Abstract
Objectives
Due to a substantial proportion of asymptomatic and mild courses, many severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infections remain unreported. Therefore, assessment of seroprevalence may detect the real burden of disease. We aimed to determine and characterize the rate of SARS-CoV-2 infections and the resulting seroprevalence in a defined population. The primary objective of the study was to assess SARS-CoV-2 antibody seroprevalence using six different IgG-detecting immunoassays. Secondary objectives of the study were: (a) to determine potential risk factors for symptomatic versus asymptomatic coronavirus disease 2019 courses, and (b) to investigate the rate of virus RNA-persistence.
Methods
CoNAN is a population-based cohort study performed in the community Neustadt am Rennsteig, Germany, which was quarantined from 22 March to 5 April after six SARS-CoV-2 cases were detected in the village's population. The SARS-CoV-2 outbreak comprised 51 cases and 3 deaths. The CoNAN study was performed from 13 May to 22 May 2020, 6 weeks after a SARS-CoV-2 outbreak.
Results
We enrolled a total of 626 participants (71% of the community population) for PCR and antibody testing in the study. All actual SARS-CoV-2 PCR tests were negative. Fifty-two out of 620 (8.4%) participants had antibodies against SARS-CoV-2 in at least two different assays. There were 38 participants with previously PCR-confirmed SARS-CoV-2 infection. Of those, only 19 (50%) displayed anti-SARS-CoV-2 antibodies. We also show that antibody-positive participants with symptoms compatible with a respiratory tract infection had significantly higher antibody levels then asymptomatic participants (EU-assay: median 2.9 versus 7.2 IgG-index, p 0.002; DS-assay: median 45.2 versus 143 AU/mL, p 0.002). Persisting viral replication was not detected.
Conclusions
Our data question the relevance and reliability of IgG antibody testing to detect past SARS-CoV-2 infections 6 weeks after an outbreak. We conclude that assessing immunity for SARS-CoV-2 infection should not rely on antibody tests alone.
Keywords: Antibody response, Immunity, Quarantine, Severe acute respiratory syndrome coronavirus 2
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a pandemic pathogen with transmission by droplets and aerosols. It has been argued that the risk of acquiring the infection is minimized if a large percentage of the population has been infected with SARS-CoV-2 and has, at least partially, developed immunity against it [1]. Several population-based cohort studies have therefore tried to determine the proportion of infected persons by measuring the seroprevalence of anti-SARS-CoV-2 antibodies. Most of these studies have used only one or two different antibody assays and omit infants, as their inclusion is a challenge in such studies. It appears that there are tremendous differences between the currently available SARS-CoV-2 antibody assays with a test specificity ranging from 84.3% to 100% in pre-coronavirus disease 2019 (COVID-19) specimens and inter-test agreements ranging from 75.7% to 94.8% [2,3].
Neustadt am Rennsteig is a village in the Ilm-district in central Thuringia, Germany (50°34′57″ N, 10°56′1″ E) with 883 inhabitants in which a SARS-CoV-2 outbreak had occurred in March and April 2020. More details on the outbreak are given in the Supplementary material (Appendix S1). Due to the isolated location of the village, the extensive testing of the population, and the clear and controlled outbreak, Neustadt am Rennsteig is well suited to study the seroprevalence and potential development of immunity of SARS-CoV-2 infections.
In order to assess seroprevalence of anti-SARS-CoV-2 antibodies in this community, we chose a population-based approach including infants applying six different IgG antibody assays in parallel.
Materials and methods
Study design and enrolment
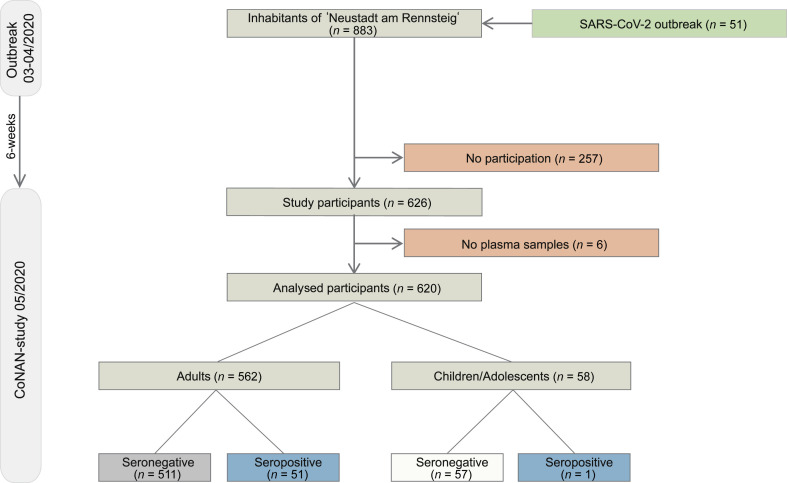
The CoNAN study (COVID-19 outbreak in Neustadt am Rennsteig) is an ongoing exploratory population-based cohort study. We here report the baseline characteristics of the participants at the time of the outbreak/quarantine initiation and at study initiation. Follow-up assessments are planned after 6 and 12 months relative to baseline assessment. Study participation is voluntary and can be withdrawn at any time, refusal to participate has no consequences. Participants were enrolled from 13 May to 16 May 2020 at a central study site, set-up in the village town hall. Participants who could not come to the study site were enrolled by the local primary care physician at their respective homes until the 22 May. This was approximately 6 weeks after a mass testing in the village was performed (Fig. 1 ). The study was set-up after the mass-testing results were obtained. After informed consent, questionnaires, blood samples and pharyngeal washes were directly taken at the study site. Pharyngeal washes were obtained by 10–20 seconds of gargling with 10 mL 0.9 NaCl% after rinsing the mouth with 50 mL non-sparkling commercial water. Pharyngeal washes were obtained under direct supervision of a study team member to ensure appropriate quality.
