Abstract
After surgery or traumatic injury, corneal wound healing can cause a scarring response that stiffens the tissue and impairs ocular function. This fibrosis is caused in part by the activation of corneal keratocytes from a native mechanically quiescent state to an activated myofibroblastic state. This transformation is tied to signaling downstream of transforming growth factor-β1 (TGF-β1). Here, to better understand how biochemical and biophysical cues interact to regulate keratocyte activation and contractility, we cultured primary rabbit corneal keratocytes on flexible substrata of varying stiffness in the presence (or absence) of TGF-β1. Time-lapse fluorescence microscopy was used to assess changes in keratocyte morphology, as well as to quantify the dynamic traction stresses exerted by cells under different experimental conditions. In other experiments, keratocytes were fixed after 5 days of culture and stained for markers of both contractility and myofibroblastic activation. Treatment with TGF-β1 elicited distinct phenotypes on substrata of different stiffnesses. Cells on soft (1 kPa) gels formed fewer stress fibers and retained a more dendritic morphology, indicative of a quiescent keratocyte phenotype. Keratocytes cultured on stiff (10 kPa) gels or collagen-coated glass coverslips, however, had broad morphologies, formed abundant stress fibers, exhibited greater levels of α-smooth muscle actin (α-SMA) expression, and exerted larger traction forces. Confocal images of phospho-myosin light chain (pMLC) immunofluorescence, moreover, revealed stiffness-dependent differences in the subcellular distribution of actomyosin contractility, with pMLC localized at the tips of thin cellular processes in mechanically quiescent cells. Importantly, keratocytes cultured in the absence of TGF-β1 showed no stiffness-dependent differences in α-SMA immunofluorescence, suggesting that a stiff microenvironment alone is insufficient to induce myofibroblastic activation. Taken together, these data suggest that changes in ECM stiffness can modulate the morphology, cytoskeletal organization, and subcellular pattern of force generation in corneal keratocytes treated with TGF-β1.
Significance
Corneal wound healing can induce a fibrotic response that stiffens the tissue and disrupts its transparent optical properties. This fibrosis is strongly associated with the TGF-β1-dependent activation of quiescent corneal keratocytes into myofibroblasts. Here, we show that changes in ECM stiffness modulate the expression of α -SMA, as well as the morphology, cytoskeletal organization, and subcellular pattern of force generation in primary corneal keratocytes treated with TGF-β1. Importantly, keratocytes cultured in the absence of TGF-β1 showed no stiffness-dependent differences, suggesting that a stiff microenvironment alone is insufficient to induce the myofibroblastic activation of this cell type. Even so, these data highlight the importance of ECM stiffness in regulating the behavior of corneal keratocytes during the repair and regeneration of corneal tissue.
Introduction
The cornea is the transparent tissue located along the anterior surface of the eye. It provides much of the refractive power required to focus light toward the retina and consists of five distinct layers: the epithelium, Bowman’s layer, stroma, Descemet’s membrane, and endothelium (1). The stromal compartment accounts for the bulk of the corneal thickness and contains a highly ordered extracellular matrix (ECM) that is essential for the optical properties of the tissue (2,3). Type I collagen fibrils of precise diameter and spacing are organized into parallel arrays (or lamellae), which are stacked orthogonally within the corneal stroma (4). This lattice-like microstructure minimizes the scattering of light and endows the tissue with its optical transparency (5).
A population of cells, termed corneal keratocytes, is distributed within the stromal compartment and maintains the precise organization of this highly ordered ECM (1,6). In the healthy cornea, these cells are sandwiched between the lamellae of aligned collagen fibrils and form a relatively sparse network interconnected by numerous dendritic cellular extensions (5,7). Upon injury, however, numerous growth factors and cytokines, including TGF-β1, PDGF-BB, and FGF-2, are released into the stromal space (8). Among these soluble cues, TGF-β1 has been shown to promote the differentiation of quiescent keratocytes into myofibroblasts (9, 10, 11). Activated myofibroblasts undergo a phenotypic transition as they become less dendritic (12), form actin stress fibers (7), express α-smooth muscle actin (α-SMA) (12), secrete fibrotic ECM proteins (10,13,14), and exert contractile forces to repair the damaged tissue (15,16). This fibrotic response distorts the highly organized ECM and causes an opacification of the cornea and, in some cases, visual impairment (13,17). As a result, there is a clinical need to understand how different cues within the stromal microenvironment modulate the myofibroblastic differentiation and contractility of corneal keratocytes. These insights may impact ongoing research efforts to suppress chronic fibrosis in the cornea (18), as well as in other tissues like the skin and lung, in which persistent myofibroblastic activity is associated with hypertrophic dermal scarring (19) and idiopathic pulmonary fibrosis (20), respectively.
During wound healing, the mechanical properties of the cornea undergo significant changes, and the stromal compartment has been shown to stiffen substantially in the days after an injury (21,22). Abundant evidence indicates that cell-ECM interactions modulate the myofibroblastic activation of corneal keratocytes (23,24), and several studies have suggested that ECM stiffness may modulate keratocyte behavior in response to different growth factors (24,25). It is unclear, however, whether stiffness itself controls directly the mechanical phenotype of these cells. TGF-β1-treated keratocytes cultured within uncompressed 3D collagen gels, for instance, exhibit fewer stress fibers and express lower levels of α-SMA than cells cultured within compressed (and thereby stiffer) collagen matrices (24,25), but more than simply ECM stiffness is variable between these culture conditions. Compressed collagen matrices are stiffer than their uncompressed counterparts, but they also have a different effective collagen concentration (e.g., 2.5 vs. 100 mg/mL for uncompressed and compressed gels, respectively (26)), as well as a different fibril network density (27). It is difficult to parse the effects of ECM stiffness as an independent variable in these experiments because changes in ECM compliance are interlinked with changes in ECM concentration and topography.
Here, we used a polyacrylamide gel system to create flexible, collagen-coated substrata of different stiffnesses, which approximate the mechanical properties of either normal or fibrotic corneal tissue (21,22). These substrata were then functionalized with the same concentration of unpolymerized type I collagen, allowing us to decouple substratum stiffness from ECM composition and topography. We then plated these gels with primary corneal keratocytes isolated from rabbit eyes and cultured them in the presence and absence of TGF-β1 to determine how changes in ECM stiffness modulate the mechanical phenotype and myofibroblastic activation of these cells.
Materials and Methods
Fabrication and functionalization of polyacrylamide substrata
Polyacrylamide (PA) substrata were fabricated on glass coverslips 31 mm in diameter, as described previously (Fig. S1; (28,29)). Briefly, glass coverslips were treated with 0.1 N NaOH for 1 h at room temperature, rinsed three times with deionized water, and then dried with compressed N2. Some of these NaOH-treated coverslips were then incubated in a 2% (v/v) solution of dichlorodimethylsilane in toluene for 30 min at room temperature. Other NaOH-treated coverslips were placed in a 2% (v/v) solution of (3-aminopropyl)trimethyloxysilane (APTMS) (Sigma-Aldrich, St. Louis, MO) in acetone, incubated for 30 min at room temperature, and then rinsed in acetone. Once completely dry, each APTMS-treated coverslip was incubated in a 0.5% (v/v) solution of glutaraldehyde (Sigma-Aldrich, St. Louis, MO) in phosphate-buffered saline (PBS) for 30 min, rinsed in deionized water, and then dried with compressed N2.
