Abstract
Selected indole-based kratom alkaloids were evaluated for their opioid and adrenergic receptor binding and functional effects, in vivo antinociceptive effects, plasma protein binding, and metabolic stability. Mitragynine, the major alkaloid in Mitragyna speciosa (kratom), had higher affinity at opioid receptors than at adrenergic receptors while the vice versa was observed for corynantheidine. The observed polypharmacology of kratom alkaloids may support its utilization to treat opioid use disorder and withdrawal.
Graphical Abstract
INTRODUCTION
Despite the use of kratom (Mitragyna speciosa) for centuries in Southeast Asia, its use has only recently received significant attention in the West. In the U.S., kratom is marketed and regulated as a dietary or herbal supplement; however, individuals use it for self-management of medical conditions such as pain, opioid use disorder (OUD), anxiety, and depression.1,2 Kratom products available in the U.S. include raw dried leaves, capsules, tablets, energy drinks, powders, and concentrated extracts which are sold on the Internet or in specialty stores.3,4 These kratom products are not subject to the same strict regulations as new drugs and thus cannot be marketed with medical claims. However, the poorly regulated botanical and dietary supplement market, which includes kratom products, may partially account for issues that are seen with adulterated products.5–9
In addition, the lack of regulation and standardization of kratom products (owing to a lack of scientific information to provide guidance) may contribute to the increased harm reported with its use.5–9 This harm has forced regulatory agencies to call for the removal of kratom products from the market.10 Case studies of fatalities wherein kratom was implicated as a contributing factor indicated that there was simultaneous use or contamination with other substances (including opioids and cannabinoids).11–13 Hence, there is a great need for the standardization of the kratom market to ensure that vendors provide products under good manufacturing practice (GMP) regulations for dietary supplements.
The major alkaloid from kratom is mitragynine (Figure 1), which acts at as a partial agonist at the mu opioid receptor (MOP).14 Interestingly, it is been shown that corynantheidine, a minor kratom alkaloid acts as a functional antagonist at the MOP and can reverse morphine induced inhibition of twitch contraction in guinea pig ileum.15 In addition, another minor alkaloid 7-hydroxymitragynine has been shown to be a potent MOP agonist and produces tolerance and physical dependence similar to other opioid agonists such as morphine and fentanyl.16,17 Other kratom alkaloids include 9-hydroxycorynantheidine, corynoxine, corynoxine B, isocorynantheidine, mitraphylline, paynantheine, speciociliatine, and speciogy-nine.18 Drug metabolism studies have shown that mitragynine is metabolized to 7-hydroxymitragynine in vivo via cytochrome P450 3A4 enzymes.19,20 Looking at the widespread use of kratom, it is essential to know the pharmacology of the individual alkaloids present, their metabolism and the pharmacology of the metabolites before policies can be enacted to limit or encourage the use of kratom. However, it is important to realize that these alkaloids have been and are being investigated as purified, individual entities. As such, the resultant data are not directly correlative to the complex plant mixture where they occur in varying concentrations and ratios that could impact each individual alkaloid’s pharmacokinetics and pharmacodynamics. Methadone and buprenorphine are the drugs mainly used in the treatment of OUD and opioid withdrawal.21 Recently the FDA approved lofexidine, an α2 adrenergic selective agonist for the treatment of opioid withdrawal.22 Our own preliminary binding data, coupled with preclinical reports, have suggested mitragynine has dual opioid and adrenergic pharmacology.4,23,24 As a result, we were interested in the extent to which this dual pharmacology of mitragynine might generalize to other kratom alkaloids. Herein, we report the binding affinities of the following selected kratom alkaloids; corynantheidine, 9-hydroxycorynantheidine, mitragynine, 7-hydroxymitragynine, and speciociliatine (Figure 1) at adrenergic and opioid receptors as well as in vitro functional effects of mitragynine and 7-hydroxymitragy-nine. The antinociceptive effects, metabolic stability, and plasma protein binding properties of selected kratom alkaloids are also investigated.
Figure 1.
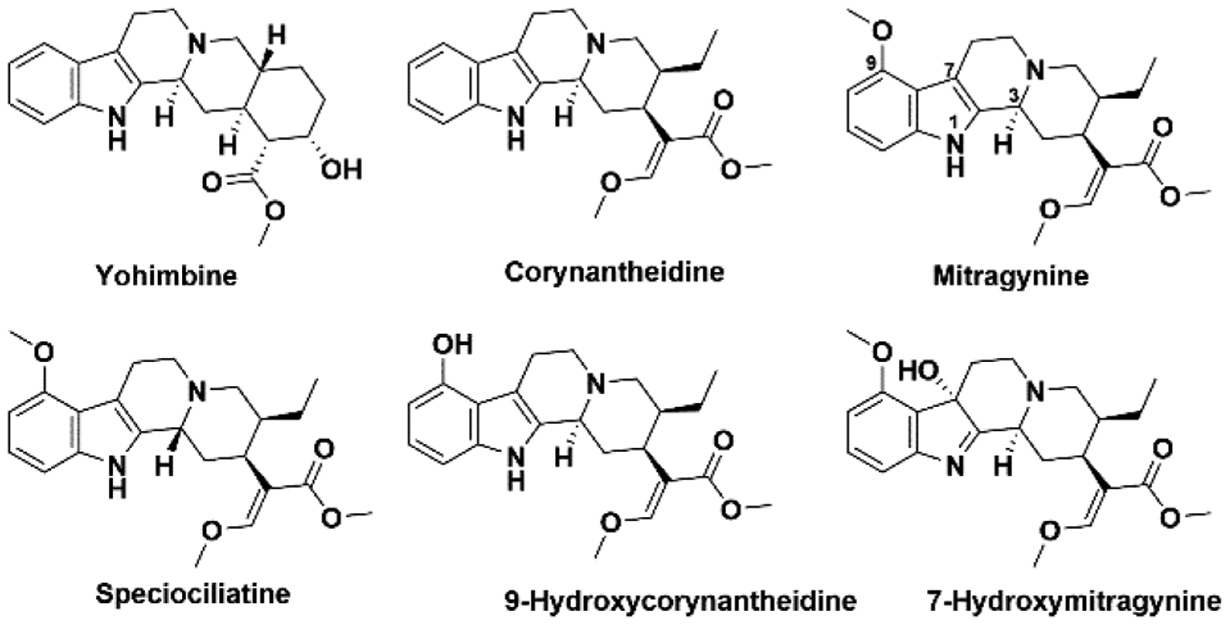
Chemical structures of yohimbine together with selected indole-based kratom alkaloids
RESULTS AND DISCUSSION
Corynantheidine, 9-hydroxycorynantheidine, mitragynine, 7-hydroxymitragynine, and speciociliatine (at two concentrations, 100 and 10,000 nM) were screened for their ability to displace bound radioligands at the delta, kappa, mu, and nociceptin opioid receptors (DOP, KOP, MOP, NOP) and at the α−1A,B,D and α−2A,B adrenergic receptors (Table 1). Compounds that showed appreciable binding at 100 nM were further screened to determine their binding affinities (Ki) at their respective receptors (Tables 2 and 3). The affinity of mitragynine was determined at all the receptors as a reference to compare to the other kratom alkaloids except at DOP and NOP, where it had poor binding at 10,000 nM. The binding affinities of selected indole-based kratom alkaloids at the DOP, KOP, and MOP are shown in Table 2 and at the α−1 and α−2 adrenergic receptors in Table 3. The studies were determined using human monoclonal receptors expressed in Chinese hamster ovary (CHO) cells (adrenergic receptors) or rat basophilic leukemia (RBL) cells (DOP and KOP) or human embryonic kidney (HEK) cells (MOP). The adrenergic and opioid affinities of these alkaloids were screened at Eurofins Cerep (Celle l’Evescault, France). 