Abstract
Research links the built environment to health outcomes, but little is known about how this affects quality of life (QOL) of African American breast cancer patients, especially those residing in disadvantaged neighborhoods. Using latent trajectory models, we examined whether the built environment using Google Street View was associated with changes in QOL over a 2-year follow-up in 228 newly diagnosed African American breast cancer patients. We measured QOL using the RAND 36-Item Health Survey subscales. After adjusting for covariates, improvement in emotional well-being and pain over time was greater for women living on streets with low-quality (vs. high-quality) sidewalks.
Keywords: breast neoplasm, residence characteristics, health-related quality of life, Geographic Information System, African Americans
Introduction
Despite the fact that health, disease, and quality of life (QOL) occur in physical and social contexts, research has often been characterized by explaining health-related outcomes exclusively in terms of individual-level, personal characteristics.(Diez-Roux, 2000) Research links neighborhood conditions (e.g., the social and built environment) to health and well-being,(Renalds et al., 2010) but little is known about how such neighborhood conditions affect cancer patients’ QOL, including those with breast cancer. With nearly four million breast cancer survivors expected to be living in the United States by 2024(American Cancer Society, 2014) and the growing number of breast cancer survivors due to improvements in early detection and breast cancer treatment,(Brinton et al., 2008; Howlader et al.; National Cancer Institute; National Cancer Institute; President’s Cancer Panel et al.; Schwartz et al., 2000) increasing attention has focused on breast cancer patients’ health-related QOL and its survival implications.(Epplein et al., 2011) A cross-sectional study of Black and Latina breast cancer patients 1–5 years following diagnosis found that neighborhood stress was associated with poor patient self-rated health.(Wu et al., 2017) And although a recent review identified important effects of the social and built environment on breast cancer incidence, stage at diagnosis, and prognosis, studies focusing on QOL were notably absent.(Gomez et al., 2015)
Some evidence supports the general importance of neighborhood conditions, and especially the built environment, in shaping the aging experience and health trajectory of late middle-aged adults.(Aneshensel et al., 2007; Kubzansky et al., 2005 ) Late middle-age is a time of life when many are retired or considering retirement. Older adults spend more time in their immediate neighborhood than younger adults and may rely more on their neighborhood for services and amenities and on their neighbors for social contact and support.(Kubzansky et al., 2005 ) Disadvantaged neighborhoods may adversely affect older adult’s psychosocial well-being through stressors in the physical environment, lower access to economic and medical resources, and a non-cohesive social environment.(Aneshensel et al., 2007; Schulz et al., 2008) Residents of disadvantaged neighborhoods may feel suspicious and distrustful of others, and thus tend to keep to themselves, resulting in feelings of social alienation, a sense of powerlessness or loss of control, increased stress, and incident or worsening depression.(Ross, 2000) In contrast, neighborhoods that are conducive to social integration and support may provide older adults with a positive identity and a sense of purpose.(Elliott, 2000) Understanding the link between neighborhood conditions and QOL outcomes among older adults has become increasingly important,(Aneshensel, 2010; Lian et al., 2014) especially among patients who reside in disadvantaged neighborhoods.
For African Americans who reside in disadvantaged urban neighborhoods, exposure to adverse neighborhood conditions may explain some observed racial disparities in health outcomes.(Schulz et al., 2008) The disparities in breast cancer incidence and prognosis between African American and White breast cancer patients are well known,(Cianfrocca and Goldstein, 2004; Demicheli et al., 2007; Deshpande et al., 2009; Elledge et al., 1994; Joslyn and West, 2000) and African American breast cancer patients have reported lower scores on many aspects of their QOL than other racial/ethnic groups.(American Cancer Society, 2013) However, few studies have examined associations between neighborhood conditions and QOL in urban-dwelling, middle-aged and older African American adults, and no such studies have been conducted in African American breast cancer patients. This study examined how the built environment affected changes in QOL over time in newly diagnosed African American breast cancer patients.
Methods
Study participants
After Institutional Review Board approval at Washington University School of Medicine, we recruited newly diagnosed African American breast cancer patients to participate in a randomized controlled trial (RCT) of a behavioral cancer-communication intervention using a touchscreen tablet-computer (clinicaltrials.gov #: NCT00929084).(Perez et al., 2014) Patients diagnosed with ductal carcinoma in situ (stage 0) or non-metastatic, invasive breast cancers (stages I-III) were enrolled between December 2009 and December 2012. Patients were identified with the help of their treating physicians at Siteman Cancer Center at Barnes-Jewish Hospital and Washington University School of Medicine or at Saint Louis University School of Medicine, both in St. Louis, Missouri. Patients were randomized either to standard of care for their breast cancer treatment (n=120) or to the intervention arm, which received standard of care plus the tablet computer containing videos of African American breast cancer survivors relating their stories about living with breast cancer (n=108). The survivor-stories video program featured 207 video clips focusing on coping, social support, healthcare experiences, follow-up care, QOL, and treatment side effects.(Perez et al., 2014) The program featured short (1–3 minute) stories told by 35 different African American breast cancer survivors and covered 12 breast cancer topics. The touchscreen tablet-computer allowed for story selection either by topic or storyteller.(Perez et al., 2014) Patients were eligible to participate in the RCT if they were ≥ 30 years of age, spoke English, had no prior history of breast cancer, did not plan a bilateral mastectomy, and did not demonstrate cognitive impairment on the Orientation-Memory-Concentration Test, administered to women ≥ 65 years of age.(Katzman et al., 1983)
Following informed consent, patients completed five telephone or in-person interviews using a computer-assisted telephone interview system to collect demographic, clinical, psychosocial, and QOL data. The baseline interview (Interview1) was planned to occur at the time of patients’ surgical post-operative visit or start of neoadjuvant treatment, depending on the course of treatment, with follow-up interviews planned one month following baseline (Interview2) and six (Interview3), 12 (Interview4), and 24 (Interview5) months after definitive surgical treatment. The intervention was administered three times over the 2-year study: the first exposure after the baseline interview and the second and third exposures 3–4 weeks before the 6-month and 12-month interviews. Clinical data were collected by patient self-report and from the medical record.
Quality of life
Patient-reported QOL was measured using the eight subscales (emotional well-being, role limitations due to emotional problems, energy/fatigue, general health, pain, physical functioning, role limitations due to physical health, and social functioning) of the RAND 36-Item Short Form Health Survey 1.0, created at RAND for the Medical Outcomes Study.(Hays et al., 1993; Healthcare) Reliability and validity of the subscales have been established in studies of both general and patient populations,(Stewart et al., 1988; Ware and Sherbourne, 1992; Wells et al., 1989) and general population norms for this instrument are available.(McHorney et al., 1994) The eight subscales are reliable measures of QOL across different racial groups.(Ashing-Giwa et al., 2004; Hays et al., 1998) Standardized scores range from 0–100 with higher scores indicating better health perceptions.
Built environment
Home address was obtained at each of the five interviews. Participants’ residential addresses were geocoded with ArcGIS 9.3 (ESRI, Redlands, CA, USA). This geocoding allowed us to identify the street segment of each patient’s residence for auditing the built environment. A street segment was defined as the section of the road between two consecutive intersections. Segments may vary by type of neighborhood.
A specially trained study team member (JCS) used each participant’s residential street address to complete the Active Neighborhood Checklist measuring neighborhood conditions through Google Street View (GSV) to assess the built environment. GSV has been found to be reliable and valid relative to the “gold standard” of in-person audits in urban areas using the Active Neighborhood Checklist.(Kelly et al., 2013b; Mooney et al., 2014; Wilson et al., 2012; Wu et al., 2014) The checklist assesses land-use characteristics (predominant land use, land-use mix, parking and recreational facilities); sidewalks (sidewalk presence/absence, buffers, continuity, width, curb cuts, misalignments and obstructions); shoulders and bike lanes (shoulder presence/absence, width, continuity, designated bike signs and obstructions); street characteristics (transit stops, number of lanes, crossing aids, traffic-calming devices, speed limit signs); and quality of the environment for pedestrians (building setback, pedestrian amenities, litter, shade trees, and hills). We selected the date of the GSV that was closest to and before the date of a participant’s baseline interview. Validity of historic and more recent GSV imagery were similar compared to in-person field audits.(Kelly et al., 2013b, a) Advantages of GSV include efficiency, researcher safety, low cost, unobtrusive data collection, and access to historical images of the same streets.
