Abstract
The cold storage of two-cell embryos is a useful technique for transporting genetically engineered mice without the shipment of live animals. However, the developmental ability of cold-stored embryos decreases with prolonged storage periods. Therefore, the transported embryos must be readily transferred to recipient mice upon arrival. The cryopreservation of cold-transported embryos may improve the flexibility of the schedule of embryo transfer. In this paper, we examined the viability and developmental ability of vitrified-warmed mouse embryos at the two-cell stage after cold storage in refrigerated temperatures for 0, 24, 48, 72, or 96 h. The viability of vitrified-warmed embryos after cold storage was comparable to vitrified-warmed embryos without cold storage. Vitrified-warmed embryos after cold storage also developed normally to pups by embryo transfer. In addition, live pups were obtained from vitrified-warmed embryos after cold-transportation from Asahikawa Medical University. In summary, cold-stored embryos can be used for the transportation and archive of genetically engineered mice.
Keywords: cold storage, cryopreservation, embryo transfer, mouse, two-cell embryos
Introduction
The cold storage of two-cell embryos is a useful technique to transport genetically engineered mice [2]. The cold-stored embryos are revived by embryo transfer at the receiving facility. By shipping cold-stored embryos, we can avoid the potential risks and difficulties associated with the shipment of live animals or cryopreserved embryos [14, 16,17,18, 23].
Two-cell mouse embryos can maintain viability and developmental ability in an optimal storage medium at refrigerated temperatures [3, 4]. Previously, we reported that M2 medium maintained the high viability and developmental ability of two-cell mouse embryos for 48 h at 4°C [18, 19]. In addition, N-acetyl cysteine (NAC) prolonged the storage period of two-cell embryos up to 96 h [6]. Therefore, improvements in the cold-storage medium prolonged the time and distance of embryo transport.
However, the cold storage of two-cell embryos remains limited by the time between embryo storage and transfer. Therefore, the appropriate number of recipient mice must be prepared in advance to ensure that the transfer of embryos to recipients is performed on the arrival date. The cryopreservation of cold-stored embryos may address this time limitation, but the viability and developmental ability of cryopreserved two-cell embryos after cold storage remain unknown.
In this study, we examined the viability and developmental ability of vitrified-warmed embryos preserved at refrigerated temperatures. In addition, we performed transport experiments of cold-stored embryos between Kumamoto University and Asahikawa Medical University to confirm the applicability of the cold-transport and cryopreservation system of two-cell embryos.
Materials and Methods
Mice
Male (10 to 18 weeks old) and female (8 to 13 weeks old) C57BL/6J mice (CLEA Japan, Inc., Tokyo, Japan) were used as sperm and oocytes donors, respectively. Twelve-week-old female ICR mice (CLEA Japan, Inc.) were used as recipients of the two-cell embryo transfer. The mice were housed in a specific pathogen-free room with a 12-h dark/light cycle (illumination from 7:00 to 19:00) at 22 ± 1°C and provided food and water ad libitum. The Animal Care and Use Committee of Kumamoto University School of Medicine approved the protocols for all animal experiments (ID: A2019-026), and all methods were performed in accordance with the relevant guidelines and regulations.
Media
Inhibin antiserum (IAS) was obtained from goats immunized with an inhibin-specific synthetic peptide coupled to keyhole limpet hemocyanin [9]. Embryo vitrification was performed using 1 M dimethyl sulfoxide (DMSO) and a solution of 2 M DMSO, 1 M acetamide, and 3 M propylene glycol (DAP213) [13]. A 0.25 M sucrose solution (37°C) was used to warm the vitrified oocytes. High-calcium human tubal fluid (mHTF) was used for in vitro fertilization (IVF) [10, 15]. Sperm were preincubated in Toyoda-Yokoyama-Hosi medium supplemented with methyl-β-cyclodextrin (cTYH) [20, 22]. M2 embryo storage medium (Sigma-Aldrich, Co., LLC., St. Luis, MO, USA) containing 1.5 mM NAC was used for the cold storage of two-cell embryos [6]. Embryos were cultured in potassium simplex medium with amino acids (KSOM/AA) [5]. All media were stored at 4°C. Fertilization, sperm, and embryo culture media were preincubated in an incubator for 30 min at 37°C in an atmosphere containing 5% CO2.
