PURPOSE
Limited data exist on the optimal duration of immunotherapy, including for non–small-cell lung cancer (NSCLC). We present an exploratory analysis of CheckMate 153, a largely community-based phase IIIb/IV study, to evaluate the impact of 1-year fixed-duration versus continuous therapy on the efficacy and safety of nivolumab.
METHODS
Patients with previously treated advanced NSCLC received nivolumab monotherapy (3 mg/kg every 2 weeks). Those still receiving treatment at 1 year, including patients perceived to be deriving benefit despite radiographic progression, were randomly assigned to continue nivolumab until disease progression or unacceptable toxicity or to stop nivolumab with the option of on-study retreatment after disease progression (1-year fixed duration).
RESULTS
Of 1,428 patients treated, 252 were randomly assigned to continuous (n = 127) or 1-year fixed-duration (n = 125) treatment (intent-to-treat [ITT] population). Of these, 89 and 85 patients in the continuous and 1-year fixed-duration arms, respectively, had not progressed (progression-free survival [PFS] population). With minimum post–random assignment follow-up of 13.5 months, median PFS was longer with continuous versus 1-year fixed-duration treatment (PFS population: 24.7 months v 9.4 months; hazard ratio [HR], 0.56 [95% CI, 0.37 to 0.84]). Median overall survival from random assignment was longer with continuous versus 1-year fixed-duration treatment in the PFS (not reached v 32.5 months; HR, 0.61 [95% CI, 0.37 to 0.99]) and ITT (not reached v 28.8 months; HR, 0.62 [95% CI, 0.42 to 0.92]) populations. Few new-onset treatment-related adverse events occurred. No new safety signals were identified.
CONCLUSION
To our knowledge, these findings from an exploratory analysis represent the first randomized data on continuous versus fixed-duration immunotherapy in previously treated advanced NSCLC and suggest that continuing nivolumab beyond 1 year improves outcomes.
INTRODUCTION
Nivolumab, a fully human immunoglobulin (Ig) G4 programmed death-1 (PD-1) immune checkpoint inhibitor antibody, is an established standard of care for previously treated patients with metastatic non–small-cell lung cancer (NSCLC) who are immunotherapy naive.1,2 CheckMate 017 (ClinicalTrials.gov identifier: NCT01642004) and 057 (ClinicalTrials.gov identifier: NCT01673867) were the first phase III trials to show that in patients with squamous and nonsquamous NSCLC, respectively, nivolumab treatment until progressive disease (PD) or unacceptable toxicity provided an overall survival (OS) benefit with a favorable safety profile when compared with docetaxel.3,4 This benefit was durable; pooled data from long-term follow-up of these studies showed a 5-year OS rate of 13% with nivolumab versus 3% with docetaxel.5 These data were supported by results from the nivolumab phase I CheckMate 003 study (ClinicalTrials.gov identifier: NCT00730639; N = 129), which has the longest survival follow-up to date among studies of immune checkpoint inhibitors in NSCLC6; a 6-year OS rate of 15% represented an unprecedented long-term survival benefit in this population.
CONTEXT
Key Objective
The optimal duration of treatment with checkpoint inhibitors is unknown. Using a largely real-world population, we explored the impact of nivolumab treatment duration on outcomes in patients with previously treated advanced non–small-cell lung cancer (NSCLC) in a randomized study. Patients who continued to receive nivolumab treatment at 1 year were randomly assigned to continue nivolumab until disease progression or unacceptable toxicity or to stop nivolumab (1-year fixed duration) with the option of on-study retreatment after disease progression.
Knowledge Generated
Patients had improved outcomes with continued nivolumab treatment compared with stopping treatment after 1 year; continuing treatment was well tolerated. Responders to nivolumab treatment seemed to derive greater survival benefit from continuing nivolumab treatment than did those with stable disease.
Relevance
These results provide valuable information on optimal treatment duration with nivolumab for patients with previously treated advanced NSCLC and could help inform clinical decisions.
The optimal duration of immunotherapy across tumor types is currently unknown. Ipilimumab, a fully human IgG1 cytotoxic T lymphocyte antigen-4 immune checkpoint inhibitor antibody, has been shown to provide long-term survival benefit in metastatic melanoma with only four induction doses administered within a 12-week period.7 In contrast, the treatment duration of anti–PD-1/programmed death ligand 1 (PD-L1) agents, indicated for several tumor types, has varied across clinical trials, some permitting treatment until PD and others requiring a fixed duration.3,4,8-10 In previously treated patients with NSCLC who were treated with nivolumab for a fixed 96-week duration in CheckMate 003, 75% who survived ≥ 5 years received no additional therapy after nivolumab and remained progression free at 5 years; 25% of these patients stopped nivolumab treatment before 96 weeks due to adverse events (AEs).8
CheckMate 153 (ClinicalTrials.gov identifier: NCT02066636) is an ongoing, phase IIIb/IV study that, unlike typical phase III studies, is largely community based and thus reflects a real-world population. The study was designed to assess the safety of nivolumab monotherapy in previously treated advanced NSCLC. The primary end point of safety, in addition to efficacy and patient-reported outcomes, from this study was reported previously.11 Here, we report an exploratory analysis that was conducted to evaluate the impact of fixed-duration therapy on the efficacy and safety of nivolumab; patients who continued to receive treatment at 1 year, regardless of response status, were randomly assigned to continue nivolumab or to stop treatment with the option of nivolumab retreatment on study after PD. To our knowledge, this is the first randomized study to evaluate fixed-duration therapy in NSCLC using a treatment that targets the PD-1 pathway. An earlier assessment of these exploratory post–random assignment outcomes was presented previously.12 Here, we report updated data with a minimum post–random assignment follow-up of at least 13 months.
METHODS
Study Design and Treatment
The methodology of CheckMate 153 has been described previously.11 Briefly, this phase IIIb/IV study evaluated nivolumab monotherapy in patients with previously treated advanced or metastatic NSCLC (Data Supplement, online only). Nivolumab 3 mg/kg was administered intravenously every 2 weeks until PD, unacceptable toxicity, or withdrawal of consent, or for 1 year (1-year fixed-duration arm). Treatment beyond initial PD, as defined by RECIST version 1.1, was permitted for patients with investigator-assessed clinical benefit, no rapid PD, and stable Eastern Cooperative Oncology Group performance status who were tolerating treatment. Before the first patient completing 1 year of treatment, the protocol was amended to randomly assign patients who continued to receive treatment at 1 year, regardless of response status, to continue nivolumab or to stop treatment with the option of receiving nivolumab retreatment on study after PD. The study was conducted in accordance with international standards of Good Clinical Practice, and the protocol was approved by the independent ethics committee or institutional review board of each participating study center. Patients provided written informed consent.
End Points
The primary end point of safety was reported previously.11 After a protocol amendment in 2014, an exploratory post–random assignment end point was added; safety and tolerability, progression-free survival (PFS), OS, and objective response rate were assessed from the time of random assignment in those patients who continued to receive treatment at 1 year.
Patients
Eligibility criteria for the overall study have been described previously11 and are reported in the Data Supplement. For the post–random assignment part of the study, patients had to be continuing nivolumab treatment at 1 year.