We examined five characteristics of the built environment of the street segment on which a patient resided. Mixed housing was present when a combination of single-family homes, multi-unit homes, or apartments was observed. Otherwise, the participant did not live on a street segment with mixed housing. We also assessed whether an abandoned building, home, or vacant lot was present or not present. A mixed-use variable was created to indicate whether or not a non-residential destination was present or not on the street segment; non-residential destinations included a grocery/convenience store, supermarket, food establishment, entertainment, library, post office, bank, dry cleaner, indoor fitness facility, park, off road walking trail, sports/playing field, basketball/tennis/volleyball court, playground, outdoor pool, park with exercise or playground facilities, and designated green space. We also observed whether or not graffiti, broken/boarded windows, litter, or broken glass were present. Sidewalk quality was based on the presence of nine characteristics of high-quality sidewalks (coded as 1 [yes] or 0 [no]), including the presence of a buffer between the curb and sidewalk, sidewalk continuity within street segment, sidewalk continuity between street segments, width of sidewalks >3 feet, no missing ramps or curb cuts, no major bumps/cracks/holes, no permanent obstructions, tree shade, and flat/gentle slope. Having no sidewalk was coded as 0. A dichotomous variable was created, with 0 indicating poor quality or no sidewalk and 1 indicating high quality sidewalks on one or more of the nine characteristics.
Potential confounders
Potential confounders consisted of variables previously associated with QOL and/or built environment, but that may not be in the causal pathway. They included individual-level sociodemographic characteristics, clinical and treatment-related variables, psychosocial factors, and neighborhood-level data from the participants’ census tracts. We also included the treatment arm to which patients were assigned in the latent growth curve models, since the study participants were part of a RCT designed to increase patients’ QOL.
Patient sociodemographics included age, marital status (married or not married), education based on the number of years (≤12 years or >12 years), employment status (employed at least part-time, homemaker/retired, unable to work/unemployed), and annual household income (<$25,000, ≥$25,000 but <$75,000, ≥$75,000, or don’t know/refused to answer). We also asked for participants’ residential address to determine whether participants moved after baseline.
Clinical and treatment-related variables included type of surgery (breast-conserving surgery or mastectomy), with or without radiation and/or chemotherapy in either the neoadjuvant or adjuvant setting. Therefore, a categorical variable was created for the extent of treatment received (surgery alone, surgery plus chemotherapy, surgery plus radiation, surgery plus radiation and chemotherapy). Cancer stage was determined by clinical staging in patients with locally advanced disease and by surgical pathology in patients with early-stage disease. Patients’ smoking history (current smoker, former smoker, never smoked), body mass index (BMI) computed in kg/m2 using patients’ height and weight collected by interview, and comorbidity, each measured at baseline, also were used. We used Katz’s validated interview adaptation of the Charlson comorbidity index(Charlson et al., 1987; Katz et al., 1996) to measure patients’ history and presence of comorbidities; each condition is weighted with higher scores indicating greater comorbidity severity.
Psychosocial factors included social support and depressive symptoms. Perceived availability of social support was assessed using the Medical Outcomes Study (MOS) Social Support Survey,(Sherbourne and Stewart, 1991) which is associated with QOL in breast cancer patients.(Jeffe et al., 2012) The instrument includes 19 items using 5-point, Likert-scaled response options, with higher scores indicating greater perceived availability of social support.(Corporation;, 2014) Among patients with chronic conditions, this measure has high discriminant and convergent validity.(Sherbourne and Stewart, 1991) Depressive symptoms were measured using the Center for Epidemiologic Studies Depression Scale (CES-D).(Radloff, 1977) The CES-D has good construct and concurrent validity and has demonstrated reliability in various populations.(Radloff, 1977; Radloff and Teri, 1986) Scores on the CES-D range from zero to 60, with higher scores indicating more severe depressed mood. For analysis, we compared patients with and without elevated depressed mood (≥16 vs. <16).(Radloff, 1977)
Neighborhood conditions Population characteristics at the census-tract level were obtained from the 2008–2012 American Community Survey and consisted of economic conditions (percentage of the population that is below the federal poverty rate, percentage of houses that are owner occupied, percentage of vacant housing units), racial segregation (percentage of African Americans), and land use (number of housing units per square mile).
Statistical analysis
Descriptive analyses were conducted to summarize each variable using SAS 9.4 (SAS Institute Inc., Cary, NC, USA). A latent trajectory model, (also called “growth curve model”), was used to examine the change in each QOL subscale over time and factors that explained any observed change in our African American breast cancer patient participants.(Curran and Hussong, 2003) All models were parameterized such that the intercepts indicated the average baseline levels of QOL subscales and the slopes denoted the trajectories of change in QOL subscales from the baseline to 24 months following definitive surgical treatment. Latent trajectory models were conducted using Mplus Version 7.1.(Muthén and Muthén, 1998–2012) Maximum likelihood method was used to accommodate non-normality and missing data. First, we estimated the relationship between the unadjusted slope and intercept of each QOL subscale. Second, we determined whether built-environment characteristics of the street segment of each patient’s residence predicted the slope and intercept of each QOL. Third, we examined whether significant built-environment variables remained significant predictors of the slope and intercept of each QOL subscale after adjusting for individual-level variables and neighborhood conditions at the census tract. Individual-level and neighborhood-level variables that did not predict either intercept or slope of QOL were not included in the final models. The Z test was used to test whether each parameter estimate was significantly different from zero at the 0.05 level of significance.
Results
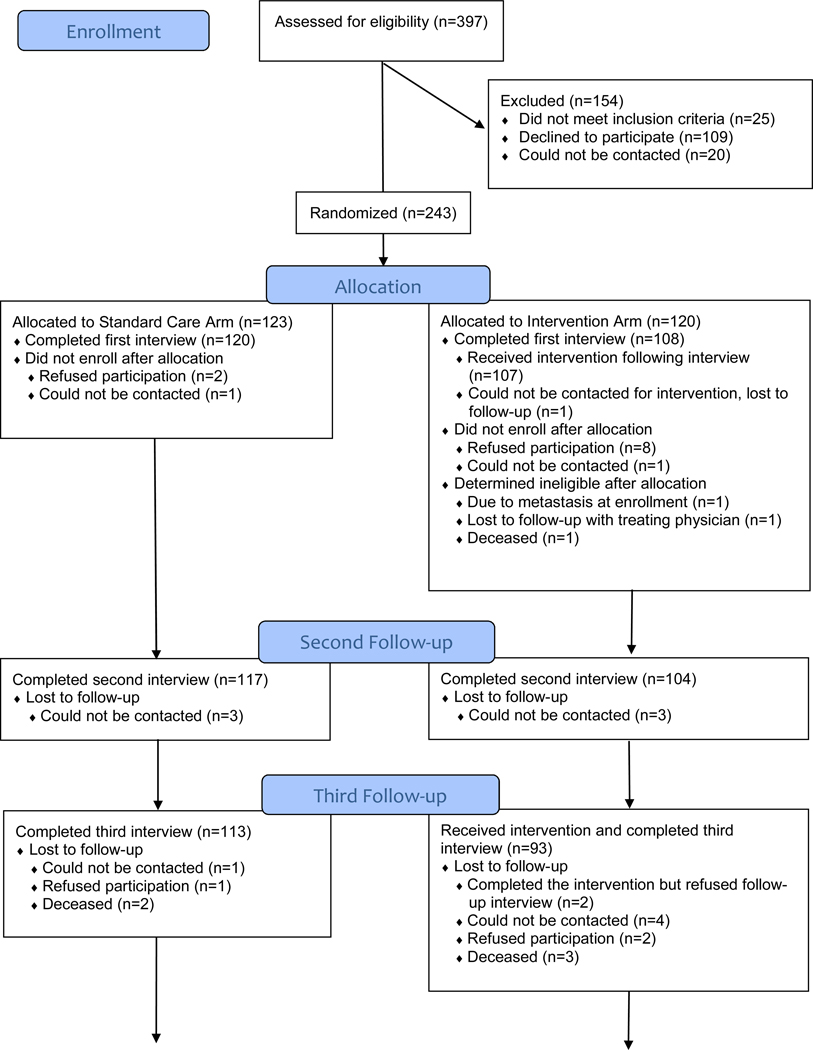
Of 397 breast cancer patients assessed for eligibility, 25 patients were deemed ineligible before randomization and three more were found to be ineligible after randomization (Figure 1). Of 369 eligible patients, 243 consented to participate and were randomized. Of these 243 patients, 228 completed the baseline interview (Interview1). Participants and nonparticipants did not differ significantly by marital status or insurance type (each p > 0.10), but participants were younger than non-participants (mean age = 55.9 vs. 62.4; p < 0.001). Retention was high, with 193 patients (85% of 228 enrolled) completing all five interviews. Patients completed the baseline interview within a mean 5.7 days (standard deviation [SD] = 13.2) of their surgical post-operative visit or start of neoadjuvant therapy, with the second interview (Interview2) a mean 32 (SD = 10.1) days after baseline. The remaining follow-up interviews were completed a mean 6.5 (SD = 0.8; Interview3), 12.7 (SD = 0.8; Interview4), and 24.5 (SD = 0.6; Interview5) months following definitive surgical treatment.
