Abstract
The non-motor symptoms (NMS) of Parkinson’s disease (PD) are found in more than 90% of patients with PD. Here, we explored the effects of electroacupuncture (EA) stimulation at Zhong wan (CV-12), Qihai (RN-7), Zusanli (ST-36) and Taichong (LR-3) on NMS and brain-gut peptides of PD. We found that EA intervention alleviated the motor deficit induced by 6-OHDA in rats indicated by the decreased abnormal involuntary movements (AIMs) scores and the net number of rotations and increased cylinder test grade. It also improved the spatial memory and attenuated anxiety-like and depression of PD model rats. EA treatment significantly inhibited neuronal apoptosis in PD model animals, as demonstrated by the increased number of TH positive cells and reduced number of apoptotic cells in the substantia nigra. The expression of cleaved caspase-3 and cleaved PARP in PD model rats was markedly suppressed by EA stimulation. Moreover, EA remarkably inhibited the inflammatory response in PD model rats, as revealed by the decreased levels of TNF-α, IL-1β, and COX-2 mRNA expression. It also attenuated the oxidative stress in rats, as indicated by the increased levels of SOD and GSH and the decreased level of MDA. EA treatment contributed to alleviating PD by regulating brain-gut peptides in rats, such as NPY, CCK, SST, GAS, and PYY. In conclusion, EA stimulation at CV-12, RN-7, ST-36, and LR-3 effectively alleviates the NMS of PD partly through regulating the levels of brain-gut peptides.
Keywords: brain-gut peptides, electroacupuncture, non-motor symptoms, Parkinson’s disease
Introduction
Parkinson’s disease (PD) is an age-associated common neurodegenerative disease. The prevalence rate is 1–2% among people over 65 years old, and the peak age of onset is 55–60 years old. There is no treatment to delay or terminate the neurodegeneration currently. One in a thousand elderly people over the age of 60 is affected by this disorder [62]. The clinical symptoms of PD patients include motor and non-motor symptoms (NMS), and the incidence of the latter could be more than 90% [35]. NMS consists of sleep disorders, neurological diseases, cognitive deficits, autonomic and sensory dysfunction, gastrointestinal dysfunction, and many others. Whether as preclinical manifestations or late complications, NMS seriously affect the life quality of PD patients [44, 45]. Therefore, it is of great significance for the early diagnosis, treatment and prognosis evaluation of PD.
Acupuncture has been used for more than 3,000 years in China [66] and it was used to treat many diseases, such as gastroenterological diseases [5], cardiovascular diseases [46], and allergic diseases [43]. Now it is widely used as a popular complementary medicine to treat a variety of disorders worldwide [9, 42, 65]. Briefly, acupuncture needles were inserted into specific acupoints to mechanically couple the connective tissues surrounding the needles. It directly or indirectly stimulates neuroreceptors through local reflexes and the central nervous system, thereby inducing neuroendocrine, endocrine, autonomic and systemic behavioral responses [19]. Compared with drug therapy, acupuncture has the advantages of quick therapeutic effect, less side effect, and low cost [14]. The therapeutic effects of acupuncture on PD-related nerve injury have been widely reported [7, 10, 38, 50, 58] and many PD patients got the improvement of their conditions after the treatment of acupuncture.
The brain-gut axis is a neuro-endocrine network in which the brain responds bidirectionally through the central nervous system, the vagus nerve, the enteric nervous system. It plays a critical role in stress and emotional cognition. A growing body of evidence indicated that peripheral hormones regulated the central nervous system function through the brain-gut axis and might induce the occurrence of neurologic diseases [28, 56]. As a neurodegenerative disease, the main pathological changes of PD, in addition to the degeneration of dopaminergic neurons in the substantia nigra and the significant decrease in the striatum dopamine level, also involve the changes in the levels of brain-gut peptides [67].
Since EA could alleviate PD to a certain extent [31] and the regulation of EA on brain-gut peptides has been reported [57], we speculated that electroacupuncture (EA) treatment can ameliorate the NMS through modulating the expression of brain-gut peptides of PD. In the present study, we first found that EA stimulation at Zhong wan (CV-12), Qihai (RN-7), Zusanli (ST-36) and Taichong (LR-3) could ameliorate the motor and non-motor symptoms, reduce the apoptosis of substantia nigra neurons, alleviate the inflammation and oxidative stress, and modulate the expression levels of several brain-gut peptides of PD model rats.
Material and Methods
Animals
Adult male (Sprague-Dawley) SD rats (260–280 g) were purchased from Liaoning Changsheng Biotechnology (Benxi, China). The animals were housed under a temperature of 25 ± 1°C and a 12 h light/dark cycle condition with no limited access to food and water. The rats were randomly divided into 4 groups: negative control (NC) group (n=6), Sham group (n=6), 6-OHDA group (n=6) and 6-OHDA+ EA group (n=6). The study involving animals was approved by the Ethics Committee of Heilongjiang Academy of Chinese Medical Sciences (the approval document is attached as Supplementary Fig.1).
Model establishment
As previously described [41, 60], the PD models were established by unilateral injection of 6-OHDA (Aladdin, Shanghai, China). After anesthetized with 3% pentobarbital sodium, the right striatum [anteroposterior (AP) 0.7 mm, mediolateral (ML) 2.6 mm, dorsoventral (DV) 4.5 mm] of rats in the 6-OHDA group and 6-OHDA+ EA group was injected with 20 µg 6-OHDA (4 µg/µl) by stereotaxic instrument for 14 days. The rats in the Sham group were injected with the same volume of saline and ascorbate (0.2% ascorbate in 0.9% sodium chloride) on the first day. Rats in the 6-OHDA + EA group were treated with electroacupuncture at a regular time every morning after 6-OHDA injection for 14 consecutive days. No treatment was performed on rats in the NC group.
EA treatment
Zhongwan (CV-12) is located 4-cun above the anterior median umbilicus. Qihai (CV-6) is positioned 1.5-cun below the umbilicus in the midline of the abdomen. Taichong (LR-3) acupoint is located between the first and second metatarsals of the back of the foot. Zusanli (ST-36) acupoint outside the knee under 3-cun. The treatment of EA was performed between 9:00 am-10:00 am for 14 days. Rats of 6-OHDA + EA group were gently immobilized. According to the previous studies [29, 51, 53], the needle handle was connected to the EA device, and the continuous wave was switched on with the frequency of 100 Hz for 20 min. The EA intensity was approximately 1 mA with the extent that animals did not struggle and kept quiet. Behavioral tests were performed after treatment for 14 days.
Abnormal involuntary movements (AIM) Score
AIM score of each rat was evaluated at 2, 7, and 14 days after the first 6-OHDA injection. As previously reported [11], AIM tests were classified into four subtypes: axial AIM, defined as upper body and neck contralateral twisted posturing; limb AIM, defined as repeated, rhythmic jerking or dystonic posturing of the forelimb on the side contralateral to the lesion; Orolingual AIM, characterized by empty jaw movements and contralateral tongue protrusion; and locomotion AIM, indicated by the increased locomotion with contralateral side bias. The total AIM score was calculated by combining each of the individual dyskinesia scores. The assessments were carried out in a blind manner.
Rotational behavior
As previously described [23], the changes in 6-OHDA-induced rotation behavior were assessed using an automatic rotometer chamber. The net number of rotations (contralateral-ipsilateral) was counted over a time span of 60 min. This behavioral test was performed blindly.
Cylinder test
Cylinder test was carried out to test the sensorimotor function. Each rat was placed into a cylinder. The number of times that two forelimbs contacted the wall during 5 min was counted.