Fig. 1.
Flow chart of the CoNAN study. ∗ PCR from pharyngeal washes obtained during the CoNAN study in May 2020.
At the study site, blood was directly centrifuged at 4°C/2000 g for 10 minutes and stored at 8°C.
All inhabitants of the community of Neustadt am Rennsteig regardless of age, gender or infection status were eligible for participation. Informed consent was provided by the participants or by the parents/legal representatives.
Individuals that did not reside in Neustadt am Rennsteig or that lived in the adjacent community of Kahlert were not eligible for inclusion.
Ethics review, data protection and data management
The study was conducted according to the current version of the Declaration of Helsinki and has been approved by the institutional ethics committees of the Jena University Hospital and the respective data protection commissioner (approval number 2020-1776) and the ethics committee of the Thuringian chamber of physicians. All data were collected with unique pseudonyms on paper case report forms. These identifiers were later used to merge the questionnaire information with the laboratory information in an electronic study database. The study is registered at the German Clinical Trials Register: DRKS00022416.
Objectives and outcomes
The primary objective was to determine the SARS-CoV-2 antibody status (seroprevalence SARS-CoV-2 antibody status was defined as positive if participants had a positive test result in two or more of the six antibody assays (details below). Participants with only one positive test were classified as uncertain. The secondary objectives of the study were: (a) to determine potential risk factors for symptomatic versus asymptomatic COVID-19 courses, and (b) to investigate the rate of virus RNA-persistence (as part of future follow-up assessments).
Questionnaire
For details see Supplementary material (Appendix S1).
SARS-CoV-2 RT-PCR
For details see Supplementary material (Appendix S1).
SARS-CoV-2 antibody testing
For details see Supplementary material (Appendix S1).
Statistical analysis
Sample size considerations
The sample size of the CoNAN-cohort is fixed by the number of inhabitants (n = 883) of the community. We aimed to include the population as completely as possible. In addition, we consulted the WHO population-based age-stratified sero-epidemiological investigation protocol for SARS-CoV-2 infection [4]. On the basis of this recommendation, we estimated that a study with 600 samples (i.e. an inclusion rate of about 70%) should be sufficient to estimate a (true) seroprevalence of 10% or smaller with a maximum expected margin of error of ±3% (defined by the expected width in percentage points of the 95% CI for the seroconversion point estimate using ‘Confidence interval for proportion using normal approximation (n large)’ of nQuery 4·0). Larger true seroprevalences would result in larger error margins.
Data analysis
All statistical analyses were performed in the analysis population, stratified by age (adults/children and adolescents) and serostatus from the serological assays. Descriptive analyses included the calculation of means with standard deviation (SD) and medians with minimum and maximum values for continuous variables, and absolute counts (n with percentages) for categorical variables. Owing to the high level of data completeness, we performed no data imputations. As inferential statistics, we applied logistic regression models exploring the associations between the participant-reported symptoms, the SARS-CoV-2 PCR results of the initial mass testing and the binary serostatus outcome. To adjust estimates for cluster effects between participants living in the same household (derived from their address information) we applied generalized estimation equations with exchangeable correlation structure and logistic link function. In addition, we adjusted some of the models for sex and age (linear). Results of logistic generalized estimation equations models are presented as odds ratio (OR) point and interval estimates. Results are presented such that OR >1 indicate increasing odds for a seropositive finding with increasing exposures. All confidence intervals were calculated with 95% coverage; CIs are Wald CIs that are not adjusted for multiple comparisons. Similarly, all reported p-values are unadjusted and two-sided. Due to the explorative nature of the study, we avoided statistical significance testing. We used the R Language for Statistical Computing (version 4.0.2; R Core Team 2019: R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria) for analyses except for the semi-quantitative antibody test results, which were compared by Wilcoxon–Mann–Whitney tests as implemented in GraphPad Prism 6 (GraphPad, San Diego, CA, USA).