7-Hydroxymitragynine had the highest affinity for the MOP (Ki = 7.16 ± 0.94 nM), followed by speciociliatine (Ki = 54.5 ± 4.42 nM), 9-hydroxycorynantheidine (Ki = 105 ± 0.60 nM), corynantheidine (Ki = 118 ± 11.8 nM), and mitragynine Ki = 161 ± 9.56 nM). Similar to the data obtained at the MOP, 7-hydroxymitragynine had the highest affinity at the KOP followed by speciociliatine, then mitragynine, while corynantheidine had the lowest binding affinity at the KOP. 7-Hydroxymitragynine, corynantheidine, and speciociliantine’s affinities at the KOP were all lower than their affinities at the MOP. The affinity of mitragynine for the KOP was similar to the affinity obtained at the MOP. Corynantheidine had a higher binding affinity at α-adrenergic receptors than opioid receptors while the vice versa was observed for mitragynine (Table 2 and Table 3). Furthermore, corynantheidine had a 131-fold higher affinity at the α−1D receptor than mitragynine (Table 3). The binding data obtained at the opioid and α-adrenergic receptors show that removal of the indole methoxy moiety on mitragynine (mitragynine vs corynantheidine) does not influence binding affinity to the MOP. However, removal of the methoxy group results in significant reduction in binding to the KOP. Molecular docking studies conducted by Váradi et al.17 indicated that the indole methoxy moiety on mitragynine was close to Trp293 and His297 residues in the MOP and close to Thr111 residue in the KOP. The loss of the hydrogen bonding interaction between the methoxy group and Thr111 in the KOP may help explain the reduction in KOP binding observed for corynantheidine. On the other hand, neither the methoxy or the hydroxy moieties appear to form strong interactions with either Trp293 and His297 in the MOP and may account for the similar binding affinities observed for corynantheidine, 9-hydroxycorynantheidine, and mitragynine at the MOP. The switch in chirality at position 3 from S (mitragynine) to R (speciociliatine) causes a significant change in the modeled 3D structure of the compound (Figure 2B). This change in conformation causes an increase in the molecular volume of speciociliatine compared to mitragynine which would allow for increased interactions with residues in the binding site, hence the increased affinity to opioid receptors observed (Table 2). Also, the switch in chirality at position 3 from S (mitragynine) to R (speciociliatine) seems to cause the β-methoxyacrylate moiety to adopt an axial position compared to mitragynine’s β-methoxyacrylate moiety which adopts an equatorial position (Figure 3). Molecular docking studies have shown that the position of the acrylate moiety influences the interaction with key residues (Gln124, Tyr128, and Trp293) important for binding to opioid receptors.14,17 Introduction of a hydroxy group at position 7 in 7-hydroxymitragynine significantly increased binding to the MOP (22.5-fold) compared to the KOP (2.7-fold). The hydroxy group causes a change in the shape of 7-hydroxymitragynine (relative to mitragynine), which results in a loss of planarity of the aromatic portion relative to the tertiary nitrogen (Figure 2C), causing 7-hydroxymitragynine to adopt a similar nonplanar conformation as morphine (Figure 3). This change in planarity results in the movement of the ligand away from Gln124 and Tyr128 toward Leu232 and Lys233, which allows for the hydroxy group of 7-hydroxymitragynine to form hydrogen bonding interactions with Tyr148 in the MOP.17 These additional interactions of 7-hydroxymitragynine with the MOP may account (in part) for the increased affinity. Although 7-hydroxymitragynine had additional interactions with hydrophilic residues such as Tyr312, Tyr320, and Thr111 in the KOP, mitragynine also had interactions with residues Ser211 and Trp124.17 This may explain why there was less change in affinity observed at the KOP compared to the MOP. Interestingly, removal of the methoxy group enhanced binding to the α−1D receptor by 131-fold (comparing affinity of corynantheidine and mitragynine at the α−1D receptor, Table 3). The enhanced binding affinity of corynantheidine compared to mitragynine at the α−1D receptor may be due to the presence of hydrophobic residues in the binding pocket, forming less favorable interactions with the methoxy group of mitragynine. Another reason could be that the region in the binding pocket of the α−1D receptor, where the methoxy group might have some interactions with, is very small which may then result in steric clashes with the methoxy group of mitragynine. In addition, corynantheidine adopts a similar 3D conformation as yohimbine, an adrenergic receptor antagonist (Figure 3). Yohimbine like corynantheidine, does not have a methoxy group on its indole ring. This further supports the idea that the presence of the methoxy group on the indole ring decreases binding to adrenergic receptors.
Table 1.
Screening of Selected Kratom Alkaloids at Adrenergic and Opioid Receptors
| binding site | percent displacement of bound radioligand | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| corynantheidine | 9-hydroxycorynanthei-dine | mitragynine | 7-hydroxymitragynine | speciociliatine | ||||||
| 100 nM | 10,000 nM | 100 nM | 10,000 nM | 100 nM | 10,000 nM 100 nM | 10,000 nM | 100 nM | 10,000 nM | ||
| adrenergic α1A | 45.9 | 100.5 | 16.4 | 62.3 | 13.2 | 83.5 | 10.1 | 3.5 | 16.8 | 81.1 |
| adrenergic α1B | 19.6 | 91.0 | −4.4 | 29.0 | −12.5 | 40.3 | 5.9 | −4.8 | 25.2 | 95.6 |
| adrenergic α1D | 73.5 | 101.2 | 12.4 | 66.3 | −2.1 | 57.9 | −1.9 | 13.5 | 6.3 | 70.5 |
| adrenergic a2A | 27.2 | 95.1 | −10.2 | 20.0 | −8.0 | 40.4 | −6.7 | −5.4 | 7.0 | 73.1 |
| adrenergic α2B | 8.5 | 79.9 | −5.7 | 14.6 | −16.0 | 25.1 | −9.9 | 1.1 | 0.0 | 67.2 |
| opioid δ | 8.6 | 31.4 | 3.4 | 25.3 | 0.4 | 18.3 | 19.6 | 93.7 | 0.6 | 69.2 |
| opioid κ | 16.7 | 59.4 | 6.7 | 39.5 | 25.2 | 88.3 | 41.0 | 97.8 | 61.9 | 98.5 |
| opioid μ | 39.6 | 96.8 | 29.3 | 95.4 | 29.0 | 93.7 | 81.8 | 100.7 | 64.7 | 98.0 |
| nociceptin NOP (ORL1) | −1.3 | 27.0 | −2.9 | 28.9 | 4.3 | 40.8 | −1.6 | 3.4 | −14.9 | 31.8 |
Table 2.
