 expression. Membranous PD-L1 expression in tumor and tumor-associated immune cells was assessed by an immunohistochemistry assay and scored on a scale of 0-5; a score ≥ 2 (staining on > 1% of cells) was considered PD-L1 positive.16
Study Design and Treatment
Registration was done centrally at the EORTC headquarters. The randomization, using a minimization technique, was stratified by AJCC-7 staging (stage IIIA v stage IIIB v stage IIIC with one to three positive nodes v stage IIIC with more than three positive nodes) and region. Only the local pharmacists were aware of trial group assignments.
Patients were randomly assigned (1:1) to receive either an intravenous infusion of pembrolizumab 200 mg or placebo every 3 weeks for a total of 18 doses for approximately 1 year or until disease recurrence, unacceptable toxicity, major protocol violation, or withdrawal of consent (Data Supplement, online only). The primary end point was RFS, as reported by the local investigators, in the overall population and in the subgroup of patients with PD-L1–positive tumors.
Assessments
Computed tomography (CT) scans and magnetic resonance imaging (MRI; full chest, abdomen, and pelvis CT and/or MRI, neck CT and/or MRI for head and neck primaries, CT and/or MRI for other localizations [eg, brain, deep soft tissue], only if clinically indicated) were performed every 12 weeks for the first 2 years and every 6 months through year 5. Recurrence or metastatic lesions had to be histologically confirmed whenever possible. The first date when recurrence was observed was taken into account.
RFS was defined as the time from random assignment until the date of first recurrence (local, regional, or distant metastasis) or death as a result of any cause. For patients without any event, the follow-up was censored at the latest disease evaluation performed according to the protocol.
Statistical Analysis
Details with regard to sample size computations, implementation of an interim analysis in an amended protocol, and dissemination of the treatment outcome results were provided in the original publication.7 The interim analysis, which became the final one, was based on 351 RFS events as reported on the clinical cutoff date of October 2, 2017. The clinical cutoff date for the current analysis was September 30, 2019. This updated analysis, with a longer follow-up, was performed to assess whether the initial findings still hold true.
RFS distribution was estimated using the Kaplan-Meier method, and the 95% CIs were estimated through the Greenwood variance formula. For treatment comparison, the log-rank test stratified by stage provided at randomization was used. The Cox model stratified by stage provided at randomization was used to estimate the HRs and the CIs, 95% for the overall population and 99% for different subgroups.
We investigated the possible predictive importance of several factors (eg, AJCC-7 and AJCC-8 staging classifications, BRAF-V600E/K mutation status) on the treatment differences with regard to RFS. Forest plots for the HRs were produced, and results of the test of interaction between each factor and the treatment group in an unstratified Cox model were indicated. The treatment HRs for each subgroup estimated using the model with the interaction term were plotted along with their 99% CIs.
The cumulative incidence of the appearance of a distant metastasis as the first RFS event was estimated by the Aalen-Johansen method, and the treatment comparison was performed using the Fine and Gray model stratified by stage at random assignment. The primary analysis of RFS included all the patients who underwent random assignment, according to the intention-to-treat (ITT) principle. Sensitivity analysis was based on the per-protocol treatment (PPT) population: Eligible patients who started the treatment were allocated by random assignment. The safety profile was assessed in patients who started treatment allocated by random assignment. All statistical analyses were performed using SAS 9.4 software (SAS Institute, Cary, NC).
Trial Oversight
The protocol was approved by the EORTC protocol review committee and independent ethics committees. The trial was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice. All patients provided written informed consent.
RESULTS
Patients and Trial Regimen
From August 2015 through November 2016, 1,019 patients were randomly assigned at 123 centers in 23 countries: 514 patients were assigned to the pembrolizumab group and 505 to the placebo group. The characteristics at baseline were similar between the two groups (Data Supplement).
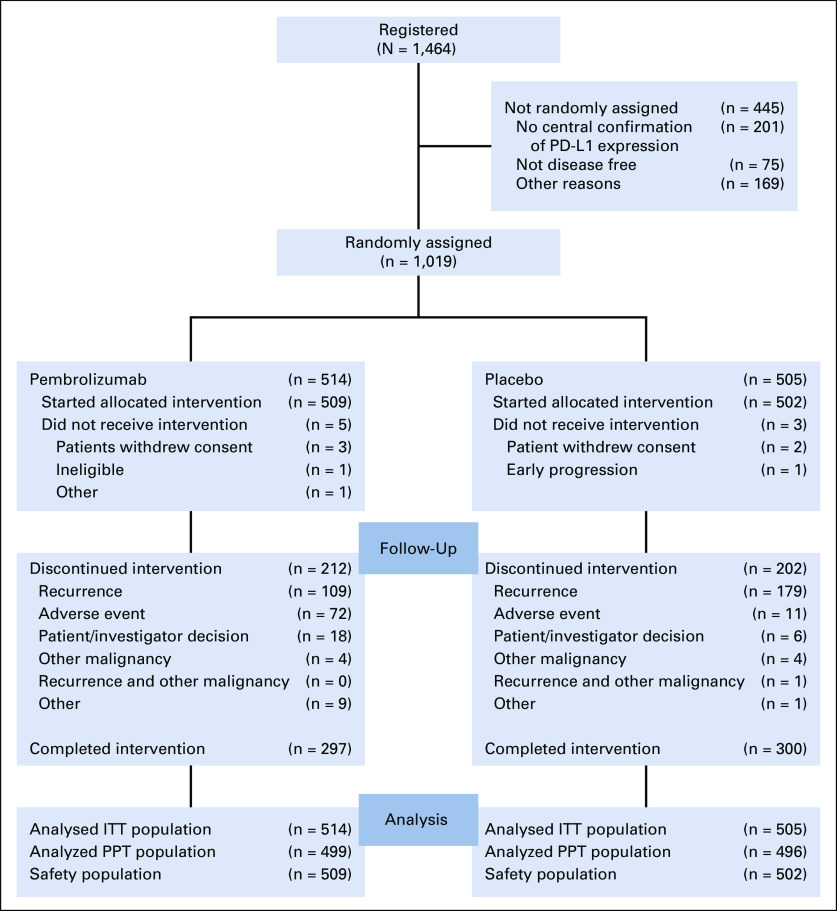
Eight patients did not start the treatment allocated by random assignment (Fig 1). Of 509 patients who started pembrolizumab, 72 (14.1%) discontinued treatment because of an adverse event (AE). Among 502 patients who received placebo, 11 (2.2%) discontinued treatment because of an AE. A total of 109 (21.4%) patients in the pembrolizumab group discontinued treatment because of disease recurrence compared with 179 (35.7%) in the placebo group. A total of 297 (58.3%) patients in the pembrolizumab group and 300 (59.8%) patients in the placebo group completed 1 year of treatment (Fig 1). The median follow-up was 36.6 months (interquartile range [IQR], 35.0-40.2 months) overall, 36.6 months (IQR, 34.9-39.8 months) in the pembrolizumab group, and 36.5 months (IQR, 35.0-40.5 months) in the placebo group.
FIG 1.
CONSORT diagram. Safety population indicates patients who started the allocated treatment. ITT, intention to treat; PD-L1, programmed cell death-ligand 1; PPT, per-protocol treatment.
Updated RFS
In the ITT overall population, the 3-year RFS rate was 63.7% (95% CI, 59.2% to 67.7%) in the pembrolizumab group and 44.1% (95% CI, 39.6% to 48.4%) in the placebo group (Fig 2A). RFS remained significantly longer in the pembrolizumab group than in the placebo group (HR stratified by stage, 0.56; 95% CI, 0.47 to 0.68; P < .001).
FIG 2.
