Abstract
Cognitive flexibility enables appropriate responses to a changing environment, and is associated with positive life outcomes. Adolescence, with its increased focus on transitioning to independent living, presents particular challenges for youth with autism spectrum disorder (ASD) who often struggle to behave in a flexible way when faced with challenges. This review focuses on brain mechanisms underlying the development of flexible cognition during adolescence, and how these neural systems are affected in ASD. Neuroimaging studies of task switching and set-shifting provide evidence for atypical lateral frontoparietal and midcingulo-insular network activation during cognitive flexibility task performance in individuals with ASD. Recent work also examines how intrinsic brain network dynamics support flexible cognition. These dynamic functional connectivity studies provide evidence for alterations in the number of transitions between brain states, as well as hyper-variability of functional connections in adolescents with ASD. Future directions for the field include addressing issues related to measurement of cognitive flexibility using a combination of metrics with ecological and construct validity. Heterogeneity of executive function ability in ASD must also be parsed in order to determine which individuals will benefit most from targeted training to improve flexibility. The influence of pubertal hormones on brain network development and cognitive maturation in adolescents with ASD is another area requiring further exploration. Finally, the intriguing possibility that bilingualism might be associated with preserved cognitive flexibility in ASD should be further examined. Addressing these open questions will be critical for future translational neuroscience investigations of cognitive and behavioral flexibility in adolescents with ASD.
Keywords: bilingualism, brain dynamics, cognitive flexibility, executive function, lateral frontoparietal network, midcingulo-insular network, restricted and repetitive behaviors
1. Cognitive flexibility: Assessment and neural mechanisms
The ability to flexibly adapt to novel circumstances is a critical feature of human cognition (1). Cognitive flexibility is the readiness with which one can selectively switch between mental processes to appropriately respond to environmental stimuli (2), and falls under the umbrella of executive function (EF), which refers to the set of abilities required to guide goal-directed behavior (3). Different aspects of EF such as updating, shifting, and inhibition are thought to be correlated, yet separable (4). EF ability and cognitive flexibility can be assessed using informant-report questionnaires, which typically have greater ecological validity, or laboratory-based measures, which can provide greater construct validity(5).
One such measure, the Behavior Rating Inventory of Executive Function (BRIEF), is an informant-report measure developed for parents and teachers to assess EF in 5–18 year-old children (6). Self-report and informant-report versions of the BRIEF (BRIEF-A) are also available for adults (7). Different subscales of the BRIEF are thought to index different EF components. The ‘shift’ and ‘emotional control’ subscales of the BRIEF load onto a factor that has been labeled ‘flexibility’ in young children (8). Similarly, the ‘shift’ scale of the BRIEF-A assesses the ability to move with ease from one situation to another as circumstances demand. Items on the ‘shift’ scale assess the ability to make transitions, tolerate change, problem-solve flexibly, switch attention, and change focus from one topic to another, thus providing an ecologically valid index of cognitive flexibility (9).
Several neuropsychological measures and test batteries have been developed to measure EF and cognitive flexibility. The Wisconsin Card Sort Task (WCST) measures the ability to infer the categories that should guide behavior, create an attentional set based on abstract categories, and switch attention and flexibly adjust behavior as task demands change (3). The Adolescent Brain and Cognitive Development (ABCD) consortium includes the Dimensional Change Card Sort (DCCS) task from the NIH Toolbox Cognition Battery to index cognitive flexibility (10). The DCCS asks participants to sort an object by either color or shape to match one of two other objects. After blocks of trials sorting solely on one dimension then another, participants alternate pseudo-randomly between sorting based on shape versus color (11). The Delis-Kaplan Executive Function System (D-KEFS) is a standardized set of tests for 8–89 year olds (12), and includes several subtests that load onto a factor labelled ‘conceptual flexibility’ (13). The NEPSY-II, specifically designed for use in 3–16 year olds, also assesses flexibility as part of its EF and attention battery (14).
It is important to note that informant and self-report measures of real-world EF and laboratory performance-based neuropsychological measures do not always converge. The BRIEF correlates with other questionnaire measures, but not with laboratory-based measures of EF, suggesting that subjective and objective measures may not assess the same underlying constructs (15). More generally, self-report and behavioral measures of the same construct are only weakly correlated, possibly due to the fact that behavioral measures tap responses during highly structured situations and typically index maximal performance, whereas self-report assesses how individuals behave across unstructured real-life situations (5). It has been suggested that measures derived from experimental cognitive psychology may provide greater insights into impaired cognition in clinical conditions than neuropsychological measures (16).
The most commonly used cognitive neuroscience paradigms for studying cognitive flexibility are those that require task switching or set-shifting (17–20). Decades of functional neuroimaging work has delineated brain networks underlying EFs that contribute to cognitive flexibility (21,22), namely the executive control/lateral frontoparietal (L-FPN) and salience/midcingulo-insular networks (M-CIN) (23–25). Cortical regions within these networks most strongly implicated in cognitive flexibility are the inferior frontal junction (IFJ), involved in the updating of task rule representations (18,26), the ventrolateral prefrontal cortex (vlPFC) involved in resolving proactive interference, response set selection, and context monitoring (27,28), and the dorsal anterior insula (dAI), which detects behaviorally-relevant stimuli and coordinates dynamic switches between brain networks (29). These regions work in the context of broader EF networks with nodes in the dorsolateral prefrontal cortex (dlPFC), dorsal anterior cingulate cortex (dACC), and superior parietal lobule (SPL) which contribute to working memory (30), motor control (31,32), and attention (33), respectively (see (25) for review) (Figure 1). Other mechanistic models of flexibility further incorporate subcortical structures like the basal ganglia, implicated in updating reward-related context representations in the prefrontal cortices. According to these models, inflexibility results from dysregulation of dopamine circuits leading to rigid behavior that is not informed by learning (34,35).
Figure 1. Brain systems underlying cognitive flexibility.
