Abstract
Tocilizumab, an interleukin-6 receptor antagonist, has been used to treat critically ill patients with coronavirus disease-2019. We present the case of a previously immunocompetent man with coronavirus disease-2019 who developed invasive pulmonary aspergillosis after treatment with tocilizumab, illustrating the importance of considering opportunistic infections when providing immune modulating therapy.
Keywords: COVID-19, opportunistic Infections, fungal infections, aspergillosis, tocilizumab, pulmonary disease
1. Background
Coronavirus disease-2019 (COVID-19), caused by severe acute respiratory coronavirus-2, has emerged as a global pandemic that has affected millions. Severe presentations of COVID-19, characterized by significant pulmonary disease and shock, are likely propagated by both direct viral effects and a cytokine-mediated inflammatory response, or “cytokine storm.” (Xu et al., 2020). Interleukin-6 (IL-6) plays a central role in the cytokine storm that is common in severe cases of COVID-19 (Zhang et al., 2020). Accordingly, IL-6 receptor antagonists such as tocilizumab, typically used in the treatment of rheumatoid arthritis, have been used in select cases of COVID-19 to suppress the suspected inflammatory damage (Xu et al., 2020).
Select governmental health organizations have recommended tocilizumab for patients with extensive and bilateral lung disease (General Office of the National Health Commission (China), 2020). Initial data on the use of these agents has been promising. Tocilizumab decreases inflammation in COVID patients, as measured with both fever and serum inflammatory markers such as C-reactive protein (Luo et al., 2020). Additionally, a small Chinese preprint study of patients with severe COVID-19 reported 75% had reduced oxygen requirement, 90% had improved computed tomography scans, and, notably, there were no reports of secondary infections or clinical deterioration (Xu et al., 2020).
Although initial data for tocilizumab in COVID-19 is promising, there are risks to immune modulating therapies, especially the development of opportunistic infections (Vallabhaneni and Chiller, 2016). Tocilizumab has been associated with invasive fungal infections including invasive candidiasis, Pneumocystis, and Cryptococcal pneumonia (Schiff et al., 2011, Campbell et al., 2011). This risk is doubled compared to controls (Hoshi et al., 2012). This case report presents a patient with severe COVID-19 pneumonia who developed invasive pulmonary aspergillosis (IPA) after treatment with tocilizumab.
2. Case report
A 72-year-old man with a 30 pack-year smoking history and a predilection for gardening presented with cough and fever. His chest X-ray (CXR) showed bilateral opacifications and nasopharyngeal polymerase chain reaction was positive for severe acute respiratory coronavirus-2 when sent to the state's public health department. Three days after admission, he developed acute hypoxemic respiratory failure, was transferred to the intensive care unit (ICU), and mechanically ventilated.
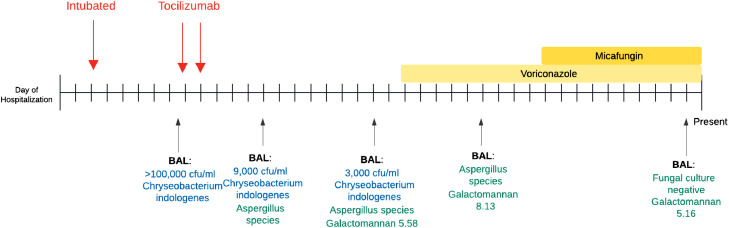
Despite lung protective ventilation, empiric treatment for hospital acquired pneumonia (azithromycin and ceftriaxone initially, then transitioned to piperacillin-tazobactam), and initiation of compassionate use remdesivir, the patient's hypoxemia continued to worsen. Serial CXRs demonstrated progressive bilateral opacifications and inflammatory makers were significantly elevated (D-dimer 15,205 ng/mL, ferritin 1945 ng/mL, C-reactive protein 22 mg/dL, LDH 425 unit/L). Serum IL-6 levels were not obtained. A bronchoalveolar lavage (BAL) performed on day 9 grew Chyrseobacterium indologenes (Fig. 1 ). Fungal cultures were not completed at this time. Ciprofloxacin was added based on Chyrseobacterium resistance to other agents and he was prone positioned.
Fig. 1.
Timeline of bronchoalveolar lavage (BAL) results in relation to clinical events.
Given persistent hypoxemia, fevers, and elevated inflammatory markers, a multidisciplinary decision was made to administer off-label 2 doses of 400 mg tocilizumab (4.4 mg/kg) on hospital days 9 and 10 (Xu et al., 2020, Luo et al., 2020). On the days directly following tocilizumab administration, his oxygenation and shock improved significantly.