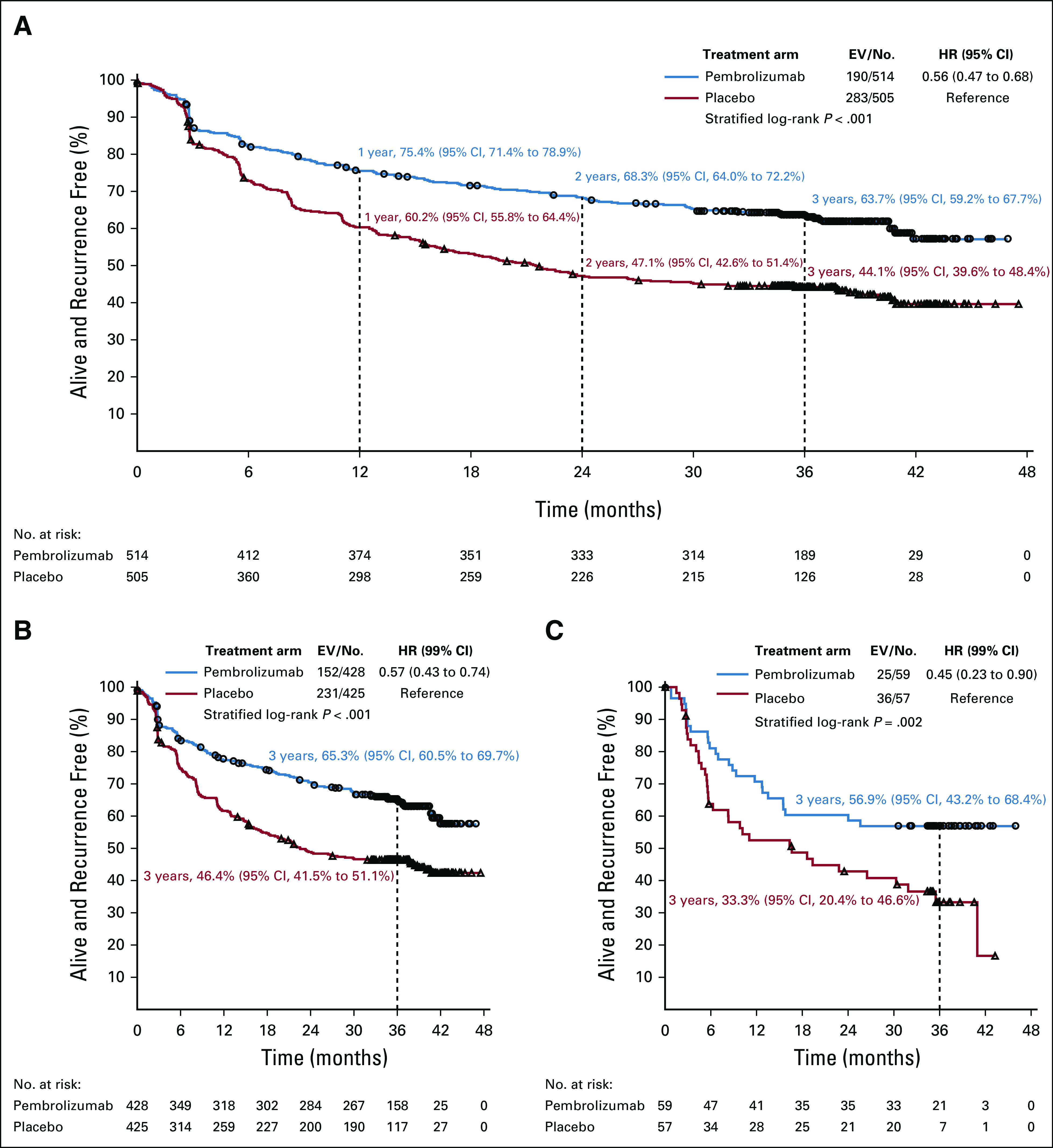
Recurrence-free survival (RFS) by treatment group. (A) In the overall population and according to programmed cell death-ligand 1 (PD-L1) tumor status. (B) PD-L1 positive. (C) PD-L1 negative. EV/No., events/number of patients; HR, hazard ratio.
During the additional follow-up period from the final analysis,7 122 new RFS events were reported—55 in the pembrolizumab group versus 67 in the placebo group. A total of 473 patients had a recurrence or died—190 (37.0%) in the pembrolizumab group and 283 (56.0%) in the placebo group (Data Supplement). Among them, 68 patients (13.2%) in the pembrolizumab group had a locoregional recurrence only versus 92 (18.2%) in the placebo group, and 117 (22.8%) patients developed distant metastases as their first recurrence (alone or combined with locoregional recurrences in the pembrolizumab group) versus 190 (37.6%) in the placebo group. The 3-year cumulative incidence rate of distant metastasis being the first site of recurrence was 22.3% (95% CI, 18.8% to 26.1%) in the pembrolizumab group and 37.3% (95% CI, 33.0% to 41.6%) in the placebo group (HR, 0.55; 95% CI, 0.44 to 0.69; Data Supplement). There were four (0.8%) deaths without recurrence (one as a result of myositis and three unrelated to treatment) in the pembrolizumab group and one (0.2%) in the placebo group. Sensitivity analysis for RFS, on the basis of the PPT population, provided similar results (HR, 0.56; 95% CI, 0.47 to 0.68).
Subgroup Analysis of RFS
The treatment difference with regard to RFS was consistently observed across subgroups determined by all baseline characteristics (Fig 3; Data Supplement).
FIG 3.
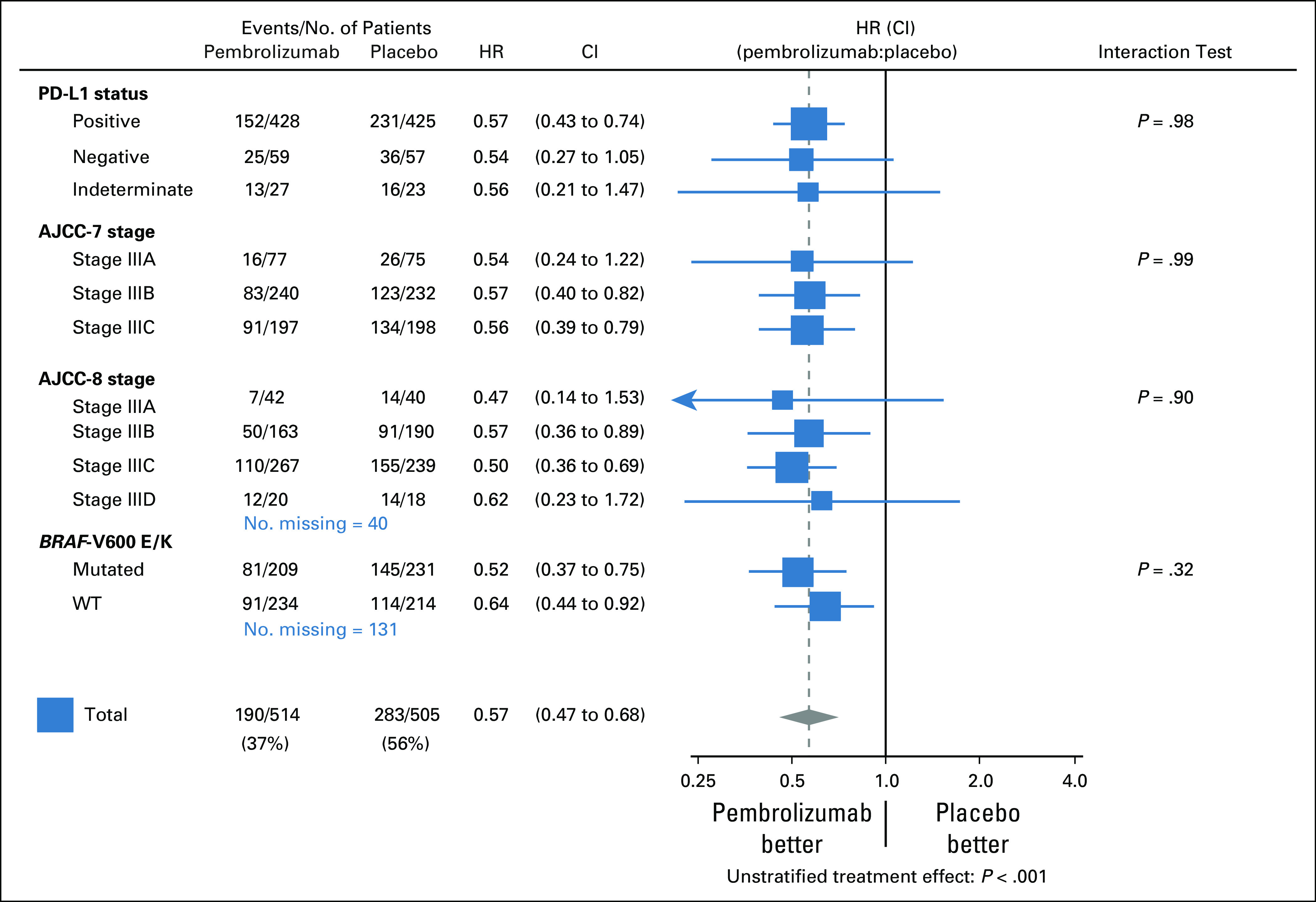
Forest plot of recurrence-free survival. AJCC, American Joint Committee on Cancer (seventh edition; AJCC-7; AJCC (eighth edition; AJCC-8), HR, hazard ratio; PD-L1, programmed cell death-ligand 1; WT, wild type.