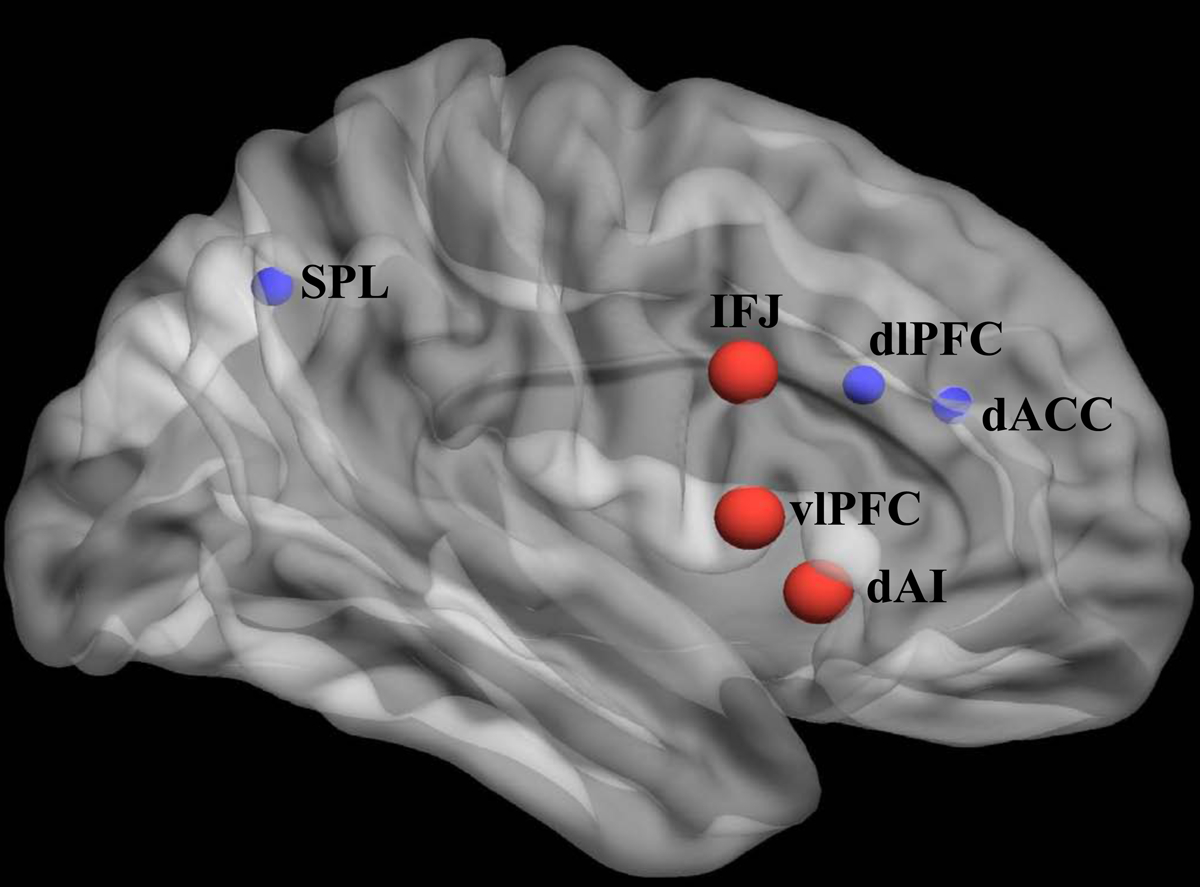
Nodes critical for cognitive flexibility (red) operate within the context of broader lateral frontoparietal (L-FPN) and midcingulo-insular (M-CIN) networks (24) supporting executive functions (blue). The inferior frontal junction (IFJ) is involved in inhibition and response set updating, the ventrolateral prefrontal cortex (vlPFC) in resolving proactive interference, response set selection, and context monitoring, the dorsal anterior insula (dAI) in switching between other large-scale functional brain networks, the dorsolateral prefrontal cortex (dlPFC) in working memory, the dorsal anterior cingulate cortex (dACC) in response selection and motor responses, and the superior parietal lobule (SPL) in visuomotor integration and attention (adapted from (25)).
Cognitive flexibility is important for promoting optimal outcomes during development, including academic achievement and employment success (36). Flexibility supports the transition to adulthood, which is associated with increased demands including navigation of new social relationships and independent living (37–39). As such, tracking the neural substrates of cognitive flexibility in typical adolescent development provides critical foundational knowledge for understanding atypical trajectories.
2. Brain network maturation underlying the development of cognitive flexibility
Cognitive flexibility emerges in early life, showing sharp increases between 7–9 years of age, then follows a protracted development throughout young adulthood, becoming largely mature by age 20 (40,41). However, this skill continues to improve throughout adolescence and adulthood, peaking between 21–30 years of age before declining in later life (40,42,43). Tasks typically used to study the neural systems supporting cognitive flexibility are summarized in Figure 2 (44–46). Several considerations must be taken into account when testing developmental populations, such as ensuring that working memory demands are not excessively high (25,47). Modifications to cognitive flexibility paradigms that render them age-appropriate often result in reduced complexity compared with those developed for adults (46).
Figure 2. Laboratory tasks for assessing cognitive flexibility in developmental populations.
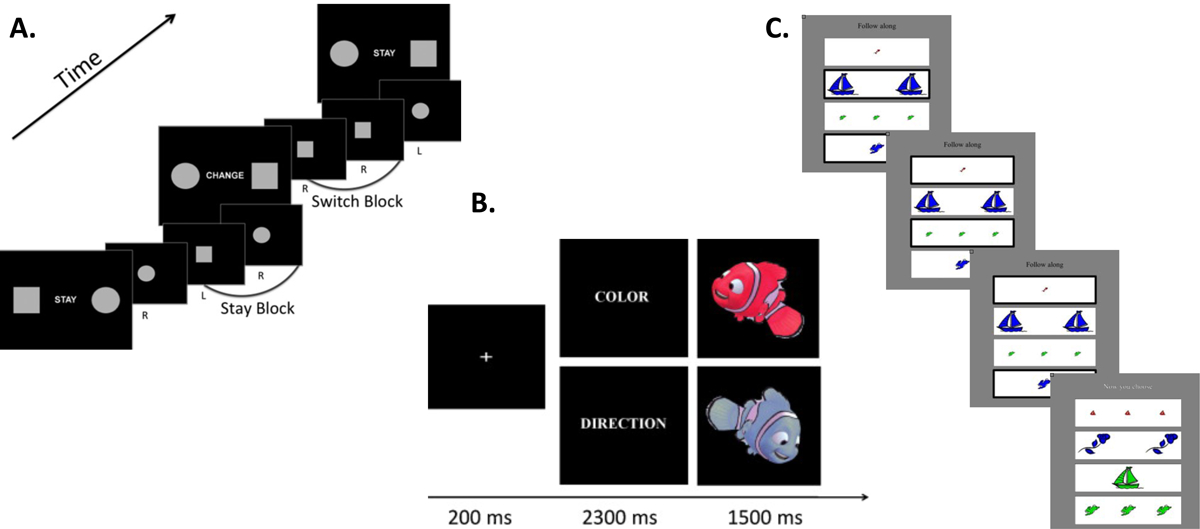
A. Example set-shifting task. Instruction cues indicate spatial mapping to stimuli to response buttons. When the circle is presented on the right side of the instruction cue, a right-handed button press to circle trials is required (44). B. Example flexible rule switch task. On Color trials, participants are instructed to press one button for red stimuli and another for blue stimuli. On Direction trials, participants are instructed to press a left button for leftward facing stimuli and right button for rightward facing stimuli (45). C. Example flexible item selection task. During Flexibility trials, participants choose three successive pairs of cards that “go together in one way” (“Now you choose”). During Control trials, the correct card pairs are highlighted by a thick black border (“Follow along”) (46).