Results
Participant characteristics
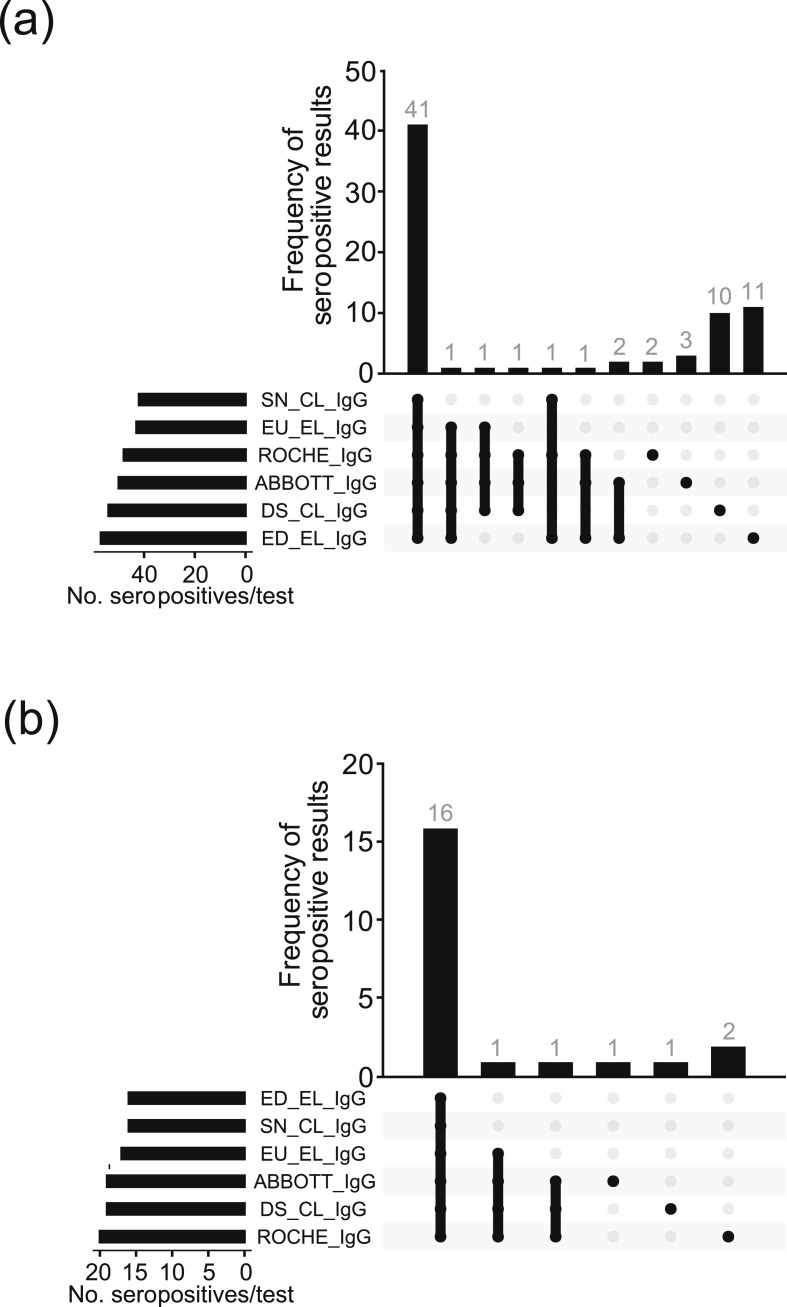
Characteristics of the participants are given in Table 1 and in the Supplementary material (Fig. S1). A total of 626 of the 883 community inhabitants (71%) participated in the study. Pharyngeal washes were obtained from 617 of 626 (98.6%) participants. All PCR tests were negative. From the 51 inhabitants that had initially been tested positive for SARS-CoV-2 by PCR, 38 participated in the CoNAN study. Plasma samples were obtained from a total of 620 (99%) participants who comprise the analysed sample cohort. All six serological assays were performed in 600 out of 626 (96%) participants. In the remaining 20 individuals (4%), five assays were used for final analysis because, either there was limited material available or the results were inconclusive in one out of the six assays. A comparison of test performance between the six serological IgG assays in the 600 participants is shown as an Upset plot in Fig. 2 .
Table 1.
Characteristics of the 562 adult participants, stratified by serostatus, and the 58 participating adolescents and children analysed (i.e. with serum samples)
| Characteristic | Adults |
Children and adolescents |
||
|---|---|---|---|---|
| Seronegative |
Seropositive |
Overall |
Overall |
|
| (n = 511) | (n = 51) | (n = 562) | (N = 58)a | |
| Size of household clusters, n (%) | ||||
| 1 person | 84 (16.4%) | 6 (11.8%) | 90 (16.0%) | 0 (0%) |
| 2 persons | 216 (42.3%) | 31 (60.8%) | 247 (44.0%) | 0 (0%) |
| 3 persons | 108 (21.1%) | 5 (9.8%) | 113 (20.1%) | 18 (31.0%) |
| 4 persons | 57 (11.2%) | 7 (13.7%) | 64 (11.4%) | 32 (55.2%) |
| 5+ persons | 44 (8.6%) | 1 (2.0%) | 45 (8.0%) | 5 (8.6%) |
| Missing | 2 (0.4%) | 1 (2.0%) | 3 (0.5%) | 3 (5.2%) |
| Sex, n (%) | ||||
| Male | 238 (46.6%) | 28 (54.9%) | 266 (47.3%) | 35 (60.3%) |
| Female | 273 (53.4%) | 23 (45.1%) | 296 (52.7%) | 22 (37.9%) |
| Missing | 1 (1.7%) | |||
| Age (years) | ||||
| Mean (SD) | 57.9 (16.8) | 60.3 (13.2) | 58.1 (16.5) | 9.62 (4.38) |
| Median (Min, Max) | 60 (18, 97) | 62 (24, 83) | 60 (18, 97) | 10 (1, 17) |
| PCR during quarantine (reported), n (%) | ||||
| Negative | 490 (95.9%) | 31 (60.8%) | 521 (92.7%) | 51 (87.9%) |
| Positive | 16 (3.1%) | 20 (39.2%) | 36 (6.4%) | 2 (3.4%) |
| Not known | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Missing | 5 (1.0%) | 0 (0%) | 5 (0.9%) | 5 (8.6%) |
| Chronic lung disease, n (%) | ||||
| Yes | 44 (8.6%) | 3 (5.9%) | 47 (8.4%) | 2 (3.4%) |
| No | 465 (91.0%) | 48 (94.1%) | 513 (91.3%) | 52 (89.7%) |
| Not known | 1 (0.2%) | 0 (0%) | 1 (0.2%) | 1 (1.7%) |
| Missing | 1 (0.2%) | 0 (0%) | 1 (0.2%) | 3 (5.2%) |
| Cardiovascular disease, n (%) | ||||
| Yes | 252 (49.3%) | 24 (47.1%) | 276 (49.1%) | 0 (0%) |
| No | 248 (48.5%) | 26 (51.0%) | 274 (48.8%) | 58 (100%) |
| Not known | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Missing | 11 (2.2%) | 1 (2.0%) | 12 (2.1%) | |
| Diabetes, n (%) | ||||
| Yes | 89 (17.4%) | 5 (9.8%) | 94 (16.7%) | 0 (0%) |
| No | 420 (82.2%) | 45 (88.2%) | 465 (82.7%) | 55 (94.8%) |
| Not known | 2 (0.4%) | 1 (2.0%) | 3 (0.5%) | 0 (0%) |
| Missing | 3 (5.2%) | |||
| Cancer, n (%) | ||||
| Yes | 34 (6.7%) | 1 (2.0%) | 35 (6.2%) | 0 (0%) |
| No | 474 (92.8%) | 50 (98.0%) | 524 (93.2%) | 55 (94.8%) |
| Not known | 3 (0.6%) | 0 (0%) | 3 (0.5%) | 0 (0%) |
| Missing | 3 (5.2%) | |||
| Autoimmune diseases/immune deficiency, n (%) | ||||
| Yes | 22 (4.3%) | 3 (5.9%) | 25 (4.4%) | 0 (0%) |
| No | 485 (94.9%) | 47 (92.2%) | 532 (94.7%) | 55 (94.8%) |
| Not known | 4 (0.8%) | 1 (2.0%) | 5 (0.9%) | 0 (0%) |
| Missing | 3 (5.2%) | |||
| Smoker, n(%) | ||||
| No | 335 (65.6%) | 42 (82.4%) | 377 (67.1%) | 55 (94.8%) |
| Current smoker | 122 (23.9%) | 5 (9.8%) | 127 (22.6%) | 0 (0%) |
| Former smoker | 52 (10.2%) | 4 (7.8%) | 56 (10.0%) | 0 (0%) |
| Missing | 2 (0.4%) | 0 (0%) | 2 (0.4%) | 3 (5.2%) |
Note that only one individual was characterized as seropositive.
