Abstract
PURPOSE
SARS-CoV-2 (COVID-19) is a systemic infection. Patients with cancer are immunocompromised and may be vulnerable to COVID-related morbidity and mortality. The objectives of this study were to determine if patients with cancer have worse outcomes compared with patients without cancer and to identify demographic and clinical predictors of morbidity and mortality among patients with cancer.
METHODS
We used data from adult patients who tested positive for COVID-19 and were admitted to two New York–Presbyterian hospitals between March 3 and May 15, 2020. Patients with cancer were matched 1:4 to controls without cancer in terms of age, sex, and number of comorbidities. Using Kaplan-Meier curves and the log-rank test, we compared morbidity (intensive care unit admission and intubation) and mortality outcomes between patients with cancer and controls. Among those with cancer, we identified demographic and clinical predictors of worse outcomes using Cox proportional hazard models.
RESULTS
We included 585 patients who were COVID-19 positive, of whom 117 had active malignancy, defined as those receiving cancer-directed therapy or under active surveillance within 6 months of admission. Presenting symptoms and in-hospital complications were similar between the cancer and noncancer groups. Nearly one half of patients with cancer were receiving therapy, and 45% of patients received cytotoxic or immunosuppressive treatment within 90 days of admission. There were no statistically significant differences in morbidity or mortality (P = .894) between patients with and without cancer.
CONCLUSION
We observed that patients with COVID-19 and cancer had similar outcomes compared with matched patients without cancer. This finding suggests that a diagnosis of active cancer alone and recent anticancer therapy do not predict worse COVID-19 outcomes and therefore, recommendations to limit cancer-directed therapy must be considered carefully in relation to cancer-specific outcomes and death.
INTRODUCTION
New York City (NYC) has been at the epicenter of the public health epidemic caused by the SARS-CoV-2 (COVID-19) pandemic. As of July 2020, there have been more than 200,000 confirmed cases in NYC. Initial reports have described the characteristics of patients with COVID-19,1,2 and although COVID-19 manifests primarily as a respiratory tract infection, emerging data indicate a more systemic infection with a variety of multisystem manifestations.3,4 As such, patients with cancer may be particularly vulnerable to COVID-19 because of immunocompromise from underlying disease and the sequalae of cancer-directed treatment.
Context
Key Objective
Patients with cancer, and particularly those receiving active treatment, are suggested to be at increased risk of poor outcomes when contracting the novel coronavirus, SARS-CoV-2 (COVID-19). However, it is unclear if this increased risk is a result of malignancy, its treatment, or other factors. This study evaluated the morbidity and mortality of hospitalized COVID-19–infected patients with cancer compared with a matched cohort of patients without cancer.
Knowledge Generated
We observed no differences in the rate of intensive care unit (ICU) admission, ventilation, or death between patients with and without cancer who were hospitalized with COVID-19. Patients with cancer undergoing anticancer therapy also had no differences in outcomes including ICU admission, ventilation, or death compared with patients who were not receiving anticancer therapy.
Relevance
Our results suggest that patients with cancer with limited comorbidities may continue their cancer care with caution.
Early reports from China regarding COVID-19 stated that patients with cancer were more susceptible to infection by COVID-19 and that they experienced higher rates of severe complications.5-9 In these studies, cancer-directed therapy within 14 days of admission resulted in increased rates of intensive care unit (ICU) admission, invasive ventilation, and death.7 Two additional cohort studies reported that patients with cancer had a higher risk of critical illness and fatalities compared with patients without cancer.10,11 However, these studies did not control for confounding factors including age, sex, and comorbidities, which are known contribute to worse patient outcomes.12
Some institutions in the United States have recently reported similar trends, including higher rates of COVID-related complications and death among patients with cancer compared with patients without cancer.12-15 However, interpretation of these studies is also limited because of heterogeneous cancer populations, small sample sizes, and limited comparisons to cohorts without cancer.16 On the basis of these sparse data, the care of patients with cancer has been altered or delayed significantly and perhaps compromised.17-18 Guidelines have further recommended caution when treating patients with cancer.19-21
However, we must accept the realization that cancer treatment can be lifesaving and often improves patients’ outcomes, making the decision of how to manage cancer in the context of COVID-19 all the more acute.17 As oncology practices cautiously decrease cancer-directed therapies because of COVID-19, we must consider the consequences that limited care for patients with cancer have on cancer-specific outcomes and mortality. Given the outstanding question of the impact of COVID-19 in patients with cancer, and those receiving anticancer therapy, the objective of this study was to determine whether patients with cancer developed higher rates of morbidity and mortality compared with a matched noncancer population.
METHODS
Study Design and Setting
We used data from a retrospective observational cohort of adult patients who presented to the emergency department (ED) at two New York–Presbyterian (NYP) hospitals (Weill Cornell Medicine and Lower Manhattan Hospital) between March 3 and May 15, 2020, and who tested positive for COVID-19, defined as a positive reverse transcription polyerase chain reaction (RT-PCR) assay. Both hospitals are academic institutions that deliver similar high-quality care. The development of this cohort has been described previously.2 This study was approved by the Weill Cornell Medicine Institutional Review Board, which waived informed consent.
Cancer cohort.
We included all consecutively hospitalized patients with an active hematologic or solid tumor malignancy who tested positive for COVID-19. Active malignancy was defined as cancer-directed therapy (eg, chemotherapy, targeted therapy, immunotherapy, radiotherapy, or surgery) or active surveillance within 6 months of COVID-19 diagnosis and ongoing management. Each record of active malignancy was independently reviewed and verified by two oncologists (G.B. and M.S.).
Noncancer controls.
Every cancer case was matched to four COVID-19–positive noncancer controls. Matching was performed in terms of age, sex, and number of comorbid conditions (obesity, diabetes, hypertension, chronic obstructive pulmonary disease [COPD], asthma, end-stage renal disease, cirrhosis, coronary artery disease, heart failure, and HIV). Comorbidities were grouped into five categories: 0, 1, 2, 3, and ≥ 4. Age was categorized into six 10-year age groups from 30 to 99 years.
Primary Outcome
Our primary study outcome was a composite incident outcome of (1) ICU admission, (2) intubation, and (3) death. Only an individual’s incident event was considered.
Covariables.
From the electronic health record, we considered age, sex, ethnicity, pre-existing comorbidities (smoking status, obesity, hypertension, COPD, asthma, end-stage renal disease, coronary artery disease, heart failure, cirrhosis, and HIV), and medications taken at home. We also included each patient’s highest level of supplemental oxygen within 3 hours of ED admission, and chest radiographic findings. First vital signs at ED presentation (body temperature, heart rate, blood pressure, and respiratory rate) and laboratory tests within 48 hours of ED admission (CBC, metabolic panel, coagulation factors, and inflammatory markers including troponin, d-dimer, ferritin, erythrocyte sedimentation rate, C-reactive protein, fibrinogen, lactase dehydrogenase (LDH), creatine kinase, and procalcitonin) were obtained from the electronic health record through an automated algorithm.22
Cancer-specific covariables.