Reduced frontal lobe contributions are thought to underlie immature control processes in children, who activate posterior but not prefrontal regions during EF tasks (48). On a range of EFs including attentional control, goal setting, and cognitive flexibility, little differentiation was observed between adolescents (age 7–16) with frontal lesions and those with extra-frontal lesions, suggesting that adolescents utilize more distributed brain networks for executive skills than adults (49). Studies of the whole-brain functional connectome demonstrate a trend towards ‘segregation’ (decrease in correlation strength between anatomically close regions) and ‘integration’ (increase in correlation strength between anatomically distant regions) across development (50,51). Diffusion imaging studies examining structural connectivity in 8–22 year-olds support the network segregation-with-age story, and further link this neurodevelopmental process with enhanced EF with age (52).
During a probabilistic reversal learning task requiring cognitive flexibility, the right anterior insular cortex (rAI) shows increased task-related responses to negative reward prediction errors in adolescents compared with adults (53). Effective connectivity work demonstrates greater causal outflow from the rAI to the L-FPN in adults compared with children (54). Findings from the task-based fMRI literature demonstrate that brain networks involved in EF are in place in children aged 8–14, with the dACC and rAI showing greater activation across more demanding task periods (55). Recent studies examining executive control networks demonstrate changes in network expression and variability with development, interpreted as increased flexibility of frontoparietal brain regions with age (56,57).
While the development of cognitive flexibility is a topic of considerable interest, links between brain network maturation and flexible behaviors are not yet firmly established. A recent study of over 2000 9–10 year olds from the ABCD dataset found that while individual differences in general cognitive ability could be predicted from functional connectivity patterns, whole brain connectomes could not reliably predict individual differences in flexibility (58). The relative immaturity of the basic science in this area is important to consider as translational research questions are addressed.
3. Cognitive flexibility deficits in autism: The importance of adolescence
Individuals with autism spectrum disorder (ASD) exhibit considerable heterogeneity in EF abilities (59). While a majority of those diagnosed with ASD experience difficulties with EF, a great deal of heterogeneity exists with respect to individual levels of impairment (60–62), and EF deficits can improve with age (63). In a meta-analysis, broad impairments across multiple EF domains were observed in children and adolescents with ASD assessed using neuropsychological measures. Deficits in cognitive flexibility decreased with age, and were observed in those with and without comorbid attention-deficit/hyperactivity disorder (ADHD) (64). Another meta-analysis including psychometric, experimental, and questionnaire-based measures of EF and a wider age range of participants also reported broad dysfunction in ASD that is relatively stable across development. Here, effect sizes were found to be largest for studies using the BRIEF questionnaire (65).
Symptom severity for restricted and repetitive behaviors (RRBs), which are considered core deficits in the disorder, are associated with measures of cognitive inflexibility in ASD (66–68). Cognitive flexibility deficits in early life can manifest as difficulties in transitioning to independent living and maintaining employment, and may contribute to the grim outcome that less than 20% of adults with ASD live independently and are fully employed (69). Despite reports of cognitive flexibility deficits in ASD (70,71), particularly in younger children (72,73), there are conflicting notions regarding the extent, nature, etiology, and neurobiology of these deficits (74).
Adolescence is a time of dramatic physical, emotional, and social change, and represents a particularly vulnerable developmental period for individuals with ASD. During this period of transition to adulthood, youth with ASD often desire independent living, employment, and social relationships, all of which can be challenging for them to achieve when dealing with persistent social, behavioral, and language deficits (75). Some children with ASD even experience deterioration in functioning in the years after the onset of puberty (76). Young adults with ASD are at increased risk for poor health outcomes, social isolation, financial adversity, and institutionalization (77).
The “two-hit” conceptual model of autism posits that early alterations in neurodevelopment lead to a “first hit”, while pubertal hormones, neural reorganization, and increasing social demands function as a “second hit” during adolescence that affects adaptive functioning and transitioning to adult roles (78). Cognitive flexibility deficits can exacerbate this difficult transition period, whereas relative sparing of flexibility may ameliorate some of the challenges typically encountered during adolescence. In youth with ASD (7–17 years), flexibility assessed by parent-report explained 22.2% of the variance in adaptive socialization skills (79), suggesting that the ability to function independently in everyday life is linked to flexibility. Longitudinal studies demonstrate that EF skills in childhood predict variance in autistic individuals’ adaptive behavior later in life (80,81). Taken together, this work highlights the need for more targeted investigation of the brain mechanisms supporting cognitive flexibility in ASD during this critical developmental stage.
4. Neural substrates of cognitive flexibility in autism: Brain activation
Despite the critical role of cognitive flexibility for supporting adaptive functioning in autism, few functional neuroimaging studies of cognitive flexibility in ASD have been conducted (Table 1). Based on the extensive cognitive neuroscience literature examining cognitive flexibility in neurotypical adults, one might expect to see differential responsivity in the L-FPN and M-CIN during such tasks in individuals with ASD (24,25). Schmitz and colleagues reported greater inferior parietal brain activation in adults with ASD as they performed a cognitive flexibility task (82). Shafritz and colleagues found reduced activation in frontal, striatal, and parietal regions during shifting trials in young adults with ASD. They also reported a negative correlation between severity of RRBs and anterior cingulate and posterior parietal activation (83). Using a reversal learning paradigm to assess behavioral flexibility, D’Cruz and colleagues found reduced activation in frontal cortex and striatum in adults with ASD (84).
Table 1.
fMRI studies of brain activation underlying cognitive flexibility in autism
| Study by publication date | Sample Size | Age Range, Years (mean, SD) | Cognitive Paradigm | Behavioral Results | Neuroimaging Results |
|---|---|---|---|---|---|
| Schmitz et al., 2005 (82) | ASD: 10 TD: 12 |
Combined: 18–52 ASD: 38±9 TD:39±6 |
Set-shifting | No significant group differences | ASD > TD activation in inferior and medial parietal cortex during switch trials |
| Shafritz et al., 2008 (83) | ASD: 15 TD: 14 |
ASD: 22.3±8.7 TD: 24.3±6.2 |
Set-shifting | ASD < TD accuracy, no significant group difference in RT | ASD < TD activation in dlPFC, ACC, IPS, BG during target-shift trials |
| Taylor et al., 2012 (85) | ASD: 14 TD: 14 |
Combined: 7–14 | Set-shifting | No significant group differences | Age x Group interaction for shift trials: insula increase activation with age in TD, decrease activation with age in ASD; vlPFC increase activation with age in ASD, no change with age in TD |
| Yerys et al., 2015 (44) | ASD: 20 TD: 19 |
Combined: 7–14 ASD: 11.32±1.84 TD: 11.36±1.54 |
Set-shifting | ASD < TD accuracy, no significant group difference in RT | ASD > TD activation in ACC, superior, middle, and inferior frontal gyrus during switch trials |
| D’Cruz et al., 2016 (84) | ASD: 17 TD: 23 |
ASD: 9–44; 17.4±8.6 TD: 7–38; 18.6±8.4 |
Reversal learning | No significant group differences | ASD < TD activation in ventral striatum, ACC, premotor cortex, posterior parietal cortex, and dlPFC during reversal |
ASD = autism spectrum disorder; TD = typically developing; dlPFC = dorsolateral prefrontal cortex; ACC = anterior cingulate cortex; IPS = intraparietal sulcus; BG = basal ganglia; vlPFC = ventrolateral prefrontal cortex
Mixed findings have also been reported in younger cohorts. Yerys and colleagues found that 7–14 year old children with ASD engaged frontal brain regions to a greater extent than typically developing (TD) peers during set-shifting (44). Examining extra-dimensional shifts in 7–14 year old children, Taylor and colleagues found an age by group interaction such that the right insula exhibited increasing activation with age in typical development, but decreasing activation with age in ASD (85). The rAI has been posited to be a locus of dysfunction in ASD (86), and functional and effective connectivity of this region and the broader M-CIN is associated with symptom severity in the domain of RRBs (87,88).