Superovulation and oocyte collection
Superovulation was performed as previously described [21]. Female mice were intraperitoneally injected with a mixed solution of 3.75 IU equine chorionic gonadotropin (eCG, ASKA Pharmaceutical Co., Ltd., Tokyo, Japan) and 0.1 ml IAS. After 48 h, the mice were injected with 7.5 IU human chorionic gonadotropin (hCG, ASKA Pharmaceutical Co., Ltd.). Seventeen hours after hCG administration, the mice were euthanized by cervical dislocation, and oviducts were immediately collected and transferred in liquid paraffin covered by a drop of fertilization medium. A dissection needle was used to collect cumulus-oocytes complexes (COCs) from the swollen ampullae in the fertilization dish. COCs were transferred into a 200-µL drop of mHTF. Oocytes were incubated in a 200-µL drop of mHTF for 1 h at 37°C in a 5% CO2 incubator.
IVF
Male mice were euthanized by cervical dislocation. Sperm were collected from the cauda epididymides and incubated in a 100-µL drop of cTYH for 1 h at 37°C in a 5% CO2 incubator. The sperm suspension was added to the fertilization medium containing oocytes. The final concentration of sperm in the fertilization medium was 800–2,000 sperm/µL. After 3 h, the oocytes were washed three times with 80-µL drops of mHTF. After 24 h coincubaiton of oocytes and sperm, two-cell embryos were counted, and the fertilization rate was calculated.
Cold storage of two-cell embryos
The cold storage of two-cell embryos was performed as described previously [6]. After IVF, two-cell embryos were washed three times in an 80-µL drop of M2 medium and then transferred into 0.6-ml tubes (Fisher brand Microcentrifuge Graduated, Flat Cap Tubes; Fisher Scientific, Pittsburgh, PA, USA) containing 600-µL of M2 medium with 1.5 mM NAC. The tubes were packed in a CARD Cold Transport Kit (Kyudo Co., Ltd., Tosu, Japan) and stored in a refrigerator at 4.0 ± 1.5°C for 0–96 h. After cold storage or shipment, the embryos were recovered from 0.6-ml tubes using a gel-loading tip.
Embryo vitrification
Embryo vitrification was performed as previously described [13]. Briefly, the embryos were transferred onto a 100-µL drop of 1 M DMSO, divided into groups of 20–100, transferred into a cryotube containing 5 µL DMSO solution, and placed on ice. After 5 min, 45 µL of DAP213 was added to the cryotube containing embryos, which was placed on ice for 5 min and then in liquid nitrogen. Vitrified embryos were warmed in 0.25 M sucrose at 37°C. Specifically, liquid nitrogen was discarded from each cryotube, and the cryotubes were allowed to stand at room temperature for 30 s. Then, 0.25 M sucrose was added to the cryotubes, and vitrified embryos were warmed quickly via pipetting. The embryos were washed three times with 100-µL drops of mHTF, and the morphologically normal embryos were counted.
Embryo culture and transfer
Embryos were washed three times and cultured with KSOM/AA in a 5% CO2 incubator until blastocyst formation. The developmental ability of cold-stored embryos was evaluated according to their developmental rates to four-cell embryos (24 h), morulae (48 h), and blastocysts (72 or 96 h) during embryo culture. After the recovery of two-cell embryos from the tubes, some embryos were transferred into each oviduct of a pseudopregnant mouse using a vaginal plug (18–20 embryos/oviduct) [12]. After 19 days, the number of pups was counted.
Transportation of two-cell embryos at refrigerated temperatures
Two-cell embryos were transported as previously described [18, 19]. In brief, storage boxes containing two-cell embryos were placed in a CARD Cold Transport Kit and transported from Asahikawa Medical University to the Center for Animal Resources and Development of Kumamoto University under refrigerated temperatures (Fig. 1). The transport kit containing the samples was arrived to Kumamoto University within 72 h via parcel delivery. The transported embryos were used for embryo cryopreservation and transfer to confirm the developmental ability of the embryos.
Fig. 1.