Cancer-specific covariables included cancer type, stage, diagnosis date, cancer treatment, and date of last cancer treatment, which were abstracted manually from the electronic health record by two oncologists.
Hospitalization events.
Hospitalization events that occurred through June 20, 2020, were identified on the basis of a review of clinical progress notes and discharge summaries. Events included cardiovascular sequelae (myocardial infarction, arrhythmias, and heart failure), need for vasopressors, venous thromboembolic events (deep vein thrombosis and pulmonary embolism), new-onset renal replacement therapy, death, and recovery events (extubation among those intubated, and hospital discharge).
Statistical Analyses
Baseline characteristics (demographics, comorbidities, clinical characteristics, and laboratory values), in-hospital treatments, and complications were compared between cancer and noncancer groups. Categorical variables were summarized using counts and percentages, and continuous variables were summarized using medians and interquartile ranges. Our primary outcome was operationalized as the number of days from ED admission date to ICU admission, intubation, or death, with participants censored at the date of event or discharge or June 20, 2020. We examined death as a secondary outcome using Kaplan-Meier plots to explore differences in the risk of death between patients with and without cancer. We examined unadjusted associations between each baseline predictor and our primary composite outcome using marginal Cox proportional hazard models to adjust for clustering by matched observations.23,24 In models that examined the cancer group separately, we used Cox models that did not account for clustering. Finally, we used multivariable marginal Cox models to estimate the association between cancer status and the composite outcome, adjusting for potential confounders. As a secondary analysis, a multivariable Cox model with the death outcome, separately, was also used. Hazard ratios (HRs) and 95% CIs were calculated for each estimate. The proportional hazards assumption was tested using Schoenfeld residuals. Finally, we used χ2 tests to examine unadjusted differences in the composite outcome between patients with cancer with hematologic and solid malignancies as well between those who did and did not receive chemotherapy within 90 days of ED presentation. All analyses were conducted in SAS version 9.4 (SAS Institute, Cary, NC). Results were considered statistically significant with a P value level of < .05.
RESULTS
Cohort Characteristics
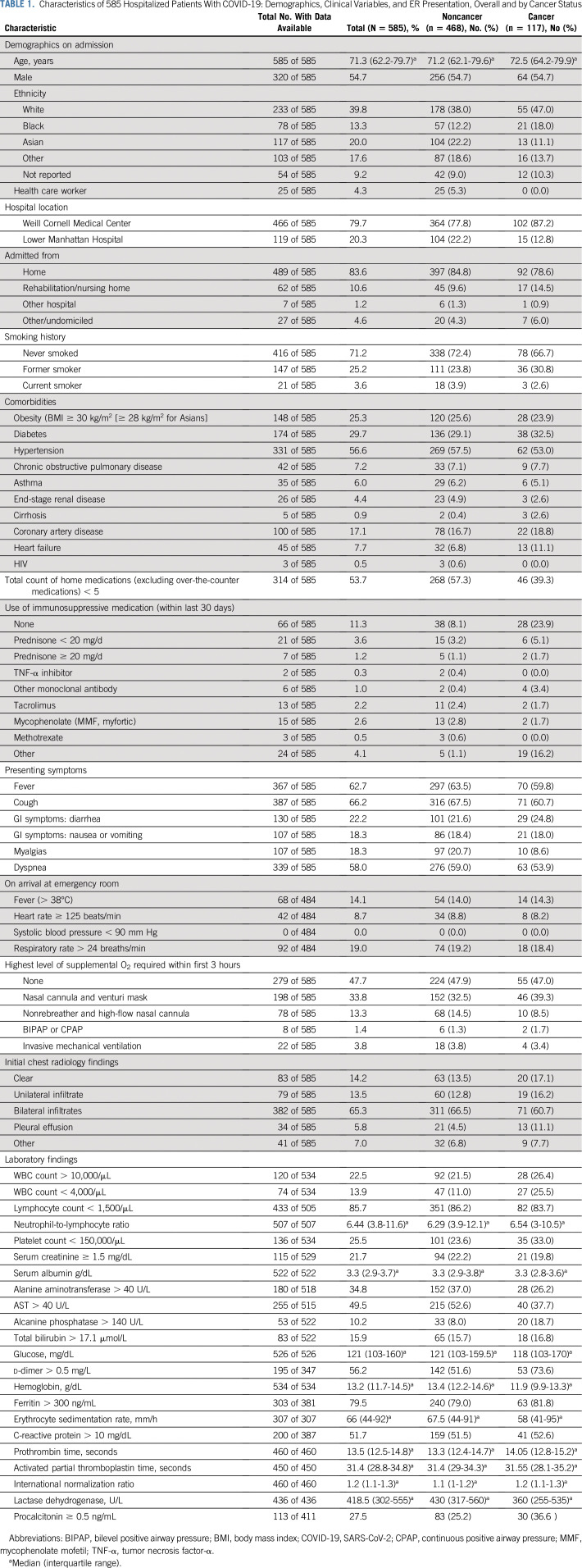
Our study included 585 adult patients who were COVID-19 positive and were admitted to two NYP hospitals. Of these, 117 patients had active malignancy and 468 were matched control patients without cancer. Characteristics of the patients with and without cancer are presented in Table 1. The median age was 71 years, and 55% were male. More patients with cancer were White (47%) compared with patients without cancer (38%). There were more Asian patients in the noncancer group (22.2%) versus the cancer group (11.1%). The majority of patients were nonsmokers (71.2%), with similar percentages of active smokers (2.6% in patients with cancer and 3.9% in patients without cancer). The prevalence of obesity was similar in both groups (23.9% of those with cancer v 25.6% of those without cancer). Comorbidities were also similar between the two groups.
TABLE 1.
Characteristics of 585 Hospitalized Patients With COVID-19: Demographics, Clinical Variables, and ER Presentation, Overall and by Cancer Status
COVID-19 Clinical Characteristics
As listed in Table 1, the most common presenting symptoms for patients with and without cancer were cough (60.7% v 67.5%), fever (59.8% v 63.5%), dyspnea (53.9% v 59%), and diarrhea (24.8% v 21.6%), respectively. Within the first 3 hours of ED presentation, more patients without cancer required nonrebreather or high-flow nasal cannula (14.5% v 8.5%), although patients with and without cancer experienced similar rates of intubation (3.8% v 3.4%). Accordingly, patients without cancer had worse initial chest radiology findings with higher rates of bilateral infiltrates (66.5% v 60.7%) compared with patients with cancer. With regard to laboratory findings, patients with cancer had more leukopenia (25.5% v 11%), thrombocytopenia (33% v 23.6%), anemia, and elevated d-dimer (73.6% v 51.6%) compared with controls without cancer.