The task-based fMRI literature has not yet converged on the neural circuitry underlying cognitive flexibility in ASD (89), though atypical L-FPN and M-CIN activation is generally observed. This is not entirely surprising, as these tasks may require different forms of flexibility, placing differential demands on various components of shifting (eg. response sets vs. context monitoring). These differences could in part drive conflicting results in terms of brain regions implicated across studies. No studies to date have focused specifically on adolescents with ASD, despite the well-characterized maturation of EF circuitry in typical development (90,91). A few studies of adolescents with ASD have noted alterations in frontoparietal activity during paradigms invoking aspects of flexibility including cognitive control (92–94) and verbal fluency (95), yet much remains unknown about this specific developmental period.
5. Neural substrates of cognitive flexibility in autism: Brain dynamics
Complementary to task-based neuroimaging, resting state fMRI paradigms, with their decreased cognitive demands and potential for data reuse, are a promising approach for exploration of typical and atypical brain networks (96,97). Beyond revealing brain regions activated in response to specific task conditions, resting state functional connectivity approaches permit analysis of how cognition emerges from brain network interactions (98). Dynamic functional connectivity approaches further enable the study of moment-to-moment, or time-varying changes in functional coupling between brain regions (99–101), and are increasingly being applied to the study of neurodevelopmental disorders (102).
One method for computing dynamic functional connectivity is the “sliding-window” approach where functional connectivity strength is computed on the order of seconds rather than minutes (103). Sliding window analyses permit the quantification of metrics including “dwell time” (the amount of time spent in a particular functional connectivity state), “frequency of occurrence” (the number of times a particular functional connectivity state occurs), and “state transitions” (the number of times transitions between functional connectivity states occur). Another method relies on the identification of critical timepoints when the BOLD signal intensity surpasses a certain threshold, giving rise to multiple stable spatial patterns or co-activation patterns (CAPs) that can be obtained by clustering of critical time frames (104). CAP analysis relies on fewer model assumptions than the sliding window approach, and allows for the examination of state alterations closer to the temporal resolution of individual time frames (105) (Figure 3). A comprehensive review of dynamic functional connectivity approaches is provided in (106).
Figure 3. Approaches for characterizing brain dynamics.
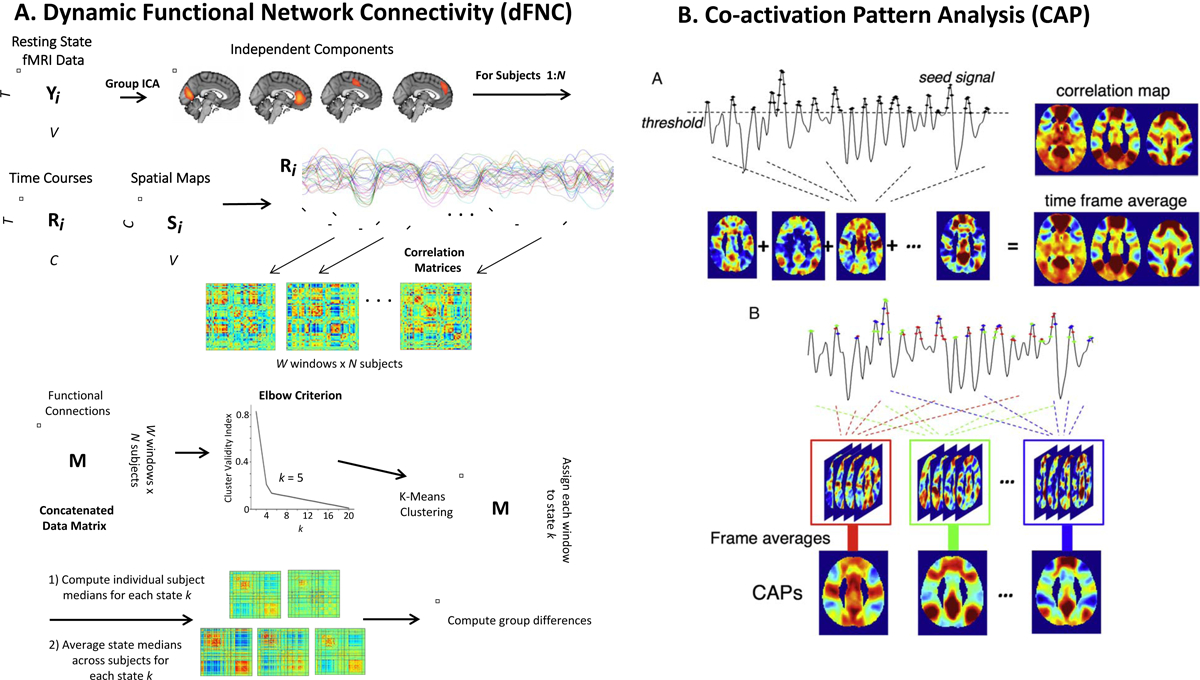
A. Example sliding window approach for computing dynamic functional network connectivity (dFNC). High-model order independent component analysis (ICA) creates functional parcellation of the brain, resulting in several independent components. B) Subject-specific timecourses are used to compute functional connectivity between pairwise components. Dynamic FNC analysis utilizes sliding windows (eg. 45 seconds in duration) to produce multiple correlation matrices for each subject (one per window). A concatenated data matrix is then subjected to k-means clustering, and the optimal k is identified using the elbow criterion (k=5 in this example). Each window is assigned to a dynamic state k regardless of subject assignment. Subject-specific medians are then back-reconstructed for each state k before they are averaged together to produce the final k dynamic states. Finally, group differences in dFNC metrics (eg. dwell time, state transitions) are computed (103). B. Example co-activation pattern (CAP) analysis. In conventional seed-based correlation analysis, functional connectivity patterns associated with a seed region-of-interest is estimated by the linear correlation between the timeseries of each gray matter voxel in the brain and the seed. The CAP method demonstrates that these patterns can be obtained by voxel-wise averaging the spatial maps of time frames when the seed signal intensity surpasses a given threshold. Temporal clustering of the extracted time frames based on their spatial similarity yields multiple spatial patterns reflecting functionally relevant CAPS across the whole brain at each individual time frame. Different colors indicate different CAPs in temporal clustering (adapted from (105)).