Affinities (Ki ± SEM nM) of Kratom Alkaloids in Specifically Binding to Opioid Receptors As Well As Subtype Selectivitya
| compd | DOP Ki ± SEM (nM) | KOP Ki ± SEM (nM) | MOP Ki ± SEM (nM) |
|---|---|---|---|
| DAMGO | ND | ND | 0.41 ± 0.04 |
| DPDPE | 1.320 ± 0.004 | ND | ND |
| U50,488 | ND | 0.300 ± 0.002 | ND |
| corynantheidine | ND | 1910 ± 50 | 118 ± 12 |
| 9-hydroxycorynantheidine | ND | ND | 105 ± 1 |
| mitragynine | ND | 198 ± 30 | 161 ± 10 |
| 7-hydroxymitragynine | 236 ± 6 | 74.1 ± 7.8 | 7.16 ± 0.94 |
| speciociliatine | ND | 116 ± 36 | 54.5 ± 4.4 |
ND: Not determined because there was less displacement of radioligand at 10,000 nM of test compound (Table 1).
Table 3.
Affinities (Ki ± SEM nM) of Kratom Alkaloids in Specifically Binding to Adrenergic Receptors As Well As Subtype Selectivitya
| compd | α−1A Ki ± SEM (nM) | α−1B Ki ± SEM (nM) | α−1D Ki ± SEM (nM) | α−2A Ki ± SEM (nM) | α−2B Ki ± SEM (nM) | α−2C Ki ± SEM (nM) |
|---|---|---|---|---|---|---|
| WB 4101b | 0.16 | 0.038 | ND | ND | ND | ND |
| prazosin | ND | ND | 0.17 ± 0.03 | ND | ND | ND |
| yohimbineb | ND | ND | ND | 3.13 | 1.16 | 0.80 |
| corynantheidine | ND | ND | 41.7 ± 4.7 | ND | ND | ND |
| mitragynine | 1340 ± 100 | 4770 ± 120 | 5480 ± 540 | 4720 ± 120 | 9290 ± 30 | 2320 ± 140 |
ND: Not determined because there was less displacement of radioligand at 10,000 nM of test compound (Table 1).
These data points were conducted once; studies are still ongoing with other kratom alkaloids and the test controls will be repeated.
Figure 2.
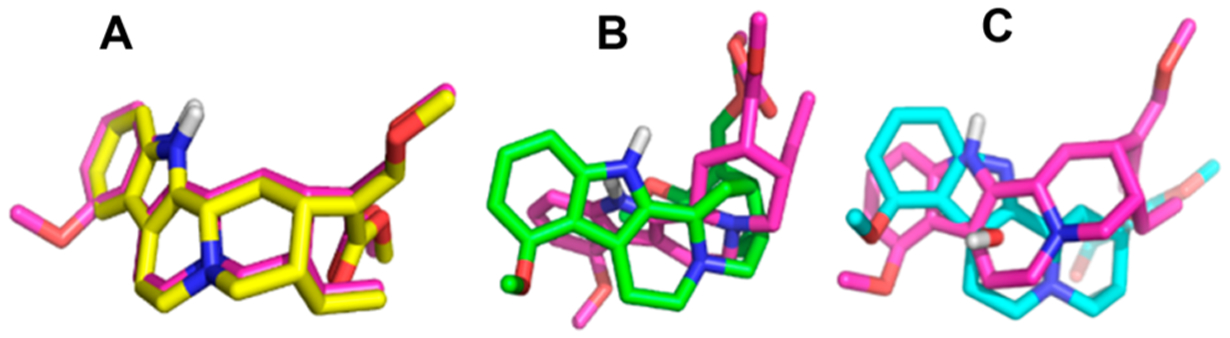
3D overlaps of mitragynine (pink) and corynantheidine (yellow) (A). 3D overlaps of mitragynine (pink) and speciociliatine (green) (B). 3D overlaps of mitragynine (pink) and 7-hydroxymitragynine (cyan) (C). Oxygen and nitrogen atoms are shown in red and blue, respectively.
Figure 3.
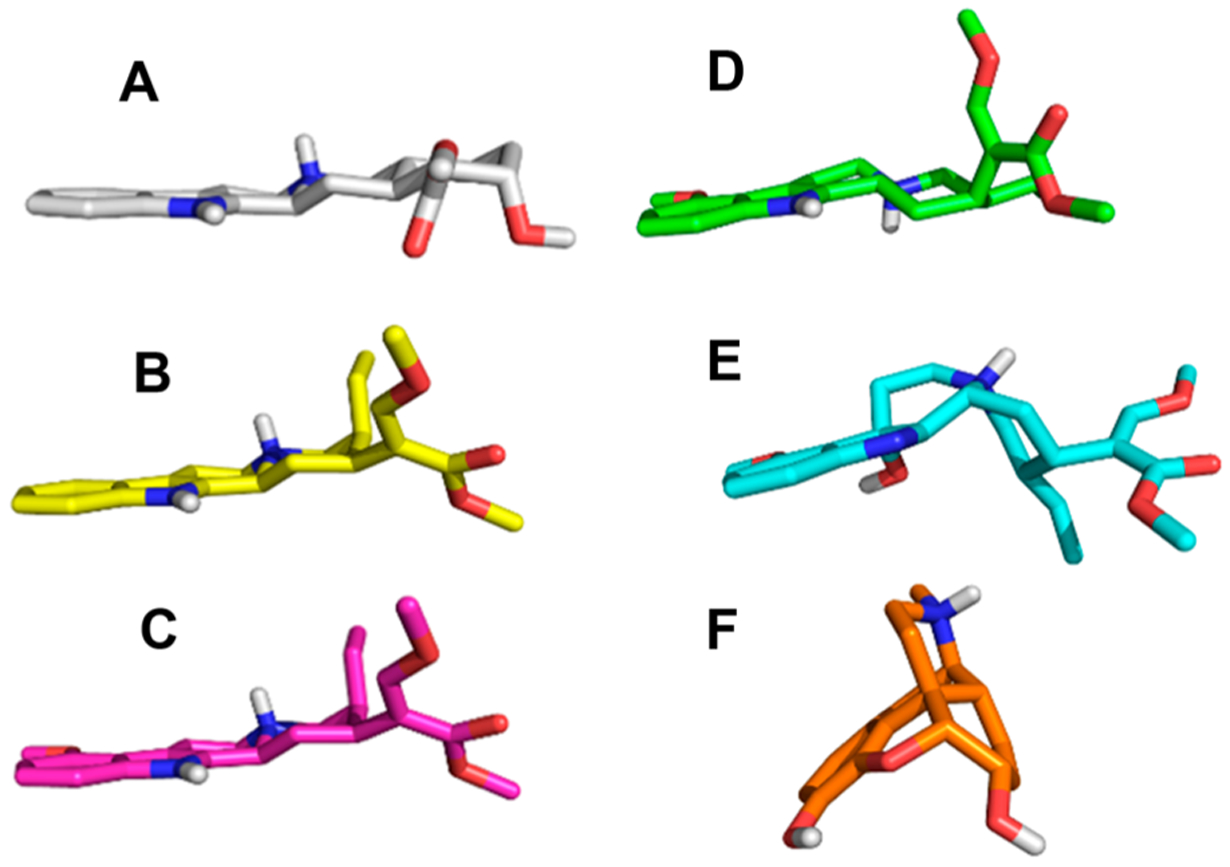
3D representations of yohimbine (gray) (A), corynantheidine (yellow) (B), mitragynine (pink) (C), speciociliatine (green) (D), 7-hydroxymitragynine (cyan) (E), and morphine (orange) (F). Oxygen and nitrogen atoms are shown in red and blue, respectively.
