Abstract
Single-particle tracking offers a method to interrogate the organization of transmembrane proteins by measuring their mobilities within a cell’s plasma membrane. Using this technique, the diffusion characteristics of the Duffy antigen (DARC), glycophorin A, band 3, and GLUT1 were compared under analogous conditions on intact human erythrocyte membranes. Microscopic diffusion coefficients revealed that the vast majority of all four transmembrane proteins exhibit very restricted movement but are not completely immobile. In fact, only 12% of GLUT1 resolved into a highly mobile subpopulation. Macroscopic diffusion coefficients and compartment sizes were also similar for all four proteins, with movements confined to the approximate dimensions of the “corrals” of the cortical spectrin cytoskeleton. Taken together, these data suggest that almost the entire populations of all four transmembrane proteins are immobilized by either the incorporation within large multiprotein complexes or entrapment within the protein network of the cortical spectrin cytoskeleton.
Significance
The architecture and activities of transmembrane proteins are crucial in maintaining cellular homeostasis. Utilizing single-particle tracking, the diffusion characteristics of Duffy antigen, glycophorin A, band 3, and GLUT1 in human erythrocyte membranes were determined. Results from these studies are in agreement with models of the erythrocyte membrane in which the majority of these proteins are associated with the major membrane-spanning protein complexes.
Introduction
The proper organization of transmembrane proteins is critical for the functioning of mammalian cells. These transmembrane proteins often form multiprotein complexes with other proteins that can be stabilized by interactions between extracellular, intracellular, and transmembrane domains (1, 2, 3). The interplay among these protein complexes along with their association with the cellular cytoskeleton (4) regulates numerous processes, including signaling events, ion transport, and cell motility, among others (5). Understanding the architecture of these transmembrane proteins and their complexes is therefore critical to understanding how these cells function.
Because of its ease of membrane isolation and compositional similarity to membranes from other cells (6,7), the erythrocyte membrane has served as a surrogate for the study of many other plasma membranes (7, 8, 9). As a consequence of contributions from many labs (8, 9, 10, 11, 12), a model of the red blood cell membrane (RBCM) has emerged that comprises two major membrane-spanning protein complexes anchored to separate cortical cytoskeleton complexes via multiple bridging proteins that can include ankyrin, adducin, protein 4.1, protein 4.2, and dematin (8, 9, 10). The first of these two prominent complexes (junctional complex) is thought to primarily contain the membrane-spanning proteins band 3; glycophorins A, C, and D, stomatin; GLUT1; Kx; Kell; and Duffy antigen tethered to the spectrin-actin cytoskeleton via adducin, protein 4.1, protein 4.2, and dematin, whereas the second (ankyrin complex) is believed to contain membrane-spanning proteins band 3, glycophorins A and B, Rh antigens, CD47, and LW, connected to the spectrin-actin cytoskeleton via ankyrin and protein 4.2 (8, 9, 10). Although this organizational structure is always depicted pictorially as static, in reality, all of the above complexes are stabilized by weak interactions that should allow proteins to associate and dissociate on a short timescale. As a consequence, a more accurate understanding of a plasma membrane’s structure not only requires a picture of each protein’s most frequent location in the mosaic of membrane complexes but also a measure of its probability of dissociating and diffusing elsewhere.
In an effort to better understand the dynamics of the RBCM’s transmembrane proteins, we have utilized single-particle-tracking methods to compare the motile properties of several prominent membrane-spanning proteins by tracking their diffusion trajectories as a function of time (13). Band 3 (AE1 and SLC4A1) was included because it constitutes the most prominent anchor of the spectrin-actin cytoskeleton to the membrane (8, 9, 10). Glycophorin A also was chosen because it serves as the most prominent member of a large group of heavily glycosylated membrane-spanning proteins (8,9). The GLUT1 was further added because it exemplifies the large group of nutrient transporters required for red blood cell (RBC) function (8,9), and the Duffy antigen was included because it can represent the many low copy number blood group antigens expressed on RBC surfaces (8,9,12). To monitor the diffusion of these proteins in intact RBCs, each protein was specifically labeled with a fluorophore via either a covalently binding ligand or a single domain antibody known as a nanobody (14). Herein, we report the diffusion properties of the Duffy antigen for the first time, to our knowledge, and compare its mobility with that of band 3, glycophorin A, and GLUT1 measured under exactly the same conditions. Our data suggest that all four major membrane-spanning proteins are comprised of subpopulations that diffuse at different but largely constrained rates, suggesting that our models of the RBCM must be viewed as the most probable arrangement of membrane proteins and that many other minor but real constellations of membrane proteins must also exist.
Materials and Methods
Materials
Isolation and characterization of recombinant camel anti-human glycophorin A VHH (15) and anti-human Duffy antigen VHH (16) nanobodies have been described previously. The photoactivatable biotin-conjugated ATB-BMPA (N-[2-[2-[2-[(N-Biotinyl-caproylamino)-ethoxy)ethoxyl]-4-[2-(trifluoromethyl)-3H-diazirin-3-yl]benzoyl]-1,3-bis(mannopyranosyl-4-yloxy)-2-propylamine) was purchased from Toronto Research Chemicals (North York, Ontario, Canada). Streptavidin-conjugated Qdot 525 and streptavidin-conjugated Alexa Fluor 488 were purchased from Invitrogen (Waltham, MA). EZ-Link N-hydroxysuccinimide (NHS)-Biotin reagent, horseradish peroxidase detection 3,3'-diaminobenzidine (DAB) Substrate Kit, streptavidin-conjugated horseradish peroxidase, and all other materials were purchased from Thermo Fisher Scientific (Waltham, MA).
Biotinylation and mass spectrometry characterization of anti-Duffy antigen nanobody
Anti-human glycophorin A VHH nanobody (16) was biotinylated with the EZ-Link NHS-Biotin reagent according to manufacturer’s instructions at a 10- or 20-fold excess biotin reagent. Biotinylated nanobody was dialyzed in phosphate-buffered saline (PBS) to remove unbound reagent and then concentrated to ∼6 mg/mL. Diluted nanobody samples were analyzed via matrix-assisted laser desorption/ionization (MALDI). Mass differences between the biotinylated antibodies were utilized to determine the average number of biotins covalently linked to the nanobody.
Preparation of human erythrocytes
Blood samples from healthy volunteers were drawn after informed consent and approval by Purdue University’s Institutional Review Board. The samples were centrifuged at 1000 × g, and both the plasma and white cell fractions (buffy coat) were discarded. The remaining erythrocytes were washed three times with PBS (pH 7.4) that lacked glucose. Cells then were diluted to 5% hematocrit in PBS.