Cancer-Specific Characteristics
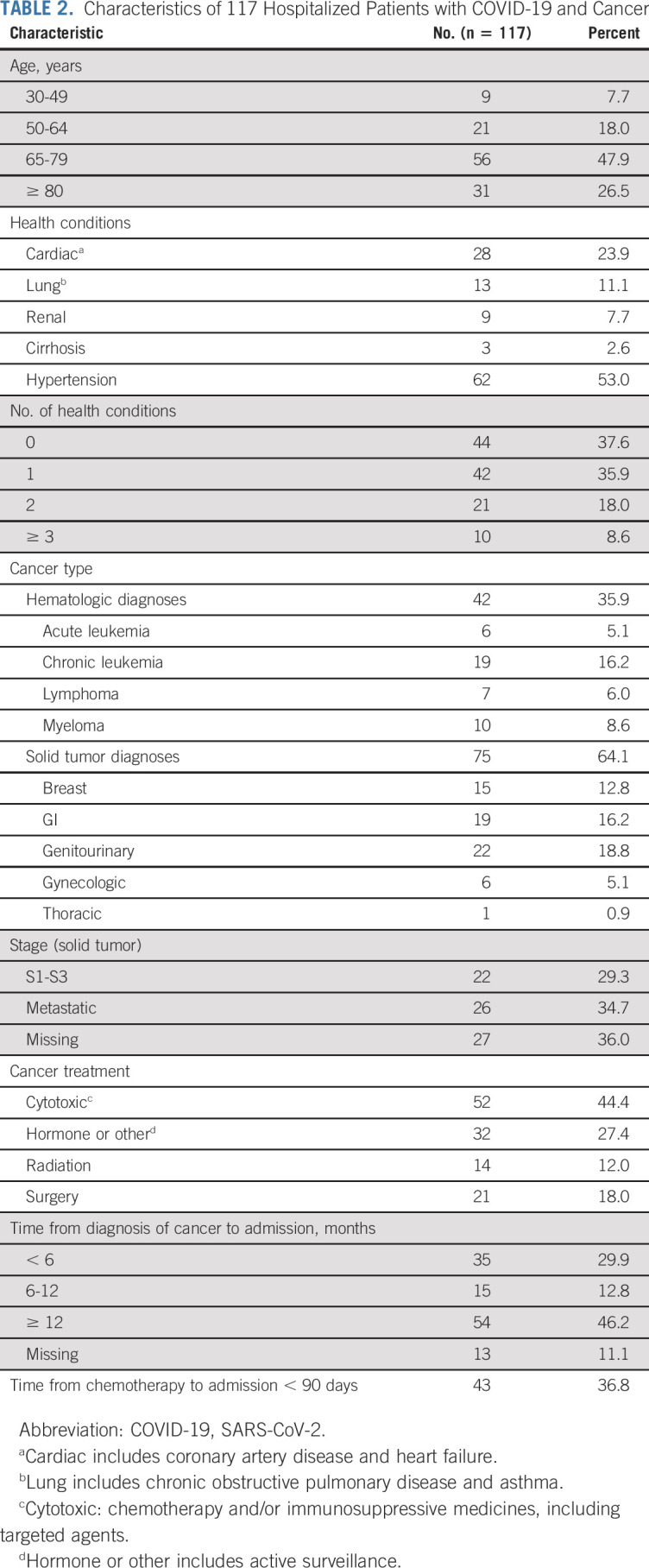
Cancer-specific characteristics are listed in Table 2. Seventy-four percent of patients with cancer were older than 65 years, and 74% of patients had zero to one comorbidity in addition to their cancer. The most common type of cancer was genitourinary (18.8%), followed by GI (16.2%), chronic leukemia (16.2%), and breast (12.8%). Nearly one half of patients (45%) were receiving active cancer therapy, including cytotoxic or immunosuppressive treatment, and 37% of patients had received cytotoxic treatment within 90 days of their ED admission.
TABLE 2.
Characteristics of 117 Hospitalized Patients with COVID-19 and Cancer
In-Hospital Treatments and Complications
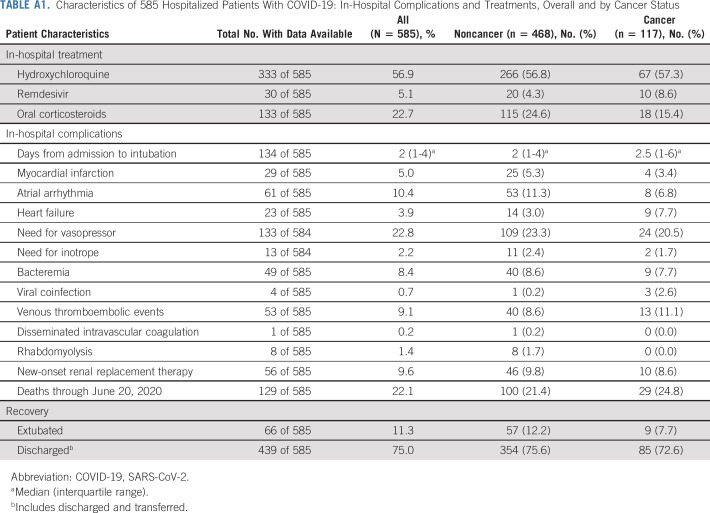
Patients both with and without cancer received hydroxychloroquine in similar proportions (57.3% v 56.8%), and more patients with cancer received remdesivir (8.6% v 4.3%). Observed complications (Appendix Table A1, online only), including myocardial infarction (3.4% v 5.3%), vasopressor requirements (20.5% v 23.3%), and bacteremia (7.7% v 8.6%), were similar for patients with and without cancer, respectively. Venous thromboembolic events were slightly higher in the cancer group (11.1%) compared with the noncancer group (8.6%).
COVID-19 Outcomes
As of June 20, 2020, similar proportions of patients with and without cancer with COVID-19 had died. We observed 29 deaths (24.8%) among hospitalized patients with cancer compared with 100 deaths (21.4%; P = .894) among patients without cancer. There was no difference in death or composite outcome (death, intubation, or ICU admission) among patients with or without cancer (Figs 1A and 1B). In addition, there were no differences in composite outcome between hematologic and solid malignancies in terms of ICU admissions, intubation, or death (P = .283). Furthermore, there was no difference in outcome if patients were treated with cytotoxic therapy within 90 days of admission (P = .446). Kaplan-Meier plots (Figs 2A and 2B) support these conclusions. We found no differences in mortality or composite outcome on the basis of patient hospital location (Appendix Figs A1A and A1B, online only), suggesting a similar quality of care at both institutions.
FIG 1.