RFS according to tumor PD-L1 expression.
In the 853 patients with PD-L1–positive tumors, the 3-year RFS rate was 65.3% (95% CI, 60.5% to 69.7%) in the pembrolizumab group and 46.4% (95% CI, 41.5% to 51.1%) in the placebo group (HR stratified by stage, 0.57; 99% CI, 0.43 to 0.74; P < .001; Fig 2B). Pembrolizumab was also consistently effective in the 116 patients with PD-L1–negative tumors, with the 3-year RFS rate being 56.9% (95% CI, 43.2% to 68.4%) in the pembrolizumab group and 33.3% (95% CI, 20.4% to 46.6%) in the placebo group (HR stratified by stage, 0.45; 99% CI, 0.23 to 0.90; Fig 2C), and in those with an undetermined tumor PD-L1 expression (Fig 3).
RFS according to AJCC-7 and AJCC-8.
The benefit of pembrolizumab was similar (P = .99) in the three AJCC-7 subgroups (Fig 3). The HRs stratified by stage as indicated at random assignment were 0.50 (99% CI, 0.22 to 1.16), 0.56 (99% CI, 0.39 to 0.81), and 0.57 (99% CI, 0.40 to 0.81) in patients with stage IIIA, IIIB, and IIIC disease, respectively (Fig 4). The 3-year RFS rates in the pembrolizumab and placebo groups were 81.2% and 66.3% in the patients with stage IIIA, 65.7% and 47.0% in those with stage IIIB, and 54.3% and 32.3% in those with stage IIIC disease, respectively. The 99% CIs of these estimates are shown in Figures 4A-4C.
FIG 4.
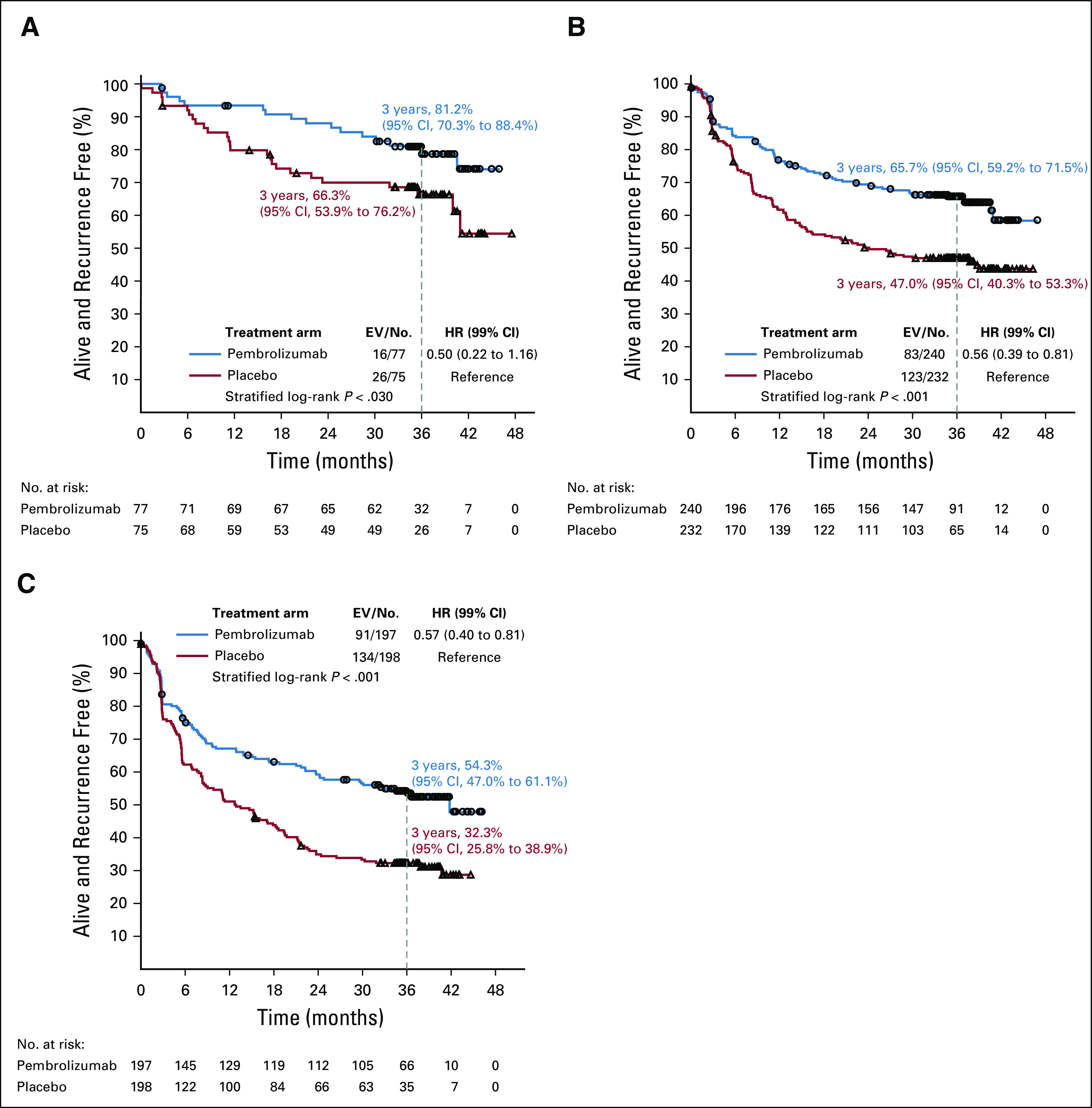
Recurrence-free survival by treatment group according to the American Joint Committee on Cancer Cancer Staging Manual (seventh edition; AJCC-7). (A) Stage IIIA. (B) Stage IIIB. (C) Stage IIIC. EV/No., events/number of patients; HR, hazard ratio.
The benefit of pembrolizumab was similar (P = .90) in the four AJCC-8 subgroups (Fig 3). The HRs stratified by stage provided at random assignment were 0.43 (99% CI, 0.13 to 1.43), 0.57 (99% CI, 0.36 to 0.90), 0.51 (99% CI, 0.37 to 0.70), and 0.68 (99% CI, 0.24 to 1.91) in patients with stage IIIA, IIIB, IIIC, and IIID disease, respectively (Fig 5). The 3-year RFS rates in the pembrolizumab and placebo groups were 82.6% and 67.4% in the patients with stage IIIA, 70.4% and 51.7% in those with stage IIIB, 59.6% and 35.2% in those with stage IIIC, and 45.0% and 22.2% in those with stage IIID disease, respectively. The 99% CIs of these estimates are shown in Figures 5A-5D.
FIG 5.
