Abstract
BACKGROUND
Manganese (Mn) is a metal ubiquitously present in nature and essential for many living organisms. As a trace element, it is required in small amounts for the proper functioning of several important enzymes, and reports of Mn deficiency are indeed rare.
METHODS
This mini-review will cover aspects of Mn toxicokinetics and its impact on brain neurotransmission, as well as its Janus-faced effects on humans and other animal’s health.
RESULTS
The estimated safe upper limit of intracellular Mn for physiological function is in anarrow range of 20 to 53μM.Therefore, intake of higher levels of Mn and the outcomes have been well documented.
CONCLUSION
The metal affects mostly the brain by accumulating in specific brain areas, altering cognitive functions and locomotion, thus severely impacting the health of the exposed organisms.
Keywords: Manganese, Trace elements, Manganism, Neurotoxicity
1. Introduction
Manganese (Mn) is a naturally occurring element, present in rocks and soils, air, water and food. It is an essential nutrient, needed for a variety of functions ranging from bone mineralization, protein and energy metabolism, and metabolic regulation to cellular protection[1, 2]. Mn serves as a co-factor for several enzymes that control the above cited functions and is easily obtained from the diet, as is present in several foods[3]. Although rare, Mn deficiency is severe and affects several organs [2].
On the other hand, Mn overexposure is a public health concern due to its widespread industrial usage and the risk for environmental contamination[4]. Anthropogenic activities as iron and stainless-steel production, formation of aluminumalloys, use of antiknock additive in gasoline, and application of fungicides, are a few sources that increase Mn levels in the environment[5, 6]. Mn is mainly absorbed by the gastrointestinal and respiratory tracts and uptake by cells through many transporters, some of them recently reported. Notably, Mn can easily cross de blood brain barrier and has a tropism by brain areas that contain dopamine, such as the basal ganglia. In these areas, Mn accumulates and causes neurotoxic effects by several mechanisms that are still being undercovered [7]. Manganism, or locura manganica, is characterized by Parkinson’s disease (PD)-like symptoms such as gait difficulties and tremors, which are not responsive to levodopa as the PD symptoms[8–10]. In addition, cognitive problems have been reported in children using milk formulas and patients using total parenteral nutrition, which then were found to have Mn levels above the recommended [11–13]. The damage caused by Mn neurotoxicity has been widely studied, thus providing impetus for reducing Mn levels in milk and nutrition formulas, prohibition of Mn-containing antiknock additives in some countries, and for more strict occupational risk assessments[14]. Studying and understanding Mn health effects is indispensable as to prevent exposure to high levels and also for the development of therapeutic strategies against poisoning.
2. Manganese toxicokinetics
When Mn in ingested from sources as water and diet, this metal is readily absorbed in the intestine through passive diffusion or active transport via the divalent metal transporter 1 (DMT1), which also transports other divalent metals such as iron (Fe) [12, 15]. Adult humans absorb approximately 3–5% of ingested Mn [1], but this rate can be modified by age [16, 17], carbohydrate source in the diet [18], presence of phytate [19] and animal protein [20], besides the content of manganese [21] and other mineral elements in diet, especially Fe, which deficiency can increase Mn absorption [18, 21]. Interestingly, high Mn intake, either through dietary or environmental exposure, causes the gastrointestinal tract to absorb less Mn while liver increases metabolism for biliary and pancreatic excretion [22, 23]. This regulation is important in preventing dyshomeostasis of Fe, since it has been reported that Mn interferes with Fe s absorption and distribution as both trace metals are known to compete for the same transporters [24, 25].
Inhalation is the primary route of entry in most occupational exposure settings, such as mining, Mn alloy production, smelting, welding, application of pesticides containing Mn and dry alkaline battery production. Mn is easily absorbed by lungs and enters the circulation,crossing to the nervous system by two zinc transporters, ZIP8 and ZIP14, bypassing the liver and BBB [26]. Since this route delivers a great amount of Mn to the brain, manganism, or locuramanganica, was primarily described and studied in workers that inhaled Mn for a long time during occupational activities [5, 27]. Because is an irritant to the pulmonary tract, it can also cause bronchitis and decrease lung function[28].
Another exposure route is intravenous, thus bypassing the gastrointestinal regulation mentioned above and therefore promoting 100% Mn absorption. Patients under total parenteral nutrition, especially infants, may receive amounts of Mn that overcome the nutritional requirements, thus leading to poisoning [12, 13]. A recent source of intravenous exposure is by the use of methcathinone (ephedrone), a synthetic drug that contains Mn dioxide and has been associated to cognitive deficits and development of parkisonism in abusers, essentially young people [29–31]. Other possible routes of exposure include in utero and dermal, which are still poorly reported.
Once in the bloodstream, Mn distribution to tissues is fast. The estimated half-life for Mn to leave plasma is 1 min [1,32], Mn is distributed from plasma to the liver (30% of total Mn), kidney (5%), pancreas (5%), colon (1%), urinary system (0.2%), bone (0.5%), brain (0.1%), erythrocytes (0.02%), and the remaining 58.18% to other soft tissues [1, 32]. Liver, pancreas, bone, kidney, and brain retain Mn more than other tissues and have the highest Mn concentrations due to the essential nature of Mn in energy production and the high energy demands of these tissues [12].
Mn transport to these tissues has been ascribed to high affinity metal transporters of Ca and Fe. In fact some of these transporters include DMT-1, ZIP8, a member of the solute carrier-39, transferrin receptor (TfR), voltageregulated and store-operated Ca2+ channels and the ionotropic glutamate receptoi Ca2+ channels [1, 12, 33]. In relation to mechanisms of cellular Mn efflux, SPCA 1 [34], ferroportin [35] and SLC30A10 [36] have been reported by playing arole. Previous data demonstrated that mutations in SPCA1 and ferroportin are known to occur in humans but do not cause a Mn toxicity phenotype [37], whereas mutations in SLC30A10 are associated to a familiar form of Parkinson’s disease [38]. This suggests that SLC30A10 is the primary Mn exporter, particularly in the digestive system, though it does not appear to be the key cellular exporter from brain.
Indeed, several studies have demonstrated that Mn can cross the blood-brain barrier (BBB). A major route of Mn influx may be mediated by TfR, the ironcarrying plasma protein [39]. In addition, Mn citrate, Mn ion and the Mn-Tf complex cross the BBB, most likely via carrier-mediated transport [40]. However, this facility in Mn across the BBB could contribute to its own toxic effect [41, 42].
Because little Mn is required to maintain physiological functions, extra Mn needs to be eliminated. The turnover of ingested Mn is relatively fast, with an average retention of 10 days [43]. Most of excess Mn is conjugated to bile by the liver and get eliminated via fecal excretion [44]. The liver plays a critical role in this process and is the main organ responsible for Mn excretion [2]. When damaged, Mn elimination is altered, resulting in its accumulation in hepatic cells and consequently increased Mn levels in the brain consistent with hepatic encephalopathy [45, 46]. Furthermore, small amounts of bile-Mn conjugates could be reabsorbed in entero-hepatic circulation and can also be detected in urine, sweat, and breast milk [15, 47].