A prepolymerization solution was created by mixing acrylamide (Bio-Rad, Hercules, CA) and bis-acrylamide (Bio-Rad, Hercules, CA) in water. Soft gels were fabricated using a preparation of 7.5% acrylamide and 3.0% bis-acrylamide, and stiff gels were fabricated using 12.5% acrylamide and 17.5% bis-acrylamide. A 10% solution of ammonium persulfate (Bio-Rad, Hercules, CA) and a 1:2000 dilution of N,N,N′,N′-tetramethylethylenediamine (Sigma-Aldrich, St. Louis, MO) were then added to initiate the polymerization reaction. A 40-μL droplet of the prepolymerization solution was then placed atop one of the APTMS-treated coverslips. This droplet was then covered by a dichlorodimethylsilane-treated coverslip to create a sandwich assembly. The mixture was allowed to polymerize between the glass coverslips for 30 min under vacuum. The top coverslip was then removed carefully using fine forceps. Each polymerized PA gel was then rinsed thoroughly with PBS.
To enable cell attachment, the surfaces of the PA substrata were functionalized with unpolymerized type I collagen through the use of the heterobifunctional cross-linker sulfosuccinimidyl 6-(4′-azido-2′-nitrophenylamino)hexanoate (sulfo-SANPAH; Pierce Biotechnology, Rockford, IL). Briefly, the gels were rinsed with a 50 mM solution of HEPES, treated with 1 mg/mL sulfo-SANPAH in sterile water, and then exposed to ultraviolet light for 10 min. The gels were then rinsed with 50 mM HEPES, treated with sulfo-SANPAH for a second time, and then rinsed three times with 50 mM HEPES to remove any excess cross-linker. The substrata were then incubated in a neutralized solution of 50 μg/mL bovine type I collagen (PureCol; Advanced Biomatrix, San Diego, CA) at 37°C for 30 min, as described previously (30,31). After incubation, the gels were rinsed with Dulbecco’s modified Eagle’s medium (DMEM; Sigma-Aldrich, St. Louis, MO) and, before plating cells, incubated with DMEM for at least 30 min at 37°C. In other experiments, to create collagen-coated glass coverslips, untreated coverslips 31 mm in diameter were incubated with a neutralized solution of 50 μg/mL bovine type I collagen at 37°C for 30 min and then rinsed twice with DMEM (30,31).
Mechanical testing of PA substrata
We used an RSA-G2 Dynamic Mechanical Analyzer (TA Instruments, New Castle, DE) to measure the mechanical properties of the soft and stiff PA preparations defined above (Fig. S2). We performed unconfined compression tests of gel specimens 10 mm in diameter, which were created by punching cylindrical plugs 10 mm in diameter from thin sheets of polymerized PA gel. A set of calipers were then used to measure the precise diameter and thickness of each specimen. All mechanical tests were performed at room temperature in a bath containing PBS. The storage modulus of the gel was measured during an isothermal strain sweep (32). The modulus evaluated at 2% strain was used to compare soft gel specimens, whereas the modulus at 0.2% strain was used to compare stiff gel specimens. For all specimens tested, these strain magnitudes remained in the linear elastic regime.
Cell culture and reagents
Primary rabbit corneal keratocytes (NRKs) were isolated from New Zealand white rabbit eyes, obtained from Pel Freez (Rogers, AR), and cultured as described previously (12,33,34). To maintain a quiescent keratocyte phenotype, the cells were cultured in serum-free (basal) media containing DMEM supplemented with 1% RPMI vitamin mix (Sigma-Aldrich, St. Louis, MO), 100 μM nonessential amino acids (Invitrogen, Carlsbad, CA), 100 μg/mL ascorbic acid (Sigma-Aldrich, St. Louis, MO), and 1% penicillin/streptomycin/amphotericin B (Lonza, Walkersville, MD) (25,33). First passage cells, cultured for 4–5 days in flasks before plating, were used in all experiments. Collagen-functionalized PA gels and collagen-coated glass coverslips were placed in a six-well plate, and primary NRKs were plated at 30,000 cells/mL in 2 mL of media, yielding a cell seeding density of ∼6300 cells/cm2. Cells were cultured in basal (serum-free) media in the presence or absence of 5 ng/mL recombinant TGF-β1 (Sigma-Aldrich, St. Louis, MO) for 4–5 days, with a media change after 48 h. In some experiments, 20 μM (−)-blebbistatin (Sigma-Aldrich, St. Louis, MO) was also added to the media to inhibit myosin II.
Immunofluorescence imaging
Samples were fixed in 4% paraformaldehyde in PBS for 20 min at room temperature, washed three times in PBS, and then permeabilized in 0.5% Triton X-100 in PBS for 30 min. Fixed cells were then blocked with either 1% bovine serum albumin fraction V (Equitech-Bio, Kerrville, TX) or 10% goat serum (Gibco) in PBS for 1 h at room temperature. Afterward, samples were washed three times and then incubated with primary antibody for 2 h at 37°C (or overnight on a shaker at 4°C). The following primary antibodies were used: anti-phospho-myosin light chain (pMLC) 2 (1:200 dilution) (Ser19; Cell Signaling, Danvers, MA) and anti-α-SMA (1:600 dilution) (Sigma-Aldrich; St. Louis, MO). After washing, the samples were incubated in Alexa-Fluor-conjugated secondary antibody (1:200 dilution) (Invitrogen, Carlsbad, CA) and/or Alexa Fluor 594 phalloidin (1:200 dilution) (Invitrogen, Carlsbad, CA), washed three times, and then incubated with 4'-6-diamidino-2-phenylindole (DAPI; 1:1000 dilution) (Sigma-Aldrich; St. Louis, MO) at room temperature for 20 min. Confocal microscopy of fixed samples was performed on a Zeiss LSM 800, controlled by Zen 2.3 (blue edition) software, using either a 20×, NA 0.8, Plan-Apochromat objective (Zeiss) or a 40×, NA 1.3, Oil DIC Plan-Apochromat objective (Zeiss).
Morphometric analysis of keratocyte geometry was performed in ImageJ using confocal fluorescence images of phalloidin staining. Each image was thresholded to generate a binarized image of cell morphologies (Fig. S3). The Analyze Particles plugin in ImageJ was then used to compute the projected area, Feret’s diameter, and aspect ratio of each cell. (Any cells located along the edge of the image were excluded from this analysis, unless the entire cell body was in view.) Feret’s diameter was used as a measure of cell length because it gives the maximal distance between any two points on the cell boundary.