Assessments
Safety assessments are described in the Data Supplement. Radiographic tumor assessments by the investigator per RECIST v1.1 were performed at baseline (1 year before random assignment); the first on-study tumor assessment was performed at week 9, and then every 8 weeks until 24 months from the first dose of study drug, followed by every 12 weeks until PD. Patients were not stratified on the basis of response status before random assignment. Of note, patients were not rebaselined at the time of random assignment; baseline measurements were only determined at the initial study screening, and PD after random assignment was determined considering nadir measurements from the full observation period before PD (per RECIST v1.1). The timing for assessments is summarized in the Data Supplement. Therefore, post–random assignment PD was determined using baseline radiographic tumor scans from screening, not from the time of random assignment. Assessment of tumor PD-L1 was not mandated per the protocol, but it was assessed if a tumor sample was available.
Statistical Analysis
After random assignment, the intent-to-treat (ITT) population comprised all patients who were receiving treatment at 1 year and were randomly assigned, regardless of response status. The post–random assignment PFS population comprised patients who did not have PD and had not initiated other systemic anticancer therapy before random assignment. Safety data were summarized for the ITT population and included events reported from random assignment to 100 days after the last dose of the study drug. PFS was analyzed in the PFS population only, and OS was analyzed in both the ITT and the PFS population. PFS and OS were estimated from the time of random assignment using the Kaplan-Meier methodology, with median values and 2-sided 95% CIs calculated using the Brookmeyer and Crowley method. A multivariable analysis was performed for PFS, adjusting for baseline patient characteristics. All analyses were based on a September 19, 2018, database lock, which provided a minimum follow-up of 1 year and approximately 1 year of additional follow-up to the previously presented data.12
RESULTS
Patients and Treatment
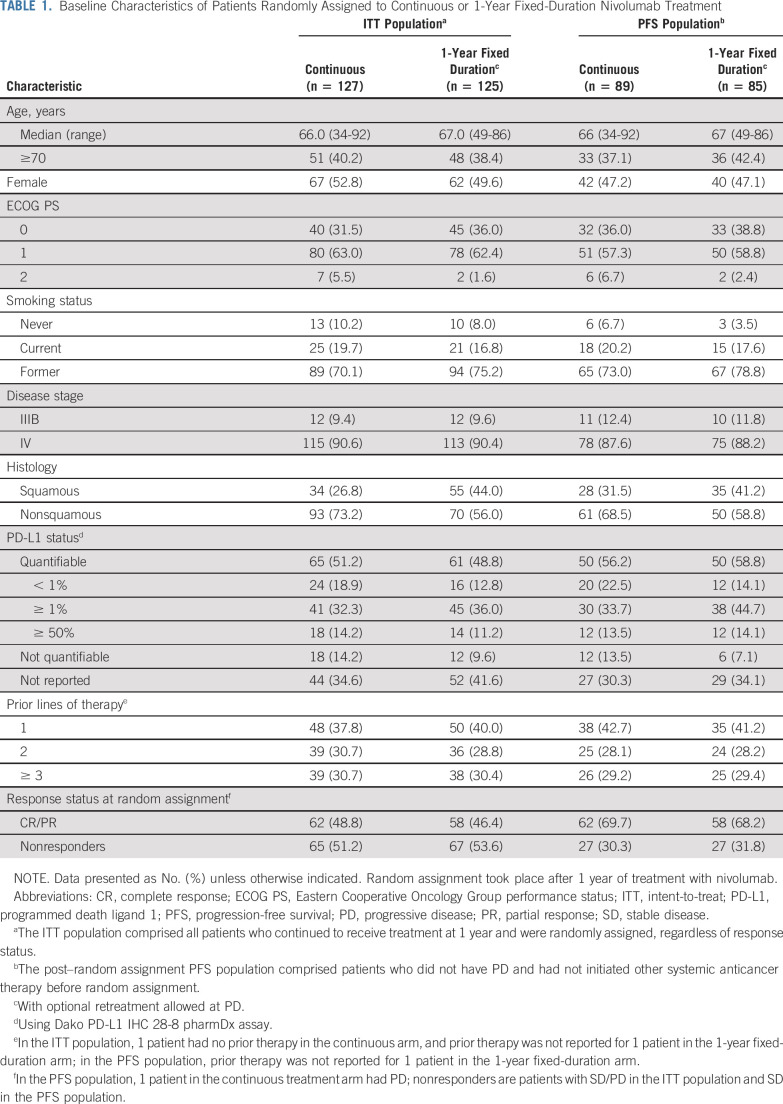
Overall, 1,428 patients initiated treatment between April 16, 2014, and September 19, 2018. At 1 year, 252 patients continued to receive treatment and were randomly assigned, regardless of response status, to receive continuous nivolumab treatment (continuous arm; n = 127) or to stop nivolumab with the potential for nivolumab retreatment at PD (1-year fixed-duration arm; n = 125; ITT population; Fig 1). This included 78 patients (38 in the continuous arm and 40 in the 1-year fixed-duration arm) who were receiving nivolumab beyond initial PD before random assignment, because they were considered by the investigator to be receiving clinical benefit. Overall, 89 and 85 patients in the continuous and 1-year fixed-duration arms, respectively, had not progressed and were included in the PFS population; 62 of these patients had an overall on-study best response of complete response (CR)/partial response (PR) and 27 had stable disease (SD) in the continuous treatment arm, and 58 patients had CR/PR and 27 had SD in the 1-year fixed-duration arm (Table 1).
FIG 1.
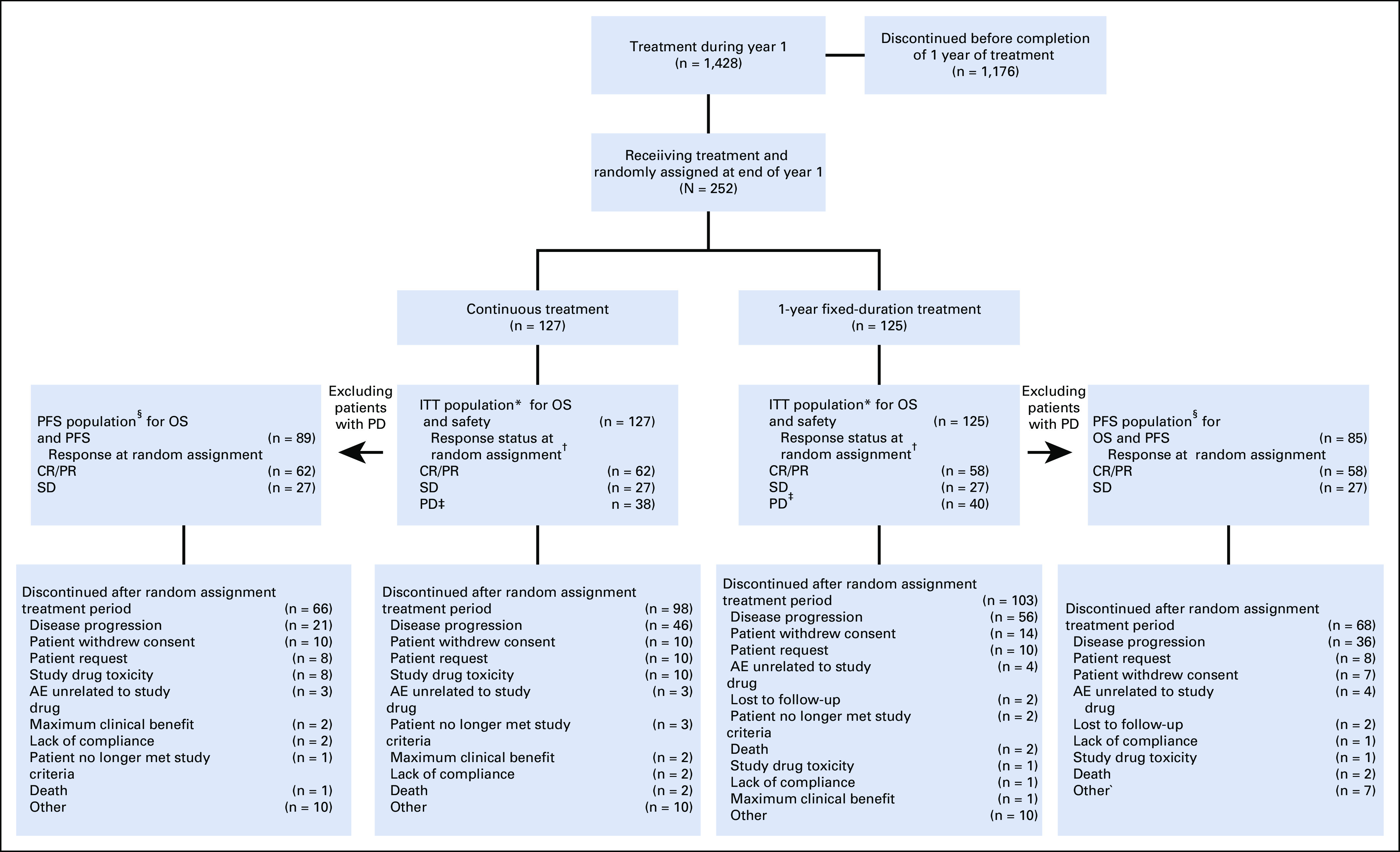
CONSORT diagram of patient disposition. AE, adverse event; CR, complete response; ITT, intent-to-treat; OS, overall survival; PD, progressive disease; PFS, progression-free survival; PR, partial response; SD, stable disease. (*) The ITT population comprised all patients who continued to receive treatment at 1 year (y) and were randomly assigned, regardless of response status. (†) Random assignment took place after 1 year of treatment with nivolumab. (‡) Patients were treated beyond progression. (§) The post–random assignment PFS population comprised patients who did not have PD and had not initiated other systemic anticancer therapy before random assignment.
