Abstract
We recently demonstrated that aspartoacylase (Aspa) and hyperpolarization-activated cyclic nucleotide-gated potassium channel 1 (Hcn1) genes were causative of essential tremor (ET) in rats. This finding was obtained using Aspaem34Kyo/Hcn1A354V double-mutant rats, but they were bred on a heterogeneous genetic background of two strains, F344 and WTC. Here, we developed an Aspaem34Kyo/Hcn1em1Kyo double-knockout rat strain with a homogenous F344 genetic background and studied the ability of glutamate receptor antagonists to suppress ET. The F344-Aspa/Hcn1 double-knockout rats exhibited spontaneous, intense body tremor equivalent to that in the double-mutant rats. N-acetyl-aspartate (NAA), a substrate of ASPA, showed accumulation in all brain regions and in the spinal cord. However, N-acetyl-aspartyl-glutamate (NAAG), which is derived from NAA and interacts with glutamatergic receptors, was decreased in the medulla oblongata of the double-knockout rats. The tremor was suppressed by 3-[(R)-2-carboxypiperazin-4-yl]-prop-2-enyl-1-phosphonic acid, an N-methyl-D-aspartate (NMDA) receptor antagonist, in F344-Aspa/Hcn1 double-knockout rats. The non-NMDA glutamate receptor antagonist NBQX weakly inhibited the tremor, while the metabotropic glutamate receptor antagonist LY341495 showed no effect. In addition, both NR2B subunit-specific (Ro 25-6981) and NR2C/NR2D subunit-specific (cis-piperidine dicarboxylic acid) NMDA receptor antagonists suppressed the tremor. These data indicated that the pathogenesis of tremor in Aspa/Hcn1 double-knockout rats involved ionotropic glutamate receptors, particularly NMDA receptors.
Keywords: essential tremor, gene knockout, NMDA receptor, rat
Introduction
Essential tremor (ET) is one of the most common movement disorders. The major manifestation of ET is a postural and/or kinetic tremor that predominantly affects the hands, head, and vocal organs [3]. The etiology of ET, however, remains poorly understood. Certain environmental factors may be at least partly involved in nonfamilial ET [10]. However, a major role for genetic factors has also been suggested in the development of ET [8].
Genetically manipulated and mutant animals have contributed to the identification of genes involved in ET development. We recently demonstrated that the aspartoacylase (Aspa) and hyperpolarization-activated cyclic nucleotide-gated potassium channel 1 (Hcn1) genes were associated with the development of ET [14, 16]. This finding was obtained using the Aspaem34Kyo/Hcn1A354V double-mutant rat strain, which was developed by crossing the F344-Aspaem34/Kyo knockout and WTC/Kyo strains. The F344-Aspaem34 knockout strain harbors a 16-bp deletion in exon 4 [14]. WTC/Kyo harbors a homozygous missense mutation (A354V) that is a loss-of-function allele of Hcn1 [16]. Thus, Aspaem34Kyo/Hcn1A354V double-mutant rats were deficient in ASPA and HCN1 functions. No abnormal phenotype was seen in F344-Aspaem34Kyo knockout or WTC/Kyo rats, but the Aspaem34Kyo/Hcn1A354V double-mutant rats exhibited spontaneous body tremor; this indicated that while neither Aspa nor Hcn1 deficiency alone could cause ET, their combination could induce ET in rats [14, 16]. Our previous study demonstrated that the tremor in Aspaem34Kyo/Hcn1A354V double-mutant rats was specifically ameliorated by medications effective against ET. Further, as in human ET, the inferior olive (IO) was identified as the primary brain region involved in generating tremor [14, 16]. Although Aspaem34Kyo/Hcn1A354V double-mutant rats were useful to examine the pathogenesis of ET, no appropriate control was available because the double-mutant rats had a heterogeneous genetic background involving both the F344 and WTC strains [14].
To address the above issue, we developed F344-Hcn1em1Kyo knockout rats that harbored a 7-bp deletion in exon 4 and exhibited no body tremor [13, 15]. In the present study, we developed the F344-Aspaem34Kyo/Hcn1em1Kyo double-knockout rat strain and examined the clinical manifestations of tremor. To identify possible pathways underlying tremor, we focused on glutamatergic neurotransmission, which would be altered by Aspa knockout, and performed behavioral pharmacology studies using various glutamate receptor antagonists.
