Abstract
Healthcare workers (HCWs) stand at the frontline for fighting coronavirus disease 2019 (COVID-19) pandemic. This puts them at higher risk of acquiring the infection than other individuals in the community. Defining immunity status among health care workers is therefore of interest since it helps to mitigate the exposure risk. This study was conducted between May 20th and 30th, 2020. Eighty-five hospitals across Kingdom of Saudi Arabia were divided into 2 groups: COVID-19 referral hospitals are those to which RT-PCR-confirmed COVID-19 patients were admitted or referred for management (Case-hospitals). COVID-19 nonaffected hospitals where no COVID-19 patients had been admitted or managed and no HCW outbreak (Control hospitals). Next, seroprevalence of severe acute respiratory syndrome coronavirus 2 among HCWs was evaluated; there were 12,621 HCWs from the 85 hospitals. There were 61 case-hospitals with 9379 (74.3%) observations, and 24 control-hospitals with 3242 (25.7%) observations. The overall positivity rate by the immunoassay was 299 (2.36%) with a significant difference between the case-hospital (2.9%) and the control-group (0.8%) (P value <0.001). There was a wide variation in the positivity rate between regions and/or cities in Saudi Arabia, ranging from 0% to 6.31%. Of the serology positive samples, 100 samples were further tested using the SAS2pp neutralization assay; 92 (92%) samples showed neutralization activity.
The seropositivity rate in Kingdom of Saudi Arabia is low and varies across different regions with higher positivity in case-hospitals than control-hospitals. The lack of neutralizing antibodies (NAb) in 8% of the tested samples could mean that assay is a more sensitive assay or that neutralization assay has a lower detection limits; or possibly that some samples had cross-reaction to spike protein of other coronaviruses in the assay, but these were not specific to neutralize severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).
Key Words: SARS-CoV-2, COVID-19, seroprevalence, serology, healthcare workers
1. Introduction
Healthcare workers (HCWs) stand at the frontline for fighting coronavirus disease 2019 (COVID-19) pandemic. This puts them at higher risk of acquiring the infection than other individuals in the community (Ferioli et al., 2020). Several hospitals, since the beginning of this pandemic, have implemented strategies to protect their HCWs that include, but not limited to, providing adequate personal protective equipment (PPE), weekly shifts system, period screening of their staff, and other infection prevetion and control (IPC) measures (Al-Tawfiq et al., 2020; Barranco and Ventura, 2020; Galan et al., 2020). Since the global emergence of this pandemic, in March 2020, many health care settings have started to report the burden of COVID-19 infection among their HCWs (Barranco and Ventura, 2020; Folgueira et al., 2020; Wei et al., 2020). However, reporting only symptomatic and infected cases among HCWs could lead to a significant underestimation of the prevalence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. Thus, many reports indicate the presence of subclinical infection among HCWs, which impose threaten risk to other patients, co-workers, and families (Ferioli et al., 2020; Korth et al., 2020). Defining immunity status among health care workers, therefore, is of interest since it helps to mitigate the exposure risk.
The evidence on COVID-19 infection among HCWs is growing and several studies had estimated the seroprevalence of SARS-CoV-2 among their HCWs. The results of those studies indicate that between 1.7% to 11% of HCWs were seropositive (Brandstetter et al., 2020; Folgueira et al., 2020; Galan et al., 2020; Garcia-Basteiro et al., 2020; Paderno et al., 2020). Importantly, a number of those studies reported the occurrence of seropositivity among individuals who did not report any symptoms by 38% to 48% (Folgueira et al., 2020; Galan et al., 2020; Garcia-Basteiro et al., 2020). The advantages of seroprevalence studies rely on the usefulness of such a method to assess the level of subclinical exposure among cases and identify high-risk groups (Al-Tawfiq and Memish, 2020). The aim of the study was to evaluate seroprevalence of SARS-CoV-2 antibodies among HCW in various hospitals in the Kingdom of Saudi Arabia (KSA) and to compare seroprevalence between HCWs in hospitals caring for COVID-19 patients and other hospitals.