Transport experiment of two-cell embryos from Asahikawa to Kumamoto. Two-cell embryos were transported from Asahikawa Medical University to CARD, Kumamoto University. Location of both institutions is depicted on a map of Japan.
Statistical analysis
Statistical analysis was performed using Prism version 7.02 (GraphPad Software Inc., San Diego, CA, USA). The results are expressed as the mean and standard deviation. Comparisons were performed using the analysis of variance following arcsine transformation of the percentages. P<0.05 was considered statistically significant.
Results
The survival rate of vitrified-warmed embryos after cold storage
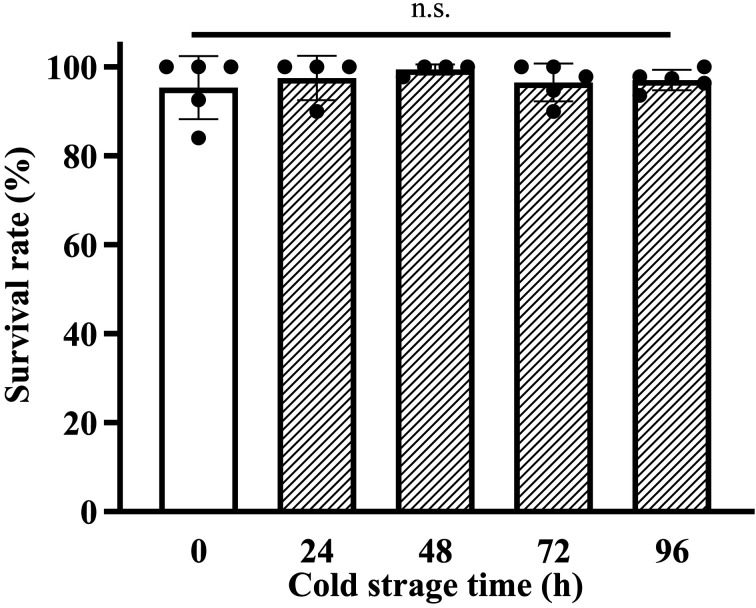
To examine the viability of vitrified-warmed two-cell embryos after cold storage, we warmed the vitrified two-cell embryos stored for 0, 24, 48, 72, or 96 h at refrigerated temperatures. The survival rates of vitrified-warmed two-cell embryos after cold storage were similar that of vitrified-warmed fresh embryos at the 0 h time point (Fig. 2, 0 h: 95.0% vs. 24 h: 97.6%, 48 h: 99.4%, 72 h: 96.8%, and 96 h: 96.9%).
Fig. 2.
The survival rate of vitrified-warmed embryos after cold storage. The embryos were stored for 0, 24, 48, 72, or 96 h before vitrification. The survival rate was calculated as follows: (Number of morphologically normal embryos/Number of collected embryos) × 100. Results are expressed as the mean ± SD (n=4–5). More than 39 embryos were analyzed in each experiment, and the total number of analyzed embryos in each experimental group was as follows (0 h: 227, 24 h: 170, 48 h: 172, 72 h: 226, and 96 h: 227).