Materials and Methods
Ethical use of animals
All animal experiments were approved by the Animal Research Committees of Kyoto University and were conducted according to their regulations on animal experimentation.
Generation of Aspa/Hcn1 double-knockout rats
F344-Aspaem34Kyo [14] and F344-Hcn1em1Kyo [13] rats were supplied by the National BioResource Project-Rat [24]. We intercrossed F1 hybrids to obtain F2 progeny. Rats homozygous for both Aspa and Hcn1 knockout alleles were selected from among F2 progeny and were used to generate the F344-Aspaem34Kyo/Hcn1em1Kyo double-knockout strain.
Measurement of N-acetyl-aspartate (NAA), N-acetyl-aspartyl-glutamate (NAAG), and glutamate
For measurement of NAA and NAAG, the hippocampus, cerebral cortex, medulla oblongata, cerebellum, thalamus, and spinal cord were harvested from 4-week-old Aspa/Hcn1 double-knockout and F344 rats. Tissues were stored at −80°C until subsequent analyses. Frozen brain tissues were homogenized with a 10-fold volume of ice-cold PBS. Homogenates were centrifuged at 12,000 × g for 15 min at 4°C, and 100 µl of the supernatant was mixed with 300 µl of acetonitrile. The mixture was centrifuged at 12,000 × g for 15 min at 4°C, and the supernatant was stored at −80°C until the liquid chromatography and mass spectrometry (LCMS) analysis. Prior to analysis, NAA-13C4 (Sigma Aldrich Japan Inc., Tokyo, Japan) and NAAG-15N2 (Sigma Aldrich) were added to the supernatant as internal standards and diluted 10-fold with 0.8% formic acid. Concentrations of internal standards were 0.25 ng/ml for NAA-13C4 and 7.5 ng/ml for NAAG-15N2.
The triple quad mass spectrometry system consisted of the ACQUITY UPLC I-Class system and the Xevo TQ-S system (Waters, MA, USA). The separation was performed on an Intrada Amino Acid column (3 µm, 2 mm × 150 mm) (Imtakt Corporation, Kyoto, Japan) maintained at 40°C. The mobile phases were defined as A (0.1 M ammonium formate/acetonitrile=80/20 (v/v) solution) and B (acetonitrile containing 0.3% formic acid). The ultra-performance liquid chromatography (UPLC) gradient program was performed as follows: 90% A from 0 to 4 min, then a transition from 90% to 25% over 8 min at a flow rate of 0.2 ml/min. The injection volume of each sample was 2 µl. Mass spectrometry was performed in positive electrospray ionization mode and detection was carried out in selected reaction monitoring mode. Monitored ions were as follows: m/z 175.97>74.03 for NAA, m/z 180.21>76.11 for NAA-13C4, m/z 305>147.86 for NAAG, m/z 307.46>89.22 for NAAG-15N2, and m/z 148.1>84.1 for glutamate. For calibration, 9 calibration standards (0.1, 0.2, 0.5, 1, 2, 5, 10, 20, 50 µM each) were used. The UPLC-MS/MS system was operated by MassLynx (Version 4.1) and data were processed with the TargetlynxTM program (Waters, MA, USA) using the internal standard method. The quantification limits of each compound were 0.5 µM for NAA, 0.1 µM for NAAG, and 1 µM for glutamate. Curve fitting was performed using the no-weighted linear least-square model, and the concentration ranges with a coefficient of determination (R2) of >0.99 were 0.5 to 50 µM for NAA, 0.1 to 50 µM for NAAG, and 1 to 50 µM for glutamate. For peak cracking of NAAG, the column was washed for 1 h after every 24 samples.
Drugs
The following were purchased from Abcam (Cambridge, UK): 3-[(R)-2-carboxypiperazin-4-yl]-prop-2-enyl-1-phosphonic acid (D-CPPene), an antagonist of N-methyl-D-aspartate receptors (NMDARs); LY341495, an antagonist of the group II metabotropic glutamate receptor (mGluR) 2/3; Ro 25-6981, a NMDAR antagonist that preferentially binds to the NR2B subunit; and cis-piperidine dicarboxylic acid (Cis-PPDA), a NMDAR antagonist that preferentially binds to NR2C/NR2D-containing receptors. NBQX (2,3-dihydroxy-6-nitro-7-sulphamoyl-benzo(F)quinoxaline), an AMPA and KA receptor antagonist, was purchased from Alomone Labs (Jerusalem, Israel).