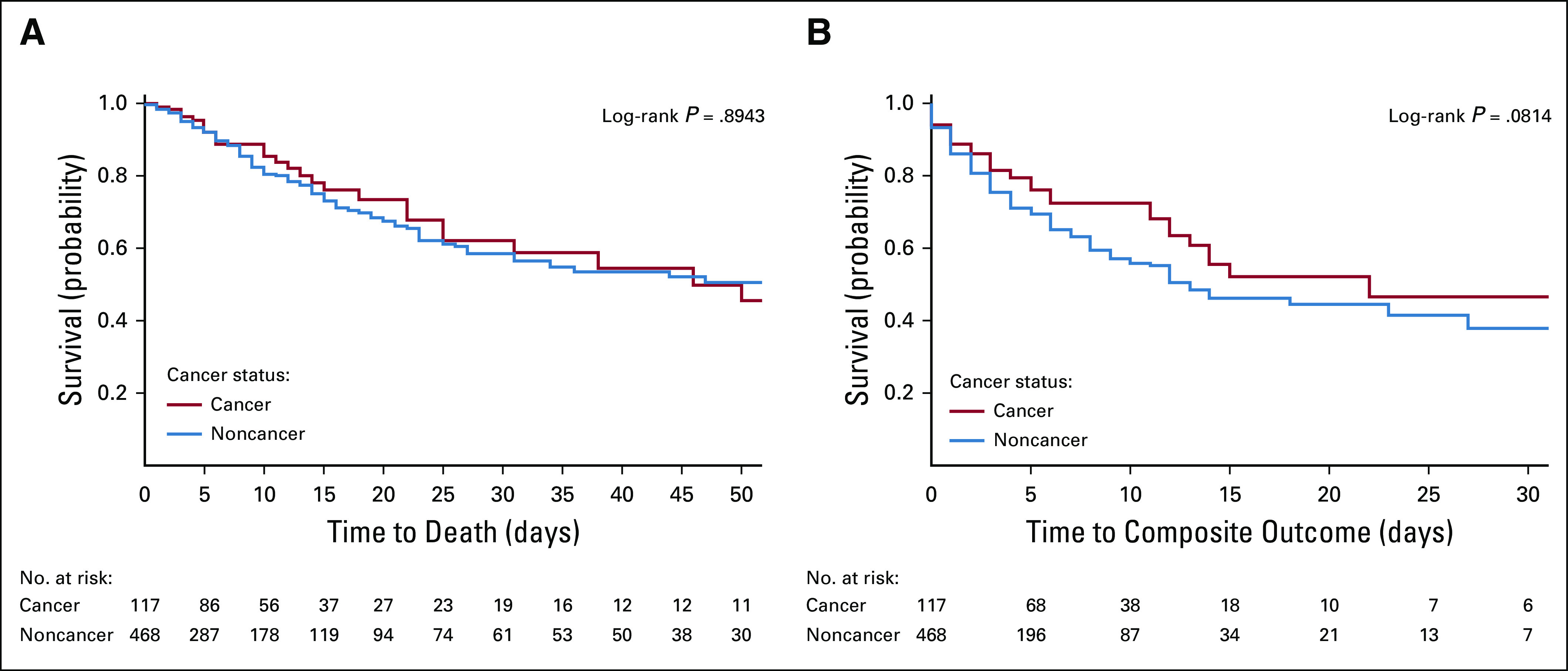
(A) Mortality between cancer and noncancer groups. (B) Composite outcome (death, intubation, or intensive care unit admission) between cancer and noncancer groups.
FIG 2.
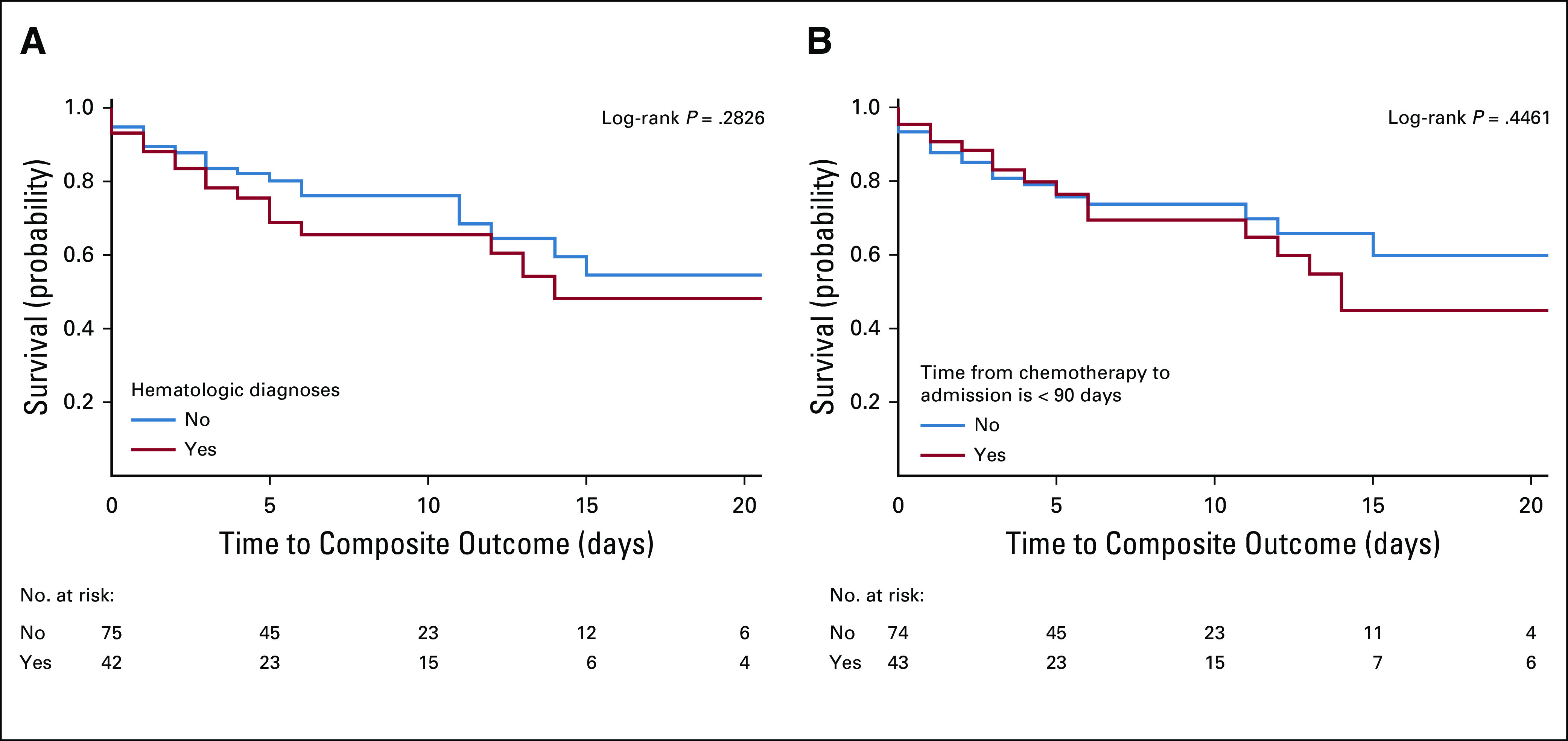
(A) Composite outcome (death, intubation, or intensive care unit ([ICU] admission) between hematologic and solid malignancies groups. (B) Composite outcome (death, intubation, or ICU admission) between patients who did and did not receive chemotherapy within 90 days of admission.