Figure 1.
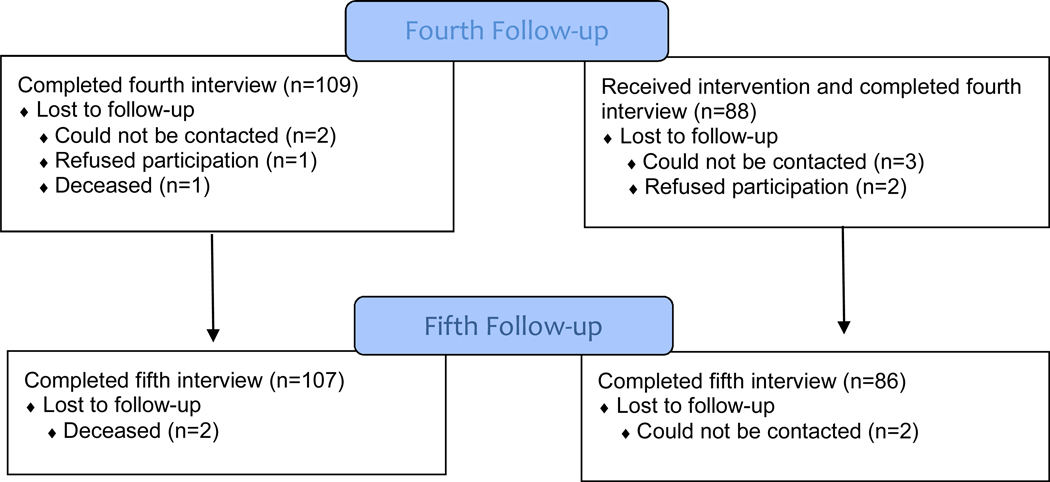
CONSORT Flow Diagram between December 2009 and January 2015.
One patient randomized to the intervention arm did not receive the intervention and was lost to follow-up after Interview1. Additionally, data about the built environment could not be collected for 12 patients because GSV data was not collected by Google, thus, baseline descriptive statistics and built-environment data shown in Table 1 are based on 215 patients. There were no significant differences in the clinical, demographic, and psychosocial variables of interest as shown in Table 1 between the 215 patients included in this secondary analysis and the 12 patients whose built-environment data could not be collected using GSV. However, a greater percentage of patients included in our analysis lived in neighborhoods that had a greater percentage of Non-Hispanic African Americans (65.5% vs. 39.8%; p < 0.01) and of residents living below the poverty level (27.2% vs. 15.9%; p = 0.01) compared to the 12 patients for whom we could not collect GSV data. We found no evidence of selection bias based on the year when the GSV imagery was obtained for the study population or on the percentage of the population living below the federal poverty level.
Table 1.
Descriptive characteristics at baseline of 215 African American study participants.
| Measure | Estimates |
|---|---|
| Quality of life a | |
| RAND 36-Item Short Form Health Survey, mean (SD) | |
| Physical functioning | 64.4 (27.6) |
| Role limitations due to physical health | 26.0 (34.5) |
| Role limitations due to emotional problems | 58.4 (43.5) |
| Energy/fatigue | 52.2 (24.5) |
| Emotional well-being | 72.9 (22.8) |
| Social functioning | 70.0 (28.8) |
| Pain | 58.2 (27.2) |
| General health | 59.7 (22.4) |
| Potential confounders | |
| Age (years), mean (SD) | 56.1 (10.0) |
| Perceived Social Support, mean (SD) | 4.3 (0.8) |
| BMI,b mean (SD) | 32.5 (8.1) |
| Comorbidity, mean (SD) | 1.0 (1.4) |
| Study Arm, n (%) | |
| Standard Care | 111 (51.6) |
| Intervention | 104 (48.4) |
| Marital status, n (%) | |
| Married | 58 (27.0) |
| Not married | 157 (73.0) |
| Household income, n (%) | |
| Less than $25,000 | 125 (58.1) |
| $25,000 to <$75,000 | 68 (31.6) |
| $75,000 or more | 17 (7.9) |
| Refused | 5 (2.3) |
| Employment, n (%) | |
| Employed | 93 (43.3) |
| Homemaker/retired | 41 (19.1) |
| Unable to work | 58 (27.0) |
| Unemployed | 23 (10.7) |
| Education, n (%) | |
| ≤ Grade 12/GED | 102 (47.4) |
| > Greater than Grade 12 | 113 (52.6) |
| Smoking Status, n (%) | |
| Nonsmoker | 105 (48.8) |
| Former Smoker | 54 (25.1) |
| Current Smoker | 56 (26.0) |
| Elevated Depressed Mood, n (%) | |
| Yes | 65 (30.2) |
| No | 150 (69.8) |
| Cancer Stage, n (%) | |
| DCIS (Stage 0) | 48 (22.3) |
| EIBC (Stage I, IIA) | 107 (49.8) |
| Late-stage (IIB, III) | 60 (27.9) |
| Type of surgery,c n (%) | |
| Breast-conserving surgery | 148 (69.8) |
| Mastectomy | 64 (30.2) |
| Built-environment characteristics | |
| Mixed residential and non-residential land used | 52 (24.2) |
| Any abandoned buildings/vacant lots,e n (%) | 20 (9.3) |
| Mixed housing, n (%) | 38 (17.7) |
| Graffiti, litter, broken windows, or glass,f n (%) | 16 (7.4) |
| At least 1 high-quality sidewalk feature,g | 150 (69.8) |
| Number of residential moves during study, n (%) | |
| 0 | 167 (77.7) |
| 1 | 35 (16.3) |
| 2 or more | 13 (6.1) |
| Census-tract characteristics | |
| % population below poverty, % (SD) | 27.2 (14.5) |
| % of owner-occupied homes, % (SD) | 53.6 (19.3) |
| % of vacant housing units, % (SD) | 18.1 (9.4) |
| % Non-Hispanic African Americans, % (SD) | 65.5 (30.9) |
| Housing density, units per square mile (SD) | 2338.2 (1667.4) |
BMI Body-mass index, GED General Education Development (High School Equivalency) Certificate, DCIS Ductal carcinoma in situ, EIBC Early-stage invasive breast cancer
Scores could range from 0–100, with higher scores indicating better quality of life for each subscale.
Weight could not be collected for 1 patient, thus, BMI could not be computed
Three patients in the intervention arm of the study did not receive definitive treatment surgery following enrollment
Three patients were missing data about mixed residential and non-residential land use
Three patients were missing data about abandoned buildings/vacant lots
Two patients were missing data about graffiti, litter, broken windows, or glass
Two patients were missing data about sidewalk quality
Most study participants were employed, had household incomes less than $25,000, were not married, and had received breast-conserving surgery for their breast cancer. Average age of participants was 56.1 years, ranging from age 33 or 81 years. Thirty-three patients in our analysis dropped out of the study and did not differ significantly from patients who completed all five interviews by household income, marital status, education, employment status, age, or cancer stage. A greater proportion of patients in our analysis who were lost to follow-up after completing Interview1 were treated with surgery only (30.0% vs. 12.7%; p = 0.02) or surgery plus chemotherapy (13.3% vs. 5.5%; p = 0.02) compared to patients who completed all intervention exposures.
The date of the GSV imagery was on average 5.4 months (SD = 15.0) before the Interview1 interview. Most women did not move during the 2-year study period, and most did not live on streets with mixed housing or abandoned buildings/vacant lots. Less than one-quarter of participants lived on streets with graffiti, broken/boarded windows, litter, or broken glass or on streets with non-residential destinations. Only 40.9% (87/213) of streets had the highest sidewalk quality on all nine characteristics of high-quality sidewalks.