Specific patterns of brain dynamics are associated with enhanced cognitive flexibility. Individuals who score higher on a card sort task exhibited whole-brain functional connectivity dynamics characterized by greater episodes of more frequently occurring brain states, and fewer episodes of less frequently occurring states associated with low vigilance and arousal (107). Older adults who performed more poorly on a battery of cognitive tests exhibited greater frequency of switching between dynamic brain states, whereas high performers exhibited a tendency to be in a state characterized by global coherence (108). Time-varying (but not static) functional connectivity of the M-CIN (109), as well as dynamics between the default mode/medial frontoparietal network (M-FPN) and L-FPN (110), has been shown to predict individual differences in cognitive flexibility. These works are beginning to reveal the links between brain dynamics and flexible cognition and behavior (111).
While several methodological issues have yet to be resolved (112), dynamic functional connectivity approaches are already revealing interesting patterns of brain dynamics that distinguish youth with ASD (Table 2). The first study of 8–18 year olds focused on the M-FPN, M-CIN, amygdala and thalamus and examined standard deviation of the sliding window correlation, which indicates intra-individual variability over time. For multiple region-of-interest (ROI) pairs, reduced static functional connectivity in adolescents with ASD was related to increased temporal variability of the BOLD signal (113).
Table 2.
fMRI studies of brain dynamics in autism
| Study by publication date | Sample Size, ABIDE or in house data | Age Range, Years (mean, SD) | Method for ssessing Dynamics | Behavioral Results | Neuroimaging Results |
|---|---|---|---|---|---|
| Falahpour et al., 2016 (113) |
Study 1 (6 ABIDE sites) ASD: 76 TD: 76 Study 2 (SDSU in house) ASD: 32 TD: 32 |
Study 1 ASD: 7–29.9; 16.1±4.9 TD: 8–29.9; 15. 8±4.5 Study 2 ASD: 9.5–17.9; 14.3 ±2. 4 TD: 8–17.5; 13.5±2.7 |
Standard deviation of sliding window correlation between select ROIs (DMN, SN, amygdala, Thal) | N/A |
Study 1 ASD > TD SD-iFC several ROI pairs Study 2 ASD > TD SD iFC one ROI pair |
| Yao et al., 2016 (115) | ASD: 31 TD: 44 (ABIDE NYU site) |
Combined: 7–18 ASD: 11.51±2.64 TD: 12.46±3.1 |
Sliding window correlation across whole- brain ICA- derived ROIs | N/A | ASD > TD mean dwell time in weak FC state |
| de Lacy et al., 2017 (116) | ASD: 423 TD: 461 (all ABIDE sites) |
Combined: 6.5–58; 16.23±7.1 | Sliding window correlation across whole- brain ICA- derived ROIS | N/A | ASD < TD transitions between brain states |
| Watanabe et al., 2017 (117) | ASD: 24 TD: 26 (ABIDE Utah site primary, Indiana and Zurich site replication) |
ASD: 18.4–38.9; 25.3±5.5 TD: 18.2–39.3; 25.3±6.3 |
Energy- landscape analysis across seven functional brain systems | Transition frequency negatively correlated with ADOS | ASD < TD transitions between brain states |
| Chen et al., 2017 (118) | ASD: 209 TD: 298 (all ABIDE sites) |
ASD: 16.5±6.2 TD: 16.8±6.2 | Standard deviation of sliding window correlation between whole- brain ROIs | Hyper-variant FC positively correlated with ADOS | ASD > TD SD-iFC for almost all ROI pairs |
| Rashid et al., 2018 (123) | Combined: 774; 560 with SRS ASD: 22 (Generation R Study) |
Combined: 6–10; 7.99±1 | Sliding window correlation across whole- brain ICA- derived ROIs | N/A | ASD traits indexed by SRS positively correlate with mean dwell time in globally disconnected state |
| Mash et al., 2019 (119) | ASD: 62 TD: 57 (SDSU in house) |
Combined: 6–18 ASD: 13.7±2.5 TD: 13.1±2.9 |
Sliding window correlation across whole- brain ICA- derived ROIs | N/A | ASD > TD SD-iFC for almost all ROI pairs |
| Li et al., 2019 (120) | ASD: 62 TD: 63 (ABIDE SDSU, Trinity, NYU, Stanford sites) |
ASD: 7.18–17.93; 11.63±2.84 TD: 7.11–17.6; 11.48±2.59 |
Sliding window correlation across whole- brain ROIs | SD-iFC between IFGoper positively correlated with SRS | ASD > TD SD-iFC for several ROI pairs; ASD > TD mean dwell time in globally hyper-connected state |
| Harlalka et al., 2019 (121) | Child ASD: 26 Child TD: 26 Adolescent ASD: 28 Adolescent TD: 28 Adult ASD: 18 Adult TD: 18 (ABIDE NYU site) |
Child ASD: 7.15–10.06; 9.51±1.12 Child TD: 6.47–10.86; 9.10±1.32 Adolescent ASD: 11.01–17.88; 13.71±1.79 Adolescent TD: 11.32–16.93; 14.01±1.74 Adult ASD: 18.58–39.1; 24.13±3.92 Adult TD: 18.5931.78; 25.41±5.87 |
Sliding window correlation across whole- brain ROIs | DMN- Attention network SD- iFC positive correlation with ADOS | ASD > TD SD-iFC for almost all ROI pairs for children, adolescents, and adults ASD > TD SD-iFC for short-range connections in adolescents |
| Guo et al., 2019 (125) | ASD: 209 TD: 298 (all ABIDE sites) |
ASD: 16.5±6.2 TD: 16.8±6.2 |
Sliding window correlation between rAI and whole brain | Decreased dynamic FC between rAI and vmPFC negative correlation with ADOS social | ASD < TD FC between rAI and vmPFC/PCC in some states |
| Fu et al., 2019 (127) | ASD: 170 TD: 195 (all ABIDE sites) |
ASD: 15.57±7.35 TD: 16.02±5.9 |
Sliding window correlation between thalamic ROIs and whole brain | Increased dynamic FC between hypothalamus and sensory region positive correlation with ADOS | ASD > TD FC between hypothalamus and sensory regions in some states ASD < TD meta-state dynamism measures |
| Guo et al., 2020 (126) | ASD: 105 TD: 102 (all ABIDE sites) |
Combined: 7–12 ASD: 10.15±1.26 TD: 10.02±1.38 |
Sliding window correlation and FCD mapping across whole- brain | Aberrant temporal variability of contralateral dynamic FCD predicted ADOS communication scores | Global alterations in dynamic FCD variability and atypical dynamics of intra- and interhemispheric FCD variability in ASD |
ABIDE = Autism Brain Imaging Data Exchange; ADOS = autism diagnostic observation schedule; ASD = autism spectrum disorder; DMN = default mode network; FCD = functional connectivity density; ICA = independent component analysis; NYU = New York University; rAI = right anterior insula; ROI = region of interest; SD-iFC = standard deviation of intrinsic functional connectivity; SN = salience network; SRS = social responsiveness scale; Thal = thalamus; SDSU = San Diego State University; TD = typically developing;
A study of 7–18 year old children from the Autism Brain Imaging Data Exchange (ABIDE) (114) found that those with ASD showed weaker whole-brain connectivity for a longer period of time compared with TD children (115). Another study using the entire available ABIDE sample (ages 6–58) found evidence for decreased state transitions in ASD (116). This finding of reduced transitions between brain states has been replicated in adults with autism (117).