3. Health effects of Mn
3.1. Essentiality and Deficiency
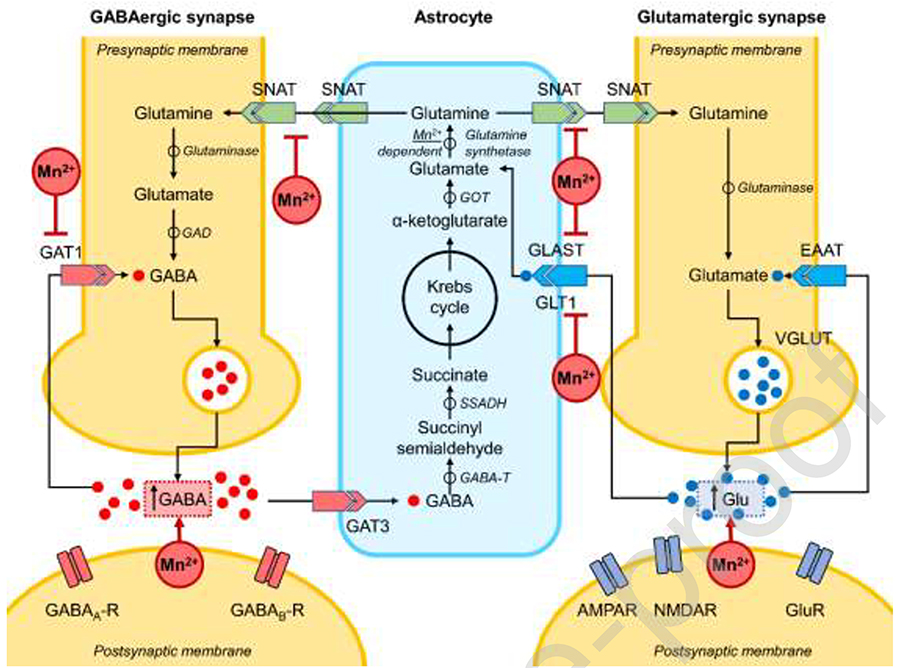
Mn is an essential nutrient for growth and development in animals, including humans, and along with other trace metals and nutrients is needed for optimal physiology. Some pathways are responsive to Mn, as those controlled by the Ataxia Telangiectasia Mutated (ATM) kinase [48], responsible for regulating immune system and the response against DNA double strand breaks. In addition, proinflammatory cytokines such as IL-6, IL-1β, and tumor necrosis factor-alpha production have been shown to be potentiated by Mn [49, 50]. In addition, metalloproteins as oxidoreductases, transferases, hydrolases, lyases, isomerases, and ligases are dependent or activated by Mn [8]. [1, 33, 51]. Particularly, the major Mn containing enzyme in mammalian brain is glutamine synthetase, which is predominantly present in astrocytes and responsible for converting the amino acid glutamate to glutamine for nitrogen clearance (Figure 1) [7, 52]. In table 1 we present the main Mn-dependent enzymes.
Figure 1. Summary of the main biological effects of Mn at recommended vs elevated levels:
Mn2+-alteration of Glu transporters (EAAT), especially GLAST and GLT1 [122], impairs glutamate uptake by astrocytes resulting in an increase in extracellular glutamate concentrations [95]. The net glutamine uptake is also inhibited due to down-regulation of of SNAT1, SNAT2, and SNAT3 expression in astrocytes [90, 96]. The overall effect of manganese on GABAergic synapses is characterized by increased extracellular GABA (GABAEC) levels that is expected to be mediated through inhibition of GAT1 [103]. However, inhibition of astrocytic GAT3 was not supported by the laboratory data [110].
AMPAR - α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor; EAAT - Excitatory amino acid transporter; GABA - Gamma-aminobutyric acid; GABA-T - GABA-transaminase; GAD - Glutamate decarboxylase; GAT - GABA transporter; GLAST1 - Glutamate aspartate transporter 1; GLT1 - Glutamate transporter 1; GluR - Glutamate receptor; GOT - Glutamate:oxaloacetate transaminase; NMDAR - N-methyl-D-aspartate receptor; SNAT - Sodium-coupled neutral amino acid transporter; SSADH - Succinic semialdehyde dehydrogenase; VGLUT - Vesicular glutamate transporter
Table 1:
Mn- dependent and Mn activated enzymes
| Enzyme | Localization | Sub-localization | Function |
|---|---|---|---|
| Arginases I [130] | Hepatocytes | Citoplasm | Amino acid metabolism |
| Arginases II [130] | Mostly in kidneys | Mitochondria | Amino acid metabolism |
| Pyruvate Carboxylase [131] | Liver, kidney and adipose tissue | Mitochondria | Gluconeogenesis and lipogenesis |
| Mn-superoxide dismutase [132] | ubiquitous | Mitochondria | Antioxidant activity |
| Glutamine Synthetase [133] | Brain, kidneys, and liver | Both | Amino acid metabolism |
| Protein serine/threonine phosphatase-1 (PP1) [134] | ubiquitous | cytosol | cell survival and differentiation |
To prevent Mn deficiency, small daily doses are necessary. The Institute of Medicine’s diary recommended intake for Mn suggests 2 mg/day as an adequate intake for adults and 1.2–1.5 mg/day for children. However, Mn essentiality in humans varies according of the life-stage and of the sex [1, 33]. Inadequate dietary intake of manganese results in impaired growth, poor bone formation and skeletal defects, abnormal glucose tolerance, and altered lipid and carbohydrate metabolism, and these changes probably occur by decreased MnSOD and glutamine synthetase (GS) activities [53].
Due to its ubiquity, dietary Mn deficiency is uncommon. Mn is easily found in animal and plant foods, with vegetables being the most abundant variety. Whole grains (wheat germ, oats, and bran), rice, and nuts (hazelnuts, almonds, and pecans), chocolate, tea, mussels, clams, legumes, fruit, leafy vegetables (spinach), seeds (flax, sesame, pumpkin, sunflower, and pine nuts), and spices (chili powder, cloves, and saffron) are all good examples of Mn-enriched foods[1, 26].
Although genetic changes in the uptake and export of Mn are rare, they may have severe consequences. Recent studies show that mutations in SLC39A8, a Mn transporter,decreased blood Mn levels causing reduced activity of Mn-dependent enzymes such as the β-1,4-galactosyltransferase and MnSOD, leading to dysglycosylation. This congenital disorder of glycosylation type lln (CDG2N) causes impaired mitochondrial function that leads to developmental delay, short statue, dwarfism, seizures, hypotonia, and dystonia. Suggested therapy is based on oral Mn and galactose supplementation [26, 54].
Two other genetic diseases are characterized by changes in Mn transport in cells: hypermanganesaemia with dystonia 1 (SLC30A10 deficiency) and hypermanganesaemia with dystonia 2 (SLC39A14 deficiency). In the first typethesystemic Mn accumulation (levels raised as ten times that of normal) leads to a distinct syndrome of hypermanganesaemia, polycythaemia, dystonia, chronic liver disease and depletion of iron stores. Although blood Mn levels do not reduce the treatment with chelation with EDTA-CaNa2 is effective for reducing the accumulation of Mn and treat neurological symptoms and prevent liver disease progression. In the second case, distinctfrom the type 1, the affected individuals mainly exhibit neurotoxic effects of Mn accumulation, such as progressive dystonia with signs of parkinsonism variables and, in general,hypermanganesaemia does not lead to systemic symptoms also seen in type 1 as liver disease or polycythaemia. In addition, chelation with EDTA-CaNa2 treatment is generally not effective to minimize neurological effects [26, 54].
Considering the set of factors that guarantee the essentiality of Mn in health, the imbalance in homeostasis, either due to its deficiency in the diet or genetic factors can be rare but with serious consequences. Furthermore, there is no effective treatment for type 2 genetic disorders yet and Mn supplementation in cases of deficiency may lead to toxicity. This underscores the importance of the search for new therapies and treatments.
3.2. Side and Toxic effects
Humans are easily exposed to Mn through air, soil and, consequently, water, being the main source of intoxication identified due to occupational exposure [9, 55]. Industry professionals in battery production, welding and mining operations are constantly exposed to Mn by inhalation, with reported neurotoxicity in these workers [27, 56–59]. Neurological damage with Mn accumulation in various areas of the midbrain such as globus pallidus, substantia nigra, subthalamic nucleus, putamen, caudate nucleus and dentate nucleus has been described [31, 60, 61]. Notably, low-level occupational exposure with airborne Mn concentrations within or below these occupational standards may also be detrimental [9, 62].
As already mentioned. Mn is necessary to control numerous metabolic functions. However, exacerbated exposure to this metal affects several biological activities, depending on the levels, routes of exposure, as well as gender and age of the exposed subject [3]. Although the main focus of toxicity is the CNS, high Mn concentrations can cause hepatic, cardiac, endocrine, male reproductive disorders and nephrotoxicity [36, 63, 64]. Symptoms of Mn intoxication consist of limb stiffness, reduced response speed, intellectual deficits, mood swings, hallucinations, tremors and balance disorders, symptoms that characterize manganism, a condition that causes neurological disorders characterized by cognitive and motor abnormalities which resemble Parkinson’s disease (PD) [65,67].