TABLE 1.
Baseline Characteristics of Patients Randomly Assigned to Continuous or 1-Year Fixed-Duration Nivolumab Treatment
In both the ITT and PFS populations, baseline characteristics for the two arms were generally well balanced, with the exception of a lower proportion of patients with squamous histology in the continuous versus 1-year fixed-duration treatment arm (ITT population, 26.8% v 44.0%; PFS population, 31.5% v 41.2%; Table 1). In the ITT population, tumor PD-L1 expression was quantifiable in 65 (51.2%) of 127 patients in the continuous arm and in 61 (48.8%) of 125 patients in the 1-year fixed-duration arm. In the PFS population, tumor PD-L1 expression was quantifiable in 50 (56.2%) of 89 patients in the continuous arm and in 50 (58.8%) of 85 patients in the 1-year fixed-duration arm (Table 1).
At database lock, minimum follow-up after random assignment in the ITT population was 13.5 months. In the ITT population, 29 (22.8%) of 127 patients in the continuous arm were receiving treatment and 22 (17.6%) of 125 patients in the 1-year fixed-duration arm were alive and on study; six of these patients were receiving retreatment after PD. In the PFS population, 23 (25.8%) of 89 patients in the continuous arm were receiving treatment and 17 (20.0%) of 85 patients in the 1-year fixed-duration arm were alive and on study; four of these patients were receiving retreatment after PD. The most frequent cause of discontinuation in both populations was PD (Fig 1). In the continuous arm after random assignment, the median duration of treatment was 13.6 months (range, 9.4-22.8 months).
Efficacy After Random Assignment
In the PFS population, median PFS was longer with continuous versus 1-year fixed-duration treatment (24.7 months v 9.4 months; hazard ratio [HR], 0.56 [95% CI, 0.37 to 0.84]; Fig 2A); PFS rates were 64.6% versus 44.0% at 1 year and 51.9% versus 30.7% at 2 years, respectively. Patients with CR/PR at random assignment had longer median PFS with continuous versus 1-year fixed-duration treatment (31.0 months v 10.6 months; HR, 0.46 [95% CI, 0.27 to 0.77]; Fig 2B). In contrast, patients with SD at random assignment had similar median PFS with continuous versus 1-year fixed-duration treatment (11.8 months v 9.4 months; HR, 1.01 [95% CI, 0.51 to 2.01]; Fig 2C). Trends in favor of longer PFS after random assignment with continuous versus 1-year fixed-duration treatment were observed across the majority of predefined subgroups (Fig 3).
FIG 2.
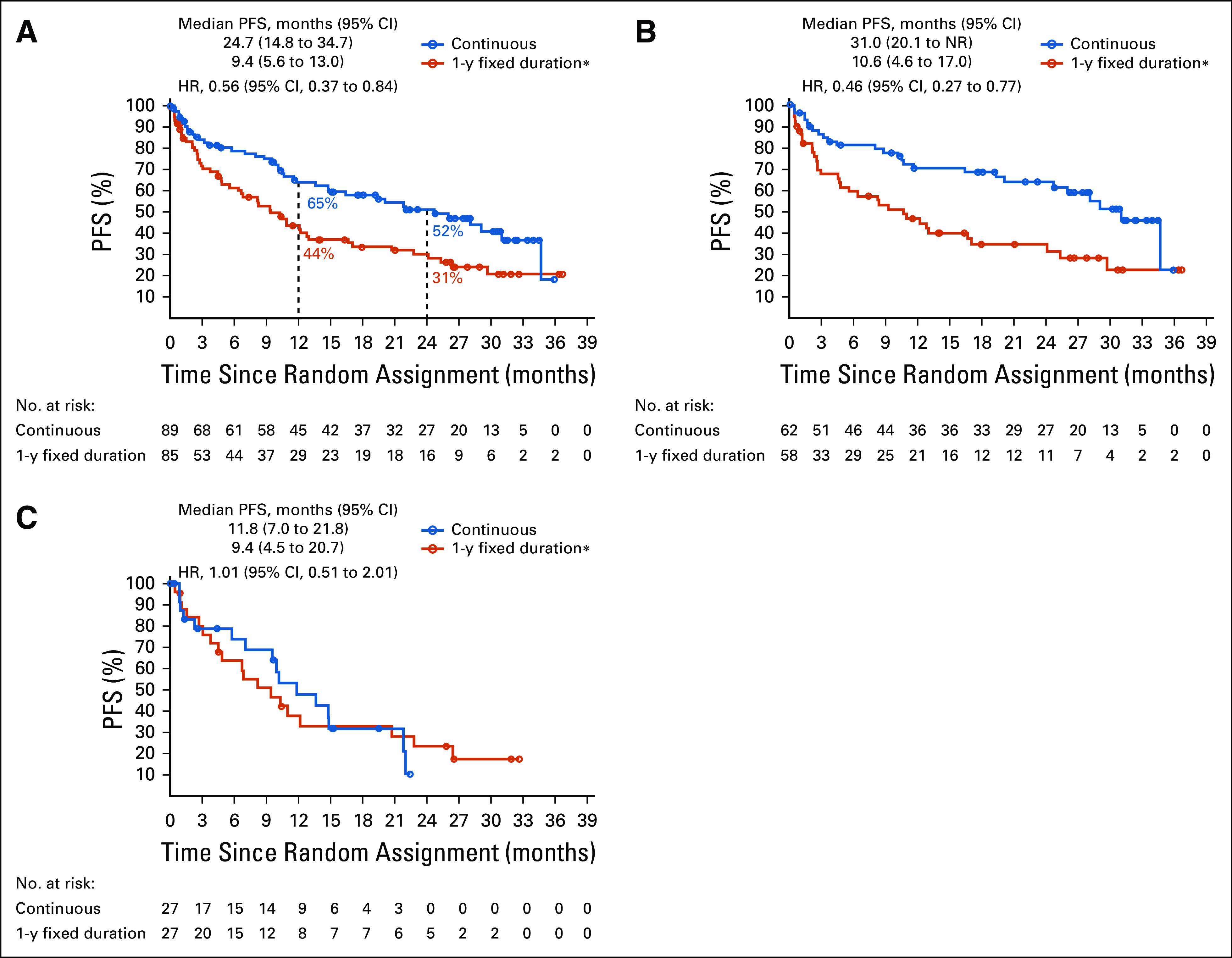
Progression-free survival (PFS) from random assignment (A) in the PFS population, (B) in patients with complete response/partial response, and (C) in patients with stable disease. Random assignment took place after 1 year of treatment with nivolumab; the post–random assignment PFS population comprised patients who did not have progressive disease (PD) and had not initiated other systemic anticancer therapy before random assignment. Minimum follow-up after random assignment in the intent-to-treat population was 13.5 months overall; median follow-up times after random assignment in the PFS population were 40.5 months and 35.2 months for the continuous arm and 1-year fixed-duration treatment arm, respectively. HR, hazard ratio. (*) Optional retreatment allowed at PD.