Behavior observation
Drug treatment and behavioral observation were performed as described previously [14, 16]. Briefly, Aspa/Hcn1 double-knockout rats (3–5 weeks of age, n=6) were administered LY341495 (5 mg/kg intraperitoneally (ip)), NBQX (15 mg/kg ip), D-CPPene (1 mg/kg ip), Ro 25-6981 (10 mg/kg ip), or cis-PPDA (10 mg/kg ip). The test dose of each drug was set to effectively block the respective target receptor, in accordance with previous reports [11, 12, 20, 25]. To evaluate tremor, animals were individually placed in an observation box (25 cm × 42 cm × 20 cm). Tremor duration and intensity were examined for 1-min periods, immediately before and 15, 30, 45, and 60 min after drug administration. The tremor intensity was evaluated using a 4-point ranked scale (0, none; 1, weak; 2, moderate; 3, marked) [16].
Statistical analysis
Data are expressed as the mean ± SEM. The statistical significance of differences among multiple groups was determined by one-way ANOVA and Tukey’s post-hoc test (tremor duration) or the Kruskal-Wallis test followed by the Steel-Dwass post-hoc test (tremor intensity). Comparisons between 2 groups (LCMS data) were determined by Student’s t-test. A P value of less than 0.05 was considered statistically significant.
Results
F344-Aspa/Hcn1 double-knockout rats exhibited body tremor
F344-Aspaem34Kyo/Hcn1em1Kyo double-knockout rats (official name; F344-Aspaem34Kyo Hcn1em1Kyo) exhibited spontaneous, intense body tremor that was comparable to that of Aspaem34Kyo/Hcn1A354V double-mutant rats, indicating the usefulness of the double-knockout rats as a model of ET (Supplementary Video 1). This finding showed that both Aspa and Hcn1 deficiencies were sufficient to provoke body tremor in rats and that the expression of this tremor was independent of genetic background.
NAA accumulated in the brain of Aspa/Hcn1 double-knockout rats
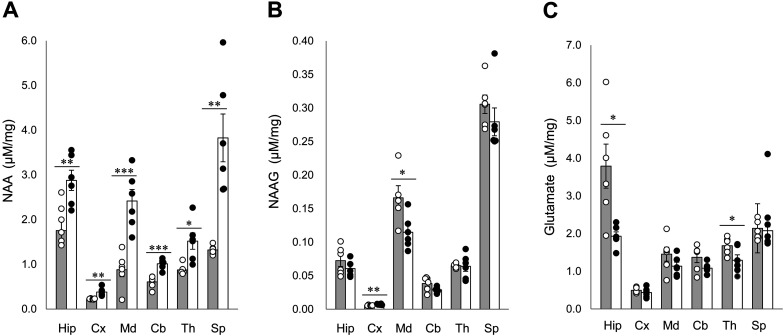
To examine the changes in NAA, NAAG, and glutamate concentrations in the brain of Aspaem34Kyo/Hcn1em1Kyo double-knockout rats, we measured each concentration in 6 brain regions (hippocampus, cerebral cortex, medulla oblongata, cerebellum, thalamus, and spinal cord). NAA significantly accumulated in every brain region and in the spinal cord of Aspa/Hcn1 double-knockout rats (Fig 1A). NAAG concentrations in the double-knockout rats increased significantly in the cortex, but decreased significantly in the medulla oblongata (Fig. 1B). Glutamate concentrations decreased significantly in the hippocampus and thalamus of Aspa/Hcn1 double-knockout rats (Fig. 1C).
Fig. 1.
NAA, NAAG, and glutamate contents in the brain regions of Aspa/Hcn1 double-knockout rats. A, NAA concentrations in various brain regions. B, NAAG concentrations in various brain regions. C, Glutamate concentrations in various brain regions. Grey and white bars represent wild-type (F344) and Aspaem34Kyo/Hcn1em1Kyo double-knockout rats, respectively. Each bar represents the mean ± SEM. of 6 animals. *; P<0.05, **; P<0.01, ***; P<0.001. Hip; hippocampus, Cx; cerebral cortex, Md; medulla oblongata, Cb; cerebellum, Th; thalamus, Sp; spinal cord.