2. Materials and methods
2.1. Study population
The study included hospitals with more than 200 beds and the study was conducted between May 20th and 30th, 2020. Study hospitals were divided into 2 groups: COVID-19 referral and/or affected hospitals are those to which real-time reverse-transcriptase polymerase chain reaction (RT-PCR)-confirmed COVID-19 patients were admitted or referred for management (Case-hospitals). COVID-19 nonaffected hospitals where no COVID-19 patients had been admitted or managed and no HCW outbreak (Control hospitals). We aimed to include 12,000 HCWs with a Case Control ratio of 2:1. HCWs who agreed to participate signed consents for participation. Health workers included physicians, nurses, pharmacists, respiratory therapists, and administrative support who agree to participate in the study. The HCWs were from departments at high risk to get exposed to COVID 19 cases: medicine, intensive care units, and emergency departments. We excluded HCWs who were experiencing any suggestive symptoms of COVID-19 at the time of enrolment.
Specimens were transported to the Saudi CDC Lab. Samples were transported and delivered within 48 hours at 4 to 8°C. When serum samples were not processed immediately, sera were stored at −80°C. Serological testing: Serum samples were screened for the presence of SARS-CoV-2 antibodies using a chemiluminescent microparticle immunoassay (CMIA) which detects IgG raised against the nucleocapsid protein of SARS-CoV-2. (Abbott Architect SARS-CoV-2 IgG kit, Abbott, IL).
2.2. SARS-CoV-2 pseudotyped viral particles (SARS2pp) neutralization assay
SARS-CoV-2 pseudotyped viral particles (SARS2pp) were produced in HEK293T cells and titrated using Huh7.5 cells as described for MERSpp previously (Alharbi et al., 2019; Almasaud et al., 2020; Grehan et al., 2015). Here, 100 samples that were positive by immunoassay (one-third of the positive samples) were prepared in a 1:20 and 1:40 dilutions to assess their neutralization activity (percent). In addition, 20 of these 100 samples were prepared in 8 serial dilutions (3-fold) starting from 1:20, and tested for neutralizing antibodies (NAbs) titre in duplicates.
A standard concentration of SARS2pp (equivalent to 200,000 RLU) and Huh7.5 cells (10,000 cells) were added to each well of 96-well opaque plate. Cells only and cells-plus-SARS2pp only (both without serum) were included in quadruplicate as controls to determine 0% and 100% neutralization activity, respectively. Following 48 hours incubation, cells were lysed and the assay was developed using Bright-Glo Luciferase Assay System (Promega, Madison, WI) and luciferase activity was measured using a luminometer. Samples were considered positive if neutralization activity was detected in both dilutions (1:20 and 1:40) and the neutralization percent was reported as compared to cell-plus-SARS2pp control. For 20 samples, a further evaluation of the NAb titres (IC50 neutralization titres) were calculated for each serum sample across 8 dilutions and plotted using GraphPad Prism.
The study was approved by the Saudi Ministry of health IRB (H-01-R-009).
2.3. Statistical method
The descriptive analysis included counts and proportions for categorical variables. For bivariable analysis, the χ2 test was conducted to assess the association of the positivity rates of serologic tests between Case-hospitals and control hospitals. Another χ2 test was conducted to assess the association between regions and the serologic test results. We employed logistic regression models to examine the relationship between the positivity of serologic testa and the exposure Case-control hospitals, as well the relationship between the outcome serology test and the exposure Regions. The magnitude of association presented as the odds. All reported 95% CI and P values in the 2 models were based on the logistic regression. We used STATA 15.1 to perform all of the analysis. The significance level for all of the statistical tests was set at 0.05.
3. Results
During the study period, there were 12,621 HCWs from 85 hospitals. There were 61 case-hospitals with 9379 (74.3%) HCWs, and 24 control-hospitals with 3242 (25.7%) HCWs. The overall positivity rate by immunoassay was 299 (2.36%) of the total 12621 HCWs (Table 1 ). Out of the 299 seropositive HCWs, 86 (28.7%) were positive by PCR for SARS-CoV-2. However, none was symptomatic as we excluded symptomatic HCWs from the study. There was a significant difference in the positivity rate between case-hospitals (2.9%) and the control-hospitals (0.8%) (table 1) with a crude Odd Ratio (OR) of 3.71 (95% confidence interval [CI]; 2.47–5.55) (P value <0.001). There was a wide variation in the positivity rate between regions and/or cities in Saudi Arabia from 0% to 6.31% (Table 2 ). The crude Odd Ratio based on Riyadh (1.06% positivity rate) is also shown in table 2. The highest rate of positivity was in Makkah (6.31%, OR 6.3) and Al-Madinah Al-Mounawarah (4.55%, OR 4.46); followed by the Eastern Region (1.55%, OR 1.47), Aseer (2.18%, OR 2.08).