FIG 3.
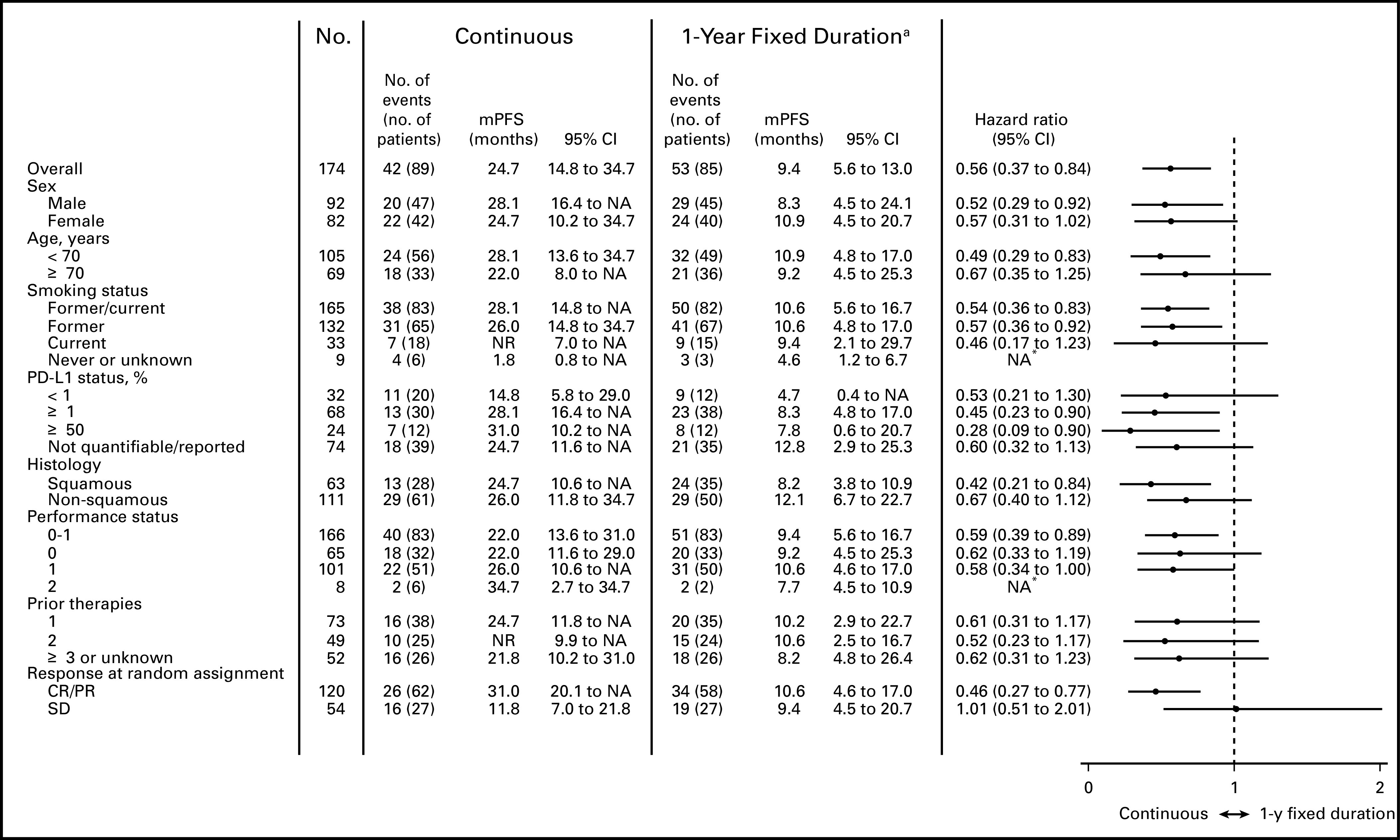
Multivariable analysis of progression-free survival (PFS) since random assignment by subgroup in the PFS population. Random assignment took place was after 1 year of treatment with nivolumab; the post–random assignment PFS population comprised patients who did not have progressive disease and had not initiated other systemic anticancer therapy before random assignment. Minimum follow-up after random assignment in the intent-to-treat population was 13.5 months overall; median follow-up times after random assignment in the PFS population were 40.5 months and 35.2 months for the continuous and 1-year fixed-duration treatment arms, respectively. A multivariable Cox proportional hazards regression analysis for PFS was also performed, whereby the effect of treatment arm was adjusted for key baseline characteristics as well as response/progression status at random assignment; the adjusted hazard ratio was 0.55 (95% CI, 0.36 to 0.85). (a) Optional retreatment was allowed at PD. CR, complete response; NA, not available; NR, not reached; PD-L1, programmed death ligand 1; PR, partial response; SD, stable disease. (*) Hazard ratio not calculated because of the small sample size.
In the ITT population, median OS after random assignment was longer with continuous versus 1-year fixed-duration treatment (not reached v 28.8 months; HR, 0.62 [95% CI, 0.42 to 0.92]; Fig 4A); OS rates were similar at 1 year (82.9% v 81.7%) but greater with continuous versus 1-year fixed-duration treatment at 2 years (70.4% v 56.8%, respectively). Similarly, in the PFS population, median OS was longer with continuous versus 1-year fixed-duration treatment (not reached v 32.5 months; HR, 0.61 [95% CI, 0.37 to 0.99]; Fig 4B); OS rates were 86.1% versus 82.0% at 1 year and 73.4% versus 60.9% at 2 years, respectively. In the PFS population, patients with CR/PR at random assignment also had longer median OS with continuous versus 1-year fixed-duration treatment (not reached v 33.5 months; HR, 0.50 [95% CI, 0.26 to 0.97]; Fig 4C). In patients with SD at random assignment, median OS was similar between treatment arms (32.2 months v 26.6 months; HR, 0.88 [95% CI, 0.42 to 1.84]; Fig 4D). In patients with PD before random assignment, median OS was not reached with continuous versus 23.8 months with 1-year fixed-duration treatment (HR, 0.70 [95% CI, 0.37 to 1.33]; Fig 4E); OS rates were 75.3% versus 81.1% at 1 year and 63.0% versus 47.8% at 2 years.