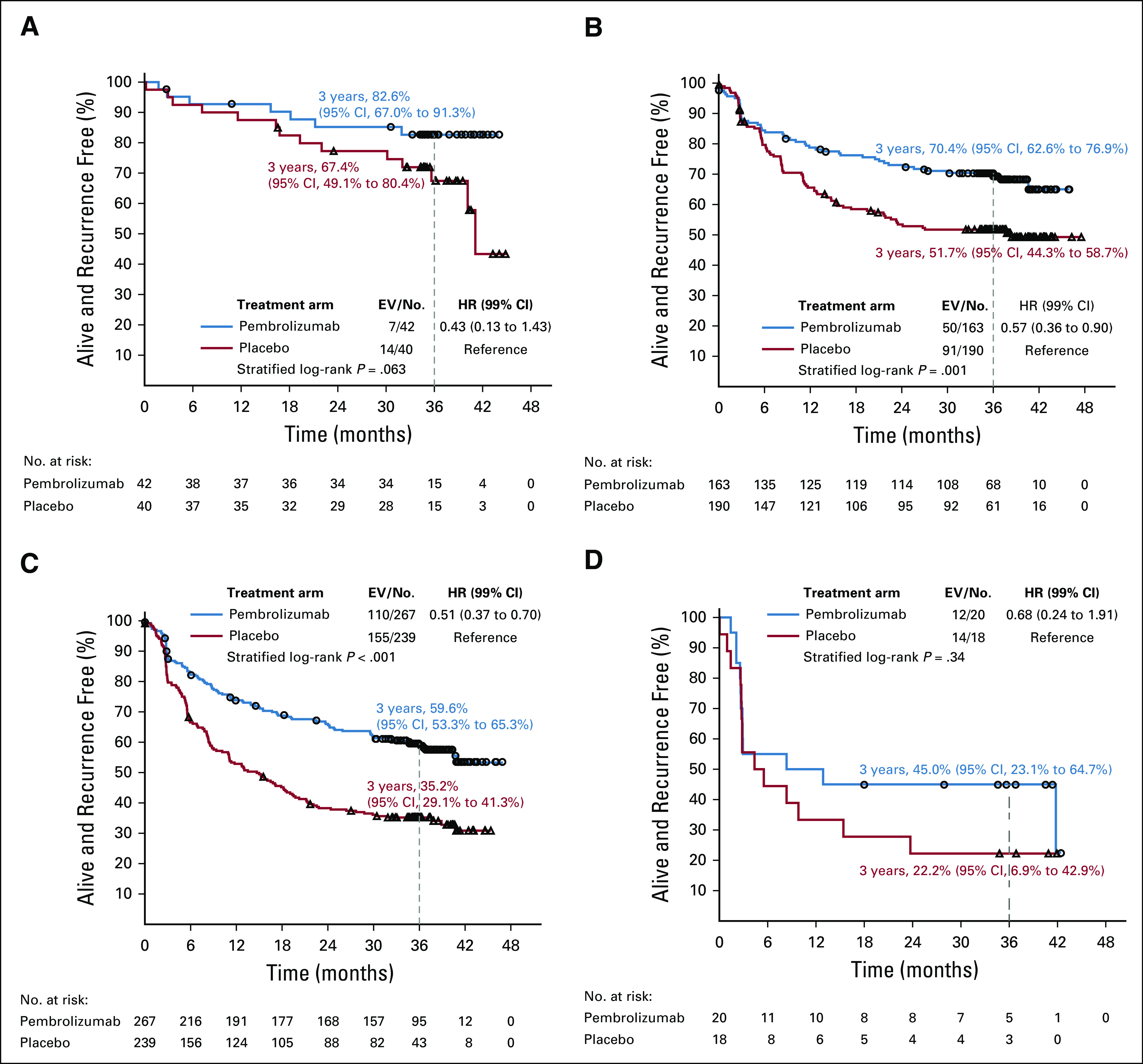
Recurrence-free survival by treatment group according to American Joint Committee on Cancer Cancer Staging Manual (eighth edition; AJCC-8). (A) Stage IIIA. (B) Stage IIIB. (C) Stage IIIC. (D) Stage IIID. EV/No., events/number of patients; HR, hazard ratio.
RFS according to BRAF-V600E/K mutation status.
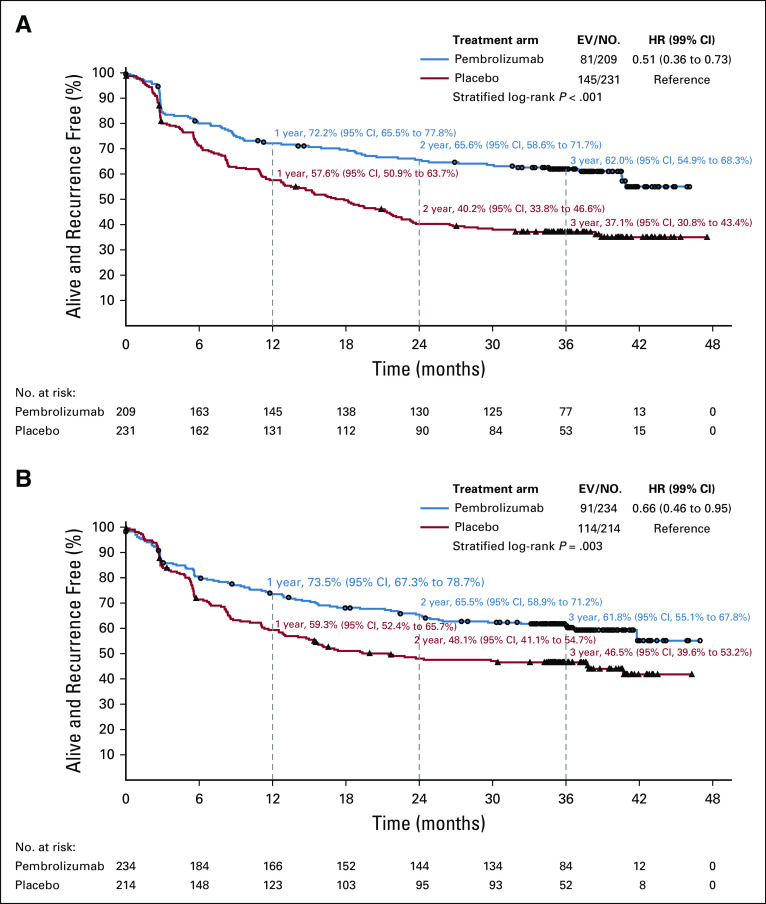
The benefit of pembrolizumab was consistent (P = .32) according to BRAF status (Fig 3). In patients with BRAF-V600E/K–mutated melanoma the HR stratified by stage was 0.51 (99% CI, 0.36 to 0.73) and the 3-year RFS rates were 62.0% (95% CI, 54.9% to 68.3%) and 37.1% (95% CI, 30.8% to 43.4%) in pembrolizumab and placebo groups, respectively (Fig 6A). The approximate 1-, 2-, and 3-year RFS rate improvements were 15% (72.2% v 57.6%), 25% (65.6% v 40.2%), and 25% (62.0% v 37.1%), respectively. In patients with BRAF wild-type melanoma the HR stratified by stage was 0.66 (99% CI, 0.46 to 0.95) and the estimated 3-year RFS rates in the pembrolizumab and placebo groups were 61.8% (95% CI, 55.1% to 67.8%) and 46.5% (95% CI, 39.6% to 53.2%), respectively (Fig 6B).
FIG 6.
Recurrence-free survival by treatment group. (A) BRAF-V600E/K mutated melanoma. (B) BRAF wild-type melanoma. EV/No., events/number of patients; HR, hazard ratio.
RFS according to other variables.
The pembrolizumab benefit was also similar in patients with microscopic and macroscopic nodal involvement (test for interaction, P = .80) and in patients with and without ulcerated melanomas (test for interaction, P = .38). Age, sex, and baseline body mass index did not significantly influence the treatment difference (Data Supplement).
Safety
At the time of the previous analysis, there were only 25 patients still receiving protocol treatment. Therefore, the incidence of the AEs already reported in the previous publication7 remained almost unchanged in the current one. For instance, the treatment-related AEs of any grade occurred in 398 (78.8%) of 509 patients (two additional patients) in the pembrolizumab group and in 333 (66.3%) of 502 patients (one additional patient) in the placebo group. Treatment-related grade 3-5 AEs were observed in 74 (14.5%) patients in the pembrolizumab group and 17 (3.4%) in the placebo group. There was one pembrolizumab-related death as a result of myositis.
Compared with the previous report, immune-related AEs (irAEs) of any grade occurred in two additional patients (ie, in 192 patients; 37.7%) in the pembrolizumab group and remained unchanged (9.0%) in the placebo group (Data Supplement). As in the previous report, an increased incidence of endocrine disorders was observed in the pembrolizumab group compared with the placebo group (23.4% v 5.0%); the most common endocrine disorders were hypothyroidism (14.5% v 2.6%) and hyperthyroidism (10.0% v 1.0%), and all were grade 1 or 2. The incidence of sarcoidosis was low (1.2% v 0%), and all occurrences were grade 1-2. The incidence of grade 3-4 irAEs remained low (7.7% v 0.6%), including colitis (2.2% v 0.2%), hypophysitis/hypopituitarism (0.6% v 0%), and type 1 diabetes mellitus (1.0% v 0%).