Predictors of Morbidity and Mortality
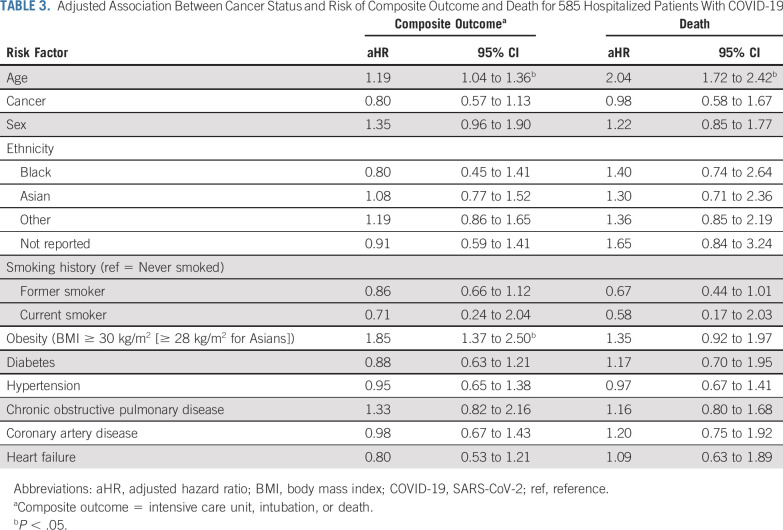
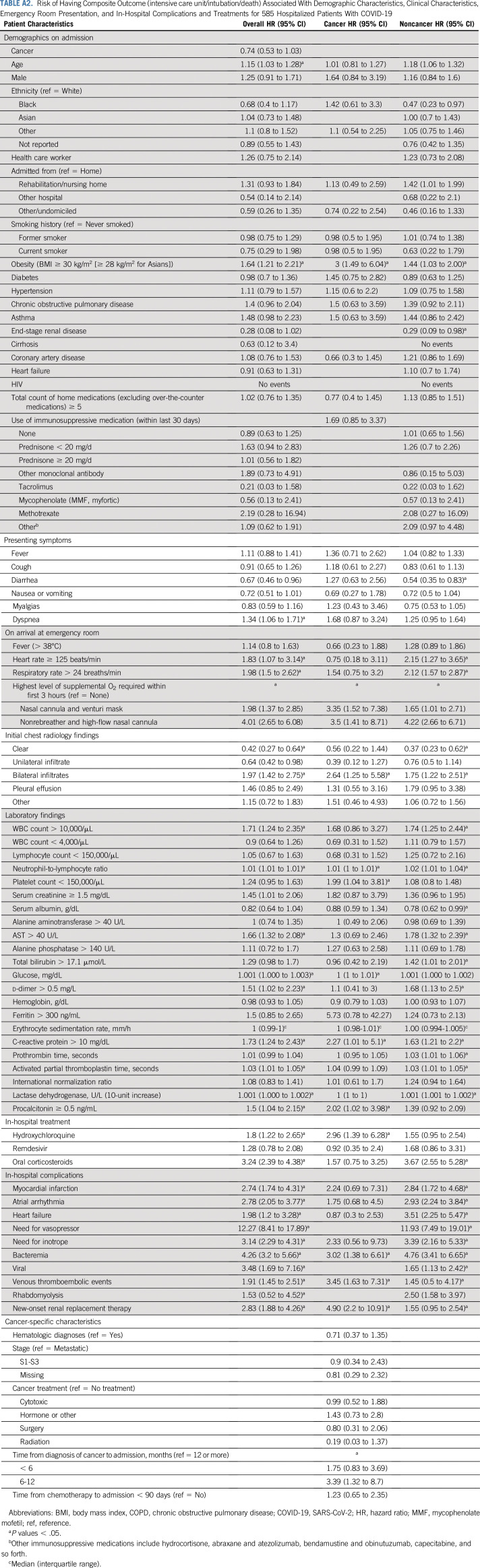
Unadjusted predictors of morbidity and mortality over the entire study cohort are listed in Appendix Table A2 (online only) The clinical factors that are associated with worse outcome include age (HR, 1.15 [95% CI, 1.03 to 1.28]) and obesity (HR, 1.64 [95% CI, 1.21 to 2.21]). Patients who presented with dyspnea (HR, 1.34 [95% CI, 1.06 to 1.71]), tachycardia (HR, 1.83 [95% CI, 1.07 to 3.14]), tachypnea (HR, 1.98 [95% CI, 1.5 to 2.62]), and bilateral lung infiltrates (HR, 1.97 [95% CI, 1.42 to 2.75]) were also predicted to have worse outcomes. The results for the cancer cohort and the noncancer controls are listed in Appendix Table A2. In patients without cancer, elevated AST (HR, 1.78 [95% CI, 1.32 to 2.39]), hyperbilirubinemia (HR, 1.42 [95% CI, 1.01 to 2.01]), and d-dimer elevation (HR, 1.68 [95% CI, 1.13 to 2.5]) predicted worse outcomes compared with patients with cancer. Cytotoxic treatment (HR, 0.99 [95% CI, 0.52 to 1.88]) or treatment within 90 days of admission (HR, 1.23 [95% CI, 0.65 to 2.35]) were not associated with worse outcomes. In a multivariable marginal Cox model, age continued to be a predictor of both composite outcome (HR, 1.19 [95% CI, 1.04 to 1.36]) and death (HR, 2.05 [95% CI, 1.72 to 2.42]), and obesity was significant only for the composite outcome (HR, 1.85 [95% CI, 1.37 to 2.50]; Table 3).
TABLE 3.
Adjusted Association Between Cancer Status and Risk of Composite Outcome and Death for 585 Hospitalized Patients With COVID-19
DISCUSSION
We demonstrate that COVID-19 hospitalized patients with active malignancies have comparable morbidity and mortality to COVID-19–infected hospitalized patients without cancer. In contrast to previous findings, we observed no differences in the risk of ICU admission, intubation, or death between patients with and without cancer. Our findings suggest that active malignancy may not be a contributive risk factor for the unfavorable prognosis of COVID-19 in patients with cancer.
By matching for age, sex, and number of comorbidities, we evaluated the impact of COVID-19 in patients with and without cancer who had a similar comorbidity burden. We found that age was a significant independent predictor of both the composite outcome and mortality in patients with COVID-19. Similarly, the case fatality rate of COVID-19 was believed to be higher in Italy than China because of the older Italian population.25 In contrast to this study, in which 75% of patients had two or more comorbidities,25 the majority of our patients had limited comorbid conditions (75% had one or fewer). This may explain why other medical conditions were not independently associated with a worse composite outcome or death.
