Abstract
Background
Nine COVID-19 (Corona Virus Disease, 2019) cases were observed in one community in Guangzhou. All the cases lived in three vertically aligned units of one building sharing the same piping system, which provided one unique opportunity to examine the transmission mode of SARS-CoV-2.
Methods
We interviewed the cases on the history of travelling and close contact with the index patients. Respiratory samples from all the cases were collected for viral phylogenetic analyses. A simulation experiment in the building and a parallel control experiment in a similar building were then conducted to investigate the possibility of transmission through air.
Results
Index patients living in Apartment 15-b had a travelling history in Wuhan, and four cases who lived in Apartment 25-b and 27-b were subsequently diagnosed. Phylogenetic analyses showed that virus of all the patients were from the same strain of the virus. No close contacts between the index cases and other families indicated that the transmission might not occur through droplet and close contacts. Airflow detection and simulation experiment revealed that flushing the toilets could increase the speed of airflow in the pipes and transmitted the airflow from Apartment 15-b to 25-b and 27-b. Reduced exhaust flow rates in the infected building might have contributed to the outbreak.
Conclusions
The outbreak of COVID-19 in this community could be largely explained by the transmission through air, and future efforts to prevent the infection should take the possibility of transmission through air into consideration. A disconnected drain pipe and exhaust pipe for toilet should be considered in the architectural design to help prevent possible virus spreading through the air.
Keywords: COVID-19, Transmission through air, China, Community outbreak
Highlights
-
•
Provide evidence of SARS-CoV-2 transmission through air.
-
•
Poor ventilation in the apartments increases the risk of transmission.
-
•
Floor drain should be sealed with water and regular disinfected.
-
•
The privately remodeled piping system might have played a role in this transmission.
1. Introduction
At the end of 2019, an unprecedented outbreak of coronavirus disease 2019 (COVID-19) infection caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has spread globally with a high burden to the society (Hauser et al., 2020; Liu et al., 2020a, Liu et al., 2020b; Pinotti et al., 2020; Wu et al., 2020a, Wu et al., 2020b). SARS-CoV-2 was spread by droplet or contact with respiratory secretions of patients (Li et al., 2020; Zhu et al., 2020), the latest evidence indicated that the contaminated air might be another important transmission mechanism (Morawska and Cao 2020; Zhang et al., 2020a, Zhang et al., 2020b; Zhou et al., 2020).
Although a few studies have suggested that the transmission though air in COVID-19 was possible in the hospital setting (Liu et al., 2020a, Liu et al., 2020b; Ye et al., 2020; Zhou et al., 2020), the direct epidemiological evidence from the community situation has not been confirmed yet, the information from other respiratory virus may lend some support for the plausibility of transmission through air. For example, during the epidemic of SARS (Severe Acute Respiratory Syndrome) in Hong Kong, transmission through air was proved to be responsible for a community clustering of the infection in Amoy Gardens, where the air carrying the SARS virus could be transmitted to the upper floors of the building (Yu et al., 2004). Previous studies on MERS (Middle East Respiratory Syndrome) (Kim et al., 2016) and influenza also provided the evidence for both short-range and longer-range transmission through air (Lindsley et al., 2016). These evidences demonstrate a potential risk for air transmission of SARS-CoV-2 to a great extent.
Thus, it is far from being enough to consider only outdoor personal protection to prevent COVID-19 (The Plos Medicine Editors 2020). With the further research on SARS-CoV-2, relatively closed room, poor ventilation condition and even contaminated drainage system might also increase the risk of indoor infection (Chen 2020; Lou et al., 2020; Morawska et al., 2020). However, the public has still not laid enough emphasis on the problem of this issue. One recent cluster of the outbreak of 9 COVID-19 cases in one community in Guangzhou, China, provided one unique opportunity to explore the possibility of the viral transmission through air. We thus conducted a series of investigations, including epidemiological survey, an airflow experiment, and a simulation experiment, in order to illuminate the potential reason of this outbreak and to raise the alarm.
2. Methods
2.1. Descriptive analysis of this cluster
We collected the relevant information with regard to the COVID-19 case clusters in the community in Guangzhou, China. The outbreak was initiated from one person of Apartment 15-b who had a travelling history in Wuhan, and was diagnosed as COVID-19 on January 27, 2020. Her four family members were subsequently confirmed as COVID-19 cases in the following days. Thereafter, four residents living in the same unit (Apartment 25-b and 27-b) began to develop symptoms of fever and chest distress, and were diagnosed with COVID-19.
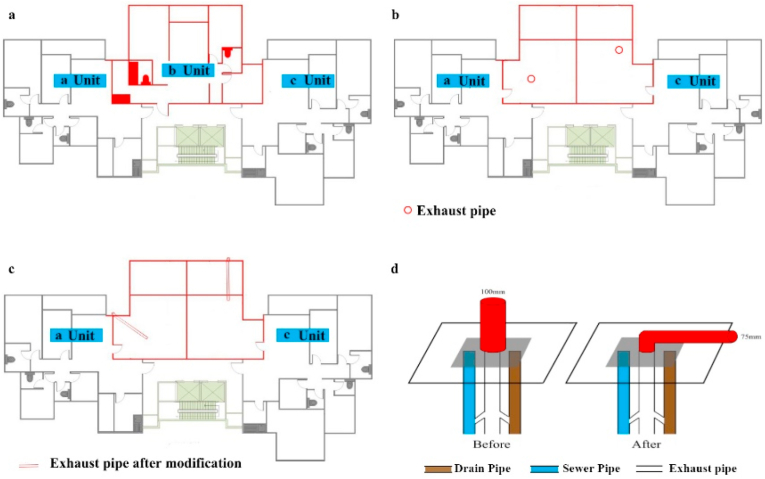
The building is a 29-storey apartment with three units (a, b, and c) on each floor, except that the top floor (29th floor) has two units (a and b) (Fig. 1). Residents of the same unit of different floors share one drain pipe, one sewer pipe and one exhaust pipe. Normally, the three pipes are connected to each other and lead to the same vent on the roof. But, the vent of the straight pipeline was replaced with a right-angle bending one, and the diameter changed from 100 mm to 75 mm (Fig. 1). The pictures of the pipes in the community were shown in Fig. S1.
Fig. 1.
Floor structure of the study building.
(a) The planar structure of Floor 2 to Floor 28. (b) The planar structure of Floor 29. (c) The planar structure of Floor 29 after modification. (d) The structure of the drain pipe, sewer pipe and exhaust pipe before and after the modification.