SDS-PAGE and Western blotting
Erythrocytes were prepared at 5% hematocrit in SDS-PAGE sample buffer containing 5% β-mercaptoethanol and separated on a 10% SDS-PAGE gels via electrophoresis. Proteins were transferred to a nitrocellulose membrane, blocked overnight in Tris-buffered saline containing 0.05% Tween 20 and 5% powdered milk, and washed three times in Tris-buffered saline. The blot was incubated with a 1:800 dilution of the anti-Duffy antigen nanobody for 1 h at room temperature. After being washed three times in Tris-buffered saline, the blot was incubated with a 1:15,000 dilution of streptavidin-conjugated horseradish peroxidase for 30 min at room temperature. Bands were imaged after using a chromogenic horseradish peroxidase detection DAB Substrate Kit per manufacturer’s instructions.
Labeling of Duffy antigen, glycophorin A, band 3, and GLUT1 in intact human erythrocytes with fluorescent tracers
For labeling of the Duffy antigen, erythrocytes at 5% hematocrit in PBS buffer containing 5 mM glucose were incubated with various concentrations of the biotinylated anti-Duffy antigen nanobody for 1 h at room temperature. Cells were then washed three times in PBS with glucose and incubated with streptavidin-conjugated Qdot 525 for 30 min. Cells were washed three times in PBS with glucose to remove any remaining unbound fluorophore. Glycophorin A was labeled with a recombinant camel anti-human glycophorin A VHH nanobody fragment and streptavidin-conjugated Qdot 515 exactly as previously described (17). Band 3 was covalently labeled with 4,4′-diisothiocyano-2,2′-stilbenedisulfonic acid biotin followed by streptavidin-conjugated Qdot 525 exactly as described previously (18). Lastly, GLUT1 was covalently labeled with biotinylated ATB-BMPA and streptavidin-conjugated Alexa Fluor 488 exactly as described previously (19).
Immunofluorescence microscopy for Duffy-antigen-labeled erythrocytes
Erythrocytes were collected and labeled as described above. Cells then were diluted to 0.1–0.2% hematocrit in PBS containing 0.1% bovine serum albumin and allowed to settle on a polylysine-coated coverslip for 10 min. Cells were then imaged on an Olympus FV1000 confocal microscope (Center Valley, PA).
Single-particle tracking via fluorescence video microscopy
Single-particle tracking was performed as described previously (17, 18, 19) and graphically depicted in Fig. 1. Briefly, labeled cells were added to a polylysine-coated coverslip located within a custom-built chamber and allowed to settle for 10 min. Unattached cells were washed away, and the chamber was filled with 500 μL PBS containing 0.1% bovine serum albumin. The exposed upper surface was imaged via oblique angle light sheet fluorescence microscopy on an Olympus IX-71 inverted microscope maintained at 37°C using a dual micro-channel plate (MCP) intensified, cooled CCD camera (XR/Turbo-120Z; Stanford Photonics, Palo Alto, CA). To obtain the oblique angle fluorescence images, an argon ion excitation laser with a 488-nm emission (Newport, Irvine, CA) was expanded and filtered using a 488/10-nm line width bandpass filter (Chroma Technology, Rockingham, VT). This light was directed toward the Olympus total internal fluorescence (TIRF) PlanApo 1.45 NA 100× oil immersed microscope objective parallel but off the optical axis through a 500-nm dichroic mirror (Chroma Technology, Rockingham, VT). The excitation beam was set just outside of the condition for total internal reflection, which allows for a deeper excitation while simultaneously reducing background fluorescence. Data were collected on a random selection of erythrocytes at 30 and 120 frames per second (FPS) for a total of 1000 frames. Only particles whose trajectories could be followed for at least 40 frames were chosen for analysis because of the possibility of large localization errors that could affect the calculation of diffusion coefficients from trajectories with fewer frames (20). In general, 200–700 erythrocytes were analyzed in each sample.
Figure 1.
Overview of single-particle tracking. A generalized protocol for obtaining single-particle tracking data is depicted along with representative particle tracks and mean-square displacement (MSD) versus time plots for the three major motion types: 1) simple Brownian diffusion, 2) diffusion within a compartment and “hopping” between compartments, and 3) confined diffusion within a cytoskeletal corral. Cartoons depicting these motion types are shown superimposed on electron micrographs from Liu et al. (34). Lastly, the particle motion is fitted to a modified diffusion equation where DM is the asymptotic macroscopic coefficient, Dμ is the short-time diffusion coefficient, L is the average spacing between barriers (compartment size), and k is a positive integer.
Analysis of mobility
For each video frame, the fluorophore position was determined using a Gaussian distribution of thresholded intensities as described previously (21). Briefly, a kernel was developed from a Gaussian distribution that was then cross correlated with each video frame of the label of interest (Fig. 1, step 3). For each frame, the cross correlated function was thresholded, and the center of mass of the thresholded correlation intensity was used as the particle’s position. Fluorophore mobility was calculated from the mean-square displacement (MSD) of each trajectory between consecutive frames (22,23). The MSD for each time interval was calculated according to the following formula:
where N is the total number of frames in the sequence, n is a positive integer that determines the time increment, j is a positive integer, δt is the time resolution (i.e., video frame time), and x(j δt + n δt) and y(j δt + n δt) describe the particle position after a time interval nδt after starting at position x(j δt), y(j δt).
Short-time diffusion coefficients (Dμ), asymptotic macroscopic coefficients (DM), and the average spacing between barriers (compartment size) were determined by fitting the MSD versus time through an infinite array of partially permeable barriers (24) using custom-designed software (Wintract and Winsat) originally developed by the Kusumi lab (22,25). The modified diffusion equation utilized is as follows:
where DM is the asymptotic macroscopic coefficient, Dμ is the short-time diffusion coefficient, L is the average spacing between barriers (compartment size), and k is a positive integer. The compartment size value represents a compartment of unknown shape but a radially averaged diameter of that value. The log of each diffusion coefficient for each trajectory was plotted in a histogram. Thus, keeping the variance as a free parameter, the distribution patterns were observed to follow a log normal distribution as found previously by numerous groups (26, 27, 28, 29, 30, 31). Mean values of these parameters were then calculated by fitting Gaussian curves using the “second derivative search for hidden peak” methodology within OriginPro 8 software (Northampton, MA). The number of distributions required to fit each histogram was chosen using the F-test with a 95% confidence level. To empirically gauge localization errors, which can affect the calculated diffusion coefficients in a positive or negative direction, and determine lower bounds for Dμ and DM, fixed cells were analyzed using the described microscopy settings and analysis. Both Dμ- and DM-values were found to be ∼5 × 10−14 μm2/s, which then were used as the lowest limit for determining the diffusion coefficients.