NMDA receptor antagonists suppressed body tremor in Aspa/Hcn1 double-knockout rats
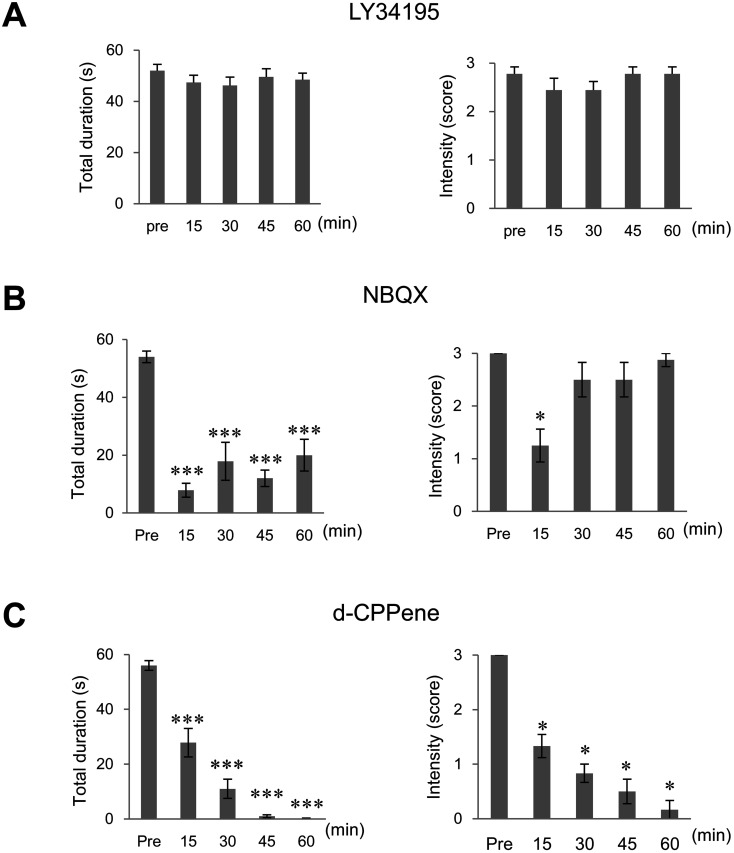
To determine which type of glutamate receptor was involved in tremor expression in Aspaem34Kyo/Hcn1em1Kyo double-knockout rats, we performed ethopharmacological analysis with glutamate receptor antagonists. LY341495, an antagonist of group II mGluR, failed to affect tremor generation in the double-mutant rats (Fig. 2A). NBQX, an antagonist at the AMPA and KA receptors, partially inhibited the tremor; specifically, it significantly reduced tremor duration, but only slightly attenuated tremor intensity (Fig. 2B). By contrast, the NMDA receptor antagonist d-CPPene clearly suppressed the tremor for at least 60 min in terms of both duration and intensity (Fig. 2C). These findings strongly suggest that ionotropic glutamate receptors, particularly NMDA receptors, are involved in tremor expression in Aspa/Hcn1 double-knockout rats.
Fig. 2.
An NMDA receptor antagonist suppressed tremor in Aspa/Hcn1 double-knockout rats. The total duration (left) and intensity (right) of body tremor in Aspaem34Kyo/Hcn1em1Kyo double-knockout rats given LY341495 (A), NBQX (B), or d-CPPene (C). d-CPPene, an NMDA receptor antagonist, significantly suppressed both the duration and intensity of tremor throughout the observation period (60 min). *; P<0.05, **; P<0.01, ***; P<0.001.