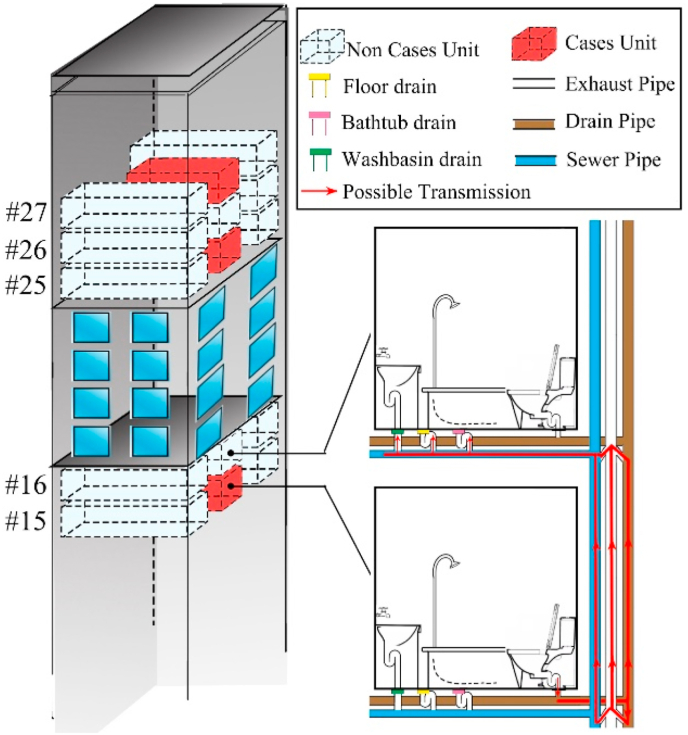
Piping system structure of the bathrooms in the study building was shown in Fig. 2. Drain pipe connected with the toilet was used to exhaust the excrement, and sewer pipe connected with floor drains was used to exhaust the sewage. Sewer pipe and drain pipe were connected with the exhaust pipe by an H-shaped joint respectively. This kind of design had better discharge capacities and was widely adopted in high rise buildings. The same apartment vertical section shared the same piping system (Guan et al., 2020). The same vertically aligned units shared the same piping system.
Fig. 2.
Piping system structure of the study building.
2.2. Coronavirus detection and characterization
Pharyngeal swabs were collected from the nine patients and used for viral testing. RNA was extracted from 200 μL of respiratory sample with the Viral Nucleic Acid Isolation Kit (Magnetic Beads) in SSNP-2000A automatic nucleic acid extraction system (bioPerfectus Technologies, Taizhou, China). Real-time reverse-transcription PCR (rRT-PCR) was carried out using the Novel Coronavirus 2019 Nucleic Acid Test Kit (bioPerfectus Technologies, Taizhou, China) in Applied Biosystems ViiA7 instruments (Applied Biosystems, Hong Kong, China), All these procedures were conducted according to the standard instructions. The TaqMan-probe-based kit was designed to detect ORF1ab and N gene of SARS-CoV-2 in one reaction. Thermal cycling was performed at 50 °C for 10 min for reverse transcription, followed by 97 °C for 1 min and then 45 cycles of 97 °C for 5 s, 58 °C for 30 s.
Conventional RT-PCR was done using the PrimeScript One Step RT-PCR Kit Ver 2.0 (TaKaRa, Dalian, China) followed by manufacturer's instructions. The forward primer (5ʹ-CCTACTAAATTAAATGATCTCTGCTTTACT-3ʹ) and the reverse primer (5ʹ-CAAGCTATAACGCAGCCTGTA-3ʹ) targeting the 158 bp of Spike (S) gene of SARS-CoV-2 were synthesized as previously described (Chan et al., 2020). PCR reaction was performed using an automated thermocycler (Applied Biosystems, Hong Kong, China) with an incubation at 50 °C for 30 min, a hot start at 94 °C for 2 min, followed by 40 cycles of 94 °C for 30 s, 55 °C for 30 s, and 72 °C for 1 min, and a final extension at 72 °C for 10 min. The PCR products were purified using QIAquick Gel Extraction Kit (Qiagen, Hilden, Germany) and sequenced by Sanger sequencing with an ABI 3730xl DNA Analyzer (Applied Biosystems, Carlsbad, USA) using the PCR primers.
MEGA X was used for multi-sequence alignment of amplicons of partial S gene regions of the samples detected in this study and other SARS-CoV-2 isolates from GISAID and related coronavirus from GenBank, and the online tool PhyML 3.0 (http://www.atgc-montpellier.fr/phyml/) was used to construct the Maximum likelihood phylogenetic tree with bootstrap values calculated from 1000 trees.
2.3. The potential transmissions in the elevators
We were unable to collect the samples in the elevators as they were disinfected and cleaned immediately after the diagnosis of the first index patient. To check the possible transmission route in the elevators, we retrospectively inspected the video records in the elevators, and verified the number of residents who had taken the elevators after the patients of Apartment 15-b took the elevators, and classified them into two groups: those from Unit b and non-Unit b. However, it was hard to exactly identify the persons who live Unit b and other units, we thus assumed that all the residents in this building had the equal access to the elevators, then estimated the numbers based on the overall number of people taking the elevators. Theoretically, the infection rate would be similar between the two groups if the infections occurred in the elevators. We applied the Fisher's exact test to examine the statistical significance of distribution of the cases between Unit b and non-Unit b. The residents from the three families (Apartment 15-b, 25-b and 27-b) were also interviewed to check whether they had close contacts during this time period.
2.4. Airflow experiment at the bathtub drain and floor drain
To investigate the dynamics of airflow in the pipes, we detected the wind speed at the bathtub drain and floor drain of different floors simultaneously (including Apartment 15-b, 16-b, 23-b, 25-b, 26-b, and 27-b) as soon as we flushed the toilet. The measuring device, Testo-425, was used for measuring the wind speed in ventilation ducts (Jankowski and Mlynarczyk 2016). We drained the floor drains in the bathrooms of the selected floors to keep the pipeline open before starting the experiment, and then flushed the toilet of Apartment 15-b and 23-b separately and detected the wind speed at the bathtub drain and floor drain of other apartments.
2.5. The tracer-gas experiment
Given that all the cases occurred in the same unit and that these households shared a common pipe system, we therefore conducted a tracer-gas experiment to simulate the process of potential transmission through air (Lim et al., 2010; Hang et al., 2014). Dry drain means the water level in the U-trap is lower than 0.5 cm or the U-trap is empty and thus can't play a protective and isolated role in stopping the contaminated air. The field survey showed that the bends of the floor drains of Apartment 8-b, 15-b, 16-b, and 26-b were dry, while the bends of the drain pipes were sealed with water in the bathroom of Apartment 21-b and 27-b.
The mixture of chloroform and carbon tetrachloride was used to mimic the spread process as they had not been used in these apartments before or existed in detergent and paint. so that the measurement might not be influenced by the background level. Considering that the chloroform and carbon tetrachloride with strong stimulate odor and unhealthy nature, this experiment was conducted after all the residents from Unit b were moved out of their apartments for quarantine. And the residents were allowed to move back their apartments after more than 14 days ventilation. We poured the mixture (50 ml) on the floor drain and into the toilet of Apartment 15-b. The residuals of the chemicals in Apartment 8-b, 21-b, 26-b, and 27-b were collected using gas sampler with aluminum foil sampling bag at different time periods-before the simulation experiment, 30 min later and 60 min later, then the corresponding concentrations were measured by gas chromatography. The situations of the four observational apartments were as follows: the floor drains of Apartment 8-b were dry and located in the downstairs of Apartment 15-b, while the drains of Apartment 21-b and 27-b were sealed by water. And Apartment 26-b located upstairs of Apartment 15-b and its floor drains were dry with the sufficient conditions for transmission through air. The other apartments were not included in this experiment due to unavailability of some empty apartments or consent of the households.