Open field test
The open field test was performed in a 100 × 100 × 40 cm box. Each rat explored the apparatus for 5 min. The behavior of each rat was recorded by video. The exploration of peripheral zone in the open field box was considered as an anxiety index.
Y maze test
The Y maze apparatus was composed of 3 black arms (50 × 10 × 20 cm). The animals were placed at the end of one arm and acclimated for 5 min. The sequence and the number of times they entered into each arm were recorded by video for 8 min. Spontaneous alternation was defined as continuous entries into three arms. At each time of the experiment, 75% of alcohol was sprayed to eliminate odor interference. The behavior of each animal was recorded by video, and the spontaneous alternations ratio (%) was calculated as a spatial memory index.
Forced swim
Each rat was placed in a glass cylinder (90 cm in height and 40 cm in diameter), which filled with water at a temperature of 22 ± 1°C and with a height of 60 cm. After swimming for 15 min, each rat was removed from a glass cylinder, gently dried and placed under a warm light for 15 min. The behavior of each animal was recorded by video, and the immobility time (the amount of time that rats had only minimal movements to keep their heads above the water surface) was measured as a depression index.
Tail suspension test
Rats were suspended and fixed at about 5 cm away from the tail tip in the tail suspension test box, and the heads were 20 cm away from the laboratory bench. Each rat was left in this position for 6 min. The behavior of each animal was recorded by video, and the immobility time (the amount of time that rats were hanging passively and motionlessly) was measured as a depression index.
Quantitate real-time PCR (qRT-PCR)
RNAs were isolated from brain tissue by using RNAsimple Total RNA Kit (TIANGEN, Beijing, China) and added with RNase inhibitor (TIANGEN). Reverse transcription was performed by using M-MLV reverse transcriptase (TIANGEN). The primers (GenScript, Nanjing, China) used in this assay were: COX-2, forward 5’- GAACACGGACTTGCTCACTT-3’, reverse 5’- ACGATGTGTAAGGTTTCAGG-3’; GAPDH(internal control), forward 5’-CGGCAAGTTCAACGGCACAG-3’, reverse 5’-CGCCAGTAGACTCCACGACAT-3’. qRT-PCR was carried out by using SYBR Green (Solarbio, China). The relative gene expression data were calculated using 2-ΔΔCt method [34].
Immunohistochemistry
For immunohistochemical staining, the tissues were fixed with 4% poly formaldehyde for 24 h. After conventional dehydrated, hyalinized and paraffin-embedded, brain tissues were cut into 5-µm-thick sections. After dewaxing to water, the sections were continuously heated in pre-boiled antigen retrieval buffer (10 mM citrate, pH 6.0) for 10 min and incubated in 3% H2O2 for 15 min at room temperature. The normal goat serum (Solarbio, Beijing, China) was used to block for 15 min. Then the slides were incubated with rabbit anti-tyrosine hydroxylase (TH) primary antibody (1:500, Abcam, Cambridge, UK ) overnight at 4°C. Next, the sections were incubated with HRP (horseradish peroxidase)-conjugated goat anti-rabbit IgG (1:500, ThermoFisher, Waltham, MA, USA) for 60 min at 37°C. After the DAB (diaminobenzidine, Solarbio) reaction, the slides were counterstained with hematoxylin (Solarbio) for 3 min, dehydrated, cleared and cover slipped. The staining results were observed under a microscope (DP73, OLYMPUS, Tokyo, Japan).
TdT mediated dUTP nick end labeling (TUNEL) assay
The tissues were fixed with 4% poly formaldehyde for 24 h. After conventional dehydrated, hyalinized and paraffin-embedded, the tissues were cut into 5-µm-thick sections. Cell apoptosis was detected by using the TUNEL Apoptosis Detection Kit (wanleibio, Shenyang, China) in accordance with the manufacturer’s protocol. The results were observed through BX53 (OLYMPUS) and photographed by DP73 (OLYMPUS).
ELISA
The tissue samples from the substantia nigra of rats were quantified, lysed, and centrifuged at 2,500 rpm for 10 min to obtain homogenate supernatant. The levels of TNF-α (tumor necrosis factor-α) and IL-1β (interleukin-1β) in the supernatant were assessed by ELISA Kits (MULTI SCIENCES, Hangzhou, China) according to the instructions. Hemagglutination of rats was performed for 30 min and centrifuged at 1,000 g for 20 min. The levels of NPY (neuropeptide Y), SST (somatostatin), CCK (cholecystokinin), PYY (peptide YY), MTL (motilin) and GAS (gastrin) in rat serum were immediately tested by ELISA Kits (Cloud-Clone Corp, Wuhan, China) according to the manufacturer’s protocols.
The levels of MDA, GSH, and SOD in rat brain tissues were detected by Malondialdehyde (MDA) assay kit (TBA method), Reduced glutathione (GSH) assay kit and Total Superoxide Dismutase (T-SOD) assay kit (Hydroxylamine method), respectively, according to the manufacturer’s instructions. These above kits were purchased from NanJing JianCheng (Nanjing, China).
Western blot
Protein quantification was measured using BCA Protein Assay Kit (Solarbio). Equal amounts of protein (10 µg) from each sample were separated by 8% or 15% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and then transferred onto PVDF (polyvinylidene difluoride, Millipore, Bedford, MA, USA) membranes. The membranes were blocked with 5% skim milk (Sangon Biotech, Shanghai, China) for 1 h at room temperature and incubated with primary antibodies: cholecystokinin rabbit polyclonal antibody (CCK, 1:1,000, NOVUS, St. Charles, MO, USA), somatostatin rabbit polyclonal antibody (SST, 1:500, ABclonal, Wuhan, China), Cleaved Caspase-3 rabbit polyclonal antibody (1:1,000, CST, Danvers, MA, USA), neuropeptide Y rabbit polyclonal antibody (NPY, 1:1,000, Affinity, Changzhou, China), Cleaved PARP rabbit polyclonal antibody (1:1,000, CST), cyclooxygenase-2 rabbit polyclonal antibody (COX-2, 1:500, ABclonal) and GAPDH mouse monoclonal antibody (internal control, 1:10,000, proteintech, Wuhan, China), overnight at 4°C. After washing, the membranes were incubated with HRP-conjugated goat anti-mouse (1:3,000, Solarbio) or goat anti-rabbit IgG (1:3,000, Solarbio) for 1 h at 37°C and visualized by using ECL Western Blotting Substrate (Solarbio).
Data statistics
All data were tested using GraphPad Prism 8.0. Data among groups were analyzed using One-Way ANOVA and post hoc Sidak’s multiple comparisons tests or post hoc Dunnett’s multiple comparisons test. The experiments were repeated at least three times. For all analyses, P<0.05 was considered significant.