The mechanisms of toxicity of Mn are not yet fully understood [6, 68]; however, some of its actions on the CNS are well-known, such as the effects on catecholamines (mainly dopamine), acetylcholine, glutamate and aminobutyric gamma acid (GABA), besides triggering mitochondrial dysfunctions by inhibiting the electron transport chain even before affecting neurons and astrocytes [6, 63, 68, 69]. Mn intoxication ordyshomeostasis is also suspected as an environmental modifier or risk factor in several neurodegenerative diseases, such as PD, amyotrophic lateral sclerosis (ALS), Huntington’s disease (HD), and Alzheimer’s disease (AD) [70]. Of note, it has been reported that excess Mn can replace copper and stabilize the prion protein (PrP), initiating its aggregation and converting it to a neurotoxic form [71, 72]. Mn can render PrP in a proteaseresistant form [71], and this has been associated with increased disease progress. Next, we will briefly discuss the main neuronal systems affected by Mn exposure.
3.2.1. Dopaminergic System
In the brain, Mn accumulates in areas rich in dopaminergic neurons, which are directly involved in motor emotional and endocrine control. Mn’spropensityto oxidize catecholamines, including dopamine (DA), decreases its concentration in the substantia nigra, leading to the onset of PD-like symptoms [73].Therefore, in the early stages of manganism, after cessation of exposure to Mn, symptoms may be reversed, whereas in patients with motor disorders, manganism is irreversible [73].
Mn-induced DA oxidation is a complex process that involves several steps in which semiquinone, aminochrome intermediates, L-cysteine, copper and NADH are involved, in its oxidation states Mn2+ and Mn3+, Mn reacts with DA through the Fenton reaction, catalyzing the auto-oxidation of the neurotransmitter, which generates reactive oxygen species (ROS) and DA-oquinone, thus leading to oxidative damage and DAergic neuronal death [73, 74].
The literature suggests that neurodegeneration caused by Mn, in addition to presenting symptoms and areas common to PD or that PD-mimicking drugs (6-hydroxydopamine / 6-OHDA, 1-methyl-4-phenylpyridide / MPP +, rotenone, paraquat), also shares multiple common effector mechanisms, causing mitochondrial dysfunction, therefore ATP depletion, oxidative stress, protein aggregation and activation of apoptotic cells [62, 73]. In C. elegans, orthologs to PD-associated genes play a role in α-synuclein toxicity and DAergic neurodegeneration, and a link between mutated α-synuclein and Mn toxicity has also been demonstrated in the worm [75, 76]. The NRAMP / DMT family of metal transporters already described in vertebrates, involving the Mn uptake pathways and toxicity, leads to developmental defects and excretory systems in C. elegans [77]. In addition, it has been shown that acute exposure to Mn leads to specific neurodegeneration in DAergic neurons sparing other neuronal systems and that this dose-dependent toxicity confirms th specificity seen in vertebrate models[78]. This same study shows that the cause of this specific neurodegeneration of DAergic neurons in the worm is the exogenous DA that potentiates Mn toxicity and its neurodegeneration and that this mechanism of toxicity requires the DAergic neuron-specific dopamine reuptake transporter, DAT-1 [78]. A summary of Mn effects on the DAergic system is illustrated in Figure 2.
Figure 2. General effects of Mn2+ exposure in dopaminergic neurons.
The net effects of Mn exposure in dopaminergic neurons are characterized by reduced dopamine levels resulting from various mechanisms [2]. Particularly, dopamine transporters, VMAT2 and DAT, are significantly down-regulated by manganese exposure leading to impaired dopamine handling in dopaminergic synapse [123]. In addition, decreased DAT activity may also occur due to DAT internalization [124]. Manganese exposure also results in increased dopamine oxidation involving Fenton chemistry, also resulting in reduced dopamine availability [125]. Recent findings also demonstrate that Mn2+ may reduce activity of tyrosine hydroxylase, a rate-limiting enzyme of dopamine synthesis [126], although the effect is shown to be dose-, time- [127], and age-dependent [6]. These data corroborate the observation of a significant reduction in tyrosine hydroxylasepositive neurons in substantia nigra pars compacta in response to manganese treatment [128]. In addition to modulation of dopamine levels, manganese exposure was also shown to modulate dopamine receptor expression and function [129].
3-MT - 3-Methoxytyramine; COMT - Catechol-O-methyltransferase; D1/2R - Dopamine receptor ½; DAT2 - Dopamine transporter; DOPA - 3,4-Dihydroxy-L-phenylalanine; DOPAC - 3,4-Dihydroxyphenylacetic acid; MAO - Monoamine oxidase; VMAT2 Vesicular monoamine transporter 2
3.2.2. Cholinergic System
Mn at neurotoxic levels also affects the cholinergic system. Acetylcholine (ACh) is an important excitatory neurotransmitter in the central and peripheral nervous systems, modulating essential cognitive functions such as learning, memory and locomotion [6, 79, 80]. Although the cholinergic system is not a primary target in Mn toxicity and several symptoms of PD and manganism are largely related to effects on the dopaminergic system, studies suggest that the cholinergic system may play an important role in both diseases, however many of these mechanisms are not well understood [6, 79, 80].
Mn alters choline uptake, Ach transferase (ChAT) activity, ACh release and postsynaptic ACh binding to clearance receptors [81–83].Studies in rats show that brain choline uptake was inhibited in the presence of Mn2+. Mn caused significant regional inhibition of choline uptake in the hippocampus, frontal and parietal cortices, caudate and putamen suggesting that choline uptake through the brain blood barrier is probably inhibited by Mn2+[81]. Different Mn exposure have shown different effects, indicating that toxic effects on cholinergic neurons occur only at specific stages of Mn poisoning [84].
The effects of Mn on ChAT in rats after life-long exposure showed that this enzyme decreased only in 2-month-old rats [39]. Since ChAT serves as a specific marker of cholinergic activity, these observations may point to a greater neurotoxic effect of manganese and greater vulnerability of cholinergic neurons in the developing brain [85]. Oxidative stress induced by Mn may mediate the decrease in ChAT activity through the denaturation of part of the enzymatic pool [86].
AChE is the degrading enzyme of ACh and therefore responsible for terminating cholinergic response in muscarinic and nicotinic ACh receptors in the brain [80]. Effects of short-term Mn exposure on adult rats have demonstrated a controversial effect on AChE activity, as both inhibition or activation have been reported [6]. Focusing on AChE inhibition, it is known that additive or synergistic mechanisms of cellular disruption caused by Mn leads to mitochondrial dysfunction and neuronal degeneration [80]. Several studies have shown that the deleterious effect of Mn on AChE and ChAT may be mediated through the induction of oxidative stress as treatment with antioxidants has been shown efficacious in reversing these inhibitions [87, 88]. The implications of Mn effects on cholinergic signaling are impairments in emotional and environmentl stimuli responses, in learning tasks and disruption of anti-inflammatory reactions. Mn-induced cortical cholinergic dysfunction is compatible with these cognitive deficits as well as those observed in dementia [79].
3.2.3. Glutamatergic System
Glutamate (Glu) is the most abundant excitatory neurotransmitter in the brain and plays several major roles in normal brain function [89]. Glu is synthesized at the nerve endings through the Krebs cycle using glucose or can also be converted from glutamine by astrocytes. Glu is highly sensitive to changes in energy supply, thus Mn-induced mitochondrial dysfunction could cause an imbalance in this neurotransmitter’s homeostasis[7, 69, 90–92]. In addition, Mn-induced ROS generation can directly inhibit Glu uptake, effectively increasing its extracellular concentrations. Studies indicate that Mn exposure may decrease the ability of astrocytes to absorb Glu, thereby increasing its excitatory potential in the synaptic cleft.This may explain the decrease in glutamate-aspartate transporter’s (GLAST) gene expression in astrocytes exposed to Mn, which would lead to a decrease in glutamate uptake, thus accumulating in the synaptic cleft and causing excitotoxicity[7, 69, 90].In nonhuman primates prolonged exposure to Mn has been associated with negative regulation of GLAST and GLT-1 transporter expression [93].
PKC stimulation significantly decreases Mn-induced astrocytic Glu uptake [91]. Studies show that increased PKC signaling by decreasing Mn-induced Glu transport has also been reported for Gin transport systems [94], The inhibitory effect of Mn on GLT-1 was reversed with the GLT-1 transcription factor mutant YY1 in astrocytes. Further studies have shown that an increase in YY1 expression upon Mn exposure was induced by NF-κB activation [95].