FIG 4.
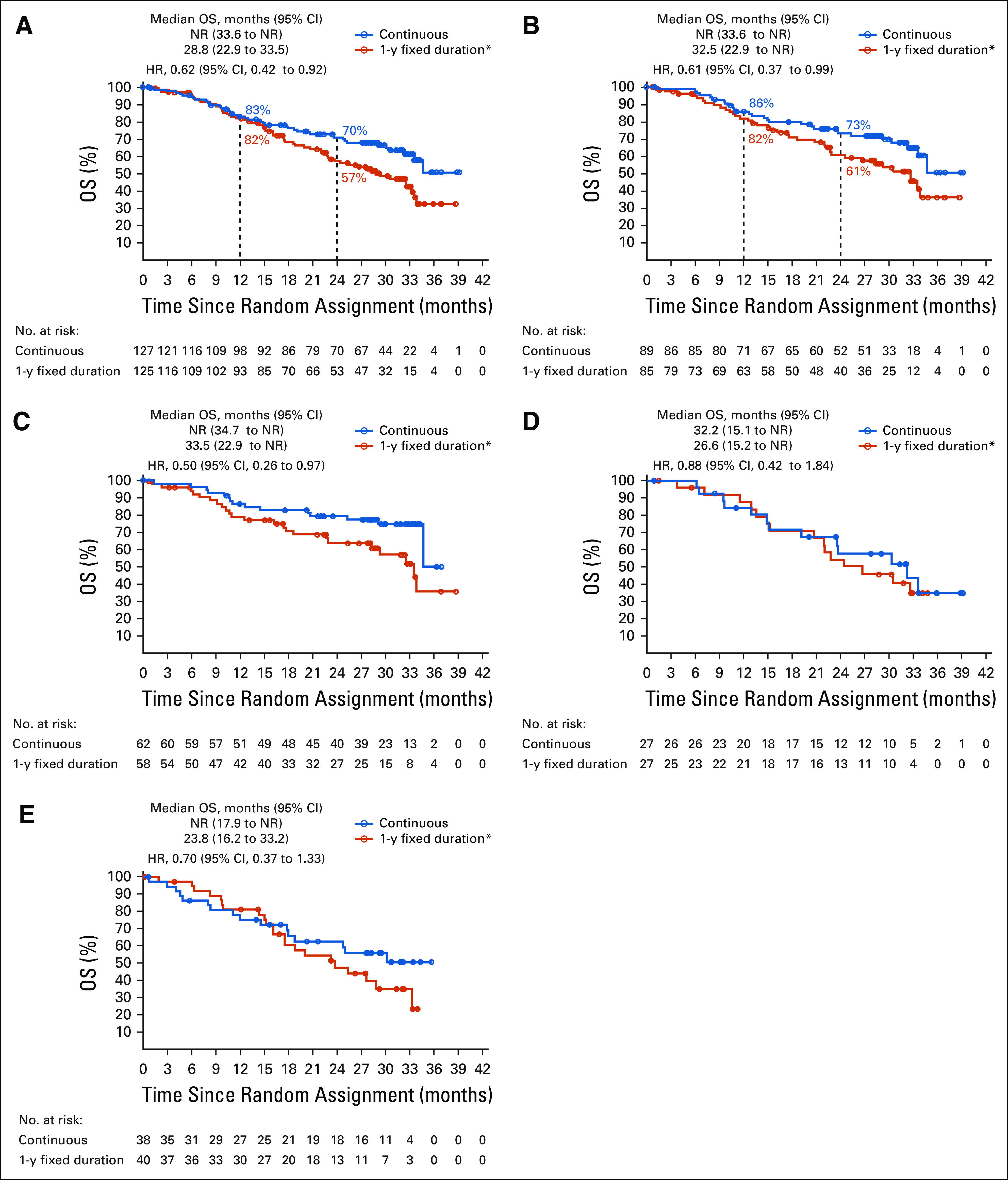
Overall survival (OS) from random assignment in (A) the intent-to-treat (ITT) population, (B) the progression-free survival (PFS) population, (C) patients with complete response/partial response (CR/PR; PFS population), (D) patients with stable disease (SD) at random assignment (PFS population), and (E) patients with progressive disease (PD) before random assignment (ITT population). Random assignment took place after 1 year (y) of treatment with nivolumab. The ITT population comprised all patients who continued to receive treatment at 1 year and were randomly assigned, regardless of response status. The post–random assignment PFS population comprised patients who did not have PD and had not initiated other systemic anticancer therapy before random assignment. Minimum follow-up after random assignment in the ITT population was 13.5 months overall; median follow-up times after random assignment in the ITT population were 39.8 months and 34.4 months for the continuous and 1-year fixed-duration treatment arms, respectively; median follow-up times after random assignment in the PFS population were 40.5 months and 35.2 months for the continuous and 1-year fixed-duration treatment arms, respectively. HR, hazard ratio; NR, not reached. (*) Optional retreatment allowed at PD.
Retreatment on Study: Reinitiation and Duration
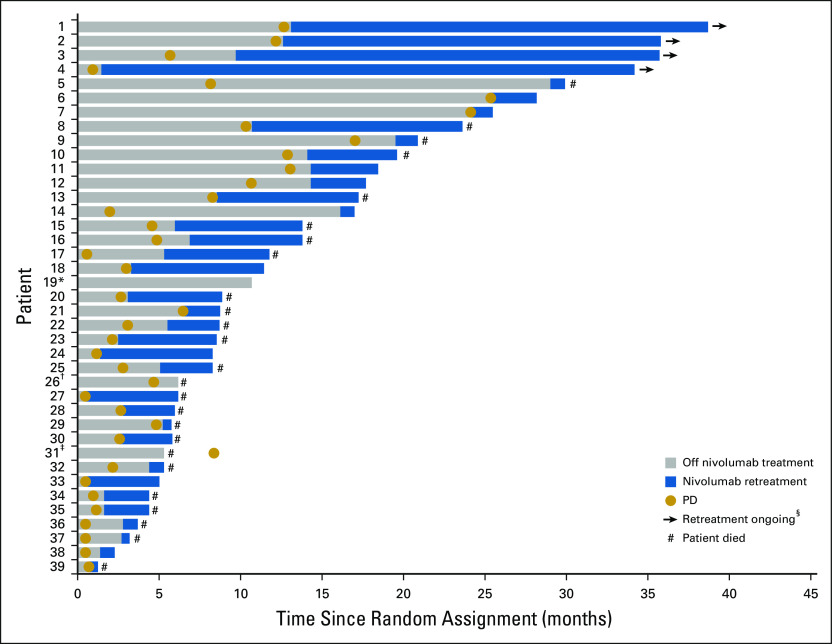
Overall, 47 patients (55.3%) who were progression free at random assignment in the 1-year fixed-duration arm had PD after random assignment. Of these, 39 (83.0%) were retreated with nivolumab (Fig 5). At database lock, four patients (10.2%) were continuing retreatment and 14 patients (35.9%) were still alive.
FIG 5.