In the parallel control experiment, we found that the vent in the top of Unit b was privately refitted into a hall by the residents in Floor 29. The original straight drain pipeline was replaced by a rectangular-curved one, with the diameter decreased from 100 mm to 75 mm. The field investigation suggested that when flushing the toilet, strong airflow might drive the virus and pollutants from the downstairs bathroom into the upstairs bathroom through the floor drain. To verify the possibility of transmission through air in an unblocked pipe, a parallel control experiment was conducted in Apartment 24-a of another building with the similar structure (Fig. S2). Compared with the exhaust line of the study building, that of the control building had a more effective ventilation and exhausting system. The less toxic n-hexane was poured on the floor drains of toilet in master bedroom and into the toilet in guest bedroom in Apartment 11-a to simulate the dynamic process of airflow, and then the concentration of the pollutants in the corresponding position of the upper apartments-Apartment 24-a was detected at different time points (0, 30, and 60 min later). To test the robustness of the results, another control experiment was implemented five days later, with the extending concentration monitoring points in Apartment 5-a, 17-a, and 24-a.
In order to simulate the real situation during the outbreak, we did not open the exhaust fan during the experiment. The measuring points were located in the center of the bathrooms at a height of 1.2 m. Duplicate VOC samples were collected on Tenax-TA sorbent tubes at 100 ml/min for 60 min and analyzed by Thermal desorption/capillary gas chromatography according to the GB/T18883-2002 method. Duplicate aldehyde samples were collected on DNPH cartridges with 500 ml/min for 60 min and analyzed by phenol reagent spectrophotometric method according to the GB/T 18204.2–2014 method. All the research personnel had worn the specialist protective masks, gloves and suits during the experiment, and avoided the unnecessary and longtime exposure in the experimental environment as well.
3. Results
3.1. Descriptive results
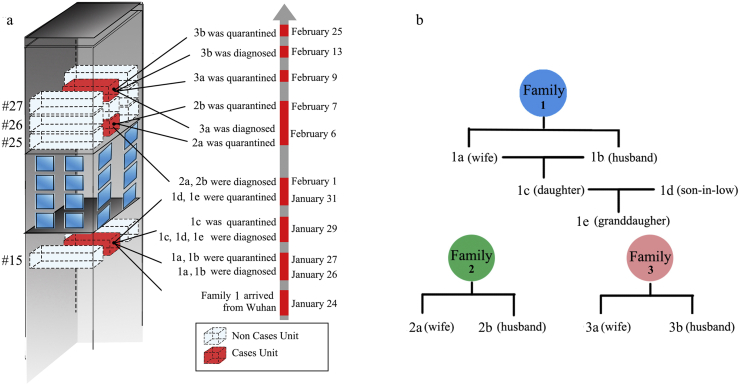
The epidemiological investigation of Family 1 (the index patients’ family) showed that every member (except for 1d) had a travel history to Wuhan. The information on the date of diagnosis and quarantine, residential location and family relationship of nine cases was shown in Fig. 3. The two COVID-19 cases (1a and 1b) living in Apartment 15-b were firstly diagnosed on January 26, 2020, following by the diagnosis of the other three members (1c, 1d, and 1e) on January 29, the quarantine measures for them started on February 1. On the same day, residents of Apartment 25-b (2a and 2b) were diagnosed with infection. From February 6 to 25, two members in Apartment 27-b (3a and 3b) were subsequently diagnosed and quarantined. Self-report from Apartments 25-b and 27-b showed that all the family members did not have a travelling history to Wuhan and any close contacts with the diagnosed cases previously. Because all the cases occurred in Unit b, the residents of Unit b were required to move to a separate hotel outside of the community for medical observation from February 8 to 22, and no new cases emerged to date.
Fig. 3.
Description of the nine cases.
(a) Onset and quarantine date for the nine cases. (b) The relationship between the nine cases. Each case was labeled with one letter and number, the number represented the family, and the letter represented sequence of the infection in each family.
3.2. Phylogenetic analyses
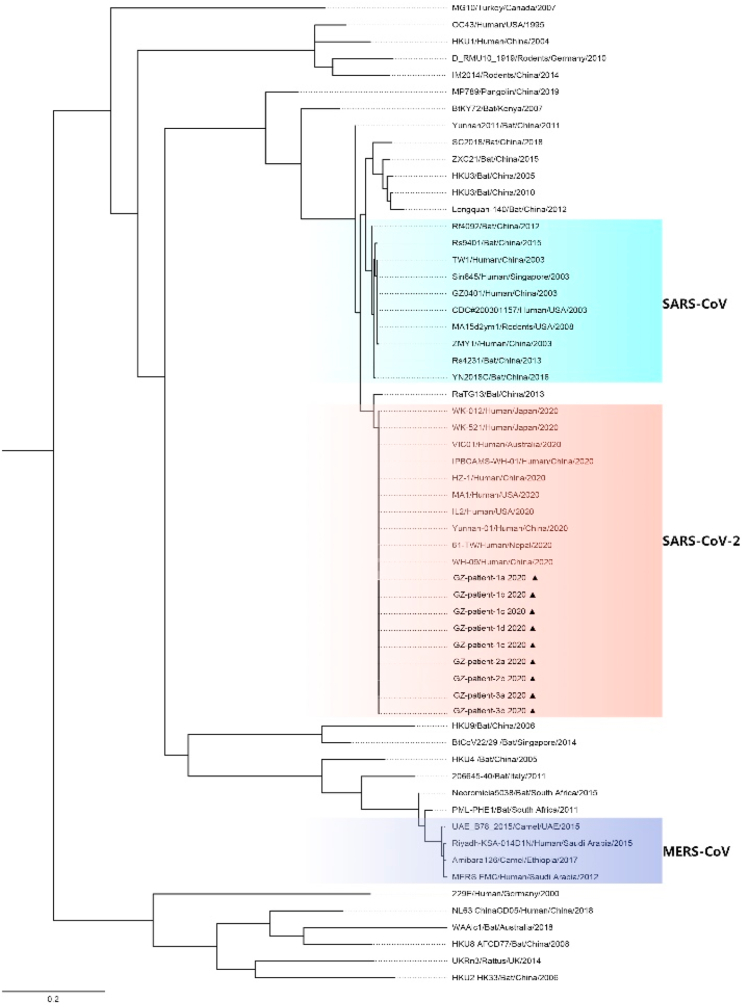
Patients from the three families were in the same viral cluster. All the S gene of SARS-CoV-2 sequences of the patients in this study had almost 100% identity, suggesting that they were infected by the same strain of the virus (Fig. 4).
Fig. 4.
Phylogenetic tree of Spike gene sequences of patients in Guangzhou(▲).