Table 1.
Rate of positive serological assay between control-hospitals and case-hospitals among healthcare workers.
| Total Number | Number Positive Serology | Percent positive Serology | |
|---|---|---|---|
| Control | 3242 | 26 | 0.8 |
| Case | 9379 | 273 | 2.9 |
| Total | 12,621 | 299 | 2.36 |
| Pearson χ2(1) = 44.2698 P = 0.0001 | |||
Table 2.
Percentage of positivity rate among healthcare workers in relation to the region/city in Saudi Arabia; Crude Odd Ratio is based on the positivity rate in Riyadh.
| Regions | Number Positive | Percent of positive | Total | Crude OR (95% CI) | (95% CI) | P value | |
|---|---|---|---|---|---|---|---|
| Hail | 1 | 0.2 | 501 | 0.18 | 0.02 | 1.36 | 0.099 |
| Najran | 1 | 0.23 | 437 | 0.21 | 0.02 | 1.56 | 0.13 |
| Baha | 1 | 0.34 | 296 | 0.31 | 0.04 | 2.32 | 0.258 |
| Qassim | 2 | 0.39 | 513 | 0.36 | 0.08 | 1.52 | 0.168 |
| Northern Border | 1 | 0.65 | 154 | 0.61 | 0.08 | 4.49 | 0.629 |
| Jazan | 3 | 0.67 | 447 | 0.63 | 0.19 | 2.06 | 0.447 |
| Riyadh | 36 | 1.06 | 3405 | 1 | |||
| Eastern Region | 29 | 1.55 | 1869 | 1.47 | 0.9 | 2.41 | 0.122 |
| Aseer | 19 | 2.18 | 870 | 2.08 | 1.19 | 3.66 | 0.01 |
| Madinah | 37 | 4.55 | 813 | 4.46 | 2.8 | 7.1 | <0.001 |
| Makkah | 169 | 6.31 | 2678 | 6.3 | 4.38 | 9.06 | <0.001 |
| Total | 299 | 2.37 | 12,621 | ||||
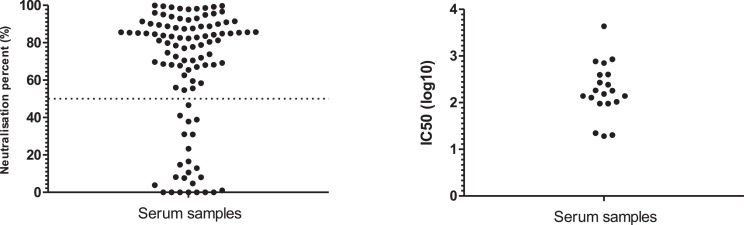
To confirm that the immunoassay test is reliable in detecting anti-SARS-CoV-2 IgG antibodies, 100 seropositive samples (1/3 of the total positive samples) were randomly selected for further testing using the SAS2pp neutralization assay. Of these samples, 92 (92%) samples showed neutralization activity; 76 of which had higher than 50% neutralization at 1:40 dilution (Fig. 1A ). Additionally, 20 of the 100 samples were further evaluated to report the IC50 NAb titres ((Fig. 1B). Some of these sera did not show NAb indicating that they could have a low level of NAb that could not be determined by the SAS2pp neutralization assay or that these samples have antibodies against related coronaviruses and might have cross-reacted with the spike antigen in immunoassay.
Fig. 1.
Neutralization assay (NA) was performed based on SARS-CoV-2 pseudotyped viral particles (SARS2pp). (A) Neutralization percentage of serum samples (n = 100) that were diluted 1:40 and run in SARS2pp NA. Dotted line shows 50% neutralization activity. (B) Few serum samples (n = 20) were further tested in SARS2pp NA in a 3-fold serial dilution to present the titre of neutralizing antibodies as 50% inhibitory concentration (IC50).