Fig. 2.
A comparison of test performance between the six serological IgG assays in 600 participants for whom all six assays were performed. (a) Upset plot of all antibody-positive participants and (b) Upset plot of previously SARS-CoV-2 PCR-positive participants. Abbreviations: SN.2019-nCoV IgG kit (Snibe Co., Ltd., Shenzhen, China); EU..SARS-COV-2 IgG ELISA kit (Euroimmun, Lübeck, Germany); DS..SARS-CoV-2 S1/S2 IgG CLIA kit (DiaSorin, Saluggia, Italy); ED..EDI Novel Coronavirus SARS-CoV-2 IgG ELISA kit (Epitope Diagnostics Inc., San Diego, USA).
Antibody assays
We found that 52/620 (8.4%) participants were seropositive. Only 19 of these individuals had tested positive by PCR during the SARS-CoV-2 outbreak (Fig. 3 a, Table 1, and see Supplementary material, Table S2).
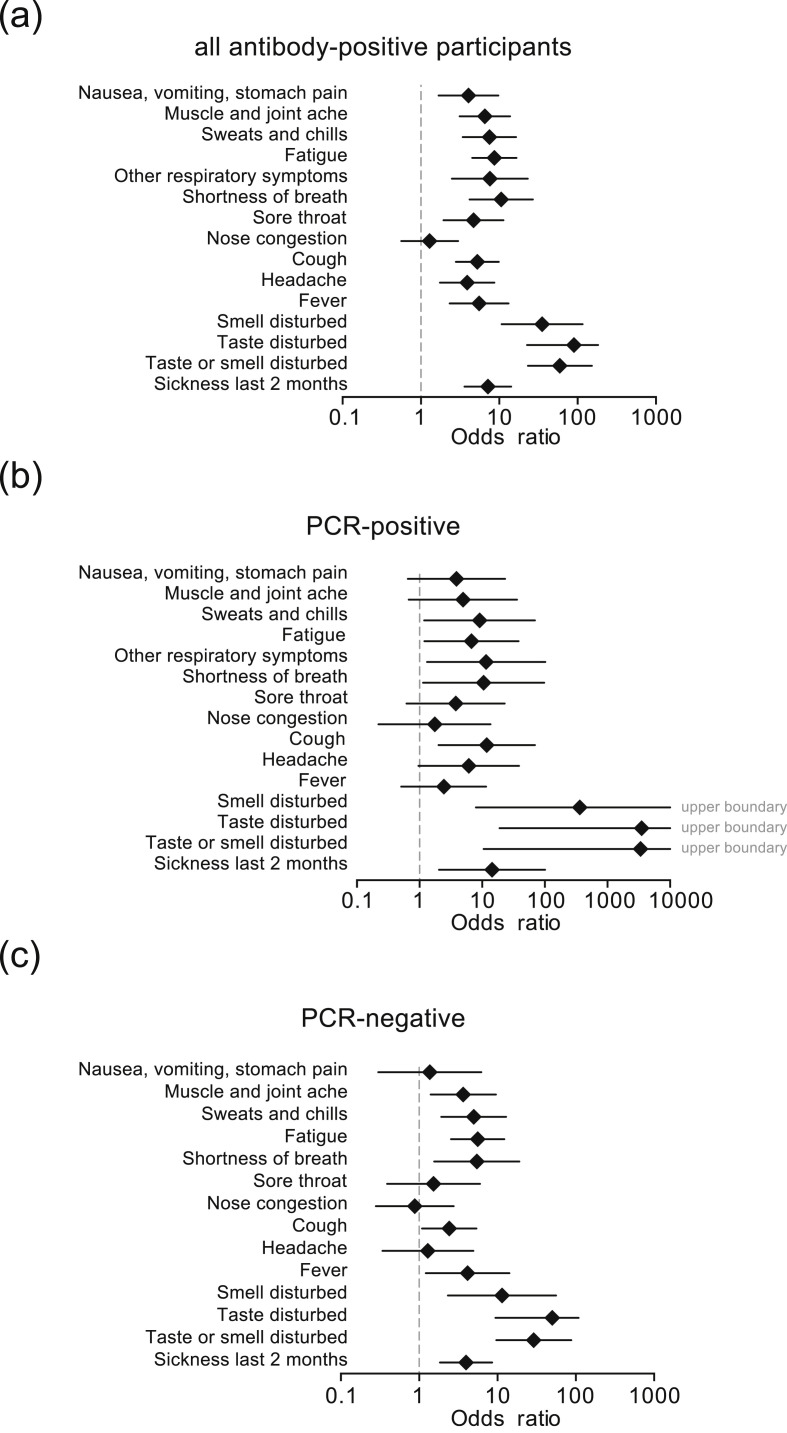
Fig. 3.