Results
Effects of EA on the motor deficit of PD model rats
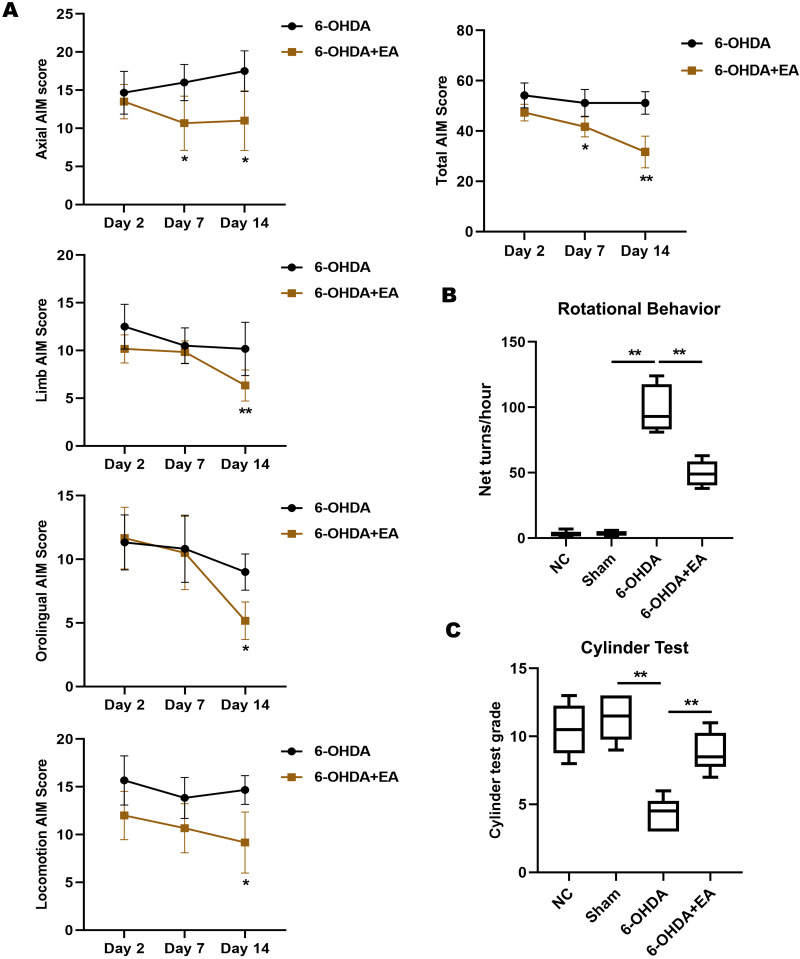
On day 2, 6-OHDA-induced AIMs scores were high and no significant differences were observed between groups. On day 7, EA stimulation led to a significant decrease in axial AIM score of PD model rats. On day 14, we found that EA treatment significantly lowered the AIMs scores in each AIM subtype analysis (axial, limb, orolingual, and locomotive AIMs). The total AIM score of rats was remarkably decreased on day 7 and 14 by EA treatment compared with 6-OHDA group (Fig. 1A). The effect of EA on 6-OHDA-induced rotational behavior was investigated. Rats of the 6-OHDA group exhibited rotational asymmetry in the direction contralateral to the lesion, while a significant decrease in the net number of rotations was found in 6-OHDA+EA group (Fig. 1B). Similarly, EA stimulation dramatically increased the cylinder test grade in PD model rats (Fig. 1C).
Fig. 1.
Effects of EA on the motor behaviors of PD model rats. A. The axial AIM, limb AIM, orolingual AIM, and locomotive AIM scores were measured on day 2, 7, and 14 after the initial 6-OHDA injection. AIM scores were assessed every 20 min over the 100 min following EA stimulation and were then integrated. B. Effects of EA treatment on 6-OHDA-induced rotations in rats from NC group, Sham group, 6-OHDA group or 6-OHDA+EA group. C. Cylinder test was performed to determine the degree of movement disorder of rats in each group. Data are expressed as mean ± SD. *P<0.05, **P<0.01.
Effects of EA on the non-motor symptoms of PD model rats
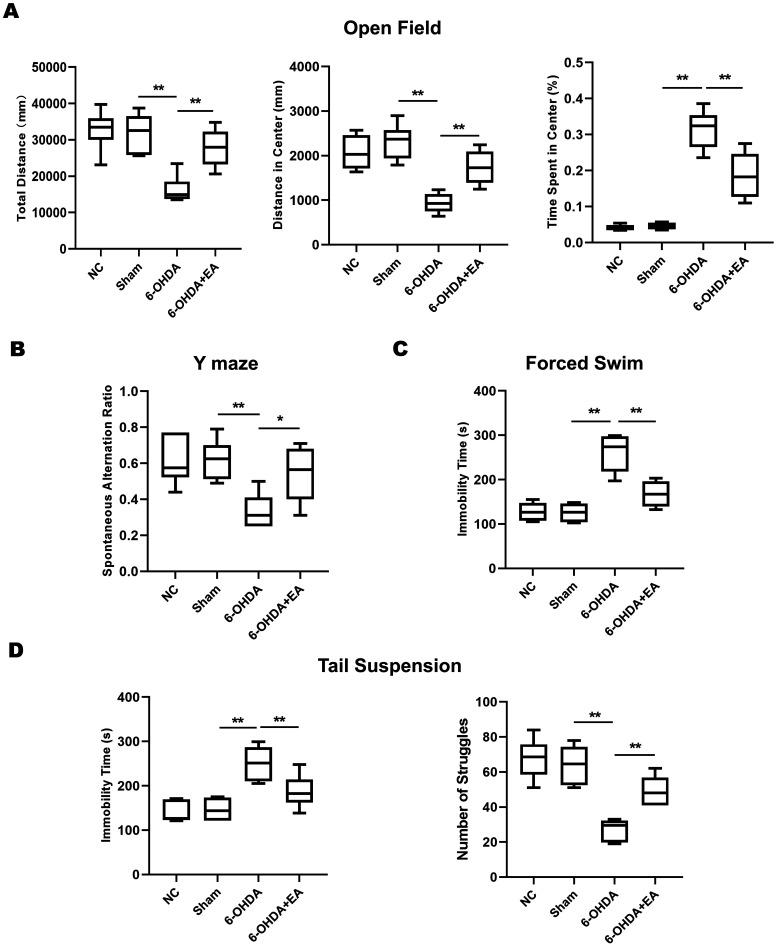
We evaluated the effect of EA on the non-motor symptoms of PD model rats. Administration with 6-OHDA in rats led to a pronounced decrease in exploration compared with rats in Sham group, as indicated by shorter total distance and distance in center and more time spent in center of the open field. However, EA stimulation significantly reversed this decreased exploratory behavior induced by 6-OHDA in rats (Fig. 2A). In Y maze test, the spontaneous alternation ratio in 6-OHDA-lesioned rats was dramatically reduced compared with the sham group, while such decrease was obviously attenuated by EA (Fig. 2B), suggesting that EA treatment contributed to restoring the spatial learning memory of PD model rats. The rats in 6-OHDA group displayed a significant longer immobility time compared to sham group in forced swim test, while the immobility time was notably decreased in rats by the treatment of EA (Fig. 2C). A similar difference was observed in tail suspension test when PD model rats were examined, meanwhile, EA markedly attenuated the decreased number of struggles induced by 6-OHDA (Fig. 2D). Taken together, the treatment of EA improved the spatial memory and attenuated the anxiety-like, depression-like behaviors in PD model rats.
Fig. 2.
Effects of EA on the non-motor behaviors of SD rats induced by 6-OHDA. A. In open field test, anxiety-like was evaluated by measuring the total distance (mm), distance in center (mm) and the time spent in center (%) of rats in the apparatus. B. In Y maze test, the spontaneous alternation ratio was calculated in rats from NC group, Sham group, 6-OHDA group or 6-OHDA+EA group. C. The immobility time (s) of rats from each group was shown in forced swim test. D. The immobility time (s) and the number of struggles of rats from each group were recorded in tail suspension test. Data are expressed as mean ± SD. *P<0.05, **P<0.01.