On average, there were 1.6 women per census tract at Interview1, ranging from one participant per tract in 59.7% of the tracts to five women in one of the 134 census tracts. There was also extensive variability in the census-tract characteristics. The average percentage of African Americans in the 134 census tracts was 59.5%, but ranged from 0 to 100%. On average, 25.7% of the population in the 134 census tracts lived below poverty, ranging from 2.0 to 67.4%. On average, 17.4% of housing units were vacant. Housing density ranged from 55 houses (suburban) to 10,649 houses (inner city) per square mile. Correlations among the census-tract variables ranged from a low correlation of 0.02 (between % African Americans and housing density) to a moderately high negative correlation of −0.68 (between % of population below poverty and % of owner-occupied housing).
Baseline and trajectories of QOL subscales
There was extensive variability in the baseline (intercept) scores across the QOL subscales (Table 2). Average scores were highest for emotional well-being and social functioning (β0= 73.84 and β0=70.00, respectively). Role limitations due to physical health had the lowest average (β0=27.15). The variance for each intercept (β0) was significantly different from zero, suggesting that the average QOL scores differed among women at baseline. Variability in the average scores was highest for role limitations due to emotional problems (variance=1201.36).
Table 2.
Growth parameter estimates from the unadjusted latent trajectory models for each of the eight subscales of the RAND 36-Item Short Form Health Survey (N=215).
| Intercept (β0) | Variance (β0) | Slope (β1) | Variance(β1) | Covariance (β0, β1) | |
|---|---|---|---|---|---|
| Emotional well-being | 73.84* | 344.78* | 0.81* | 6.66** | −21.02* |
| Role limitations due to emotional problems | 62.22* | 1201.36* | 1.43* | 41.89** | −115.40* |
| Energy/fatigue | 52.15* | 380.00* | 0.61* | 6.43** | −20.10* |
| General health | 59.94* | 373.87* | −0.83* | 6.55** | −11.21 |
| Pain | 59.83* | 405.68* | 0.50 | 15.07** | −15.23 |
| Physical functioning | 64.85* | 593.91* | −1.20* | 12.27** | −8.98 |
| Role limitations due to physical health | 27.15* | 604.90* | 4.89** | 36.76* | −0.12 |
| Social functioning | 70.00* | 499.50* | 1.25* | 15.19** | −26.43* |
p < 0.05.
p < 0.01.
Change in QOL for each subscale also is shown in Table 2. The slope (β1) was significantly different from zero for seven of the eight subscales. QOL improved over time for five subscales (emotional well-being, role limitations due to emotional problems, energy/fatigue, role limitations due to physical health, and social functioning), declined for physical functioning and general health subscales, and remained stable for the pain subscale. The variance for the slope (β1) was significantly different from zero for each of the eight subscales, suggesting that there were differences in the trajectories of each subscale for different women. The covariance between the intercept and the slope (β0, β1) was statistically significant for emotional well-being, role limitations due to emotional problems, energy/fatigue, and social functioning. The significant, negative parameter estimates for these subscales indicate that women who reported lower QOL scores at baseline showed greater improvement in their QOL scores over time. The rate of improvement for the other subscales was independent of their intercept.
Associations between the built environment and each QOL subscale
Table 3 shows the average adjusted QOL subscale baseline scores and the average trajectories of those QOL subscales. In general, the built-environment factors showed stronger associations with the baseline (intercept) QOL scores than with the QOL trajectories in these models that were adjusted for other built-environment factors but not adjusted for other potential confounding variables. Different built-environment factors were associated with the baseline levels of different QOL subscales. The presence of abandoned buildings/vacant lots was associated with poorer QOL at baseline on role limitations due to emotional problems (β=−25.40). Moving at least once over the study period was associated with significantly poorer QOL on six subscales at baseline, but not with role limitations due to emotional problems and pain. Presence of graffiti or broken windows or glass was associated with poorer QOL at baseline on role limitations due to emotional problems. Living on streets with high (vs. poor)-quality sidewalks was associated with better QOL on emotional well-being (β=5.00), role limitations due to emotional problems (β=6.81), and social functioning (β=5.34) subscales at baseline. Neither mixed use nor mixed housing was associated with any of the QOL subscales at baseline.
Table 3.
Growth parameter estimates of built environment from latent trajectory models (N = 215).
| RAND 36-Item Short Form Health Survey | ||||||||
|---|---|---|---|---|---|---|---|---|
| Emotional well-being | Role limitations due to emotional problems | Energy/fatigue | General health | Pain | Physical functioning | Role limitations due to physical health | Social functioning | |
| Intercept (β0)† | 71.36* | 59.49* | 53.37* | 62.80* | 58.40* | 70.21* | 26.46* | 71.41* |
| Mixed use | −1.53 | −2.18 | −2.19 | −2.35 | −0.67 | −2.72 | 1.60 | −3.96 |
| Abandoned buildings/vacant lots | −5.93 | −25.40* | −4.88 | −5.06 | −0.47 | 1.66 | −5.22 | −4.50 |
| Mixed housing | 1.46 | −1.99 | 2.57 | −0.50 | 1.01 | 0.32 | −5.16 | −0.78 |
| Graffiti/litter/broken windows or glass | 6.85 | 15.24* | 3.39 | 5.70 | −0.23 | −0.73 | 5.59 | 4.68 |
| Sidewalk quality | 5.00* | 6.81* | 3.27 | 1.41 | 2.99 | 0.24 | 1.58 | 5.34* |
| Moved at least once | −10.64* | −8.23 | −11.93* | −10.04* | −6.39 | −11.16* | −12.40* | −9.86* |
| Slope (β1)‡ | 3.17* | 6.05* | 1.64* | −0.09 | 2.92* | −0.16 | 7.60* | 3.73* |
| Mixed use | −1.08 | −1.80 | −0.78 | −0.43 | −0.61 | −0.25 | −0.90 | −1.81 |
| Abandoned buildings/vacant lots | −0.99 | 1.81 | −1.33 | 0.26 | −1.89 | −1.46 | 0.82 | −2.33 |
| Mixed housing | 0.10 | 2.94 | −0.24 | 0.099 | −0.44 | 0.76 | 1.91 | 0.56 |
| Graffiti/litter/broken windows or glass | −0.03 | −3.41 | 1.07 | 0.28 | −0.45 | 0.77 | −2.61 | 1.14 |
| Sidewalk quality | −0.82* | −1.57 | −0.28 | −0.36 | −1.05* | −0.73 | −0.63 | −0.46 |
| Moved at least once | 0.30 | −2.27 | 0.86 | 0.58 | −0.06 | 0.22 | −3.10 | 0.58 |
Note: All regression coefficients of built-environment factors were adjusted for other built-environment factors simultaneously.
Z test: p < 0.05.
Reflects the average score of each RAND SF-36 subscale at the baseline.
Reflects the trajectory of each RAND SF-36 subscale over 2-year follow-up.
Also shown in Table 3, the trajectories (slope) of emotional well-being (β= 3.17), role limitations due to emotional problems (β= 6.05), energy/fatigue (β=1.64), pain (β=2.92), role limitations due to physical health (β= 7.60), and social functioning (β= 3.73) improved significantly over time. However, of the built-environment variables, only sidewalk quality was associated with trajectories of any of the QOL subscales. Living on streets with high (vs. poor)-quality sidewalks was associated with the trajectories of emotional well-being and pain. That is, the effect of sidewalk quality on improvement in these two subscales was smaller for women living on streets with high- (vs. poor)-quality sidewalks, since women living on streets with high-quality sidewalks reported better QOL at baseline on the emotional well-being than women living on streets with poor-quality sidewalks.
Table 4 displays the effects of sidewalk quality on the intercept (baseline) and trajectories of three QOL subscales (emotional well-being, pain, and social functioning), adjusted for age, education, smoking status, and elevated depressed mood, which were significantly associated with the trajectory of QOL subscales. We did not adjust for study arm or other potential confounders (i.e., social support, census-tract variables, comorbidity, disease stage, type of surgery, marital status, BMI, treatment received, household income, employment status, moved at least once, and the other built-environment characteristics), which were not associated with the trajectory of any of the QOL subscales (data not shown). As shown in Table 4, after adjusting for confounders, living on streets with high-quality sidewalks was significantly associated with higher baseline QOL on the emotional well-being (β=5.35) and social functioning (β=6.47) subscales. Living on streets with high-quality sidewalks was negatively associated with the slope for emotional well-being (β=−1.40) and pain (β=−1.94). Women living on streets with high-quality sidewalks reported better QOL on the emotional well-being (β=−1.40) and pain subscales (β=−1.94) at baseline than women living on streets with poor-quality sidewalks. As emotional well-being and pain scores improved over time for all women, the estimates shown here indicate that the improvement in these QOL scores after adjusting for covariates was less dramatic for women living on streets with high-quality sidewalks than the improvement observed among women living on streets with poor-quality sidewalks.