4. Discussion
COVID-19 is an emerging disease caused by the SARS-CoV-2 and was declared as a pandemic in March 2020. The first case of COVID-19 was reported in Saudi Arabia on March 2nd, 2020 and the current study was conducted between May 20th and 30th, 2020 at the times when the KSA had 62,000 to 80,000 cases. The study was conducted just before the peak of cases in the country. Being front liners in the control of the disease, HCWs represent 3.5% to 16% of all cases in China and USA respectively (European Center for Disease Control and Preventio). Previously, there were several Middle East Respiratory Syndrome Coronavirus (MERS-CoV) hospital outbreaks described in KSA (Al-Tawfiq and Auwaerter, 2019). The most important sero-epidemiological study among HCW has shown that 20 out of 250 cases were diagnosed by serology with various attack rate between various departments based on extent of exposure to MERS (Alraddadi et al., 2016) and only 25% of cases had positive PCR. There is 1 report describing the seroprevalence of MERS-CoV among HCWs during the Korean outbreak with a seropositivity of 0.7% in HCW who did not use PPE and 0% in those who used PPE (Kim et al., 2016). With the ongoing outbreak and concern of asymptomatic transmission, several asymptomatic HCWs can infect patients in addition several asymptomatic patients can infect HCW. With the increasing number of HCWs that are isolated at home following exposure to COVID-19 cases and with the increasing number of COVID-19 patients requiring more care from HCWs, better strategies were suggested to assess the immunity of HCWs to protect both patients and HCWs; especially that the COVID-19 PCR testing may have detection limitations.
At the national level, studying the prevalence of SARs-CoV-2 among Saudi HCWs is important to understand the exposure risk among this population, and to compare different risk factors of the infection, which can influence infection control measures and policies. Our aim was to study the seroprevalence of COVID-19 among HCW in various hospitals in KSA using a screening serological tests followed by a confirmatory neutralization test. However, it may be important to note that screening serological tests are lacking specificity as advised by the WHO.
In this study the overall rate of positive serology was 2.3% among HCWs. In a study in Tennessee, USA, of 249 front-line HCWs who cared for COVID-19 patients, 8% tested positive for COVID-19 antibodies by serology (Stubblefield et al., 2020). In a study of 2507 HCWs in Italy, the positivity rate was 0% for IgM and 0.7% for IgG antibodies (Lahner et al., 2020). We observed a higher positivity rate among case-hospitals of 2.9% vs 0.8% in the control hospitals. Thus, the overall rate of IgG positivity in this study is 3.3 times that seen in the study from Italy. However, there are variable rates of positivity among HCWs worldwide (Barrett et al., 2020; Brandstetter et al., 2020; Folgueira et al., 2020; Galan et al., 2020; Garcia-Basteiro et al., 2020; Hains et al., 2020; Korth et al., 2020; Paderno et al., 2020; Sandri et al., 2020). Antibody response may be related to the level of exposure in hospitals as exemplified by the differences in the control-hospitals vs the case-hospitals in this study. In an Infectious Diseases specialized setting in Naples, Southern Italy, antibody seroprevalence was 1.7% among tested HCWs (Fusco et al., 2020), indicating low rate despite the possible high exposure. Therefore, additional explanations are required; and one such explanation is the possibility of the short-lived antibody response or the timing of the antibody tests post exposure (Seow et al., 2020).
The study showed variable seroprevalence in different regions. Case-hospitals were more likely to have positive serology compared to the control-hospitals. Although most of the control hospitals were in the regions where the number of cases is low, control hospitals from regions with higher community cases were also included. Multiple factors may play a role in the occurrence of higher seroprevalence among HCWs in the case-hospitals at different regions. One factor is of course the number of COVID-19 cases in these hospitals. Another factor is the number of COVID-19 cases in the community. As indicated above, this study was conducted between May 20th and 30th, 2020 at the time when the Kingdom had 62,000 to 80,000 cases. And there were different positivity rates and cases between the different regions at that time. Of the total reported cases at that time, Makkah region accounted for 41% of all the reported cases indicating a higher prevalence of cases in the region. In addition, 15% of the cases were in Madinah, 20% in Riyadh, 19% in the eastern region and less than 2% in other regions. There was a high number of community cases in the regions that have high HCW seroprevalence for