Associations for reported clinical symptoms for the outcome ‘positive antibody status’ for (a) all participants; (b) previously SARS-CoV-2 PCR-positive participants and (c) previously SARS-CoV-2 PCR-negative participants. Odds ratio and corresponding 95% CI are derived from the logistic generalized estimation equations model adjusted for household clustering and sex and age (linear); the plots display the complete cases.
In 26 participants, only one out of six assays was positive. These patients were judged to reflect uncertain cases and assessed as seronegative for the comparison. Of those, three had previously been tested SARS-CoV-2 positive by PCR. In all three, none of the additional serological assays were positive (not reported in this manuscript, (2 × IgM/1 × IgA), and only one person had symptoms compatible with a respiratory tract infection. From the remaining 23 participants, four participants reported symptoms compatible with respiratory tract infection and none had IgA or IgM antibodies.
Thirty-three of the antibody-positive participants had no previous positive PCR result. Of those, 12 lived in the same household with SARS-CoV-2-positive people and a further five had direct contact or contact with a contact of a SARS-CoV-2-infected person, suggestive of direct transmission. Eight of the remaining participants had symptoms compatible with a respiratory tract infection while eight participants did not.
Antibody assays and self-reported symptoms
Table 2 displays a summary of the self-reported symptoms and summarizes any of the 14 questions related to symptoms into one variable. Thirteen participants with PCR-proven SARS-CoV-2 infection reported no symptoms consistent with a respiratory infection or sickness. In contrast, any symptom was reported by 181 PCR-negative participants and 168 antibody-negative participants during the same period, potentially reflecting common respiratory infections in spring (Table 2). In the seropositive group, 13/52 participants (25%) had no symptoms (Table 2). Fig. 3 is a more detailed depiction of individual symptoms in all participants (Fig. 3a) or stratified by the initial SARS-CoV-2 PCR results (Fig. 3b,c). Disturbances of smell and/or taste were the best predictors of later seropositivity irrespective of stratification with OR point estimates ≥10. Interestingly, in individuals who knew that they were initially PCR negative, perceived muscle and joint pain, sweats and chills, shortness of breath or fatigue turned out to be predictors of later seropositivity as well. Variables were strongly associated with OR 15.38 (95% CI 7.00–33.80; p = 0.001) for PCR versus antibody status, OR 5.17 (95% CI 2.35–11.37; p 0.001) for PCR versus any reported symptom and OR 9.06 (95% CI 4.23–19.37; p 0.001) for antibody status versus any reported symptom.
Table 2.
Three-way cross-table of the initial mass testing SARS-CoV-2 PCR results (PCRinitial) against antibody status 6 weeks later and the self-reporting of any symptoms in all 620 participants of the CoNAN study
| Antibody positive |
Antibody negative |
|||
|---|---|---|---|---|
| Any symptom positive | Any symptom negative | Any symptom positive | Any symptom negative | |
| PCRinitial positive |
n = 18 (2.9%) |
n = 1 (0.2%) |
n = 7 (1.1%) |
n = 12 (1.9%) |
| PCRinitial negative |
n = 21 (3.4%) |
n = 12 (1.9%) |
n = 160 (25.8%) |
n = 382 (61.6%) |
| PCRinitial missing |
n = 0 (0.0%) |
n = 0 (0.0%) |
n = 1 (0.2%) |
n = 6 (1.0%) |
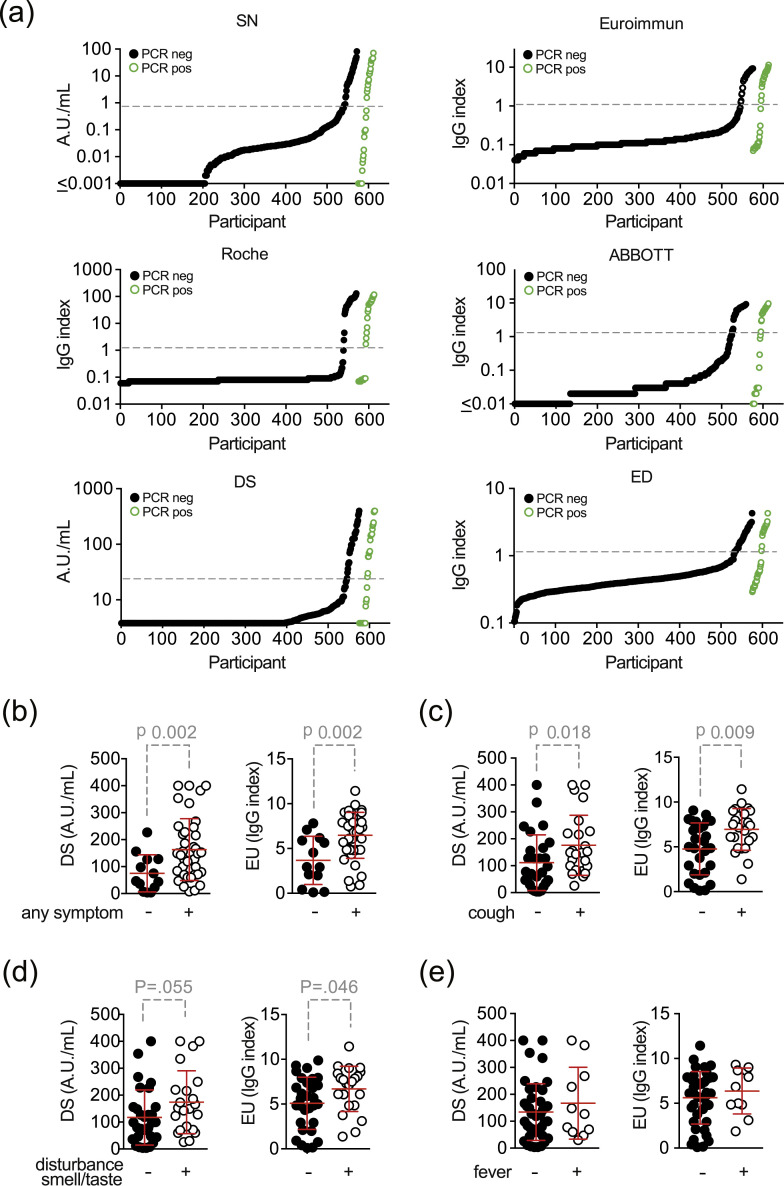
Individual assay results for all six IgG tests performed were stratified by previous PCR test results and are shown in Fig. 4 (a). For seropositive participants, we also assessed whether the extent of the antibody response differed between symptomatic and asymptomatic individuals. Data are shown for two of the assays (Fig. 4b–e). Symptomatic individuals showed a significantly higher antibody response when all symptoms were considered (EU-assay: median 2.9 versus 7.2 IgG-index, p 0.002; DS-assay: median 45.2 versus 143 AU/mL, p 0.002) or cough only was considered (EU-assay: median 4.9 versus 7.3 IgG-index, p 0.009; DS-assay: median 76.30 versus 148.5 AU/mL; p 0.018) (Fig. 4b,c). The increase was borderline for disturbance of smell or taste (Fig. 4d) and non-significant for fever (Fig. 4e). This suggests a relationship between the development of symptoms and the extent of the antibody response.