Obesity, which accounted for 25% of our patient population, was found to be independently associated with an increased risk of composite outcome and death in both unadjusted and adjusted models. The prevalence of obesity in the United States is rising, and its impact in the context of COVID-19 specifically has not been well described. In France, a small study of 124 patients with COVID-19 found a strong correlation between higher body mass index (BMI) and need for invasive mechanical ventilation as a marker for disease severity.27 In NYC, a BMI of > 25 kg/m2 was associated with a risk of hospital admission with COVID-19, but only a BMI > 40 kg/m2 predicted critical illness, which accounted for 6% of the population studied.28 Obesity is a well-known pro-inflammatory condition, and immune dysregulation may be a reason why obesity is linked with poor outcomes.29-31
In addition, a difference in demographics, including ethnicity and socioeconomic status, as well as discrepancies in health care system practices, may account for the reduced mortality we observed in our hospitalized patients with cancer when compared with others.13 Although the majority of our population identified as White, there was no association between ethnicity and outcome when adjusted for other factors. Other confounding factors, such as smoking, which may adversely affect outcomes from COVID-1911,32 and was not accounted for in previous studies,13 may further explain the differences in patient outcome. In our study, we did not find any statistical significance in patients who were current smokers (HR, 0.75 [95% CI, 0.29 to 1.98]); however, this likely reflects a small subset of patients who were active smokers when analyzed.
Currently, there are limited published data regarding presenting symptoms and hospital complications rates in patients with COVID-19 and cancer. In our matched cohort, presenting symptoms and serious hospital complication rates in patients with and without cancer were similar, including vasopressor requirements, bacteremia and venous thromboembolism. Tachycardia, dyspnea, and bilateral infiltrates were all associated with an increased risk of composite outcome. We observed a lower incidence of infection in both groups, which may have contributed to improved outcomes compared with other data sets.11
Although previous reports have suggested that COVID-19–positive patients with hematologic malignancies have worse outcomes than do patients with solid tumors,11,13 our data showed no differences between the two groups. A predominance of chronic lymphocytic leukemia on active treatment within our hematologic population could account for the improved outcomes. A recent publication characterized protective anti-inflammatory benefits of Bruton’s tyrosine kinase inhibitors in patients with hematologic malignancy and COVID-19 infection.33 Furthermore, we did not observe differences in ICU admission, intubation, or death between patients with cancer who received cytotoxic chemotherapy or immunosuppressive therapy within 3 months of hospitalization versus more than 3 months, consistent with previous data.13,34
Our study has some limitations. The observational nature of this study does not allow us to determine causality. Although sizable, our cancer cohort was heterogeneous and limited to hospitalized patients, thus reducing generalizability to patients with cancer in the outpatient setting. We did not account for socioeconomic status or differences in health care systems that may explain the variations in our results compared with others. Although it is not clear how these imbalances could affect the interpretation of our results, we acknowledge that these imbalances could bias our data and must be considered. Finally, given the long length of study of many hospitalized patients with COVID-19, some outcomes may not have been observed at the date of analysis.
In summary, we showed that hospitalized patients with COVID-19 and cancer have a similar presentation and have similar rates of complications and death compared with patients without cancer. COVID-19–infected patients with cancer were reported initially to have worse outcomes; however, these studies were not controlled for comorbidities15,35,36 that have been shown to significantly influence outcomes.2,12,15 Our findings suggest that older age is the most contributive risk factor for poor outcomes in patients with COVID-19 and that a diagnosis of cancer itself, or its treatment, may not influence severe outcomes.
Our data have important implications about the impact of COVID-19 on patients with cancer. We should consider the consequences of limiting care for patients with cancer on cancer-specific outcomes and mortality in the context of COVID-19. During the COVID-19 pandemic, we may be able to deliver anticancer care safely to patients who are younger with limited comorbidities; however, it is important to balance the risks and benefits of safely delivering cancer-directed therapy. We may consider a more conservative approach in older patients with metastatic cancer and multiple comorbidities, for whom the efficacy of continued lines of therapy may be limited. Conversely, we would consider cautious continuation of therapy in patients with fewer comorbidities and in whom therapy is associated with significant benefit. Our findings do not mitigate the importance of the continued screening and heightened vigilance that will be required to deliver safe quality care to our patients.
ACKNOWLEDGMENT
We thank the following Weill Cornell Medicine medical students for their contributions to the COVID-19 Registry through medical chart abstraction: Zara Adamou, BA; Haneen Aljayyousi, BA; Mark N. Alshak, BA (student leader); Bryan K. Ang, BA; Elena Beideck, BS; Orrin S. Belden, BS; Sharmi Biswas, MD; Anthony F. Blackburn, BS; Joshua W. Bliss, PharmD; Kimberly A. Bogardus, BA; Chelsea D. Boydstun, BA; Clare A. Burchenal, MPH; Eric T. Caliendo, BS; John K. Chae, BA; David L. Chang, BS; Frank R. Chen, BS; Kenny Chen, BA; Andrew Cho, PhD; Alice Chung, BA; Alisha N. Dua, MRes; Andrew Eidelberg, BS; Rahmi S. Elahjji, BA; Mahmoud Eljaby, MMS; Emily R. Eruysal, BS; Kimberly N. Forlenza, MS; Rana Khan Fowlkes, BA; Rachel L. Friedlander, BA; Gary George, BS; Shannon Glynn, BS; Leora Haber, BA; Janice Havasy, BS; Alex Huang, BA; Hao Huang, BS; Jennifer H. Huang, BS; Sonia Iosim, BS; Mitali Kini, BS; Rohini V. Kopparam, BS; Jerry Y. Lee, BA; Mark Lee, BS, BA; Aretina K. Leung, BA; Han A. Li, BA (student leader); Bethina Liu, AB; Charalambia Louka, BS; Brienne Lubor, BS; Dianne Lumaquin, BS; Matthew L. Magruder, BA; Ruth Moges, MS; Prithvi M. Mohan, BS; Max F. Morin, BS; Sophie Mou, BA; J. J. Nario, BS; Yuna Oh, BS; Noah Rossen, BA; Emma M. Schatoff, PhD; Pooja D. Shah, BA; Sachin P. Shah, BA; Daniel Skaf, BS; Shoran Tamura, BS; Ahmed Toure, BA; Camila M. Villasante, BA; Gal Wald, BA; Graham T. Wehmeyer, BS (student leader); Samuel Williams, BA; Ashley Wu, BS; Andrew L. Yin, BA; and Lisa Zhang, BA.
Appendix
FIG A1.