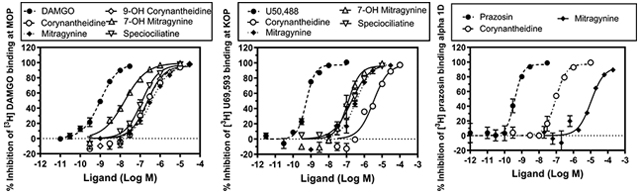
The functional effects of mitragynine and 7-hydroxymitragynine were evaluated at MOP, KOP, and DOP (Figure 4). Mitragynine was further evaluated at adrenergic receptors for agonist and antagonist effects (SI, Figures S1,S2). 7-Hydroxymitragynine was found to be a full agonist at MOP and a competitive antagonist at DOP and KOP, while mitragynine and speciociliatine were partial agonists at MOP (Figure 4). Speciociliatine had no agonist or antagonist effects at the KOP. Mitragynine was a partial agonist at α1A,D (SI, Figure S1) and produced competitive antagonist effects at α−1A,B,D,2C (SI, Figures S1,S2). Interestingly, α1 adrenergic antagonists have been shown to be effective in reversing the rigidity in the diaphragm, chest wall, and upper airway (wooden chest syndrome) produced by fentanyl, which suggests that mitragynine may be useful in curbing fentanyl related overdose.25 Further studies conducted to investigate the in vivo functional effects of mitragynine, 7-hydroxymitragynine, and speciociliatine using the hot plate test at 52 ± 0.1 °C revealed that 7-hydroxymitragynine and speciociliatine produced maximum response (100% MPE), with 7-hydroxymitragynine being more potent than speciociliatine and morphine but less potent than fentanyl (Figure 5). Speciociliatine had a similar potency to morphine, and mitragynine had the least efficacy among the compounds tested at the highest dose assayed. The antinociceptive effect of 7-hydroxymitragynine was reversed by 0.1 mg/kg naltrexone (Figure 5), which suggests that 7-hydroxymitragynine may be acting through the MOP as demonstrated by the binding and in vitro functional assays. Speciociliatine had antinociceptive effects at 10 mg/kg; however, this dose produced lethality in 5/21 rats tested, indicating a narrow therapeutic window. Because the ED50 of speciociliatine to produce antinociception is close to its LD50 value, in this acute antinociception assay (i.e., hot plate test), it was not feasible to evaluate the extent to which the antinociception observed was due to activation of opioid receptors by conducting antagonism tests with opioid subtype antagonists. In addition, speciociliatine similar to U69, 593 produced hypothermia but not 7-hydroxymitragynine or mitragynine.27 Collectively, this profile of in vivo activity may indicate that speciociliatine is acting, at least in part, through nonopioid receptors. Our results together with other studies have shown that 7-hydroxymitragynine is more potent at the MOP than mitragynine (7-hydroxymitragynine EC50 = 53 ± 4 nM, mitragynine EC50 = 203 ± 13 nM,17 7-hydroxymitragynine EC50 = 34.5 ± 4.5, mitragynine EC50 = 339 ± 178 nM14). These results together with previous studies which showed that mitragynine has a lower efficacy than 7-hydroxymitragynine may help explain the lower abuse liability observed for mitragynine compared to 7-hydroxymitragy-nine.14,16,17,28 The functional results obtained for speciociliatine at the MOP (Figure 4, upper panels) are contrary to what was previously reported by Kruegel et al., where they showed that speciociliatine had no measurable agonist activity at any of the human opioid receptors and had only weak antagonist effects.14 Our results together with previous studies by Takayama et al. (speciociliatine produced maximum inhibition of electrically induced twitch contraction of guinea pig ileum similar to morphine) show that speciociliatine may be acting as an opioid agonist.15 These differences in the in vitro functional effects observed may be due to the different assay types used to evaluate speciociliatine. In the Kruegel et al. study, a bioluminescence resonance energy transfer (BRET) assay was used, while in our study a homogeneous time-resolved fluorescence (HTRF) assay was used. Different types of functional assays may result in different agonistic effects as reported by Niedernberg et al.29
Figure 4.