Fig. 4.
Semi-quantitative test results of (a) all six assays from all participants with serology shown for previously PCR-positive (black dots) and PCR-negative (green circles) participants. Dashed grey-line indicates threshold for positive test results according to the manufacturer. (b–e) Semi-quantitative test results of participants with self-reported symptoms (with two-sided p values of the Wilcoxon–Mann–Whitney test): (b) any symptom, (c) cough, (d) taste/smell disorder and (e) fever. When results were below or above the detection limit of the assay, values were set to the respective lower or upper boundaries. Abbreviations: SN..2019-nCoV IgG kit (Snibe Co., Ltd., Shenzhen, China); EU..SARS-COV-2 IgG ELISA kit (Euroimmun, Lübeck, Germany); DS..SARS-CoV-2 S1/S2 IgG CLIA kit (DiaSorin, Saluggia, Italy); ED..EDI Novel Coronavirus SARS-CoV-2 IgG ELISA kit (Epitope Diagnostics Inc., San Diego, USA).
Discussion
Our data show strikingly lower numbers of seropositive participants than we had expected based on the initial mass screening and the estimates of asymptomatic infections previously reported [5,6]. Only 8.4% of the tested population were seropositive for anti-SARS-CoV-2 antibodies. In the cohort, 6.2% (38/610) had a previously proven SARS-CoV-2 infection. Characteristics of the 38 participants (adults, adolescents and children combined) analysed (i.e. with serum samples) with a positive PCR test for SARS-CoV-2 during quarantine stratified by serostatus are shown in the Supplementary material (Table S3). Assuming that all antibody-positive participants were also infected, an estimate for the minimum crude infection rate in the cohort of 620 participants is 71/620 (11.5%; 95% CI 9.1%–14.2%). Our data clearly show, that the extent of a SARS-CoV-2 outbreak in the community is underestimated when PCR tests or seroprevalence are being used alone. When assessing the extent of a SARS-CoV-2 outbreak in a community, a combinatory approach with both approaches could be favourable.
It has been shown that most patients develop serum antibodies against SARS-CoV-2 within approximately 1 week after infection [7]. Several investigators have reported 100% anti-SARS-CoV-2 IgG seropositivity in patients or in convalescent individuals [[8], [9], [10], [11]]. However, our findings confirm studies in which IgG against different SARS-CoV-2 antigens was not detectable in a fraction of patients who were examined at least 14 days after disease onset or in convalescents [10,12]. It is currently unknown why specific antibodies cannot be detected in some patients with previously PCR-proven SARS-CoV-2 infection. It has been suggested that less severe clinical manifestations might be associated with lower or absent antibody titres [7]. Long et al. show that asymptomatic patients might develop a weaker detectable antibody response against SARS-CoV-2 infection, as indicated by an early decrease of IgG and neutralizing antibodies [13]. In fact, this is confirmed by our data. However, there are also reports on asymptomatic individuals in whom specific neutralizing antibodies against SARS-CoV-2 could be detected [14]. Specific antibodies could have been produced, potentially at low concentrations, and declined rapidly, especially as waning of specific antibodies after infection is a common feature observed in coronavirus infections [15,16]. Whether the low rate of seroprevalence reflects early waning of the specific (and assay-detectable) antibody response or whether these individuals in fact did not develop antibodies that could be detected with the applied assays, remains speculative.
This post-outbreak seroprevalence cohort study differs from similar studies [17], first by the ‘complete’ cohort approach including children and infants instead of a representative sample, and second by the extensive use of different antibody assays.
Overall, all current evidence indicates that antibody responses alone do not suffice to overcome SARS-CoV-2 infection. Data from severe acute respiratory syndrome coronavirus and Middle East respiratory syndrome coronavirus suggest that T-cell responses are required for protection and may last longer than antibody titres [[18], [19], [20], [21], [22]]. In addition, potent neutralizing antibodies were found in patients with high or low serum concentrations of specific antibodies measured by ELISA [12]. Consequently, we are currently analysing the neutralization capacity in cell culture systems and SARS-CoV-2-specific T-cell responses in our study participants.
