Abstract
Objective:
To study [i] the relationship between Body Mass Index (BMI) and knee synovial inflammation using non-contrast enhanced MRI and [ii] the association of synovial inflammation versus degenerative abnormalities and pain.
Materials and Methods:
Subjects with risk for and mild to moderate radiographic osteoarthritis were selected from the Osteoarthritis Initiative. Subjects were grouped into three BMI categories with 87 subjects per group: normal weight (BMI 20–24.9 kg/m2), overweight (BMI 25–29.9 kg/m2), and obese (BMI ≥ 30 kg/m2), frequency matched for age, sex, race, Kellgren-Lawrence grade, history of knee surgery and injury. Semi-quantitative synovial inflammation imaging biomarkers were obtained including effusion-synovitis, size and intensity of infrapatellar fat pad signal abnormality and synovial proliferation score. Cartilage composition was measured using T2 relaxation time and structural abnormalities using the whole-organ magnetic resonance imaging score (WORMS). The Western Ontario and McMasters (WOMAC) Osteoarthritis Index was used for pain assessment. Intra- and inter-reader reproducibility was assessed by kappa values.
Results:
Overweight and obese groups had higher prevalence and severity of all synovial inflammatory markers (p≤0.03). Positive associations were found between synovial inflammation imaging biomarkers and average T2 values, WORMS maximum scores and total WOMAC pain scores (p<0.05). Intra-reader and inter-reader kappa values for imaging biomarkers were high (0.76–1.00 and 0.60–0.94 respectively).
Conclusion:
Being overweight or obese was significantly associated with a greater prevalence and severity of synovial inflammation imaging biomarkers. Substantial reproducibility and high correlation with knee structural, cartilage compositional degeneration, and WOMAC pain scores validate the synovial inflammation biomarkers used in this study.
Keywords: Knee, osteoarthritis, synovitis, body mass index, magnetic resonance imaging
Introduction
Osteoarthritis (OA) is one of the leading causes of disability and reduced quality of life among older adults; it is characterized by pain and declining physical function [1], and frequently leads to joint replacement. The global prevalence of OA has been estimated to be over 300 million individuals [2]. According to a previous study, a 63.1% increase in years lived with disability (YLD) from 1990 to 2007 was followed by a further 31.4% increase from 2007 to 2017 [2]. Given our increasing aging population with greater amount of obesity [3], the burden of OA is expected to rise leading to major challenges for health care and public health systems.
OA has both systemic and local (periarticular) risk factors. Inflammation or synovitis, in the knee is a recognized local risk factor for knee OA [4, 5]. Magnetic Resonance Imaging (MRI) detected synovitis of the knee has been independently associated with the incidence of radiographic OA, cartilage loss and clinical progression of the disease [5]. Moreover, change in synovitis over time has been shown to correlate with changes in knee pain [5, 6]. While contrast-enhanced (CE) MRI is the gold standard for imaging-based synovitis assessment, more recently non-contrast enhanced (NCE) MRI has been used with promising results to measure the degree of synovitis [6]. Studies of active inflammatory processes in the knee using valid and reliable measures are needed to characterize a potential inflammatory OA phenotype [4, 5] and define patient populations for whom targeting synovitis may have the greatest benefits.
Obesity is one of the well-known risk factors in OA [7–9]. However, it is not fully understood how obesity is associated with OA. Historically, this association was ascribed to excessive joint loading as a result of increased body weight. However, the association between obesity and OA is also observed in non-weight-bearing joints [10, 11] suggesting a more complex etiology for obesity-induced OA. Similarly, metabolic syndrome has been found in some studies to be associated with an increased risk of knee OA but not hip OA, suggesting mechanical factors may not be solely responsible [12]. Inflamed adipose tissue and obesity-related dyslipidemia may cause causing low grade intraarticular inflammation thus playing a role in obesity-induced OA [13]. Furthermore, despite reports supporting the role of obesity and synovial inflammation, there is limited evidence from existing studies on the effects of BMI on synovial inflammation using MRI based biomarkers of inflammation.
This study sought to [i] examine the influence of Body Mass Index (BMI) on the prevalence and severity of synovial inflammation assessed with existing as well as novel and recently validated methods for use with NCE-MRI and [ii] determine the cross-sectional association between synovial inflammation and degenerative knee abnormalities including semi-quantitative whole organ MRI scores (WORMS) and T2 relaxation time measurements as well as pain symptoms.
Materials and Methods
Database and Subjects
The study participants were selected from the OA Initiative (OAI, https://oai.nih.gov). The OAI is a longitudinal, multi-center cohort study that recruited 4796 individuals and is sponsored by the US National Institutes of Health (NIH). Study protocol, amendments, and informed consent documentation were reviewed and approved by all local institutional review boards. Data used in the preparation of this manuscript were obtained from the publicly available OAI database (http://www.oai.ucsf.edu/). The specific OAI datasets used for this study were the baseline clinical dataset 0.2.2, baseline imaging datasets 0.E.1 and 0.C.2.
We studied the right knee in individuals from the OAI database examined at baseline. The right knee was chosen because the full imaging complement was available including T2 relaxation time measurements. The following inclusion criteria were used: [i] availability and acceptable quality of baseline right knee MRIs (with coronal and sagittal FSE sequences, and available T2-values) and [ii] Kellgren-Lawrence (KL) grade 0–3 in the study knee at the baseline visit. Subjects with a history of all inflammatory arthritis diagnosed during follow-up and arthroscopy for meniscectomy and ligament repair were excluded. We divided study participants into three groups based on BMI: a normal-weight group (BMI = 20–24.9 kg/m2), an overweight group (BMI > 25 and ≤ 29.9 kg/m2) and an obese group (BMI ≥ 30 kg/m2). The subjects were randomly selected in each group by using frequency matching for age, gender, race, KL grades, history of a knee injury and surgery (apart from arthroscopy) resulting in 87 subjects for each group (total n = 261). History of knee injury was assessed through a questionnaire in which subjects were asked whether they had a history of knee injury, causing difficulty to walk for at least one week. The subject selection process is illustrated in Fig. 1 and subject characteristics are listed in Table 2.
Fig. 1.
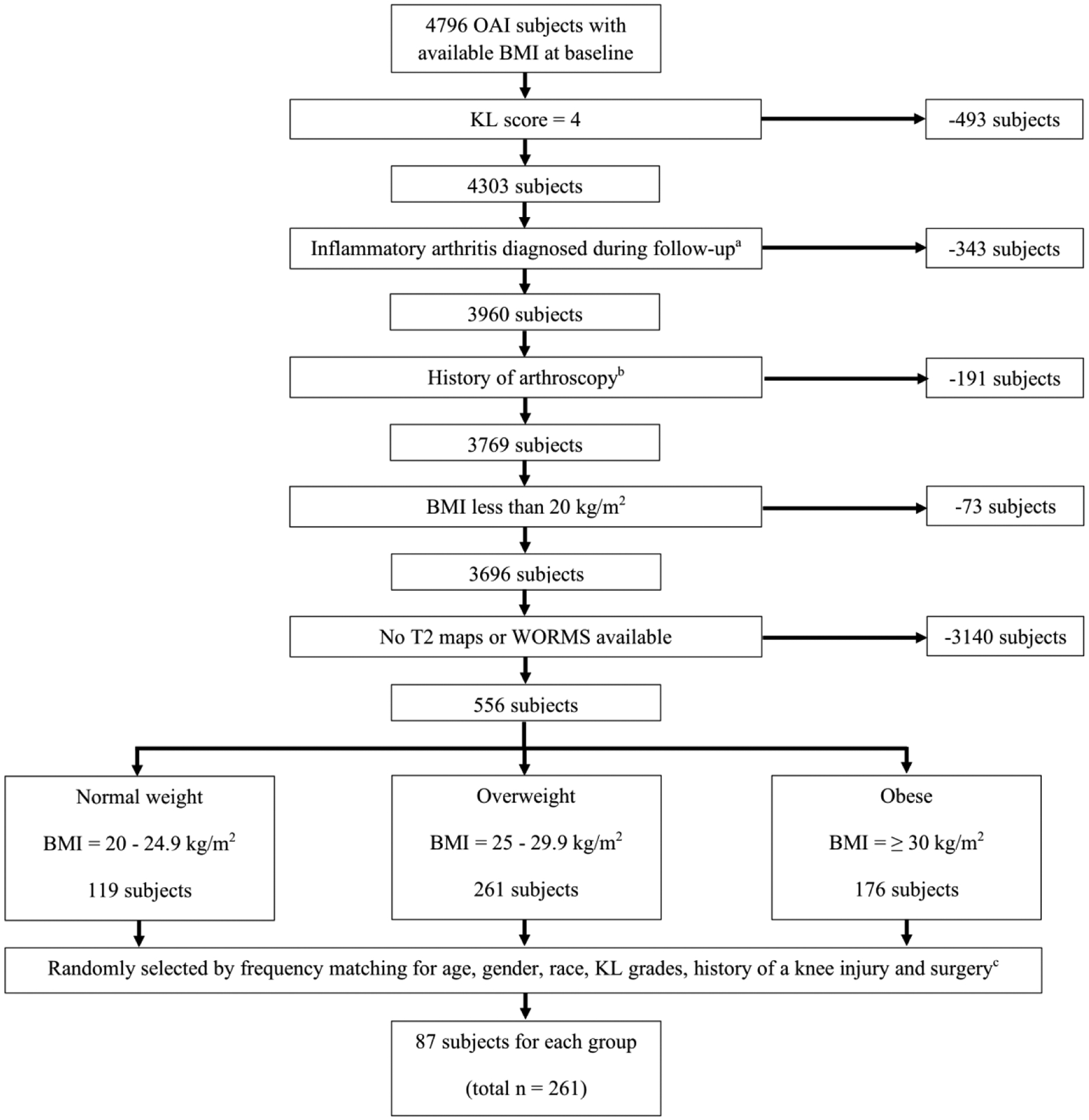
Patient selection from the OAI database.