In vitro developmental ability of cold-vitrified embryos
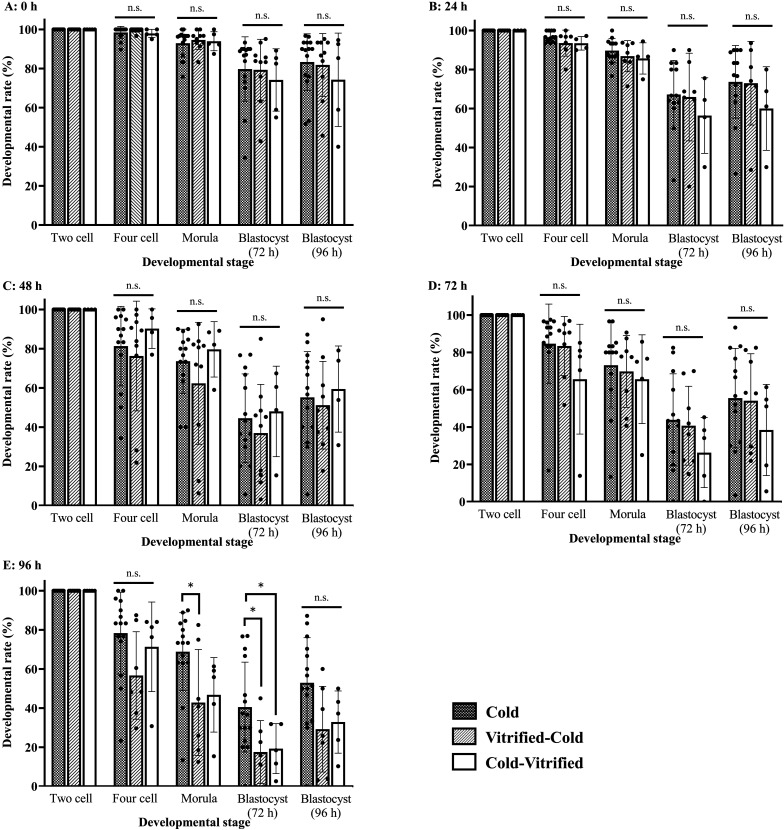
To examine the developmental ability of vitrified-warmed two-cell embryos after cold storage in vitro, we stored the two-cell embryos for 0, 24, 48, 72, 96 h in refrigerated temperatures before vitrification. After warming the cold-vitrified two-cell embryos, the embryos were transferred to the KSOM drop, and we counted the four-cell embryos (24 h), morulae (48 h), and blastocysts (72 and 96 h). The developmental rates of cold-vitrified embryos were similar those of cold and vitrified-cold embryos (Fig. 3).
Fig. 3.
The developmental ability of vitrified-warmed embryos after cold storage. The embryos stored in the refrigerator for 0, 24, 48, 72, or 96 h (A: 0 h, B: 24 h, C: 48 h, D: 72 h, E: 96 h) and then vitrified. The developmental rate was calculated as follows: (Number of four-cell embryos, morulae, or blastocysts/Number of two-cell embryos) × 100. Results are expressed as the mean ± SD (n=4–15). *P<0.05 compared with Cold-stored embryos. More than 27 embryos were analyzed in each experiment, and the total number of analyzed embryos in each experimental group was as follows (0 h-Cold: 457, 0 h-Vitrified-Cold: 328, 0h-Cold-Vitrified: 197, 24 h-Cold: 397, 24 h-Vitrified-Cold: 236, 24 h: Cold-Vitrified: 166, 48 h-Cold: 426, 48 h-Vitrified-Cold: 324, 48 h-Cold-Vitrified: 167, 72 h-Cold: 397, 72 h-Vitrified-Cold: 257, 72 h-Cold-Vitrified: 205, 96 h-Cold: 456, 96 h-Vitrified-Cold: 227, and 96 h-Cold-Vitrified: 208).
Birth rate of cold-vitrified embryos after embryo transfer
We also examined the birth rate of cold-vitrified embryos by embryo transfer. Cold-vitrified embryos developed normally to pups. The birth rate of cold-vitrified embryos was similar those of cold and vitrified-cold embryos (Table 1).
Table 1. Developmental ability of cold-vitrified embryos by embryo transfer.
| Stored period (h) | Two-cell embryos | Number of recipients | Number oftransferred embryos | Number of live pups (%) |
|---|---|---|---|---|
| 72 | Cold | 4 | 80 | 35 (43.8 ± 4.8) |
| Vitrified-Cold | 4 | 80 | 34 (42.5 ± 22.2) | |
| Cold-Vitrified | 3 | 60 | 27 (45.0 ± 10.0) | |
| 96 | Cold | 7 | 140 | 36 (25.7 ± 9.8) |
| Vitrified-Cold | 4 | 80 | 25 (31.3 ± 16.5) | |
| Cold-Vitrified | 4 | 78 | 17 (21.8 ± 6.2) |
Two-cell embryos were transferred to each oviduct of the recipient mice (18–20 embryos/recipient), and the number of pups was recorded after 19 days. The birth rate was calculated as follows: (Number of pups/Number of transferred two-cell embryos) × 100. Results are expressed as the mean ± SD (n=3–7).