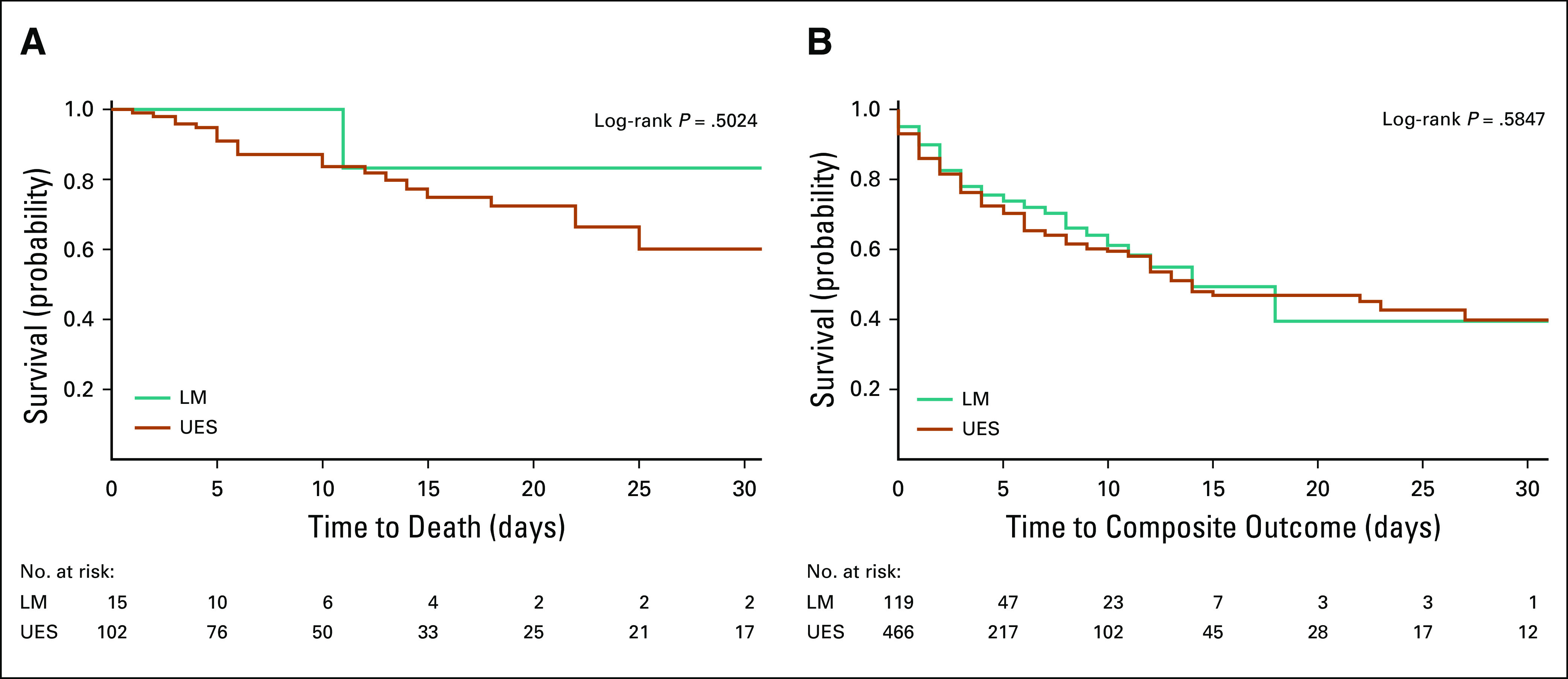
(A) Mortality by hospital location. (B) Composite outcome (death, intubation, or intensive care unit admission) by hospital location. LM, New York/Presbyterian -Lower Manhattan Hospital; UES, New York/Presbyterian-Upper East Side Hospital.
TABLE A1.
Characteristics of 585 Hospitalized Patients With COVID-19: In-Hospital Complications and Treatments, Overall and by Cancer Status
TABLE A2.
Risk of Having Composite Outcome (intensive care unit/intubation/death) Associated With Demographic Characteristics, Clinical Characteristics, Emergency Room Presentation, and In-Hospital Complications and Treatments for 585 Hospitalized Patients With COVID-19
SUPPORT
Supported by New York–Presbyterian Hospital and Weill Cornell Medical College, including the Clinical and Translational Science Center (UL1 TR000457) and the Joint Clinical Trials Office.
AUTHOR CONTRIBUTIONS
Conception and design: Gagandeep Brar, Laura C. Pinheiro, Michael Shusterman, Brandon Swed, Doru Paul, Manuel Hidalgo, Manish A. Shah
Financial support: Manuel Hidalgo, Manish A. Shah
Administrative support: Frank Chen, Manish A. Shah
Provision of study material or patients: Frank Chen, Manish A. Shah
Collection and assembly of data: Gagandeep Brar, Michael Shusterman, Brandon Swed, Frank Chen, Samuel Yamshon, John Vaughn, Manish A. Shah
Data analysis and interpretation: Gagandeep Brar, Laura C. Pinheiro, Michael Shusterman, Evgeniya Reshetnyak, Orysya Soroka, Frank Chen, Peter Martin, Doru Paul, Manuel Hidalgo, Manish A. Shah
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
COVID-19 Severity and Outcomes in Patients With Cancer: A Matched Cohort Study
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Laura C. Pinheiro
Employment: Johnson and Johnson (I)
Peter Martin
Consulting or Advisory Role: Celgene, Janssen, Bayer, Kite Pharma, BeiGene, IMAB, MorphoSys, Sandoz, TeneoBio, Karyopharm, Kite/Gilead, Verastem, Cellectar, Regeneron
Research Funding: Karyopharm (Inst)
Travel, Accommodations, Expenses: Janssen
Manuel Hidalgo
Stock and Other Ownership Interests: Champions Oncology, Pharmacyte Biotech, BioOncotech, Nelum, Agenus
Honoraria: Agenus, Pharmacyte Biotech, InxMed, Takeda, Oncomatrix, Sumitomo Group
Consulting or Advisory Role: Pharmacyte Biotech, Agenus, Oncomatrix, Bayer, Takeda
Research Funding: BiolineRx, Erytech
Patents, Royalties, Other Intellectual Property: Royalties from Myriad for PALB2 patent
Expert Testimony: Myriad Genetics
Travel, Accommodations, Expenses: BiolineRx
Manish A. Shah
Consulting or Advisory Role: Astellas Pharma, Lilly Japan
Research Funding: Merck (Inst), Oncolys BioPharma (Inst), Bristol Myers Squibb (Inst)
No other potential conflicts of interest were reported.