Results and Discussion
Biotinylation, characterization, and optimization of an anti-Duffy antigen nanobody for use in single-particle tracking
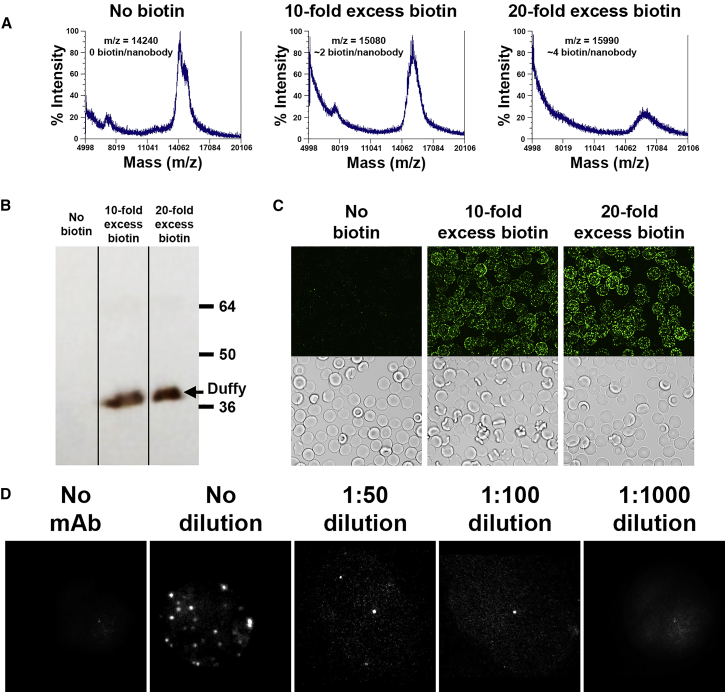
To gain insight into the dynamics of the erythrocyte’s major membrane-spanning protein complexes, we performed single-particle tracking experiments on Duffy antigen, glycophorin A, band 3, and GLUT1 under identical conditions. The design, preparation, and optimization of fluorescent labels for single-particle tracking of glycophorin A (17), band 3 (18), and GLUT1 (19) have been previously reported; however, single-particle tracking of Duffy antigen has never been described, to our knowledge. To specifically label Duffy antigen on intact erythrocytes, a well-characterized nanobody against human Duffy antigen (16) was covalently labeled with biotin using methods developed for labeling glycophorin A (15). As shown in Fig. 2 A, the reaction of the nanobody with a 10-fold molar excess of EZ-Link NHS-Biotin reagent (MW = 454 Da) yielded a conjugate containing ∼2 biotins covalently attached per nanobody, whereas a 20-fold excess yielded ∼4 biotins per nanobody. To ensure that the biotinylation of the nanobody did not impact its ability to bind Duffy antigen, a Western blot of whole erythrocytes was performed to determine whether the labeled nanobody could still bind Duffy antigen. As shown in Fig. 2 B, the biotinylated nanobody recognized only a single protein band at the expected MW of the Duffy antigen, confirming both its affinity and selectivity for Duffy antigen. Next, the biotinylated nanobody was incubated with intact human erythrocytes followed by labeling with streptavidin-conjugated Qdot 525. As visualized in the fluorescent micrographs of Fig. 2 C, erythrocytes were readily labeled with the biotinylated nanobody regardless of the density of biotinylation. Because both preparations yielded similar results, the nanobody labeled with 10-fold excess biotinylation reagent was employed for all further experiments.
Figure 2.
Characterization and optimization of biotinylated anti-Duffy antigen nanobody for single-particle tracking. (A) Anti-Duffy antigen nanobody was incubated with multiple concentrations of the reactive NHS-linked biotin. MALDI mass spectrometry was performed to determine the average mass and number of biotin molecules covalently attached to each nanobody. (B) A Western blot was performed using lysed erythrocytes and probed with the biotinylated nanobodies followed by visualization with streptavidin-conjugated horse radish peroxidase. (C) Erythrocytes were treated with biotinylated nanobodies, labeled with streptavidin-conjugated Qdot 525, and visualized via fluorescent microscopy. (D) Erythrocytes were treated with various dilutions of the biotinylated nanobodies, labeled with streptavidin-conjugated Qdot 525, and visualized via fluorescent microscopy. A representative image of a single erythrocyte at each dilution level is shown.
To perform these single-particle tracking studies with the least possible perturbation of the membrane and to ensure that the streptavidin-fluorescent probe would be highly unlikely to cross-link two biotinylated proteins present on the same cell, a probe concentration was sought when only a single or no labeled proteins were present per erythrocyte. Under these conditions, the chances of labeling and cross-linking two adjacent copies of the same desired membrane-spanning protein are very small, and with trajectories on 200–700 different cells measured for each histogram, the data would not be expected to be biased even if a few cross-linked proteins were to have been monitored. It is also highly improbable that intercellular cross-linking could occur because each cell is immobilized on a glass slide, and the physical distances between cells were always too great for streptavidin to bridge. Therefore, various RBC labeling concentrations were tested to identify conditions that would place no more than a single quantum dot per cell. Thus, as shown in Fig. 2 D, many fluorophores per cell were observed when an undiluted nanobody was utilized. Moreover, a 1:50 dilution of nanobody still exhibited >1 fluorophore on many erythrocytes, whereas essentially all cells labeled with a 1:100 dilution were either unlabeled or bound a single fluorophore. Because further dilution yielded very few labeled cells, a 1:100 dilution was used for all further Duffy antigen experiments.
Analysis of Duffy antigen mobility
With labeling conditions established, the next task was to select the optimal video microscopy frame rates for characterizing the motions of the different diffusing membrane-spanning proteins. Historically, a frame rate of 120 FPS has been utilized for monitoring the diffusion of erythrocyte membrane proteins (17, 18, 19); however, collection of data sets using different frame rates allows for obtaining multiple perspectives on protein motion. The reason for this can be best understood by envisioning two populations of a membrane protein in which one population is tethered to the spectrin-actin cytoskeleton and thus has restricted movement around a point in space, whereas the second population is free to diffuse across the membrane. At very fast frame rates in which each protein’s movement is captured over a very short time increment, a highly restricted protein may move the same distance or frame as a protein traversing across the membrane even though the restricted protein may simply be moving away for a single frame and then back to its starting position during the second frame. Thus, at this high frame rate, the two populations would appear to exhibit the same microscopic diffusion coefficient (Dμ). However, when the time increment for each frame is significantly lengthened (i.e., a slower frame rate such as 30 FPS), the same restricted protein will appear to have moved very little because it will have only moved back and forth around its point of origin, i.e., essentially “freezing” it in place from the camera’s perspective. In contrast, the freely diffusing protein will have continued to move away from its original position during each successive frame, thus yielding a faster Dμ-value. Because the macroscopic diffusion coefficient (DM) takes into account the entire captured movement of the protein, it should not be influenced as strongly by such changes in the frame rate.