DISCUSSION
The analysis at 3-year median follow-up of the EORTC 1325/KEYNOTE-054 trial comparing adjuvant therapy with pembrolizumab with placebo in patients with resected high-risk stage III melanoma demonstrates a sustained RFS benefit. The updated HR estimate was 0.56, which is in line with the previous estimate of 0.57 as assessed at the 1.25-year median follow-up.7 With regard to safety and irAEs, there were only a small number of additional cases compared with the initial report in 2018.7 Therefore, we did not re-analyze the positive association between irAEs and outcome in pembrolizumab-treated patients.17
The absolute difference in RFS rates between the pembrolizumab group and the placebo group increased from approximately 15% at 1 year to approximately 20% at 2 and 3 years. The benefit is consistent across all subgroups, in particular according to PD-L1 status, AJCC-7 and -8 staging, and BRAF-V600E/K status as illustrated in the forest plot (Fig 3). Predictive importance of ulceration status was weak; the HR observed in patients with ulcerated melanoma (0.54) was similar to the one in those with nonulcerated melanoma (0.64). This contrasts with adjuvant therapy with interferons (IFNs), where IFN sensitivity is probably limited to ulcerated melanoma as observed in retrospective studies.18-20 This was recently substantiated by the results of the randomized adjuvant PEG-IFN EORTC 18081 trial in ulcerated stage II melanoma.21 With regard to subgroup staging, it is interesting to observe that the estimated HR observed in the AJCC-7 stage IIIA subgroup (0.50) was lower than in the stage IIIB or IIIC subgroups and that among the four AJCC-8 subgroups, the lowest HR was reported in the best prognostic AJCC-8 IIIA subgroup (0.43). This observation of a clear benefit is important because the indication of adjuvant therapy with anti–programmed death 1 in these best prognostic AJCC subgroups is debated because of the risk of chronic irAEs.22
Of note, in our EORTC 1325/KEYNOTE-054 trial, the estimated improvement of RFS as a result of pembrolizumab was larger in patients with BRAF-V600E/K mutant melanoma (HR, 0.51) than in BRAF wild-type melanoma (HR, 0.66), with an increased difference in 3-year RFS rate of approximately 25% (62.0% v 37.1%) versus 15% (61.8% v 46.5%), respectively, versus placebo. It also indicates a lack of prognostic importance of BRAF-V600 mutation status in the pembrolizumab group. In the advanced melanoma setting, Larkin et al23 also showed that the nivolumab group had a 5-year overall survival (OS) rate of 46% and 43% in patients with BRAF-V600 mutant and wild-type melanoma, respectively, whereas in the nivolumab plus ipilimumab group, it was 60% and 48%, respectively. Of note, in patients with BRAF-V600 melanoma recruited in the COMBI-v trial (ClinicalTrials.gov identifier: NCT01597908), dabrafenib and trametinib combination yielded a lower 5-year OS rate (34%).24 In addition, in patients with BRAF-V600 mutation in the pembrolizumab group of our EORTC 1325/KEYNOTE-054 trial, the 3-year RFS rate of 62% was practically identical to the 59% observed in the adjuvant dabrafenib plus trametinib combination arm of the COMBI-AD trial,8 whereas the 3-year RFS rates were practically identical in the respective placebo groups as well (37% v 40%). However, in each trial, the absolute RFS rate improvement changed over time: In the COMBI-AD trial,8 the estimated RFS benefit of the BRAF and MEK inhibitor combination versus placebo was larger than in our trial at 1 year (32% v 15%) but was approximately the same at 2 years (23% v 25%) and inferior at 3 years (19% v 25%). This retrospective, indirect comparison would suggest a crossing of the RFS curve of dabrafenib plus trametinib combination with the one of pembrolizumab at approximately 30 months from the start of treatment, which would be similar to the crossing of the progression-free survival and OS curves at approximately 16 months in advanced melanoma in pooled analyses.2,25 Long-term RFS results are required to ascertain these preliminary findings.
Whether the association of RFS benefit and OS benefit in adjuvant trials in melanoma as established with IFNs and ipilimumab26 will be upheld in the adjuvant trials with the more active drugs nivolumab, pembrolizumab, and the combination of dabrafenib and trametinib seems likely but has not been formally demonstrated at this point in time because of a lack of mature OS follow-up and a sufficient number of events. The EORTC 1325/KEYNOTE-054 trial is the only trial in which patients from the placebo arm could cross over at the time of recurrence and receive experimental treatment as part of the study protocol and, thus, will play an important role in addressing this question. Currently, CheckMate-915 is assessing the value of adjuvant combination therapy with nivolumab and ipilimumab versus nivolumab in resected stage IIIB/CIV melanoma. A press release communicated that at interim analysis, the primary end point of RFS in the PD-L1–negative patient population was not met.27 In resected stage IV melanoma, the combination nivolumab plus ipilimumab therapy seemed superior to nivolumab monotherapy in a randomized phase II trial, and both therapies were better than placebo.28 Moreover, the current developments with neoadjuvant immunotherapy create new opportunities in a constantly changing landscape of (neo)adjuvant therapy in melanoma.29-34
In conclusion, pembrolizumab adjuvant therapy in resected high-risk stage III melanoma provided at the 3-year median follow-up a sustained and clinically meaningful improvement in RFS. This finding was consistent across subgroups.
ACKNOWLEDGMENT
We are grateful to Merck & Co for supporting this independent European Organisation for Research and Treatment of Cancer (EORTC) study. We thank the patients and their families for participating in this study. We thank the investigators who participated in this study and who have not been included among the co-author list of this article (Data Supplement). We warmly thank all EORTC headquarters team members who have not been included among the co-author list of this article and who contributed to the study success (S. Janssen, R. Louis, N. Ealut, L. Wijnen, G. de Schaetzen, N. Jha, and S. Rivrain) as well as contributors from Merck & Co (V. Rivas, S. Cornfield, J. DeWald, R. Kloss Silverman, S, Patel, A, Rahman, and S. Diede).
PRIOR PRESENTATION
Presented at the American Society of Clinical Oncology Annual Meeting, May 29-31, 2020.
SUPPORT
Supported by Merck & Co, Kenilworth, NJ.
CLINICAL TRIAL INFORMATION
NCT02362594 (MK-3475-054/1325-MG/KEYNOTE-054)
EQUAL CONTRIBUTION
S.S. and C.R. contributed equally to this article.