NR2B and NR2C/2D NMDA antagonists suppressed body tremor in Aspa/Hcn1 double-knockout rats
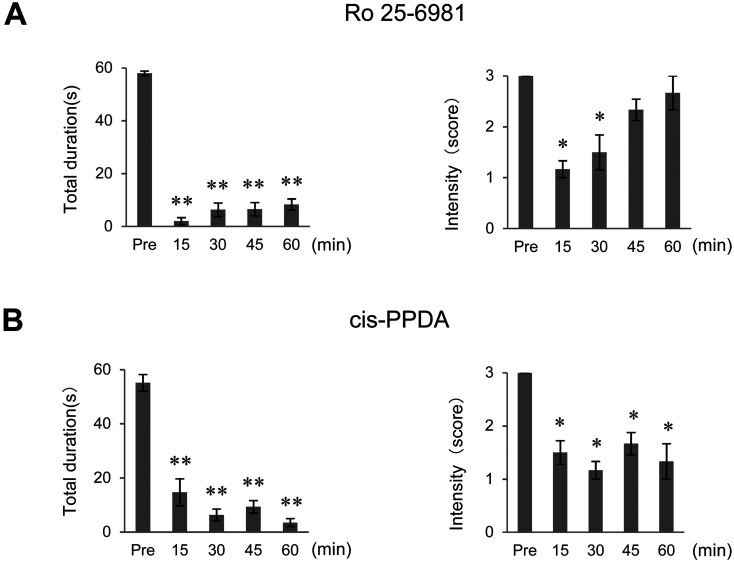
To characterize the NMDA receptors involved in tremor expression in Aspaem34Kyo/Hcn1em1Kyo double-knockout rats, we conducted ethopharmacological analysis with subunit-specific NDMA antagonists. Ro 25-6981, which preferentially antagonizes the NR2B-containing NMDA receptor, significantly suppressed the duration of tremors, but its inhibition of tremor intensity was weak and lasted only 30 min (Fig. 3A). By contrast, cis-PPDA, an antagonist at the NR2C/NR2D-containing NMDA receptors, markedly suppressed both the duration and intensity of tremors, for at least 60 min (Fig. 3B). These findings suggest that the NMDA receptors, which contain NR2B and NR2C/NR2D subunits, are involved in the expression of tremors in Aspa/Hcn1 double-knockout rats.
Fig. 3.
An NR2C/NR2D NMDA receptor antagonist suppressed tremor in Aspa/Hcn1 double-knockout rats. The total duration (left) and intensity (right) of body tremor in Aspaem34Kyo/Hcn1em1Kyo double-knockout rats given Ro 25-6981 (A) or cis-PPDA (B). cis-PPDA, an antagonist of NMDA receptors containing the NR2C and NR2D subunits, significantly suppressed both the duration and intensity of tremor throughout the observation period (60 min). *; P<0.05, **; P<0.001.
Discussion
The F344-Aspaem34Kyo/Hcn1em1Kyo double-knockout rat has a homogenous genetic background and exhibited spontaneous body tremor equivalent to that observed in Aspa/Hcn1 double-mutant rats. This finding indicates that F344-Aspa/Hcn1 double-knockout rats can be used as an improved animal model of ET.
ASPA (EC 3.5.1.15) hydrolyzes NAA to aspartate and acetate, and the concentration of NAA is elevated in Aspaem34Kyo-knockout rats [14]. NAA serves as a precursor of the neuroactive peptide NAAG, and is converted to NAAG by NAAG synthetase, which forms a peptide bond between NAA and glutamate. Consistent with our previous study [14], F344-Aspa/Hcn1 double-knockout rats exhibited a marked elevation in NAA concentrations throughout the brain and the spinal cord; however, this elevation was not accompanied by increases in NAAG concentrations. NAAG was slightly elevated in the cerebral cortex, but was decreased in the medulla oblongata (Fig. 1). Although the mechanism of the lack of widespread NAAG elevation remains unclear, it may be linked to reduced glutamate content in several brain regions in the double-knockout rats.
NAAG acts as an agonist at mGluR 3 [28] and also inhibits the function of NMDA receptors [23]. Thus, the reduced NAAG concentrations in F344-Aspa/Hcn1 double-knockout rats may decrease mGluR 3 activity and increase NMDA receptor activity. In addition, NAA has been shown to cause neural excitation through the activation of ionotropic glutamate receptors, which include NMDA receptors [2, 22]; this suggests that ionotropic glutamate receptors are activated in F344-Aspa/Hcn1 double-knockout rats. Consistent with this hypothesis, the NMDA antagonist d-CPPene suppressed tremor generation in F344-Aspa/Hcn1 double-knockout rats, and the non-NMDA ionotropic glutamate receptor antagonist NBQX also weakly inhibited tremor. Meanwhile, the mGluR 2/3 antagonist LY341495 showed no effects, suggesting that ionotropic glutamate receptors, particularly NMDA receptors, are involved in tremor expression in Aspa/Hcn1 double-knockout rats.
NMDA receptors are oligomeric ligand-gated ion channels formed by the assembly of an essential NR1 subunit and 4 different NR2 subunits (NR2A–D) [7]. Our ethopharmacological experiments suggested that NR2C/NR2D-containing NMDA receptors, and to a lesser extent, NR2B-containing NMDA receptors, were involved in tremor pathogenesis in the double-knockout rats.
