TRANSLATIONAL PERSPECTIVE
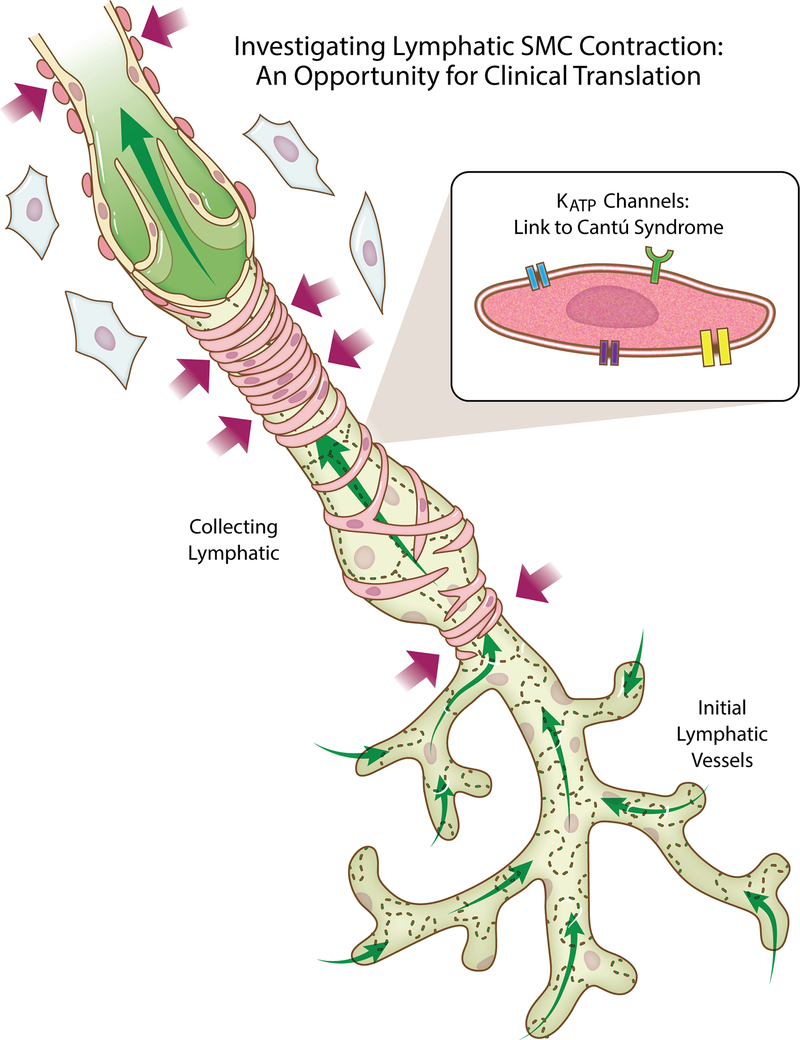
Lymphatic function is critical for maintaining interstitial fluid balance and is linked to multiple pathological conditions. Over the past 15 years lymphatic research has re-emerged as an area of emphasis for vascular biologists wanting to understand the causes of edema and the poor fluid drainage that are common denominators for various diseases (Breslin et al. 2018). Research has focused on two big questions: 1) How does fluid enter the lymphatic system? 2) How does fluid move through an initial lymphatic network? Interstitial fluid is thought to enter the lymphatic system through specialized intercellular junctions between endothelial cells of the initial lymphatic vessels. The fluid then passes into upstream collecting lymphatic vessels, also termed contractile vessels. These vessel segments, referred to as lympangions, are separated by intraluminal valves that prevent backflow of lymph. While current research has expanded to include coordination of lymphangion development, the active mechanism responsible for fluid flow from initial lymphatics to the collecting vessels, and the involvement of immune cells and other cell types, the mechanisms that control the frequency and force of contractions by lymphatic smooth muscle cells remain critical foci of investigation. Spontaneous smooth muscle cell contraction involves cell-surface receptors, ion fluxes and actin-myosin dynamics. As these mechanisms are essential for driving collecting vessel constriction and lymph flow, the discovery of new information has led to advancements in our understanding of how the lymphatic system works (Figure 1).
Figure 1:
Development of lymphatic smooth muscle cell (SMC specific mechanisms represent therapeutic targets for clinical translation. Interstitial fluid enters initial lymphatics and drains through collecting vessels, which are wrapped by SMCs. SMC constriction regulated by ion fluxes, receptor-mediated signaling, and interrelationships with other cell types is critical for lymphatic pumping and function. As highlighted by the work by Davis et al. in the current Journal of Physiology issue, basic science discovery of SMC mechanisms motivate new opportunities for translating an advanced understanding to treatment Cantú Syndrome and other diseases associated with lymphedema.
Basic science discoveries now motivate the challenge of linking new information to diseases. Perhaps, the more important question for lymphatic researchers and clinicians should be related to how our advanced understanding can impact treatment. In the current issue of the Journal of Physiology, work by Davis et al. provides an example of meeting this challenge by linking ATP sensitive smooth muscle specific potassium channel (KATP) dysfunction to Cantú Syndrome, a rare disease associated with genetic mutation that alters potassium channel function and leads to lymphedema. In the context of lymphatic function, the genetic mutation is considered to cause the potassium channels to be preferentially in the open state (Davis et a., 2020). The results by Davis et al. suggest that gain-of-function KATP subunit mutant mice also exhibit lymphatic contractile dysfunction, and that the inhibition of over-activity represents a therapeutic approach for reversing the effects. While a few studies have implicated the presence and a role for KATP channels in lymphatic vessels, the work by Davis et al. contributes cell-specific information regarding their role and a link to a disease state. The incorporation of studies with mutant mice, isolated vessels from humans, in vitro experiments, and multiple methodological approaches serve to provide novel mechanistic understanding of how a disease scenario can influence lymphatic fluid flow and in turn highlights why clinicians should be mindful about new basic research findings.
Acknowledgments
FUNDING
Funding was provided by NIH 1R01AG049821 awared to W.L.M. and NIH 1R01GM120774 awarded to J.W.B.
Footnotes
CONFLICTS OF INTEREST
The author does not have any conflicts of interests on the submission.
REFERENCES
- Breslin JW, Yang Y, Scallan JP, Sweat RS, Adderley SP, Murfee WL. (2018). Lymphatic Vessel Network Structure and Physiology. Compr Physiol 13;9(1):207–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M, Kim H, Zawieja S, Castorena-Gonzalez J, Gui P, Li M, Saunders B, Zinselmeyer B, Randolph G, Remedi M, Nichols C. (2020). Kir6.1-Dependent KATP Channels in Lymphatic Smooth Muscle and Vessel Dysfunction in Mice with Kir6.1 Gain-of-Function. Journal of Physiology. (JP-RP-2020–279612R1) [DOI] [PMC free article] [PubMed] [Google Scholar]