Traction force microscopy
To perform traction force microscopy (TFM) experiments, a population of fluorescent polystyrene microspheres 200 nm in diameter (FluoSpheres, 0.2 μm, dark red; ThermoFisher, Waltham, MA) were suspended within the prepolymerization solution (0.04% solids) before fabricating the PA substrata. The embedded beads were used as fiducial markers to track gel deformations during time-lapse culture. Customized multiwell plates containing collagen-functionalized PA substrata with fluorescent microspheres were plated with NRK cells, as described above. After 48 h of culture, the plate was transferred to a humidified stage incubator on a Zeiss AxioObserver 7 microscope, equipped with a motorized stage and an ApoTome.2 structured illumination module, and cultured at 37°C with 5% CO2. Phase contrast and epifluorescence image stacks were captured at 30-min intervals for an additional 48 h of culture using a 20×, NA 0.8, Ph2 Plan-Apochromat objective (Zeiss) to capture both the bead displacements at the surface of the PA substratum as well as changes in cell morphology. At the end of the experiment, the NRKs were lysed using a 5% solution of Triton X-100 in PBS to capture the undeformed configuration of the substratum. We then used the Particle Image Velocimetry and Fourier Transform Traction Cytometry (FTTC) plugins in ImageJ (35) combined with the measured mechanical properties of the PA substrata to compute cellular traction stresses. Traction stresses were reconstructed at a regular grid spacing of ∼6.5 μm. The traction force at each grid location was calculated by multiplying the traction stress vector by the unit grid area (42 μm2). The net traction force for each cell was then computed by summing the magnitudes of the traction force acting at each grid point (36). Only isolated cells were used for this analysis, so cell boundaries were not considered when evaluating the net traction force.
Statistical analysis
Data represent means ± SD for at least three independent experiments. Statistical comparisons were made using either a Student’s t-test or a one- or two-way ANOVA followed by a Tukey post hoc test, with p-values as specified in the figure legends.
Results
Substratum stiffness modulates the morphology and cytoskeletal organization of cultured corneal keratocytes
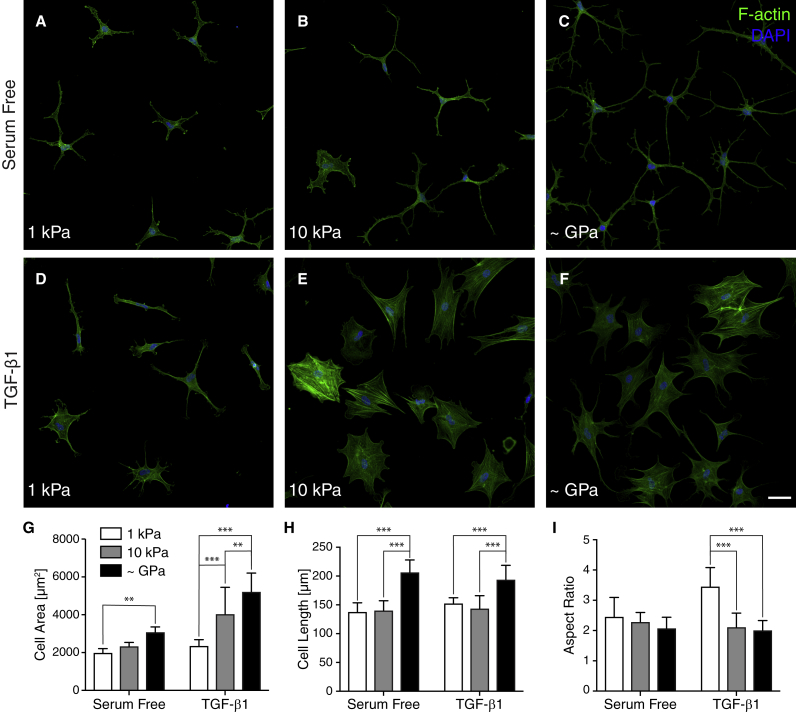
Primary corneal keratocytes (NRKs) were cultured on PA substrata of varying stiffness in the presence or absence of TGF-β1. The compliant PA substrata were functionalized with unpolymerized type I collagen and exhibited Young’s moduli of either 1 kPa or 10 (Fig. S2), approximating the mechanical properties of normal and fibrotic corneal tissue (21,22) Collagen-coated glass coverslips were used as controls because these substrata are the standard assay used to study the behavior of corneal keratocytes in 2D culture (10,30,31,37).
After 5 days of culture in serum-free media, NRKs cultured on glass coverslips exhibited a morphology characteristic of quiescent keratocytes in vivo: cells exhibited thin, highly branched dendritic processes extending outward from the cell body. On both soft (1 kPa) and stiff (10 kPa) substrata, the cultured NRKs retained this highly branched morphology in serum-free conditions, although cell area and cell length exhibited modest decreases with substratum stiffness (Fig. 1, A–C). In contrast, NRKs treated with TGF-β1 and cultured on either glass coverslips or stiff (10 kPa) PA substrata exhibited morphologies indicative of myofibroblastic activation. Cells were spread out with broad morphologies and clearly visible stress fibers (Fig. 1, E and F). On soft (1 kPa) substrata, however, the TGF-β1-treated NRKs exhibited fewer of these morphological hallmarks, as the cells exhibited less spreading, formed fewer stress fibers, and often had several thin cellular processes, characteristics indicative of a more quiescent keratocyte phenotype (Fig. 1 D). Quantitative morphometric analysis of cell geometry showed significant decreases in both cell length and cell area on substrata of decreasing stiffness (Fig. 1, G and H), particularly in the presence of TGF-β1, in which NRKs on soft substrata were indistinguishable from their counterparts in serum-free media. We also observed a significant increase in the aspect ratio of TGF-β1-treated cells on soft substrata, indicative of a more elongated morphology (Fig. 1 I).
Figure 1.
Substratum stiffness modulates the morphology of TGF-β1-treated corneal keratocytes. (A–F) Characteristic confocal fluorescence images of fixed corneal keratocytes cultured on either functionalized 1-kPa (A and D) or 10-kPa (B and E) PA substrata or collagen-coated glass (C and F) cover slips are shown. Before fixation, cells were cultured in either serum-free media (A–C) or media containing exogenous TGF-β1 (D–F) for 5 days. Keratocytes were stained using phalloidin (green) and DAPI (blue) to visualize F-actin and nuclei, respectively. (G–I) Quantification of projected cell area (G), cell length (H), and cell aspect ratio (I) are shown. Mean ± SD is shown for n = 9 substrates from five experimental replicates. A two-way ANOVA with a Tukey post hoc test was used to evaluate significance among groups. ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001. Scale bars, 50 μm.
Substratum stiffness regulates the expression of α-SMA only in the presence of TGF-β1
To determine whether the observed stiffness-dependent differences in morphology and stress fiber formation were associated with changes in myofibroblastic differentiation, we also stained cultured NRKs for α-SMA immunofluorescence (Fig. 2). In serum-free media, very few α-SMA-positive cells were detected (Fig. 2, A, C, and E), regardless of the stiffness of the underlying substratum, indicating extremely low levels of myofibroblasts (Fig. 2 G). In the presence of TGF-β1, however, we observed marked increases in the number of α-SMA-positive cells on both stiff PA substrata (Fig. 2 D) and collagen-coated glass coverslips (Fig. 2 F). But when NRKs were cultured on soft PA substrata, even in the presence of TGF-β1 (Fig. 2 B), we observed significantly lower levels of α-SMA immunofluorescence (Fig. 2 G). This result suggests that a sufficiently compliant microenvironment can suppress the myofibroblastic activation of corneal keratocytes.
Figure 2.