Table 4.
Growth parameter estimates (standard error) of sidewalk quality from unadjusted and adjusted latent trajectory models (N=215).
| RAND 36-Item Short Form Health Survey | |||
|---|---|---|---|
| Emotional well-being | Pain | Social functioning | |
| Intercept | |||
| Unadjusted Model | |||
| Sidewalk quality | 6.27 (3.11)* | 4.49 (3.49) | 8.05 (3.45) |
| Adjusted Model | |||
| Sidewalk quality | 5.35 (2.35)* | 3.90 (3.38) | 6.47 (3.16)* |
| Age | 0.19 (0.11) | 0.45 (0.16)* | 0.38 (0.15)* |
| Education: <12 years vs. >12 years | −2.49 (3.10) | 0.03 (4.48) | 1.11 (4.18) |
| 12 years vs. >12 years | 1.15 (2.45) | 6.86 (3.53) | 6.33 (3.30) |
| Smoking history | −1.75 (2.23) | −10.88 (3.25)* | −5.43 (3.00) |
| Depressed mood | −28.68 (2.45)* | −14.78 (3.51)* | −26.66 (3.28)* |
| Slope | |||
| Unadjusted model | |||
| Sidewalk quality | −1.62 (0.62)* | −2.37 (0.90)* | −1.44 (0.83) |
| Adjusted model | |||
| Sidewalk quality | −1.40 (0.59)* | −1.94 (0.90)* | −1.06 (0.82) |
| Age | −0.04 (0.03) | −0.11 (0.04)* | −0.08 (0.04)* |
| Education: <12 years vs. >12 years | −0.02 (0.76) | 0.08 (1.18) | 0.69 (1.08) |
| 12 years vs. >12 years | 0.68 (0.62) | −2.14 (0.95)* | −0.48 (0.87) |
| Smoking history | −1.38 (0.55)* | 0.39 (0.85) | −1.27 (0.78) |
| Depressed mood | 2.61 (0.61)* | 0.05 (0.92) | 1.00 (0.85) |
Z test: p<0.05.
Discussion
To our knowledge, this is the first study to examine the extent to which the built environment affected changes in QOL over time in a sample of newly diagnosed African American breast cancer patients. QOL improved over time for seven of the eight subscales and remained stable for one subscale, pain. Our findings showed that living on streets with high-quality sidewalks was associated with higher baseline emotional well-being and social functioning scores in adjusted models. Sidewalk quality was negatively associated with the slope for emotional well-being and pain in adjusted models. As emotional well-being and pain scores improved over time for all women, the negative estimates for these trajectories indicate that the improvement in these QOL scores was larger for women living on streets with poor-quality sidewalks than the improvement observed among women living on streets with high-quality sidewalks. As previous work has shown greater mobility among older adults in neighborhoods with better sidewalks (Clarke and Gallagher, 2013) as well as health benefits from walking outside, (Simonsick et al., 2005; Wong et al., 2003) attention to the built environment, especially sidewalk quality, seems to be critical for understanding why QOL might, or might not, improve following a breast cancer diagnosis.
Our data in newly diagnosed breast cancer patients showed that sidewalk quality at baseline was associated with improvements in emotional well-being and pain over a 2-year follow-up. While studies have examined neighborhood conditions related to QOL, few have been conducted in African American breast cancer survivors, and according to one review article, most studies have used a cross-sectional design.(Gomez et al., 2015) For example, perceived neighborhood stress and neighborhood social disorder were found to be associated with poorer self-rated health among ethnic minority breast cancer survivors.(Tejeda et al., 2017; Wu et al., 2018) It is important to understand how sidewalk quality “gets under the skin” and can affect QOL. The mechanism through which sidewalk quality affects changes in QOL may include physical activity and BMI.(Pruitt et al., 2012) Although BMI was not associated with trajectories of any of the QOL subscales, and was not included as a confounder in our models, physical activity after diagnosis could be a mechanism through which sidewalk quality affects change in both QOL subscales, as even moderate levels of physical activity (e.g., walking at least 3–5 hours/week at a pace of 2.0–2.9 mph on average) was observed to have survival benefits after a breast cancer diagnosis.(Holmes et al., 2005) Additional studies should be conducted to focus on the relevance of neighborhoods in promoting wellness and QOL among breast cancer survivors. Because QOL is associated with survival,(Coates et al., 1992) and African American breast cancer patients have poorer survival than other racial/ethnic groups,(Chlebowski et al., 2005; DeSantis et al., 2014) it is important to identify ways to improve QOL in African American patients. Unfortunately, our computer-based cancer-communication intervention using African American survivor story videos did not affect QOL trajectories.(Thompson et al., 2019)
Strengths of our study include its longitudinal design as part of a randomized controlled trial to examine effects of the observed built environment on African American breast cancer patients’ QOL. We used GSV, which has been found to yield valid and reliable information about the built environment. Advantages include efficiency, researcher safety, low cost, unobtrusive data collection, and access to historical images of the same streets. By not using self-reported data about neighborhood conditions, potential bias is reduced. In addition, we focused on African-American women only who often report lower scores on many aspects of their QOL than other racial/ethnic groups.(American Cancer Society, 2013; Paskett et al., 2008) Moreover, 85 percent of enrolled women completed all five interviews, reducing the likelihood of selection bias.
We also recognize the limitations of our study. Our participation rate for enrollment into the study included 62% of 369 eligible patients identified, yet this percentage is similar to the number of African American women enrolled in a prior longitudinal QOL study of patients with early-stage breast cancer and age-matched controls.(Jeffe et al., 2012) Although participants and nonparticipants did not differ significantly by marital status or insurance type, nonparticipants were an average six years older than participants. Another limitation is that we did not have data on the street addresses of study participants prior to their enrollment in the study. Neighborhood conditions and life experiences over a woman’s life course prior to a breast cancer diagnosis may affect various health outcomes. We only found a significant association between sidewalk and the QOL trajectory over time. A possible explanation is because the prevalence of other built-environment factors was too low to show an effect on the QOL trajectory in this sample. Future studies with larger samples are needed to further examine the associations between those other built-environment factors and the QOL trajectory. In addition, we only audited the street segment of each study participant’s residence. Although this limited geographic area that was audited does not capture the entire neighborhood of study participants (however defined), our study shows important associations of the built environment with changes in QOL in very close proximity to where participants lived. Because adjacent street segments tend to have similar characteristics,(Mooney et al., 2014) our findings likely extend beyond the street segment of the participants.
In conclusion, sidewalk quality was associated with changes in emotional well-being and pain QOL measures in newly diagnosed African American breast cancer patients. The improvement in these QOL scores over 2-year follow-up was larger for women living on streets with poor-quality sidewalks than the improvement observed among women living on streets with high-quality sidewalks. The extent to which high-quality sidewalks can promote mobility and healthy aging is important,(Clarke and Gallagher, 2013) as even a small amount of habitual walking outside the home has health benefits.(Simonsick et al., 2005; Wong et al., 2003)
Highlights.
Many African American breast cancer patients live on low sidewalk quality streets.
High sidewalk quality had an impact on improvements in emotional well-being and pain.
Sidewalk quality may affect changes in QOL via physical activity or stress.
Acknowledgements
We thank our patient participants, the interviewers, Ms. Lori Grove in Oncology Data Services for assistance with data collection from the medical record, and Ms. Julianne Sefko for geocoding services provided through the Health Behavior, Communication and Outreach Core, both at Washington University School of Medicine. We also thank the physicians in addition to Dr. Margenthaler, who helped recruit their patients for this study, including Drs. Timothy Eberlein, William Gillanders, Rebecca Aft, and Amy Cyr at Washington University School of Medicine and Dr. Theresa Schwartz and Pam Hunborg, RN, at Saint Louis University School of Medicine.
Funding Source
This study was supported by the National Cancer Institute (grant 2P50 CA095815-06; Principal Investigator: Matthew W. Kreuter) and the National Cancer Institute Cancer Center Support Grant to the Alvin J. Siteman Cancer Center at Barnes-Jewish Hospital and Washington University School of Medicine in St. Louis, Missouri (grant P30 CA091842; Principal Investigator: T. Eberlein) for services provided by the Health Behavior, Communication and Outreach Core.
Appendix 1.