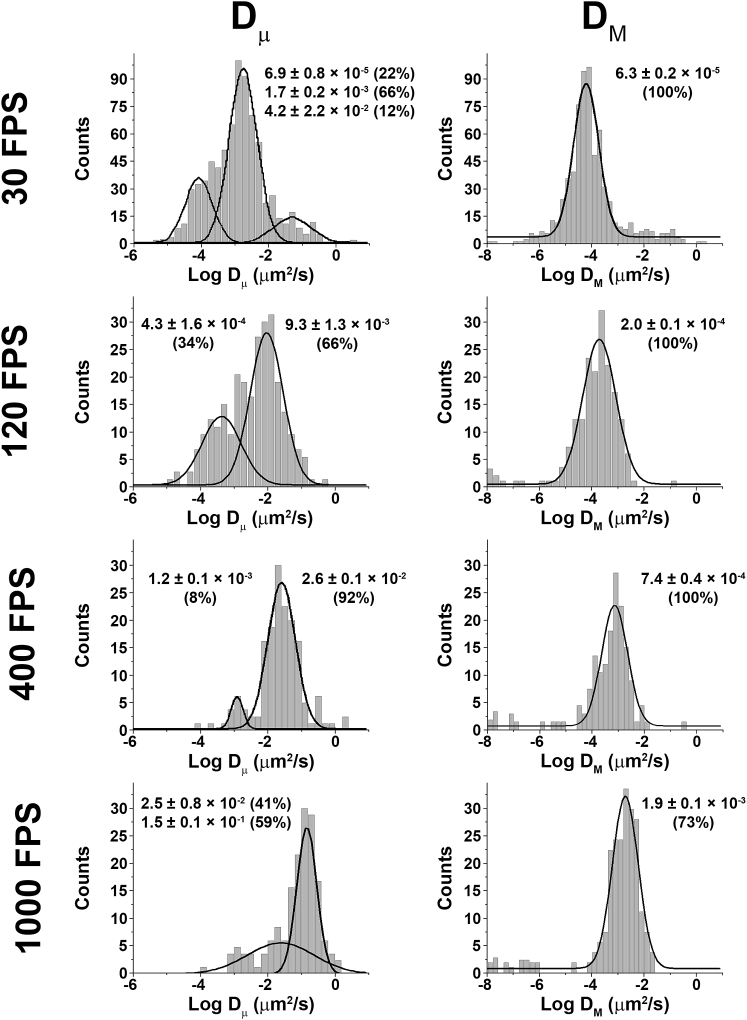
In practice, when a protein with known restricted and mobile subpopulations, such as GLUT1 (19), is imaged at different frame rates, the differences in Dμ-values become apparent. As shown in Fig. 3, at 30 FPS, GLUT1 Dμ-values separate into three different populations, with the slowest moving population centered at 7 × 10−5 μm2/s. However, at 1000 FPS, the slowest population is 2.5 × 10−2 μm2/s, i.e., nearly a three-log difference. This difference occurs because of the fact that the protein is still able to move short distances even though it is probably tethered to a fixed point on the cortical spectrin cytoskeleton. After considerable analysis of different video frame rates, we selected the standard 120 FPS and the slower 30 FPS to obtain the most complete understanding of the mobilities of the four selected membrane-spanning proteins.
Figure 3.
The effect of the frame rate on microscopic and macroscopic diffusion coefficients. Erythrocytes were incubated with biotinylated ATB-BMPA and streptavidin-conjugated Alexa Fluor 488 to selectively and covalently label GLUT1 proteins. Then, cells with a single fluorophore were imaged at various FPS, and the microscopic (Dμ) and macroscopic (DM) diffusion coefficients were determined.
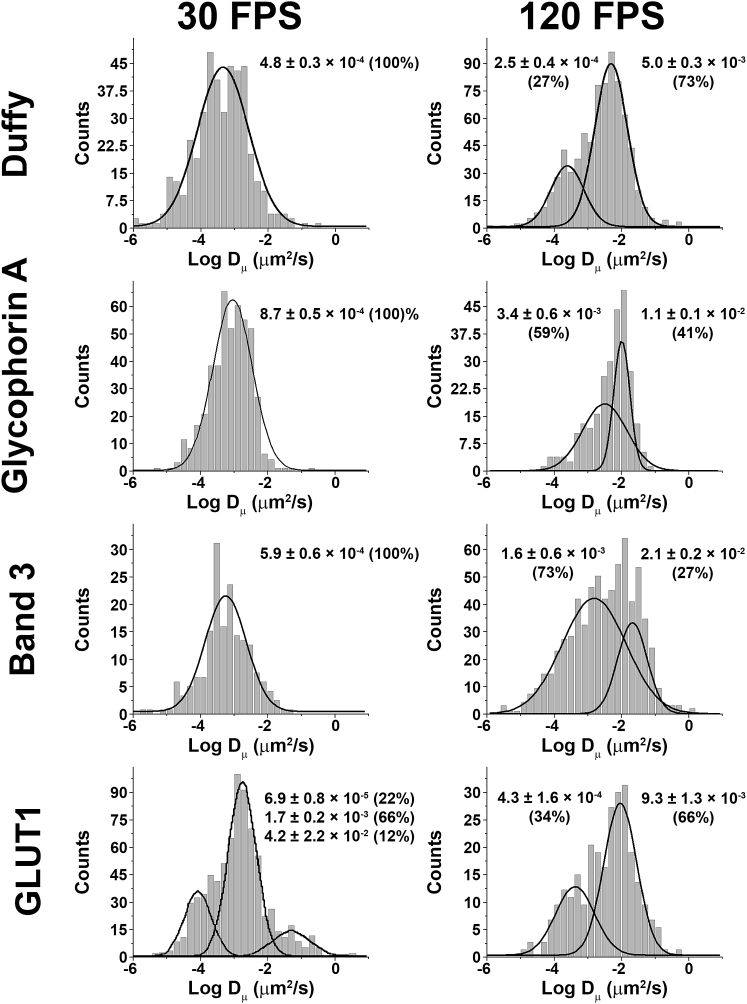
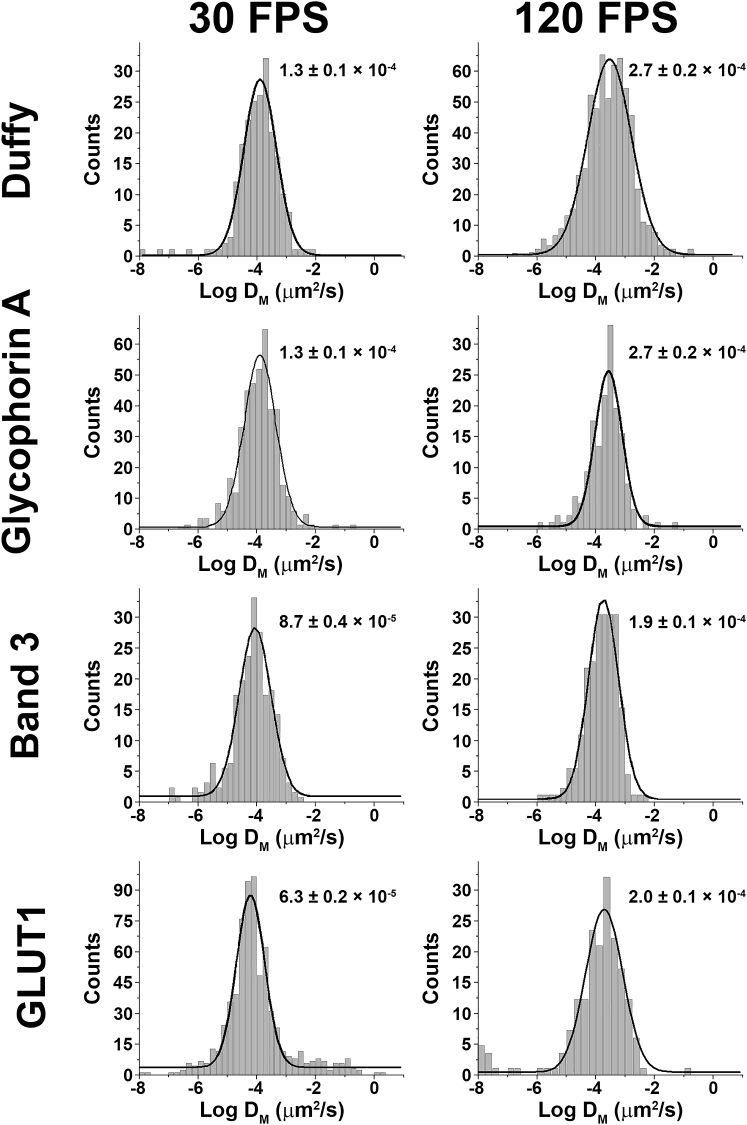
As shown in Fig. 4 and Table 1, at 30 FPS, the Dμ histogram for the Duffy antigen yielded a single peak centered at 4.8 × 10−4 μm2/s, whereas at 120 FPS, two peaks were resolved at 2.5 × 10−4 and 5.0 × 10−3 μm2/s. Macroscopic diffusion coefficients (DM) for Duffy antigen were 1.3 × 10−4 and 2.7 × 10−4 μm2/s at 30 and 120 FPS, respectively (Fig. 5; Table 1). Overall, these diffusion coefficients suggest strong immobilization for nearly all copies of the protein because no freely mobile population was observed. This conclusion is consistent with our understanding of the Duffy antigen, namely that it is primarily associated with the junctional complex (8,9) through its interaction with protein 4.1R (32). When considered with the low expression level of Duffy antigen (<1 Duffy antigen per junctional complex (8)), the very low Dμ-values strongly suggest that nearly all copies of Duffy antigen are probably bound in a junctional complex.