aHistory of rheumatoid arthritis or other inflammatory arthritis
bHistory of knee arthroscopy for meniscectomy or ligament repair
cHistory of other types of knee surgery (all subjects with arthroscopy excluded)
Table 2.
Subject characteristics according to BMI category
| Normal weight (n=87) |
Overweight (n=87) |
Obesity (n=87) |
P-valuec | |
|---|---|---|---|---|
| BMI, mean ± SD kg/m2 | 22.76 ± 1.62 | 27.33 ± 1.40 | 33.03 ± 2.29 | <0.001b |
| Age, mean ± SD years | 55.64 ± 7.17 | 56.56 ± 8.67 | 57.72 ± 8.04 | 0.23b |
| Males (Percent) | 39 (44.83%) | 46 (52.87%) | 40 (45.98%) | 0.52a |
| History of knee injuryd (Percent) | 36 (41.38%) | 25 (28.74%) | 31 (35.63%) | 0.22a |
| History of knee surgerye (Percent) | 12 (13.79%) | 19 (21.84%) | 18 (20.69%) | 0.34a |
| Knee Kellgren-Lawrence scores | 0.97a | |||
| Grade 0 (Percent) | 57 (65.52%) | 53 (60.92%) | 52 (59.77%) | |
| Grade 1 (Percent) | 13 (14.94%) | 17 (19.54%) | 18 (20.69%) | |
| Grade 2 (Percent) | 12 (13.79%) | 11 (12.64%) | 12 (13.79%) | |
| Grade 3 (Percent) | 5 (5.75%) | 6 (6.90) | 5 (5.75) | |
| Racial composition | 0.90a | |||
| White or Caucasian (Percent) | 69 (79.31%) | 70 (80.46%) | 68 (78.16%) | |
| Black or African American (Percent) | 14 (16.09%) | 14 (16.09%) | 17 (19.54%) | |
| Other non-white (Percent) | 4 (4.60%) | 3 (3.45%) | 2 (2.30%) | |
| Total WOMAC knee pain, mean ± SD | 1.52 ± 2.60 | 2.43 ± 3.29 | 1.71 ± 2.53 | 0.08b |
Pearson’s chi-squared test
Linear regression analysis
Significant values are in bold (p value < 0.05)
History of knee injury causing difficult to walk for at least one week
History of other types of knee surgery (all subjects with arthroscopy excluded)
BMI, body mass index; WOMAC, Western Ontario and McMasters Osteoarthritis Index
MR Imaging Protocol
MR images were obtained at four different clinical sites of the OAI with cross calibrated 3.0-T scanners (Magnetom Trio, Siemens, Erlangen, Germany) using identical quadrature transmit-receive coils (USA Instruments, Aurora, Ohio). To semi-quantitatively assess structural abnormalities of the right knee three sequences were used: (a) a coronal 2D intermediate-weighted (IW) turbo spin-echo (TSE) sequence (repetition time [TR]/echo time [TE] = 3700/29 ms), (b) a sagittal 3D dual-echo steady-state (DESS) sequence with water excitation and coronal and axial reformations (TR/TE = 16.3/4.7 ms, flip angle = 25°) and (c) a sagittal 2D IW fat-suppressed TSE sequence (TR/TE = 3200/30 ms).
Quantitative measurements based on T2 relaxation time were obtained of the right knee using a sagittal 2D multislice multiecho (MSME) spin-echo (SE) sequence (TR = 2700ms, TEs = 10, 20, 30, 40, 50, 60 and 70ms, field of view = 12cm, slice thickness = 3mm with 0.5 mm gap, in-plane spatial resolution = 0.31 × 0.45 mm2). This sequence was only performed at the right knee. Additional information about the above sequences is available in the OAI MR protocol [14].
MR Image Analysis
Synovial inflammatory markers
Right knee baseline images were assessed by one radiologist (T.K.; 5 years of experience) blinded to subject characteristics and under supervision of a board-certified musculoskeletal radiologist (T.M.L.; 24 years of experience). Specifically, six sub-features were graded per knee as described in previous publications (Table 1) [15–18]. As it is not always possible to differentiate synovial thickening from intraarticular joint fluid on NCE-MRI, we used the term effusion-synovitis as previously described [16, 18]. First, the extent of effusion-synovitis was graded by measuring the maximum anteroposterior (AP) diameter of the suprapatellar recess in mm at a midline slice on sagittal fat-saturated intermediated weighted (IW) or DESS images according to the Anterior Cruciate Ligament OsteoArthritis Score (ACLOAS) [18]. In detail, effusion-synovitis was graded from 0 to 3 according to the degree of capsular distension with grade 0 being equivalent to a < 2 mm anteroposterior diameter of the effusion. A joint distension spanning ≥ 2 to < 5 mm in the AP diameter on the mid-slice sagittal image was graded as 1, while a joint effusion between ≥ 5 and < 10 mm was graded as 2. Any joint distension measuring ≥ 10 mm in the AP-diameter was scored as grade 3. Second, effusion-synovitis was also graded on axial fat-saturated DESS images according to the MRI Osteoarthritis Knee Score (MOAKS) [16]. In detail, effusion-synovitis was graded by using a 4-point scale (0 = physiologic amount of fluid; 1= small amount of fluid continuously extending into the retropatellar space; 2 = medium - with slight convexity of the suprapatellar bursa; 3= large - evidence of capsular distention). Third and fourth, we assessed the size and highest signal intensity on the sagittal IW fat-suppressed images of Hoffa or the infrapatellar fat pad (IPFP) abnormality as previously published [17]. The size of the IPFP signal abnormalities was graded as follows: grade 0 = no signal abnormality; grade 1 ≤ 33% of the region showing signal abnormality; grade 2 = 34 – 66% of the region; grade 3 ≥ 66% of the region. Grade of signal intensity (SI) was characterized as follows: grade 0 = none; grade 1 = mild (lower than cartilage); grade 2 = moderate (equal to or higher than cartilage but lower than fluid); grade 3 = severe (equal to fluid). Fifth, the presence and severity of synovial proliferations in the knee were assessed on fat-saturated DESS and IW images, if the effusion-synovitis score was ≥ 1 by either ACLOAS or MOAKS methods, in the suprapatellar recess and other visible areas of the joint [15]. Grade 1 corresponded to a smooth synovium, with no proliferation or synovial bands visible; grade 2 was defined as a mild irregularity of the synovium, either focal or diffuse, and the presence of some synovial bands or small bodies; grade 3 was defined as extensive synovial thickening with irregular villonodular proliferation. Finally, the same synovial proliferation score was used in knees with a popliteal cyst. We did not evaluate the suprapatellar fat pad in our study because our group previously showed that suprapatellar fat pad (SPFP) signal alteration or its mass effect was not associated with baseline degenerative changes of the knee joint [19]. Furthermore, SPFP abnormalities were rarely associated with knee pain and tended to occur in highly active young subjects [20] who are typically not obese or overweight.