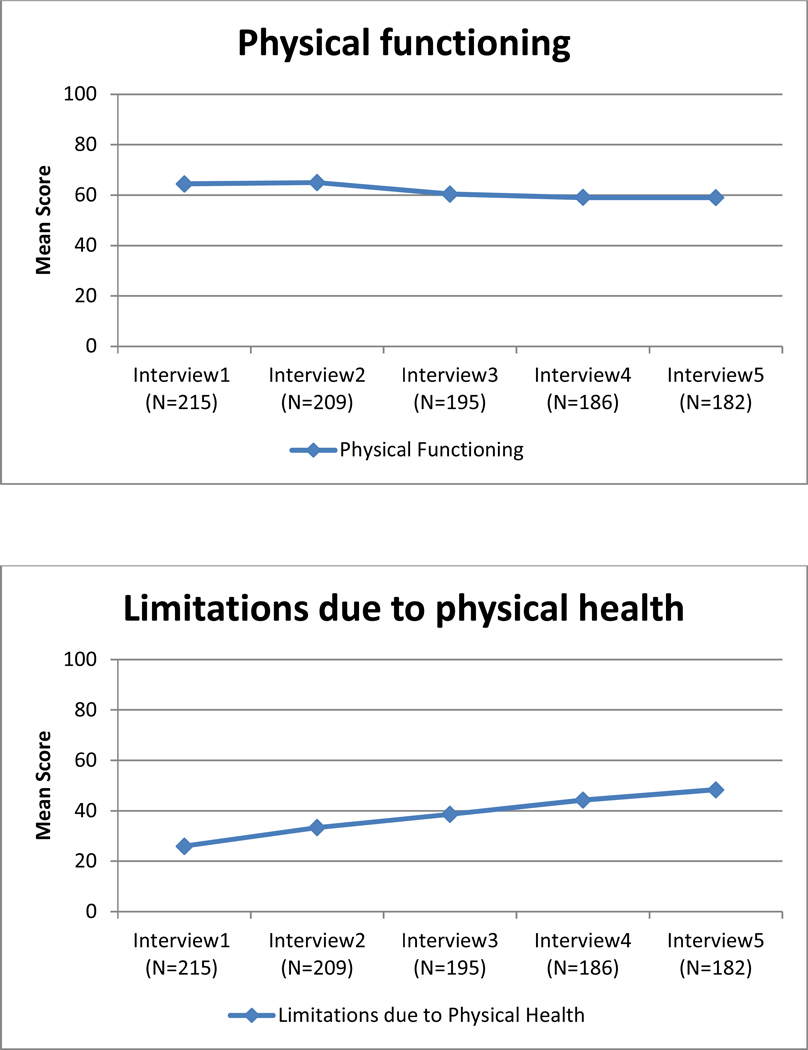
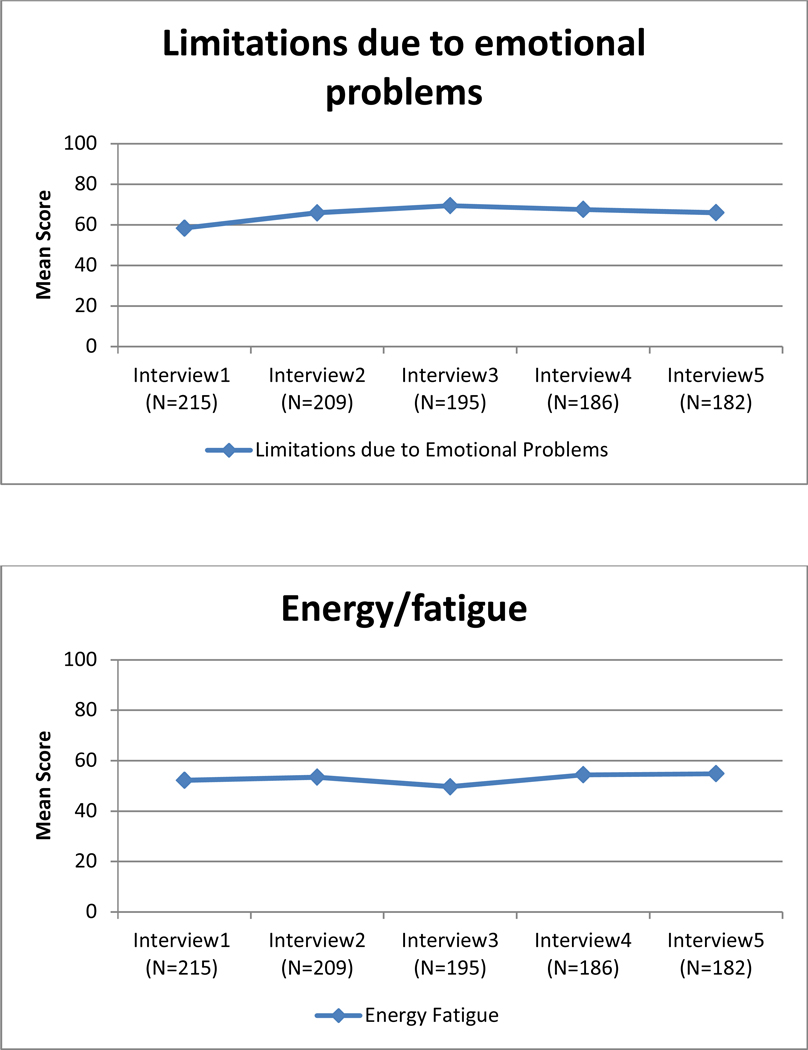
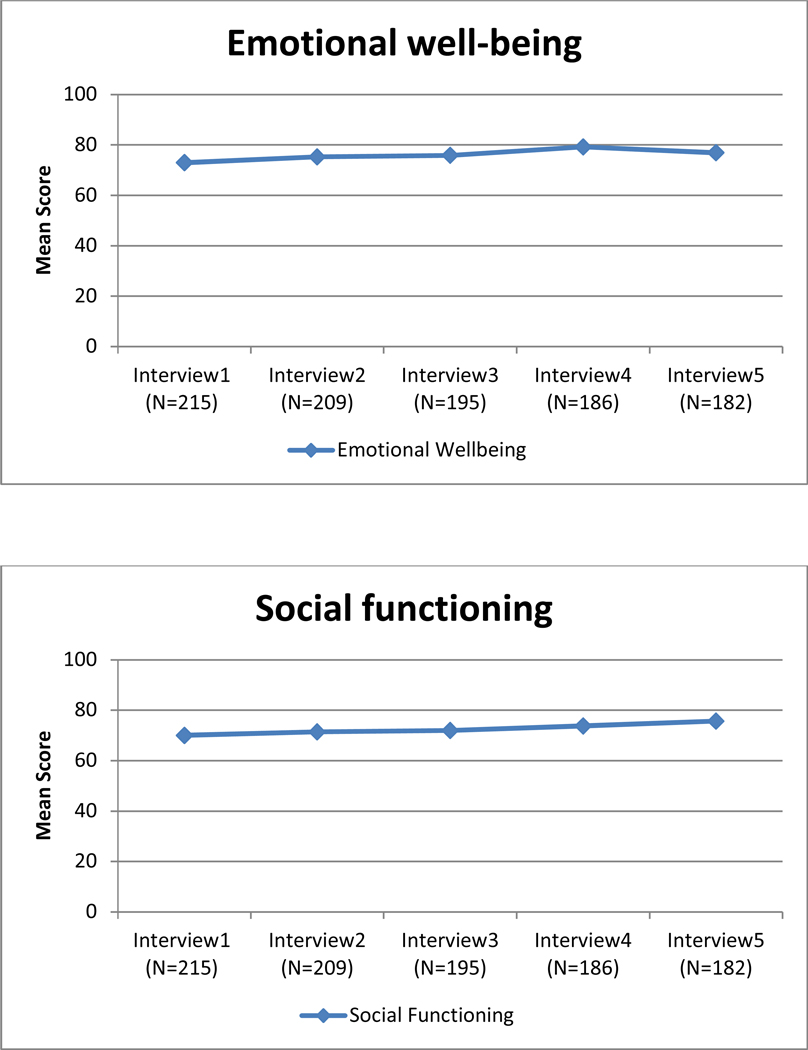
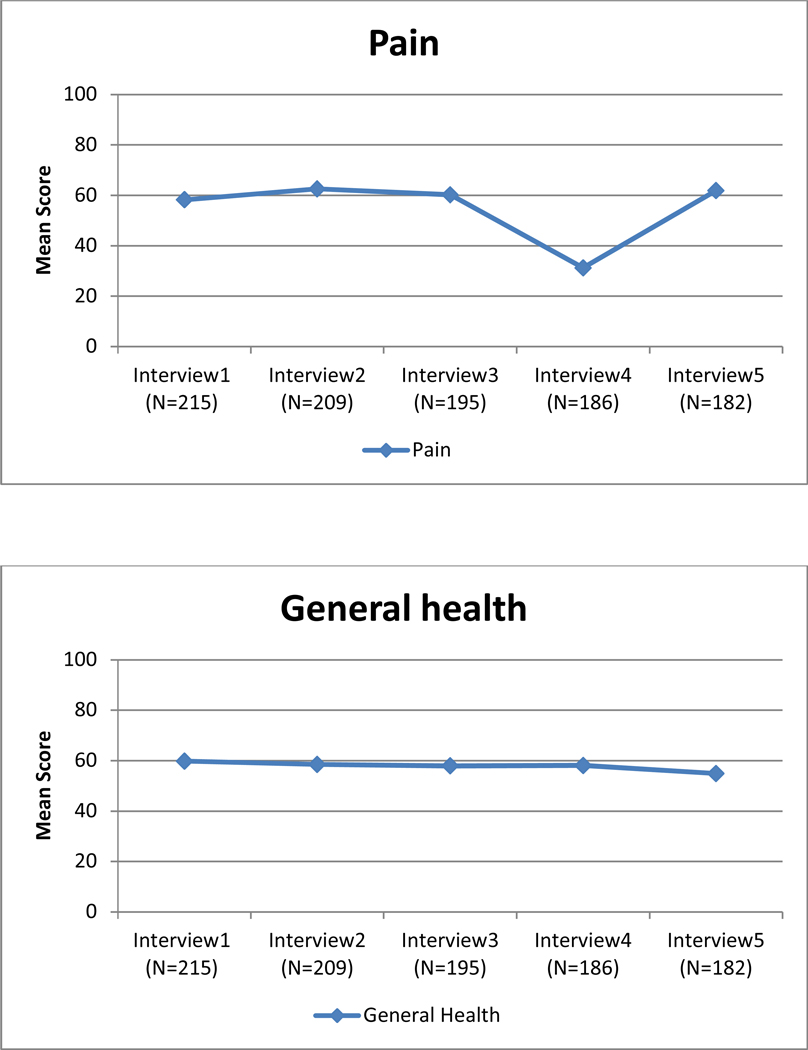
Unadjusted mean quality of life scores for each of the eight subscales of the SF-36 over 2-year follow-up.