COVID-19 as seen in Makkah and Madinah compared to Riyadh and Eastern region. There were relatively high number of community cases in Riyadh and Eastern province than other areas in the country but these showed low HCW seroprevelance. The findings suggest that the high seroprevalence may be secondary to community exposure although other factors cannot be excluded such hospital environment and compliance with infection control measures. One study showed that HCW positivity for SARS-CoV-2 was associated with working in COVID-19 units, (RR = 2.449, CI = 1.062–5.649, P = 0.027), a positive household, inappropriate use of PPE, staying in the same break room where a positive HCW is staying without a medical mask for more than 15 minutes, consuming food within 1 minute of an HCW, and noncompliance with social distancing (Çelebi et al., 2020). It was found that exposure of HCWs to SARS-CoV-2 may be in the hospital, from household members, or community-acquired (Burrer et al., 2020). In 1 study, 137 (27%) of 500 HCW were positive for SARS-CoV-2 antibody with much higher rate among symptomatic participants (75%) and of all the positive HCWs 34% had community exposure (Venugopal et al., 2020). Seroprevalence is also related to the level of HCWs exposure and 1 study showed that seroprevalence was higher in an intermediate-risk-group vs high-risk-group (5.4 % vs 1.2 %) (Korth et al.,2020). Another study showed that enzyme immunoassay and microneutralization assay were positive in 17.14% (18/105) of HCWs who were contacts of COVID-19 patients despite negative SARS-CoV-2 (Chen et al., 2020). The risk of seropositivity was lower with wearing face mask (OR, 0.127, 95% CI 0.017, 0.968) (Chen et al., 2020). We showed that HCWs working in case-hospitals had higher positivity rate and this was similar to a study that showed 19 (7.6%) of 249 HCWs who worked in COVID-19 units for 1 month were positive (Stubblefield et al., 2020). However, in another study of 202 HCWs the positivity rate was 14.4% for IgM and 7.4% for IgG with no relationship to COVID-19 exposure (Sotgiu et al., 2020). It was interesting to note that 1 study showed similar seropositivity among HCWs with heavy and low COVID-19 exposure (Hunter et al., 2020). Thus, a multicenter study is needed to elucidate carefully factors contributing to the seropositivity of HCWs to SARS-CoV-2. Such identification would aid in better understanding and facilitate the introduction of preventive measures for HCWs.
The lack of NAb in 8% of the tested samples could mean that the used antibody assay is a more sensitive or that neutralization assay has a lower detection limit. It could possibly be that some sera had cross-reactive antibodies to the spike protein in the serological assay originating from an exposure to other coronaviruses; especially that MERS is endemic in Saudi Arabia and the asymptomatic rate of MERS infections in understudied. The ELISA or CMIA assays are not 100% specific and positive reactions were obtained for subjects with antibodies against other coronaviruses. The assay used is a CMIA with higher specificity compare to ELISA. The specificity of the assay based on the available studies is 99.2 -100% (Bryan et al., 2020; Perkmann et al., 2020; Public Health England, 2020). The tested samples without Nab activities is more likely to have lower level of antibody titre to be detected by Nab assay or that these samples have antibodies against related coronaviruses and might have cross-reacted with the spike antigen in immunoassay.
This study is the first seroprevalence of HCWs in Saudi Arabia and was conducted in May 2020. The hospitals were classified based on the available national surveillance data (National Health electronic surveillance system). The control hospitals implemented the same criteria and indication for COVID-19 testing as the case hospitals. Indications for repeating the test were similar in both types of hospitals. Control Hospitals were defined as COVID-19 non-affected hospitals as there was no management of COVID-19 patients and there were no COVID-19 outbreaks among HCWs. And thus, we could not exclude completely the diagnosis of a single COVID-19 case in the control hospitals.
In conclusion, this is a national serosurvey of SARS-CoV-2 among HCWs working in hospitals with and without COVID-19 patients. The study was based on a random sample of HCWs and was conducted during the early time of cases in Saudi Arabia. Since the duration of detectable antibodies is variable no recommendation can be generated for those who have negative SARS-COV2 antibodies. The findings may indicate a large number of HCWs are still at high risk of acquiring infection in different setting and thus strong strategies are need to strengthen infection control measures, developement of vaccination strategies (once available) and to continuation of staff training.