3.3. Possible transmission in the elevators
Our retrospective inspection of the video records in the elevators suggested that a total of 83 residents (28 from Unit b and 55 from non-Unit b) had taken the elevators after the index patients from Family 1 used them (Table 1). In addition, we found that the occupants of Apartments 25-b and 27-b did not have close contact with others according to the video records in the elevators. Among them, four cases were from Unit b, while 24 of the 79 non-cases were from Unit b. Under the circumstances that both residents in Unit b and non-unit b have the same possibility to enter the elevator after Family 1 taking the elevator, Fisher's exact test showed that there was a significant difference in the morbidity of COVID-19 between different units (p < 0.05), with a higher infected rate in residents living in Unit b than in non-unit b.
Table 1.
Distribution of cases and non-cases by Unit b and non-Unit b.
| Cases | Non-cases | Total | |
|---|---|---|---|
| Unit b | 4 (14.3%) | 24 (85.7%) | 28 |
| Non-Unit b | 0 (0.0%) | 55 (100.0%) | 55 |
| Total | 4 | 79 | 83 |
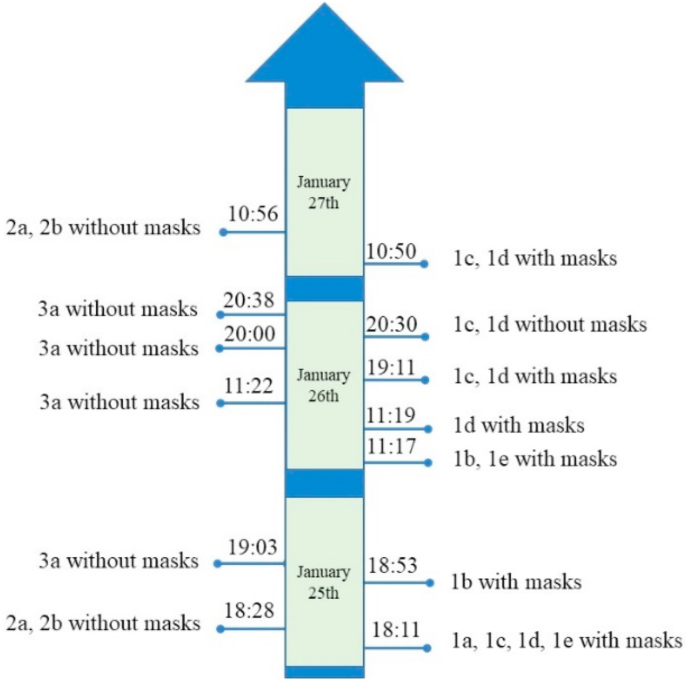
The time points of elevator used by Family 1, Family 2, and Family 3 were recorded from the video records in the elevators from January 25 to 27 (Fig. 5). Before the confirmation and quarantine of the index patient, all the members of Family 1 wore masks, except that 1c and 1d were recorded in the elevator without wearing masks at 20:30 on January 26. Eight minutes later, 3a entered the same elevator without wearing a mask. At that time, 1c and 1d did not have any symptoms, such as cough and sneeze. In addition, 3a and 1c, 1d had no direct contact with each other according to the video records in the elevators.
Fig. 5.
The time of elevator usage by Family 1, 2 and 3.
According to the incubation period of the infection and the fact that 3a′s symptoms began on February 6 (Backer et al., 2020), we estimated that its possible infection date was between January 30 and February 3. In addition, both Family 2 and 3 reported that they did not have close contacts with Family 1 or other cases. It was thus speculated that Family 2 and 3 were unlikely to get infected by Family 1 through close contacts in the elevators.
3.4. Detection of wind speed at the bathtub drain and floor drain
The results of wind speed after flushing the toilet were depicted in Table 2 and Table 3. We observed an increased wind speed both at the bathtub drain and floor drain after flushing the toilet. For example, when flushing the toilet of Apartment 15-b, the wind speed at the bathtub drain increased by 0.24 m/s, 0.28 m/s, 0.29 m/s, 0.28 m/s, and 0.19 m/s in Apartment 15-b, 16-b, 25-b, 26-b, and 27-b, while the wind speed at floor drain increased by 0.16 m/s, 0.44 m/s, 0.23 m/s, 0.10 m/s, and 0.56 m/s, respectively. Consistent changes were observed for Apartment 23-b. The results indicated that the airflow produced by flushing the toilet in one floor can influence the whole building due to the interlinked pipes that connects floors upstairs and downstairs.
Table 2.
Wind speed at the bathtub draina.
| Flush the toilet: #15-b |
Flush the toilet: #23-b |
||||
|---|---|---|---|---|---|
| Background at the bathtub drain (m/s) |
Wind speed at the bathtub drain (m/s) |
Differences (m/s) |
Wind speed at the bathtub drain (m/s) |
Differences (m/s) |
|
| #15-b | 0.12 | 0.36 | 0.24 | 0.20 | 0.08 |
| #16-b | 0.01 | 0.29 | 0.28 | 0.46 | 0.45 |
| #25-b | 0.22 | 0.51 | 0.29 | 0.66 | 0.44 |
| #26-b | 0.02 | 0.30 | 0.28 | 0.77 | 0.75 |
| #27-b | 0.04 | 0.23 | 0.19 | 0.15 | 0.11 |
Instrument used for detection: Testo-425; # Residential apartment.
Table 3.
Wind speed at the floor draina.
| Flush the toilet: #15-b |
Flush the toilet: #23-b |
||||
|---|---|---|---|---|---|
| Background at the floor drain (m/s) |
Wind speed at the floor drain (m/s) |
Differences (m/s) |
Wind speed at the floor drain (m/s) |
Differences (m/s) |
|
| #15-b | 0.06 | 0.22 | 0.16 | 0.20 | 0.14 |
| #16-b | 0.01 | 0.45 | 0.44 | 0.58 | 0.57 |
| #25-b | 0.10 | 0.33 | 0.23 | 0.19 | 0.09 |
| #26-b | 0.02 | 0.12 | 0.10 | 0.13 | 0.11 |
| #27-b | 0.63 | 1.19 | 0.56 | 0.90 | 0.27 |
Instrument used for detection: Testo-425; # Residential apartment.
3.5. The tracer-gas experiment
The results of the tracer-gas experiment were shown in Table 4. Before the experiments, there was minimal chloroform and carbon tetrachloride in the master bathroom and guest bathroom of all the Apartments (the concentrations were below the detection limit). While we observed a detectable concentration of the organics in the master bathroom and guest bathroom of Apartment 26-b and 27-b, after 30 and 60 min later. For Apartment 26-b, the concentration of chloroform was 0.12 mg/L and 0.074 mg/L in master bathroom, and 0.087 mg/L and 0.096 mg/L in guest bathroom (corresponding to 30 and 60 min later), while the corresponding concentration of carbon tetrachloride was 0.26 mg/L and 0.13 mg/L in master bathroom, and 0.17 mg/L and 0.14 mg/L in guest bathroom.
Table 4.