In addition, glutamate (Glu) and γ-aminobutyric acid (GABA) homeostasis is dependent on the metabolic interaction between neurons and astrocytes. The interaction, characterized by the glutamine (Gin)/Glu cycle (GGC) involves the synthesis of Gin by specific astrocytes and their subsequent release from the astrocytes into the extracellular space (Figure 1) [89, 90]. Impairment of the GGC has been previously described in response to Mn exposure [96, 97], since astrocytes accumulate Mn and show mitochondrial dysfunction [98]. As a consequence of the breakdown in their function and supportive role of neurons, increased glutamate levels in the synaptic cleft has been shown to cause excitotoxicity [99]. Disruption of GGC has been reported in numerous pathological conditions, such as epilepsy, cerebral ischemia, AD, PD and manganism [89].
3.2.4. GABAergic System
GABA (γ-aminobutyric acid) is known to be the major inhibitory neurotransmitter and this GABAergic system is affected by Mn; however the exact mechanisms underlying this effect are not well-known [69, 100, 101]. Several studies have suggesteda possible increase in GABA release upon exposure to Mn [102]. In contrast, it has been proposed that GABA uptake isinhibited by Mn exposure [103]. The mechanisms of Mn toxicity in the GABAergic system are poorly understood, but most likely factors such as concentration, time and exposure conditions are related to the toxic effects of Mn[100, 101, 104, 105].
GABA plays a key rolemediating the direct and indirect pathway of the basal ganglia, both with GABAergic thalamus, which then propagate to cortical regions [106, 107]. The ganglion-thalamus-cortical pathway is mainly involved in voluntary movement, fine motor control as well as in emotions, motivation and cognition that drive the movement [108]. Studies show high levels of thalamic GABA in a group of foundry workers with high Mn exposure levels [102]; however, data may vary depending on the animal model used [101, 109]. These discrepancies suggest that the effects of Mn on the GABAergic system are complex and that species differences, duration of exposure and routeof exposure may play a role in the effect of Mn neurotoxicity [6]. GABA clearance is partially performed by astrocytes, whichexpress the GABA transporter GAT3 isoform that is required to eliminate excess GABA from the synapse. The relatively high Km of GATS suggests that it plays a critical role in eliminating GABA from the synaptic cleft during Mn neurotoxicity [110]; however, with increasing Mn concentrations, the protective function of astrocytes may be compromised [110].
It is know that GABA biosynthesis depends strictly on the adequate supply of Glu within GABAergic neurons and subsequent conversion of Glu to GABA by Glu decarboxylase (GAD) [111]. Therefore, the mechanisms responsible for GGC between astrocytes and neurons are fundamental to brain physiology [7]. There are still many questions regarding the exact mechanism of Mn toxicity on the GABAergic system; however the dependence that exists between Glu and GABA suggests they are intertwined.
4. Perspectives and Concluding Remarks
The research on Mn’s effects in different animals has provided scientific basis for the understanding of its role in several biochemical and physiological processes. Research in this field has also enabled the understanding of the effects caused by Mn exposure and its putative mechanisms. These opposing biological effects are summarized in Figure 1.
The interaction between Mn exposure and genetic models for neurodegenerative diseases has been a subject of recent attention. In a C. elegans model, it has been demonstrated that wildtype human α-synuclein has a neuroprotective effect when mutants for PD genes were exposed to Mn [112]. On the other hano, evidence from human neuroblastoma cell lines SH-SY5Y and SKN-MC demonstrated that Mn exposure resulted in synergistic exacerbation of cellular toxicity with concomitant overexpression of a-synuclein [113, 114]. In addition, in vitro and in vivo models for Huntington’s disease (HD) have demonstrated that mutated huntingtin may affect Mn homeostasis by reducing its levels, impairing urea cycle (contributing to HD striatal urea-cycle pathophysiology) [115], and inhibiting autophagy (which increases protein aggregation and cell death) [116]. Striatal cells expressing mutant huntingtin appeared to be more resistant to Mn than wildtype cells secondary to reduced Mn accumulation, a finding that was also reproduced in vivo [117, 118]. In addition, Mn supplementation has been shown to restore autophagic function and promote aggregate clearance[116].
Other focus areas that have been subject to recent studies are the molecular targets of Mn, in hope of developing new pharmaceutical modalities to counteract the neurotoxic effects of Mn. Such mechanisms include transporters [119], transcriptional factors and modulating proteins [120, 121]. In addition, neuroprotective small molecules which might be effective in attenuating intracellular Mn levels, have been studied and recently reviewed by Peres et al [6].
There remain many gaps to be filled regarding Mn kinetics, additional mechanisms of toxicity and its role in triggering and propagating neurodegenerative diseases. The impacts of this metal needs to be further explored and elucidated since its deficiency or excessive levels can cause serious health conditions.
Acknowledgements
MA and ABB were supported in part by grants from the National Institute of Environmental Health Sciences, NIEHS, R01ES07331, R01ES10563. MA was also supported in part bygrant #R01ES020852. DSA is recipient of CNPq researcher scholarship and supported by CNPq (Universal Grant) 453963/2014-5, FAPERGS/PqG # 18/2551-0000434-0, CNPq/FAPERGS/DECIT/SCTIE-MS/PRONEM #16/25510000248-7 and PROPPI/UNIPAMPA. ATGS is recipiente of schollarship from Coordenaçäo de Aperfeiçoamento de Pessoal de Nivel Superior - Brasil (CAPES) Finance Code 001.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Aschner M, Erikson K, Manganese, Advances in nutrition 8(3) (2017) 520–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Chen P, Bornhorst J, Aschner M, Manganese metabolism in humans, Frontiers in bioscience 23 (2018) 1655–1679. [DOI] [PubMed] [Google Scholar]
- [3].Ávila DS, Gubert P, Roos DH, Puntel RL, Aschner M, Manganese, in: Caballero B (Ed.), Encyclopedia of Food and Health, Elsevier; 2015, pp. 637–641. [Google Scholar]
- [4].Ade KK, Janssen MJ, Ortinski P.l., Vicini S, Differential tonic GABA conductances in striatal medium spiny neurons, The Journal of neuroscience: the official journal of the Society for Neuroscience 28(5) (2008) 1185–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Levy BS, Nassetta WJ, Neurologic effects of manganese in humans: a review, International journal of occupational and environmental health 9(2) (2003) 153–63. [DOI] [PubMed] [Google Scholar]
- [6].Peres TV, Schettinger MR, Chen P, Carvalho F, Avila DS, Bowman AB, Aschner M, "Manganese-induced neurotoxicity: a review of its behavioral consequences and neuroprotective strategies", BMC pharmacology & toxicology 17(1) (2016) 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Ke T, Sidoryk-Wegrzynowicz M, Pajarillo E, Rizor A, Soares FAA, Lee E, Aschner M, Role of Astrocytes in Manganese Neurotoxicity Revisited, Neurochemical research 44(11) (2019) 2449–2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Aschner M, Guilarte TR, Schneider JS, Zheng W, Manganese: recent advances in understanding its transport and neurotoxicity, Toxicology and applied pharmacology 221(2) (2007) 131–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].O'Neal SL, Zheng W, Manganese Toxicity Upon Overexposure: a Decade in Review, Current environmental health reports 2(3) (2015) 315–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Santamaria AB, Manganese exposure, essentiality & toxicity, The Indian journal of medical research 128(4) (2008) 484–500. [PubMed] [Google Scholar]