Focusing on functional connectivity variance in 6–36 year-olds within ABIDE, greater variance of widespread long-range dynamic functional connections in ASD was reported, and linked with symptom severity indexed by the Autism Diagnostic Observation Schedule (ADOS) (118). Similar findings of hyper-variability of functional connections in ASD have been observed in studies focusing on the adolescent period (119,120). The only study to stratify participants into child, adolescent, and adult groups found evidence for greater hyper-variability of short-range functional connections that distinguished adolescents with ASD (121).
In a very large sample including 774 6–10 year-old children from the Generation R Study (122), higher levels of autistic traits and ASD diagnosis were associated with longer dwell times in a functional connectivity state characterized by global disconnection (123). These findings suggest that atypical brain dynamics in ASD may be present at earlier points in development than adolescence.
Dynamic functional connectivity research has rapidly accelerated (124), in part due to the availability of data made possible through the ABIDE initiative (125–128). The studies reviewed here have utilized different subsets from the larger ABIDE datasets, with minimal sample overlap across studies (Table 2). The data thus far provides evidence for both alterations in the number of transitions between brain states, and hyper-variability of functional connections in adolescents with ASD. Limitations and inconsistencies in this literature could be attributed to variable MRI data acquisition parameters, participant demographics, and data analytic pipelines. Increased sample inhomogeneity due to data pooling across sites can introduce biases that must be considered. Efforts to overcome these limitations include cross-site replication or leave-one-site-out cross validation (118,129).
To date, no studies have explicitly explored the link between atypical brain dynamics and cognitive flexibility in ASD. The hope is that with greater methods development in the field of machine learning (130), these types of neuroimaging markers may eventually be used to parse heterogeneity, monitor treatment response, and predict individual outcomes in ASD (131).
6. Outstanding Issues and Future Directions
Ecological validity and measurement
Measurement issues still complicate the study of cognitive flexibility in ASD. Performance-based measures such as the WCST can hone in on specific cognitive constructs (132), whereas more ecologically-valid measures such as the BRIEF are sensitive indices of real-world behaviors (6) but may be subject to reporter bias. Although individuals with ASD appear behaviorally rigid in daily activities, neuropsychological and laboratory-based measures of cognitive flexibility provide mixed results with respect to patterns of EF deficits (74). Poor convergence between these two types of measures might influence findings of cognitive flexibility impairments in ASD and relationships with outcomes such as symptom severity and adaptive functioning. Measures with high reliability such as informant- or self-report may better predict individual differences in real-life outcomes, whereas behavioral measures that are sensitive to within-person experimental manipulations may be important for studying processes that underlie task performance (5). A clearer neuroimaging story might emerge if cognitive neuroscientifically-derived measures are used alongside informant reports of cognitive flexibility in future studies.
In parallel to the identification of neural circuits involved in cognitive flexibility, standardized assessments of flexible behaviors in daily life that potentially have greater ecological validity must be developed and validated. The Flexibility Scale, based on data collected from 300 6–17 year olds, is an informant report that densely samples cognitive aspects of flexibility in everyday settings and has been shown to discriminate participants with ASD and controls. Exploratory factor analysis revealed evidence for five factors related to Routines/Rituals, Transitions/Change, Special Interests, Social Flexibility, and Generativity, and the scale demonstrated convergent and divergent validity with comparative domains in other measures including the ‘shift’ subscale of the BRIEF and D-KEFS performance (133). Once further validated, specific measures of cognitive flexibility such as the Flexibility Scale could be used in future research to promote standardization and replicability in the field.
Neuroimaging of individual differences and heterogeneity
Children and adolescents with ASD may have difficulties with flexibility that can persist into adulthood (134). Understanding the neural basis of individual differences in cognitive flexibility in ASD will pave the way for development of more targeted early interventions to improve the lives of those affected. Specifically, young children exhibiting impaired EF abilities who are identified early in life may benefit from targeted training in this area. Unstuck and On Target (UOT) is an EF intervention designed for children with ASD that can be implemented in school and at home. UOT targets insistence on sameness, flexibility, goal-setting and planning through a cognitive-behavioral program involving self-regulatory scripts, guided practice, and cueing, and has been shown to be effective for improving classroom behavior, flexibility and problem-solving in children with ASD (135). The neural mechanisms associated with successful implementation of this intervention are yet unknown.
Importantly, not all children with ASD exhibit the same level and profile of EF deficits. While some studies provide evidence of uniform patterns of abilities across EF domains (60,61), others suggest that distinct EF subtypes exist (136). This heterogeneity of EF ability and underlying brain network organization makes accurate characterization of EF in ASD all the more challenging. Neuroimaging can provide a means for understanding neurobiological mechanisms underlying heterogeneous symptom presentation in ASD. Future directions include further attempts at stratifying youth with ASD based on EF profiles and individual connectomes (136–138).
Future studies must work to overcome the limited generalizability afforded by small sample sizes (139) and further consider females with ASD and individuals of varied socioeconomic status, who are largely under-represented in neuroimaging research. Co-occurring conditions that are associated with cognitive inflexibility have also not been adequately considered, despite initial evidence that EF impairment is more severe in children with comorbid ASD and ADHD (60).
Consideration of adolescence and puberty
As Table 1 and Table 2 demonstrate, very few neuroimaging studies have focused on the adolescent period specifically in ASD. Additionally, it is completely unknown how puberty, which marks the beginning of adolescence, influences the development of brain systems underlying cognitive flexibility in autism. Dissociable effects of pubertal hormones and age on the adolescent brain have been documented, suggesting that pubertal stage may be a better predictor of cognitive and behavioral maturity than chronological age (140). Hormonal effects on brain, behavior, and cognition constitute an active area of research (141) that must be incorporated into future cognitive neuroscience work on adolescent ASD.
Alternative approaches for bolstering cognitive flexibility in ASD
The bilingual advantage refers to the phenomenon that individuals who speak two languages fluently often perform better on tasks of EF than monolingual individuals (142, but see 143). Despite the potential advantages that bilingualism may confer for EF, clinical practitioners commonly advise against providing children with developmental disabilities a bilingual environment (144), believing that concentrating on one language will better support language development (145). Yet, a growing body of work suggests there are no negative effects of being raised in a bilingual environment (146,147). A study of 6–16 year olds with average IQ levels suggests that second language exposure in children with ASD is associated with reduced clinical impact in the domains of functional communication and EF (148). Bilingualism may even mitigate set-shifting difficulties in children with ASD of average IQ (149). If bilingualism is indeed found to confer an EF advantage in ASD, then encouraging parents to speak two languages in the home may be one “natural intervention” strategy for bolstering cognitive flexibility in high-functioning autism.