Authors’ Contributions
Haleema Alserehi: Conceptualization, Design and Methodology Validation, Analysis, data/evidence collection, Resources, Data Curation, Writing Original Draft, Supervision; Ada Mohammed Alqunaibet: Conceptualization, Design and Methodology Validation, Analysis, Data Curation, Writing - Original Draft; Jaffar A. Al-Tawfiq: Analysis, Writing - Original Draft, Writing - Review & Editing; Naif Khalaf Alharbi: Analysis, Resources, Data Curation, Writing - Original Draft, Writing - Review & Editing; Abeer Nizar Alshukairi: Conceptualization, Design and Methodology, Validation; Khalid Hamdan Alanazi: Conceptualization, data/evidence collection, Resources, Data Curation; Ghada Mohammed Bin Saleh: Analysis, data/evidence collection, Resources, Data Curation; Amer Mohammed Alshehri: Analysis, data/evidence collection, Resources, Data Curation; Abdulrahman Almasoud: Analysis, Resources, Data Curation; Anwar M. Hashem: Analysis, Resources, Data Curation; Amaal Rabie Alruwaily: data/evidence collection, Resources, Data Curation, Writing - Original Draft; Rehab Habeeb Alaswad: data/evidence collection, Data Curation; Hind Mohammed Al-Mutlaq: data/evidence collection, Data Curation; Abdulllah Ali Almudaiheem: data/evidence collection, Resources, Data Curation; Fatmah Mahmoud Othman: data/evidence collection, Resources, Writing - Original Draft; Sumyah Abdullah Aldakeel: Analysis, Resources; Mouath Rashid Abu Ghararah: Analysis, Resources; Hani Abdulaziz Jokhdar: Conceptualization, Design and Methodology, Resources; Abdullah Rshoud Algwizani: Conceptualization, Design and Methodology, Resources; Sami Saeed Almudarra: Conceptualization, Design and Methodology, Design and Methodology, Writing - Review & Editing, Supervision; Ahmed Mohammed Albarrag: Conceptualization, Design and Methodology, Analysis, Resources, Writing - Review & Editing. All authors approved the final manuscript.
Acknowledgment
We would like to thank Abbott company for sponsoring the laboratory testing kits used in this study.
REFERENCES
- Alharbi NK, Qasim I, Almasoud A, Aljami HA, Alenazi MW, Alhafufi A. Humoral immunogenicity and efficacy of a single dose of ChAdOx1 MERS vaccine candidate in dromedary camels. Sci Rep. 2019;8(9):16292. doi: 10.1038/s41598-019-52730-4. https://pubmed.ncbi.nlm.nih.gov/31705137/ Available: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almasaud A, Alharbi NK, Hashem AM. Methods in molecular biology [Internet] Humana Press Inc; 2020. Generation of MERS-CoV pseudotyped viral particles for the evaluation of neutralizing antibodies in mammalian sera; pp. 117–126.https://pubmed.ncbi.nlm.nih.gov/31883092/ 23rd July 2020. Available: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alraddadi BM, Al-Salmi HS, Jacobs-Slifka K, Slayton RB, Estivariz CF, Geller AI. Risk factors for middle east respiratory syndrome coronavirus infection among healthcare personnel. Emerg Infect Dis. 2016;22(11):1915–1920. doi: 10.3201/eid2211.160920. http://www.ncbi.nlm.nih.gov/pubmed/27767011 Available: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Tawfiq JA, Auwaerter PG. Healthcare-associated infections: the hallmark of Middle East respiratory syndrome coronavirus with review of the literature. J Hosp Infect. 2019;101(1):20–29. doi: 10.1016/j.jhin.2018.05.021. http://linkinghub.elsevier.com/retrieve/pii/S019567011830286X Available: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Tawfiq JA, Garout MA, Gautret P. Preparing for emerging respiratory pathogens such as SARS-CoV, MERS-CoV, and SARS-CoV-2. Le Infez Med. 2020;28(suppl 1):64–70. [PubMed] [Google Scholar]
- Al-Tawfiq JA, Memish ZA. Serologic testing of coronaviruses from MERS-CoV to SARS-CoV-2: Learning from the past and anticipating the future. Travel Med Infect Dis. 2020;37:101785. doi: 10.1016/j.tmaid.2020.101785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barranco R, Ventura F. Covid-19 and infection in health-care workers: an emerging problem. Med Leg J. 2020;88(2):65–66. doi: 10.1177/0025817220923694. [DOI] [PubMed] [Google Scholar]