- [11].Erikson KM, Thompson K, Aschner J, Aschner M, Manganese neurotoxicity: a focus on the neonate, Pharmacol Ther 113(2) (2007) 369–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Aschner JL, Aschner M, Nutriťonal aspects of manganese homeostasis, Molecular aspects of medicine 26(4–5) (2005) 353–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Aschner JL, Anderson A, Slaughter JC, Aschner M, Steele S, Beller A, Mouvery A, Furlong HM, Maitre NL, Neuroimaging identifies increased manganese deposition in infants receiving parenteral nutrition, The American journal of clinical nutrition 102(6) (2015) 1482–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Aschner M, Lukey B, Tremblay A, The Manganese Health Research Program (MHRP): status report and future research needs and directions, Neurotoxicology 27(5) (2006) 733–6. [DOI] [PubMed] [Google Scholar]
- [15].Horning KJ, Caito SW, Tipps KG, Bowman AB, Aschner M, Manganese Is Essential for Neuronal Health, Annual review of nutrition 35 (2015) 71–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Keen CL, Bell JG, Lonnerdal B, The effect of age on manganese uptake and retention from milk and infant formulas in rats, The Journal of nutrition 116(3) (1986) 395–402. [DOI] [PubMed] [Google Scholar]
- [17].Zlotkin SH, Atkinson S, Lockitch G, Trace elements in nutrition for premature infants, Clinics in perinatology 22(1) (1995) 223–40. [PubMed] [Google Scholar]
- [18].Lee DY, Johnson PE, Factors affecting absorption and excretion of 54Mn in rats, The Journal of nutrition 118(12) (1988) 1509–16. [DOI] [PubMed] [Google Scholar]
- [19].Davidsson L, Almgren A, Juillerat MA, Hurrell RF, Manganese absorption in humans: the effect of phytic acid and ascorbic acid in soy formula, The American journal of clinical nutrition 62(5) (1995) 984–7. [DOI] [PubMed] [Google Scholar]
- [20].Wapnir RA, Protein nutrition and mineral absorption, CRC Press; 1990. [Google Scholar]
- [21].Davis CD, Wolf TL, Greger JL, Varying levels of manganese and iron affect absorption and gut endogenous losses of manganese by rats, The Journal of nutrition 122(6) (1992) 1300–8.. [DOI] [PubMed] [Google Scholar]
- [22].Dorman DC, Struve MF, James RA, McManus BE, Marshall MW, Wong BA, Influence of dietary manganese on the pharmacokinetics of inhaled manganese sulfate in male CD rats, Toxicological sciences: an official journal of the Society of Toxicology 60(2) (2001) 242–51. [DOI] [PubMed] [Google Scholar]
- [23].Malecki EA, Radzanowski GM, Radzanowski TJ, Gallaher DD, Greger JL, Biliary manganese excretion in conscious rats is affected by acute and chronic manganese intake but not by dietary fat, The Journal of nutrition 126(2) (1996) 489–98. [DOI] [PubMed] [Google Scholar]
- [24].Gunshin H, Mackenzie B, Berger UV, Gunshin Y, Romero MF, Boron WF, Nussberger S, Gollan JL, Hediger MA, Cloning and characterization of a mammalian proton-coupled metalion transporter, Nature 388(6641) (1997) 482–8. [DOI] [PubMed] [Google Scholar]
- [25].Moldovan N, Al-Ebraheem A, Miksys NA, Farquharson MJ, Bock NA, Altered transition metal homeostasis in mice following manganese injections for manganese-enhanced magnetic resonance imaging, Biometals: an international journal on the role of metal ions in biology, biochemistry, and medicine 26(1) (2013) 179–87. [DOI] [PubMed] [Google Scholar]
- [26].Chen P, Chakraborty S, Mukhopadhyay S, Lee E, Paoliello MM, Bowman AB, Aschner M, Manganese homeostasis in the nervous system, Journal of neurochemistry 134(4) (2015) 601–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Couper J, On the effects of black oxide of manganese when inhaled into the lungs, British Annals of Medicine 1 (1837) 41–42. [Google Scholar]
- [28].Roels H, Lauwerys R, Buchet JP, Genet P, Sarhan MJ, Hanotiau I, de Fays M, Bernard A, Stanescu D, Epidemiological survey among workers exposed to manganese: effects on lung, central nervous system, and some biological indices, American journal of industrial medicine 11(3) (1987) 307–27. [DOI] [PubMed] [Google Scholar]
- [29].de Bie RM, Gladstone RM, Strafella AP, Ko JH, Lang AE, Manganese-induced Parkinsonism associated with methcathinone (Ephedrone) abuse, Archives of neurology 64(6) (2007) 886–9. [DOI] [PubMed] [Google Scholar]
- [30].Sikk K, Taba P, Haldre S, Bergquist J, Nyholm D, Askmark H, Danfors T, Sorensen J, Thurfjell L, Raininko R, Eriksson R, Flink R, Farnstrand C, Aquilonius SM, Clinical, neuroimaging and neurophysiological features in addicts with manganese-ephedrone exposure, Acta neurologica Scandinavica 121(4) (2010) 237–43. [DOI] [PubMed] [Google Scholar]
- [31].Ennok M, Sikk K, Haldre S, Taba P, Cognitive profile of patients with manganesemethcathinone encephalopathy, Neurotoxicology 76 (2020) 138–143. [DOI] [PubMed] [Google Scholar]
- [32].Li X, Xie J, Lu L, Zhang L, Zhang L, Zou Y, Wang Q, Luo X, Li S, Kinetics of manganese transport and gene expressions of manganese transport carriers in Caco-2 cell monolayers, Biometals: an international journal on the role of metal ions in biology, biochemistry, and medicine 26(6) (2013) 941–53. [DOI] [PubMed] [Google Scholar]
- [33].Avila DS, Puntel RL, Aschner M, Manganese in health and disease, Interrelations between essential metal ions and human diseases, Springer; 2013, pp. 199–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Mukhopadhyay S, Linstedt AD, Identification of a gain-of-function mutation in a Golgi Ptype ATPase mat enhances Mn2+ efflux and protects against toxicity, Proceedings of the National Academy of Sciences of the United States of America 108(2) (2011) 858–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Yin Z, Jiang H, Lee ES, Ni M, Erikson KM, Milatovic D, Bowman AB, Aschner M, Ferroportin is a manganese-responsive protein that decreases manganese cytotoxicity and accumulation, Journal of neurochemistry 112(5) (2010) 1190–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Leyva-lllades D, Chen P, Zogzas CE, Hutchens S, Mercado JM, Swaim CD, Morrisett RA, Bowman AB, Aschner M, Mukhopadhyay S, SLC30A10 is a cell surface-localized manganese efflux transporter, and parkinsonism-causing mutations block its intracellular trafficking and efflux activity, The Journal of neuroscience: the official journal of the Society for Neuroscience 34(42) (2014) 14079–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Brissot P, Bardou-Jacquet E, Jouanolle AM, Loreal O, Iron disorders of genetic origin: a changing world, Trends in molecular medicine 17(12) (2011) 707–13. [DOI] [PubMed] [Google Scholar]
- [38].Quadri M, Federico A, Zhao T, Breedveld GJ, Battisti C, Delnooz C, Severijnen LA, Di Toro Mammarella L, Mignarri A, Monti L, Sanna A, Lu P, Punzo F, Cossu G, Willemsen R, Rasi F, Oostra BA, van de Warrenburg BP, Bonifati V, Mutations in SLC30A10 cause parkinsonism and dystonia with hypermanganesemia, polycythemia, and chronic liver disease, American journal of human genetics 90(3) (2012) 467–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Aschner M, Aschner JL, Manganese transport across the blood-brain barrier: relationship to iron homeostasis, Brain research bulletin 24(6) (1990) 857–60. [DOI] [PubMed] [Google Scholar]
- [40].Crossgrove JS, Allen DD, Bukaveckas BL, Rhineheimer SS, Yokel RA, Manganese distribution across the blood-brain barrier. I. Evidence for carrier-mediated influx of managanese citrate as well as manganese and manganese transferrin, Neurotoxicology 24(1) (2003)3–13. [DOI] [PubMed] [Google Scholar]
- [41].dos Santos AP, Milatovic D, Au C, Yin Z, Batoreu MC, Aschner M, Rat brain endothelial cells are a target of manganese toxicity, Brain research 1326 (2010) 152–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Mishra G, Shukla R, Flasan M, Khanna SK, Das M, Potentiation of neurotoxicity of Lathyrus sativus by manganese: alterations in blood-brain barrier permeability, Toxicology mechanisms and methods 19(4) (2009) 318–26. [DOI] [PubMed] [Google Scholar]