Initiation and duration of nivolumab retreatment in patients in the progression-free survival population who progressed after random assignment. (*) Patient was last reported as progression free and had one dose during retreatment. (†) Patient had one dose during retreatment. (‡) Patient had confirmed disease progression after beginning retreatment; patient had one dose during retreatment. (§) Two additional patients with ongoing retreatment had progressive disease (PD) before random assignment.
Subsequent Therapy off Study
In the PFS population, subsequent therapy off study was received by 37.1% and 48.2% of patients in the continuous and 1-year fixed-duration treatment arms, respectively (Data Supplement). Subsequent systemic therapy use was lower in the continuous arm (24.7%) versus the 1-year fixed-duration treatment arm (40.0%). The most frequently administered systemic therapy was post-study nivolumab, used by 14.6% of the continuous and 17.6% of the 1-year fixed-duration treatment arm. Usage of subsequent therapies in the ITT population showed similar trends to that of the PFS population, although overall usage was generally higher across categories (Data Supplement).
Post–Random Assignment Safety
After random assignment, patients in the continuous versus the 1-year fixed-duration arm had higher incidences of any-grade treatment-related select AEs (32.3% v 15.2%), treatment-related adverse events (TRAEs; 48.0% v 26.4%), and TRAEs leading to discontinuation (9.4% v 1.6%; Data Supplement), consistent with the longer treatment duration. Grade 3-4 TRAEs occurred in 9.4% in the continuous arm and in 3.2% in the 1-year fixed-duration treatment arm (Data Supplement). Few new-onset treatment-related events occurred in either arm, and no new safety signals were iden-tified after random assignment. One treatment-related death was reported, as a result of myelodysplastic syndrome, in the continuous treatment arm.
DISCUSSION
To our knowledge, CheckMate 153 is the first randomized study in NSCLC to evaluate fixed-duration versus continuous treatment with a therapy that targets the PD-1 pathway. Findings indicated that continuing nivolumab provided a clinically meaningful benefit versus stopping treatment at 1 year in patients with previously treated NSCLC; after random assignment, median PFS and OS were longer with continuous treatment. Interestingly, the trend in OS benefit with continuous versus 1-year fixed-duration treatment was similar in the ITT and PFS populations. Furthermore, in the PFS population, patients with CR/PR at random assignment seemed to derive greater benefit with continuous versus 1-year fixed-duration treatment. In contrast, patients with SD at random assignment seemed to derive similar benefit in both arms. Patients who were treated beyond progression seemed to derive longer-term OS benefit with continuous versus 1-year fixed-duration treatment; however, results should be interpreted with caution because of the small number of patients. In patients who were randomly assigned to the 1-year fixed-duration arm and subsequently progressed, retreatment with nivolumab did not seem to provide the same level of clinical benefit as that observed before random assignment. Continuous nivolumab showed no new or emerging safety signals. Grade 3-4 TRAE rates after random assignment were low in both treatment arms.
When CheckMate 153 was initiated, the magnitude of survival benefit with immunotherapy versus chemotherapy was unknown, and survival beyond 1 year was not expected. However, subsequent studies, including those with nivolumab, showed a survival benefit beyond 1 year across a large proportion of previously treated patients with NSCLC.3,4,13,14 Nonetheless, the appropriate treatment duration in NSCLC was unknown and was determined arbitrarily for clinical trials because of a lack of clinical evidence and because the available data were from nonrandomized studies; at that time, nivolumab was administered until PD or unacceptable toxicity, whereas treatment duration for other agents varied.3,4,13,14 Recently, a number of immunotherapy studies in NSCLC have shown that a fixed duration of therapy may provide durable clinical benefit.8,10 Nevertheless, there has been some concern about stopping immunotherapy in patients with response or SD because of limited data on response after retreatment across tumor types.15 For example, in advanced melanoma, patients with a PR or SD when anti–PD-1 therapy was discontinued (1-year median treatment duration) were shown to be at a higher risk of progression than were those patients with CR.16 It is plausible that early relapse observed with discontinuation of immunotherapy, regardless of tumor type, may be caused by loss of PD-1/PD-L1 inhibition,17 potentially because of rapid antibody clearance resulting in a short on-target treatment lifespan in the local tumor environment.18 Resistance mechanisms may also develop when chronic PD-1/PD-L1 blockade is removed.19
Inconsistent findings have also been reported from fixed-duration immunotherapy studies in NSCLC that retrospectively evaluated retreatment after PD. Some studies have shown that 70% to 79% of patients with tumors positive for PD-L1, who were treated for a 2-year fixed duration without progression, experienced clinical benefit from retreatment after relapse after first-line20 and second-line treatment.10 However, a study assessing 1-year fixed-duration treatment found that only 52% of previously treated patients who relapsed responded to retreatment.21 In the current randomized study, although responses to retreatment were not assessed, 36% of patients retreated at relapse after 1-year fixed-duration treatment were still alive after a minimum follow-up of 13.5 months. Comparisons among studies should be interpreted with caution because of the different study designs, treatment durations, patient populations, and limited patient numbers. Additional investigation is needed to fully assess the benefit of retreatment after a fixed duration of therapy.
Although we show that continuous nivolumab treatment beyond 1 year provided survival benefit, it would be interesting to assess the value of a longer fixed-duration treatment period, beyond 1 year, in patients who remain progression free. With 13.5 months’ minimum follow-up, a proportion of patients in the continuous arm continued to receive nivolumab treatment and were progression free, with 1 patient receiving nivolumab for approximately 23 months after random assignment (approximately 3 years overall).
Although the primary objective of CheckMate 153 was to assess safety in a largely real-world population, the corresponding efficacy data reported at that time showed that nivolumab provided clinical benefit in this setting.11 Given the change in the NSCLC treatment landscape over the course of the study, this large nivolumab-treated population consequently presented the unique opportunity to assess treatment duration in a randomized setting. It was recognized that even in a study of this size, only a small number of patients would still be receiving treatment at 1 year. Therefore, to observe a marked difference in efficacy between continuous and fixed-duration treatment, the difference would have to be substantial.
The post–random assignment analysis in CheckMate 153 had several limitations. Patients were not rebaselined at random assignment, the time point at which post–random assignment survival outcome assessment began, or at PD in the fixed-treatment cohort, limiting assessment of response after retreatment. Furthermore, patients were not stratified by response status at random assignment. Per protocol, although random assignment was permitted for patients who had progressed before random assignment, these patients were not included in the PFS analysis. In addition, the protocol did not require assessments to be conducted by blinded independent central review, which may have biased the estimate of the treatment effect. Finally, because the study was not powered for efficacy analyses, these were exploratory end points. Despite these limitations, this study provides key information on treatment duration for patients with NSCLC. Moreover, it is unlikely that this study, which had to enroll and treat 1,428 patients to randomly assign 252 patients who continued to receive nivolumab treatment at 1 year, can or will be replicated in the future; CheckMate 153 may represent the definitive study on fixed versus continuous nivolumab treatment.
In conclusion, this exploratory randomized analysis of the CheckMate 153 study supports, overall, the continuous use of nivolumab beyond 1 year in previously treated patients with advanced NSCLC, even in those who achieve a response. A survival benefit was observed when compared with a 1-year fixed-duration treatment, and a tolerable safety profile was maintained with the longer treatment duration. Although additional analyses are warranted, this study provides valuable insight into treatment duration for patients with NSCLC, and may inform treatment decisions for clinicians.