Substratum stiffness regulates the expression of α-SMA in corneal keratocytes treated with TGF-β1. (A–F) Characteristic confocal fluorescence images of cells cultured on either functionalized 1-kPa (A and B) or 10-kPa (C and D) PA substrata or collagen-coated glass (E and F) coverslips are shown. Before fixation, cells were cultured in either serum-free media (A, C, and E) or media containing exogenous TGF-β1 (B, D, and F) for 5 days. Keratocytes were stained for α-SMA immunofluorescence (green) as well as F-actin (red) and DAPI (blue). (G) The percentage of cells positive for α-SMA immunofluorescence is shown. Mean ± SD is shown for n = 16 substrates from four experimental replicates. A two-way ANOVA with a Tukey post hoc test was used to evaluate significance among groups. ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001. Scale bars, 50 μm.
Subcellular patterns of actomyosin contractility and force generation depend on substratum stiffness
Myofibroblasts are characterized by an elevated contractile phenotype and, during wound healing, exert the mechanical forces necessary to repair damaged tissue (18,38,39). In the cornea, these forces can distort the highly aligned ECM and compromise the transparent optical properties of the tissue. To determine how changes in ECM stiffness might influence the contractile behavior of cultured NRKs, we stained the cells for pMLC immunofluorescence, which identifies regions of active contractility (Fig. 3). In serum-free conditions, pMLC was localized primarily at the tips of dendritic cellular extensions within cells cultured on substrata of all stiffnesses (Fig. 3, A–C). In the presence of TGF-β1, however, keratocytes exhibited broad pMLC immunofluorescence on stiff PA substrata or collagen-coated glass coverslips, with pMLC colocalizing with the actin stress fibers that spanned the cell body (Fig. 3, E and F). But when TGF-β1-treated cells were cultured on soft PA substrata, they showed a strikingly different phenotype: pMLC was concentrated primarily at the distal tips of cellular extensions, with only low levels present in the main cell body (Fig. 3 D). This result was similar to the subcellular patterns of pMLC immunofluorescence observed within keratocytes cultured in serum-free media alone, suggesting that a compliant microenvironment helps maintain the quiescent mechanical phenotype associated with inactivated NRKs.
Figure 3.
Subcellular localization of pMLC immunofluorescence depends on substratum stiffness in TGF-β1-treated keratocytes. (A–F) Characteristic confocal fluorescence images of corneal keratocytes cultured in either serum-free media (A–C) or media containing exogenous TGF-β1 (D–F) on substrata of varying stiffnesses are shown. Keratocytes were stained with phalloidin (red), DAPI (blue), and anti-pMLC (green) to visualize F-actin, cell nuclei, and phosphorylated myosin light chain immunofluorescence, respectively. Scale bars, 25 μm. (Boxes with white dashed lines indicate image insets. Inset scale bars, 10 μm.)
Although pMLC immunofluorescence provides information regarding subcellular patterns of contractility, it is not necessarily a proxy for the magnitude and distribution of cellular traction forces. To determine how changes in substratum stiffness might modulate the traction forces exerted by cultured NRKs, we embedded fluorescent microspheres within our PA substrata and performed TFM experiments in the presence or absence of TGF-β1 (Fig. 4). After 4 days of culture, NRKs in serum-free media exhibited a mechanically quiescent phenotype on either soft or stiff PA substrata (Fig. 4, A and B). When treated with TGF-β1, however, the NRKs exhibited stiffness-dependent differences in contractile behavior (Fig. 4, C and D). Cells cultured on stiff PA substrata exerted high contractile forces (Fig. 4 D), as quantified by peak traction stress (Fig. 4 E) and net traction force (Fig. 4 F), a result consistent with their abundant stress fiber formation and elevated α-SMA immunofluorescence in stiff microenvironments (Fig. 2). A few outlier cells exhibited especially high traction stresses (Fig. 4, E and F), owing perhaps to relative differences in their levels of α-SMA expression, as a strong positive correlation has been reported between α-SMA expression and traction force magnitude for corneal fibroblasts cultured in different concentration of TGF-β1 (16). This TGF-β1-induced increase in contractile stress, however, was markedly reduced in NRKs cultured on soft PA substrata (Fig. 4 C).
Figure 4.
Compliant substrata suppress the traction forces exerted by TGF-β1-treated keratocytes. (A–D) Computed traction stresses for representative NRKs cultured on either 1-kPa (A and C) or 10-kPa (B and D) PA substrata are shown. Cells were cultured in either serum-free media (A and B) or media containing exogenous TGF-β1 (C and D) for 4 days. Scale bars, 25 μm. (E and F) Quantification of peak traction stress (E) and net traction force (F) after 96 h of culture is shown. Mean ± SD is shown for cells analyzed from five experimental replicates. A two-way ANOVA with a Tukey post hoc test was used to evaluate significance among groups. ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001.
These data, however, provide only a snapshot of the contractile behavior of keratocytes after 4 days of culture, so we also performed dynamic TFM experiments between 48 and 96 h of culture to determine how the patterns of traction stress evolve over time (Fig. 5). (This time-frame was selected to capture the transition of cultured NRKs to a myofibroblastic phenotype.) When treated with TGF-β1, NRKs cultured on soft substrata displayed relatively low traction stresses (<100 Pa) at 48 h. Between 48 and 96 h of culture, as these cells extended and retracted their numerous processes, the traction stress maps underwent dynamic spatial variations (Fig. 5, A–C; Video S1), but the largest stresses remained localized at the tips of these dynamic cellular extensions. The magnitude of the peak traction stress (Fig. 5 G) and net traction force (Fig. 5 H), however, did not change significantly with time. In contrast, TGF-β1-treated keratocytes cultured on stiff PA gels showed a striking increase in contractility over time (Fig. 5, D–F; Video S2). We observed significant increases in both the peak traction stress and net traction force exerted by cells between 48 and 96 h of culture (Fig. 5, G and H). But, strikingly, the spatial distribution of these traction stresses remained relatively static (Fig. 5, D–F), as the cells formed stable contacts with the underlying substratum and pulled with increasing levels of force.
Figure 5.
Substratum stiffness modulates time-varying patterns of NRK traction stress in the presence of TGF-β1. (A–F) Representative traction stresses exerted by an individual TGF-β1-treated NRK after 48 (A and D), 72 (B and E), or 96 (C and F) hours of culture are shown. NRKs were cultured on either 1-kPa (A–C) or 10-kPa (D–F) PA substrata. (Please note the difference in scale between A–C and D–F.) (G and H) Quantification of time-varying changes in peak traction stress (G) and net traction force (H) is shown. Mean ± SD is shown for cells analyzed from five experimental replicates. A two-way ANOVA with a Tukey post hoc test was used to evaluate significance among groups. ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001.
After 48 h of culture in TGF-β1-containing media, phase contrast (left panel) and epifluorescence (right panel) images were captured at 30 min intervals for an additional 48 h of culture to visualize changes in cell morphology, as well as the displacements of fluorescent microspheres embedded within the PA gel. Tracked bead motion was used to compute the traction forces.
After 48 h of culture in TGF-β1-containing media, phase contrast (left panel) and epifluorescence (right panel) images were captured at 30 min intervals for an additional 48 h of culture to visualize changes in cell morphology, as well as the displacements of fluorescent microspheres embedded within the PA gel. Tracked bead motion was used to compute the traction forces.