Figure 4.
Comparison of microscopic diffusion rates of Duffy antigen, glycophorin A, band 3, and GLUT1 at 30 and 120 frames per second (FPS). Erythrocytes were incubated with appropriate fluorescent tags, and cells with a single fluorophore were imaged at either 30 or 120 FPS to determine the microscopic diffusion coefficient(s) (Dμ).
Table 1.
Summary of Microscopic Diffusion Rates, Macroscopic Diffusion Rates, and Compartment Sizes of the Duffy Antigen, Glycophorin A, Band 3, and GLUT1 from Single-Particle-Tracking Measurements
| Camera Speed | Protein | Dμ (μm2/s) |
DM (μm2/s) |
Compartment Size (nm) | ||
|---|---|---|---|---|---|---|
| Fraction (%) | Dμ | Fraction (%) | DM | |||
| 30 FPS | Duffy | 100 | 4.8 ± 0.3 × 10−4 | 100 | 1.3 ± 0.1 × 10−4 | 40 |
| glycophorin A | 100 | 8.7 ± 0.5 × 10−4 | 100 | 1.3 ± 0.6 × 10−4 | 43 | |
| band 3 | 100 | 5.9 ± 0.6 × 10−4 | 100 | 8.7 ± 0.4 × 10−5 | 35 | |
| 71 | ||||||
| GLUT1 | 22 | 6.9 ± 0.8 × 10−5 | 100 | 6.3 ± 0.2 × 10−5 | 36 | |
| 66 | 1.7 ± 0.2 × 10−3 | |||||
| 12 | 4.2 ± 2.2 × 10−2 | |||||
| 120 FPS | Duffy | 27 | 2.5 ± 0.4 × 10−4 | 100 | 2.7 ± 0.1 × 10−4 | 38 |
| 73 | 5.0 ± 0.3 × 10−3 | |||||
| glycophorin A | 59 | 3.4 ± 0.6 × 10−2 | 100 | 2.7 ± 0.2 × 10−4 | 36 | |
| 41 | 1.1 ± 0.1 × 10−4 | |||||
| band 3 | 73 | 1.6 ± 0.6 × 10−3 | 100 | 1.9 ± 0.1 × 10−4 | 35 | |
| 27 | 2.1 ± 0.2 × 10−2 | 66 | ||||
| GLUT1 | 34 | 4.3 ± 1.6 × 10−4 | 100 | 2.0 ± 0.1 × 10−4 | 39 | |
| 66 | 9.3 ± 1.3 × 10−3 | |||||
Figure 5.
Comparison of macroscopic diffusion rates of Duffy antigen, glycophorin A, band 3, and GLUT1 at 30 and 120 FPS. Erythrocytes were incubated with appropriate fluorescent tags, and cells with a single fluorophore were imaged at either 30 or 120 FPS to determine the macroscopic diffusion coefficient(s) (DM).