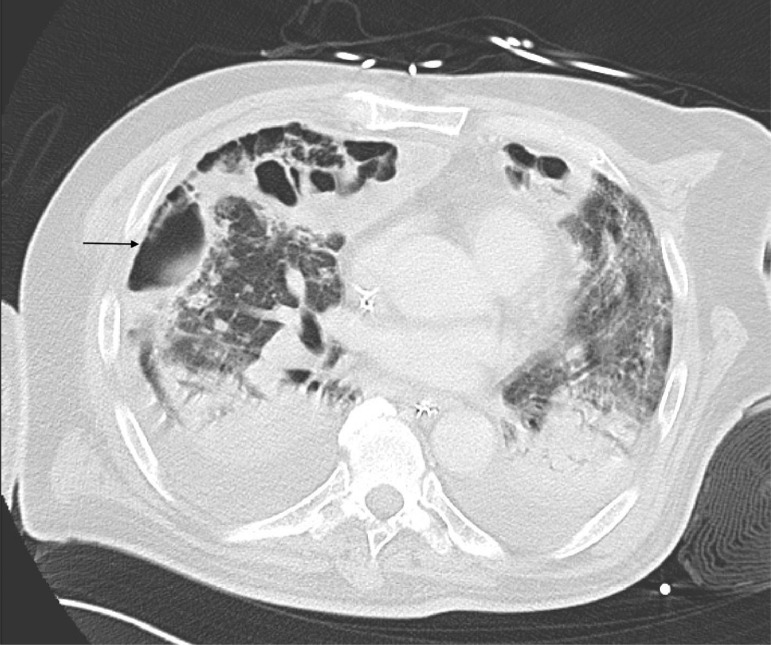
However, beginning on day 13, he developed recurrent shock and fever. His CXR showed increased opacification at the lung bases, and fungal cultures on day 14 BAL grew Aspergillus. A subsequent BAL confirmed Aspergillus (species unknown) and BAL galactomannan was 5.58. Computed tomography scan demonstrated large bilateral cavitary lesions with aspergillomas (Fig. 2 ). After initiation of dual therapy voriconazole and micafungin, the patient had improvement in his oxygenation and BAL results, but remained ventilator dependent and eventually received a tracheostomy. Since initial submission of this report, the patient was decannulated (day 69) and discharged (day 80), and has recovered well at home with physical therapy.
Fig. 2.
Chest CT scan illustrating extensive bilateral cavitary lung disease, air-fluid levels, and an aspergilloma in the right middle lobe (indicated by an arrow).
3. Discussion
In this case, we describe new onset invasive fungal infection after treatment with tocilizumab in a patient with acute hypoxemic respiratory failure secondary to COVID-19.
Immune modulating agents are known to be a major risk factor for IPA (Baddley, 2011). Immunosuppressive drugs such as steroids are occasionally used to treat severe H1N1 infections, and they are strongly associated with IPA (Huang et al., 2019). Ultimately, the risk of invasive fungal infections with tocilizumab treatment is incompletely understood, in part because fungal infections are generally rare compared to other opportunistic infections, and the scientific community is still collecting data about many monoclonal antibody treatments (Vallabhaneni and Chiller, 2016). The risk of IPA is most well-established with the older TNF-alpha inhibitors, but newer biologics are now being implicated as well (Nedel et al., 2009). All patients who receive tocilizumab are recommended to be tested for latent tuberculosis (General Office of the National Health Commission (China), 2017), but there are not yet standardized recommendations for screening for other opportunistic infections.
It appears that patients with severe COVID-19 are at increased risk for IPA, even without the use of immune suppressing medication. Recent preprint research suggests that critically ill patients with COVID-19 are at high risk for IPA even without tocilizumab or other immune modulating medication (Alanio et al., 2020, Blaize et al., 2020). This phenomenon is also seen in severe H1N1influenza infections, as prior work has shown that H1N1-associated IPA occurs in up to 28% of patients in the ICU, and carries a mortality of 61% (Vanderbeke et al., 2018, Wauters et al., 2012).
Although it is possible that our patient would have developed IPA despite tocilizumab treatment, COVID- and H1N1-associated IPA both tend to appear at a mean of 3 days after intubation (Alanio et al., 2020, Wauters et al., 2012). Our patient's deterioration and diagnosis of IPA occurred on day 13, suggesting an alternate etiology of the fungal infection. While the causality of tocilizumab with our patient's IPA is speculative as there was no fungal culture on the BAL prior to tocilizumab administration, there is a known association between immune modulating medications and opportunistic respiratory infections including IPA (Campbell et al., 2011, Hoshi et al., 2012; Schiff et al., 2011). It is therefore important to consider de novo opportunistic infections prior to initiating treatment with tocilizumab. Additionally, clinical deterioration after starting such an immune modulating agent warrants investigation for an opportunistic infection; early identification and prompt administration of antifungals improves survival in IPA (General Office of the National Health Commission (China), 2017).
Because critically ill patients in the ICU are already at increased risk for IPA (Baddley, 2011), prior to the administration of immunomodulatory agents for severe cases of COVID-19, appropriate patients should be evaluated for latent fungal infections (e.g. cultures, serologic testing, galactomannan). It is also wise to consider other risk factors that would make a patient prone to invasive fungal infections, such as underlying immune compromise (i.e. malignancy, chemotherapy, transplant patients) as well as patients with organ dysfunction (i.e., pulmonary disease, renal, or liver failure) (Baddley, 2011). Although the causal link between agents like tocilizumab and invasive fungal infections remains unclear, this case illustrates the importance of maintaining a high degree of clinical suspicion for opportunistic infections when treating COVID-19 patients with immune modulating therapy.