EA reduces neuronal apoptosis in the substantia nigra of PD model rats
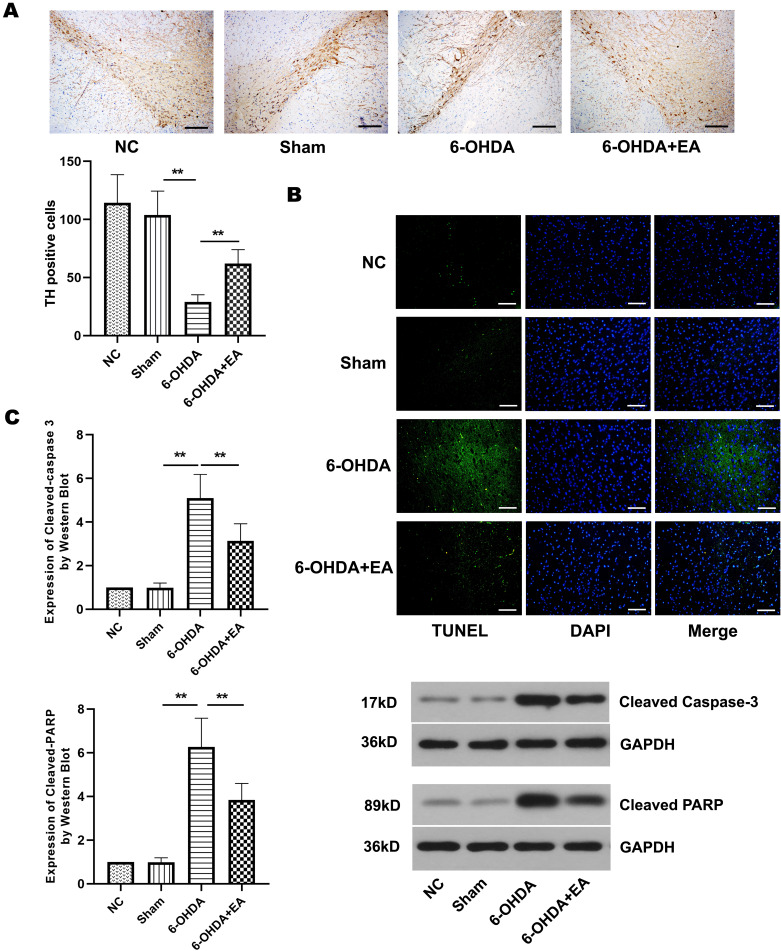
Considering studies showed that apoptosis plays an important role in PD [2, 18, 20], we investigated the effect of electropuncture on the neuronal apoptosis induced by 6-OHDA in PD model rats. Here, we evaluated the effect of EA on neuronal apoptosis in the substantia nigra of PD model rats. Immunohistochemistry showed that 6-OHDA caused a significant decrease in the number of TH positive cells in the substantia nigra of PD model rats, but EA treatment dramatically attenuated this decrease (Fig. 3A). The results of TUNEL assay illustrated that the treatment of EA reduced the number of apoptotic cells caused by 6-OHDA in the substantia nigra of rats (Fig. 3B). Next, we examined the effect of EA on apoptosis-related proteins in the substantia nigra of PD model rats. The expression of cleaved caspase-3 and cleaved PARP was notably enhanced by 6-OHDA in rats, Nevertheless, EA stimulation markedly decreased the expression of these two proteins (Fig. 3C). These results demonstrated that EA treatment reduced neuronal apoptosis in the substantia nigra of PD model rats.
Fig. 3.
Effect of EA on neuronal apoptosis in the substantia nigra of 6-OHDA-induced rats. A. TH positive cells in the substantia nigra of rats from NC group, Sham group, 6-OHDA group, and 6-OHDA+EA group were stained after 6-OHDA lesioning. Scale bar=200 µm. B. Photomicrographs showed the TUNEL positive cells (green) in the substantia nigra of rats from each group. DAPI (2-4-Amidinophenyl-6-indolecarbamidine dihydrochloride) was used to stain nuclei (blue) for light microscopy. Scale bar=100 µm. C. Western blot showed the expression of cleaved caspase-3 and cleaved PARP in the substantia nigra of rats from each group. Data are expressed as mean ± SD. **P<0.01.
EA inhibits the inflammatory response and oxidative stress of the substantia nigra in PD model rats
To explore the effect of EA on inflammatory response induced by 6-OHDA in rats, we detected the levels of TNF-α and IL-1β by ELISA. As shown in Fig. 4A, TNF-α and IL-1β levels were significantly elevated in the 6-OHDA group compared with the sham group, and their levels were markedly reduced by stimulation of EA. The expression of COX-2 at mRNA level and protein level was notably increased by 6-OHDA in rats. Although no significant effect of EA on COX-2 protein expression was observed, the elevation of COX-2 at mRNA level was dramatically decreased by EA treatment in rats (Fig. 4B). Additionally, EA could inhibit the oxidative stress reaction of the substantia nigra in 6-OHDA-lesioned rats, as indicated by the significant decrease of MDA level and the considerable increases of SOD and GSH levels (Fig. 4C). Collectively, EA treatment inhibited the inflammatory response and oxidative stress of the substantia nigra in PD model rats.
Fig. 4.
Effect of EA on the inflammatory response and oxidative stress of the substantia nigra in 6-OHDA-induced rats. A. TNF-α and IL-1β levels in the substantia nigra of rats from NC group, Sham group, 6-OHDA group, and 6-OHDA+EA group were detected by ELASA. B. The expression of COX-2 at mRNA level or protein level was assessed by qRT-PCR or Western blot, respectively. C. The levels of MDA, SOD, and GSH in the brain substantia nigra of rats from each group. Data are expressed as mean ± SD. *P<0.05, ** P<0.01.
Effects of EA on the levels of brain-gut peptides in PD model rats
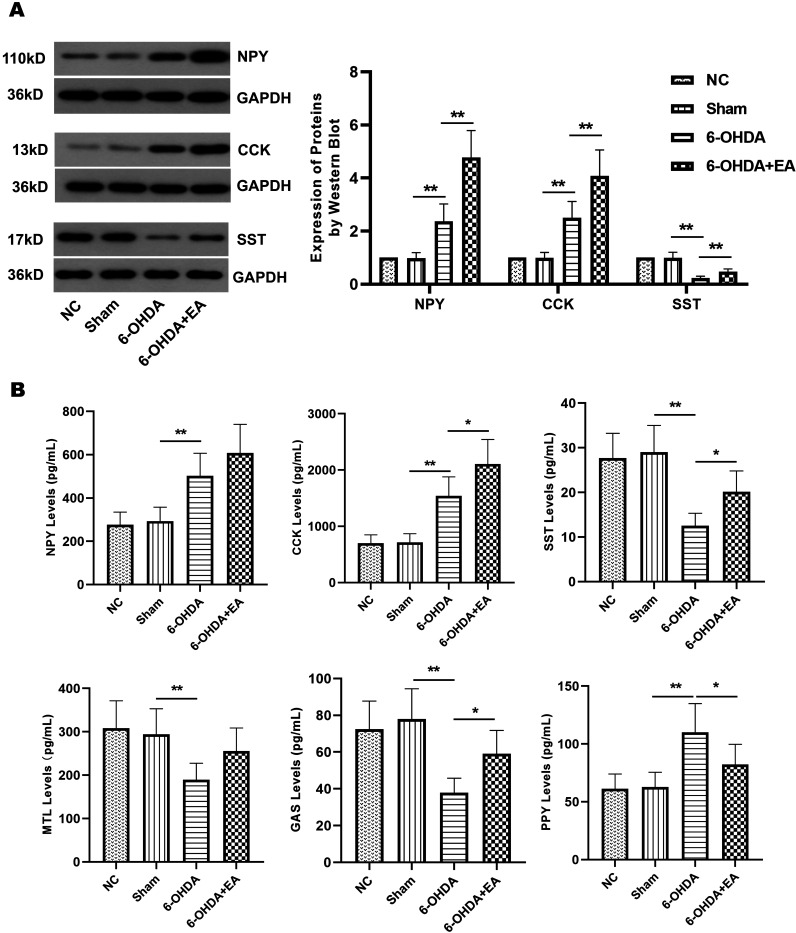
As a neurodegenerative disease, PD can also lead to changes in the content of brain-gut peptides, even some of which are closely related to the severity of PD [55]. Therefore, we determined the effect of EA on the expression of brain-gut peptides. neuropeptide Y (NPY) is a potent orexigenic peptide, which plays an important role as a central regulator of energy homeostasis and appetite in the hypothalamus [4]. In the brain substantia nigra and serum of PD model rats, the level of NPY was notably elevated (Figs. 5A and B), which was considered to be a brain neuroprotective mechanism [39]. Although EA stimulation did not significantly increase NPY level in serum, it promoted this protective effect by remarkably enhancing NPY protein expression in PD model rats (Figs. 5A and B). Cholecystokinin (CCK) is a hormone produced within the gastrointestinal tract. It is a potent inhibitor of gastric acid secretion. Similarly, EA dramatically improved CCK level in the brain substantia nigra and serum of PD model animals (Figs. 5A and B). Somatostatin (SST) is a naturally occurring peptide hormone with a number of effects in living organisms. EA also markedly reversed the decreased level of SST induced by 6-OHDA in the substantia nigra and serum of PD model rats (Figs. 5A and B). The effect of EA on several gastrointestinal peptides was investigated. Motilin (MTL) is synthesized in the upper gastrointestinal tract and has the prokinetic activity on gastrointestinal motility [37]. Similarly, gastrin (GAS) is synthesized from neuroendocrine G cells and stimulates gastrointestinal motility and gastric emptying [59]. Peptide YY (PYY) is secreted post-prandially from endocrine L cells of the hindgut, and it is thought to be a satiety signal [49]. Although no significant difference was observed in the elevation of MTL level, the level of GAS or PYY in the serum of PD model rats was notably improved or reduced by EA stimulation, respectively (Fig. 5B). These data implied that EA treatment contributed to alleviate PD by regulating brain-gut peptides in rats.