Comparison of the mobilities of Duffy antigen, glycophorin A, band 3, and GLUT1
To compare the mobility of the Duffy antigen with other membrane proteins, single-particle tracking of glycophorin A (17), band 3 (18), and GLUT1 (19) were also performed on parallel preparations of erythrocytes, exactly as described above. In general, diffusion constants of membrane-spanning proteins are bounded by a lower value of ∼10−6 μm2/s, the mobility of fixed proteins (18) and a theoretical upper limit of ∼5 μm2/s, the diffusion coefficient of most phospholipids (25). In practice, however, the upper limit of most erythrocyte membrane-spanning proteins is only ∼10−2 μm2/s, which corresponds to membrane-spanning proteins whose interaction with the cortical cytoskeleton has been broken (33). Therefore, to more easily compare the four membrane proteins analyzed, their mobilities were broadly classified into three categories that correspond to the diffusion coefficients groupings previously seen with GLUT1 (19): 1) highly immobilized (Dμ ∼10−4 μm2/s), 2) moderately immobilized (Dμ ∼10−3 μm2/s), and 3) relatively unrestrained (Dμ ∼10−2 μm2/s).
As shown in Fig. 4 and Table 1, when diffusion was analyzed over longer time intervals (i.e., 30 FPS), glycophorin A, Duffy antigen, and band 3 all appeared to remain fairly stationary, suggesting that few if any copies of these proteins were free to move long distances across the membrane. In contrast, GLUT1, which surprisingly displayed two immobilized populations, with one diffusing ∼25 times slower than the other, further distinguished itself by also having a small (12% of the total) population that exhibited relatively unrestricted diffusion at 30 FPS (Dμ = 4.2 × 10−2 μm2/s).
Although the above data argue that virtually all copies of the four membrane-spanning proteins (except for 12% of the GLUT1 population) are constrained in their mobilities, the probability still exists that they undergo shorter range constrained movement while tethered to the flexible spectrin-actin cortical cytoskeleton. To compare these short-range motions of Duffy antigen, glycophorin A, band 3, and GLUT1, the trajectories of all four proteins were again measured at 120 FPS, when such small localized movements are readily measured. As noted in the right panel of Fig. 4, all four membrane-spanning proteins separated into two populations. Overall, Duffy antigen and GLUT1 showed a smaller range of motion compared with glycophorin A and band 3. This suggests that Duffy antigen and GLUT1 may either be bound more tightly to the spectrin-actin cortical cytoskeleton or attached to the anastomosing cytoskeletal mesh at a region that experiences less mobility (e.g., the junctional complex).
The compartment size measurements and macroscopic diffusion coefficients both support the idea that the majority of these proteins are immobile and essentially “corralled” within the repeating arrangement of the cortical spectrin cytoskeleton. As shown in Fig. 1, step 4, the cortical spectrin cytoskeleton is arranged into repeating triangular structures. In this artificially stretched preparation of the spectrin network, the length of each side is ∼200 nm (34). However, in situ, spectrin tetramers are thought to be compacted to ∼1/3 of their contour lengths, resulting in spectrin corrals of only ∼70 nm on a side (18,35). The maximal diameter of a circle that can fit within these triangular corrals is ∼35 nm, i.e., corresponding approximately to the compartment sizes reported for all four proteins (Table 1). In fact, the only real variation in compartment size distribution was seen with band 3, which exhibited a second subpopulation with a larger size of 66–71 nm (Table 1), probably corresponding to the minor subpopulation of band 3 known to be unattached to the spectrin-actin cytoskeleton (8).
Conclusions
With the mobilities of four prominent erythrocyte membrane proteins now characterized under identical conditions, it is now possible to offer support for a few generalizations regarding the structure and organization of the RBCM. First, because the majority of the copies of Duffy antigen, glycophorin A, band 3, and GLUT1 are constrained in their mobilities, one might extrapolate that most membrane-spanning proteins are either anchored to the cortical cytoskeleton or associated with another protein that is tethered to the cytoskeleton. This would imply that although the erythrocyte plasma membrane may in fact be a fluid mosaic, most of the fluidity must reside in the lipid fraction of the membrane because the majority of the membrane-spanning proteins appear to associate into large cytoskeletally tethered complexes within this sea of fluid lipids. This conclusion is in fact congruent with previous single-particle-tracking studies performed on glycophorin A (17), band 3 (18,25), and GLUT1 (19) in which the data were interpreted to indicate that the majority of these proteins were bound within either the ankyrin or spectrin-actin junctional complex. In a previous study in which orthovanadate-induced tyrosine phosphorylation of band 3 was used to rupture the interactions of band 3 with adducin and ankyrin, at least 84% of band 3 became freely diffusing, with a Dμ of ∼1.5 × 10−2 μm2/s (33). In these studies, only a single protein, GLUT1, displayed a subpopulation with a similar freely diffusing Dμ-value (4.2 × 10−2 μm2/s). However, this diffusion rate is still ∼100-fold slower than the Dμ of most phospholipids (1.6–9.4 μm2/s) (25), suggesting that even the most freely diffusing of these erythrocyte membrane proteins is still retarded in its movement.
When compared with the diffusion properties of membrane-spanning proteins from other mammalian cells, many of which have diffusion coefficients between 1.3 × 10−1 and 2.5 × 10−2 μm2/s (Table 2), the erythrocyte’s membrane-spanning proteins would appear to exhibit reduced mobilities (Table 1). Although part of this apparent discrepancy could derive from sampling biases, it is tempting to speculate that the erythrocyte has evolved to form a more stable and durable plasma membrane. Thus, whereas most other mammalian plasma membranes are undergoing constant replacement and repair via recycling through endosomal compartments, the human erythrocyte must survive for ∼120 days in circulation without rejuvenation from any newly synthesized proteins or lipids. Moreover, the RBCM must withstand enormous shear stresses because it passes approximately every 60 s through capillaries with diameters ∼1/3 of that of the RBC (36). Added to this are the damages deriving from constant exposure to oxidative stresses (36) and one can speculate that the RBCM must be assembled with more structurally durable components than the average plasma membrane of other somatic cells. Perhaps the strong tethering of membrane-spanning proteins to each other and to the spectrin-actin cytoskeleton provides some of this added resilience and stability.
Table 2.