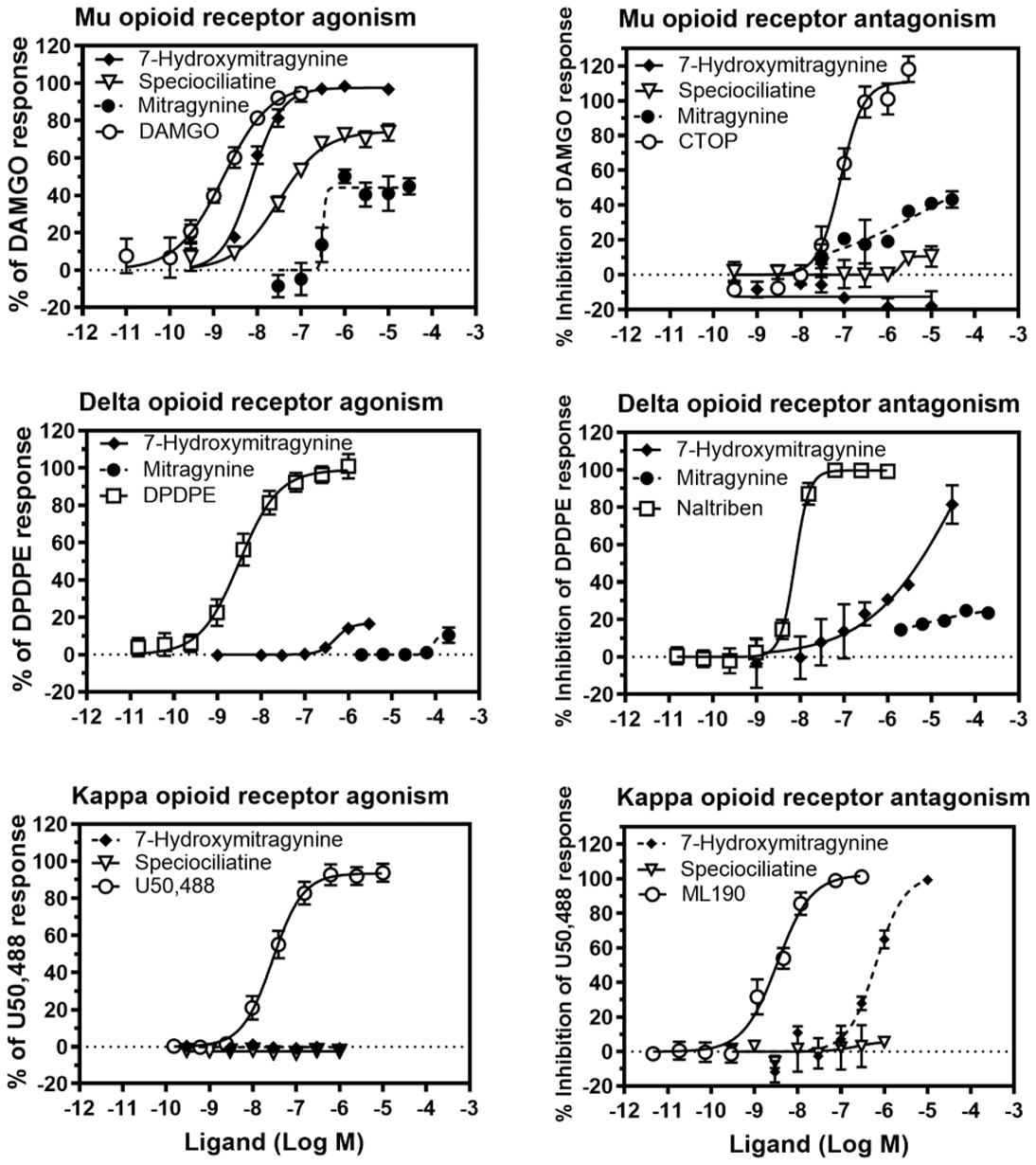
Concentration effect curves of mitragynine and 7-hydroxymitragynine at MOP (top left), DOP (middle left), and KOP (bottom left). Concentration % inhibition of control agonist effect curves of mitragynine and 7-hydroxymitragynine at MOP (top right), DOP (middle right), and KOP (bottom right). The EC50 of 7-hydroxymitragynine, speciociliatine, and mitragynine at MOP were determined as 7.6, 39.2, and 307.5 nM, respectively. The KB of 7-hydroxymitragynine at DOP and KOP were determined as 550.2 and 115.0 nM, respectively. The KB of mitragynine at MOP was determined as 179.2 nM.
Figure 5.
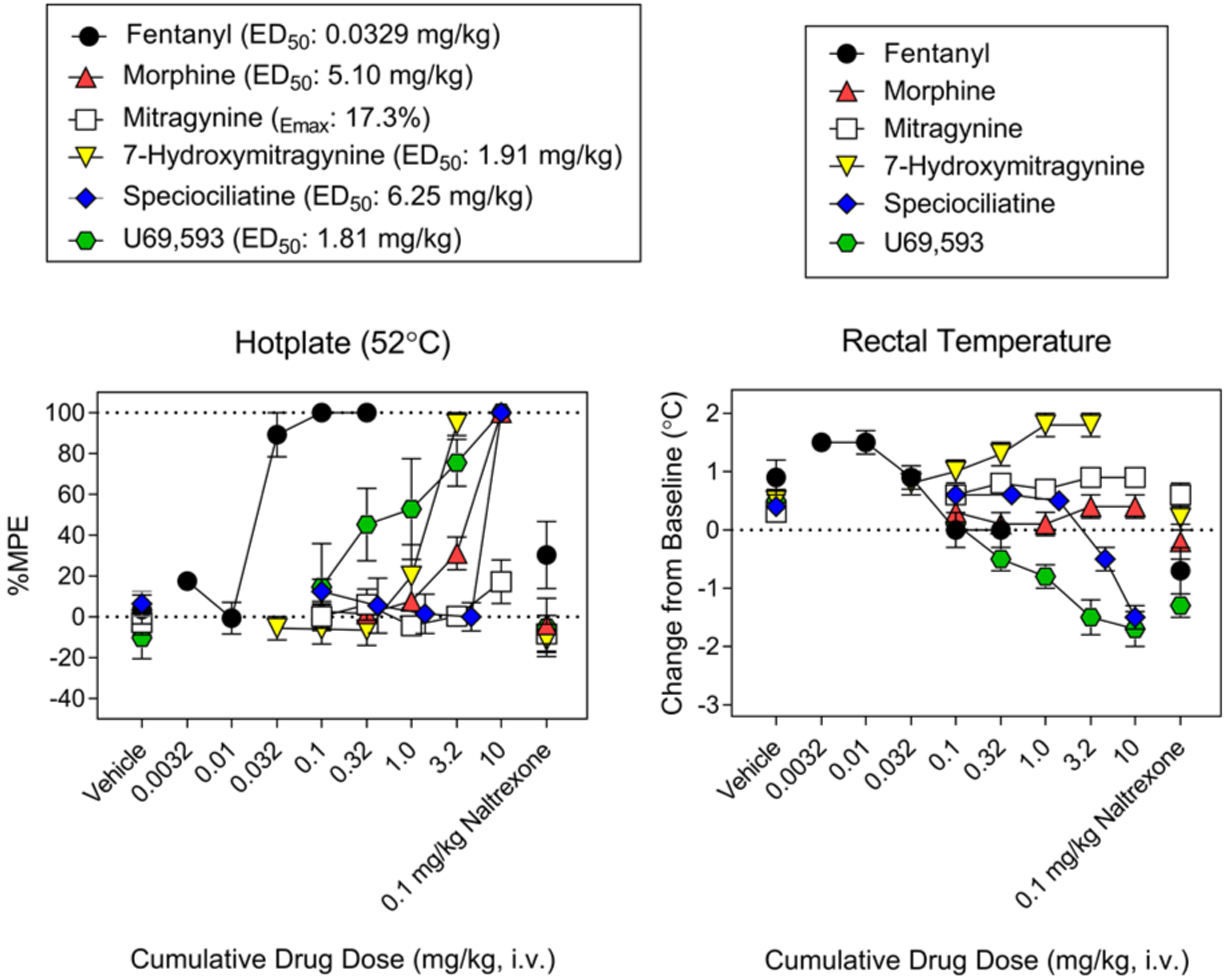
Hot plate test in rats (n = 6) at 52 ± 0.1 °C (left) showing antinociceptive effects of mitragynine, 7-hydroxymitragynine, speciociliatine, fentanyl, morphine, and U69, 593. Change in body temperature (right) produced by mitragynine, 7-hydroxymitragynine, speciociliatine, fentanyl, morphine, and U69, 593. Treatment with 0.1 mg/kg naltrexone antagonized antinociception of all the compounds tested. All drugs were administered intravenously (iv).