ACKNOWLEDGMENT
We thank the patients and families for making this trial possible, and the investigators (Data Supplement) and clinical study teams who participated in the trial. We also thank Tanisha Bryant for contributions as protocol manager of this trial; Dako for collaborative development of the PD-L1 IHC 28-8 pharmDx assay; and Mhairi Laird, PhD, of Caudex, for their assistance in the preparation of the manuscript. D.M.W., E.B.G., J.C., M.M., M.H., R.J., D.B.D., G.K., S.B., C.R., G.B. Jr, V.G., and D.R.S. are members of the Immuno-Oncology Integrated Community Oncology Network.
PRIOR PRESENTATION
Presented in part at the European Society for Medical Oncology Congress, Madrid, Spain, September 8-12, 2017.
SUPPORT
Supported by Bristol Myers Squibb Company and Ono Pharmaceutical.
CLINICAL TRIAL INFORMATION
NCT02066636 (CheckMate 153)
See accompanying editorial on page 3830
Bristol Myers Squibb Company policy on data sharing may be found at https://www.bms.com/researchers-and-partners/clinical-trials-and-research/disclosure-commitment.html.
AUTHOR CONTRIBUTIONS
Conception and design: David M. Waterhouse, Robert Jotte, Lawrence H. Einhorn, Craig Reynolds, Vijay Gunuganti, Ang Li, Nivedita Aanur, David R. Spigel
Provision of study material or patients: David M. Waterhouse, Edward B. Garon, Michael McCleod, Maen Hussein, Robert Jotte, Leora Horn, Davey B. Daniel, George Keogh, Ben Creelan, Lawrence H. Einhorn, Petros Nikolinakos, Felix Couture, Natasha B. Leighl, Craig Reynolds, David R. Spigel
Collection and assembly of data: David M. Waterhouse, Edward B. Garon, Jason Chandler, Michael McCleod, Maen Hussein, Robert Jotte, Leora Horn, Davey B. Daniel, George Keogh, Ben Creelan, Lawrence H. Einhorn, Justin Baker, Samer Kasbari, Petros Nikolinakos, Felix Couture, Natasha B. Leighl, Craig Reynolds, George Blumenschein Jr, Vijay Gunuganti, Ang Li, Nivedita Aanur, David R. Spigel
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Continuous Versus 1-Year Fixed-Duration Nivolumab in Previously Treated Advanced Non–Small-Cell Lung Cancer: CheckMate 153
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
David M. Waterhouse
Consulting or Advisory Role: Bristol Myers Squibb Company, AZTherapies, AbbVie, Amgen, Celgene, McGivenny Global, CTI, Janssen Oncology, Seattle Genetics
Speakers' Bureau: Bristol Myers Squibb, Janssen Oncology
Travel, Accommodations, Expenses: Bristol Myers Squibb Company
Edward B. Garon
Consulting or Advisory Role: Dracen, EMD Serono, Novartis, GlaxoSmithKline
Research Funding: Merck (Inst), Genentech (Inst), AstraZeneca (Inst), Novartis (Inst), Lilly (Inst), Bristol Myers Squibb (Inst), Mirati Therapeutics (Inst), Dynavax (Inst), Iovance Biotherapeutics (Inst), Neon Therapeutics (Inst), EMD Serono (Inst)
Jason Chandler
Employment: George Clinical
Consulting or Advisory Role: Janssen Oncology, AbbVie, Bristol Myers Squibb
Speakers' Bureau: Amgen
Maen Hussein
Stock and Other Ownership Interests: Celgene (I)
Consulting or Advisory Role: Guardant Health
Speakers' Bureau: Boehringer Ingelheim, Pfizer, Incyte, Bristol Myers Squibb, AMAG Pharmaceuticals, Heron
Robert Jotte
Honoraria: Bristol Myers Squibb, Roche/Genentech
Speakers' Bureau: Bristol Myers Squibb, Roche/Genentech
Travel, Accommodations, Expenses: Bristol Myers Squibb, Roche/Genentech
Leora Horn
Consulting or Advisory Role: Merck, Xcovery, Genentech, AstraZeneca, Incyte, EMD Serono, Tesaro, Pfizer, Amgen, Bayer, Puma Biotechnology
Research Funding: Boehringer Ingelheim (Inst), Xcovery (Inst)
Travel, Accommodations, Expenses: Xcovery
Davey B. Daniel
Research Funding: AstraZeneca (Inst), Genentech (Inst), Guardant Health (Inst), Janssen Research and Development (Inst), Bristol Myers Squibb (Inst), G1 Therapeutics (Inst), Genentech (Inst), Merck (Inst), Novartis (Inst), AbbVie (Inst), ARMO BioSciences (Inst), Genentech (Inst), G1 Therapeutics (Inst), Novartis (Inst), Immunomedics (Inst), Lilly (Inst), Merus NV (Inst), Daiichi Sankyo (Inst)
George Keogh
Research Funding: Pfizer (Inst), Medivation (Inst), Bristol Myers Squibb (Inst), Dendreon (Inst), Merck (Inst), Gilead Sciences (Inst), Pfizer (Inst), Astellas Medivation (Inst), Bayer (Inst), Zeno Pharmaceuticals (Inst), Seattle Genetics (Inst), Incyte (Inst), Amgen (Inst)
Ben Creelan
Consulting or Advisory Role: Boehringer Ingelheim, AbbVie, KSQ Therapeutics, BerGenBio, AstraZeneca/MedImmune, ARMO BioSciences, E.R. Squibb Sons, LLC, Gilead Sciences, GlaxoSmithKline
Speakers' Bureau: AstraZeneca/MedImmune, ARIAD, Genentech/Roche, Takeda, Foundation Medicine
Research Funding: Boehringer Ingelheim (Inst), Bristol Myers Squibb (Inst), Iovance Biotherapeutics (Inst), Prometheus Laboratories (Inst), NeoGenomics Laboratories (Inst), Biodesix (Inst)
Travel, Accommodations, Expenses: AstraZeneca
Lawrence H. Einhorn
Stock and Other Ownership Interests: Amgen, Biogen
Samer Kasbari
Speakers' Bureau: Bristol Myers Squibb, Lilly
Travel, Accommodations, Expenses: Bristol Myers Squibb
Felix Couture
Consulting or Advisory Role: Bristol Myers Squibb
Natasha B. Leighl
Consulting or Advisory Role: Excovery
Research Funding: Novartis (Inst), Roche Canada (Inst), Guardant (Inst), Array BioPharma (Inst)
Travel, Accommodations, Expenses: Merck Sharp & Dohme, Bristol Myers Squibb, Nektar, GlaxoSmithKline, Roche, AstraZeneca
Craig Reynolds
Leadership: American Oncology Network
Stock and Other Ownership Interests: Gilead Sciences, American Oncology Network
Honoraria: Lilly, Celgene, Genentech, Bristol Myers Squibb, Merck Sharp & Dohme
Consulting or Advisory Role: Genentech, Lilly, Bristol Myers Squibb
Speakers' Bureau: Celgene, Genentech, Merck Sharp & Dohme
George Blumenschein Jr
Employment: Janssen (I). Johnson & Johnson (I)
Stock and Other Ownership Interests: Virogin Biotech
Consulting or Advisory Role: Bristol Myers Squibb, Bayer, Celgene, Clovis Oncology, AbbVie, ARIAD, Merck, Genentech, Novartis, Xcovery, Adicet, Amgen, AstraZeneca, Roche, MedImmune, Maverick Therapeutics, Johnson & Johnson (I), Virogin Biotech
Research Funding: Merck, Celgene, Genentech, Xcovery, Novartis, Bristol Myers Squibb, GlaxoSmithKline, Adaptimmune, Macrogenics, Kite Pharma, Immatics, Torque, Incyte, MedImmune, Exelixis, Immunocore, Roche, AstraZeneca, Bayer, Tmunity Therapeutics, Regeneron, BeiGene, Repertoire Immune Medicines
Nivedita Aanur