Summary of Frame Rates, Diffusion Coefficients, and Compartment Sizes of Various Mammalian Transmembrane Proteins from Single-Particle-Tracking Measurements
| Camera Speed | Protein | Dμ (μm2/s) | Compartment Size (nm) | Reference |
|---|---|---|---|---|
| 150 FPS | glycine receptor GlyR α3K | 1.5 × 10−1 | 153 | (37) |
| 20 FPS | glycine receptor GlyR α3K | 1.3 × 10−1 | N.D. | (38) |
| 20 FPS | glycine receptor GlyR α3K | 4.4 × 10−2 | N.D. | (38) |
| 33 FPS | EGFR | 1.3 × 10−1 | N.D. | (39) |
| 50 FPS | TNF-R1 | 1.2 × 10−1 | N.D. | (40) |
| 33 FPS | MHC class II (TM-I-Ek) | 4.5 × 10−2 | 42 | (41) |
| 66 | ||||
| 200 FPS | immunoglobulin E receptor (IgE-FcεRI) | 1.6 × 10−1 | 513 | (42) |
| 4.0 × 10−2 | 204 | |||
| 10 FPS | Her2 | 5.5 × 10−2 | 640 | (43) |
| 50 FPS | aquaporin-3 | 1.5 × 10−2 | N.D. | (44) |
| 33 FPS | mGluR3 | 7.8 × 10−2 | N.D. | (45) |
| 33 FPS | ADRB2 | 7.9 × 10−2 | N.D. | (45) |
| 33 FPS | HTR2A | 6.4 × 10−2 | N.D. | (45) |
| 33 FPS | HRH1 | 6.6 × 10−1 | N.D. | (45) |
| 33 FPS | ADORA2A | 8.3 × 10−2 | N.D. | (45) |
| 33 FPS | FFAR4 | 8.7 × 10−2 | N.D. | (45) |
| 33 FPS | CXCR4 | 8.4 × 10−2 | N.D. | (45) |
| 33 FPS | F2R | 7.5 × 10−2 | N.D. | (45) |
| 33 FPS | GCGR | 4.7 × 10−1 | N.D. | (45) |
| 4.7 × 10−2 |
N.D., not determined.
Author Contributions
P.S.L., G.C.K., and K.G. were responsible for designing the research. G.C.K. and K.G. performed the experimental studies. G.C.K., K.G., and K.S.P. analyzed the data. K.S.P. and P.S.L. wrote the manuscript.
Acknowledgments
This work was supported by the National Institutes of Health grant number GM024417 (P.S.L.).
Editor: Gerhard Schuetz.
References
- 1.Popot J.L., Engelman D.M. Membrane protein folding and oligomerization: the two-stage model. Biochemistry. 1990;29:4031–4037. doi: 10.1021/bi00469a001. [DOI] [PubMed] [Google Scholar]
- 2.Zhou F.X., Merianos H.J., Engelman D.M. Polar residues drive association of polyleucine transmembrane helices. Proc. Natl. Acad. Sci. USA. 2001;98:2250–2255. doi: 10.1073/pnas.041593698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gratkowski H., Lear J.D., DeGrado W.F. Polar side chains drive the association of model transmembrane peptides. Proc. Natl. Acad. Sci. USA. 2001;98:880–885. doi: 10.1073/pnas.98.3.880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nicolson G.L. The fluid-mosaic model of membrane structure: still relevant to understanding the structure, function and dynamics of biological membranes after more than 40 years. Biochim. Biophys. Acta. 2014;1838:1451–1466. doi: 10.1016/j.bbamem.2013.10.019. [DOI] [PubMed] [Google Scholar]
- 5.Roca-Cusachs P., Iskratsch T., Sheetz M.P. Finding the weakest link: exploring integrin-mediated mechanical molecular pathways. J. Cell Sci. 2012;125:3025–3038. doi: 10.1242/jcs.095794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alberts B., Johnson A., Walter P. Fourth Edition. Garland Science; New York, NY: 2002. Molecular Biology of the Cell. [Google Scholar]
- 7.Gascard P., Mohandas N. New insights into functions of erythroid proteins in nonerythroid cells. Curr. Opin. Hematol. 2000;7:123–129. doi: 10.1097/00062752-200003000-00009. [DOI] [PubMed] [Google Scholar]
- 8.Lux S.E., IV Anatomy of the red cell membrane skeleton: unanswered questions. Blood. 2016;127:187–199. doi: 10.1182/blood-2014-12-512772. [DOI] [PubMed] [Google Scholar]
- 9.Mankelow T.J., Satchwell T.J., Burton N.M. Refined views of multi-protein complexes in the erythrocyte membrane. Blood Cells Mol. Dis. 2012;49:1–10. doi: 10.1016/j.bcmd.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mohandas N., Gallagher P.G. Red cell membrane: past, present, and future. Blood. 2008;112:3939–3948. doi: 10.1182/blood-2008-07-161166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morrison M., Mueller T.J., Edwards H.H. Protein architecture of the erythrocyte membrane. Prog. Clin. Biol. Res. 1981;51:17–34. [PubMed] [Google Scholar]
- 12.Daniels G. Functions of red cell surface proteins. Vox Sang. 2007;93:331–340. doi: 10.1111/j.1423-0410.2007.00970.x. [DOI] [PubMed] [Google Scholar]
- 13.Shen H., Tauzin L.J., Landes C.F. Single particle tracking: from theory to biophysical applications. Chem. Rev. 2017;117:7331–7376. doi: 10.1021/acs.chemrev.6b00815. [DOI] [PubMed] [Google Scholar]
- 14.Muyldermans S. Nanobodies: natural single-domain antibodies. Annu. Rev. Biochem. 2013;82:775–797. doi: 10.1146/annurev-biochem-063011-092449. [DOI] [PubMed] [Google Scholar]
- 15.Habib I., Smolarek D., Bertrand O. V(H)H (nanobody) directed against human glycophorin A: a tool for autologous red cell agglutination assays. Anal. Biochem. 2013;438:82–89. doi: 10.1016/j.ab.2013.03.020. [DOI] [PubMed] [Google Scholar]
- 16.Smolarek D., Hattab C., Bertrand O. A recombinant dromedary antibody fragment (VHH or nanobody) directed against human Duffy antigen receptor for chemokines. Cell. Mol. Life Sci. 2010;67:3371–3387. doi: 10.1007/s00018-010-0387-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Giger K., Habib I., Low P.S. Diffusion of glycophorin A in human erythrocytes. Biochim. Biophys. Acta. 2016;1858:2839–2845. doi: 10.1016/j.bbamem.2016.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kodippili G.C., Spector J., Low P.S. Imaging of the diffusion of single band 3 molecules on normal and mutant erythrocytes. Blood. 2009;113:6237–6245. doi: 10.1182/blood-2009-02-205450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kodippili G.C., Putt K.S., Low P.S. Evidence for three populations of the glucose transporter in the human erythrocyte membrane. Blood Cells Mol. Dis. 2019;77:61–66. doi: 10.1016/j.bcmd.2019.03.005. [DOI] [PubMed] [Google Scholar]
- 20.Michalet X. Mean square displacement analysis of single-particle trajectories with localization error: Brownian motion in an isotropic medium. Phys. Rev. E Stat. Nonlin. Soft Matter Phys. 2010;82:041914. doi: 10.1103/PhysRevE.82.041914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gelles J., Schnapp B.J., Sheetz M.P. Tracking kinesin-driven movements with nanometre-scale precision. Nature. 1988;331:450–453. doi: 10.1038/331450a0. [DOI] [PubMed] [Google Scholar]