AUTHOR CONTRIBUTIONS
Conception and design: Alexander M. M. Eggermont, Georgina V. Long, Dirk Schadendorf, Alexander C. J. van Akkooi, Clemens Krepler, Sandrine Marreaud, Stefan Suciu, Caroline Robert
Provision of study material or patients: Alexander M. M. Eggermont, Christian U. Blank, Mario Mandala, Georgina V. Long, Victoria G. Atkinson, Shahneen Sandhu, James Larkin, Dirk Schadendorf, Anna Maria Di Giacomo, Ralf Gutzmer, Rahima Jamal, Paul C. Lorigan, Caroline Robert
Collection and assembly of data: Alexander M. M. Eggermont, Christian U. Blank, Mario Mandala, Georgina V. Long, Victoria G. Atkinson, Stéphane Dalle, Andrew M. Haydon, Andrey Meshcheryakov, Adnan Khattak, Matteo S. Carlino, Shahneen Sandhu, Susana Puig, Piotr Rutkowski, Dirk Schadendorf, Rutger Koornstra, Leonel Hernandez-Aya, Anna Maria Di Giacomo, Jean-Jacques Grob, Ralf Gutzmer, Alexander C. J. van Akkooi, Clemens Krepler, Nageatte Ibrahim, Sandrine Marreaud, Stefan Suciu, Caroline Robert
Data analysis and interpretation: Alexander M. M. Eggermont, Christian U. Blank, Georgina V. Long, Victoria G. Atkinson, Stéphane Dalle, Andrew M. Haydon, Andrey Meshcheryakov, Adnan Khattak, Matteo S. Carlino, James Larkin, Paolo A. Ascierto, Piotr Rutkowski, Dirk Schadendorf, Leonel Hernandez-Aya, Anna Maria Di Giacomo, Alfonsus J. M. van den Eertwegh, Jean-Jacques Grob, Rahima Jamal, Paul C. Lorigan, Alexander C. J. van Akkooi, Clemens Krepler, Nageatte Ibrahim, Sandrine Marreaud, Michal Kicinski, Stefan Suciu, Caroline Robert
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Longer Follow-Up Confirms Recurrence-Free Survival Benefit of Adjuvant Pembrolizumab in High-Risk Stage III Melanoma: Updated Results From the EORTC 1325-MG/KEYNOTE-054 Trial
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Alexander M. M. Eggermont
Stock and Other Ownership Interests: RiverD, Skyline Diagnostics, Theranovir
Honoraria: Ellipses Pharma, GlaxoSmithKline, ISA Pharmaceuticals, MSD, Novartis, Pfizer, Sellas Life Sciences, Skyline Diagnostics, BIOCAD, CatalYm, BioInvent, IO Biotech, Nektar
Consulting or Advisory Role: Ellipses Pharma, GlaxoSmithKline, ISA Pharmaceuticals, MSD, Novartis, Pfizer, Sellas Life Sciences, Skyline Diagnostics, BioInvent, IO Biotech, CatalYm, Nektar
Speakers’ Bureau: MSD, BIOCAD
Expert Testimony: Novartis
Travel, Accommodations, Expenses: Bristol Myers Squibb
Christian U. Blank
Stock and Other Ownership Interests: Uniti Cars, Forty Seven, Neon Therapeutics
Consulting or Advisory Role: Roche (Inst), Genentech (Inst), MSD Oncology (Inst), Bristol Myers Squibb (Inst), Novartis (Inst), GlaxoSmithKline (Inst), Pfizer (Inst), AstraZeneca (Inst), Eli Lilly (Inst), Pierre Fabre (Inst), GenMab (Inst), Third Rock Ventures
Research Funding: Bristol Myers Squibb (Inst), Novartis (Inst), NanoString Technologies (Inst)
Travel, Accommodations, Expenses: Bristol Myers Squibb
Mario Mandala
Honoraria: MSD Oncology, Novartis, Pierre Fabre
Consulting or Advisory Role: Bristol Myers Squibb, MSD Oncology, Novartis, Pierre Fabre
Research Funding: Novartis (Inst)
Georgina V. Long
Honoraria: Bristol Myers Squibb, Merck, Pierre Fabre
Consulting or Advisory Role: Bristol Myers Squibb, Merck, Novartis, Pierre Fabre, Aduro Biotech, OncoSec, Roche, Amgen, Hexal AG (Sandoz), Mass-Array, Highlight Therapeutics, MSD, QBiotics, Skyline DX
Victoria G. Atkinson
Honoraria: Bristol Myers Squibb, Novartis, Merck Sharp & Dohme, Pierre Fabre, Roche, Genentech, Merck Serono, Nektar
Consulting or Advisory Role: Bristol Myers Squibb, Merck Sharp & Dohme, Novartis, Merck Serono, Pierre Fabre, Roche
Speakers’ Bureau: Roche, Genentech, Bristol Myers Squibb, Novartis, Merck Sharp & Dohme, Merck Serono
Travel, Accommodations, Expenses: Bristol Myers Squibb, OncoSec, Merck Sharp & Dohme, Pierre Fabre
Stéphane Dalle
Employment: Sanofi Pasteur (I)
Research Funding: Bristol Myers Squibb (Inst), Merck Sharp & Dohme (Inst),
Travel, Accommodations, Expenses: Bristol Myers Squibb, Pierre Fabre, Merck Sharp & Dohme
Andrew M. Haydon
Honoraria: Novartis, Merck
Consulting or Advisory Role: Novartis, Pierre Fabre, Merck Sharp & Dohme
Speakers’ Bureau: Novartis, Merck
Andrey Meshcheryakov
Honoraria: Amgen, Bayer AG, BIOCAD, Bristol Myers Squibb, Eli Lilly, Merck, SERVIER, Takeda Pharmaceuticals, Eisai, AstraZeneca, Sanofi, Aventis
Consulting or Advisory Role: Amgen, Bayer AG, BIOCAD, Bristol Myers Squibb, Eli Lilly, Merck, SERVIER, Takeda Pharmaceuticals, Eisai, AstraZeneca, Sanofi, Aventis
Research Funding: Sanofi, AstraZeneca
Travel, Accommodations, Expenses: BIOCAD, SERVIER, Merck Sharp & Dohme, Sanofi, Merck
Matteo S. Carlino
Honoraria: Bristol Myers Squibb, MSD, Novartis
Consulting or Advisory Role: Bristol Myers Squibb, MSD, Amgen, Novartis, Pierre Fabre, Roche, IDEAYA Biosciences, Sanofi, Merck Serono, Regeneron Pharmaceuticals, QBiotics, Nektar, Eisai
Shahneen Sandhu
Honoraria: Bristol Myers Squibb (Inst), Merck (Inst), Merck Serono (Inst), AstraZeneca (Inst)
Consulting or Advisory Role: Amgen
Speakers’ Bureau: Bristol Myers Squibb, Merck, Roche, Genentech
Research Funding: Amgen (Inst), Bristol Myers Squibb (Inst), AstraZeneca (Inst), Merck (Inst), Endocyte (Inst), AAA (Inst), Genentech (Inst), Roche (Inst)
Travel, Accommodations, Expenses: Roche, Genentech
James Larkin
Honoraria: Eisai, Bristol Myers Squibb, MSD, GlaxoSmithKline, Pfizer, Novartis, Roche, Genentech, Pierre Fabre, EUSA Pharma, Achilles Therapeutics, AstraZeneca, Boston Biomedical, Ipsen, Imugene, Incyte, iOnctura, Merck Serono, Nektar, Vitaccess, Kymab, Secarna
Consulting or Advisory Role: Eisai, Bristol Myers Squibb, MSD, GlaxoSmithKline, Pfizer, Novartis, Roche, Genentech, Pierre Fabre, EUSA Pharma, Achilles Therapeutics, AstraZeneca, Boston Biomedical, Ipsen, Imugene, Incyte, iOnctura, Merck Serono, Nektar, Vitaccess, Secarna, Kymab
Research Funding: Pfizer (Inst), Novartis (Inst), MSD (Inst), Bristol Myers Squibb (Inst), Achilles Therapeutics (Inst), Roche (Inst), Nektar (Inst), Covance (Inst), Immunocore (Inst), AVEO Oncology (Inst)
Travel, Accommodations, Expenses: Bristol Myers Squibb, Pfizer, Novartis, Roche, Genentech, AstraZeneca, Boston Biomedical, Incyte, GlaxoSmithKline, Pierre Fabre, Merck Serono