- [43].Kodama H, Shimojo N, Suzuki KT, Distribution of manganese in rat pancreas and identification of its primary binding protein as pro-carboxypeptidase B, The Biochemical journal 278 ( Pt 3) (1991) 857–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Rahil-Khazen R, Bolann BJ, Myking A, Ulvik RJ, Multi-element analysis of trace element levels in human autopsy tissues by using inductively coupled atomic emission spectrometry technique (ICP-AES), Journal of trace elements in medicine and biology: organ of the Society for Minerals and Trace Elements 16(1) (2002) 15–25. [DOI] [PubMed] [Google Scholar]
- [45].Rivera-Mancia S, Rios C, Montes S, Manganese accumulation in the CNS and associated pathologies, Biometals: an international journal on the role of metal ions in biology, biochemistry, and medicine 24(5) (2011) 811–25. [DOI] [PubMed] [Google Scholar]
- [46].Zeron FIM, Rodriguez MR, Montes S, Castaneda CR, Blood manganese levels in patients with hepatic encephalopathy, Journal of trace elements in medicine and biology: organ of the Society for Minerals and Trace Elements 25(4) (2011) 225–9. [DOI] [PubMed] [Google Scholar]
- [47].Schroeder FIA, Balassa JJ, Tipton IH, Essential trace metals in man: manganese. A study in homeostasis, Journal of chronic diseases 19(5) (1966) 545–71. [DOI] [PubMed] [Google Scholar]
- [48].Chan DW, Son SC, Block W, Ye R. Khanna KK, Wold MS, Douglas P, Goodarzi AA, Pelley J, Taya Y, Lavin MF, Lees-Miller SP, Purification and characterization of ATM from human placenta. A manganese-dependent, wortmannin-sensitive serine/threonine protein kinase, The Journal of biological chemistry 275(11) (2000) 7803–10. [DOI] [PubMed] [Google Scholar]
- [49].Zhang P, Lokuta KM, Turner DE, Liu B, Synergistic dopaminergic neurotoxicity of manganese and lipopolysaccharide: differential involvement of microglia and astroglia, Journal of neurochemistry 112(2) (2010) 434–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Haase H, Innate Immune Cells Speak Manganese, Immunity 48(4) (2018) 616–618. [DOI] [PubMed] [Google Scholar]
- [51].Kaletta T, Hengartner MO, Finding function in novel targets: C. elegans as a model organism, Nature reviews. Drug discovery 5(5) (2006) 387–98. [DOI] [PubMed] [Google Scholar]
- [52].Albrecht J, Sidoryk-Wegrzynowicz M, Zielinska M, Aschner M, Roles of glutamine in neurotransmission, Neuron Glia Biol 6(4) (2010) 263–76. [DOI] [PubMed] [Google Scholar]
- [53].Li L, Yang X, The Essential Element Manganese, Oxidative Stress, and Metabolic Diseases: Links and Interactions, Oxidative medicine and cellular longevity 2018 (2018) 7580707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Anagianni S, Tuschl K, Genetic Disorders of Manganese Metabolism, Current neurology and neuroscience reports 19(6) (2019) 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Yang FI, Wang J, Yang X, Wu F, Qi Z, Xu B, Liu W, Deng Y, Occupational manganese exposure, reproductive hormones, and semen quality in male workers: A cross-sectional study, Toxicology and industrial health 35(1) (2019) 53–62. [DOI] [PubMed] [Google Scholar]
- [56].Cowan DM, Fan Q, Zou Y, Shi X, Chen J, Aschner M, Rosenthal FS, Zheng W, Manganese exposure among smelting workers: blood manganese-iron ratio as a novel tool for manganese exposure assessment, Biomarkers: biochemical indicators of exposure, response, and susceptibility to chemicals 14(1) (2009) 3–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Flynn MR, Susi P, Neurological risks associated with manganese exposure from welding operations--a literature review, International journal of hygiene and environmental health 212(5) (2009) 459–69. [DOI] [PubMed] [Google Scholar]
- [58].Fluang CC, Chu NS, Lu CS, Wang JD, Tsai JL, Tzeng JL, Wolters EC, Caine DB, Chronic manganese intoxication, Archives of neurology 46(10) (1989) 1104–6. [DOI] [PubMed] [Google Scholar]
- [59].Ono K, Komai K, Yamada M, Myoclonic involuntary movement associated with chronic manganese poisoning, Journal of the neurological sciences 199(1–2) (2002) 93–6. [DOI] [PubMed] [Google Scholar]
- [60].Balachandran RC, Mukhopadhyay S, McBride D, Veevers J, Harrison FE, Aschner M, Haynes EN, Bowman AB, Brain Manganese and the Balance between Essential Roles and Neurotoxicity, The Journal of biological chemistry (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Stepens A, Groma V, Skuja S, Platkajis A, Aldins P, Eksteina I, Martinsone I, Bricis R, Donaghy M, The outcome of the movement disorder in methcathinone abusers: clinical, MRI and manganesemia changes, and neuropathology, Eur J Neurol 21(2) (2014) 199–205. [DOI] [PubMed] [Google Scholar]
- [62].Chen P, Chakraborty S, Peres TV, Bowman AB, Aschner M, Manganese-induced Neurotoxicity: From C. elegans to Humans, Toxicology research 4(2) (2015) 191–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Avila DS, Puntel RL, Aschner M, Manganese in Health and Disease, 13 (2013) 199–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].DeWitt MR, Chen P, Aschner M, Manganese efflux in Parkinsonism: insights from newly characterized SLC30A10 mutations, Biochemical and biophysical research communications 432(1) (2013) 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Aschner M, Erikson K, Manganese, Advances in Nutrition: An International Review Journal 8(3)(2017)520–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Barbeau A, Manganese and extrapyramidal disorders (a critical review and tribute to Dr. George C. Cotzias), Neurotoxicology 5(1) (1984) 13–35. [PubMed] [Google Scholar]
- [67].Mena I, Marin O, Fuenzalida S, Cotzias GC, Chronic manganese poisoning. Clinical picture and manganese turnover, Neurology 17(2) (1967) 128–36. [DOI] [PubMed] [Google Scholar]
- [68].Farina M, Avila DS, da Rocha JB, Aschner M, Metals, oxidative stress and neurodegeneration: a focus on iron, manganese and mercury, Neurochemistry international 62(5) (2013) 575–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Fitsanakis VA, Au C, Erikson KM, Aschner M, The effects of manganese on glutamate, dopamine and gamma-aminobutyric acid regulation, Neurochemistry international 48(6–7) (2006) 426–33. [DOI] [PubMed] [Google Scholar]
- [70].Avila DS, Benedetto A, Au C, Rocha JBT, Aschner M, Bowman AB, Manganese and brain function, in: Avigliano L, Rossi L (Eds.), Biochemical Aspects of Human Nutrition, Research Signpost; 2010, pp. 197–222. [Google Scholar]
- [71].Brown DR, Hafiz F, Glasssmith LL, Wong BS, Jones IM, Clive C, Haswell SJ, Consequences of manganese replacement of copper for prion protein function and proteinase resistance, EM BO J 19(6) (2000) 1180–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Giese A, Levin J, Bertsch U, Kretzschmar H, Effect of metal ions on de novo aggregation of full-length prion protein, Biochemical and biophysical research communications 320(4) (2004) 1240–6. [DOI] [PubMed] [Google Scholar]
- [73].Benedetto A, Au C, Aschner M, Manganese-induced dopaminergic neurodegeneration: insights into mechanisms and genetics shared with Parkinson's disease, Chemical reviews 109(10) (2009) 4862–84. [DOI] [PubMed] [Google Scholar]
- [74].Graumann R, Paris I, Martinez-Alvarado P, Rumanque P, Perez-Pastene C, Cardenas SP, Marin P, Diaz-Grez F, Caviedes R, Caviedes P, Segura-Aguilar J, Oxidation of dopamine to aminochrome as a mechanism for neurodegeneration of dopaminergic systems in Parkinson's disease. Possible neuroprotective role of DT-diaphorase, Pol J Pharmacol 54(6) (2002) 573–9. [PubMed] [Google Scholar]