Limitations
This study has several limitations. First, our study was a population-based cohort study. We were able to recruit 71% of the community population, but 29% of the population did not participate for unknown reasons, which could introduce bias in the assessment. Second, the study was carried out 6 weeks after the end of the 14-day quarantine. This could have missed a number of participants who had a rapidly waning antibody response. Third, there was no baseline of the antibody status before the quarantine as some participants might have been exposed earlier during the pandemic, as a result, we here report seroprevalence and not ‘true’ seroconversion rates. Finally, symptoms were retrospectively assessed up to several weeks after the outbreak. This self-reported assessment might be prone to recall bias so that the rate of symptomatic or true asymptomatic infections and the respective associations should be judged with caution.
Conclusions
Our data question the relevance and reliability of IgG antibody testing to detect past SARS-CoV-2 infections. In our completely PCR-tested community, only half of the participants with proven SARS-CoV-2 infection developed detectable IgG antibody levels with six different assays. We conclude that assessing the extent of an outbreak of and immunity for SARS-CoV-2 infection should not rely on antibody assays alone.
CoNAN study group
Technische Universität Ilmenau, Ilmenau, Germany: Thomas Hotz; Local Cooperation partners: Petra Enders, Renate Koch, Steffen Mai, Matthias Ullrich; Institute of Clinical Chemistry and Laboratory Diagnostics and Integrated Biobank Jena (IBBJ), Jena University Hospital – Friedrich Schiller University, Jena, Germany: Cora Richert, Cornelius Eibner, Bettina Meinung, Kay Stötzer, Julia Köhler; Children's Hospital, Jena University Hospital – Friedrich Schiller University, Jena, Germany: Hans Cipowicz, Christine Pinkwart; Department of Anesthesiology and Intensive Care Medicine Jena University Hospital – Friedrich Schiller University, Jena, Germany: Michael Bauer, Petra Dickmann, Annika Licht, Juliane Scholz, Wibke Wetzker; Institute for Infectious Disease and Infection Control, Jena University Hospital – Friedrich Schiller University, Jena, Germany: Anita Hartung, Daniel Weiss, Lara Thieme, Gabi Hanf, Clara Schnizer, Jasmin Müller, Jennifer Kosenkow, Franziska Röstel; Institute of Immunology, Jena University Hospital – Friedrich Schiller University, Jena, Germany: Nico Andreas, Raphaela Marquardt; Institute of Medical Microbiology, Jena University Hospital – Friedrich Schiller University, Jena, Germany: Stefanie Deinhardt-Emmer, Sebastian Kuhn.
Author contributions
MWP and SW had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis; they conceived and designed the study. Data acquisition was by MB, SG, JG, MK, AK, BL, OM, HP and DR. All authors contributed to the analysis and interpretation of data. SG, TK, MWP, AS and SW drafted the manuscript. All authors contributed to critical revision of the manuscript and additional important intellectual content and data interpretation. AS carried out the statistical analyses. MWP and SW obtained funding. JA, TK, SK and BL provided administrative, technical, or material support. MWP and SW supervised the study.
Transparency declaration
SW received speaker fees from MSD and Infectopharm. SH received speaker fees from Pfizer, MSD and Astra Zeneca. TK received speaker fees from Roche MP and has participated in international advisory boards from Pfizer, Novartis, Basilea and Cubist and received speaker fees from the same companies. CB has participated in advisory boards from GSK and received speaking fees from Pfizer. All other authors report that they have no conflict of interest. Funding: CoNAN was funded by the Sondervermögen ‘Corona’ of the Thuringian Ministry for Economic Affairs, Science and Digital Society. The funding agency had no role in the design and conduct of the study; collection, management, analyses, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Acknowledgements
The project was carried out in cooperation with the district administration and the health department of the Ilm district and is funded by the Thuringian Ministry for Economic Affairs, Science and Digital Society from the, Thüringer Corona-Pandemie-Hilfsfonds“, SV-Kapitel 82 30, Titel 68205 # 5526/32-4-2.
Editor: F. Allerberger
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cmi.2020.11.009.
Contributor Information
The CoNAN Study Group:
Thomas Hotz, Local Cooperation Partners, Petra Enders, Renate Koch, Steffen Mai, Matthias Ullrich, Cora Richert, Cornelius Eibner, Bettina Meinung, Kay Stötzer, Julia Köhler, Hans Cipowicz, Christine Pinkwart, Michael Bauer, Petra Dickmann, Annika Licht, Juliane Scholz, Wibke Wetzker, Anita Hartung, Daniel Weiss, Lara Thieme, Gabi Hanf, Clara Schnizer, Jasmin Müller, Jennifer Kosenkow, Franziska Röstel, Nico Andreas, Raphaela Marquardt, Stefanie Deinhardt-Emmer, and Sebastian Kuhn
Appendix A. Supplementary data
The following is the Supplementary data to this article:
Fig. S1. Age distribution of the CoNAN cohort.
Table S1. Sensitivities and specificities of the applied tests shown as provided by the manufacturer.
Table S2. Self-reported symptoms of the 562 adult participants stratified by serostatus and 58 participating adolescents and children analysed (i.e. with serum samples).
Table S3. Characteristics of the 38 participants (adults, adolescents and children combined) with a positive mass PCR test for SARS-CoV-2 during quarantine stratified by serostatus.