Transport experiment of embryos between Kumamoto University and Asahikawa Medical University
We confirmed whether vitrified/warmed two-cell embryos after cold storage have normal developmental ability after transport from Asahikawa Medical University. We compared the birth rates of Cold, Vitrified-Cold, and Cold-Vitrified embryos after embryo transfer (Table 2). Pups were successfully obtained from the transported embryos in all groups. There was no significant difference in the developmental ability between Cold and Cold-Vitrified two-cell embryos (Table 3).
Table 2. Preparation of transported 2-cell embryos for embryo transfer.
| Two-cell embryos | Number of cold stored 2-cell embryos | Number of survival 2-cell embryos (%) | Number ofcryopreserved 2-cell embryos | Number of retrieved 2-cell embryos (%) |
|---|---|---|---|---|
| Cold | 80 | 80 (100) | - | - |
| Vitrified-Cold | 117 | 112 (95.7 ± 5.5) | - | - |
| Cold-Vitrified | 81 | 81 (100) | 81 | 79 (97.5 ± 3.4) |
Two-cell embryos were transported from Asahikawa Medical University to CARD, Kumamoto University. Results are expressed as the mean ± SD (n=2–4).
Table 3. Developmental ability of cold-vitrified embryos transported from Asahikawa Medical University.
| Two-cell embryos | Number of recipients | Number oftransferred embryos | Number of live pups (%) |
|---|---|---|---|
| Cold | 4 | 80 | 38 (47.5 ± 13.2) |
| Vitrified-Cold | 6 | 112 | 28 (25.0 ± 18.2) |
| Cold-Vitrified | 4 | 79 | 24 (30.4 ± 12.9) |
Two-cell embryos were transferred to each oviduct of the recipient mice (17–20 embryos/recipient), and the number of pups was recorded after 19 days. Two-cell embryos stored in refrigerated temperatures for 3 days were used as controls (Cold). The birth rate was calculated as follows: (Number of pups/Number of transferred two-cell embryos) × 100. Results are expressed as the mean ± SD (n=4–6).
Discussion
In this study, vitrified-warmed mouse embryos at the two-cell stage after cold storage showed comparable viability to vitrified-warmed fresh embryos. In addition, the cold-vitrified embryos developed normally to blastocysts and pups. Furthermore, we confirmed that this cold-storage technique could be used for the transportation and cryopreservation of mouse embryos between animal facilities in Japan.
The cold-transport system of two-cell embryos is a useful technique to transport genetically engineered mice without the shipment of live animals. There are several studies that have improved the storage medium of two-cell mouse embryos [1, 3, 4, 7, 8, 11, 18, 19]. Previously, we reported that NAC improved the developmental rate of two-cell embryos after cold storage and prolonged the storage period up to 96 h [6]. However, after the 96-h storage, the developmental rate decreased in a time-dependent manner. Therefore, we had to prepare enough recipients and perform embryo transfer using the cold-stored embryos on the arrival date. In this study, we confirmed that cold-stored embryos tolerated cryopreservation (Fig. 3) and that cold-vitrified embryos developed to pups (Table 1). These results suggest that the cold-stored technique can potentially be used to transport and cryopreserve genetically engineered mice.
The cold storage and cryopreservation of two-cell embryos would be useful for efficient animal production by embryo transfer. The number of recipient mice is difficult to control because the number of pseudopregnant female mice depends on the estrous cycle and copulating rate. Using the cold storage and cryopreservation system, we can easily change the date and number of embryo transfers. The combination of cold storage and cryopreservation of embryos would allow a flexible schedule of embryo transfer based on the number of staff and recipients.
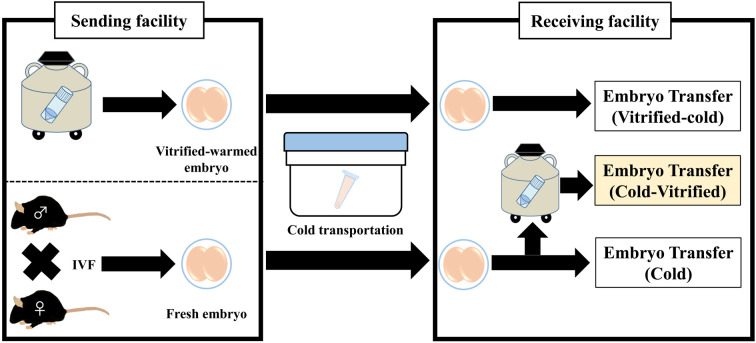
In summary, we demonstrated that cold-stored mouse embryos can be cryopreserved with high developmental ability. The cold storage of two-cell embryos is a useful technique for the shipment of genetically engineered mice, avoiding the shipment of live mice. The transport technique of mouse embryos is also helpful to conduct collaborative research project under the travel restrictions due to novel coronavirus pandemic. This technique will provide a simple transportation and archive system of genetically engineered mice and contribute to a seamless network to conduct multi-institutional research using laboratory animals (Fig. 4).