- [75].Gitler AD, Chesi A, Geddie ML, Strathearn KE, Hamamichi S, Hill KJ, Caldwell KA, Caldwell GA, Cooper AA, Rochet JC, Lindquist S, Alpha-synuclein is part of a diverse and highly conserved interaction network that includes PARK9 and manganese toxicity, Nature genetics 41(3) (2009) 308–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Settivari R, Levora J, Nass R, The divalent metal transporter homologues SMF-1/2 mediate dopamine neuron sensitivity in caenorhabditis elegans models of manganism and parkinson disease, The Journal of biological chemistry 284(51) (2009) 35758–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Au C, Benedetto A, Anderson J, Labrousse A, Erikson K, Ewbank JJ, Aschner M, SMF-1, SMF-2 and SMF-3 DMT1 orthologues regulate and are regulated differentially by manganese levels in C. elegans, PLoS One 4(11) (2009) e7792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Benedetto A, Au C, Avila DS, Milatovic D, Aschner M, Extracellular dopamine potentiates mn-induced oxidative stress, lifespan reduction, and dopaminergic neurodegeneration in a BLI3-dependent manner in Caenorhabditis elegans, PLoS genetics 6(8) (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Finkelstein Y, Milatovic D, Aschner M, Modulation of cholinergic systems by manganese, Neurotoxicology 28(5) (2007) 1003–14. [DOI] [PubMed] [Google Scholar]
- [80].Milatovic D, Gupta RC, Aschner M, Anticholinesterase toxicity and oxidative stress, TheScientificWorldJournal 6 (2006) 295–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Eriksson H, Morath C, Heilbronn E, Effects of manganese on the nervous system Acta neurologica Scandinavica. Supplementum 100 (1984) 89–93. [PubMed] [Google Scholar]
- [82].King RG, Sharp JA, Boura AL, The effects of Al3+, Cd2+ and Mn2+ on human erythrocyte choline transport, Biochemical pharmacology 32(23) (1983) 3611–7. [DOI] [PubMed] [Google Scholar]
- [83].Lockman PR, Roder KE, Allen DD, Inhibition of the rat blood-brain barrier choline transporter by manganese chloride, Journal of neurochemistry 79(3) (2001) 588–94. [DOI] [PubMed] [Google Scholar]
- [84].Lai JC, Leung TK, Guest JF, Davison AN, Lim L, The effects of chronic manganese chloride treatment expressed as age-dependent, transient changes in rat brain synaptosomal uptake of amines, Journal of neurochemistry 38(3) (1982) 844–7. [DOI] [PubMed] [Google Scholar]
- [85].Lai JC, Leung TK, Lim L, Differences in the neurotoxic effects of manganese during development and aging: some observations on brain regional neurotransmitter and nonneurotransmitter metabolism in a developmental rat model of chronic manganese encephalopathy, Neurotoxicology 5(1) (1984) 37–47. [PubMed] [Google Scholar]
- [86].Moyano P, Garcia JM, Anadon MJ, Lobo M, Garcia J, Frejo MT, Sola E, Pelayo A, Pino JD, Manganese induced ROS and AChE variants alteration leads to SN56 basal forebrain cholinergic neuronal loss after acute and long-term treatment, Food and chemical toxicology: an international journal published for the British Industrial Biological Research Association 125 (2019) 583–594. [DOI] [PubMed] [Google Scholar]
- [87].Liapi C, Zarros A, Galanopoulou P, Theocharis S, Skandali N, Al-Humadi H, Anifantaki F, Gkrouzman E, Mellios Z, Tsakiris S, Effects of short-term exposure to manganese on the adult rat brain antioxidant status and the activities of acetylcholinesterase, (Na,K)-ATPase and MgATPase: modulation by L-cysteine, Basic Clin Pharmacol Toxicol 103(2) (2008) 171–5. [DOI] [PubMed] [Google Scholar]
- [88].Adedara IA, Ego VC, Subair T.l., Oyediran O, Farombi EO, Quercetin Improves Neurobehaviorai Performance Through Restoration of Brain Antioxidant Status and Acetylcholinesterase Activity in Manganese-Treated Rats, Neurochemical research 42(4) (2017) 1219–1229. [DOI] [PubMed] [Google Scholar]
- [89].Bak LK, Schousboe A, Waagepetersen HS, The glutamate/GABA-glutamine cycle: aspects of transport, neurotransmitter homeostasis and ammonia transfer, Journal of neurochemistry 98(3) (2006) 641–53. [DOI] [PubMed] [Google Scholar]
- [90].Sidoryk-Wegrzynowicz M, Aschner M, Manganese toxicity in the central nervous system: the glutamine/glutamate-gamma-aminobutyric acid cycle, Journal of internal medicine 273(5) (2013) 466–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Sidoryk-Wegrzynowicz M, Lee E, Aschner M, Mechanism of Mn(ll)-mediated dysregulation of glutamine-glutamate cycle: focus on glutamate turnover, Journal of neurochemistry 122(4) (2012) 856–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Yudkoff M, Daikhin Y, Nissim I, Horyn O, Lazarow A, Luhovyy B, Wehrli S, Response of brain amino acid metabolism to ketosis, Neurochemistry international 47(1–2) (2005) 119–28. [DOI] [PubMed] [Google Scholar]
- [93].Erikson KM, Dorman DC, Lash LH, Aschner M, Duration of airborne-manganese exposure in rhesus monkeys is associated with brain regional changes in biomarkers of neurotoxicity, Neurotoxicology 29(3) (2008) 377–85. [DOI] [PubMed] [Google Scholar]
- [94].Sidoryk-Wegrzynowicz M, Lee E, Mingwei N, Aschner M, Disruption of astrocytic glutamine turnover by manganese is mediated by the protein kinase C pathway, Glia 59(11) (2011) 1732–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Karki P, Webb A, Smith K, Johnson J Jr., Lee K, Son DS, Aschner M, Lee E, Yang Yin 1 is a repressor of glutamate transporter EAAT2, and it mediates manganese-induced decrease of EAAT2 expression in astrocytes, Molecular and cellular biology 34(7) (2014) 1280–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Milatovic D, Yin Z, Gupta RC, Sidoryk M, Albrecht J, Aschner JL, Aschner M, Manganese induces oxidative impairment in cultured rat astrocytes, Toxicological sciences: an official journal of the Society of Toxicology 98(1) (2007) 198–205. [DOI] [PubMed] [Google Scholar]
- [97].Lee ES, Sidoryk M, Jiang H, Yin Z, Aschner M, Estrogen and tamoxifen reverse manganese-induced glutamate transporter impairment in astrocytes, Journal of neurochemistry 110(2) (2009) 530–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Hazell AS, Normandin L, Norenberg MD, Kennedy G, Yi JH, Alzheimer type II astrocytic changes following sub-acute exposure to manganese and its prevention by antioxidant treatment, Neuroscience letters 396(3) (2006) 167–71. [DOI] [PubMed] [Google Scholar]
- [99].Gobbo OL, Petit F, Gurden H, Dhenain M, In vivo detection of excitotoxicity by manganese-enhanced MRI: comparison with physiological stimulation, Magn Reson Med 68(1) (2012) 234–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Bonilla E, Increased GABA content in caudate nucleus of rats after chronic manganese chloride administration, Journal of neurochemistry 31(2) (1978) 551–2. [DOI] [PubMed] [Google Scholar]
- [101].Gwiazda RH, Lee D, Sheridan J, Smith DR, Low cumulative manganese exposure affects striatal GABA but not dopamine, Neurotoxicoiogy 23(1) (2002) 69–76. [DOI] [PubMed] [Google Scholar]
- [102].Dydak U, Jiang YM, Long LL, Zhu H, Chen J, Li WM, Edden RA, Hu S, Fu X, Long Z, Mo XA, Meier D, Harezlak J, Aschner M, Murdoch JB, Zheng W, In vivo measurement of brain GABA concentrations by magnetic resonance spectroscopy in smelters occupationally exposed to manganese, Environmental health perspectives 119(2) (2011) 219–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Anderson JG, Fordahl SC, Cooney PT, Weaver TL, Colyer CL, Erikson KM, Manganese exposure alters extracellular GABA, GABA receptor and transporter protein and mRNA levels in the developing rat brain, Neurotoxicoiogy 29(6) (2008) 1044–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Kersante F, Rowley SC, L Pavlov M, Gutierrez-Mecinas A, Semyanov JM, Reul MC, Walker AC Linthorst, A functional role for both -aminobutyric acid (GABA) transporter-1 and GABA transporter-3 in the modulation of extracellular GABA and GABAergic tonic conductances in the rat hippocampus, The Journal of physiology 591(10) (2013) 2429–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Long Z, Li XR, Xu J, Edden RA, Qin WP, Long LL, Murdoch JB, Zheng W, Jiang YM, Dydak U, Thalamic GABA predicts fine motor performance in manganese-exposed smelter workers, PLoS One 9(2) (2014) e88220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Graybiel AM, The basal ganglia, Current biology: CB 10(14) (2000) R509–11. [DOI] [PubMed] [Google Scholar]