Pharmacokinetic studies were conducted to investigate the metabolism and plasma protein binding properties of the selected indole-based kratom alkaloids (Table 4). The in vitro metabolic half-life (t1/2) values were used to estimate the in vitro intrinsic clearance and further extrapolated to determine the hepatic clearance using the well-stirred model as described by Obach.30 Corynantheidine (t1/2 = 6.1 min) was found to be unstable in human liver microsomes compared to mitragynine (t1/2= 20 min) and speciociliatine (t1/2= 41.8 min). All examined alkaloids exhibited high plasma protein binding of >97% in human plasma except 7-hydroxymitragynine, which was reported to be 90% bound to plasma protein.26 The extrapolation of the in vitro intrinsic clearance to hepatic clearance suggests that corynantheidine would possess poor systemic exposure in vivo compared to mitragynine and speciociliatine. However, the plasma and microsomal protein binding corrected hepatic ratio suggests that the alkaloids have low hepatic extraction ratios (<0.3). In general, the binding correction for clearance prediction resulted in an under-prediction of clearance. Therefore, it is important to perform in vivo pharmacokinetic studies in animal models and establish a correlation to better predict the human hepatic clearance of these alkaloids.31 Interestingly, the hydroxylated kratom alkaloids, 9-hydroxycorynantheidine (t1/2= 181 min) and 7-hydroxymitragynine (t1/2 = 170 min), were found to be resistant toward oxidative metabolism in human liver microsomes, and there is a likelihood that these could undergo phase II conjugative metabolism, thus resulting in higher metabolic clearance.
Table 4.
In Vitro Pharmacokinetic Parameters of Kratom Alkaloidsa
| compounds | parameters | ||||||
|---|---|---|---|---|---|---|---|
| k (min −1) | t1/2 (min) | fu | fu, mic | CLint,H (mL/min/kg) | CLH (mL/min/kg) | E | |
| corynantheidine | 0.1145 | 6.1 | 0.02 | 0.4 | 103.1 | 4.1 | 0.20 |
| 9-hydroxycorynantheidine | 0.0038 | 181.1 | 0.027 | 0.534 | 3.4 | 0.2 | 0.01 |
| 7-hydroxymitragynine | 0.0041 | 170.1 | 0.1b | 0.852 | 3.7 | 0.4 | 0.02 |
| mitragynine | 0.0347 | 20.0 | 0.02 | 0.307 | 31.2 | 1.9 | 0.09 |
| speciociliatine | 0.0166 | 41.8 | 0.01 | 0.276 | 14.9 | 0.5 | 0.03 |
Abbreviations: k = elimination rate constant; t1/2 = in vitro half-life; fu = fraction unbound in human plasma; fu, mic = fraction unbound in human liver microsomes; CLint,H = in vitro hepatic intrinsic clearance; CLint,H = hepatic clearance; E = hepatic extraction ratio. Each value represents mean of triplicate experiments.
Fraction unbound in human plasma for 7-hydroxymitragynine was obtained from the literature.26
CONCLUSION
In summary, 7-hydroxymitragynine had the highest affinity at opioid receptors when compared to mitragynine and corynantheidine. Corynantheidine had the highest affinity at adrenergic receptors. Speciociliatine had dual affinity to both the KOP and MOP, produced agonistic effects at the MOP, and produced antinociceptive effects and hypothermia. 7-Hydroxymitragynine was more potent than morphine and speciociliatine in the hot plate antinociceptive test and a full agonist in the in vitro functional assay. All the alkaloids exhibited high plasma protein binding of >97% in human plasma except 7-hydroxymitragynine. In addition, the hydroxylated kratom alkaloids, 9-hydroxycorynantheidine and 7-hydroxymitragynine, were found to be resistant toward oxidative metabolism in human liver microsomes compared to the nonhydroxylated alkaloids. The polypharmacology ex hibited by the kratom alkaloids may support the claims made by patients taking kratom for the self-management of numerous diseases such as pain, OUD, and opioid withdrawal.
EXPERIMENTAL SECTION
General Chemistry.
The compounds were available in our alkaloid library and were isolated and structural elucidated through 1H NMR, 13C NMR, and HRMS using Bruker model AMX 500 and Avance NEO 600 NMR spectrometers operating at 500 and 600 MHz in 1H and 126 and 151 MHz in 13C, respectively. HRMS and purity (≥95%) were determined using an Agilent 1290 Infinity series ultraperformance liquid chromatography (UPLC) system equipped with photodiode array detector and quadrupole-time-of-flight (QTOF) Agilent 6540 mass spectrometer. The isolation of mitragynine, corynantheidine, and speciociliatine were done following the procedure described in Sharma et al., 2019.18 7-Hydroxymitragynine and 9-hydroxycorynantheidine were obtained by semisynthesis from mitragynine following the procedure reported by Kruegel et al., 2016.14 The detailed experimental procedures and characterization of the kratom alkaloids are available in the Supporting Information (SI).
Radioligand Binding and Functional Assays.
The kratom alkaloids were screened at Eurofins Cerep (Celle l’Evescault, France) for their in vitro binding affinity and efficacy at alpha adrenergic and opioid receptors. Briefly, each cell membrane homogenate was incubated with a radioligand in the absence or presence of the kratom alkaloids in a buffer. Nonspecific binding was determined in the presence of a specific agonist or antagonist at the target receptor. Following incubation, the samples were filtered rapidly under vacuum through glass fiber filters presoaked in a buffer and rinsed several times with an ice-cold buffer using a 48- or 96-sample cell harvester. The cAMP and calcium mobilization assays were used to evaluate the functional effects of the kratom alkaloids. The experimental conditions that were used for the binding and functional assays are summarized in SI, Tables S6, S7.
Hot Plate and Hypothermia Test.