Susana Puig
Honoraria: Amgen (I), Avene, Avene (I), Almirall, Almirall (I), Bristol Myers Squibb, ISDIN, La Roche Posay, Roche, Genentech, Sanofi, Regeneron Pharmaceuticals, Sun Pharma Industries (I), Sun Pharma Industries, Pfizer, Canfield Scientific (I)
Consulting or Advisory Role: ISDIN, Almirall, Almirall (I), Sun Pharma Industries, Sun Pharma Industries (I), Roche, Genentech, Sanofi, Regeneron Pharmaceuticals
Speakers’ Bureau: Sanofi, Regeneron Pharmaceuticals, Roche, Genentech, Almirall, Pfizer, ISDIN, La Roche Posay, Fotofinder (I)
Research Funding: La Roche Posay (Inst), Sun Pharma Industries (Inst), Roche (Inst)
Travel, Accommodations, Expenses: Almirall, Almirall (I), ISDIN, ISDIN (I), BIODERMA, BIODERMA (I)
Paolo A. Ascierto
Stock and Other Ownership Interests: PrimeVax
Consulting or Advisory Role: Bristol Myers Squibb, Roche, Genentech, Merck Sharp & Dohme, Novartis, Array BioPharma, Merck Serono, Pierre Fabre, Incyte, MedImmune, AstraZeneca, Sun Pharma Industries, Sanofi, Idera, Ultimovacs, Sandoz, Immunocore, 4SC, Alkermes, Italfarmaco, Nektar, Boehringer Ingelheim, Eisai, Regeneron Pharmaceuticals
Research Funding: Bristol Myers Squibb (Inst), Roche (Inst), Genentech (Inst), Array BioPharma (Inst)
Travel, Accommodations, Expenses: Merck Sharp & Dohme
Piotr Rutkowski
Honoraria: Bristol Myers Squibb, MSD, Novartis, Roche, Eli Lilly, Pfizer, Pierre Fabre
Consulting or Advisory Role: Novartis, Blueprint Medicines, Bristol Myers Squibb, Pierre Fabre, MSD, Amgen
Speakers’ Bureau: Pfizer, Novartis, Eli Lilly
Research Funding: Novartis (Inst), Roche (Inst), Bristol Myers Squibb (Inst)
Travel, Accommodations, Expenses: Orphan Europe, Pierre Fabre
Dirk Schadendorf
Honoraria: Roche, Genentech, Novartis, Bristol Myers Squibb, Merck Sharp & Dohme, Immunocore, Merck Serono, Array BioPharma, Incyte, Pfizer, Pierre Fabre, Philogen, Regeneron Pharmaceuticals, 4SC, Mologen, Sanofi, NeraCare, Sun Pharma Industries, Inflarx, Ultimovacs, Sandoz
Consulting or Advisory Role: Roche, Genentech, Novartis, Bristol Myers Squibb, Merck Sharp & Dohme, Merck Serono, Incyte, 4SC, Pierre Fabre, Mologen, Sanofi, Regeneron Pharmaceuticals
Speakers’ Bureau: Roche, Bristol Myers Squibb, Merck Sharp & Dohme, Novartis, Incyte, Pierre Fabre, Sanofi, Regeneron Pharmaceuticals, Merck KGaA
Research Funding: Bristol Myers Squibb (Inst), Novartis (Inst)
Travel, Accommodations, Expenses: Roche, Genentech, Bristol Myers Squibb, Merck Serono, Novartis, Merck Sharp & Dohme, Pierre Fabre, Sanofi, Regeneron Pharmaceuticals
Rutger Koornstra
Consulting or Advisory Role: Bristol Myers Squibb, MSD Oncology, Sanofi, AstraZeneca, Pfizer, Pierre Fabre
Research Funding: Roche (Inst)
Leonel Hernandez-Aya
Consulting or Advisory Role: Massive Bio, Bristol Myers Squibb
Speakers’ Bureau: Sanofi, Regeneron Pharmaceuticals
Research Funding: Bristol Myers Squibb (Inst), Regeneron Pharmaceuticals (Inst), Immunocore (Inst), Merck (Inst), Polynoma (Inst), Corvus Pharmaceuticals (Inst), Roche (Inst), Genentech (Inst), Merck Serono (Inst), Amgen (Inst), MedImmune (Inst), Takeda Pharmaceuticals (Inst), Moderna Therapeutics (Inst)
Travel, Accommodations, Expenses: Sanofi, Regeneron Pharmaceuticals, Bristol Myers Squibb
Anna Maria Di Giacomo
Consulting or Advisory Role: Bristol Myers Squibb, Incyte, Pierre Fabre, MSD Oncology, Sanofi, GlaxoSmithKline
Travel, Accommodations, Expenses: Pierre Fabre, Bristol Myers Squibb
Alfonsus J. M. van den Eertwegh
Honoraria: Bristol Myers Squibb
Consulting or Advisory Role: Bristol Myers Squibb, MSD Oncology, Amgen, Roche, Novartis, Sanofi, Pfizer, Ipsen, Merck, Pierre Fabre
Research Funding: Roche, Sanofi, Bristol Myers Squibb
Travel, Accommodations, Expenses: MSD Oncology, Roche, Pfizer, Sanofi
Jean-Jacques Grob
Consulting or Advisory Role: Bristol Myers Squibb, MSD Oncology, Roche, Genentech, Novartis, Amgen, Pierre Fabre, Sun Pharma Industries, Merck KGaA
Speakers’ Bureau: Novartis
Travel, Accommodations, Expenses: Bristol Myers Squibb, MSD Oncology, Novartis, Pierre Fabre
Ralf Gutzmer
Honoraria: Bristol Myers Squibb, Merck Sharp & Dohme, Roche, Genentech, Novartis, Merck Serono, Almirall, Amgen, Sun Pharma Industries, Pierre Fabre, Sanofi, Regeneron Pharmaceuticals, Bayer AG, Immunocore
Consulting or Advisory Role: Bristol Myers Squibb, Merck Sharp & Dohme, Roche, Genentech, Novartis, Almirall, 4SC, Amgen, Pierre Fabre, Merck Serono, Sun Pharma Industries, Sanofi, Immunocore
Research Funding: Pfizer (Inst), Novartis (Inst), Johnson & Johnson (Inst), Amgen (Inst), Merck Serono (Inst), Sun Pharma Industries (Inst), Sanofi (Inst)
Travel, Accommodations, Expenses: Bristol Myers Squibb, Roche, Merck Serono, Pierre Fabre, Sun Pharma Industries
Rahima Jamal
Research Funding: Merck Sharp & Dohme (Inst), Bristol Myers Squibb (Inst)
Paul C. Lorigan
Honoraria: Novartis, Pierre Fabre, Merck, Bristol Myers Squibb, MSD, NeraCare, Amgen, Roche, Oncology Education Canada
Consulting or Advisory Role: Merck Sharp & Dohme, Bristol Myers Squibb, Novartis, Amgen, GlaxoSmithKline, NeraCare, Pierre Fabre,
Speakers’ Bureau: Merck Sharp & Dohme, Novartis, Bristol Myers Squibb, Roche
Research Funding: Bristol Myers Squibb
Travel, Accommodations, Expenses: Merck Sharp & Dohme, Bristol Myers Squibb
Alexander C. J. van Akkooi
Consulting or Advisory Role: Amgen (Inst), Novartis (Inst), MSD Oncology (Inst), Merck (Inst), Bristol Myers Squibb (Inst), 4SC (Inst), Sanofi (Inst)
Research Funding: Amgen (Inst), Novartis (Inst), Bristol Myers Squibb (Inst)
Travel, Accommodations, Expenses: Novartis
Clemens Krepler
Employment: Merck & Co
Stock and Other Ownership Interests: Merck & Co
Nageatte Ibrahim
Employment: Merck
Stock and Other Ownership Interests: Merck, GlaxoSmithKline
Michal Kicinski
Research Funding: Merck (Inst), Pierre Fabre (Inst), Bristol Myers Squibb (Inst), Janssen Pharmaceuticals (Inst)
Stefan Suciu
Research Funding: Merck (Inst), Bristol Myers Squibb (Inst)
Caroline Robert
Consulting or Advisory Role: Bristol Myers Squibb, Roche, Amgen, Novartis, Pierre Fabre, MSD, Sanofi, Biothera, CureVac, Merck
No other potential conflicts of interest were reported.