- [107].Kropotov JD, Etlinger SC, Selection of actions in the basal ganglia-thalamocortical circuits: review and model, International journal of psychophysiology: official journal of the International Organization of Psychophysiology 31(3) (1999) 197–217. [DOI] [PubMed] [Google Scholar]
- [108].Ma RE, Ward EJ, Yeh CL, Snyder S, Long Z, Gokalp Yavuz F, Zauber SE, Dydak U, Thalamic GABA levels and occupational manganese neurotoxicity: Association with exposure levels and brain MRI, Neurotoxicoiogy 64 (2018) 30–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Burton NC, Guilarte TR, Manganese neurotoxicity: lessons learned from longitudinal studies in nonhuman primates, Environmental health perspectives 117(3) (2009) 325–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Fordahl SC, Erikson KM, Manganese accumulation in membrane fractions of primary astrocytes is associated with decreased gamma-aminobutyric acid (GABA) uptake, and is exacerbated by oleic acid and palmitate, Environmental toxicology and pharmacology 37(3) (2014) 1148–56. [DOI] [PubMed] [Google Scholar]
- [111].Waagepetersen HS, Sonnewald U, Larsson OM, Schousboe A, Synthesis of vesicular GABA from glutamine involves TCA cycle metabolism in neocortical neurons, Journal of neuroscience research 57(3) (1999) 342–9. [PubMed] [Google Scholar]
- [112].Bornhorst J, Chakraborty S, Meyer S, Lohren H, Brinkhaus SG, Knight AL, Caldwell KA, Caldwell GA, Karst U, Schwerdtle T, Bowman A, Aschner M, The effects of pdrl, djrl.l and pinkl loss in manganese-induced toxicity and the role of alpha-synuclein in C. elegans, Metallomics: integrated biometal science 6(3) (2014) 476–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Pifl C, Khorchide M, Kattinger A, Reither H, Hardy J, Hornykiewicz O, alpha-Synuclein selectively increases manganese-induced viability loss in SK-N-MC neuroblastoma cells expressing the human dopamine transporter, Neuroscience letters 354(1) (2004) 34–7. [DOI] [PubMed] [Google Scholar]
- [114].Li Y, Sun L, Cai T, Zhang Y, Lv S, Wang Y, Ye L, alpha-Synuclein overexpression during manganese-induced apoptosis in SH-SY5Y neuroblastoma cells, Brain research bulletin 81(4–5) (2010) 428–33. [DOI] [PubMed] [Google Scholar]
- [115].Bichell TJV, Wegrzynowicz M, Tipps KG, Bradley EM, Uhouse MA, Bryan M, Horning K, Fisher N, Dudek K, Halbesma T, Umashanker P, Stubbs AD, Holt HK, Kwakye GF, Tidball AM, Colbran RJ, Aschner M, Neely MD, Di Pardo A, Maglione V, Osmand A, Bowman AB, Reduced bioavailable manganese causes striatal urea cycle pathology in Huntington's disease mouse model, Biochimica et biophysica acta. Molecular basis of disease 1863(6) (2017) 1596–1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Bryan MR, O'Brien MT, Nordham KD, Rose DIR, Foshage AM, Joshi P, Nitin R, Uhouse MA, Di Pardo A, Zhang Z, Maglione V, Aschner M, Bowman AB, Acute manganese treatment restores defective autophagic cargo loading in Huntington's Disease cell lines, Human molecular genetics (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Kwakye GF, Li D, Bowman AB, Novel high-throughput assay to assess cellular manganese levels in a striatal cell line model of Huntington's disease confirms a deficit in manganese accumulation, Neurotoxicology 32(5) (2011) 630–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Williams BB, Li D, Wegrzynowicz M, Vadodaria BK, Anderson JG, Kwakye GF, Aschner M, Erikson KM, Bowman AB, Disease-toxicant screen reveals a neuroprotective interaction between Huntington's disease and manganese exposure, Journal of neurochemistry 112(1) (2010) 227–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Taylor CA, Hutchens S, Liu C, Jursa T, Shawlot W, Aschner M, Smith DR, Mukhopadhyay S, SLC30A10 transporter in the digestive system regulates brain manganese under basal conditions while brain SLC30A10 protects against neurotoxicity, The Journal of biological chemistry 294(6) (2019) 1860–1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Wollenhaupt SG, Soares AT, Salgueiro WG, Noremberg S, Reis G, Viana C, Gubert P, Soares FA, Affeldt RF, Ludtke DS, Santos FW, Denardin CC, Aschner M, Avila DS, Selenoand telluro-xylofuranosides attenuate Mn-induced toxicity in C. elegans via the DAF-16/FOXO pathway, Food and chemical toxicology: an international journal published for the British Industrial Biological Kesearch Association 64 (2014) 192–9. [DOI] [PubMed] [Google Scholar]
- [121].Peres TV, Arantes LP, Miah MR, Bornhorst J, Schwerdtle T, Bowman AB, Leal RB, Aschner M, Role of Caenorhabditis elegans AKT-1/2 and SGK-1 in Manganese Toxicity, Neurotoxicity research 34(3) (2018) 584–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Lee E, Karki P, Johnson J Jr., Hong P, Aschner M, Manganese Control of Glutamate Transporters' Gene Expression, Adv Neurobiol 16 (2017) 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Guilarte TR, Burton NC, McGlothan JL, Verina T, Zhou Y, Alexander M, Pham L, Griswold M, Wong DF, Syversen T, Schneider JS, Impairment of nigrostriatal dopamine neurotransmission by manganese is mediated by pre-synaptic mechanism(s): implications to manganese-induced parkinsonism, Journal of neurochemistry 107(5) (2008) 1236–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Roth J, Ponzoni S, Aschner M, Manganese homeostasis and transport, Met Ions Life Sci 12(2013)169–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Sistrunk SC, Ross MK, Filipov NM, Direct effects of manganese compounds on dopamine and its metabolite Dopac: an in vitro study, Environmental toxicology and pharmacology 23(3) (2007) 286–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Kumasaka MY, Yajima I, Ohgami N, Ninomiya H, lida M, Li X, Oshino R, Tanihata H, Yoshinaga M, Kato M, Manganese-Mediated Decrease in Levels of c-RET and Tyrosine Hydroxylase Expression In Vitro, Neurotoxicity research 32(4) (2017) 661–670. [DOI] [PubMed] [Google Scholar]
- [127].Zhang D, Kanthasamy A, Anantharam V, Kanthasamy A, Effects of manganese on tyrosine hydroxylase (TH) activity and TH-phosphorylation in a dopaminergic neural cell line, Toxicology and applied pharmacology 254(2) (2011) 65–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Stanwood GD, Leitch DB, Savchenko V, Wu J, Fitsanakis VA, Anderson DJ, Stankowski JN, Aschner M, McLaughlin B, Manganese exposure is cytotoxic and alters dopaminergic and GABAergic neurons within the basal ganglia, Journal of neurochemistry 110(1) (2009) 378–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Kern CH, Stanwood GD, Smith DR, Preweaning manganese exposure causes hyperactivity, disinhibition, and spatial learning and memory deficits associated with altered dopamine receptor and transporter levels, Synapse 64(5) (2010) 363–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Yu H, Iyer RK, Kern RM, Rodriguez W.l., Grody WW, Cederbaum SD, Expression of arginase isozymes in mouse brain, Journal of neuroscience research 66(3) (2001) 406–22. [DOI] [PubMed] [Google Scholar]
- [131].Scrutton MC, Utter MF, Mildvan AS, Pyruvate carboxylase. VI. The presence of tightly bound manganese, The Journal of biological chemistry 241(15) (1966) 3480–7. [PubMed] [Google Scholar]
- [132].Buettner GR, Ng CF, Wang M, Rodgers VG, Schafer FQ, A new paradigm: manganese superoxide dismutase influences the production of H202 in cells and thereby their biological state, Free Radic Biol Med 41(8) (2006) 1338–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Wedler FC, Denman RB, Glutamine synthetase: the major Mn(II) enzyme in mammalian brain, CurrTop Cell Regul 24 (1984) 153–69. [DOI] [PubMed] [Google Scholar]
- [134].Xiao L, Gong LL, Yuan D, Deng M, Zeng XM, Chen LL, Zhang L, Yan Q, Liu JP, Hu XH, Sun SM, Liu J, Ma HL, Zheng CB, Fu H, Chen PC, Znao JQ, Xie SS, Zou LJ, Xiao YM, Liu WB, Zhang J, Liu Y, Li DW, Protein phosphatase-1 regulates Aktl signal transduction pathway to control gene expression, ceil survival and differentiation, Cell Death Differ 17(9) (2010) 1448–62. [DOI] [PubMed] [Google Scholar]