- Barrett ES, Horton DB, Roy J, Gennaro ML, Brooks A, Tischfield J. Prevalence of SARS-CoV-2 infection in previously undiagnosed health care workers at the onset of the U.S. COVID-19 epidemic. medRxiv. 2020 doi: 10.1101/2020.04.20.20072470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandstetter S, Roth S, Harner S, Buntrock-Döpke H, Toncheva A, Borchers N. Symptoms and immunoglobulin development in hospital staff exposed to a SARS-CoV-2 outbreak. Pediatr Allergy Immunol. 2020 doi: 10.1111/pai.13278. [DOI] [PubMed] [Google Scholar]
- Bryan A, Pepper G, Wener MH, Fink SL, Morishima C, Chaudhary A. Performance characteristics of the abbott architect sars-cov-2 igg assay and seroprevalence in Boise, Idaho. J Clin Microbiol. 2020;58(8):e00941-20. doi: 10.1128/JCM.00941-20. https://pubmed.ncbi.nlm.nih.gov/32381641/ Available: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burrer SL, de Perio MA, Hughes MM, Kuhar DT, Luckhaupt SE, McDaniel CJ. Characteristics of health care personnel with COVID-19 — United States, february 12–april 9, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(15):477–481. doi: 10.15585/mmwr.mm6915e6. https://pubmed.ncbi.nlm.nih.gov/32298247/ Available: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Çelebi G, Pişkin N, Çelik Bekleviç A, Altunay Y, Salcı Keleş A, Tüz MA. Specific risk factors for SARS-CoV-2 transmission among health care workers in a university hospital. Am J Infect Control. 2020;48(10):1225–1230. doi: 10.1016/j.ajic.2020.07.039. pmc/articles/PMC7409872/?report=abstract Available: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Tong X, Wang J, Huang W, Yin S, Huang R. High SARS-CoV-2 antibody prevalence among healthcare workers exposed to COVID-19 patients. J Infect. 2020;81(3):420–426. doi: 10.1016/j.jinf.2020.05.067. https://pubmed.ncbi.nlm.nih.gov/32504745/ Available: [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Center for Disease Control and Prevention. An overview of the rapid test situation for COVID-19 diagnosis in the EU/EEA [Internet]. [22nd October 2020]. Available: https://www.ecdc.europa.eu/en/publications-data/overview-rapid-test-situation-covid-19-diagnosis-eueea
- Ferioli M, Cisternino C, Leo V, Pisani L, Palange P, Nava S. Protecting healthcare workers from SARS-CoV-2 infection: practical indications. Eur Respir Rev. 2020;29(155):200068. doi: 10.1183/16000617.0068-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folgueira MD, Munoz-Ruiperez C, Alonso-Lopez MA, Delgado R. SARS-CoV-2 infection in health care workers in a large public hospital in Madrid, Spain, during march 2020. medRxiv. 2020 doi: 10.1101/2020.04.07.20055723. [DOI] [Google Scholar]
- Fusco FM, Pisaturo M, Iodice V, Bellopede R, Tambaro O, Parrella G. COVID-19 infections among healthcare workers in an infectious diseases specialized setting in Naples, Southern Italy: results of a cross-sectional surveillance study. J Hosp Infect. 2020;105(4):596–600. doi: 10.1016/j.jhin.2020.06.021. https://pubmed.ncbi.nlm.nih.gov/32565367/ Available: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galan I, Velasco M, Casas ML, Goyanes MJ, Rodriguez-Caravaca G, Losa JE. SARS-CoV-2 seroprevalence among all workers in a teaching hospital in Spain: unmasking the risk. medRxiv. 2020 doi: 10.1101/2020.05.29.20116731. [DOI] [Google Scholar]
- Garcia-Basteiro A, Moncunill G, Tortajada M, Vidal M, Guinovart C, Jimenez A. Seroprevalence of antibodies against SARS-CoV-2 among health care workers in a large Spanish reference hospital. Nat Commun. 2020;11(1):3500. doi: 10.1038/s41467-020-17318-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grehan K, Ferrara F, Temperton N. An optimised method for the production of MERS-CoV spike expressing viral pseudotypes. MethodsX. 2015;2:379–384. doi: 10.1016/j.mex.2015.09.003. https://pubmed.ncbi.nlm.nih.gov/26587388/ . eCollection 2015. Available: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hains DS, Schwaderer AL, Carroll AE, Starr MC, Wilson AC, Amanat F. Asymptomatic Seroconversion of Immunoglobulins to SARS-CoV-2 in a pediatric dialysis unit. JAMA. 2020;323(23):2424–2425. doi: 10.1001/jama.2020.8438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter BR, Dbeibo L, Weaver C, Beeler C, Saysana M, Zimmerman M. Seroprevalence of SARS-CoV-2 antibodies among healthcare workers with differing levels of COVID-19 patient exposure. Infect Control Hosp Epidemiol. 