We previously showed that the IO of the brain stem was a primary region involved in tremor generation in a rat model of ET. In this model, IO neurons were activated and IO lesioning inhibited the tremor [16]. IO neurons exhibit synchronous oscillation activity and send their output signals to the cerebellum via climbing fibers [9]. Extreme oscillations have been postulated to be functionally related to the manifestations of pathological rhythmic bursts, such as myoclonus, convulsion, and tremor [18, 19, 26].
Interestingly, the NMDA receptor plays an important role in the oscillatory activity of IO neurons [21]. Blockage of NMDA receptors abolishes 2 different oscillations found in IO neurons. One is the spontaneous subthreshold oscillation that manifests at the normal resting membrane potential, while the other is an oscillation that is invariably induced by activation of NMDA receptors at depolarized membrane potentials. Indeed, Chen et al. demonstrated NR2A/B immunoreactivity in the IO of adult rats, although they did not examine the immunoreactivities of NR2C or NR2D [5]. In addition, NR2D was shown to be markedly expressed in the brain stem, including in the IO [27]. These findings support our hypothesis that NR2B- and NR2C/2D-containing NMDA receptors are involved in tremor generation in rats.
HCN channels can actively dampen both inhibitory and excitatory stimuli arriving at the cell membrane, and thereby help to stabilize the membrane potential [4]. We previously showed that Hcn1-knockout rats exhibited a marked decrease of Ih, which restores hyperpolarized membrane potentials to be the resting state [13]. Thus, the membrane potentials of IO neurons may be unstable in Aspa/Hcn1 double-knockout rats. As both types of oscillations mentioned above are greatly influenced by the membrane potential [21], we hypothesize that highly unstable membrane potentials may disturb, and possibly exaggerate, the oscillations in the IO, and thereby induce tremor.
Finally, our results suggest that NMDA receptor blockers have the potential to treat ET. Indeed, NMDA receptor antagonists have been used for the treatment of depression, Alzheimer’s disease, dementia, Parkinson’s disease, and neuropathic pain [1, 6, 17]. Therefore, F344-Aspaem34Kyo/Hcn1em1Kyo rats can serve as a good animal model for evaluating novel therapeutic candidates and improving our understanding of the pathophysiology of ET.
In summary, we developed an improved rat model of ET, the F344-Aspaem34Kyo Hcn1em1Kyo rat strain. In this model, ionotropic glutamate receptors, particularly NMDA receptors containing the NR2C and NR2D subunits, were fundamentally involved in tremor generation in rats. Further studies are required to clarify the detailed mechanisms underlying tremor induction and enhanced activity of glutamate receptors in the F344-Aspaem34Kyo/Hcn1em1Kyo rat.
Acknowledgments
The authors would like to thank the National BioResource Project-Rat in Japan (http://www.anim.med.kyoto-u.ac.jp/nbr) for distributing the F344-Aspaem34Kyo (NBRP Rat No: 0806) and F344-Hcn1em1Kyo rats (NBRP Rat No: 0821). F344-Aspaem34Kyo Hcn1em1Kyo double-knockout rats (NBRP Rat No: 0884) are available from the project.