REFERENCES
- 1.Eggermont AMM, Spatz A, Robert C: Cutaneous melanoma. Lancet 383:816-827, 2014 [DOI] [PubMed] [Google Scholar]
- 2.Ugurel S Röhmel J Ascierto PA, et al. : Survival of patients with advanced metastatic melanoma: The impact of MAP kinase pathway inhibition and immune checkpoint inhibition - Update 2019. Eur J Cancer 130:126-138, 2020 [DOI] [PubMed] [Google Scholar]
- 3.Eggermont AM Chiarion-Sileni V Grob JJ, et al. : Adjuvant ipilimumab versus placebo after complete resection of high-risk stage III melanoma (EORTC 18071): A randomised, double-blind, phase 3 trial. Lancet Oncol 16:522-530, 2015 [DOI] [PubMed] [Google Scholar]
- 4.Eggermont AM Chiarion-Sileni V Grob JJ, et al. : Prolonged survival in stage III melanoma with ipilimumab as adjuvant therapy. N Engl J Med 375:1845-1855, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eggermont AMM Chiarion-Sileni V Grob JJ, et al. : Adjuvant ipilimumab versus placebo after complete resection of stage III melanoma: Long-term follow-up results of the European Organisation for Research and Treatment of Cancer 18071 double-blind phase 3 randomised trial. Eur J Cancer 119:1-10, 2019 [DOI] [PubMed] [Google Scholar]
- 6.Weber J Mandala M Del Vecchio M, et al. : Adjuvant nivolumab versus ipilimumab in resected stage III or IV melanoma. N Engl J Med 377:1824-1835, 2017 [DOI] [PubMed] [Google Scholar]
- 7.Eggermont AMM Blank CU Mandala M, et al. : Adjuvant pembrolizumab versus placebo in resected stage III melanoma. N Engl J Med 378:1789-1801, 2018 [DOI] [PubMed] [Google Scholar]
- 8.Long GV Hauschild A Santinami M, et al. : Adjuvant dabrafenib plus trametinib in stage III BRAF-mutated melanoma. N Engl J Med 377:1813-1823, 2017 [DOI] [PubMed] [Google Scholar]
- 9.Hauschild A Dummer R Schadendorf D, et al. : Longer follow-up confirms relapse-free survival benefit with adjuvant dabrafenib plus trametinib in patients with resected BRAF V600-mutant stage III melanoma. J Clin Oncol 36:3441-3449, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Akkooi ACJ Nowecki ZI Voit C, et al. : Sentinel node tumor burden according to the Rotterdam criteria is the most important prognostic factor for survival in melanoma patients: A multicenter study in 388 patients with positive sentinel nodes. Ann Surg 248:949-955, 2008 [DOI] [PubMed] [Google Scholar]
- 11.van der Ploeg APT van Akkooi ACJ Rutkowski P, et al. : Prognosis in patients with sentinel node-positive melanoma is accurately defined by the combined Rotterdam tumor load and Dewar topography criteria. J Clin Oncol 29:2206-2214, 2011 [DOI] [PubMed] [Google Scholar]
- 12.van der Ploeg AP van Akkooi AC Haydu LE, et al. : The prognostic significance of sentinel node tumour burden in melanoma patients: An international, multicenter study of 1539 sentinel node-positive melanoma patients. Eur J Cancer 50:111-120, 2014 [DOI] [PubMed] [Google Scholar]
- 13.Balch CM Gershenwald JE Soong SJ, et al. : Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol 27:6199-6206, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gershenwald JE, Scolyer RA, Hess KR, et al: Melanoma staging: Evidence-based changes in the American Joint Committee on Cancer Eighth Edition Cancer Staging Manual. CA Cancer J Clin 67:472-492, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eggermont AMM Blank CU Mandala M, et al. : Prognostic and predictive value of AJCC-8 staging in the phase III EORTC1325/KEYNOTE-054 trial of pembrolizumab vs placebo in resected high-risk stage III melanoma. Eur J Cancer 116:148-157, 2019 [DOI] [PubMed] [Google Scholar]
- 16.Daud AI Wolchok JD Robert C, et al. : Programmed death-ligand 1 expression and response to the anti-programmed death 1 antibody pembrolizumab in melanoma. J Clin Oncol 34:4102-4109, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eggermont AMM Kicinski M Blank CU, et al. : Association between immune-related adverse events and recurrence-free survival among patients with stage III melanoma randomized to receive pembrolizumab or placebo: A secondary analysis of a randomized clinical trial. JAMA Oncol 6:519-527, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eggermont AMM Suciu S Testori A, et al. : Ulceration and stage are predictive of interferon efficacy in melanoma: Results of the phase III adjuvant trials EORTC 18952 and EORTC 18991. Eur J Cancer 48:218-225, 2012 [DOI] [PubMed] [Google Scholar]
- 19.Eggermont AM Suciu S Rutkowski P, et al. : Long term follow up of the EORTC 18952 trial of adjuvant therapy in resected stage IIB-III cutaneous melanoma patients comparing intermediate doses of interferon-alpha-2b (IFN) with observation: Ulceration of primary is key determinant for IFN-sensitivity. Eur J Cancer 55:111-121, 2016 [DOI] [PubMed] [Google Scholar]
- 20.Ives NJ Suciu S Eggermont AMM, et al. : Adjuvant interferon-α for the treatment of high-risk melanoma: An individual patient data meta-analysis. Eur J Cancer 82:171-183, 2017 [DOI] [PubMed] [Google Scholar]
- 21.Eggermont AMM Rutkowski P Dutriaux C, et al. : Adjuvant therapy with pegylated interferon-alfa2b vs observation in stage II B/C patients with ulcerated primary: Results of the European Organisation for Research and Treatment of Cancer 18081 randomised trial. Eur J Cancer 133:94-103, 2020 [DOI] [PubMed] [Google Scholar]
- 22.Higham CE Chatzimavridou-Grigoriadou V Fitzgerald CT, et al. : Adjuvant immunotherapy: The sting in the tail. Eur J Cancer 132:207-210, 2020 [DOI] [PubMed] [Google Scholar]
- 23.Larkin J Chiarion-Sileni V Gonzalez R, et al. : Five-year survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med 381:1535-1546, 2019 [DOI] [PubMed] [Google Scholar]
- 24.Robert C Grob JJ Stroyakovskiy D, et al. : Five-year outcomes with dabrafenib plus trametinib in metastatic melanoma. N Engl J Med 381:626-636, 2019 [DOI] [PubMed] [Google Scholar]
- 25.Eggermont AMM, Robert C, Ribas A: The new era of adjuvant therapies for melanoma. Nat Rev Clin Oncol 15:535-536, 2018 [DOI] [PubMed] [Google Scholar]
- 26.Suciu S Eggermont AMM Lorigan P, et al. : Relapse-free survival as a surrogate for overall survival in the evaluation of stage II-III melanoma adjuvant therapy. J Natl Cancer Inst 110:87-96, 2018 [DOI] [PubMed] [Google Scholar]
- 27.https://news.bms.com/press-release/corporatefinancial-news/bristol-myers-squibb-announces-update-checkmate-915-opdivo-niv Bristol Myers Squibb: Bristol Myers Squibb announces update on CheckMate-915 for Opdivo (nivolumab) plus Yervoy (ipilimumab) versus Opdivo alone in patients with resected high-risk melanoma and PD-L1 <1%, 2019.
- 28.Zimmer L Livingstone E Hassel JC, et al. : Adjuvant nivolumab plus ipilimumab or nivolumab monotherapy versus placebo in patients with resected stage IV melanoma with no evidence of disease (IMMUNED): A randomised, double-blind, placebo-controlled, phase 2 trial. Lancet 395:1558-1568, 2020 [DOI] [PubMed] [Google Scholar]
- 29.Ascierto PA, Eggermont AMM: Neoadjuvant therapy in melanoma: The next step? Lancet Oncol 19:151-153, 2018 [DOI] [PubMed] [Google Scholar]
- 30.Blank CU Rozeman EA Fanchi LF, et al. : Neoadjuvant versus adjuvant ipilimumab plus nivolumab in macroscopic stage III melanoma. Nat Med 24:1655-1661, 2018 [DOI] [PubMed] [Google Scholar]
- 31. doi: 10.1038/s41591-018-0197-1. Amaria RN, Reddy SM, Tawbi HA, et al: Neoadjuvant immune checkpoint blockade in high-risk resectable melanoma. Nat Med 24:1649-1654, 2018 [Erratum: Nat Med 24:1941-1942, 2018] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Amaria RN Menzies AM Burton EM, et al. : Neoadjuvant systemic therapy in melanoma: Recommendations of the International Neoadjuvant Melanoma Consortium. Lancet Oncol 20:e378-e389, 2019 [DOI] [PubMed] [Google Scholar]
- 33. Blank CU, Reijers ILM, Pennington T, et al: First safety and efficacy results of PRADO: A phase II study of personalized response-driven surgery and adjuvant therapy after neoadjuvant ipilimumab (IPI) and nivolumab (NIVO) in resectable stage III melanoma. J Clin Oncol 38, 2020 (suppl; abstr 10002) [Google Scholar]
- 34.Rozeman EA Menzies AM van Akkooi ACJ, et al. : Identification of the optimal combination dosing schedule of neoadjuvant ipilimumab plus nivolumab in macroscopic stage III melanoma (OpACIN-neo): A multicentre, phase 2, randomised, controlled trial. Lancet Oncol 20:948-960, 2019 [DOI] [PubMed] [Google Scholar]