Table 1.
Grading of MRI-synovial inflammatory markers
| MRI-inflammatory markers | Criteria |
|---|---|
| Effusion-synovitis according to ACLOAS | Anteroposterior diameter of joint distension on the mid-slice sagittal image |
| 0 | < 2 mm |
| 1 | ≥ 2 to < 5 mm |
| 2 | ≥ 5 and < 10 mm |
| 3 | ≥ 10 mm |
| Effusion-synovitis according to MOAKS | Graded on axial image |
| 0 | Physiologic amount of fluid |
| 1 | Small amount of fluid continuously extending into the retropatellar space |
| 2 | Medium - with slight convexity of the suprapatellar bursa |
| 3 | Large - evidence of capsular distention |
| Infrapatellar fat pad (IPFP) abnormality | Hyperintense signal intensity in IPFP on IW fat-suppressed images |
| The size of IPFP abnormality | |
| 0 | No signal abnormality |
| 1 | ≤ 33% of the region |
| 2 | 34 – 66% of the region |
| 3 | ≥ 66% of the region |
| The highest signal intensity in IPFP abnormality | |
| 0 | None |
| 1 | Mild (lower than cartilage) |
| 2 | Moderate (equal to or higher than cartilage but lower than fluid) |
| 3 | Severe (equal to fluid) |
| Synovial proliferation score in kneea and popliteal cystb | Graded on fat-saturated DESS and IW images |
| 1 | Smooth synovium, with no proliferation or synovial bands visible |
| 2 | Mild irregularity of the synovium, either focal or diffuse, and the presence of some synovial bands or small bodies |
| 3 | Extensive synovial thickening with irregular villonodular proliferation |
If the synovitis-effusion score was ≥ 1 by either ACLOAS or MOAKS methods
Used only in knees with a popliteal cyst
ACLOAS, Anterior Cruciate Ligament OsteoArthritis Score; MOAKS, MRI Osteoarthritis Knee Score; IW, Intermediate-Weighted sequence
Other semi-quantitative structural abnormalities in knee
The remaining features of OA related abnormalities were assessed using the WORMS semi-quantitative analysis [21, 22]. The following parameters were evaluated separately: meniscal lesions were graded from 0 to 4 in each of 6 regions (medial / lateral and anterior / body / posterior); cartilage defects were graded from 0 to 6, bone marrow edema like lesions (BML) and subarticular cysts were scored from 0 to 3 in each of 6 regions (patella, trochlea, medial / lateral femur, and medial / lateral tibia). Other lesions including ligamentous abnormalities and popliteal cysts were also scored from 0 to 3. The WORMS max score for each lesions was defined as the maximum score in any knee region, for each lesion, respectively.
T2 relaxation time measurements
The software used for the T2 analysis is a spline-based algorithm written in MATLAB (The Mathworks, Natick, Massachusetts), that has been described previously [23]. The cartilage of five compartments (patella, lateral femur, medial femur, lateral tibia, medial tibia) was semi-automatically segmented. The trochlear region was excluded from the analysis due to flow-artifacts caused by the popliteal artery. Mean baseline T2 values were computed separately for each compartment and globally (average of all compartments combined) of the segmented regions of interest.
Intra- / inter-reader reproducibility
Reproducibility for composite MRI-synovial inflammatory scores was assessed in 20 randomly selected subjects. For reproducibility analysis we included 20 subjects based on a previous publication by Sim et al [24]. All gradings were performed twice by one musculoskeletal radiologist (T.K.) on two separate occasions (at least 3 weeks apart) for intra-observer reproducibility and also compared with the gradings performed by a second musculoskeletal radiologist (T.M.L.) for inter-reader reproducibility.
Reproducibility results of the WORMS gradings and the knee cartilage T2 relaxation time measurements have been described and validated by our group in multiple previous studies [21, 23, 25, 26]. For WORMS readings (all lesion combined), the Cohen’s κ value for intra-reader reproducibility was 0.64 and values for inter-reader reproducibility ranged from 0.73 to 0.77 [26]. For the reproducibility of cartilage T2 relaxation time measurements, coefficients of variation (CV) were calculated on a percentage basis as the root mean square average, with CVs ranging from 1.66% to 1.76% for intra-reader reproducibility and 1.12% to 2.06% for inter-reader reproducibility.
Pain assessment
The Western Ontario and McMasters (WOMAC) Osteoarthritis Index is a well-established clinical tool used to quantify OA symptoms in the knee, including pain, stiffness, and physical function [27]. We assessed pain symptoms in the right knee within the past seven days prior to the MRI by using the WOMAC pain subscale. There are five questions in the WOMAC asking the person to describe the pain they have experienced during certain activities: during walking, stair climbing, rest, nocturnal, and weight-bearing with five possible responses (none, mild, moderate, severe, and extreme, scored as 0 – 4). Total WOMAC knee pain was calculated by summation of scores from all five questions.
Statistical analysis
Statistical analyses were performed with STATA version 14 software (StataCorp LP, College Station, TX), using a two-sided, 0.05 level of significance. We considered a trend toward statistical significance if the p-value was equal to 0.05 – 0.10 [28]. The differences in baseline subject characteristics between groups were assessed using linear regression analysis (continuous variables) and Pearson’s chi-square tests (categorical variables). For the cross-sectional analysis, linear regression models were used to assess the differences in six MRI-synovial inflammatory markers (synovitis-effusion by two methods, highest signal and size of IPFP abnormalities and synovial proliferation score in knee and popliteal cyst) between overweight and obese groups compared to normal weight group as well as the obese group compared to the overweight group. A linear regression model was also used to assess associations between composite MRI-synovial inflammatory markers and T2 (average T2 value), morphologic parameters (WORMS) and pain symptoms (WOMAC). All linear regression analyses were adjusted for baseline age, gender and race. The additional analyses for association between MRI-synovial inflammatory markers, degenerative knee abnormalities and WOMAC pain score were done with adjustment for age, gender, race and BMI. This additional adjustment was performed in order to assess whether the association between MRI-based synovial inflammation markers and degenerative knee abnormalities as well as WOMAC pain score were not affected by baseline BMI.
Inter- and intra-reader reproducibility measurements for MRI-synovial inflammation markers were tested by weighted kappa values. According to Landis and Koch [29], a Kappa value of less than 0.00 indicates poor agreement; a value of 0.00–0.20, slight agreement; a value of 0.21–0.40, fair agreement; a value of 0.41–0.60, moderate agreement; a value of 0.61–0.80, substantial agreement; and a value of 0.81–1.00, almost perfect agreement.
Results
Subject characteristics and BMI
Subjects had a mean age of 56.64 ± 8.00 years and an average BMI of 27.70 ± 4.58 kg/m2. Of note, 69 subjects (25.46%) had popliteal cysts. Table 2 outlines the baseline distribution of patient characteristics and OA risk factors according to the BMI category. There were no significant differences in age, sex, history of knee injury, history of knee surgery, KL scores, and race between the three BMI groups. Total WOMAC knee pain scores for overweight and obese group compared with normal weight group showed no significant difference (p = 0.081) adjusted for age, gender and race. When compared with the overweight group, the WOMAC pain score was significantly lower in the normal weight group (Difference = −0.92 [95%CI = −1.74 – −0.07], p = 0.03) but there was no significant difference in WOMAC pain score between the obese and overweight group (−0.72 [−1.68 – 0.43], p = 0.09). WOMAC pain score between the obese and normal weight group was not statistically significant (0.16 [−0.66 – 0.97], p = 0.70).