References
- 1.Aiyer R., Mehta N., Gungor S., Gulati A.2018. A systematic review of NMDA receptor antagonists for treatment of neuropathic pain in clinical practice. Clin. J. Pain 34: 450–467. [DOI] [PubMed] [Google Scholar]
- 2.Akimitsu T., Kurisu K., Hanaya R., Iida K., Kiura Y., Arita K., Matsubayashi H., Ishihara K., Kitada K., Serikawa T., Sasa M.2000. Epileptic seizures induced by N-acetyl-L-aspartate in rats: in vivo and in vitro studies. Brain Res. 861: 143–150. doi: 10.1016/S0006-8993(00)02028-X [DOI] [PubMed] [Google Scholar]
- 3.Benito-León J., Louis E.D.2006. Essential tremor: emerging views of a common disorder. Nat. Clin. Pract. Neurol. 2: 666–678, quiz 2p, 691. doi: 10.1038/ncpneuro0347 [DOI] [PubMed] [Google Scholar]
- 4.Biel M., Wahl-Schott C., Michalakis S., Zong X.2009. Hyperpolarization-activated cation channels: from genes to function. Physiol. Rev. 89: 847–885. doi: 10.1152/physrev.00029.2008 [DOI] [PubMed] [Google Scholar]
- 5.Chen L.W., Tse Y.C., Li C., Guan Z.L., Lai C.H., Yung K.K.L., Shum D.K.Y., Chan Y.S.2006. Differential expression of NMDA and AMPA/KA receptor subunits in the inferior olive of postnatal rats. Brain Res. 1067: 103–114. doi: 10.1016/j.brainres.2005.10.054 [DOI] [PubMed] [Google Scholar]
- 6.Dang Y.H., Ma X.C., Zhang J.C., Ren Q., Wu J., Gao C.G., Hashimoto K.2014. Targeting of NMDA receptors in the treatment of major depression. Curr. Pharm. Des. 20: 5151–5159. doi: 10.2174/1381612819666140110120435 [DOI] [PubMed] [Google Scholar]
- 7.Furukawa H., Singh S.K., Mancusso R., Gouaux E.2005. Subunit arrangement and function in NMDA receptors. Nature 438: 185–192. doi: 10.1038/nature04089 [DOI] [PubMed] [Google Scholar]
- 8.Jiménez-Jiménez F.J., Alonso-Navarro H., García-Martín E., Lorenzo-Betancor O., Pastor P., Agúndez J.A.G.2013. Update on genetics of essential tremor. Acta Neurol. Scand. 128: 359–371. doi: 10.1111/ane.12148 [DOI] [PubMed] [Google Scholar]
- 9.Llinás R., Yarom Y.1986. Oscillatory properties of guinea-pig inferior olivary neurones and their pharmacological modulation: an in vitro study. J. Physiol. 376: 163–182. doi: 10.1113/jphysiol.1986.sp016147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Louis E.D.2001. Etiology of essential tremor: should we be searching for environmental causes? Mov. Disord. 16: 822–829. doi: 10.1002/mds.1183 [DOI] [PubMed] [Google Scholar]
- 11.Mikics E., Toth M., Biro L., Bruzsik B., Nagy B., Haller J.2017. The role of GluN2B-containing NMDA receptors in short- and long-term fear recall. Physiol. Behav. 177: 44–48. doi: 10.1016/j.physbeh.2017.04.005 [DOI] [PubMed] [Google Scholar]
- 12.Ngomba R.T., Biagioni F., Casciato S., Willems-van Bree E., Battaglia G., Bruno V., Nicoletti F., van Luijtelaar E.L.J.M.2005. The preferential mGlu2/3 receptor antagonist, LY341495, reduces the frequency of spike-wave discharges in the WAG/Rij rat model of absence epilepsy. Neuropharmacology 49:(Suppl 1): 89–103. doi: 10.1016/j.neuropharm.2005.05.019 [DOI] [PubMed] [Google Scholar]
- 13.Nishitani A., Kunisawa N., Sugimura T., Sato K., Yoshida Y., Suzuki T., Sakuma T., Yamamoto T., Asano M., Saito Y., Ohno Y., Kuramoto T.2019. Loss of HCN1 subunits causes absence epilepsy in rats. Brain Res. 1706: 209–217. doi: 10.1016/j.brainres.2018.11.004 [DOI] [PubMed] [Google Scholar]