Footnotes
Disclaimers
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or their staff members.
Disclosures
The authors declare that they have no conflicts of interest, financial or otherwise.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- American Cancer Society, 2013. Facts & figures for American Americans, Atlanta, GA. [Google Scholar]
- American Cancer Society, 2014. Cancer treatment and survivorship facts & figures 2014–2015., Atlanta, GA. [Google Scholar]
- Aneshensel C, 2010. Neighborhood as a social context of the stress process , Advances in the Conceptualization of the Stress Process. Springer, pp. 35–52. [Google Scholar]
- Aneshensel CS, Wight RG, Miller-Martinez D, Botticello AL, Karlamangla AS, Seeman TE, 2007. Urban Neighborhoods and Depressive Symptoms Among Older Adults. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences 62, S52S59. [DOI] [PubMed] [Google Scholar]
- Ashing-Giwa KT, Padilla GV, Tejero JS, Kim J, 2004. Breast cancer survivorship in a multiethnic sample: challenges in recruitment and measurement. Cancer 101, 450–465. [DOI] [PubMed] [Google Scholar]
- Brinton LA, Sherman ME, Carreon JD, Anderson WF, 2008. Recent trends in breast cancer among younger women in the United States. J Natl Cancer Inst 100, 1643–1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlson ME, Pompei P, Ales K, MacKenzie CR, 1987. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. Journal of Chronic Diseases 40, 373–383. [DOI] [PubMed] [Google Scholar]
- Chlebowski RT, Chen Z, Anderson GL, Rohan T, Aragaki A, Lane D, Dolan NC, Paskett ED, McTiernan A, Hubbell FA, Adams-Campbell LL, Prentice R, 2005. Ethnicity and Breast Cancer: Factors Influencing Differences in Incidence and Outcome. J. Natl. Cancer Inst. 97, 439–448. [DOI] [PubMed] [Google Scholar]
- Cianfrocca M, Goldstein LJ, 2004. Prognostic and Predictive Factors in Early-Stage Breast Cancer. Oncologist 9, 606–616. [DOI] [PubMed] [Google Scholar]
- Clarke P, Gallagher NA, 2013. Optimizing mobility in later life: the role of the urban built environment for older adults aging in place. J Urban Health 90, 997–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coates A, Gebski V, Signorini D, Murray P, McNeil D, Byrne M, Forbes (for the Australian New Zealand Breast Cancer Trials Group), J.F., 1992. Prognostic value of quality-of-life scores during chemotherapy for advanced breast cancer. Journal of Clinical Oncology 10, 1833–1838. [DOI] [PubMed] [Google Scholar]
- Corporation;, R., 2014. Medical Outcomes Study: Social Support Survey Scoring Instructions. . [Google Scholar]
- Curran PJ, Hussong AM, 2003. The use of latent trajectory models in psychopathology research. Journal of Abnormal Psychology 112, 526–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demicheli R, Retsky M, Hrushesky W, Baum M, Gukas I, Jatoi I, 2007. Racial disparities in breast cancer outcome. Cancer 110, 1880–1888. [DOI] [PubMed] [Google Scholar]
- DeSantis C, Ma J, Bryan L, Jemal A, 2014. Breast cancer statistics, 2013. CA Cancer J Clin, 52–62. [DOI] [PubMed] [Google Scholar]
- Deshpande AD, Jeffe DB, Gnerlich J, Iqbal AZ, Thummalakunta A, Margenthaler JA, 2009. Racial disparities in breast cancer survival: an analysis by age and stage. Journal of Surgical Research 153, 105–113. PMCID: PMC3240670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diez-Roux AV, 2000. Multilevel analysis in public health research. Annual Review of Public Health 21, 171–192. [DOI] [PubMed] [Google Scholar]
- Elledge RM, Clark GM, Chamness GC, Osborne CK, 1994. Tumor biologic factors and breast cancer prognosis among white, Hispanic, and black women in the United States. J Natl Cancer Inst 86, 705–712. [DOI] [PubMed] [Google Scholar]
- Elliott M, 2000. The stress process in neighborhood context. Health Place 6, 287–299. [DOI] [PubMed] [Google Scholar]
- Epplein M, Zheng Y, Zheng W, Chen Z, Gu K, Penson D, Lu W, Shu XO, 2011. Quality of life after breast cancer diagnosis and survival. J Clin Oncol 29, 406–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez SL, Shariff-Marco S, DeRouen M, Keegan THM, Yen IH, Mujahid M, Satariano WA, Glaser SL, 2015. The impact of neighborhood social and built environment factors across the cancer continuum: Current research, methodological considerations, and future directions. Cancer 121, 2314–2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hays RD, Alonso J, Coons SJ, 1998. Possibilities for summarizing health-related quality of life when using a profile instrument, in: Staquet MJ, Hays RD, Fayers PM (Eds.), Quality of life assessment in clinical trials: Methods and practice. Oxford University Press, Oxford, England, pp. 143–153. [Google Scholar]
- Hays RD, Sherbourne CD, Mazel RM, 1993. The RAND 36-item health survey 1.0. Health Economics 2, 217–227. [DOI] [PubMed] [Google Scholar]
- Healthcare, R., 36-Item Short Form Survey Instrument (SF-36). RAND 36-Item Health Survey 1.0 Questionnaire Items. [Google Scholar]
- Holmes MD, Chen WY, Feskanich D, Kroenke CH, Colditz GA, 2005. Physical activity and survival after breast cancer diagnosis. JAMA 293, 2479–2486. [DOI] [PubMed] [Google Scholar]
- Howlader N, Noone AM, Krapcho M, Neyman N, Aminou R, Waldron W, Altekruse SF, Kosary CL, Ruhl J, Tatalovich Z, Cho H, Mariotto A, Eisner MP, Lewis DR, Chen HS, Feuer EJ, Cronin KA, Edwards B.K.e., SEER cancer statistics review, 1975–2008. Bethesda, MD: National Cancer Institute. Available at: http://seer.cancer.gov/csr/1975_2008/, based on November 2010 SEER data submission, posted to the SEER web site, 2011. [Google Scholar]
- Jeffe DB, Pérez M, Liu Y, Collins KK, Aft RL, Schootman M, 2012. Quality of life changes over time in women diagnosed with ductal carcinoma in situ, early-stage invasive breast cancer, and age-matched controls. Breast Cancer Research and Treatment 134, 379–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joslyn SA, West MM, 2000. Racial differences in breast carcinoma survival. Cancer 1, 114–123. [DOI] [PubMed] [Google Scholar]
- Katz JN, Chang LC, Sangha O, Fossel AH, Bates DW, 1996. Can comorbidity be measured by questionnaire rather than medical record review? Medical Care 34, 73–84. [DOI] [PubMed] [Google Scholar]
- Katzman R, Brown T, Fuld P, Peck A, Schechter R, Schimmel H, 1983. Validation of a short Orientation-Memory-Concentration test of cognitive impairment. American Journal of Psychiatry 140, 734–739. [DOI] [PubMed] [Google Scholar]
- Kelly CM, Wilson JS, Baker EA, Miller DK, Schootman M, 2013a. Using Google Street View to audit the built environment: inter-rater reliability results. Ann Behav Med 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly CM, Wilson JS, Baker EA, Miller DK, Schootman M, 2013b. Using Google Street View to audit the built environment: inter-rater reliability results. Ann Behav Med 45 Suppl 1, S108–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubzansky LD, Subramanian SV, Kawachi I, Fay ME, Soobader M-J, Berkman LF, 2005. Neighborhood contextual Influences on depressive symptoms in the elderly. Am J Epidemiol 162, 253–260. [DOI] [PubMed] [Google Scholar]