References
- 1.Randolph H.E., Barreiro L.B. Herd immunity: understanding COVID-19. Immunity. 2020;52:737–741. doi: 10.1016/j.immuni.2020.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Whitman J.D., Hiatt J., Mowery C.T., Shy B.R., Yu R., Yamamoto T.N. Evaluation of SARS-CoV-2 serology assays reveals a range of test performance. Nat Biotechnol. 2020;38:s1174–s1183. doi: 10.1038/s41587-020-0659-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jaaskelainen A.J., Kuivanen S., Kekalainen E., Ahava M.J., Loginov R., Kallio-Kokko H. Performance of six SARS-CoV-2 immunoassays in comparison with microneutralisation. J Clin Virol. 2020;129:104512. doi: 10.1016/j.jcv.2020.104512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.WHO-2019 https://apps.who.int/iris/bitstream/handle/10665/331656/WHO-2019-nCoV-Seroepidemiology-2020.1-eng.pdf?sequence=1&isAllowed=y; 2019 Available from:
- 5.Hains D.S., Schwaderer A.L., Carroll A.E., Starr M.C., Wilson A.C., Amanat F. Asymptomatic seroconversion of immunoglobulins to SARS-CoV-2 in a pediatric dialysis unit. JAMA. 2020;323:2424–2425. doi: 10.1001/jama.2020.8438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shields A., Faustini S.E., Perez-Toledo M., Jossi S., Aldera E., Allen J.D. SARS-CoV-2 seroprevalence and asymptomatic viral carriage in healthcare workers: a cross-sectional study. Thorax. 2020 doi: 10.1136/thoraxjnl-2020-215414. thoraxjnl-2020-215414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang A.T., Garcia-Carreras B., Hitchings M.D.T., Yang B., Katzelnick L.C., Rattigan S.M. A systematic review of antibody mediated immunity to coronaviruses: kinetics, correlates of protection, and association with severity. Nat Commun. 2020;11:4704. doi: 10.1038/s41467-020-18450-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sun B., Feng Y., Mo X., Zheng P., Wang Q., Li P. Kinetics of SARS-CoV-2 specific IgM and IgG responses in COVID-19 patients. Emerg Microb Infect. 2020;9:940–948. doi: 10.1080/22221751.2020.1762515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jiang H.W., Li Y., Zhang H.N., Wang W., Men D., Yang X. Global profiling of SARS-CoV-2 specific IgG/IgM responses of convalescents using a proteome microarray. Nat Commun. 2020;11:3581. doi: 10.1038/s41467-020-17488-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.To K.K., Tsang O.T., Leung W.S., Tam A.R., Wu T.C., Lung D.C. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: an observational cohort study. Lancet Infect Dis. 2020;20:565–574. doi: 10.1016/S1473-3099(20)30196-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xiang F., Wang X., He X., Peng Z., Yang B., Zhang J. Antibody detection and dynamic characteristics in patients with COVID-19. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa461. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Robbiani D.F., Gaebler C., Muecksch F., Lorenzi J.C.C., Wang Z., Cho A. Convergent antibody responses to SARS-CoV-2 in convalescent individuals. Nature. 2020 doi: 10.1038/s41586-020-2456-9. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Long Q.X., Tang X.J., Shi Q.L., Li Q., Deng H.J., Yuan J. Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections. Nat Med. 2020 doi: 10.1038/s41591-020-0965-6. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 14.Choe P.G., Kang C.K., Suh H.J., Jung J., Kang E., Lee S.Y. Antibody responses to SARS-CoV-2 at 8 weeks postinfection in asymptomatic patients. Emerg Infect Dis. 2020;26:2484–2487. doi: 10.3201/eid2610.202211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Callow K.A., Parry H.F., Sergeant M., Tyrrell D.A. The time course of the immune response to experimental coronavirus infection of man. Epidemiol Infect. 1990;105:435–446. doi: 10.1017/s0950268800048019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu L.P., Wang N.C., Chang Y.H., Tian X.Y., Na D.Y., Zhang L.Y. Duration of antibody responses after severe acute respiratory syndrome. Emerg Infect Dis. 2007;13:1562–1564. doi: 10.3201/eid1310.070576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Russell T.W., Hellewell J., Jarvis C.I., van Zandvoort K., Abbott S., Ratnayake R. Estimating the infection and case fatality ratio for coronavirus disease (COVID-19) using age-adjusted data from the outbreak on the Diamond Princess cruise ship, February 2020. Euro Surveill. 2020;25 doi: 10.2807/1560-7917.ES.2020.25.12.2000256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu W.J., Zhao M., Liu K., Xu K., Wong G., Tan W. T-cell immunity of SARS-CoV: implications for vaccine development against MERS-CoV. Antivir Res. 2017;137:82–92. doi: 10.1016/j.antiviral.2016.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vabret N., Britton G.J., Gruber C., Hegde S., Kim J., Kuksin M. Immunology review, immunology of COVID-19: current state of the science. Immunity. 2020;52:910–941. doi: 10.1016/j.immuni.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen J., Subbarao K. The immunobiology of SARS∗. Annu Rev Immunol. 2007;25:443–472. doi: 10.1146/annurev.immunol.25.022106.141706. [DOI] [PubMed] [Google Scholar]
- 21.Zhao J., Zhao J., Mangalam A.K., Channappanavar R., Fett C., Meyerholz D.K. Airway memory CD4+ T cells mediate protective immunity against emerging respiratory coronaviruses. Immunity. 2016;44:1379–1391. doi: 10.1016/j.immuni.2016.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tang F., Quan Y., Xin Z.T., Wrammert J., Ma M.J., Lv H. Lack of peripheral memory B cell responses in recovered patients with severe acute respiratory syndrome: a six-year follow-up study. J Immunol. 2011;186:7264–7268. doi: 10.4049/jimmunol.0903490. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Age distribution of the CoNAN cohort.
Table S1. Sensitivities and specificities of the applied tests shown as provided by the manufacturer.
Table S2. Self-reported symptoms of the 562 adult participants stratified by serostatus and 58 participating adolescents and children analysed (i.e. with serum samples).
Table S3. Characteristics of the 38 participants (adults, adolescents and children combined) with a positive mass PCR test for SARS-CoV-2 during quarantine stratified by serostatus.