Fig. 5.
Effects of EA on the levels of brain-gut peptides. A. The expression of NPY, CCK, and SST in the brain substantia nigra of rats from NC group, Sham group, 6-OHDA group, and 6-OHDA+EA group was determined by Western blot. B. The levels of NPY, CCK, SST, MTL, GAS, and PYY in the serum of rats from each group were detected by ELSA. Data are expressed as mean ± SD. *P<0.05, **P<0.01.
Discussion
Acupuncture was originated in China and gradually became one of the popular therapies used in China. Later, acupuncture was introduced to other regions, including Asia, Europe, and the United States [54]. It was proven to be effective for a variety of neurological diseases, including PD [6, 24]. PD is a chronic neurodegenerative disease characterized by selective loss of dopaminergic neurons in the substantia nigra, resulting in abnormal motor behavior. Acupuncture at CV-12 can strengthen the function of spleen and stomach [52]. Stimulation at CV-6 has the function of alleviating depression [33]. Acupuncture at LR-3 can not only reduce depression but also treat some brain diseases [30, 63]. ST-36 is used to relieve pain, numbness, and dysfunctions of the digestive system, restricted movement, and to strengthen the entire body [27]. Our study focused on the NMS of Parkinson’s disease, including depression, gastrointestinal dysfunction, et al. This complex disease requires the joint application of multiple acupoints, and the selection of these four acupoints is also in line with the clinical practice of traditional Chinese medicine. In this work, we found that EA stimulation at CV-12, RN-7, ST-36, and LR-3 could ameliorate the motor symptoms, indicated by the decreased AIMs scores, net number of rotations, and increased cylinder test grade.
With the increasing awareness of the presence of NMS in PD, it is recognized that these non-motor characteristics play an extremely important, and sometimes even dominant, role in the diagnosis and treatment of this disorder. NMS can occur at all stages of PD and is considered to be a frequent and important component of PD. Therefore, the NMS of PD are the hotspots and difficulties of current studies. It has been demonstrated that the striatum administration of 6-OHDA in animals could lead to a progressive deterioration of dopaminergic neurons in the substantia nigra as seen in PD patients over a course of a few weeks [3]. Studies showed that acupuncture could relieve the depression and anxiety of PD patients [8, 48]. According to the results of behavioral tests in the present study, the treatment of EA at CV-12, RN-7, ST-36, and LR-3 appears to restrain the depressive and anxiety-like behaviors and improve the spatial learning memory ability of PD model animals. Many current studies have used open field, forced swimming and tail suspension tests to explore the NMS of PD, but some researchers pointed out that the immobility time might be the result of hypokinesia rather than depression [1]. Although there is currently no better method for the assessment of NMS, this issue is still worthy of further exploration in the subsequent research.
The action mechanism of 6-OHDA in animals includes cell apoptosis, inflammatory response, oxidative stress, and others [25]. The pathological and biochemical manifestations of this model are similar to human PD, such as degeneration, death, and deletion of neurons in the substantia nigra and decreased TH activity in the substantia nigra and striatum. The previous study showed that treatment at acupoints Yanglingquan (GB-43) and LR3 markedly increased the number of TH-positive neurons in the substantia nigra [18]. Our data indicated that EA at CV-12, CV-6, LR-3, and ST-36 effectively alleviated 6-OHDA-induced neuronal loss and significantly alleviates neuronal apoptosis in PD model animals. Therefore, we speculated that EA treatment at least partially inhibits neuronal loss by suppressing apoptosis. Necrosis may also be involved in the pathogenesis of PD, the role of necrosis and the effect of EA stimulation on necrosis will be explored in future research.
Inflammatory response and oxidative stress are closely related to the development of depression. Animal experiments have confirmed that some inflammatory factors, such as TNF-α and IL-1β, led to the pleasure of animals to disappear and induced their depression-like behavior [21]. In human clinical studies, it has also been found that patients with depression might have immunoinflammatory responses [61]. The productions of inflammatory cytokines, such as TNF-α and IL-1β, are elevated in the substantia nigra of PD patients [64]. In this study, EA treatment at CV-12, RN7, ST36, and LR3 significantly reduced the levels of TNF-α and IL-1β. Moreover, the inhibitors of COX-2, the key enzyme for the inflammatory response, have achieved good therapeutic effects in the treatment of depression [36]. EA also markedly decreased the expression of COX-2 protein. A prior study also reported that acupuncture at Sanyinjiao (SP-6) and Shenmen (HT-7) reduced the inflammation-associated depression-like behavior in animals [26]. A growing number of studies showed that microglia-mediated neuronal apoptosis through inflammatory and oxidative stress responses and were involved in the pathogenesis of neurodegenerative diseases [22, 47]. SOD is the main enzyme for scavenging free radicals in the body, the decrease of its activity leads to the disorder of defense mechanism [15]. Excessive free radicals and increased lipid peroxidation cause the production changes of MDA and GSH. Acupuncture stimulation at Yanglingquan (GB-34), Taichong (LR-3), Zusanli (ST-36), and Xuehai (SP-10) acupoints displayed anti-oxidative property in the nigrostriatal system in the PD model animals [60]. In our study, EA stimulation at CV-12, RN-7, ST-36, and LR-3 suppressed the oxidative stress of PD model rats.