Simulations of transmission via sewer pipes in field experimenta.
| Apartment | Room | Time (min) |
Chloroform (mg/L) |
Carbon tetrachloride (mg/L) |
Field observation |
|---|---|---|---|---|---|
| #8-b | Master Bathroom | background | 0.00 | 0.00 | Dry floor drains |
| 30 | 0.00 | 0.00 | |||
| 60 | 0.00 | 0.00 | |||
| Guest Bathroom | background | 0.00 | 0.00 | ||
| 30 | 0.00 | 0.00 | |||
| 60 | 0.00 | 0.00 | |||
| #21-b | Master Bathroom | background | 0.00 | 0.00 | Water seal in the floor drains |
| 30 | 0.00 | 0.00 | |||
| 60 | 0.00 | 0.00 | |||
| Guest Bathroom | background | 0.00 | 0.00 | ||
| 30 | 0.00 | 0.00 | |||
| 60 | 0.00 | 0.00 | |||
| #26-b | Master Bathroom | background | 0.00 | 0.00 | Dry floor drains |
| 30 | 0.12 | 0.26 | |||
| 60 | 0.074 | 0.13 | |||
| Guest Bathroom | background | 0.00 | 0.00 | ||
| 30 | 0.087 | 0.17 | |||
| 60 | 0.096 | 0.14 | |||
| #27-b | Master Bathroom | background | 0.00 | 0.00 | Water seal in the floor drains |
| 30 | 0.00 | 0.00 | |||
| 60 | 0.00 | 0.0025 | |||
| Guest Bathroom | background | 0.00 | 0.00 | ||
| 30 | 0.00 | 0.00 | |||
| 60 | 0.00 | 0.0024 |
Instrument used for detection: Agilent 7890A GC; # Residential apartment.
We only detected minor concentration of carbon tetrachloride (0.0025 mg/L and 0.0024 mg/L) both in the master bathroom and guest bathroom of Apartment 27-b. In the sewer pipes of Apartment 8-b which straight below Apartment 15-b, we did not detect any organic solvent.
Before the parallel simulation experiments, the concentration of n-hexane was under the detect limit on the floor and the toilet of master bathroom in control Apartment 5-a, 17-a, and 24-a, nor 30 min and 60 min after the experiment (Table S1). The results in both Experiment 1 and 2 indicated a relatively lower ventilation efficiency of sewage system in Unit b due to the modification of the pipes, compared with the control building. The tracer gas could not be detected in 30 min and 60 min in the control building, while in the study building, tracer gas could remain in the pipes after 60 min or even longer.
4. Discussion
There is an increasing concern on the possibility of the SARS-CoV-2 transmission through air, in this respect, our study provided the timely evidence from a field survey in a community with an outbreak of SARS-CoV-2. The consistent findings from the epidemiological investigation, the airflow experiment, the simulation experiment, as well as the parallel control experiment, supported that the virus might be transmitted by the contaminated air in some special circumstances.
In view of seriousness of this community outbreak, local government and the department of health had timely cleaned and disinfected the apartment and promptly transferred all the occupants to a hotel for quarantine. Meanwhile, with the improvement of self-protection consciousness, residents had generally and consciously worn the masks and avoided going outside. Thus, the outbreak was timely stopped and did not cause a wider range of infection. However, the causes behind this community outbreak deserve to pay enough attention and take warning.
Several different mechanisms have been proposed to explain the transmission of the case clustering in this community. Genetic relatedness of SARS-CoV-2 sequence showed that the virus from the nine patients were highly homologous, supporting a person-to-person transmission. The investigations at early stage were thus mainly focused on the potential transmission through droplet and person-to-person contacts in the elevators. However, we were unable to determine whether person-to-person transmission occurred through respiratory droplets or close contacts. As our retrospective inspection did not find close contacts between the index patients in Apartment 15-b and others according to video records in the elevators, and epidemiologic interview records showed that diagnosed cases except for those who came from Apartment 15-b have no direct or indirect contact with COVID-19 cases or travelling history to high-risk areas. Furthermore, after the first patient was diagnosed, property department of the apartment quickly sterilized the public places including the elevators. Considering the observation that the cases all occurred in Unit b as the index patient, we thus speculated that viral transmission through air might be a more likely route among the patients. The tracer gas experiment indicated that virus could be spread through the building drainage and vent systems, and water seal in the trap could largely reduce but not completely block this transmission. Meanwhile, wind speed experiment also verified that when flushing the toilet, strong airflow will produce to drive the pollutants or virus through the drainage and exhaust system for vertical transmission. These results explain the possibility that SARS-CoV-2 might spread through the connecting piping system from the cases unit to non-cases units, however, infection through this way might be opportunistic. Firstly, the environmental conditions (such as temperature and humidity) of piping system are complicated and changeable which lead an uncertain survival time of SARS-CoV-2. Secondly, the spread distance of SARS-CoV-2 was largely determined by the speed of airflow in the piping system. In addition, the point-in-time of using the bathroom is also unpredictable in different floors of the apartment. Thus, based on the complexity and dynamicity of the airflow in the piping system, infection through this way is opportunistic to a large extent. We surely believed that transmission through the piping system is the most probable explanation to this series of infection. However, we are not sure whether the cases of Apartment 27-b were directly infected by the patients of Apartment 15-b, or were indirectly infected by the patients of Apartment 25-b that were firstly infected by the patients of Apartment 15-b.
The airflow experiment showed the air could quickly pass through the pipe line system across the different floors of the building, probably by means of negative pressure in the pipe system generated by the exhaust fan or the chimney effect (Chen et al., 2017). This result supported our hypothesis that the air could move upwards due to the buoyancy of the warm, humid air within the pipes and could be transferred to the apartment units with an unsealed floor drain. Our simulation experiment also confirmed this hypothesis, as we detected the corresponding chemical residuals in Apartment 16-b, 26-b, and 27-b a few minutes after pouring the organic solvents on the ground of Apartment 15-b.
However, one may wonder why there was not any infected individuals in the a units or c units or in other buildings. Our parallel control experiment suggested that the transformed exhaust pipe (became curved and the diameter was reduced to about 75% of the original pipe) may lead to a poor ventilation rate from indoor to outside, so the contaminated air could stay in the sewage pipe for longer, thus increasing the transmission through air in of the same unit, while hindered the horizontal spread of the virus.
Our findings shared some similar features with the clustering of SARS cases in Amoy Community of Hong Kong (Yu et al., 2004). For example, the U-shaped traps in the floor drains in some apartments have not been filled with water for a long time, the seals in the traps thus dried out, and as a result, a connection was opened to the contaminated water. In addition, the chimney effect would make the transmission of virus through air in the pipeline continuously advected upward, thus polluting the air in the bathrooms of other households of the same unit. Another investigation for typhimurium gastroenteritis outbreak from the United Kingdom also suggested that the blocked sewage-disposal system could be a reservoir of bacteria and the blocked U-shaped bend would contribute to the spread of contaminated air (Mair-Jenkins et al., 2017).