Inhibition of myosin II contractility disrupts stiffness-dependent differences in keratocyte behavior
To determine how changes in myosin II contractility might affect the observed stiffness-dependent differences in NRK activation, we cultured keratocytes on substrata of varying stiffness in the presence of both TGF-β1 and the nonmuscle myosin II inhibitor blebbistatin. As described above (Fig. 1), NRKs treated with TGF-β1 alone displayed a morphology redolent of mechanical quiescence when cultured on soft substrata or of myofibroblastic activation when cultured in stiff microenvironments (Fig. 6, A–C). When the keratocytes were treated with blebbistatin simultaneously, however, these stiffness-dependent differences diminished significantly. In the absence of myosin II-based contractility, NRKs treated with TGF-β1 displayed a dendritic morphology on substrata of all stiffnesses, with numerous thin projections, and showed no evidence of stress fiber formation, behaviors consistent with a more quiescent keratocyte phenotype (Fig. 6, D–F). Quantification of keratocyte geometry showed decreased stiffness-dependent differences in cell area, length, and aspect ratio when NRKs were treated simultaneously with blebbistatin (Fig. 6, G–I). On stiff PA substrata and collagen-coated glass coverslips, the amount of cell spreading decreased significantly in the presence of blebbistatin, but interestingly, if the NRKs were cultured on soft substrata, myosin II inhibition did not significantly alter cell area, suggesting that soft microenvironments promote a mechanically quiescent phenotype in the corneal stroma.
Figure 6.
Inhibition of myosin II contractility disrupts stiffness-dependent differences in NRK morphology. (A–F) Characteristic confocal fluorescence images of NRKs cultured on either functionalized 1-kPa (A and D) or 10-kPa (B and E) PA substrata or collagen-coated glass (C and F) coverslips are shown. Before fixation, cells were cultured for 5 days in media containing either TGF-β1 (A–C) or TGF-β1 with 20 μM blebbistatin (D–F). Keratocytes were stained using phalloidin (green) and DAPI (blue) to visualize F-actin and nuclei, respectively. (G–I) Quantification of projected cell area (G), cell length (H), and cell aspect ratio (I) is shown. Mean ± SD is shown for n = 8 substrates from three experimental replicates. A two-way ANOVA with a Tukey post hoc test was used to evaluate significance among groups. ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001. Scale bars, 50 μm.
We also performed TFM experiments to determine how treatment with blebbistatin influences the distribution of TGF-β1-induced traction forces (Fig. 7). As described above (Fig. 4), NRKs treated with TGF-β1 alone generated significantly larger traction forces when cultured on stiff, as opposed to soft, PA substrata (Fig. 7, A and B). These stiffness-dependent differences, however, were disrupted completely when the keratocytes were treated with blebbistatin (Fig. 7, C and D). Interestingly, in the presence of both TGF-β1 and blebbistatin, the stresses became localized primarily within the tips of thin cellular extensions, a result in striking agreement with the traction forces exerted by NRKs cultured in serum-free conditions. Quantification of peak traction stress and net traction force corroborated these observations (Fig. 7, E and F). Inhibiting myosin II-based contractility produced a significant drop in peak traction stress independently of whether the cells were cultured on stiff or soft PA substrata (Fig. 7 E). It is interesting to note that the peak traction stresses exerted by keratocytes treated with TGF-β1 and blebbistatin (14 ± 4 or 22 ± 7 Pa for NRKs cultured on soft or stiff substrata, respectively (Fig. 7 E)) were similar to those exerted by cells cultured in serum-free media (25 ± 17 or 25 ± 13 Pa for cells cultured on soft or stiff substrata, respectively (Fig. 4 E)). Similar trends were observed when comparing net traction force (Fig. 7 F). Taken together, these results suggest that inhibiting myosin II-based contractility is sufficient to disrupt the stiffness-dependent myofibroblastic activation of cultured corneal keratocytes.
Figure 7.
Myosin II inhibition blocks stiffness-dependent differences in NRK traction force. (A–D) Computed traction stresses for NRKs cultured on either 1-kPa (A and C) or 10-kPa (B and D) PA substrata after 96 h of culture are shown. Cells were treated with either TGF-β1 alone (A and B) or TGF-β1 and blebbistatin (C and D) for 4 days. Cell outlines are indicated by white dashed lines. Scale bars, 25 μm. (E and F) Quantification of peak traction stress (E) and net contractile force (F) after 96 h of culture is shown. Mean ± SD is shown for cells from n = 5 substrates from three experimental replicates. A two-way ANOVA with a Tukey post hoc test was used to evaluate significance among groups. ∗∗∗p < 0.001.
Discussion
In this study, we used a PA gel system to study the effects of substratum stiffness on the myofibroblastic differentiation and contractility of cultured primary corneal keratocytes in the presence of TGF-β1. PA substrata were fabricated with a small-strain Young’s modulus similar to that of either normal (1 kPa) or fibrotic (10 kPa) corneal tissue (21,22) and were functionalized with unpolymerized type I collagen because keratocytes are situated between lamellae of collagen fibrils within the corneal stroma (4). Importantly, primary keratocytes were cultured in defined serum-free media, which has been shown, in the absence of exogenous growth factors, to maintain the mechanically quiescent phenotype observed in vivo (12). Culture in serum-containing media promotes the activation of keratocytes into corneal fibroblasts (12).
NRKs cultured on stiff (10 kPa) substrata exhibited broad morphologies, had an abundance of stress fibers, and expressed elevated levels of α-SMA when treated with TGF-β1, a result consistent with those of previous studies using keratocytes cultured on either tissue-culture plastic or collagen-coated glass coverslips (10,40). On soft (1 kPa) substrata, however, cultured NRKs expressed low levels of α-SMA and formed thin cellular extensions with very few observable stress fibers, even in the presence of TGF-β1, characteristics redolent of the mechanical quiescence observed of inactivated keratocytes (12). Importantly, keratocytes cultured in the absence of TGF-β1 did not exhibit stiffness-dependent differences in myofibroblastic activation. On substrata of all stiffnesses, NRKs cultured in serum-free conditions retained a dendritic morphology and expressed negligible amounts of α-SMA, suggesting that a stiff microenvironment alone is insufficient to induce the myofibroblastic differentiation of corneal keratocytes. This result differs from that of Dreier and colleagues, who, using a similar PA gel system, reported that corneal fibroblasts cultured in serum-containing media express stiffness-dependent differences of α-SMA in both the presence and absence of TGF-β1 (41). Similar TGF-β1-independent changes in the expression of α-SMA on substrata of different stiffnesses have been reported for dermal fibroblasts (42), valvular interstitial cells (43), and lung fibroblasts (44). Grinnell and colleagues have argued that fibroblast behavior depends on the tension state of cell-matrix interactions, with low tension promoting a more dendritic morphology and quiescent phenotype (45,46). Inactivated keratocytes do not generate significant traction forces, as compared with corneal fibroblasts (16), and likely experience a more low-tension environment. Keratocytes also do not form stress fibers. Even on rigid substrata, keratocytes exhibit predominately cortical F-actin (12), whereas fibroblasts of numerous cell types have been shown to form abundant stress fibers, as well as prominent focal adhesions, when cultured on stiff substrata (37,47). We suspect that these biophysical differences influence the dissimilar response of keratocytes and fibroblasts to changes in substratum stiffness.