- 22.Kusumi A., Sako Y., Yamamoto M. Confined lateral diffusion of membrane receptors as studied by single particle tracking (nanovid microscopy). Effects of calcium-induced differentiation in cultured epithelial cells. Biophys. J. 1993;65:2021–2040. doi: 10.1016/S0006-3495(93)81253-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sheetz M.P., Turney S., Elson E.L. Nanometre-level analysis demonstrates that lipid flow does not drive membrane glycoprotein movements. Nature. 1989;340:284–288. doi: 10.1038/340284a0. [DOI] [PubMed] [Google Scholar]
- 24.Powles J.G., Mallett M.J.D., Evans W.A.B. Exact analytic solutions for diffusion impeded by an infinite array of partially permeable barriers. Proc. R. Soc. Lond. 1992;436:391–403. [Google Scholar]
- 25.Fujiwara T., Ritchie K., Kusumi A. Phospholipids undergo hop diffusion in compartmentalized cell membrane. J. Cell Biol. 2002;157:1071–1081. doi: 10.1083/jcb.200202050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim D.-H., Kim D.-K., Ryu S.H. Single particle tracking-based reaction progress kinetic analysis reveals a series of molecular mechanisms of cetuximab-induced EGFR processes in a single living cell. Chem. Sci. 2017;8:4823–4832. doi: 10.1039/c7sc01159h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Normanno D., Boudarène L., Dahan M. Probing the target search of DNA-binding proteins in mammalian cells using TetR as model searcher. Nat. Commun. 2015;6:7357. doi: 10.1038/ncomms8357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rossier O., Octeau V., Giannone G. Integrins β1 and β3 exhibit distinct dynamic nanoscale organizations inside focal adhesions. Nat. Cell Biol. 2012;14:1057–1067. doi: 10.1038/ncb2588. [DOI] [PubMed] [Google Scholar]
- 29.Knight S.C., Xie L., Tjian R. Dynamics of CRISPR-Cas9 genome interrogation in living cells. Science. 2015;350:823–826. doi: 10.1126/science.aac6572. [DOI] [PubMed] [Google Scholar]
- 30.Zhen C.Y., Tatavosian R., Ren X. Live-cell single-molecule tracking reveals co-recognition of H3K27me3 and DNA targets polycomb Cbx7-PRC1 to chromatin. eLife. 2016;5:e17667. doi: 10.7554/eLife.17667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saxton M.J. Single-particle tracking: the distribution of diffusion coefficients. Biophys. J. 1997;72:1744–1753. doi: 10.1016/S0006-3495(97)78820-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Salomao M., Zhang X., An X. Protein 4.1R-dependent multiprotein complex: new insights into the structural organization of the red blood cell membrane. Proc. Natl. Acad. Sci. USA. 2008;105:8026–8031. doi: 10.1073/pnas.0803225105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ferru E., Giger K., Low P.S. Regulation of membrane-cytoskeletal interactions by tyrosine phosphorylation of erythrocyte band 3. Blood. 2011;117:5998–6006. doi: 10.1182/blood-2010-11-317024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu S.C., Derick L.H., Palek J. Visualization of the hexagonal lattice in the erythrocyte membrane skeleton. J. Cell Biol. 1987;104:527–536. doi: 10.1083/jcb.104.3.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Paramore S., Ayton G.S., Voth G.A. Extending a spectrin repeat unit. I: linear force-extension response. Biophys. J. 2006;90:92–100. doi: 10.1529/biophysj.105.066969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mohanty J.G., Nagababu E., Rifkind J.M. Red blood cell oxidative stress impairs oxygen delivery and induces red blood cell aging. Front. Physiol. 2014;5:84. doi: 10.3389/fphys.2014.00084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Notelaers K., Rocha S., Ameloot M. Analysis of alpha3 GlyR single particle tracking in the cell membrane. Biochim. Biophys. Acta. 2014;1843:544–553. doi: 10.1016/j.bbamcr.2013.11.019. [DOI] [PubMed] [Google Scholar]
- 38.Notelaers K., Smisdom N., Ameloot M. Ensemble and single particle fluorimetric techniques in concerted action to study the diffusion and aggregation of the glycine receptor α3 isoforms in the cell plasma membrane. Biochim. Biophys. Acta. 2012;1818:3131–3140. doi: 10.1016/j.bbamem.2012.08.010. [DOI] [PubMed] [Google Scholar]
- 39.Li Y., Hu Y., Cang H. Light sheet microscopy for tracking single molecules on the apical surface of living cells. J. Phys. Chem. B. 2013;117:15503–15511. doi: 10.1021/jp405380g. [DOI] [PubMed] [Google Scholar]
- 40.Heidbreder M., Zander C., Heilemann M. TNF-α influences the lateral dynamics of TNF receptor I in living cells. Biochim. Biophys. Acta. 2012;1823:1984–1989. doi: 10.1016/j.bbamcr.2012.06.026. [DOI] [PubMed] [Google Scholar]
- 41.Umemura Y.M., Vrljic M., Kusumi A. Both MHC class II and its GPI-anchored form undergo hop diffusion as observed by single-molecule tracking. Biophys. J. 2008;95:435–450. doi: 10.1529/biophysj.107.123018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Spendier K., Lidke K.A., Thomas J.L. Single-particle tracking of immunoglobulin E receptors (FcεRI) in micron-sized clusters and receptor patches. FEBS Lett. 2012;586:416–421. doi: 10.1016/j.febslet.2012.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xiao Z., Ma X., Fang X. Single-molecule study of lateral mobility of epidermal growth factor receptor 2/HER2 on activation. J. Phys. Chem. B. 2008;112:4140–4145. doi: 10.1021/jp710302j. [DOI] [PubMed] [Google Scholar]
- 44.Marlar S., Arnspang E.C., Nejsum L.N. Elevated cAMP increases aquaporin-3 plasma membrane diffusion. Am. J. Physiol. Cell Physiol. 2014;306:C598–C606. doi: 10.1152/ajpcell.00132.2013. [DOI] [PubMed] [Google Scholar]
- 45.Yanagawa M., Hiroshima M., Sako Y. Single-molecule diffusion-based estimation of ligand effects on G protein-coupled receptors. Sci. Signal. 2018;11:eaao1917. doi: 10.1126/scisignal.aao1917. [DOI] [PubMed] [Google Scholar]