There is plausible biological evidence for the SARS-CoV-2 transmission through air(Christian et al., 2004; Jones and Brosseau 2015). For example, the novel coronavirus was detected in the air samples in the health care settings in China and Singapore (Ge et al., 2020; Ong et al., 2020). Similarly, the SARS-CoV can be detected in the air samples from SARS-patients admitted to hospitals in both Beijing and Hong Kong (Xiao et al., 2004; Booth et al., 2005). One US study reported that the stool specimens in one infected patient was tested positive for SARS-CoV-2 using RT-PCR (Holshue et al., 2020). The virus-laden feces could enter the sewer and got stuck and dried, the contaminated air then spread to other households through sewage pipes, or air carried virus could be sucked through the drain pipe of the index patient's unit and exhausted into the other units.
Although the exact survival time of SARS-CoV-2 in the environment is not clear, one study demonstrated that SARS-CoV can survive for as long as two weeks (Chan et al., 2011). Since the cases occurred in the same unit, the sewage disposal system could serve as a reservoir for the virus due to diarrheal excretion. Once the toilet flushed, virus-laden air could be generated and diffused in the sanitary plumbing system due to pressure fluctuations (Li et al., 2007; Johnson et al., 2013), resulting in contamination among other adjacent floors through the unsealed floor drain and connected exhaust pipe (Gormley et al., 2017). When the residents contacted with contamination areas or inhaled the contaminated air, they might be infected (Otter et al., 2016). Another contributing factor may be related to the time point of the event, low outdoor temperature (ranged between 11 °C and 18 °C), which prevented the residents from opening their windows for ventilation. What's more, while using the bathroom, residents tended to open the electric heater rather than the windows or exhaust fan due to the cold weather. Narrow space, dry drains and poor ventilation thus contributing to the increased concentration of the virus.
Identification of transmission route of the infection has important implications for preventing the rapid spread of virus in certain circumstances, especially in a relatively closed space, such as hospitals and residential buildings. In a confined space, the air can be easily polluted by the virus-laden particles. Different from droplets that cannot be spread over a long distance due to large particles, the air transmission mode observed in this study is considered as another important mechanism of transmission, which can be usually suspended in the atmosphere for a long time, more importantly, the contaminated air can spread over a long distance (MacIntyre et al., 2019). However, it should be noted that the long-distance air transmission could be possible only if a few important conditions exist, such as limited space, prolonged exposure, and high concentrations of virus in the air due to the poor ventilation, therefore, the author think that, though our study supported existence of this transmission mode in the novel coronavirus, the transmission can only occur opportunistically.
In terms of the architecture design, we suggest that design of the pipes should ensure that the water is always kept in the U-bend (Drinka et al., 2004). As for the older buildings, the government and the community should regularly dredge the drainage pipeline, prohibit the private modification of the exhaust port. The procedures conducted in those closed space may generate localized virus, which make the transmission through air become possible (Ge et al., 2020; Wax and Christian 2020). Recently, the SARS-CoV-2 outbreak among the passengers and crew of the quarantined Princess Cruises' ship, Diamond Princess caught global attention, the spread had been continuing even after taking the quarantine and other protective measures (Zhang et al., 2020a, Zhang et al., 2020b), some patients also reported no history of close contact with confirmed cases. The suitable environment for the transmission through air caused by closed space and central air-conditioning may account for the rapid infection. What's more, displacement ventilation, which means the air intakes are at low level and extracts are at high level, is also an efficient measure to prevent contaminated air (Bhagat and Linden 2020; Chen 2020).
Our study possessed a few strengths. The comprehensive field survey provided a timely evidence on the implications of possible transmission mode, and the simulation experiment provided a solid test for our hypothesis. On the other hand, several limitations should be noted. The sample size was too small (only nine cases) to illustrate the correlation of the transmission through air among the cases in this community. We cannot obtain the precise numbers of the residents who had direct or indirect contacts with index case in the elevator and the probability of the droplet transmission could not be absolutely excluded. Furthermore, the simulation experiments were conducted only in a few apartments due to limited consent of residents, which may affect the representativeness of the study. Only one time of measurement was conducted, which might have limited our ability to give more information, such as the standard deviation. Although the airflow experiment validated that strong airflow will be induced in the piping system by flushing the toilet and this might drive the spread of virus through the connected pipes among the bathroom of different floors. Due to the limitation of the anemometer, we cannot ascertain the direction of the wind flow. What we can confirm was that infection among families was caused by the transmission through the contaminated air of the piping system, however, the infection within the family might also existed as it is hard to get more specific detail information about the personal infection source. However, this did not affect our conclusion. Finally, it should be emphasized that reproducing the trace-gas experiment by using chloroform and carbon tetrachloride as tracers should only be used in unoccupied spaces. Before the residents move in, make sure that the concentration of the chemicals would not bring short-term or long-term influence to the health of the residents.
5. Conclusion
In summary, our epidemiologic analysis and the simulation experiments supported the possibility of SARS-CoV-2 transmission through air in this community. Dried-up seals of the floor-drain traps, chimney effect and retrofitting of exhaust vent have contributed to the spread of the virus from one household to another. This hypothesis should have important implications for the prevention and control of COVID-19 infection and similar respiratory viruses. In addition, a disconnected drain pipe and exhaust pipe for toilet should be recommended in the architectural design to help prevent cross-pollution.
Funding
The investigation was supported by The National Natural Science Foundation of China (grant numbers: 81773403 and 81741101 to Wei Zhu, grant numbers: 82041021 to Hualiang Lin), The Bill & Melinda Gates Foundation (grant numbers: INV-006371 to Hualiang Lin), and The Project for Key Medicine Discipline Construction of Guangzhou Municipality (grant numbers: 2017-2019-07 to Zhoubin Zhang). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
CRediT authorship contribution statement
Guozhen Lin: Supervision, Writing - original draft. Shiyu Zhang: Methodology, Writing - original draft. Yi Zhong: Data curation, Writing - original draft. Lin Zhang: Data curation. Siqi Ai: Formal analysis. Kuibiao Li: Data curation. Wenzhe Su: Data curation. Lan Cao: Data curation. Yuteng Zhao: Supervision. Fei Tian: Formal analysis. Jinrong Li: Visualization. Yinglin Wu: Visualization. Chongshan Guo: Writing - review & editing. Rongfei Peng: Writing - review & editing. Xinwei Wu: Writing - review & editing. Pingsheng Gan: Writing - review & editing. Wei Zhu: Conceptualization, Funding acquisition, Project administration, Writing - review & editing. Hualiang Lin: Conceptualization, Formal analysis, Methodology, Project administration, Writing - review & editing. Zhoubin Zhang: Conceptualization, Funding acquisition, Project administration, Writing - review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We acknowledge the contributions made by the staff from Guangzhou Center for Disease Control and Prevention, for help with the field survey and epidemiological investigation.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.atmosenv.2020.118083.
Contributor Information
Wei Zhu, Email: zhuw@gzcdc.org.cn.
Hualiang Lin, Email: linhualiang@mail.sysu.edu.cn.