We also observed a clear relationship between substratum stiffness and keratocyte morphology, especially when cells were treated with TGF-β1. In other studies, cultured fibroblasts have been shown to exhibit morphological differences on substrata of varying stiffness, with cells typically rounding up on the most compliant substrata (48,49). In our experiments, however, TGF-β1-treated keratocytes exhibited a decrease in cell area on soft substrata but had an elongated, dendritic morphology (not a rounded one). This geometry, redolent of quiescent keratocytes maintained in serum-free conditions (12,25), may indicate further differences in the mechanical phenotype of corneal keratocytes and fibroblasts (34,50).
Consistent with this idea, we observed a strong correlation between levels of α-SMA immunofluorescence and cellular traction force. In previous work, elevated α-SMA expression has been shown to promote increased traction stress in cultured corneal fibroblasts (16), but it remained unclear how changes in ECM stiffness might affect this relationship. In our experiments, TGF-β1-treated keratocytes cultured on compliant PA substrata exhibited lower levels of both α-SMA immunofluorescence and cellular traction force as compared with their counterparts on either stiff PA substrata or collagen-coated glass coverslips. We report peak traction stresses of ∼200–300 Pa for TGF-β1-treated keratocytes on stiff PA substrata, values consistent with those reported previously for corneal fibroblasts in the presence of TGF-β (16). Even so, NRKs maintained in defined serum-free media exerted substantially lower traction stresses, further evidence of a phenotypic difference between keratocytes and fibroblasts (7,25,51).
Disrupted contractility can block the myofibroblastic activation of both corneal fibroblasts (52) and primary corneal keratocytes (40). In addition, Rho kinase inhibition has been shown to reduce stress fiber formation in keratocytes cultured within 3D collagen gels (25). Consistent with these results, we demonstrated that direct inhibition of myosin II is sufficient to disrupt any stiffness-dependent differences in TGF-β1-induced myofibroblastic differentiation. We also observed substantially different subcellular distributions of both contractile stress and pMLC immunofluorescence on substrata of varying stiffnesses. MLC phosphorylation is regulated in part by the Rho pathway (53), but it is unclear how these subcellular differences in actomyosin contractility influence the downstream expression of α-SMA and control the myofibroblastic activation of corneal keratocytes.
Previous studies have suggested that signaling pathways involving the transcriptional activator, Yes-associated protein (YAP) (54) and those downstream of integrin-based focal adhesions (47) may regulate stiffness-dependent differences in myofibroblastic activation. Treatment with TGF-β1 is thought to induce myofibroblastic differentiation via the activation and nuclear localization of Smad3 (38), but it is not necessarily clear how these mechanotransductive pathways interact with signaling downstream of TGF-β1 (55). Recent work using renal fibroblasts, however, has suggested that stiffness-dependent differences in the nuclear localization of YAP modulate Smad2/3 nuclear accumulation and, consequently, levels of myofibroblastic differentiation (56). It would be interesting to determine whether a similar mechanotransductive pathway regulates the TGF-β1-driven activation of corneal keratocytes.
Finally, in addition to TGF-β1, numerous other growth factors are released into the stromal compartment and elicit changes in keratocyte behavior after an injury (8). Both PDGF-BB and FGF-2, for instance, have been shown to stimulate the proliferation of corneal keratocytes (57). PDGF-BB also promotes a migratory keratocyte phenotype (58), whereas FGF-2 modulates the expression of keratan sulfate proteoglycans, a class of secreted molecules that help maintain corneal transparency (59). It is unclear how changes in ECM stiffness regulate the response of corneal keratocytes to these other soluble growth factors. Determining how biophysical and biochemical cues are integrated to control keratocyte behavior will be essential to developing new treatment strategies for the repair and regeneration of corneal tissue.
Conclusions
Taken together, these data suggest that ECM stiffness plays a key role in the myofibroblastic activation and contractility of primary corneal keratocytes. Even in the presence of TGF-β1, a compliant microenvironment promotes a dendritic cell morphology and suppresses both stress fiber formation and levels of α-SMA immunofluorescence in primary keratocytes cultured in defined serum-free media. Changes in substratum stiffness can also modulate subcellular distributions of contractile force and actomyosin contractility, and inhibition of myosin II disrupts any stiffness-dependent differences in keratocyte behavior. Importantly, we show that primary keratocytes cultured in the absence of TGF-β1 exhibit no clear stiffness-dependent behaviors, a result that suggests a stiff microenvironment alone is not sufficient to promote the myofibroblastic differentiation of corneal keratocytes. Even so, these data further highlight the importance of ECM stiffness in regulating the mechanical phenotype of keratocytes during corneal wound healing.
Author Contributions
V.D.V., W.M.P., and D.W.S. conceived the study and designed the experiments. D.P.M. conducted all experiments and analyzed all experimental data. M.M.-M. and P.B.K. assisted with the isolation of primary corneal keratocytes. D.P.M. and J.M.H. performed the mechanical testing of PA substrata. All authors discussed and interpreted results. D.P.M., V.D.V., W.M.P., and D.W.S. wrote the manuscript with feedback from all authors.
Acknowledgments
The authors thank Taylor Ware for the use of the Dynamic Mechanical Analyzer.
This work was supported by the National Institutes of Health grants R01 EY030190, R01 EY013322, and P30 EY030413, as well as an unrestricted grant from Research to Prevent Blindness.
Editor: Guy Genin.
Footnotes
Supporting Material can be found online at https://doi.org/10.1016/j.bpj.2020.08.040.