BMI and MRI-synovial inflammation abnormalities
Table 3 shows the scores for the MRI-synovial inflammatory markers across all BMI groups. Overall overweight and obese groups had significantly higher synovial inflammatory markers compared with the normal weight group (all p < 0.05; Table 2, Figure 1). Overweight and obese groups had a greater score for synovial proliferation (0.75 [0.51 – 1.00], p < 0.001 and 0.78 [0.54 – 1.02], p < 0.001 respectively) and also for subjects with popliteal cysts (0.26 [0.02 – 0.51], p = 0.04 and 0.31 [0.02 – 0.51], p = 0.01 respectively). There was no significant difference for effusion-synovitis and synovial proliferation scores between overweight and obese groups. Only the size of IPFP signal abnormality in the obese group showed no significant difference compared to the normal weight group (0.14 [−0.03 – 0.31], p = 0.11). In comparison to the overweight group, the obese group had a lower proportion of IPFP signal abnormalities with significant difference for size (−0.21 [−0.38 – 0.03], p = 0.02) but no statistical significance for higher signal intensity (−0.14 [−0.38 – 0.09], p = 0.24). On the other hand, there were no significant differences in the remaining synovial inflammatory markers between the overweight and obese groups.
Table 3.
Differences in MRI-synovial inflammatory biomarkers between the overweight and obese groups compared to the normal weight group (All p-values were statistically significant except for size of IPFP abnormality in the obese vs. normal weight group)
| MRI-Inflammatory markers |
Normal weight | Overweight | Obese | Overall P-valuea,b |
||||
|---|---|---|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Difference compared to normal (95% CI) |
P-valuea,b | Mean ± SD | Difference compared to normal (95% CI) |
P-valuea,b | ||
| Effusion-synovitis | ||||||||
| - ACLOAS method | 0.37 ± 0.07 | 0.64 ± 0.07 | 0.27 (0.07 – 0.47) |
0.01 | 0.71 ± 0.07 | 0.34 (0.14 – 0.53) |
0.001 | 0.002 |
| - MOAKS method | 0.56 ± 0.07 | 0.94 ± 0.07 | 0.38 (0.19 – 0.58) |
<0.001 | 1.05 ± 0.07 | 0.50 (0.30 – 0.69) |
<0.001 | <0.001 |
| IPFP abnormality | ||||||||
| - Highest SI | 0.93 ± 0.09 | 1.35 ± 0.09 | 0.42 (0.18 – 0.66) |
0.001 | 1.21 ± 0.09 | 0.28 (0.04 – 0.52) |
0.02 | 0.002 |
| - Size | 0.76 ± 0.06 | 1.11 ± 0.06 | 0.35 (0.17 – 0.52) |
<0.001 | 0.91 ± 0.06 | 0.14 (−0.03 – 0.31) |
0.11 | 0.001 |
| Synovial proliferation score | ||||||||
| - Knee | 0.82 ± 0.09 | 1.57 ± 0.09 | 0.75 (0.51 – 1.00) |
<0.001 | 1.60 ± 0.09 | 0.78 (0.54 – 1.02) |
<0.001 | <0.001 |
| - Popliteal cyst | 0.30 ± 0.09 | 0.56 ± 0.09 | 0.26 (0.02 – 0.51) |
0.04 | 0.61 ± 0.09 | 0.31 (0.06 – 0.56) |
0.01 | 0.03 |
Linear regression analysis adjusted for age, gender and race
Significant p-values are in bold (p-value < 0.05)
ACLOAS, Anterior Cruciate Ligament OsteoArthritis Score; MOAKS, MRI Osteoarthritis Knee Score; IPFP, InfraPatellar Fat Pad; SI, signal intensity
MRI-synovial inflammation abnormalities, T2 and structural knee abnormalities
Subjects with MRI-synovial inflammatory markers had significantly higher T2-values adjusted for age, gender and race (all p < 0.01) (Table 4). Even after additional adjustment for BMI, IPFP abnormalities and synovial proliferation score in popliteal cysts still remained statistically significant (p = 0.001– 0.03), and effusion-synovitis by both methods demonstrated a trend toward statistical significance (p = 0.07). Only the synovial proliferation score in the knee did not reach statistical significance after additional adjustment for BMI (p = 0.14).
Table 4.
Association between MRI-based synovial inflammation markers in the knee and T2/WORMS
| Synovitis -Effusion | IPFP abnormality | Synovial proliferation score | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ACLOAS method | MOAKS method | Highest SI | Size | Knee | Popliteal cyst | |||||||
| Coef. (95% CI) | P-valuea,b | Coef. (95% CI) | P-valuea,b | Coef. (95% CI) | P-valuea,b | Coef. (95% CI) | P-valuea,b | Coef. (95% CI) | P-valuea,b | Coef. (95% CI) | P-valuea,b | |
| Average T2 value | 0.52 (0.15 – 0.89) |
0.01 | 0.62 (0.25 – 0.98) |
0.001 | 0.57 (0.27 – 0.88) |
<0.001 | 0.48 (0.07 – 0.90) |
0.02 | 0.45 (0.17 – 0.74) |
0.002 | 0.46 (0.16 – 0.76) |
0.003 |
| WORMS max scoresc | ||||||||||||
| Meniscus | 0.17 (− 0.03 – 0.38) |
0.09 | 0.28 (0.08 – 0.48) |
0.01 | 0.27 (0.10 – 0.43) |
0.002 | 0.15 (−0.08 – 0.37) |
0.20 | 0.22 (0.07 – 0.37) |
0.01 | 0.17 (0.01 – 0.34) |
0.04 |
| Ligament | 0.08 (−0.06 – 0.22) |
0.27 | 0.14 (0.01 – 0.28) |
0.04 | 0.16 (0.04 – 0.28) |
0.01 | 0.18 (0.03 – 0.34) |
0.02 | 0.07 (−0.04 – 0.18) |
0.22 | −0.05 (−0.17 – 0.06) |
0.37 |
| Cartilage | 0.73 (0.46 – 1.0) |
<0.001 | 0.47 (0.19 – 0.75) |
0.001 | 0.29 (0.06 – 0.53) |
0.01 | 0.24 (−0.08 – 0.56) |
0.14 | 0.38 (0.16 – 0.59) |
0.001 | 0.15 (−0.08 – 0.38) |
0.20 |
| Bone marrow lesion | 0.23 (0.06 – 0.41) |
0.01 | 0.19 (0.01 – 0.36) |
0.04 | 0.15 (0.01 – 0.30) |
0.04 | 0.09 (−0.11 – 0.29) |
0.37 | 0.05 (−0.08 – 0.19) |
0.43 | 0.07 (−0.08 – 0.21) |
0.36 |
| Subchondral bone cyst | 0.24 (0.06 – 0.41) |
0.01 | 0.32 (0.15 – 0.49) |
<0.001 | 0.05 (−0.09 – 0.20) |
0.48 | 0.04 (−0.16 – 0.24) |
0.69 | 0.11 (−0.03 – 0.24) |
0.12 | 0.11 (−0.03 – 0.25) |
0.13 |
Linear regression analysis adjusted for age, gender and race
Significant p-values are in bold (p-value < 0.05)
WORM = semi-quantitative whole organ MRI scores; WORMS max scores were defined as the maximum lesion score in any knee region
ACLOAS, Anterior Cruciate Ligament OsteoArthritis Score; MOAKS, MRI Osteoarthritis Knee Score; IPFP, InfraPatellar Fat Pad; SI, signal intensity; WOMAC, Western Ontario and McMasters Osteoarthritis Index
A similar analysis demonstrated that overall MRI-synovial inflammation abnormalities had a positive correlation with the severity of WORMS maximum scores but there were different degrees of correlation as shown in Table 4. None of the MRI-synovial inflammation abnormalities demonstrated a negative correlation with WORMS maximum scores. Effusion-synovitis scores using the MOAKS methods were significantly correlated with all WORMS maximum scores, including meniscus, cartilage, BML, and subchondral bone cysts (p < 0.01 – 0.04). Subjects with higher signal change in the IPFP had significantly higher meniscus, ligament, cartilage and BML max scores (p = < 0.01 – 0.04) but subjects with greater size of IPFP signal abnormality had only more significant ligament max scores (p = 0.02). Subjects with higher synovial proliferation scores in the popliteal cyst had only significantly higher meniscus maximum scores (p = 0.04). These findings still remained significant after adjustment for BMI.