- Lian M, Perez M, Liu Y, Schootman M, Frisse A, Foldes E, Jeffe DB, 2014. Neighborhood socioeconomic deprivation, tumor subtypes, and causes of death after non-metastatic invasive breast cancer diagnosis: a multilevel competing-risk analysis. Breast Cancer Res Treat 147, 661–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHorney CA, Kosinski M, Ware JE, 1994. Comparisons of the costs and quality of norms for the SF-36 health survey collected by mail versus telephone interview: Results from a national survey. Medical Care 32, 551–567. [DOI] [PubMed] [Google Scholar]
- Mooney SJ, Bader MDM, Lovasi GS, Neckerman KM, Teitler JO, Rundle AG, 2014. Validity of an ecometric neighborhood physical disorder measure constructed by virtual street audit. Am J Epidemiol 180, 626–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthén LK, Muthén BO, 1998–2012. Mplus user’s guide. Sixth Edition. Muthén & Muthén, Los Angeles, CA. [Google Scholar]
- National Cancer Institute, Estimated US cancer prevalence counts: Who are our cancer survivors in the US? Bethesda, MD: National Cancer Institute, 2009. Available at: http://cancercontrol.cancer.gov/ocs/prevalence/index.html. Accessed April 26, 2012. [Google Scholar]
- National Cancer Institute, SEER cancer statistics in review. Table 4.25: Cancer of the breast (invasive): estimated United States cancer prevalence counts on January 1, 2009: by race/ethnicity, sex and years since diagnosis. Available at: http://seer.cancer.gov/csr/1975_2009_pops09/results_single/sect_04_table.25.pdf. Accessed May 10, 2012.
- Paskett ED, Alfano CM, Davidson MA, Andersen BL, Naughton MJ, Sherman A, McDonald PG, Hays J, 2008. Breast cancer survivors’ health-related quality of life : racial differences and comparisons with noncancer controls. Cancer 113, 3222–3230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez M, Sefko JA, Ksiazek D, Golla B, Casey C, Margenthaler JA, Colditz G, Kreuter MW, Jeffe DB, 2014. A novel intervention using interactive technology and personal narratives to reduce cancer disparities: African American breast cancer survivor stories. J Cancer Surviv 8, 21–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- President’s Cancer Panel, US Department of Health and Human Services, National Institutes of Health, National Cancer Institute, Living beyond cancer: finding a new balance. Available at: http://deainfo.nci.nih.gov/advisory/pcp/annualReports/pcp03-04rpt/Survivorship.pdf. Accessed April 26, 2012.
- Pruitt SL, McQueen A, Deshpande AD, Jeffe DB, Schootman M, 2012. Mediators of the effect of neighborhood poverty on physical functioning among breast cancer survivors: a longitudinal study. Cancer Causes and Control 23, 1529–1540. PMCID: PMC3425435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff LS, 1977. The CES-D scale: A self-report depression scale for research in the general population. Applied Psychological Measurement 1, 385–401. [Google Scholar]
- Radloff LS, Teri L, 1986. Use of the Center for Epidemiological Studies-Depression Scale with older adults. Clinical Gerontologist 5, 119–136. [Google Scholar]
- Renalds A, Smith TH, Hale PJ, 2010. A systematic review of built environment and health. Fam Community Health 33, 68–78. [DOI] [PubMed] [Google Scholar]
- Ross C, 2000. Neighborhood disadvantage and adult depression. Journal of Health and Social Behavior 41, 177–187. [PubMed] [Google Scholar]
- Schulz A, Zenk S, Israel B, Mentz G, Stokes C, Galea S, 2008. Do neighborhood economic characteristics, racial composition, and residential stability predict perceptions of stress associated with the physical and social environment? Findings from a multilevel analysis in Detroit. Journal of Urban Health 85, 642–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz GF, Solin LJ, Olivotto IA, Ernster VL, Pressman PI, Consensus Conference Committee, 2000. Consensus conference on the treatment of in situ ductal carcinoma of the breast, April 22–25, 1999 Cancer 88, 946–954. [PubMed] [Google Scholar]
- Sherbourne C, Stewart A, 1991. The MOS social support survey. Social Science and Medicine 32, 705–714. [DOI] [PubMed] [Google Scholar]
- Simonsick EM, Guralnik JM, Volpato S, Balfour J, Fried LP, 2005. Just get out the door! Importance of walking outside the home for maintaining mobility: findings from the women’s health and aging study. Journal of the American Geriatrics Society 53, 198–203. [DOI] [PubMed] [Google Scholar]
- Stewart AL, Hays R, Ware J, 1988. The MOS short-form general health survey: Reliability and validity in a patient population. Medical Care 26, 724–735. [DOI] [PubMed] [Google Scholar]
- Tejeda S, Stolley MR, Vijayasiri G, Campbell RT, Estwing Ferrans C, Warnecke RB, Rauscher GH, 2017. Negative psychological consequences of breast cancer among recently diagnosed ethnically diverse women. Psychooncology 26, 2245–2252. [DOI] [PubMed] [Google Scholar]
- Thompson T, Pérez M, Yan Y, Kreuter M, Margenthaler J, Colditz G, Jeffe D, 2019. Survivor stories: Quality of life results from a randomized controlled trial of a video narrative intervention. Presented at the 40th Annual Meeting and Scientific Sessions of the Society of Behavioral Medicine, Washington, DC, March 6–9, 2019 Ann Behav Med 53 (suppl.), S225. [Google Scholar]
- Ware JE, Sherbourne CD, 1992. The MOS 36-Item Short-Form Health Survey (SF-36). Medical Care 30, 473–483. [PubMed] [Google Scholar]
- Wells KB, Stewart AL, Hays RD, Burnam MA, Rogers W, Daniels M, Berry S, Greenfield S, Ware JE, 1989. The functioning and well-being of depressed patients: Results from the medical outcomes study. Journal of the American Medical Association 262, 914–919. [PubMed] [Google Scholar]
- Wilson J, Kelly C, Schootman M, Baker E, Banerjee A, Clennin M, Miller D, 2012. Assessing the built environment using omnidirectional imagery. Am J Prev Med 42, 193–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong CH, Wong SF, Pang WS, Azizah MY, Dass MJ, 2003. Habitual walking and its correlation to better physical function: implications for prevention of physical disability in older persons. The journals of gerontology. Series A, Biological sciences and medical sciences 58, 555–560. [DOI] [PubMed] [Google Scholar]
- Wu C, Ashing KT, Jones VC, Barcelo L, 2017. The association of neighborhood context with health outcomes among ethnic minority breast cancer survivors. J Behav Med. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C, Ashing KT, Jones VC, Barcelo L, 2018. The association of neighborhood context with health outcomes among ethnic minority breast cancer survivors. J Behav Med 41, 52–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu YT, Nash P, Barnes LE, Minett T, Matthews FE, Jones A, Brayne C, 2014. Assessing environmental features related to mental health: a reliability study of visual streetscape images. BMC Public Health 14, 1094. [DOI] [PMC free article] [PubMed] [Google Scholar]