Zhoubin Zhang, Email: gzcdczzb@gzcdc.org.cn.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Backer J.A., Klinkenberg D., Wallinga J. Incubation period of 2019 novel coronavirus (2019-nCoV) infections among travellers from Wuhan, China, 20–28 January 2020. Euro Surveill. 2020;25(5):2000062. doi: 10.2807/1560-7917.ES.2020.25.5.2000062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhagat R.K., Linden P.F. Displacement ventilation: a viable ventilation strategy for makeshift hospitals and public buildings to contain COVID-19 and other airborne diseases. Roy. Soc. Open Sci. 2020;7(9) doi: 10.1098/rsos.200680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth T.F., Kournikakis B., Bastien N., Ho J., Kobasa D., Stadnyk L., Li Y., Spence M., Paton S., Henry B. Detection of airborne severe acute respiratory syndrome (SARS) coronavirus and environmental contamination in SARS outbreak units. J. Infect. Dis. 2005;191(9):1472–1477. doi: 10.1086/429634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan J.F., Yuan S., Kok K.H., To K.K., Chu H., Yang J., Xing F., Liu J., Yip C.C., Poon R.W., Tsoi H.W., Lo S.K., Chan K.H., Poon V.K., Chan W.M., Ip J.D., Cai J.P., Cheng V.C., Chen H., Hui C.K., Yuen K.Y. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395(10223):514–523. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan K.H., Peiris J.S., Lam S.Y., Poon L.L., Yuen K.Y., Seto W.H. The effects of temperature and relative humidity on the viability of the SARS coronavirus. Adv. Virol. 2011:734690. doi: 10.1155/2011/734690. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q.Y. Can we migrate COVID-19 spreading risk? Front. Environ. Sci. Eng. 2020;15(3) doi: 10.1007/s11783-020-1328-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z., Chen X., Zheng Y., Sun J., Chen F., Shi L., Li F., Dong Y., Zhang Z. Air ingress analysis of chimney effect in the 200 MWe pebble-bed modular high temperature gas-cooled reactor. Ann. Nucl. Energy. 2017;106:143–153. [Google Scholar]
- Christian M.D., Loutfy M., McDonald L.C., Martinez K.F., Ofner M., Wong T., Wallington T., Gold W.L., Mederski B., Green K. Possible SARS coronavirus transmission during cardiopulmonary resuscitation. Emerg. Infect. Dis. 2004;10(2):287. doi: 10.3201/eid1002.030700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drinka P.J., Krause P., Nest L., Tyndall D. Report of an outbreak: nursing home architecture and influenza‐A attack rates: update. J. Am. Geriatr. Soc. 2004;52(5):847–848. doi: 10.1111/j.1532-5415.2004.52230_6.x. [DOI] [PubMed] [Google Scholar]
- Ge X.Y., Pu Y., Liao C.H., Huang W.F., Zeng Q., Zhou H., Yi B., Wang A.M., Dou Q.Y., Zhou P.C., Chen H.L., Liu H.X., Xu D.M., Chen X., Huang X. Evaluation of the exposure risk of SARS-CoV-2 in different hospital environment. Sustain. Cities Soc. 2020;61:102413. doi: 10.1016/j.scs.2020.102413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gormley M., Aspray T.J., Kelly D.A., Rodriguez-Gil C. Pathogen cross-transmission via building sanitary plumbing systems in a full scale pilot test-rig. PloS One. 2017;12(2) doi: 10.1371/journal.pone.0171556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan Y.X., Fang Z., Tang Z., Yuan J.P. Influence of the vent pipe diameter on the discharge capacity of a circuit vent building drainage system. Build. Serv. Eng. Technol. 2020;41(1):5–24. [Google Scholar]
- Hang J., Li Y.G., Jin R.Q. The influence of human walking on the flow and airborne transmission in a six-bed isolation room: tracer gas simulation. Build. Environ. 2014;77:119–134. doi: 10.1016/j.buildenv.2014.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser A., Counotte M.J., Margossian C.C., Konstantinoudis G., Low N., Althaus C.L., Riou J. Estimation of SARS-CoV-2 mortality during the early stages of an epidemic: a modeling study in Hubei, China, and six regions in Europe. PLoS Med. 2020;17(7) doi: 10.1371/journal.pmed.1003189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holshue M.L., DeBolt C., Lindquist S., Lofy K.H., Wiesman J., Bruce H., Spitters C., Ericson K., Wilkerson S., Tural A. First case of 2019 novel coronavirus in the United States. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowski T., Mlynarczyk M. AN IMPACT OF the efficient FUNCTIONING OF the ventilation and air-conditioning system ON THERMAL COMFORT OF the medical staff IN the OPERATING room. J. Ecol. Eng. 2016;17(5):114–119. [Google Scholar]
- Johnson D., Lynch R., Marshall C., Mead K., Hirst D. Aerosol generation by modern flush toilets. Aerosol. Sci. Technol. 2013;47(9):1047–1057. doi: 10.1080/02786826.2013.814911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R.M., Brosseau L.M. Aerosol transmission of infectious disease. J. Occup. Environ. Med. 2015;57(5):501–508. doi: 10.1097/JOM.0000000000000448. [DOI] [PubMed] [Google Scholar]
- Kim S.-H., Chang S.Y., Sung M., Park J.H., Bin Kim H., Lee H., Choi J.-P., Choi W.S., Min J.-Y. Extensive viable Middle East respiratory syndrome (MERS) coronavirus contamination in air and surrounding environment in MERS isolation wards. Clin. Infect. Dis. 2016;63(3):363–369. doi: 10.1093/cid/ciw239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q., Guan X., Wu P., Wang X., Zhou L., Tong Y., Ren R., Leung K.S.M., Lau E.H.Y., Wong J.Y., Xing X., Xiang N., Wu Y., Li C., Chen Q., Li D., Liu T., Zhao J., Liu M., Tu W., Chen C., Jin L., Yang R., Wang Q., Zhou S., Wang R., Liu H., Luo Y., Liu Y., Shao G., Li H., Tao Z., Yang Y., Deng Z., Liu B., Ma Z., Zhang Y., Shi G., Lam T.T.Y., Wu J.T., Gao G.F., Cowling B.J., Yang B., Leung G.M., Feng Z. Early transmission dynamics in wuhan, China, of novel coronavirus–infected pneumonia. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Leung G.M., Tang J., Yang X., Chao C., Lin J.Z., Lu J., Nielsen P.V., Niu J., Qian H. Role of ventilation in airborne transmission of infectious agents in the built environment-a multidisciplinary systematic review. Indoor Air. 2007;17(1):2–18. doi: 10.1111/j.1600-0668.2006.00445.x. [DOI] [PubMed] [Google Scholar]
- Lim T., Cho J., Kim B.S. The predictions of infection risk of indoor airborne transmission of diseases in high-rise hospitals: tracer gas simulation. Energy Build. 2010;42(8):1172–1181. [Google Scholar]