Higher WOMAC knee pain scores were found in subjects with higher effusion-synovitis by both methods (ACLOAS method; 0.89 [0.40 – 1.38], p < 0.01 and MOAKS method; 0.57 [0.08 – 1.06], p = 0.02), higher signal in the IPFP (0.47 [0.06 – 0.88], p = 0.03), and higher synovial proliferation score (0.49 [0.11 – 0.87], p = 0.01). No significant correlation was observed for the size of IPFP abnormality and synovial proliferation score in the popliteal cyst (p-value 0.09 and 0.21 respectively). These findings remained unchanged after adjustment for BMI.
Reproducibility of clinical readings
Observed agreement and weighted Cohen’s κ values were calculated to compare each score separately. Intra-reader agreement/κ values were 97.50%/0.94 and 95%/0.84 for effusion-synovitis using ACLOAS and MOAKS methods respectively, 93.33%/0.76 and 95%/0.81 for IPFP signal and size respectively, 96.67%/0.91 and 100%/1.00 for synovial proliferation score of the knee and popliteal cyst respectively. Inter-reader agreement/κ values were 97.50%/0.94 and 98.33%/0.90 for effusion-synovitis using ACLOAS and MOAKS methods respectively, 97.50%/0.93 and 87.50%/0.60 for IPFP signal intensity and size respectively, 95.00%/0.86 and 96.67%/0.89 for synovial proliferation score of the knee and popliteal cyst respectively. These findings demonstrated moderate inter-reader agreement for the size of IPFP abnormality, substantial intra-reader agreement of the signal of IPFP abnormality and almost perfect agreement for the other MRI-synovial inflammation abnormalities.
Discussion
This study examined the association of BMI and the prevalence of knee synovial inflammatory abnormalities diagnosed on NCE MRI and found an increased prevalence and severity of MRI-synovial inflammation biomarkers (effusion-synovitis, IPFP abnormalities and synovial proliferation score) with BMI. Our study also found that MRI-synovial inflammatory biomarkers were positively correlated with knee joint structural and cartilage compositional changes (WORMS and T2-values) as well as pain symptoms (WOMAC pain score), which validated our inflammatory biomarkers as parameters of increased joint degeneration. Moreover, high intra-reader and inter-reader reliabilities for MRI-synovial inflammatory biomarkers were demonstrated.
The link between obesity and OA is multifactorial; in addition to wear and tear due to excess weight, low grade systemic inflammation from inflamed adipose tissue and dyslipidemia in obesity as well as IPFP aggravating local inflammation can contribute to progression of OA in obesity [13]. Inflammation, within the joint, or synovitis is a predictive biomarker of joint damage and the clinical outcomes [5, 30, 31]. Synovial inflammation can promote cartilage degeneration by secreting catabolic and pro-inflammatory mediators [31]. Cartilage breakdown products in turn amplify synovial inflammation, creating a vicious circle [32, 33]. Hung et al. studied symptomatic knee OA patients and showed that obesity was associated with a higher incidence of moderate effusion at baseline and also more progression of effusion over 3 years on NCE-MRI but no statistically significant association in overweight subjects was observed by adjusted for age, gender and KL grades [34]. Our results confirm the observations by showing greater prevalence and severity in all six synovial inflammatory markers in both overweight and obese groups as compared to normal weight group. Moreover, this study was conducted with more control over potential covariates of synovial inflammation than the previous study and included more patients with obesity. Our results highlight the role of inflammation in patients with increased BMI as a potential target for therapeutic intervention in OA.
In comparison to obese subjects, overweight subjects in our study trended to have more IPFP signal alteration, in particular a larger size of signal abnormality than obese subjects. One possible explanation is that overweight subjects have a smaller IPFP resulting in relative increase in size of IPFP signal alteration as compared to obese subjects which had larger IPFP [35]. Pan et al. showed that a smaller IPFP maximal area measured on a sagittal T2 weighted sequence was significantly associated with greater IPFP signal intensity alteration [36]. However, the association between BMI and IPFP volume is still inconclusive and findings are inconsistent in previous studies [35, 37, 38]. Burda et al. showed a positive association of IPFP volume (measuring from all MRI sections) with BMI in healthy men and women [35]. A small study (n = 15 with knee OA and n = 15 control subjects, all female) did not show a significant associaton between IPFP volume and BMI between groups [37]. Another study, assessed IPFP area in sagittal MRI sequences, also failed to demonstrate a significant relationship between IPFP size and BMI [38].
Our observation of an association between synovial inflammation and symptoms in patients with knee OA has been reported earlier [39]. Hill et al. showed that moderate to large synovitis-effusion and synovial thickening, but not popliteal cysts, detected by NCE-MRI were highly correlated with knee pain measured on a visual analog scale (VAS) [40]. Also signal intensity alterations in the IPFP predicted knee pain in patients with knee OA [41, 42]. Other studies examined the relationship between pain and NCE-MRI-detected synovitis and noted that changes in pain scores over time varied with changes in effusion and synovitis, strengthening this hypothesis [42–45]. Moreover, some studies found BMI to be a predictor of knee pain, independent of radiographic features [46, 47]. Our results are consistent with these findings as we found higher total WOMAC knee pain scores in overweight subjects compared to normal weight subjects. Interestingly associations were higher in overweight than in obese subjects which is in line with the more pronounced IPFP signal abnormalities in overweight subjects. Moreover, normal weight and obese groups in our study presented no WOMAC pain score differences. These conflicting results are likely due to the complex etiology of knee pain in osteoarthritis, which cannot be explained by only one abnormality. One potential explanation may be that our obese subjects were slightly more physically active than our overweight subjects which was measured using the Physical Activity Scale for the Elderly (PASE) in the OAI (data not shown in this study). A previous study found a significant association between inactivity and self-reported knee dysfunction and pain [48]. The results of this study also support the potential role of being active in overweight and obese patients as treatment intervention in OA patients with synovial inflammation.
NCE-MRI can be used as a non-invasive tool to evaluate the synovial inflammatory activity. In a recent meta-analysis [6] synovitis scores obtained from contrast enhanced MRI demonstrated higher correlations with histologic assessment of synovial tissue inflammation than synovitis-effusion detected on NCE-MRI but differences were not statistically significant. In this meta-analysis, there were only two available studies for NCE-MRI and both of them evaluated only effusion-synovitis by using MOAKS method [49, 50]. Moreover, the signal alterations in the IPFP on NCE-MRI are sensitive but not specific for synovitis assessed on CE-MRI as the reference [51]. The signal change in IPFP is not only related to synovitis, but may also be seen in post-traumatic edema, patellar maltracking, cysts or Hoffa’s ganglion [43, 51]. However, in large cohort studies, such as the OAI, with longitudinal assessment of intra-articular structures over a long follow-up interval, CE-MRI is not routinely used due to concerns about nephrogenic systemic fibrosis and gadolinium deposition in the brain, especially with repeated injections.
A small number of studies have reported that semi-quantitative assessment of effusion-synovitis and Hoffa synovitis was associated with structural changes found on MRI including cartilage defects, cartilage volume and BMLs [33, 41]. Our results support the observation that NCE-MRI synovial inflammation biomarkers are positively corelated with with T2-values, which are a sensitive parameter for evaluation of early degeneration of articular cartilage, and all joint structural abnormalities using WORMS, not only cartilage and BML as previously reported. These associations still remained statistically significant even after we adjusted for BMI.