Level changes in brain-gut peptides secreted by the nervous system and the digestive system could be used as indicators of certain neurodegenerative diseases and gastrointestinal dysfunction. Brain-gut peptides might exert a two-way regulation by affecting the interaction between enteric microorganisms and the gut-microbe-brain axis [13]. Human cognitive function is closely related to NPY. NPY has an obvious regulatory effect on the learning and memory processes of animals [16]. EA stimulation at CV-12, RN-7, ST-36, and LR-3 significantly enhanced the expression of NPY protein in the substantia nigra of 6-OHDA rats, which was one of the important ways to improve the ability of learning and memory of rats. In addition, NPY was also described to stimulate neuronal proliferation and survival, suppress excitotoxicity, attenuate neuroinflammation [12] and anxiety-like behavior [40]. CCK is a peptide hormone secreted by small intestine mucosa, which has the functions of early satiety, regulating pain, body temperature, and analgesia, as well as information transmission and memory and anti-inflammatory effects [28]. In this work, the level of CCK in the substantia nigra and serum of rats was significantly increased by 6-OHDA, and EA treatment led to a further increase in its expression. SST is widely distributed in the central and peripheral nervous systems as well as in the gastrointestinal system. It functions as a neurotransmitter/neuromodulator, which is associated with learning, memory and cognition. In PD model rats, EA stimulation markedly restored the expression level of SST in the substantia nigra and serum of 6-OHDA lesioned rats. It has been shown that the secretion of MTL and GAS was inhibited in patients with constipation [17]. Although EA did not obviously enhance MTL level, a significant increase in the level of GAS induced by EA treatment was observed in the serum of PD model rats, which suggested that EA relived constipation caused by PD. PYY was reported to be elevated in the serum of depressed mice [56], and it had the potential to alleviate depression-like behavior [40]. In this work, we found that EA stimulation dramatically decreased PYY level in the serum of PD model animals. The prior study illustrated that EA at acupoints of Foot-Yangming Meridian enhanced the gastric motility and regulated the content of motilin and somatostatin in sinus ventriculi and bulbus [32]. Our results are consistent with the previous study. Taken together, EA treatment at CV-12, RN-7, ST-36, and LR-3, at least in part, ameliorates the NMS of PD in rats through regulating the brain-gut peptides.
Conclusion
In summary, our study for the first time demonstrated that EA treatment at CV-12, RN-7, ST-36, and LR-3 could effectively alleviate not only the motor symptoms but also the NMS of PD, including the improvement of spatial memory, the decreases of depression-like and anxiety-like behaviors. EA stimulation also reduced neuronal apoptosis, inflammatory response, and oxidative stress, and it ameliorated PD at least in part by regulating the levels of brain-gut peptides in rats.
Conflict of Interests
None.
Acknowledgments
This study was supported by a grant from the National Natural Science Foundation of China (No. 81674091).
References
- 1.Asakawa T., Fang H., Sugiyama K., Nozaki T., Hong Z., Yang Y., Hua F., Ding G., Chao D., Fenoy A.J., Villarreal S.J., Onoe H., Suzuki K., Mori N., Namba H., Xia Y.2016. Animal behavioral assessments in current research of Parkinson’s disease. Neurosci. Biobehav. Rev. 65: 63–94. doi: 10.1016/j.neubiorev.2016.03.016 [DOI] [PubMed] [Google Scholar]
- 2.Banati R.B., Daniel S.E., Blunt S.B.1998. Glial pathology but absence of apoptotic nigral neurons in long-standing Parkinson’s disease. Mov. Disord. 13: 221–227. doi: 10.1002/mds.870130205 [DOI] [PubMed] [Google Scholar]
- 3.Blum D., Torch S., Lambeng N., Nissou M., Benabid A.L., Sadoul R., Verna J.M.2001. Molecular pathways involved in the neurotoxicity of 6-OHDA, dopamine and MPTP: contribution to the apoptotic theory in Parkinson’s disease. Prog. Neurobiol. 65: 135–172. doi: 10.1016/S0301-0082(01)00003-X [DOI] [PubMed] [Google Scholar]
- 4.Brown L.M., Clegg D.J.2010. Central effects of estradiol in the regulation of food intake, body weight, and adiposity. J. Steroid Biochem. Mol. Biol. 122: 65–73. doi: 10.1016/j.jsbmb.2009.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cahn A.M., Carayon P., Hill C., Flamant R.1978. Acupuncture in gastroscopy. Lancet 311: 182–183. doi: 10.1016/S0140-6736(78)90614-1 [DOI] [PubMed] [Google Scholar]
- 6.Cai W., Shen W.D.2018. Anti-apoptotic mechanisms of acupuncture in neurological diseases: A review. Am. J. Chin. Med. 46: 515–535. doi: 10.1142/S0192415X1850026X [DOI] [PubMed] [Google Scholar]
- 7.Chan Y.Y., Lo W.Y., Yang S.N., Chen Y.H., Lin J.G.2015. The benefit of combined acupuncture and antidepressant medication for depression: A systematic review and meta-analysis. J. Affect. Disord. 176: 106–117. doi: 10.1016/j.jad.2015.01.048 [DOI] [PubMed] [Google Scholar]
- 8.Chen F.P., Chang C.M., Shiu J.H., Chiu J.H., Wu T.P., Yang J.L., Kung Y.Y., Chen F.J., Chern C.M., Hwang S.J.2015. A clinical study of integrating acupuncture and Western medicine in treating patients with Parkinson’s disease. Am. J. Chin. Med. 43: 407–423. doi: 10.1142/S0192415X15500263 [DOI] [PubMed] [Google Scholar]
- 9.Choi K.H., Flynn K.2017. Korean Sa-Ahm Acupuncture for Treating Canine Oral Fibrosarcoma. J. Acupunct. Meridian Stud. 10: 211–215. doi: 10.1016/j.jams.2017.04.005 [DOI] [PubMed] [Google Scholar]
- 10.Dong B., Chen Z., Yin X., Li D., Ma J., Yin P., Cao Y., Lao L., Xu S.2017. The Efficacy of Acupuncture for Treating Depression-Related Insomnia Compared with a Control Group: A Systematic Review and Meta-Analysis. BioMed Res. Int. 2017: 9614810. doi: 10.1155/2017/9614810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Doo A.R., Kim S.N., Hahm D.H., Yoo H.H., Park J.Y., Lee H., Jeon S., Kim J., Park S.U., Park H.J.2014. Gastrodia elata Blume alleviates L-DOPA-induced dyskinesia by normalizing FosB and ERK activation in a 6-OHDA-lesioned Parkinson’s disease mouse model. BMC Complement. Altern. Med. 14: 107. doi: 10.1186/1472-6882-14-107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duarte-Neves J., Pereira de Almeida L., Cavadas C.2016. Neuropeptide Y (NPY) as a therapeutic target for neurodegenerative diseases. Neurobiol. Dis. 95: 210–224. doi: 10.1016/j.nbd.2016.07.022 [DOI] [PubMed] [Google Scholar]
- 13.Mayer E.A., Tillisch K.2011. The brain-gut axis in abdominal pain syndromes. Annu. Rev. Med. 62: 381–396. doi: 10.1146/annurev-med-012309-103958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eshkevari L.2003. Acupuncture and pain: a review of the literature. AANA J. 71: 361–370. [PubMed] [Google Scholar]