- 14.Nishitani A., Tanaka M., Shimizu S., Kunisawa N., Yokoe M., Yoshida Y., Suzuki T., Sakuma T., Yamamoto T., Kuwamura M., Takenaka S., Ohno Y., Kuramoto T.2016. Involvement of aspartoacylase in tremor expression in rats. Exp. Anim. 65: 293–301. doi: 10.1538/expanim.16-0007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nishitani A., Yoshihara T., Tanaka M., Kuwamura M., Asano M., Tsubota Y., Kuramoto T.2020. Muscle weakness and impaired motor coordination in hyperpolarization-activated cyclic nucleotide-gated potassium channel 1-deficient rats. Exp. Anim. 69: 11–17. doi: 10.1538/expanim.19-0067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ohno Y., Shimizu S., Tatara A., Imaoku T., Ishii T., Sasa M., Serikawa T., Kuramoto T.2015. Hcn1 is a tremorgenic genetic component in a rat model of essential tremor. PLoS One 10: e0123529. doi: 10.1371/journal.pone.0123529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Olivares D., Deshpande V.K., Shi Y., Lahiri D.K., Greig N.H., Rogers J.T., Huang X.2012. N-methyl D-aspartate (NMDA) receptor antagonists and memantine treatment for Alzheimer’s disease, vascular dementia and Parkinson’s disease. Curr. Alzheimer Res. 9: 746–758. doi: 10.2174/156720512801322564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Palmer G.C., Stagnitto M.L., Ray R.K., Knowles M.A., Harvey R., Garske G.E.1993. Anticonvulsant properties of calcium channel blockers in mice: N-methyl-D-,L-aspartate- and Bay K 8644-induced convulsions are potently blocked by the dihydropyridines. Epilepsia 34: 372–380. doi: 10.1111/j.1528-1157.1993.tb02424.x [DOI] [PubMed] [Google Scholar]
- 19.Park Y.G., Park H.Y., Lee C.J., Choi S., Jo S., Choi H., Kim Y.H., Shin H.S., Llinas R.R., Kim D.2010. CaV3.1 is a tremor rhythm pacemaker in the inferior olive. Proc. Natl. Acad. Sci. USA 107: 10731–10736. doi: 10.1073/pnas.1002995107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Paterson N.E., Malekiani S.A., Foreman M.M., Olivier B., Hanania T.2009. Pharmacological characterization of harmaline-induced tremor activity in mice. Eur. J. Pharmacol. 616: 73–80. doi: 10.1016/j.ejphar.2009.05.031 [DOI] [PubMed] [Google Scholar]
- 21.Placantonakis D., Welsh J.2001. Two distinct oscillatory states determined by the NMDA receptor in rat inferior olive. J. Physiol. 534: 123–140. doi: 10.1111/j.1469-7793.2001.t01-1-00123.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rubin Y., LaPlaca M.C., Smith D.H., Thibault L.E., Lenkinski R.E.1995. The effect of N-acetylaspartate on the intracellular free calcium concentration in NTera2-neurons. Neurosci. Lett. 198: 209–212. doi: 10.1016/0304-3940(95)12014-U [DOI] [PubMed] [Google Scholar]
- 23.Sekiguchi M., Okamoto K., Sakai Y.1989. Low-concentration N-acetylaspartylglutamate suppresses the climbing fiber response of Purkinje cells in guinea pig cerebellar slices and the responses to excitatory amino acids of Xenopus laevis oocytes injected with cerebellar mRNA. Brain Res. 482: 87–96. doi: 10.1016/0006-8993(89)90545-3 [DOI] [PubMed] [Google Scholar]
- 24.Serikawa T., Mashimo T., Takizawa A., Okajima R., Maedomari N., Kumafuji K., Tagami F., Neoda Y., Otsuki M., Nakanishi S., Yamasaki K., Voigt B., Kuramoto T.2009. National BioResource Project-Rat and related activities. Exp. Anim. 58: 333–341. doi: 10.1538/expanim.58.333 [DOI] [PubMed] [Google Scholar]
- 25.Szczurowska E., Mareš P.2015. Different action of a specific NR2B/NMDA antagonist Ro 25-6981 on cortical evoked potentials and epileptic afterdischarges in immature rats. Brain Res. Bull. 111: 1–8. doi: 10.1016/j.brainresbull.2014.11.001 [DOI] [PubMed] [Google Scholar]
- 26.Welsh J.P.1998. Systemic harmaline blocks associative and motor learning by the actions of the inferior olive. Eur. J. Neurosci. 10: 3307–3320. doi: 10.1046/j.1460-9568.1998.00334.x [DOI] [PubMed] [Google Scholar]
- 27.Wenzel A., Fritschy J.M., Mohler H., Benke D.1997. NMDA receptor heterogeneity during postnatal development of the rat brain: differential expression of the NR2A, NR2B, and NR2C subunit proteins. J. Neurochem. 68: 469–478. doi: 10.1046/j.1471-4159.1997.68020469.x [DOI] [PubMed] [Google Scholar]
- 28.Wroblewska B., Wroblewski J.T., Pshenichkin S., Surin A., Sullivan S.E., Neale J.H.1997. N-acetylaspartylglutamate selectively activates mGluR3 receptors in transfected cells. J. Neurochem. 69: 174–181. doi: 10.1046/j.1471-4159.1997.69010174.x [DOI] [PubMed] [Google Scholar]