Fig. 4.
Transport and cryopreservation of cold-stored mouse embryos. At the sending facility, two-cell embryos were prepared from cryopreserved samples or produced by in vitro fertilization (IVF). The embryos were transported at refrigerated temperatures. At the receiving facility, the embryos were transferred to recipient mice or cryopreserved in a liquid nitrogen tank before use.
Acknowledgments
We thank Chihiro Hino and Norihiko Shimizu (Department of Advanced Medical Science, Asahikawa Medical University, Asahikawa, Japan) for technical support in transport experiment. The authors would like to thank Enago (www.enago.jp) for the English language review.
References
- 1.de Dios Hourcade J., Pérez-Crespo M., Serrano A., Gutiérrez-Adán A., Pintado B.2012. In vitro and in vivo development of mice morulae after storage in non-frozen conditions. Reprod. Biol. Endocrinol. 10: 62. doi: 10.1186/1477-7827-10-62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Du Y., Xie W., Liu C.2010. Strategies and considerations for distributing and recovering mouse lines. Methods Enzymol. 476: 37–52. doi: 10.1016/S0076-6879(10)76003-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Herr C.M., Wright R., Jr.1988. Cold culture of different stage mouse embryos in bicarbonated and bicarbonate-free media. Theriogenology 30: 159–168. doi: 10.1016/0093-691X(88)90273-7 [DOI] [PubMed] [Google Scholar]
- 4.Herr C.M., Wright R.W., Jr.1988. Cold storage of mouse embryos of different stages of development. Theriogenology 29: 765–770. doi: 10.1016/S0093-691X(88)80021-9 [DOI] [PubMed] [Google Scholar]
- 5.Ho Y., Wigglesworth K., Eppig J.J., Schultz R.M.1995. Preimplantation development of mouse embryos in KSOM: augmentation by amino acids and analysis of gene expression. Mol. Reprod. Dev. 41: 232–238. doi: 10.1002/mrd.1080410214 [DOI] [PubMed] [Google Scholar]
- 6.Horikoshi Y., Takeo T., Nakagata N.2016. N-acetyl cysteine prolonged the developmental ability of mouse two-cell embryos against oxidative stress at refrigerated temperatures. Cryobiology 72: 198–204. doi: 10.1016/j.cryobiol.2016.05.002 [DOI] [PubMed] [Google Scholar]
- 7.Kasai M.1986. Nonfreezing technique for short-term storage of mouse embryos. J. In Vitro Fert. Embryo Transf. 3: 10–14. doi: 10.1007/BF01131374 [DOI] [PubMed] [Google Scholar]
- 8.Kasai M., Niwa K., Iritani A.1983. Protective effect of sucrose on the survival of mouse and rat embryos stored at 0 degree C. J. Reprod. Fertil. 68: 377–380. doi: 10.1530/jrf.0.0680377 [DOI] [PubMed] [Google Scholar]
- 9.Kishi H., Okada T., Otsuka M., Watanabe G., Taya K., Sasamoto S.1996. Induction of superovulation by immunoneutralization of endogenous inhibin through the increase in the secretion of follicle-stimulating hormone in the cyclic golden hamster. J. Endocrinol. 151: 65–75. doi: 10.1677/joe.0.1510065 [DOI] [PubMed] [Google Scholar]
- 10.Kito S., Ohta Y.2008. In vitro fertilization in inbred BALB/c mice II: effects of lactate, osmolarity and calcium on in vitro capacitation. Zygote 16: 259–270. doi: 10.1017/S0967199408004619 [DOI] [PubMed] [Google Scholar]
- 11.Miyoshi I., Ishikawa K., Kasai M., Kasai N.1992. Useful short-range transport of mouse embryos by means of a nonfreezing technique. Lab. Anim. Sci. 42: 198–201. [PubMed] [Google Scholar]