Conclusion
Cognitive flexibility may facilitate optimal functioning in ASD during the volatile period of adolescence. Studies of the neural mechanisms underlying the development of flexible cognition and behavior in ASD provide initial evidence for altered brain activation and dynamics in diagnosed individuals. Moving forward, issues of measurement and sample heterogeneity must be adequately addressed in order to maximize ecological and construct validity in studies of cognitive flexibility in ASD. Development of interventions to enhance flexibility, and neuroimaging studies exploring the mechanisms underlying training effects, will be important future directions.
Acknowledgments:
This work was supported by the National Institute of Mental Health [R01MH107549], the Canadian Institute for Advanced Research, and a University of Miami Gabelli Senior Scholar Award to LQU.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: Dr. Uddin reports no biomedical financial interests or potential conflicts of interest.
References
- 1.Cole MW, Laurent P, Stocco A (2013): Rapid instructed task learning: a new window into the human brain’s unique capacity for flexible cognitive control. Cogn Affect Behav Neurosci 13: 1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Scott WA (1962): Cognitive Complexity and Cognitive Flexibility. Sociometry 25: 405–414. [Google Scholar]
- 3.Banich MT (2009): Executive Function: The Search for an Integrated Account. Curr Dir Psychol Sci 18: 89–94. [Google Scholar]
- 4.Miyake A, Friedman NP (2012): The Nature and Organization of Individual Differences in Executive Functions: Four General Conclusions. Curr Dir Psychol Sci 21: 8–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dang J, King KM, Inzlicht M (2020): Why Are Self-Report and Behavioral Measures Weakly Correlated? Trends Cogn Sci 24: 267–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gioia GA, Isquith PK, Guy SC, Kenworthy L (2000): Behavior Rating Inventory of Executive Function. Child Neuropsychol 6: 235–238. [DOI] [PubMed] [Google Scholar]
- 7.Roth RM, Isquith PK, Gioia GA (2005): BRIEF-A: Behavior Rating Inventory of Executive Function--Adult Version: Professional Manual. Psychological Assessment Resources. [Google Scholar]
- 8.Skogan AH, Egeland J, Zeiner P, Øvergaard KR, Oerbeck B, Reichborn-Kjennerud T, Aase H (2016): Factor structure of the Behavior Rating Inventory of Executive Functions (BRIEF-P) at age three years. Child Neuropsychol 22: 472–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Isquith PK, Roth RM, Gioia GA, Par S (2006): Behavior Rating Inventory of Executive Function--Adult Version (BRIEF-A) Interpretive Report. Lutz, FL: Psychological Assessment Resources. [Google Scholar]
- 10.Luciana M, Bjork JM, Nagel BJ, Barch DM, Gonzalez R, Nixon SJ, Banich MT (2018): Adolescent neurocognitive development and impacts of substance use: Overview of the adolescent brain cognitive development (ABCD) baseline neurocognition battery. Developmental Cognitive Neuroscience, vol. 32 pp 67–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zelazo PD (2006): The Dimensional Change Card Sort (DCCS): a method of assessing executive function in children. Nat Protoc 1: 297–301. [DOI] [PubMed] [Google Scholar]
- 12.Delis DC, Kaplan E, Kramer JH (2001): Delis-Kaplan Executive Function System. Retrieved from https://psycnet.apa.org/doiLanding?doi=10.1037/t15082-000 [DOI] [PubMed]
- 13.Latzman RD, Markon KE (2010): The Factor Structure and Age-Related Factorial Invariance of the Delis-Kaplan Executive Function System (D-KEFS). Assessment, vol. 17 pp 172–184. [DOI] [PubMed] [Google Scholar]
- 14.Brooks BL, Sherman EMS, Strauss E (2009): NEPSY-II: A Developmental Neuropsychological Assessment, Second Edition. Child Neuropsychology, vol. 16 pp 80–101. [Google Scholar]
- 15.Toplak ME, West RF, Stanovich KE (2013): Practitioner review: do performance-based measures and ratings of executive function assess the same construct? J Child Psychol Psychiatry 54: 131–143. [DOI] [PubMed] [Google Scholar]
- 16.MacDonald AW 3rd, Carter CS (2002): Cognitive experimental approaches to investigating impaired cognition in schizophrenia: a paradigm shift. J Clin Exp Neuropsychol 24: 873–882. [DOI] [PubMed] [Google Scholar]
- 17.Worringer B, Langner R, Koch I, Eickhoff SB, Eickhoff CR, Binkofski FC (2019): Common and distinct neural correlates of dual-tasking and task-switching: a meta-analytic review and a neuro-cognitive processing model of human multitasking. Brain Struct Funct 224: 1845–1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Derrfuss J, Brass M, Neumann J, von Cramon DY (2005): Involvement of the inferior frontal junction in cognitive control: Meta-analyses of switching and Stroop studies. Hum Brain Mapp 25: 22–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Monsell S (2003): Task switching. Trends Cogn Sci 7: 134–140. [DOI] [PubMed] [Google Scholar]
- 20.Ravizza SM, Carter CS (2008): Shifting set about task switching: behavioral and neural evidence for distinct forms of cognitive flexibility. Neuropsychologia 46: 2924–2935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim C, Cilles SE, Johnson NF, Gold BT (2012): Domain general and domain preferential brain regions associated with different types of task switching: a meta-analysis. Hum Brain Mapp 33: 130–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Niendam TA, Laird AR, Ray KL, Dean YM, Glahn DC, Carter CS (2012): Meta-analytic evidence for a superordinate cognitive control network subserving diverse executive functions. Cogn Affect Behav Neurosci 12: 241–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, et al. (2007): Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci 27: 2349–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Uddin LQ, Yeo BTT, Spreng RN (2019): Towards a Universal Taxonomy of Macro-scale Functional Human Brain Networks. Brain Topogr 32: 926–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dajani DR, Uddin LQ (2015): Demystifying cognitive flexibility: Implications for clinical and developmental neuroscience. Trends Neurosci 38: 571–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Armbruster DJ, Ueltzhoffer K, Basten U, Fiebach CJ (2012): Prefrontal cortical mechanisms underlying individual differences in cognitive flexibility and stability. J Cogn Neurosci 24: 2385–2399. [DOI] [PubMed] [Google Scholar]
- 27.Badre D, Wagner AD (2006): Computational and neurobiological mechanisms underlying cognitive flexibility. Proc Natl Acad Sci U S A 103: 7186–7191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dippel G, Beste C (2015): A causal role of the right inferior frontal cortex in implementing strategies for multi-component behaviour. Nat Commun 6: 6587. [DOI] [PubMed] [Google Scholar]
- 29.Uddin LQ (2015): Salience processing and insular cortical function and dysfunction. Nat Rev Neurosci 16: 55–61. [DOI] [PubMed] [Google Scholar]