Synovitis can also be assessed indirectly on NCE-MRI by using the synovial proliferation score. Our group reported the synovial proliferation score as a synovial inflammation marker in young healthy patients with acute ACL rupture and results showed no significant association with average T2 value and synovial fluid biomarkers obtained at the time of surgery [15]. However, this study showed that synovial proliferation scores at the knee were positively correlated with T2 values, meniscus and cartilage maximum WORMS grades. Interestingly, synovial proliferation in popliteal cysts also showed significant and positive correlations with T2 values and meniscus lesions by WORMS. One potential explanation for the synovial fluid biomarker findings in the previous ACL tear study [15] could be that synovial proliferation scores measure a chronic change that needs a longer time to develop, so synovial proliferation on MRI would be well-demonstrated in a chronic process such as OA rather than in an acute trauma setting. It should also be noted that a previous study showed that synovial thickening detected by on NCE-MRI of the knee corresponded to mild chronic synovitis on histo-pathology [52]. To the best of our knowledge, this is the first study that evaluated the association of the synovial proliferation score on MRI with WORMS and WOMAC pain scores as well as inter-reader reproducibility. Future longitudinal studies are needed to further evaluate the association between synovial proliferation scores and the development of OA.
Our study has several limitations. We graded size and highest signal of IPFP signal alterations as previously reported [17] but most previous studies focused only on size of signal alterations by using the MOAKS grading system [16], a subjective score differentiating mild to severe grades, and another study used a more detailed grading with 0=normal; 1=<10% signal abnormality of the region; grade 2=10–20% of the region and grade 3>20% of the region [41]. However, we demonstrated that both size and degree of signal intensity of IPFP abnormalities potentially contributed to a good surrogate marker for synovial inflammation. Also we used semi-quantitative grades for synovial inflammatory markers which have less accuracy and sensitivity than quantitative scores. Moreover, despite adjusting for potential covariates such as age, gender, and well-established synovial inflammation risk factors, other unknown confounding factors may not have been accounted for. The lack of data about timing and type of knee injury and surgery is another limitation of our study, though we excluded subjects with arthroscopy. These findings could have had an effect on the MRI biomarkers that were evaluated, especially if injury was recent. However, there were no statistical significances in history of knee injury and surgery between the three BMI groups in our study. Lastly, the cross-sectional nature of this study precluded any inference about predicting OA progression by these MRI biomarkers. Longitudinal studies are needed to address causality issues.
In conclusion, this study showed that BMI was associated with a higher prevalence and severity of synovial inflammation detected on NCE-MRI in adults. We also found significant correlations between synovial inflammation imaging biomarkers with knee structural and cartilage compositional changes as well as WOMAC pain scores validating these biomarkers in a cross-sectional setting. Moreover, high reproducibilities demonstrate the potential value of these biomarkers in a clinical setting. Overall our findings highlight that increased BMI and synovial inflammation may be rational targets for therapeutic intervention in OA.
Fig. 2.
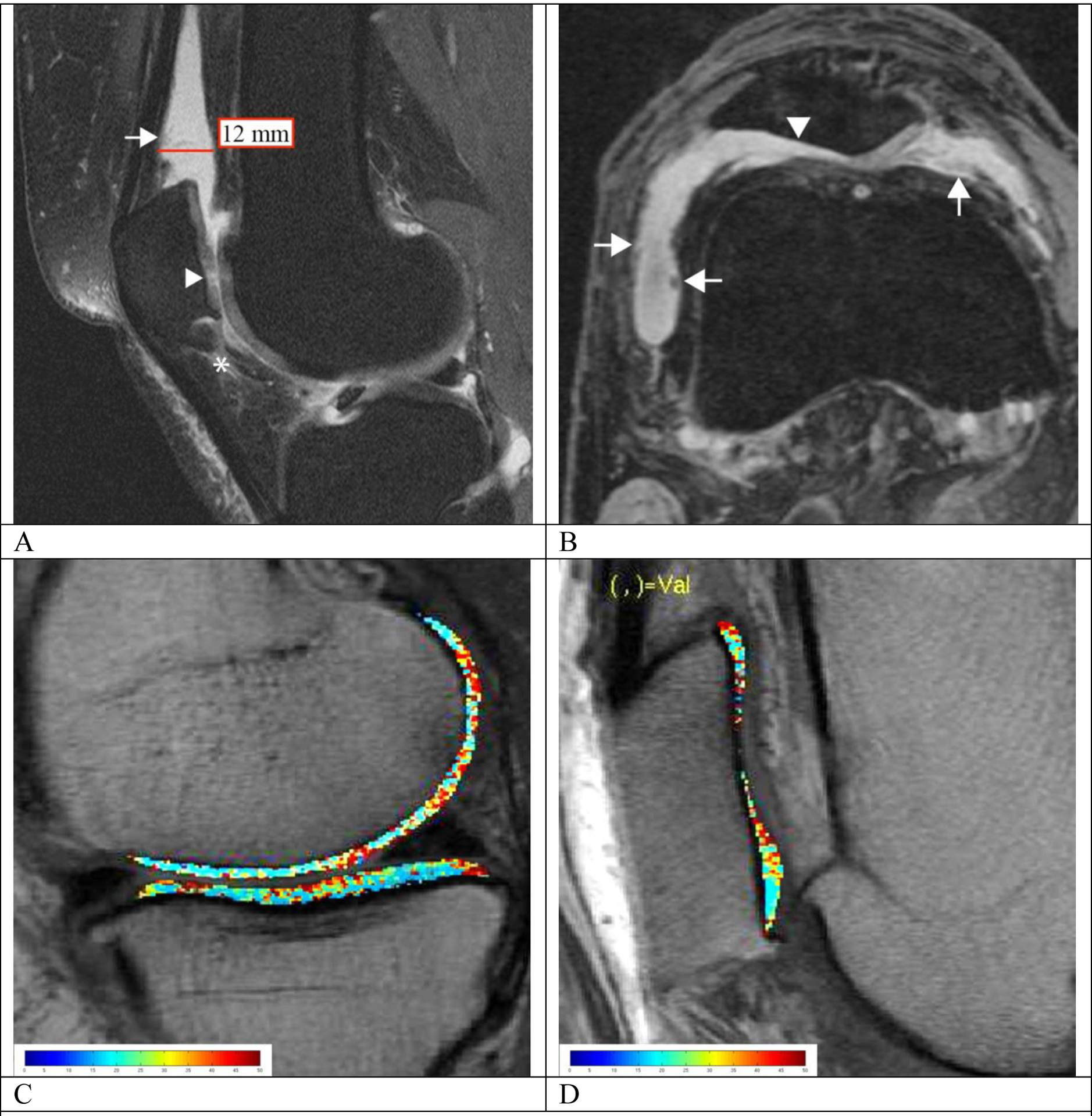
MRI-synovial inflammatory biomarkers (A, B) and representative T2 maps (C, D) in overweight and obese subjects. (A) Mid-sagittal intermediate-weighted fat-suppressed image of a 47-year-old obese woman (Total WOMAC pain subscale = 5) with focal increased signal intensity within the IPFP (grade 2 for signal which is equal to cartilage and grade 1 for size with less than 33% IPFP involvement; asterisk), effusion-synovitis in the suprapatellar recess (grade 3 according to ACLOAS) and mild irregularity of the synovium with synovial thickening (grade 2 for synovial proliferation score; arrow). There is focal cartilage loss at the patella (WORM score = 2.5; arrowhead). The T2 color map is shown in (C) which shows heterogeneously increased T2 relaxation time throughout the entire medial femoral condyle and tibia (41.13 and 35.93 ms, respectively). (B) Axial reformatted 3D DESS image of a 62-year-old overweight man (Total WOMAC pain subscale = 4) demonstrating irregular and villonodular synovial thickening (grade 3 for synovial proliferation score; arrow), and effusion-synovitis (grade 2 according to MOAKS). Full thickness cartilage loss at the lateral facet of the patella was also noted (WORMS = 6; arrowhead). The T2 color map demonstrates heterogeneously elevated mean T2 relaxation time (34.12 ms) throughout the entire patella, highest near the cartilage defects (D)
Acknowledgements
We would like to thank the participants and staff of the Coordinating Center of the OAI for their invaluable assistance with patient selection, statistical analysis, and technical support.