Employment: Bristol Myers Squibb
Stock and Other Ownership Interests: Bristol Myers Squibb
David R. Spigel
Leadership: ASCO Adjuvant Lung Cancer Guideline Committee (Inst)
Consulting or Advisory Role: Genentech/Roche (Inst), Novartis (Inst), Celgene (Inst), Bristol Myers Squibb (Inst), AstraZeneca (Inst), Pfizer (Inst), GlaxoSmithKline (Inst), Merck (Inst), Nektar (Inst), Takeda (Inst), TRM Oncology (Inst), Evelo Therapeutics (Inst), Illumina (Inst), PharmaMar (Inst), Aptitude Health (Inst), Bayer (Inst), Dracen Pharmaceuticals (Inst), EMD Serono (Inst), Iksuda Therapeutics (Inst), Molecular Templates (Inst), Seattle Genetics (Inst), TRIPTYCH Health Partners (Inst), Williams and Connolly LLP (Inst)
Research Funding: Genentech/Roche (Inst), Novartis (Inst), Celgene (Inst), Bristol Myers Squibb (Inst), Lilly (Inst), AstraZeneca (Inst), University of Texas Southwestern Medical Center - Simmons Cancer Center (Inst), Merck (Inst), G1 Therapeutics (Inst), Neon Therapeutics (Inst), Takeda (Inst), Nektar (Inst), Celldex (Inst), Clovis Oncology (Inst), Daiichi Sankyo (Inst), EMD Serono (Inst), Astellas Pharma (Inst), GRAIL (Inst), Transgene (Inst), Aeglea Biotherapeutics (Inst), Ipsen (Inst), BIND Therapeutics (Inst), Eisai (Inst), ImClone Systems (Inst), Immunogen (Inst), Janssen Oncology (Inst), MedImmune (Inst), Molecular Partners (Inst)
Travel, Accommodations, Expenses: AstraZeneca, Celgene, Bristol Myers Squibb, Genentech, Merck, Pfizer, Spectrum Pharmaceuticals, Amgen, Daiichi Sankyo, GlaxoSmithKline, Janssen Oncology, Novartis, Seattle Genetics, Takeda
No other potential conflicts of interest were reported.
REFERENCES
- 1. Bristol Myers Squibb Company: Opdivo (nivolumab) prescribing information, June 2020. https://packageinserts.bms.com/pi/pi_opdivo.pdf.
- 2.National Comprehensive Cancer Network : Non-small Cell Lung Cancer, Volume 1. National Comprehensive Cancer Network 2019. https://www.nccn.org/professionals/physician_gls/default.aspx
- 3.Borghaei H Paz-Ares L Horn L, et al. : Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med 373:1627-1639, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brahmer J Reckamp KL Baas P, et al. : Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med 373:123-135, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borghaei H Brahmer JR Chow LQM, et al. : Five-year outcomes from the randomized, phase 3 trials CheckMate 017/057: Nivolumab vs docetaxel in previously treated NSCLC. Presented at the IASLC 20th World Conference on Lung Cancer, Barcelona, Spain, September 10, 2019 [Google Scholar]
- 6.Antonia SJ Borghaei H Ramalingam SS, et al. : Four-year survival with nivolumab in patients with previously treated advanced non-small-cell lung cancer: A pooled analysis. Lancet Oncol 20:1395-1408, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schadendorf D Hodi FS Robert C, et al. : Pooled analysis of long-term survival data from phase II and phase III trials of ipilimumab in unresectable or metastatic melanoma. J Clin Oncol 33:1889-1894, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gettinger S Horn L Jackman D, et al. : Five-year follow-up of nivolumab in previously treated advanced non-small-cell lung cancer: Results from the CA209-003 study. J Clin Oncol 36:1675-1684, 2018 [DOI] [PubMed] [Google Scholar]
- 9.Antonia SJ Villegas A Daniel D, et al. : Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. N Engl J Med 377:1919-1929, 2017 [DOI] [PubMed] [Google Scholar]
- 10. Herbst RS, Garon EB, Kim D, et al: Long-term follow-up in the KEYNOTE-010 study of pembrolizumab (pembro) for advanced NSCLC, including in patients (pts) who completed 2 years of pembro and pts who received a second course of pembro. Ann Oncol 28:x39-x43, 2018. [Google Scholar]
- 11.Spigel DR McCleod M Jotte RM, et al. : Safety, efficacy, and patient-reported health-related quality of life and symptom burden with nivolumab in patients with advanced non-small cell lung cancer, including patients aged 70 years or older with poor performance status (CheckMate 153). J Thorac Oncol 14:1628-1639, 2019 [DOI] [PubMed] [Google Scholar]
- 12.Spigel DR McLeod M Hussein MA, et al. : Randomized results of fixed-duration (1-yr) vs continuous nivolumab in patients (pts) with advanced non-small cell lung cancer (NSCLC). Ann Oncol 28:v461-v496, 2017 [Google Scholar]
- 13.Herbst RS Baas P Kim DW, et al. : Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): A randomised controlled trial. Lancet 387:1540-1550, 2016 [DOI] [PubMed] [Google Scholar]
- 14.Rittmeyer A Barlesi F Waterkamp D, et al. : Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): A phase 3, open-label, multicentre randomised controlled trial. Lancet 389:255-265, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baik CS Rubin EH Forde PM, et al. : Immuno-oncology clinical trial design: Limitations, challenges, and opportunities. Clin Cancer Res 23:4992-5002, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jansen YJL Rozeman EA Mason R, et al. : Discontinuation of anti-PD-1 antibody therapy in the absence of disease progression or treatment limiting toxicity: Clinical outcomes in advanced melanoma. Ann Oncol 30:1154-1161, 2019 [DOI] [PubMed] [Google Scholar]
- 17. Spigel DR: Do we treat patients with immunotherapy until progression? Immunotherapy until progressive disease. European Lung Cancer Congress. Geneva, Switzerland, April 10-13, 2019. [Google Scholar]
- 18. doi: 10.1126/scitranslmed.aal3604. Arlauckas SP, Garris CS, Kohler RH, et al: In vivo imaging reveals a tumor-associated macrophage-mediated resistance pathway in anti-PD-1 therapy. Sci Transl Med 9:eaal3604, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ramos P, Bentires-Alj M: Mechanism-based cancer therapy: Resistance to therapy, therapy for resistance. Oncogene 34:3617-3626, 2015 [DOI] [PubMed] [Google Scholar]
- 20.Reck M Rodríguez-Abreu D Robinson AG, et al. : KEYNOTE-024 3-year survival update: Pembrolizumab versus platinum-based chemotherapy for advanced NSCLC. World Conference on Lung Cancer. Barcelona, Spain, September 7-10, 2019 [Google Scholar]
- 21. doi: 10.1136/jitc-2020-000650. Sheth S, Gow C, Mueller N, et al: Durvalumab activity in previously treated patients who stopped durvalumab without disease progression. Ann Oncol 30:v475-v532, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]