2020:1–2. doi: 10.1017/ice.2020.390. https://pubmed.ncbi.nlm.nih.gov/32741406/ Available: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim C-J, Choi WS, Jung Y, Kiem S, Seol HY, Woo HJ. Surveillance of the Middle East respiratory syndrome (MERS) coronavirus (CoV) infection in healthcare workers after contact with confirmed MERS patients: incidence and risk factors of MERS-CoV seropositivity. Clin Microbiol Infect. 2016;22(10):880–886. doi: 10.1016/j.cmi.2016.07.017. http://linkinghub.elsevier.com/retrieve/pii/S1198743X16302415 Available: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korth J, Wilde B, Dolff S, Anastasiou OE, Krawczyk A, Jahn M. SARS-CoV-2-specific antibody detection in healthcare workers in Germany with direct contact to COVID-19 patients. J Clin Virol. 2020;128:104437. doi: 10.1016/j.jcv.2020.104437. http://www.ncbi.nlm.nih.gov/pubmed/32434708 Available: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahner E, Dilaghi E, Prestigiacomo C, Alessio G, Marcellini L, Simmaco M. Prevalence of SARS-CoV-2 infection in health workers (HWs) and diagnostic test performance: the experience of a teaching hospital in central Italy. Int J Environ Res Public Health. 2020;17(12):4417. doi: 10.3390/ijerph17124417. https://pubmed.ncbi.nlm.nih.gov/32575505/ Available: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paderno A, Fior M, Berretti G, Schreiber A, Grammatica A, Mattavelli D. SARS-CoV-2 infection in health care workers: cross-sectional analysis of an otolaryngology unit. Otolaryngol Head Neck Surg. 2020;163(4):671–672. doi: 10.1177/0194599820932162. https://pubmed.ncbi.nlm.nih.gov/32482123/ Available: [DOI] [PubMed] [Google Scholar]
- Perkmann T, Perkmann-Nagele N, Breyer M-K, Breyer-Kohansal R, Burghuber OC, Hartl S. Side by side comparison of three fully automated SARS-CoV-2 antibody assays with a focus on specificity. Clin Chem. 2020;66(11):1405–1413. doi: 10.1093/clinchem/hvaa198. https://pubmed.ncbi.nlm.nih.gov/32777031/ Available: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Public Health England. Evaluation of the Abbott SARS-CoV-2 IgG for the detection of anti-SARS-CoV-2 antibodies evaluation of Abbott SARS-CoV-2 IgG assay for the detection of anti-SARS-CoV-2 antibodies 2 uncontrolled document when printed or downloaded. CURRENT VERSION CAN BE AC [Internet]. 2020. Available: www.facebook.com/PublicHealthEngland
- Sandri MT, Azzolini E, Torri V, Carloni S, Tedeschi M, Castoldi M. IgG serology in health care and administrative staff populations from 7 hospital representative of different exposures to SARS-CoV-2 in Lombardy, Italy. medRxiv. 2020 doi: 10.1101/2020.05.24.20111245. [DOI] [Google Scholar]
- Seow J, Graham C, Merrick B, Acors S, Steel KJA, Hemmings O. Longitudinal evaluation and decline of antibody responses in SARS-CoV-2 infection. medRxiv [Internet] 2020 http://medrxiv.org/content/early/2020/07/11/2020.07.09.20148429.abstract 24th July 20202020.07.09.20148429. Available: [Google Scholar]
- Sotgiu G, Barassi A, Miozzo M, Saderi L, Piana A, Orfeo N. SARS-CoV-2 specific serological pattern in healthcare workers of an Italian COVID-19 forefront hospital. BMC Pulm Med [Internet] 2020;20(1) doi: 10.1186/s12890-020-01237-0. https://pubmed.ncbi.nlm.nih.gov/32727446/ 23rd October 2020. Available: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stubblefield WB, Talbot HK, Feldstein L, Tenforde MW, Rasheed MAU, Mills L. Seroprevalence of SARS-CoV-2 among frontline healthcare personnel during the first month of caring for COVID-19 patients - Nashville, Tennessee. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa93. http://www.ncbi.nlm.nih.gov/pubmed/32628750 Available: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venugopal U, Jilani N, Rabah S, Shariff MA, Jawed M, Batres AM. SARS-CoV-2 seroprevalence among health care workers in a New York City Hospital: a cross-sectional analysis during the COVID-19 pandemic. Int J Infect Dis. 2020;102:63–69. doi: 10.1016/j.ijid.2020.10.036. https://linkinghub.elsevier.com/retrieve/pii/S1201971220322402 Available: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei XS, Wang XR, Zhang JC, Yang WB, Ma WL, Yang BH. A cluster of health care workers with COVID-19 pneumonia caused by SARS-CoV-2. J Microbiol Immunol Infect. 2020;S1684(1182):30107–301090. doi: 10.1016/j.jmii.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]