Funding information
This project was supported by the Osteoarthritis Initiative (OAI), a public-private partnership comprised of five contracts (National Institute of Arthritis and Musculoskeletal and Skin Diseases contracts N01-AR-2-2258, N01-AR-2-2259, N01-AR-2-2260, N01- AR-2-2261, and N01-AR-2-2262) funded by the National Institutes of Health (NIH), with research conducted by the OAI Study Investigators. Private funding partners include Merck Research Laboratories; Novartis Pharmaceuticals Corporation, GlaxoSmithKline; and Pfizer, Inc. Private sector funding for the OAI is managed by the Foundation for the National Institutes of Health. The analyses performed in this study were supported by NIH/NIAMS grant R01AR064771.
Glossary
- ACLOAS
Anterior Cruciate Ligament OsteoArthritis Score
- MOAKS
MRI Osteoarthritis Knee Score
- IPFP
InfraPatellar Fat Pad
- SI
signal intensity
- WOMAC
The Western Ontario and McMasters Osteoarthritis Index
- WORM
semi-quantitative whole organ MRI scores
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Conflict of interest: All authors declare that they have no conflicts of interest.
Ethical approval: Informed consent was obtained from all individual participants included in the study. The OAI study is compliant with the Health Insurance Portability and Accountability Act and was approved by the local institutional review board of each OAI participating center. All procedures performed in this study were in accordance with the ethical standards of the local institutional review board and with the 1964 Helsinki declaration and its later amendments.
References
- 1.Guccione AA, Felson DT, Anderson JJ, Anthony JM, Zhang Y, Wilson PW, et al. The effects of specific medical conditions on the functional limitations of elders in the Framingham Study. Am J Public Health. 1994; 84(3):351–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Disease GBD, Injury I, Prevalence C. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018; 392(10159):1789–1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Flegal KM, Kruszon-Moran D, Carroll MD, Fryar CD, Ogden CL. Trends in Obesity Among Adults in the United States, 2005 to 2014. JAMA. 2016; 315(21):2284–2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dell’Isola A, Allan R, Smith SL, Marreiros SSP, Steultjens M. Identification of clinical phenotypes in knee osteoarthritis: a systematic review of the literature. BMC Musculoskeletal Disorders. 2016; 17(1):425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang X, Hunter DJ, Jin X, Ding C. The importance of synovial inflammation in osteoarthritis: current evidence from imaging assessments and clinical trials. Osteoarthritis Cartilage. 2018; 26(2):165–174. [DOI] [PubMed] [Google Scholar]
- 6.Shakoor D, Demehri S, Roemer FW, Loeuille D, Felson DT, Guermazi A. Are contrast-enhanced and non-contrast MRI findings reflecting synovial inflammation in knee osteoarthritis: a meta-analysis of observational studies. Osteoarthritis Cartilage. 2020; 28(2):126–136. [DOI] [PubMed] [Google Scholar]
- 7.Blagojevic M, Jinks C, Jeffery A, Jordan KP. Risk factors for onset of osteoarthritis of the knee in older adults: a systematic review and meta-analysis. Osteoarthritis Cartilage. 2010; 18(1):24–33. [DOI] [PubMed] [Google Scholar]
- 8.Laberge MA, Baum T, Virayavanich W, Nardo L, Nevitt MC, Lynch J, et al. Obesity increases the prevalence and severity of focal knee abnormalities diagnosed using 3T MRI in middle-aged subjects--data from the Osteoarthritis Initiative. Skeletal Radiol. 2012; 41(6):633–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lementowski PW, Zelicof SB. Obesity and osteoarthritis. Am J Orthop (Belle Mead NJ). 2008; 37(3):148–151. [PubMed] [Google Scholar]
- 10.Carman WJ, Sowers M, Hawthorne VM, Weissfeld LA. Obesity as a risk factor for osteoarthritis of the hand and wrist: a prospective study. Am J Epidemiol. 1994; 139(2):119–129. [DOI] [PubMed] [Google Scholar]
- 11.Grotle M, Hagen KB, Natvig B, Dahl FA, Kvien TK. Obesity and osteoarthritis in knee, hip and/or hand: an epidemiological study in the general population with 10 years follow-up. BMC Musculoskelet Disord. 2008; 9:132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Engstrom G, Gerhardsson de Verdier M, Rollof J, Nilsson PM, Lohmander LS. C-reactive protein, metabolic syndrome and incidence of severe hip and knee osteoarthritis. A population-based cohort study. Osteoarthritis Cartilage. 2009; 17(2):168–173. [DOI] [PubMed] [Google Scholar]
- 13.Thijssen E, van Caam A, van der Kraan PM. Obesity and osteoarthritis, more than just wear and tear: pivotal roles for inflamed adipose tissue and dyslipidaemia in obesity-induced osteoarthritis. Rheumatology. 2014; 54(4):588–600. [DOI] [PubMed] [Google Scholar]
- 14.Peterfy CG, Schneider E, Nevitt M. The osteoarthritis initiative: report on the design rationale for the magnetic resonance imaging protocol for the knee. Osteoarthritis Cartilage. 2008; 16(12):1433–1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heilmeier U, Mamoto K, Amano K, Eck B, Tanaka M, Bullen JA, et al. Infrapatellar fat pad abnormalities are associated with a higher inflammatory synovial fluid cytokine profile in young adults following ACL tear. Osteoarthritis Cartilage. 2020; 28(1):82–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hunter DJ, Guermazi A, Lo GH, Grainger AJ, Conaghan PG, Boudreau RM, et al. Evolution of semi-quantitative whole joint assessment of knee OA: MOAKS (MRI Osteoarthritis Knee Score). Osteoarthritis Cartilage. 2011; 19(8):990–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu Y, Foreman SC, Joseph GB, Neumann J, Tien PC, Li X, et al. Is treated HIV infection associated with knee cartilage degeneration and structural changes? A longitudinal study using data from the osteoarthritis initiative. BMC Musculoskelet Disord. 2019; 20(1):190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roemer FW, Frobell R, Lohmander LS, Niu J, Guermazi A. Anterior Cruciate Ligament OsteoArthritis Score (ACLOAS): Longitudinal MRI-based whole joint assessment of anterior cruciate ligament injury. Osteoarthritis Cartilage. 2014; 22(5):668–682. [DOI] [PubMed] [Google Scholar]
- 19.Schwaiger BJ, Mbapte Wamba J, Gersing AS, Nevitt MC, Facchetti L, McCulloch CE, et al. Hyperintense signal alteration in the suprapatellar fat pad on MRI is associated with degeneration of the patellofemoral joint over 48 months: data from the Osteoarthritis Initiative. Skeletal Radiol. 2018; 47(3):329–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tsavalas N, Karantanas AH. Suprapatellar fat-pad mass effect: MRI findings and correlation with anterior knee pain. AJR Am J Roentgenol. 2013; 200(3):W291–296. [DOI] [PubMed] [Google Scholar]
- 21.Baum T, Joseph GB, Arulanandan A, Nardo L, Virayavanich W, Carballido-Gamio J, et al. Association of magnetic resonance imaging-based knee cartilage T2 measurements and focal knee lesions with knee pain: data from the Osteoarthritis Initiative. Arthritis Care Res (Hoboken). 2012; 64(2):248–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peterfy CG, Guermazi A, Zaim S, Tirman PF, Miaux Y, White D, et al. Whole-Organ Magnetic Resonance Imaging Score (WORMS) of the knee in osteoarthritis. Osteoarthritis Cartilage. 2004; 12(3):177–190. [DOI] [PubMed] [Google Scholar]