Antinociceptive testing was performed in the hot plate test, as previously described.32 Sprague-Dawley rats were placed on a heated (52 °C) enclosed Hot Plate Analgesia Meter (Columbus Instruments, Columbus, OH), and latency to jump or lick/shake the back paws was determined. If there was no response within 60 s, the rat was removed from the apparatus. All compounds were administered intravenously using cumulative dosing every 5 min until the rats maxed out on the hot plate (60 s). The rectal temperature was taken immediately after measuring the latency. Once they maxed out, 0.1 mg/kg naltrexone (iv) was then administered to antagonize opioid effects. All rat studies were conducted in accordance with the National Institutes of Health Guidelines for Animal Care and Use and with approved animal protocols from the University of Florida Animal Care and Use Committee.
3D Chemical Representation of Opioid Alkaloids.
Chemical structures of the compounds in the figures overlapped were sketched in SybylX2.1.1, and Gasteiger-Hückel charges were assigned before energy minimization (100,000 iterations) with Tripos Force Fields. Chemical structures in the 3D representation of the compounds were built in Chimera 1.13.1 using the isomeric SMILES from Pubchem. Gasteiger charges were assigned before energy minimization (100,000 conjugate gradient steps). Pictures of the compounds were generated using PyMOL Molecular Graphics System, version 1.3.0.0, Schrödinger, LLC.
Human Plasma and Microsomal Binding.
Alkaloids at 1.0 μM concentration were mixed with human plasma or inactivated human liver microsomes. The mixtures were subjected to equilibrium dialysis versus 50 mM phosphate buffer pH 7.4 at 37 °C for 4 h using an HT from Dialysis LLC (Groton, Connecticut) as previously described.33 The dialysis membrane of molecular weight cutoff 12–14 kDa was used. Dialysis experiments were done in triplicate. On completion of the dialysis period, the plasma, microsomal, and buffer samples were removed. Recovery through the dialysis procedure was determined by analyzing samples of the mixtures that were not subjected to dialysis, and recovery values were found to be ≥86%.
Metabolic Stability of Kratom Alkaloids in Human Liver Microsomes.
The in vitro metabolic stability of each alkaloid was performed using human liver microsomes in triplicate. The incubation mixtures consisted of human liver microsomes, substrate, and NADPH. Reactions were initiated with the addition of NADPH and kept in an incubator shaker at 37 °C. Aliquots were withdrawn at 0, 5, 10, 15, 30, and 60 min and the reaction terminated with acetonitrile containing phenacetin (internal standard) and then filtered. The filtrates were subjected to UPLC-mass spectrometry (MS/MS) analysis. The in vitro elimination half-life (t1/2), intrinsic hepatic clearance, and extrapolated hepatic clearance were determined as described by Obach.30 The equations used for the calculation of the in vitro pharmacokinetic parameters are shown in the SI.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by National Institute on Drug Abuse grants DA47855 and DA48353. This work was also supported by funds from the State of Florida and a generous gift through the University of Florida Foundation.
ABBREVIATIONS USED
- CHO
Chinese hamster ovary
- DOP
delta opioid receptor
- FDA
Food and Drug Administration
- HEK
human embryonic kidney
- KOP
kappa opioid receptor
- MOP
mu opioid receptor
- OUD
opioid use disorder
- RBL
rat basophilic leukemia
Footnotes
The authors declare no competing financial interest.
Supporting Information
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.jmedchem.9b01465.
Experimental procedures and spectroscopic data for the kratom alkaloids, details of the PK studies, and assay protocols for the binding and in vivo studies (PDF)
Molecular formula strings (CSV)
REFERENCES
- (1).Grundmann O Patterns of Kratom Use and Health Impact in the US—Results from an Online Survey. Drug Alcohol Depend. 2017, 176, 63. [DOI] [PubMed] [Google Scholar]
- (2).Coe MA; Pillitteri JL; Sembower MA; Gerlach KK; Henningfield JE Kratom as a Substitute for Opioids: Results from an Online Survey. Drug Alcohol Depend. 2019, 202, 24. [DOI] [PubMed] [Google Scholar]
- (3).Adkins JE; Boyer EW; McCurdy CR Mitragyna Speciosa, A Psychoactive Tree from Southeast Asia with Opioid Activity. Curr. Top. Med. Chem 2011, 11, 1165. [DOI] [PubMed] [Google Scholar]
- (4).Prozialeck WC; Jivan JK; Andurkar SV Pharmacology of Kratom: An Emerging Botanical Agent with Stimulant, Analgesic and Opioid-like Effects. J. Am. Osteopath. Assoc 2012, 112, 792. [PubMed] [Google Scholar]
- (5).Aggarwal G; Robertson E; McKinlay J; Walter E Death from Kratom Toxicity and the Possible Role of Intralipid. J. Intensive Care Soc 2018, 19, 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Matson M; Schenk N Fatality of 33-Year-Old Man Involving Kratom Toxicity. J. Forensic Sci 2019, 64, 1933. [DOI] [PubMed] [Google Scholar]
- (7).Eggleston W; Stoppacher R; Suen K; Marraffa JM; Nelson LS Kratom Use and Toxicities in the United States. Pharmacotherapy 2019, 39, 775. [DOI] [PubMed] [Google Scholar]
- (8).Kuehn B Kratom-Related Deaths. JAMA 2019, 321, 1966. [DOI] [PubMed] [Google Scholar]
- (9).Olsen EO; O’Donnell J; Mattson CL; Schier JG; Wilson N Notes from the Field: Unintentional Drug Overdose Deaths with Kratom Detected — 27 States, July 2016-December 2017. MMWR.Morb. Mortal. Wkly. Rep 2019, 68, 326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Statement from FDA Commissioner Scott Gottlieb, M.D., on the agency’s scientific evidence on the presence of opioid compounds in kratom, underscoring its potential for abuse; U.S. Food and Drug Administration, 2018; https://www.fda.gov/news-events/press-announcements/statement-fda-commissioner-scott-gottlieb-mdagencys-scientific-evidence-presence-opioid-compounds (accessed Jun 24, 2019). [Google Scholar]
- (11).Karinen R; Fosen JT; Rogde S; Vindenes V An Accidental Poisoning with Mitragynine. Forensic Sci. Int 2014, 245, e29. [DOI] [PubMed] [Google Scholar]
- (12).Kronstrand R; Roman M; Thelander G; Eriksson A Unintentional Fatal Intoxications with Mitragynine and O-Desmethyltramadol from the Herbal Blend Krypton. J. Anal. Toxicol 2011, 35, 242. [DOI] [PubMed] [Google Scholar]