- Lindsley W.G., Blachere F.M., Beezhold D.H., Thewlis R.E., Noorbakhsh B., Othumpangat S., Goldsmith W.T., McMillen C.M., Andrew M.E., Burrell C.N., Noti J.D. Viable influenza A virus in airborne particles expelled during coughs versus exhalations. Influenza Other Respir. Viruses. 2016;10(5):404–413. doi: 10.1111/irv.12390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu K., Ai S., Song S., Zhu G., Tian F., Li H., Gao Y., Wu Y., Zhang S., Shao Z., Liu Q., Lin H. Population movement, city closure in Wuhan and geographical expansion of the 2019-nCoV pneumonia infection in China in January 2020. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Ning Z., Chen Y., Guo M., Liu Y., Gali N.K., Sun L., Duan Y., Cai J., Westerdahl D., Liu X., Xu K., Ho K.F., Kan H., Fu Q., Lan K. Aerodynamic analysis of SARS-CoV-2 in two Wuhan hospitals. Nature. 2020;582(7813):557–560. doi: 10.1038/s41586-020-2271-3. [DOI] [PubMed] [Google Scholar]
- Lou M., Liu S., Gu C., Hu H., Tang Z., Zhang Y., Xu C., Li F. The bioaerosols emitted from toilet and wastewater treatment plant: a literature review. Environ. Sci. Pollut. Res. Int. 2020 doi: 10.1007/s11356-020-11297-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacIntyre C.R., Das A., Chen X., Charitha S.D., Con D. Evidence of long-distance aerial Convection of Variola virus and implications for disease control. Viruses. 2019;12(1) doi: 10.3390/v12010033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mair-Jenkins J., Borges-Stewart R., Harbour C., Cox-Rogers J., Dallman T., Ashton P., Johnston R., Modha D., Monk P., Puleston R. Investigation using whole genome sequencing of a prolonged restaurant outbreak of Salmonella Typhimurium linked to the building drainage system, England, February 2015 to March 2016. Eur. Commun. Dis. Bull. 2017;22(49) doi: 10.2807/1560-7917.ES.2017.22.49.17-00037. 17-00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morawska L., Cao J. Airborne transmission of SARS-CoV-2: the world should face the reality. Environ. Int. 2020;139:105730. doi: 10.1016/j.envint.2020.105730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morawska L., Tang J.L.W., Bahnfleth W., Bluyssen P.M., Boerstra A., Buonanno G., Cao J.J., Dancer S., Floto A., Franchimon F., Haworth C., Hogeling J., Isaxon C., Jimenez J.L., Kurnitski J., Li Y.G., Loomans M., Marks G., Marr L.C., Mazzarella L., Melikov A.K., Miller S., Milton D.K., Nazaroff W., Nielsen P.V., Noakes C., Peccia J., Querol X., Sekhar C., Seppanen O., Tanabe S., Tellier R., Tham K.W., Wargocki P., Wierzbicka A., Yao M.S. How can airborne transmission of COVID-19 indoors be minimised? Environ. Int. 2020;142 doi: 10.1016/j.envint.2020.105832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong S.W.X., Tan Y.K., Chia P.Y., Lee T.H., Ng O.T., Wong M.S.Y., Marimuthu K. Air, surface environmental, and personal protective Equipment contamination by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) from a symptomatic patient. J. Am. Med. Assoc. 2020 doi: 10.1001/jama.2020.3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otter J., Donskey C., Yezli S., Douthwaite S., Goldenberg S., Weber D. Transmission of SARS and MERS coronaviruses and influenza virus in healthcare settings: the possible role of dry surface contamination. J. Hosp. Infect. 2016;92(3):235–250. doi: 10.1016/j.jhin.2015.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinotti F., Di Domenico L., Ortega E., Mancastroppa M., Pullano G., Valdano E., Boëlle P.-Y., Poletto C., Colizza V. Tracing and analysis of 288 early SARS-CoV-2 infections outside China: a modeling study. PLoS Med. 2020;17(7) doi: 10.1371/journal.pmed.1003193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Plos Medicine Editors Pandemic responses: Planning to neutralize SARS-CoV-2 and prepare for future outbreaks. PLoS Med. 2020;17(4) doi: 10.1371/journal.pmed.1003123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wax R.S., Christian M.D. Practical recommendations for critical care and anesthesiology teams caring for novel coronavirus (2019-nCoV) patients. Can. J. Anesth. 2020 doi: 10.1007/s12630-020-01591-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu P., Hao X., Lau E.H., Wong J.Y., Leung K.S., Wu J.T., Cowling B.J., Leung G.M. Real-time tentative assessment of the epidemiological characteristics of novel coronavirus infections in Wuhan, China, as at 22 January 2020. Euro Surveill. 2020;25(3):2000044. doi: 10.2807/1560-7917.ES.2020.25.3.2000044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu R., Ai S., Cai J., Zhang S., Qian Z., Zhang Y., Wu Y., Chen L., Tian F., Li H., Li M., Lin H. Predictive model and risk factors for case fatality of COVID-19: a cohort of 21,392 cases in Hubei, China. Innovation. 2020;1(2):100022. doi: 10.1016/j.xinn.2020.100022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao W., Wang M., Wei W., Wang J., Zhao J., Yi B., Li J. Detection of SARS-CoV and RNA on aerosol samples from SARS-patients admitted to hospital. Zhonghua liu xing bing xue za zhi= Zhonghua liuxingbingxue zazhi. 2004;25(10):882–885. [PubMed] [Google Scholar]
- Ye G., Lin H., Chen S., Wang S., Zeng Z., Wang W., Zhang S., Rebmann T., Li Y., Pan Z., Yang Z., Wang Y., Wang F., Qian Z., Wang X. Environmental contamination of SARS-CoV-2 in healthcare premises. J. Infect. 2020;81(2):e1–e5. doi: 10.1016/j.jinf.2020.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu I.T., Li Y., Wong T.W., Tam W., Chan A.T., Lee J.H., Leung D.Y., Ho T. Evidence of airborne transmission of the severe acute respiratory syndrome virus. N. Engl. J. Med. 2004;350(17):1731–1739. doi: 10.1056/NEJMoa032867. [DOI] [PubMed] [Google Scholar]
- Zhang L., Shen M., Ma X., Su S., Gong W., Wang J., Tao Y., Zou Z., Zhao R., Lau J.T.F., Li W., Liu F., Ye K., Wang Y., Zhuang G., Fairley C.K. What is required to prevent a second Major outbreak of SARS-CoV-2 upon Lifting quarantine in wuhan city, China. Innovation. 2020;1(1) doi: 10.1016/j.xinn.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S., Diao M., Yu W., Pei L., Lin Z., Chen D. Estimation of the reproductive number of Novel Coronavirus (COVID-19) and the probable outbreak size on the Diamond Princess cruise ship: a data-driven analysis. Int. J. Infect. Dis. 2020 doi: 10.1016/j.ijid.2020.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L., Yao M., Zhang X., Hu B., Li X., Chen H., Zhang L., Liu Y., Du M., Sun B., Jiang Y., Zhou K., Hong J., Yu N., Ding Z., Xu Y., Hu M., Morawska L., Grinshpun S.A., Biswas P., Flagan R.C., Zhu B., Liu W., Zhang Y. Breath-, air- and surface-borne SARS-CoV-2 in hospitals. J. Aerosol Sci. 2020 doi: 10.1016/j.jaerosci.2020.105693. 105693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., Zhao X., Huang B., Shi W., Lu R., Niu P., Zhan F., Ma X., Wang D., Xu W., Wu G., Gao G.F., Tan W. A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.