Supporting Material
References
- 1.Fini M.E. Keratocyte and fibroblast phenotypes in the repairing cornea. Prog. Retin. Eye Res. 1999;18:529–551. doi: 10.1016/s1350-9462(98)00033-0. [DOI] [PubMed] [Google Scholar]
- 2.Hogan M.J., Alvarado J.A., Weddell J.E. Saunders; Philadelphia, PA: 1971. Histology of the Human Eye: An Atlas and Textbook. [Google Scholar]
- 3.Meek K.M. Corneal collagen-its role in maintaining corneal shape and transparency. Biophys. Rev. 2009;1:83–93. doi: 10.1007/s12551-009-0011-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Meek K.M., Boote C. The use of X-ray scattering techniques to quantify the orientation and distribution of collagen in the corneal stroma. Prog. Retin. Eye Res. 2009;28:369–392. doi: 10.1016/j.preteyeres.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 5.Hassell J.R., Birk D.E. The molecular basis of corneal transparency. Exp. Eye Res. 2010;91:326–335. doi: 10.1016/j.exer.2010.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nishida T., Yasumoto K., Desaki J. The network structure of corneal fibroblasts in the rat as revealed by scanning electron microscopy. Invest. Ophthalmol. Vis. Sci. 1988;29:1887–1890. [PubMed] [Google Scholar]
- 7.Jester J.V., Barry P.A., Cavanagh H.D. Corneal keratocytes: in situ and in vitro organization of cytoskeletal contractile proteins. Invest. Ophthalmol. Vis. Sci. 1994;35:730–743. [PubMed] [Google Scholar]
- 8.Torricelli A.A.M., Singh V., Wilson S.E. The corneal epithelial basement membrane: structure, function, and disease. Invest. Ophthalmol. Vis. Sci. 2013;54:6390–6400. doi: 10.1167/iovs.13-12547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kurosaka H., Kurosaka D., Tanaka Y. Transforming growth factor-beta 1 promotes contraction of collagen gel by bovine corneal fibroblasts through differentiation of myofibroblasts. Invest. Ophthalmol. Vis. Sci. 1998;39:699–704. [PubMed] [Google Scholar]
- 10.Jester J.V., Huang J., Cavanagh H.D. Transforming growth factor(beta)-mediated corneal myofibroblast differentiation requires actin and fibronectin assembly. Invest. Ophthalmol. Vis. Sci. 1999;40:1959–1967. [PubMed] [Google Scholar]
- 11.Nakamura K. Interaction between injured corneal epithelial cells and stromal cells. Cornea. 2003;22(7 Suppl):S35–S47. doi: 10.1097/00003226-200310001-00006. [DOI] [PubMed] [Google Scholar]
- 12.Jester J.V., Barry-Lane P.A., Petroll W.M. Induction of alpha-smooth muscle actin expression and myofibroblast transformation in cultured corneal keratocytes. Cornea. 1996;15:505–516. [PubMed] [Google Scholar]
- 13.Chen C., Michelini-Norris B., Schultz G. Measurement of mRNAs for TGFss and extracellular matrix proteins in corneas of rats after PRK. Invest. Ophthalmol. Vis. Sci. 2000;41:4108–4116. [PubMed] [Google Scholar]
- 14.Blalock T.D., Duncan M.R., Schultz G.S. Connective tissue growth factor expression and action in human corneal fibroblast cultures and rat corneas after photorefractive keratectomy. Invest. Ophthalmol. Vis. Sci. 2003;44:1879–1887. doi: 10.1167/iovs.02-0860. [DOI] [PubMed] [Google Scholar]
- 15.Roy P., Petroll W.M., Jester J.V. Exertion of tractional force requires the coordinated up-regulation of cell contractility and adhesion. Cell Motil. Cytoskeleton. 1999;43:23–34. doi: 10.1002/(SICI)1097-0169(1999)43:1<23::AID-CM3>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 16.Chen J., Li H., Wang J.H. Alpha-smooth muscle actin expression enhances cell traction force. Cell Motil. Cytoskeleton. 2007;64:248–257. doi: 10.1002/cm.20178. [DOI] [PubMed] [Google Scholar]
- 17.Boote C., Du Y., Meek K.M. Quantitative assessment of ultrastructure and light scatter in mouse corneal debridement wounds. Invest. Ophthalmol. Vis. Sci. 2012;53:2786–2795. doi: 10.1167/iovs.11-9305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hinz B. Myofibroblasts. Exp. Eye Res. 2016;142:56–70. doi: 10.1016/j.exer.2015.07.009. [DOI] [PubMed] [Google Scholar]
- 19.Desmoulière A., Chaponnier C., Gabbiani G. Tissue repair, contraction, and the myofibroblast. Wound Repair Regen. 2005;13:7–12. doi: 10.1111/j.1067-1927.2005.130102.x. [DOI] [PubMed] [Google Scholar]
- 20.King T.E., Jr., Pardo A., Selman M. Idiopathic pulmonary fibrosis. Lancet. 2011;378:1949–1961. doi: 10.1016/S0140-6736(11)60052-4. [DOI] [PubMed] [Google Scholar]
- 21.Thomasy S.M., Raghunathan V.K., Murphy C.J. Elastic modulus and collagen organization of the rabbit cornea: epithelium to endothelium. Acta Biomater. 2014;10:785–791. doi: 10.1016/j.actbio.2013.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Raghunathan V.K., Thomasy S.M., Murphy C.J. Tissue and cellular biomechanics during corneal wound injury and repair. Acta Biomater. 2017;58:291–301. doi: 10.1016/j.actbio.2017.05.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miron-Mendoza M., Koppaka V., Petroll W.M. Techniques for assessing 3-D cell-matrix mechanical interactions in vitro and in vivo. Exp. Cell Res. 2013;319:2470–2480. doi: 10.1016/j.yexcr.2013.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Petroll W.M., Miron-Mendoza M. Mechanical interactions and crosstalk between corneal keratocytes and the extracellular matrix. Exp. Eye Res. 2015;133:49–57. doi: 10.1016/j.exer.2014.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lakshman N., Petroll W.M. Growth factor regulation of corneal keratocyte mechanical phenotypes in 3-D collagen matrices. Invest. Ophthalmol. Vis. Sci. 2012;53:1077–1086. doi: 10.1167/iovs.11-8609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Petroll W.M., Lakshman N. Fibroblastic transformation of corneal keratocytes by Rac inhibition is modulated by extracellular matrix structure and stiffness. J. Funct. Biomater. 2015;6:222–240. doi: 10.3390/jfb6020222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abou Neel E.A., Cheema U., Nazhat S.N. Use of multiple unconfined compression for control of collagen gel scaffold density and mechanical properties. Soft Matter. 2006;2:986–992. doi: 10.1039/b609784g. [DOI] [PubMed] [Google Scholar]
- 28.Lee K., Chen Q.K., Nelson C.M. Matrix compliance regulates Rac1b localization, NADPH oxidase assembly, and epithelial-mesenchymal transition. Mol. Biol. Cell. 2012;23:4097–4108. doi: 10.1091/mbc.E12-02-0166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Simi A.K., Anlaş A.A., Nelson C.M. A soft microenvironment protects from failure of midbody abscission and multinucleation downstream of the EMT-promoting transcription factor Snail. Cancer Res. 2018;78:2277–2289. doi: 10.1158/0008-5472.CAN-17-2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miron-Mendoza M., Graham E., Petroll W.M. The role of thrombin and cell contractility in regulating clustering and collective migration of corneal fibroblasts in different ECM environments. Invest. Ophthalmol. Vis. Sci. 2015;56:2079–2090. doi: 10.1167/iovs.15-16388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kivanany P.B., Grose K.C., Petroll W.M. An in vitro model for assessing corneal keratocyte spreading and migration on aligned fibrillar collagen. J. Funct. Biomater. 2018;9:54. doi: 10.3390/jfb9040054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boothby J.M., Ware T.H. Dual-responsive, shape-switching bilayers enabled by liquid crystal elastomers. Soft Matter. 2017;13:4349–4356. doi: 10.1039/c7sm00541e. [DOI] [PubMed] [Google Scholar]