- (13).McIntyre IM; Trochta A; Stolberg S; Campman SC Mitragynine ‘Kratom’ Related Fatality: A Case Report with Postmortem Concentrations. J. Anal. Toxicol 2015, 39, 152. [DOI] [PubMed] [Google Scholar]
- (14).Kruegel AC; Gassaway MM; Kapoor A; Váradi A; Majumdar S; Filizola M; Javitch JA; Sames D Synthetic and Receptor Signaling Explorations of the Mitragyna Alkaloids: Mitragynine as an Atypical Molecular Framework for Opioid Receptor Modulators. J. Am. Chem. Soc 2016, 138, 6754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Takayama H; Ishikawa H; Kurihara M; Kitajima M; Aimi N; Ponglux D; Koyama F; Matsumoto K; Moriyama T; Yamamoto LT; Watanabe K; Murayama T; Horie S Studies on the Synthesis and Opioid Agonistic Activities of Mitragynine-Related Indole Alkaloids: Discovery of Opioid Agonists Structurally Different from Other Opioid Ligands. J. Med. Chem 2002, 45, 1949. [DOI] [PubMed] [Google Scholar]
- (16).Hemby SE; McIntosh S; Leon F; Cutler SJ; McCurdy CR Abuse Liability and Therapeutic Potential of the Mitragyna Speciosa (Kratom) Alkaloids Mitragynine and 7-Hydroxymitragynine. Addict. Biol 2019, 24, 874. [DOI] [PubMed] [Google Scholar]
- (17).Váradi A; Marrone GF; Palmer TC; Narayan A; Szabó MR; Le Rouzic V; Grinnell SG; Subrath JJ; Warner E; Kalra S; Hunkele A; Pagirsky J; Eans SO; Medina JM; Xu J; Pan Y-X; Borics A; Pasternak GW; McLaughlin JP; Majumdar S Mitragynine/Corynantheidine Pseudoindoxyls As Opioid Analgesics with Mu Agonism and Delta Antagonism, Which Do Not Recruit β-Arrestin-2. J. Med. Chem 2016, 59, 8381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Sharma A; Kamble SH; León F; Chear NJ-Y; King TI; Berthold EC; Ramanathan S; McCurdy CR; Avery BA Simultaneous Quantification of Ten Key Kratom Alkaloids in Mitragyna Speciosa Leaf Extracts and Commercial Products by Ultra-performance Liquid Chromatography-tandem Mass Spectrometry. Drug Test. Anal 2019, 11, 1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Kruegel AC; Uprety R; Grinnell SG; Langreck C; Pekarskaya EA; Le Rouzic V; Ansonoff M; Gassaway MM; Pintar JE; Pasternak GW; Javitch JA; Majumdar S; Sames D 7-Hydroxymitragynine Is an Active Metabolite of Mitragynine and a Key Mediator of Its Analgesic Effects. ACS Cent. Sci 2019, 5, 992–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Kamble SH; Sharma A; King TI; León F; McCurdy CR; Avery BA Metabolite Profiling and Identification of Enzymes Responsible for the Metabolism of Mitragynine, the Major Alkaloid of Mitragyna Speciosa (Kratom). Xenobiotica 2019, 49, 1279. [DOI] [PubMed] [Google Scholar]
- (21).Connock M; Juarez-Garcia A; Jowett S; Frew E; Liu Z; Taylor RJ; Fry-Smith A; Day E; Lintzeris N; Roberts T; Burls A; Taylor RS Methadone and Buprenorphine for the Management of Opioid Dependence: A Systematic Review and Economic Evaluation. Health Technol. Assess 2007, 11, 1–171 iii-iv. [DOI] [PubMed] [Google Scholar]
- (22).Gorodetzky CW; Walsh SL; Martin PR; Saxon AJ; Gullo KL; Biswas K A Phase III, Randomized, Multi-Center, Double Blind, Placebo Controlled Study of Safety and Efficacy of Lofexidine for Relief of Symptoms in Individuals Undergoing Inpatient Opioid Withdrawal. Drug Alcohol Depend. 2017, 176, 79. [DOI] [PubMed] [Google Scholar]
- (23).Boyer EW; Babu KM; Adkins JE; McCurdy CR; Halpern JH Self-Treatment of Opioid Withdrawal Using Kratom (Mitragynia Speciosa Korth). Addiction 2008, 103, 1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Kruegel AC; Grundmann O The Medicinal Chemistry and Neuropharmacology of Kratom: A Preliminary Discussion of a Promising Medicinal Plant and Analysis of Its Potential for Abuse. Neuropharmacology 2018, 134, 108. [DOI] [PubMed] [Google Scholar]
- (25).Torralva PR; Janowsky A Noradrenergic Mechanisms in Fentanyl-Mediated Rapid Death Explain Failure of Naloxone in the Opioid Crisis. J. Pharmacol. Exp. Ther 2019, 371, 453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Manda V; Avula B; Ali Z; Khan I; Walker L; Khan S Evaluation of In Vitro Absorption, Distribution, Metabolism, and Excretion (ADME) Properties of Mitragynine, 7-Hydroxymitragynine, and Mitraphylline. Planta Med. 2014, 80, 568. [DOI] [PubMed] [Google Scholar]
- (27).Minervini V; Dahal S; France CP Behavioral Characterization of k Opioid Receptor Agonist Spiradoline and Cannabinoid Receptor Agonist CP55940 Mixtures in Rats S. J. Pharmacol. Exp. Ther 2017, 360, 280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Yue K; Kopajtic TA; Katz JL Abuse Liability of Mitragynine Assessed with a Self-Administration Procedure in Rats. Psychopharmacology (Berl). 2018, 235, 2823. [DOI] [PubMed] [Google Scholar]
- (29).Niedernberg A; Tunaru S; Blaukat A; Harris B; Kostenis E Comparative Analysis of Functional Assays for Characterization of Agonist Ligands at G Protein-Coupled Receptors. J. Biomol. Screening 2003, 8, 500. [DOI] [PubMed] [Google Scholar]
- (30).Obach RS Prediction of Human Clearance of Twenty-Nine Drugs from Hepatic Microsomal Intrinsic Clearance Data: An Examination of In Vitro Half-Life Approach and Nonspecific Binding to Microsomes. Drug Metab. Dispos 1999, 27, 1350–1359. [PubMed] [Google Scholar]
- (31).Wood FL; Houston JB; Hallifax D Clearance Prediction Methodology Needs Fundamental Improvement: Trends Common to Rat and Human Hepatocytes/Microsomes and Implications for Experimental Methodology. Drug Metab. Dispos 2017, 45, 1178. [DOI] [PubMed] [Google Scholar]
- (32).Hiranita T; Leon F; Felix JS; Restrepo LF; Reeves ME; Pennington AE; Obeng S; Avery BA; McCurdy CR; McMahon LR; Wilkerson JL The Effects of Mitragynine and Morphine on Schedule-Controlled Responding and Antinociception in Rats. Psychopharmacology (Berl). 2019, 236, 2725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Zamek-Gliszczynski MJ; Ruterbories KJ; Ajamie RT; Wickremsinhe ER; Pothuri L; Rao MVS; Basavanakatti VN; Pinjari J; Ramanathan VK; Chaudhary AK Validation of 96-Well Equilibrium Dialysis with Non-Radiolabeled Drug for Definitive Measurement of Protein Binding and Application to Clinical Development of Highly-Bound Drugs. J. Pharm. Sci 2011, 100, 2498. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