REFERENCES
- 1.Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goyal P, Choi JJ, Pinheiro LC, et al. Clinical characteristics of Covid-19 in New York City. N Engl J Med. 2020;382:2372–2374. doi: 10.1056/NEJMc2010419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study Lancet 3951054–1062.2020[Erratum: Lancet 395:1038, 2020] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jazieh AR, Alenazi TH, Alhejazi A, et al. Outcome of oncology patients infected with coronavirus. JCO Glob Oncol. 2020;6:471–475. doi: 10.1200/GO.20.00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. doi: 10.1001/jamaoncol.2020.0980. Yu J, Ouyang W, Chua MLK, et al: SARS-CoV-2 transmission in patients with cancer at a tertiary care hospital in Wuhan, China. JAMA Oncol 6:1108-1110, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liang W, Guan W, Chen R, et al. Cancer patients in SARS-CoV-2 infection: A nationwide analysis in China. Lancet Oncol. 2020;21:335–337. doi: 10.1016/S1470-2045(20)30096-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. doi: 10.1016/j.annonc.2020.03.296. Zhang L, Zhu F, Xie L, et al: Clinical characteristics of COVID-19-infected cancer patients: A retrospective case study in three hospitals within Wuhan, China. Ann Oncol 31:894-901, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sidaway P. COVID-19 and cancer: What we know so far. Nat Rev Clin Oncol. 2020;17:336. doi: 10.1038/s41571-020-0366-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deng G, Yin M, Chen X, et al. Clinical determinants for fatality of 44,672 patients with COVID-19. Crit Care. 2020;24:179. doi: 10.1186/s13054-020-02902-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.He W, Chen L, Chen L, et al. COVID-19 in persons with haematological cancers. Leukemia. 2020;34:1637–1645. doi: 10.1038/s41375-020-0836-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dai M, Liu D, Liu M, et al. Patients with cancer appear more vulnerable to SARS-COV-2: A multicenter study during the COVID-19 outbreak. Cancer Discov. 2020;10:783–791. doi: 10.1158/2159-8290.CD-20-0422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area JAMA 3232052.2020[Erratum: doi: 10.1001/jama.2020.7681] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mehta V, Goel S, Kabarriti R, et al. Case fatality rate of cancer patients with COVID-19 in a New York hospital system. Cancer Discov. 2020;10:935–941. doi: 10.1158/2159-8290.CD-20-0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miyashita H, Mikami T, Chopra N, et al. Do patients with cancer have a poorer prognosis of COVID-19? An experience in New York City. Ann Oncol. 2020;31:1088–1089. doi: 10.1016/j.annonc.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mehra MR, Desai SS, Kuy S, et al. Retraction: Cardiovascular disease, drug therapy, and mortality in Covid-19. N Engl J Med. 2020;382:2582. doi: 10.1056/NEJMoa2007621. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 16.Robinson AG, Gyawali B, Evans G. COVID-19 and cancer: Do we really know what we think we know? Nat Rev Clin Oncol. 2020;17:386–388. doi: 10.1038/s41571-020-0394-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lewis MA. Between Scylla and Charybdis - Oncologic decision making in the time of Covid-19. N Engl J Med. 2020;382:2285–2287. doi: 10.1056/NEJMp2006588. [DOI] [PubMed] [Google Scholar]
- 18.Cannistra SA, Haffty BG, Ballman K. Challenges faced by medical journals during the COVID-19 pandemic. J Clin Oncol. 2020;38:2206–2207. doi: 10.1200/JCO.20.00858. [DOI] [PubMed] [Google Scholar]
- 19.ASCO COVID-19 provider & practice informationhttps://www.asco.org/asco-coronavirus-information/provider-practice-preparedness-covid-19
- 20.0 Lou E, Beg S, Bergsland E, et al: Modifying practices in GI oncology in the face of COVID-19: Recommendations from expert oncologists on minimizing patient risk. JCO Oncol Pract 16:383-388, 202. [Google Scholar]
- 21.Shah MA, Emlen MF, Shore T, Mayer S, Leonard JP, Rossi A, et al. Hematology and oncology clinical care during the coronavirus disease 2019 pandemic. CA Cancer J Clin. 2020 doi: 10.3322/caac.21627. [epub ahead of print] July 14, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sholle ET, Kabariti J, Johnson SB, et al. Secondary Use of Patients’ Electronic Records (SUPER): An approach for meeting specific data needs of clinical and translational researchers. AMIA Annu Symp Proc. 2018;2017:1581–1588. [PMC free article] [PubMed] [Google Scholar]
- 23.Lee E.W., Wei L.J., Amato D.A., Leurgans S. Cox-Type Regression Analysis for Large Numbers of Small Groups of Correlated Failure Time Observations. In: Klein J.P., Goel P.K., editors. Survival Analysis: State of the Art. Nato Science (Series E: Applied Sciences) vol 211. Springer; Dordrecht: (1992). pp. p237–247.https://doi.org/10.1007/978-94-015-7983-4_14 [Google Scholar]
- 24.Lin DY. Cox regression analysis of multivariate failure time data: The marginal approach. Stat Med. 1994;13:2233–2247. doi: 10.1002/sim.4780132105. [DOI] [PubMed] [Google Scholar]
- 25.Onder G, Rezza G, Brusaferro S. Case-fatality rate and characteristics of ptients dying in relation to COVID-19 in Italy. JAMA. 2020;323:1775–1776. doi: 10.1001/jama.2020.4683. [DOI] [PubMed] [Google Scholar]
- 26. Reference deleted.
- 27.Simonnet A, Chetboun M, Poissy J, et al. High prevalence of obesity in severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) requiring invasive mechanical ventilation. Obesity (Silver Spring) 2020;28:1195–1199. doi: 10.1002/oby.22831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Petrilli CM, Jones SA, Yang J, et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: Prospective cohort study. BMJ. 2020;369:m1966. doi: 10.1136/bmj.m1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sattar N, McInnes IB, McMurray JJV. Obesity is a risk factor for severe COVID-19 infection: Multiple potential mechanisms. Circulation. 2020;142:4–6. doi: 10.1161/CIRCULATIONAHA.120.047659. [DOI] [PubMed] [Google Scholar]
- 30.Schmidt FM, Weschenfelder J, Sander C, et al. Inflammatory cytokines in general and central obesity and modulating effects of physical activity. PLoS One. 2015;10:e0121971. doi: 10.1371/journal.pone.0121971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Caër C, Rouault C, Le Roy T, et al. Immune cell-derived cytokines contribute to obesity-related inflammation, fibrogenesis and metabolic deregulation in human adipose tissue. Sci Rep. 2017;7:3000. doi: 10.1038/s41598-017-02660-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu W, Tao ZW, Wang L, et al. Analysis of factors associated with disease outcomes in hospitalized patients with 2019 novel coronavirus disease. Chin Med J (Engl) 2020;133:1032–1038. doi: 10.1097/CM9.0000000000000775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Treon SP, Castillo JJ, Skarbnik AP, et al. The BTK inhibitor ibrutinib may protect against pulmonary injury in COVID-19-infected patients. Blood. 2020;135:1912–1915. doi: 10.1182/blood.2020006288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Luo J, Rizvi H, Egger JV, et al. Impact of PD-1 blockade on severity of COVID-19 in patients with lung cancers. Cancer Discov. 2020;10:1121–1128. doi: 10.1158/2159-8290.CD-20-0596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang B, Li R, Lu Z, et al. Does comorbidity increase the risk of patients with COVID-19: Evidence from meta-analysis. Aging (Albany NY) 2020;12:6049–6057. doi: 10.18632/aging.103000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang J, Zheng Y, Gou X, et al. Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: A systematic review and meta-analysis. Int J Infect Dis. 2020;94:91–95. doi: 10.1016/j.ijid.2020.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]