- 33.Jester J.V., Ho-Chang J. Modulation of cultured corneal keratocyte phenotype by growth factors/cytokines control in vitro contractility and extracellular matrix contraction. Exp. Eye Res. 2003;77:581–592. doi: 10.1016/s0014-4835(03)00188-x. [DOI] [PubMed] [Google Scholar]
- 34.Lakshman N., Kim A., Petroll W.M. Characterization of corneal keratocyte morphology and mechanical activity within 3-D collagen matrices. Exp. Eye Res. 2010;90:350–359. doi: 10.1016/j.exer.2009.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Martiel J.-L., Leal A., Théry M. Measurement of cell traction forces with ImageJ. Methods Cell Biol. 2015;125:269–287. doi: 10.1016/bs.mcb.2014.10.008. [DOI] [PubMed] [Google Scholar]
- 36.Maruthamuthu V., Sabass B., Gardel M.L. Cell-ECM traction force modulates endogenous tension at cell-cell contacts. Proc. Natl. Acad. Sci. USA. 2011;108:4708–4713. doi: 10.1073/pnas.1011123108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jester J.V., Huang J., Cavanagh H.D. TGFbeta induced myofibroblast differentiation of rabbit keratocytes requires synergistic TGFbeta, PDGF and integrin signaling. Exp. Eye Res. 2002;75:645–657. doi: 10.1006/exer.2002.2066. [DOI] [PubMed] [Google Scholar]
- 38.Hinz B. Formation and function of the myofibroblast during tissue repair. J. Invest. Dermatol. 2007;127:526–537. doi: 10.1038/sj.jid.5700613. [DOI] [PubMed] [Google Scholar]
- 39.Hinz B. The myofibroblast: paradigm for a mechanically active cell. J. Biomech. 2010;43:146–155. doi: 10.1016/j.jbiomech.2009.09.020. [DOI] [PubMed] [Google Scholar]
- 40.Chen J., Guerriero E., SundarRaj N. Rho-mediated regulation of TGF-β1- and FGF-2-induced activation of corneal stromal keratocytes. Invest. Ophthalmol. Vis. Sci. 2009;50:3662–3670. doi: 10.1167/iovs.08-3276. [DOI] [PubMed] [Google Scholar]
- 41.Dreier B., Thomasy S.M., Murphy C.J. Substratum compliance modulates corneal fibroblast to myofibroblast transformation. Invest. Ophthalmol. Vis. Sci. 2013;54:5901–5907. doi: 10.1167/iovs.12-11575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Achterberg V.F., Buscemi L., Hinz B. The nano-scale mechanical properties of the extracellular matrix regulate dermal fibroblast function. J. Invest. Dermatol. 2014;134:1862–1872. doi: 10.1038/jid.2014.90. [DOI] [PubMed] [Google Scholar]
- 43.Ma H., Killaars A.R., Anseth K.S. Myofibroblastic activation of valvular interstitial cells is modulated by spatial variations in matrix elasticity and its organization. Biomaterials. 2017;131:131–144. doi: 10.1016/j.biomaterials.2017.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Asano S., Ito S., Hasegawa Y. Matrix stiffness regulates migration of human lung fibroblasts. Physiol. Rep. 2017;5:e13281. doi: 10.14814/phy2.13281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Grinnell F., Ho C.-H., Skuta G. Dendritic fibroblasts in three-dimensional collagen matrices. Mol. Biol. Cell. 2003;14:384–395. doi: 10.1091/mbc.E02-08-0493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rhee S., Grinnell F. Fibroblast mechanics in 3D collagen matrices. Adv. Drug Deliv. Rev. 2007;59:1299–1305. doi: 10.1016/j.addr.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Goffin J.M., Pittet P., Hinz B. Focal adhesion size controls tension-dependent recruitment of α-smooth muscle actin to stress fibers. J. Cell Biol. 2006;172:259–268. doi: 10.1083/jcb.200506179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Georges P.C., Janmey P.A. Cell type-specific response to growth on soft materials. J. Appl. Physiol. 2005;98:1547–1553. doi: 10.1152/japplphysiol.01121.2004. [DOI] [PubMed] [Google Scholar]
- 49.Solon J., Levental I., Janmey P.A. Fibroblast adaptation and stiffness matching to soft elastic substrates. Biophys. J. 2007;93:4453–4461. doi: 10.1529/biophysj.106.101386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Grinnell F., Petroll W.M. Cell motility and mechanics in three-dimensional collagen matrices. Annu. Rev. Cell Dev. Biol. 2010;26:335–361. doi: 10.1146/annurev.cellbio.042308.113318. [DOI] [PubMed] [Google Scholar]
- 51.Roy P., Petroll W.M., Jester J.V. An in vitro force measurement assay to study the early mechanical interaction between corneal fibroblasts and collagen matrix. Exp. Cell Res. 1997;232:106–117. doi: 10.1006/excr.1997.3511. [DOI] [PubMed] [Google Scholar]
- 52.Vishwanath M., Ma L., Petroll W.M. Modulation of corneal fibroblast contractility within fibrillar collagen matrices. Invest. Ophthalmol. Vis. Sci. 2003;44:4724–4735. doi: 10.1167/iovs.03-0513. [DOI] [PubMed] [Google Scholar]
- 53.Tomasek J.J., Gabbiani G., Brown R.A. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat. Rev. Mol. Cell Biol. 2002;3:349–363. doi: 10.1038/nrm809. [DOI] [PubMed] [Google Scholar]
- 54.Liu F., Lagares D., Tschumperlin D.J. Mechanosignaling through YAP and TAZ drives fibroblast activation and fibrosis. Am. J. Physiol. Lung Cell. Mol. Physiol. 2015;308:L344–L357. doi: 10.1152/ajplung.00300.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Piersma B., Bank R.A., Boersema M. Signaling in fibrosis: TGF-β, WNT, and YAP/TAZ converge. Front. Med. (Lausanne) 2015;2:59. doi: 10.3389/fmed.2015.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Szeto S.G., Narimatsu M., Yuen D.A. YAP/TAZ are mechanoregulators of TGF-β-Smad signaling and renal fibrogenesis. J. Am. Soc. Nephrol. 2016;27:3117–3128. doi: 10.1681/ASN.2015050499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Etheredge L., Kane B.P., Hassell J.R. The effect of growth factor signaling on keratocytes in vitro and its relationship to the phases of stromal wound repair. Invest. Ophthalmol. Vis. Sci. 2009;50:3128–3136. doi: 10.1167/iovs.08-3077. [DOI] [PubMed] [Google Scholar]
- 58.Kim A., Lakshman N., Petroll W.M. Growth factor regulation of corneal keratocyte differentiation and migration in compressed collagen matrices. Invest. Ophthalmol. Vis. Sci. 2010;51:864–875. doi: 10.1167/iovs.09-4200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Long C.J., Roth M.R., Funderburgh J.L. Fibroblast growth factor-2 promotes keratan sulfate proteoglycan expression by keratocytes in vitro. J. Biol. Chem. 2000;275:13918–13923. doi: 10.1074/jbc.275.18.13918. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
After 48 h of culture in TGF-β1-containing media, phase contrast (left panel) and epifluorescence (right panel) images were captured at 30 min intervals for an additional 48 h of culture to visualize changes in cell morphology, as well as the displacements of fluorescent microspheres embedded within the PA gel. Tracked bead motion was used to compute the traction forces.
After 48 h of culture in TGF-β1-containing media, phase contrast (left panel) and epifluorescence (right panel) images were captured at 30 min intervals for an additional 48 h of culture to visualize changes in cell morphology, as well as the displacements of fluorescent microspheres embedded within the PA gel. Tracked bead motion was used to compute the traction forces.