- 15.Fergusson D., Doucette S., Glass K.C., Shapiro S., Healy D., Hebert P., Hutton B.2005. Association between suicide attempts and selective serotonin reuptake inhibitors: systematic review of randomised controlled trials. BMJ 330: 396–399. doi: 10.1136/bmj.330.7488.396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gackenheimer S.L., Schober D.A., Gehlert D.R.2001. Characterization of neuropeptide Y Y1-like and Y2-like receptor subtypes in the mouse brain. Peptides 22: 335–341. doi: 10.1016/S0196-9781(01)00335-7 [DOI] [PubMed] [Google Scholar]
- 17.Giladi N., Manor Y., Hilel A., Gurevich T.2014. Interdisciplinary teamwork for the treatment of people with Parkinson’s disease and their families. Curr. Neurol. Neurosci. Rep. 14: 493. doi: 10.1007/s11910-014-0493-1 [DOI] [PubMed] [Google Scholar]
- 18.He Y., Lee T., Leong S.K.2000. 6-Hydroxydopamine induced apoptosis of dopaminergic cells in the rat substantia nigra. Brain Res. 858: 163–166. doi: 10.1016/S0006-8993(99)02459-2 [DOI] [PubMed] [Google Scholar]
- 19.Langevin H.M., Churchill D.L., Wu J., Badger G.J., Yandow J.A., Fox J.R., Krag M.H.2002. Evidence of connective tissue involvement in acupuncture. FASEB J. 16: 872–874. doi: 10.1096/fj.01-0925fje [DOI] [PubMed] [Google Scholar]
- 20.Jellinger K.A.2000. Cell death mechanisms in Parkinson’s disease. J. Neural Transm. (Vienna) 107: 1–29. doi: 10.1007/s007020050001 [DOI] [PubMed] [Google Scholar]
- 21.Moskal J.R., Burch R., Burgdorf J.S., Kroes R.A., Stanton P.K., Disterhoft J.F., Leander J.D.2014. GLYX-13, an NMDA receptor glycine site functional partial agonist enhances cognition and produces antidepressant effects without the psychotomimetic side effects of NMDA receptor antagonists. Expert Opin. Investig. Drugs 23: 243–254. doi: 10.1517/13543784.2014.852536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim Y.S., Joh T.H.2006. Microglia, major player in the brain inflammation: their roles in the pathogenesis of Parkinson’s disease. Exp. Mol. Med. 38: 333–347. doi: 10.1038/emm.2006.40 [DOI] [PubMed] [Google Scholar]
- 23.Kim Y.S., Joo W.S., Jin B.K., Cho Y.H., Baik H.H., Park C.W.1998. Melatonin protects 6-OHDA-induced neuronal death of nigrostriatal dopaminergic system. Neuroreport 9: 2387–2390. doi: 10.1097/00001756-199807130-00043 [DOI] [PubMed] [Google Scholar]
- 24.Kong K.H., Ng H.L., Li W., Ng D.W., Tan S.I., Tay K.Y., Au W.L., Tan L.C.S.2017. Acupuncture in the treatment of fatigue in Parkinson’s disease: A pilot, randomized, controlled, study. Brain Behav. 8: e00897. doi: 10.1002/brb3.897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kumar R., Agarwal A.K., Seth P.K.1995. Free radical-generated neurotoxicity of 6-hydroxydopamine. J. Neurochem. 64: 1703–1707. doi: 10.1046/j.1471-4159.1995.64041703.x [DOI] [PubMed] [Google Scholar]
- 26.Kwon S., Lee B., Yeom M., Sur B.J., Kim M., Kim S.T., Park H.J., Lee H., Hahm D.H.2012. Modulatory effects of acupuncture on murine depression-like behavior following chronic systemic inflammation. Brain Res. 1472: 149–160. doi: 10.1016/j.brainres.2012.07.009 [DOI] [PubMed] [Google Scholar]
- 27.Kwon S., Seo B.K., Kim S.2016. Acupuncture points for treating Parkinson’s disease based on animal studies. Chin. J. Integr. Med. 22: 723–727. doi: 10.1007/s11655-016-2525-y [DOI] [PubMed] [Google Scholar]
- 28.Lach G., Schellekens H., Dinan T.G., Cryan J.F.2018. Anxiety, Depression, and the Microbiome: A Role for Gut Peptides. Neurotherapeutics 15: 36–59. doi: 10.1007/s13311-017-0585-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li M., Li L., Wang K., Su W., Jia J., Wang X.2017. The effect of electroacupuncture on proteomic changes in the motor cortex of 6-OHDA Parkinsonian rats. Brain Res. 1673: 52–63. doi: 10.1016/j.brainres.2017.07.027 [DOI] [PubMed] [Google Scholar]
- 30.Liang P., Wang Z., Qian T., Li K.2014. Acupuncture stimulation of Taichong (Liv3) and Hegu (LI4) modulates the default mode network activity in Alzheimer’s disease. Am. J. Alzheimers Dis. Other Demen. 29: 739–748. doi: 10.1177/1533317514536600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lin J.G., Chen C.J., Yang H.B., Chen Y.H., Hung S.Y.2017. Electroacupuncture promotes recovery of motor function and reduces dopaminergic neuron degeneration in rodent models of parkinson’s disease. Int. J. Mol. Sci. 18: 1846. doi: 10.3390/ijms18091846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lin Y.P., Yi S.X., Yan J., Chang X.R.2007. Effect of acupuncture at Foot-Yangming Meridian on gastric mucosal blood flow, gastric motility and brain-gut peptide. World J. Gastroenterol. 13: 2229–2233. doi: 10.3748/wjg.v13.i15.2229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu J., Wang A., Nie G., Wang X., Huang J.2018. [Acupuncture for female depression: a randomized controlled trial]. Zhongguo Zhenjiu 38: 375–378.(in Chinese) [DOI] [PubMed] [Google Scholar]
- 34.Livak K.J., Schmittgen T.D.2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Δ Δ C(T)) Method. Methods 25: 402–408. doi: 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- 35.McDowell K., Chesselet M.F.2012. Animal models of the non-motor features of Parkinson’s disease. Neurobiol. Dis. 46: 597–606. doi: 10.1016/j.nbd.2011.12.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Myint A.M., Steinbusch H.W.M., Goeghegan L., Luchtman D., Kim Y.K., Leonard B.E.2007. Effect of the COX-2 inhibitor celecoxib on behavioural and immune changes in an olfactory bulbectomised rat model of depression. Neuroimmunomodulation 14: 65–71. doi: 10.1159/000107420 [DOI] [PubMed] [Google Scholar]
- 37.Ohno T., Mochiki E., Kuwano H.2010. The roles of motilin and ghrelin in gastrointestinal motility. Int. J. Pept. 2010: 820794. doi: 10.1155/2010/820794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ostrovsky D.A., Ehrlich A.2019. Bee Venom Acupuncture in Addition to Anti-Parkinsonian Medications may Improve Activities of Daily Living and Motor Symptoms More Than Medication Alone in Idiopathic Parkinson’s Disease. Explore (NY) 15: 71–73. doi: 10.1016/j.explore.2018.10.013 [DOI] [PubMed] [Google Scholar]
- 39.Pain S., Vergote J., Gulhan Z., Bodard S., Chalon S., Gaillard A.2019. Inflammatory process in Parkinson disease: neuroprotection by neuropeptide Y. Fundam. Clin. Pharmacol. 33: 544–548. doi: 10.1111/fcp.12464 [DOI] [PubMed] [Google Scholar]
- 40.Painsipp E., Herzog H., Sperk G., Holzer P.2011. Sex-dependent control of murine emotional-affective behaviour in health and colitis by peptide YY and neuropeptide Y. Br. J. Pharmacol. 163: 1302–1314. doi: 10.1111/j.1476-5381.2011.01326.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Park H.J., Lim S., Joo W.S., Yin C.S., Lee H.S., Lee H.J., Seo J.C., Leem K., Son Y.S., Kim Y.J., Kim C.J., Kim Y.S., Chung J.H.2003. Acupuncture prevents 6-hydroxydopamine-induced neuronal death in the nigrostriatal dopaminergic system in the rat Parkinson’s disease model. Exp. Neurol. 180: 93–98. doi: 10.1016/S0014-4886(02)00031-6 [DOI] [PubMed] [Google Scholar]