- 12.Nakagata N.1992. [Embryo transfer through the wall of the fallopian tube in mice]. Jikken Dobutsu 41: 387–388. [DOI] [PubMed] [Google Scholar]
- 13.Nakao K., Nakagata N., Katsuki M.1997. Simple and efficient vitrification procedure for cryopreservation of mouse embryos. Exp. Anim. 46: 231–234. doi: 10.1538/expanim.46.231 [DOI] [PubMed] [Google Scholar]
- 14.Quigley C.2013. Lost in transit - a forgotten rodent welfare issue? Altern. Lab. Anim. 41: 8–9. doi: 10.1177/026119291304100118 [DOI] [PubMed] [Google Scholar]
- 15.Quinn P., Kerin J.F., Warnes G.M.1985. Improved pregnancy rate in human in vitro fertilization with the use of a medium based on the composition of human tubal fluid. Fertil. Steril. 44: 493–498. doi: 10.1016/S0015-0282(16)48918-1 [DOI] [PubMed] [Google Scholar]
- 16.Shaw J.M., Nakagata N.2002. Cryopreservation of transgenic mouse lines. Methods Mol. Biol. 180: 207–228. [DOI] [PubMed] [Google Scholar]
- 17.Swallow J., Anderson D., Buckwell A.C., Harris T., Hawkins P., Kirkwood J., Lomas M., Meacham S., Peters A., Prescott M., Owen S., Quest R., Sutcliffe R., Thompson K., Transport Working Group, Laboratory Animal Science Association (LASA)0. 2005. Guidance on the transport of laboratory animals. Lab. Anim. 39: 1–39. doi: 10.1258/0023677052886493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takeo T., Kaneko T., Haruguchi Y., Fukumoto K., Machida H., Koga M., Nakagawa Y., Takeshita Y., Matsuguma T., Tsuchiyama S., Shimizu N., Hasegawa T., Goto M., Miyachi H., Anzai M., Nakatsukasa E., Nomaru K., Nakagata N.2009. Birth of mice from vitrified/warmed 2-cell embryos transported at a cold temperature. Cryobiology 58: 196–202. doi: 10.1016/j.cryobiol.2008.12.011 [DOI] [PubMed] [Google Scholar]
- 19.Takeo T., Kondo T., Haruguchi Y., Fukumoto K., Nakagawa Y., Takeshita Y., Nakamuta Y., Tsuchiyama S., Shimizu N., Hasegawa T., Goto M., Miyachi H., Anzai M., Fujikawa R., Nomaru K., Kaneko T., Itagaki Y., Nakagata N.2010. Short-term storage and transport at cold temperatures of 2-cell mouse embryos produced by cryopreserved sperm. J. Am. Assoc. Lab. Anim. Sci. 49: 415–419. [PMC free article] [PubMed] [Google Scholar]
- 20.Takeo T., Nakagata N.2010. Combination medium of cryoprotective agents containing L-glutamine and methyl-beta-cyclodextrin in a preincubation medium yields a high fertilization rate for cryopreserved C57BL/6J mouse sperm. Lab. Anim. 44: 132–137. doi: 10.1258/la.2009.009074 [DOI] [PubMed] [Google Scholar]
- 21.Takeo T., Nakagata N.2015. Superovulation using the combined administration of inhibin antiserum and equine chorionic gonadotropin increases the number of ovulated oocytes in C57BL/6 female mice. PLoS One 10: e0128330. doi: 10.1371/journal.pone.0128330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Toyoda Y.1971. Studies on fertilization of mouse eggs in vitro. I. In vitro fertilization of eggs by fresh epididymal sperm. Jpn. J. Anim. Reprod. 16: 147–151. doi: 10.1262/jrd1955.16.152 [DOI] [Google Scholar]
- 23.Wadman M.2012. Lab-animal flights squeezed. Nature 489: 344–345. doi: 10.1038/489344a [DOI] [PubMed] [Google Scholar]