Declaration of competing interest
None of the authors have conflicts of interest to disclose, or a financial relationship with a commercial entity that has interest in the subject of the manuscript.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- Actemra [Package Insert]; South San Francisco, CA: 2017. Genentech, Inc. [Google Scholar]
- Alanio A, Dellière S, Fodil S, Bretagne S, Mégarbane B. High prevalence of putative invasive pulmonary aspergillosis in critically ill COVID-19 patients. [Preprint]. 2020 [15th April 2020]. Available https://papers.ssrn.com/sol3/papers.cfm?abstract_id=3575581 [DOI] [PMC free article] [PubMed]
- Baddley JW. Clinical risk factors for invasive aspergillosis. Med Mycol. 2011;49(Suppl 1):S7–S12. doi: 10.3109/13693786.2010.505204. [DOI] [PubMed] [Google Scholar]
- Blaize M, Mayaux J, Nabet C, Lampros A, Marcelin AG, Thellier M. Fatal invasive aspergillosis and coronavirus disease in an immunocompetent patient. Emerg Infect Dis. 2020;26(7):1636–1637. doi: 10.3201/eid2607.201603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell L, Chen C, Bhagat SS, Parker RA, Ostor AJ. Risk of adverse events including serious infections in rheumatoid arthritis patients treated with tocilizumab: a systematic literature review and meta-analysis of randomized controlled trials. Rheumatology (Oxford) 2011;50(3):552–562. doi: 10.1093/rheumatology/keq343. [DOI] [PubMed] [Google Scholar]
- General Office of the National Health Commission (China) 7th ed. 2020. Novel coronavirus pneumonia diagnosis and treatment plan. [Google Scholar]
- Hoshi D, Nakajima A, Inoue E, Shidara K, Sato E, Kitahama M. Incidence of serious respiratory infections in patients with rheumatoid arthritis treated with tocilizumab. Mod Rheumatol. 2012;22(1):122–127. doi: 10.1007/s10165-011-0488-6. [DOI] [PubMed] [Google Scholar]
- Huang L, Zhang N, Huang X, Xiong S, Feng Y, Zhang Y. Invasive pulmonary aspergillosis in patients with influenza infection: a retrospective study and review of the literature. Clin Resp J. 2019;13(4):202–211. doi: 10.1111/crj.12995. [DOI] [PubMed] [Google Scholar]
- Luo P, Liu Y, Qiu L, Liu X, Liu D, Li J. Tocilizumab treatment in COVID-19: a single center experience. J Med Virol 92(7):814–818. [DOI] [PMC free article] [PubMed]
- Nedel WL, Kontoyiannis DP, Pasqualotto AC. Aspergillosis in patients treated with monoclonal antibodies. Rev Iberoam Micol. 2009;26(3):175–783. doi: 10.1016/j.riam.2009.04.001. [DOI] [PubMed] [Google Scholar]
- Schiff MH, Kremer JM, Jahreis A, Vernon E, Isaacs JD, van Hollenhoven RF. Integrated safety in tocilizumab clinical trials. Arthritis Res Ther. 2011;13(5):R141. doi: 10.1186/ar3455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallabhaneni S, Chiller TM. Fungal infections and new biologic therapies. Curr Rheumatol Rep. 2016;18(5):29. doi: 10.1007/s11926-016-0572-1. [DOI] [PubMed] [Google Scholar]
- Vanderbeke L, Spriet I, Breynaert C, Rijnders BJA, Verweij PE, Wauters J. Invasive pulmonary aspergillosis complicating severe influenza: epidemiology, diagnosis and treatment. Curr Opin Infect Dis. 2018;31(6):471–480. doi: 10.1097/QCO.0000000000000504. [DOI] [PubMed] [Google Scholar]
- Wauters J, Baar I, Meersseman P, Meersseman W, Dams K, De Paep R. Invasive pulmonary aspergillosis is a frequent complication of critically ill H1N1 patients: a retrospective study. Intensive Care Med. 2012;38(11):1761–1768. doi: 10.1007/s00134-012-2673-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Han M, Li T, Sun W, Wang D, Fu B. Effective treatment of severe COVID-19 patients with Tocilizumab. ChinaXiv. 2020 doi: 10.1073/pnas.2005615117. http://chinaxiv.org/abs/202003.00026 [Preprint][15th April 2020]Available. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Wu Z, Li JW, Zhao H, Wang GQ. The cytokine release syndrome (CRS) of severe COVID-1 and Interleukin-6 receptor (IL-6R) antagonist Tocilizumab may be the key to reduce the mortality. Int J Antimicrob Agents. 2020;55(5):105954. doi: 10.1016/j.ijantimicag.2020.105954. [DOI] [PMC free article] [PubMed] [Google Scholar]