- 42.Penn Y.Y.2018. Acupuncture treatment for dysfunctional uterine bleeding in an adolescent. BMJ Case Rep. 2018: 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pfab F., Kirchner M.T., Huss-Marp J., Schuster T., Schalock P.C., Fuqin J., Athanasiadis G.I., Behrendt H., Ring J., Darsow U., Napadow V.2012. Acupuncture compared with oral antihistamine for type I hypersensitivity itch and skin response in adults with atopic dermatitis: a patient- and examiner-blinded, randomized, placebo-controlled, crossover trial. Allergy 67: 566–573. doi: 10.1111/j.1398-9995.2012.02789.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ravina B., Camicioli R., Como P.G., Marsh L., Jankovic J., Weintraub D., Elm J.2007. The impact of depressive symptoms in early Parkinson disease. Neurology 69: 342–347. doi: 10.1212/01.wnl.0000268695.63392.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Reijnders J.S.A.M., Ehrt U., Weber W.E.J., Aarsland D., Leentjens A.F.G.2008. A systematic review of prevalence studies of depression in Parkinson’s disease. Mov. Disord. 23: 183–189, quiz 313. doi: 10.1002/mds.21803 [DOI] [PubMed] [Google Scholar]
- 46.Richter A., Herlitz J., Hjalmarson A.1991. Effect of acupuncture in patients with angina pectoris. Eur. Heart J. 12: 175–178. doi: 10.1093/oxfordjournals.eurheartj.a059865 [DOI] [PubMed] [Google Scholar]
- 47.Saijo K., Winner B., Carson C.T., Collier J.G., Boyer L., Rosenfeld M.G., Gage F.H., Glass C.K.2009. A Nurr1/CoREST pathway in microglia and astrocytes protects dopaminergic neurons from inflammation-induced death. Cell 137: 47–59. doi: 10.1016/j.cell.2009.01.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Subramanian I.2017. Complementary and Alternative Medicine and Exercise in Nonmotor Symptoms of Parkinson’s Disease. Int. Rev. Neurobiol. 134: 1163–1188. doi: 10.1016/bs.irn.2017.05.037 [DOI] [PubMed] [Google Scholar]
- 49.Ueno H., Yamaguchi H., Mizuta M., Nakazato M.2008. The role of PYY in feeding regulation. Regul. Pept. 145: 12–16. doi: 10.1016/j.regpep.2007.09.011 [DOI] [PubMed] [Google Scholar]
- 50.Verástegui C.2018. Evidence for the Use of Acupuncture in Treating Parkinson’s Disease: Update of Information From the Past 5 Years, a Mini Review of the Literature. Rev. Int. Acupunt. 12: 74–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang H., Pan Y., Xue B., Wang X., Zhao F., Jia J., Liang X., Wang X.2011. The antioxidative effect of electro-acupuncture in a mouse model of Parkinson’s disease. PLoS One 6: e19790. doi: 10.1371/journal.pone.0019790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang L., Shen G., Wang H., Hu M., Yao Y., Ye S.2018. [The effects of electroacupuncture at shu and mu points of stomach on gastric motility, the NMDA of vagus nerve dorsal nucleus and serum NO expression in functional dyspepsia rats]. Zhongguo Zhenjiu 38: 285–290.(in Chinese) [DOI] [PubMed] [Google Scholar]
- 53.Wang S.J., Ma J., Gong Y.X., Wang Y.C., Zeng X.L., Liang Y., Sun G.J.2014. [Effect of electroacupuncture intervention on ERK 1/2 signaling and TNF-α and IL-1β protein levels in the substantia Nigra in rats with Parkinson’s Disease]. Zhen Ci Yan Jiu 39: 456–460. [PubMed] [Google Scholar]
- 54.White A., Ernst E.2004. A brief history of acupuncture. Rheumatology (Oxford) 43: 662–663. doi: 10.1093/rheumatology/keg005 [DOI] [PubMed] [Google Scholar]
- 55.Witjas T., Kaphan E., Azulay J.P.2007. [Non-motor fluctuations in Parkinson’s disease]. Rev. Neurol. (Paris) 163: 846–850. doi: 10.1016/S0035-3787(07)91470-8 [DOI] [PubMed] [Google Scholar]
- 56.Yamada C., Mogami S., Kanno H., Hattori T.2018. Peptide YY Causes Apathy-Like Behavior via the Dopamine D2 Receptor in Repeated Water-Immersed Mice. Mol. Neurobiol. 55: 7555–7566. doi: 10.1007/s12035-018-0931-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yang Y., Xu P.D., Xin Y., Kang Z.X., Zhang H.X.Z.L., Zhou L.2016. [Involvement of Neurotensin-mediated Brain-gut Axis in Electroacupuncture Intervention Induced Improvement of Functional Dyspepsia in Rats]. Zhen Ci Yan Jiu 41: 35–39, 50.(in Chinese) [PubMed] [Google Scholar]
- 58.Yeo S., van den Noort M., Bosch P., Lim S.2018. A study of the effects of 8-week acupuncture treatment on patients with Parkinson’s disease. Medicine (Baltimore) 97: e13434. doi: 10.1097/MD.0000000000013434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang Q., Yu J.C., Kang W.M., Zhu G.J.2011. Effect of ω-3 fatty acid on gastrointestinal motility after abdominal operation in rats. Mediators Inflamm. 2011: 152137. doi: 10.1155/2011/152137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yu Y.P., Ju W.P., Li Z.G., Wang D.Z., Wang Y.C., Xie A.M.2010. Acupuncture inhibits oxidative stress and rotational behavior in 6-hydroxydopamine lesioned rat. Brain Res. 1336: 58–65. doi: 10.1016/j.brainres.2010.04.020 [DOI] [PubMed] [Google Scholar]
- 61.Li Y.F., Huang Y., Amsdell S.L., Xiao L., O’Donnell J.M., Zhang H.T.2009. Antidepressant- and anxiolytic-like effects of the phosphodiesterase-4 inhibitor rolipram on behavior depend on cyclic AMP response element binding protein-mediated neurogenesis in the hippocampus. Neuropsychopharmacology 34: 2404–2419. doi: 10.1038/npp.2009.66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zeng B.Y., Zhao K.2016. Effect of Acupuncture on the Motor and Nonmotor Symptoms in Parkinson’s Disease--A Review of Clinical Studies. CNS Neurosci. Ther. 22: 333–341. doi: 10.1111/cns.12507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang L., Zhong Y., Quan S., Liu Y., Shi X., Li Z., Wang J.2017. [Acupuncture combined with auricular point sticking therapy for post stroke depression:a randomized controlled trial] Zhongguo Zhenjiu 37: 581–585.(in Chinese) [DOI] [PubMed] [Google Scholar]
- 64.Zhang Q.S., Heng Y., Yuan Y.H., Chen N.H.2017. Pathological α-synuclein exacerbates the progression of Parkinson’s disease through microglial activation. Toxicol. Lett. 265: 30–37. doi: 10.1016/j.toxlet.2016.11.002 [DOI] [PubMed] [Google Scholar]
- 65.Zhao Y.X., Yao M.J., Liu Q., Xin J.J., Gao J.H., Yu X.C.2020. Electroacupuncture treatment attenuates paclitaxel-induced neuropathic pain in rats via inhibiting spinal glia and the TLR4/NF-κB pathway. J. Pain Res. 13: 239–250. doi: 10.2147/JPR.S241101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhuang Y., Xing J.J., Li J., Zeng B.Y., Liang F.R.2013. History of acupuncture research. Int. Rev. Neurobiol. 111: 1–23. doi: 10.1016/B978-0-12-411545-3.00001-8 [DOI] [PubMed] [Google Scholar]
- 67.Ziemssen T., Reichmann H.2007. Non-motor dysfunction in Parkinson’s disease. Parkinsonism Relat. Disord. 13: 323–332. doi: 10.1016/j.parkreldis.2006.12.014 [DOI] [PubMed] [Google Scholar]