- 23.Neumann J, Hofmann FC, Heilmeier U, Ashmeik W, Tang K, Gersing AS, et al. Type 2 diabetes patients have accelerated cartilage matrix degeneration compared to diabetes free controls: data from the Osteoarthritis Initiative. Osteoarthritis Cartilage. 2018; 26(6):751–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sim J, Wright CC. The kappa statistic in reliability studies: use, interpretation, and sample size requirements. Phys Ther. 2005; 85(3):257–268. [PubMed] [Google Scholar]
- 25.Chanchek N, Gersing AS, Schwaiger BJ, Nevitt MC, Neumann J, Joseph GB, et al. Association of diabetes mellitus and biochemical knee cartilage composition assessed by T2 relaxation time measurements: Data from the osteoarthritis initiative. J Magn Reson Imaging. 2018; 47(2):380–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pan J, Pialat JB, Joseph T, Kuo D, Joseph GB, Nevitt MC, et al. Knee cartilage T2 characteristics and evolution in relation to morphologic abnormalities detected at 3-T MR imaging: a longitudinal study of the normal control cohort from the Osteoarthritis Initiative. Radiology. 2011; 261(2):507–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol. 1988; 15(12):1833–1840. [PubMed] [Google Scholar]
- 28.Thiese MS, Ronna B, Ott U. P value interpretations and considerations. J Thorac Dis. 2016; 8(9):E928–E931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977; 33(1):159–174. [PubMed] [Google Scholar]
- 30.Berenbaum F Osteoarthritis as an inflammatory disease (osteoarthritis is not osteoarthrosis!). Osteoarthritis Cartilage. 2013; 21(1):16–21. [DOI] [PubMed] [Google Scholar]
- 31.Hunter DJ, Zhang W, Conaghan PG, Hirko K, Menashe L, Li L, et al. Systematic review of the concurrent and predictive validity of MRI biomarkers in OA. Osteoarthritis Cartilage. 2011; 19(5):557–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.de Lange-Brokaar BJ, Ioan-Facsinay A, van Osch GJ, Zuurmond AM, Schoones J, Toes RE, et al. Synovial inflammation, immune cells and their cytokines in osteoarthritis: a review. Osteoarthritis Cartilage. 2012; 20(12):1484–1499. [DOI] [PubMed] [Google Scholar]
- 33.Wang X, Blizzard L, Halliday A, Han W, Jin X, Cicuttini F, et al. Association between MRI-detected knee joint regional effusion-synovitis and structural changes in older adults: a cohort study. Ann Rheum Dis. 2016; 75(3):519–525. [DOI] [PubMed] [Google Scholar]
- 34.Hung A, Sayre EC, Guermazi A, Esdaile JM, Kopec JA, Thorne A, et al. Association of Body Mass Index With Incidence and Progression of Knee Effusion on Magnetic Resonance Imaging and on Knee Examination. Arthritis Care Res (Hoboken). 2016; 68(4):511–516. [DOI] [PubMed] [Google Scholar]
- 35.Burda B, Steidle-Kloc E, Dannhauer T, Wirth W, Ruhdorfer A, Eckstein F. Variance in infra-patellar fat pad volume: Does the body mass index matter?-Data from osteoarthritis initiative participants without symptoms or signs of knee disease. Ann Anat. 2017; 213:19–24. [DOI] [PubMed] [Google Scholar]
- 36.Pan F, Han W, Wang X, Liu Z, Jin X, Antony B, et al. A longitudinal study of the association between infrapatellar fat pad maximal area and changes in knee symptoms and structure in older adults. Annals of the rheumatic diseases. 2014; 74. [DOI] [PubMed] [Google Scholar]
- 37.Chuckpaiwong B, Charles HC, Kraus VB, Guilak F, Nunley JA. Age-associated increases in the size of the infrapatellar fat pad in knee osteoarthritis as measured by 3T MRI. J Orthop Res. 2010; 28(9):1149–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Han W, Cai S, Liu Z, Jin X, Wang X, Antony B, et al. Infrapatellar fat pad in the knee: is local fat good or bad for knee osteoarthritis? Arthritis Res Ther. 2014; 16(4):R145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Garnero P, Piperno M, Gineyts E, Christgau S, Delmas PD, Vignon E. Cross sectional evaluation of biochemical markers of bone, cartilage, and synovial tissue metabolism in patients with knee osteoarthritis: relations with disease activity and joint damage. Ann Rheum Dis. 2001; 60(6):619–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hill CL, Gale DG, Chaisson CE, Skinner K, Kazis L, Gale ME, et al. Knee effusions, popliteal cysts, and synovial thickening: association with knee pain in osteoarthritis. J Rheumatol. 2001; 28(6):1330–1337. [PubMed] [Google Scholar]
- 41.Han W, Aitken D, Zhu Z, Halliday A, Wang X, Antony B, et al. Signal intensity alteration in the infrapatellar fat pad at baseline for the prediction of knee symptoms and structure in older adults: a cohort study. Ann Rheum Dis. 2016; 75(10):1783–1788. [DOI] [PubMed] [Google Scholar]
- 42.Zhang Y, Nevitt M, Niu J, Lewis C, Torner J, Guermazi A, et al. Fluctuation of knee pain and changes in bone marrow lesions, effusions, and synovitis on magnetic resonance imaging. Arthritis Rheum. 2011; 63(3):691–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hill CL, Hunter DJ, Niu J, Clancy M, Guermazi A, Genant H, et al. Synovitis detected on magnetic resonance imaging and its relation to pain and cartilage loss in knee osteoarthritis. Ann Rheum Dis. 2007; 66(12):1599–1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lo GH, McAlindon TE, Niu J, Zhang Y, Beals C, Dabrowski C, et al. Bone marrow lesions and joint effusion are strongly and independently associated with weight-bearing pain in knee osteoarthritis: data from the osteoarthritis initiative. Osteoarthritis Cartilage. 2009; 17(12):1562–1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang X, Jin X, Han W, Cao Y, Halliday A, Blizzard L, et al. Cross-sectional and Longitudinal Associations between Knee Joint Effusion Synovitis and Knee Pain in Older Adults. J Rheumatol. 2016; 43(1):121–130. [DOI] [PubMed] [Google Scholar]
- 46.Goulston LM, Kiran A, Javaid MK, Soni A, White KM, Hart DJ, et al. Does obesity predict knee pain over fourteen years in women, independently of radiographic changes? Arthritis Care Res (Hoboken). 2011; 63(10):1398–1406. [DOI] [PubMed] [Google Scholar]
- 47.Oliveria SA, Felson DT, Cirillo PA, Reed JI, Walker AM. Body weight, body mass index, and incident symptomatic osteoarthritis of the hand, hip, and knee. Epidemiology. 1999; 10(2):161–166. [PubMed] [Google Scholar]
- 48.Lee J, Song J, Hootman JM, Semanik PA, Chang RW, Sharma L, et al. Obesity and other modifiable factors for physical inactivity measured by accelerometer in adults with knee osteoarthritis. Arthritis Care Res (Hoboken). 2013; 65(1):53–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Loeuille D, Sauliere N, Champigneulle J, Rat AC, Blum A, Chary-Valckenaere I. Comparing non-enhanced and enhanced sequences in the assessment of effusion and synovitis in knee OA: associations with clinical, macroscopic and microscopic features. Osteoarthritis Cartilage. 2011; 19(12):1433–1439. [DOI] [PubMed] [Google Scholar]
- 50.Riis RG, Gudbergsen H, Simonsen O, Henriksen M, Al-Mashkur N, Eld M, et al. The association between histological, macroscopic and magnetic resonance imaging assessed synovitis in end-stage knee osteoarthritis: a cross-sectional study. Osteoarthritis Cartilage. 2017; 25(2):272–280. [DOI] [PubMed] [Google Scholar]
- 51.Roemer FW, Guermazi A, Zhang Y, Yang M, Hunter DJ, Crema MD, et al. Hoffa’s Fat Pad: Evaluation on Unenhanced MR Images as a Measure of Patellofemoral Synovitis in Osteoarthritis. AJR Am J Roentgenol. 2009; 192(6):1696–1700. [DOI] [PubMed] [Google Scholar]
- 52.Fernandez-Madrid F, Karvonen RL, Teitge RA, Miller PR, An T, Negendank WG. Synovial thickening detected by MR imaging in osteoarthritis of the knee confirmed by biopsy as synovitis. Magn Reson Imaging. 1995; 13(2):177–183. [DOI] [PubMed] [Google Scholar]