- 30.Nee DE, D’Esposito M (2016): The representational basis of working memory Behavioral Neuroscience of Learning and Memory. Springer, pp 213–230. [DOI] [PubMed] [Google Scholar]
- 31.Hoffstaedter F, Grefkes C, Caspers S, Roski C, Palomero-Gallagher N, Laird AR, et al. (2014): The role of anterior midcingulate cortex in cognitive motor control: evidence from functional connectivity analyses. Hum Brain Mapp 35: 2741–2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Margulies DS, Uddin LQ (2019): Network convergence zones in the anterior midcingulate cortex. Handb Clin Neurol 166: 103–111. [DOI] [PubMed] [Google Scholar]
- 33.Wager TD, Jonides J, Reading S (2004): Neuroimaging studies of shifting attention: a meta-analysis. NeuroImage, vol. 22 pp 1679–1693. [DOI] [PubMed] [Google Scholar]
- 34.Solomon M, Smith AC, Frank MJ, Ly S, Carter CS (2011): Probabilistic reinforcement learning in adults with autism spectrum disorders. Autism Research, vol. 4 pp 109–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Solomon M, Frank MJ, Daniel Ragland J, Smith AC, Niendam TA, Lesh TA, et al. (2015): Feedback-Driven Trial-by-Trial Learning in Autism Spectrum Disorders. American Journal of Psychiatry, vol. 172 pp 173–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Diamond A, Lee K (2011): Interventions shown to aid executive function development in children 4 to 12 years old. Science 333: 959–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bailey CE (2007): Cognitive accuracy and intelligent executive function in the brain and in business. Ann N Y Acad Sci 1118: 122–141. [DOI] [PubMed] [Google Scholar]
- 38.Burt KB, Paysnick AA (2012): Resilience in the transition to adulthood. Dev Psychopathol 24: 493–505. [DOI] [PubMed] [Google Scholar]
- 39.Kapp SK, Gantman A, Laugeson EA (2011): Transition to adulthood for high-functioning individuals with autism spectrum disorders. A comprehensive book on autism spectrum disorders 451–478. [Google Scholar]
- 40.Anderson P (2002): Assessment and development of executive function (EF) during childhood. Child Neuropsychol 8: 71–82. [DOI] [PubMed] [Google Scholar]
- 41.Dick AS (2014): The development of cognitive flexibility beyond the preschool period: an investigation using a modified Flexible Item Selection Task. J Exp Child Psychol 125: 13–34. [DOI] [PubMed] [Google Scholar]
- 42.Hunter SJ, Sparrow EP (2012): Executive Function and Dysfunction: Identification, Assessment and Treatment Cambridge University Press. [Google Scholar]
- 43.Cepeda NJ, Kramer AF, Gonzalez de Sather JC (2001): Changes in executive control across the life span: examination of task-switching performance. Dev Psychol 37: 715–730. [PubMed] [Google Scholar]
- 44.Yerys BE, Antezana L, Weinblatt R, Jankowski KF, Strang J, Vaidya CJ, et al. (2015): Neural Correlates of Set-Shifting in Children With Autism. Autism Res 8: 386–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wendelken C, Munakata Y, Baym C, Souza M, Bunge SA (2012): Flexible rule use: common neural substrates in children and adults. Dev Cogn Neurosci 2: 329–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dajani DR, Odriozola P, Winters M, Voorhies W, Marcano S, Baez A, Gates KM, Dick AS, Uddin LQ (2020): Measuring cognitive flexibility with the Flexible Item Selection Task: From MRI adaptation to individual connectome mapping. J Cogn Neurosci. [DOI] [PubMed] [Google Scholar]
- 47.Dirks B, Voorhies W, Romero C, Kupis L, Nomi JS, Dajani DR, Odriozola P, Burrows CA, Beaumont A, Cardona S, Parlade M, Alessandri M, Britton JC, Uddin LQ (2020): Neural responses to a set-shifting task in children with autism spectrum disorder. Under Review. [DOI] [PubMed] [Google Scholar]
- 48.Bunge SA, Dudukovic NM, Thomason ME, Vaidya CJ, Gabrieli JD (2002): Immature frontal lobe contributions to cognitive control in children: evidence from fMRI. Neuron 33: 301–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jacobs R, Simon Harvey A, Anderson V (2011): Are executive skills primarily mediated by the prefrontal cortex in childhood? Examination of focal brain lesions in childhood. Cortex, vol. 47 pp 808–824. [DOI] [PubMed] [Google Scholar]
- 50.Fair DA, Cohen AL, Power JD, Dosenbach NU, Church JA, Miezin FM, et al. (2009): Functional brain networks develop from a “local to distributed” organization. PLoS Comput Biol 5: e1000381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Satterthwaite TD, Ciric R, Roalf DR, Davatzikos C, Bassett DS, Wolf DH (2019): Motion artifact in studies of functional connectivity: Characteristics and mitigation strategies. Hum Brain Mapp 40: 2033–2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Baum GL, Ciric R, Roalf DR, Betzel RF, Moore TM, Shinohara RT, et al. (2017): Modular Segregation of Structural Brain Networks Supports the Development of Executive Function in Youth. Curr Biol 27: 1561–1572.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hauser TU, Iannaccone R, Walitza S, Brandeis D, Brem S (2015): Cognitive flexibility in adolescence: neural and behavioral mechanisms of reward prediction error processing in adaptive decision making during development. Neuroimage 104: 347–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Uddin LQ, Supekar KS, Ryali S, Menon V (2011): Dynamic reconfiguration of structural and functional connectivity across core neurocognitive brain networks with development. J Neurosci 31: 18578–18589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Engelhardt LE, Paige Harden K, Tucker-Drob EM, Church JA (2019): The neural architecture of executive functions is established by middle childhood. NeuroImage, vol. 185 pp 479–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chai LR, Khambhati AN, Ciric R, Moore TM, Gur RC, Gur RE, et al. (2017): Evolution of brain network dynamics in neurodevelopment. Netw Neurosci 1: 14–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Medaglia JD, Satterthwaite TD, Kelkar A, Ciric R, Moore TM, Ruparel K, et al. (2018): Brain state expression and transitions are related to complex executive cognition in normative neurodevelopment. Neuroimage 166: 293–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sripada C, Rutherford S, Angstadt M, Thompson WK, Luciana M, Weigard A, et al. (2019): Prediction of neurocognition in youth from resting state fMRI. Mol Psychiatry. 10.1038/s41380-019-0481-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Demetriou EA, DeMayo MM, Guastella AJ (2019): Executive Function in Autism Spectrum Disorder: History, Theoretical Models, Empirical Findings, and Potential as an Endophenotype. Front Psychiatry 10: 753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dajani DR, Llabre MM, Nebel MB, Mostofsky SH, Uddin LQ (2016): Heterogeneity of executive functions among comorbid neurodevelopmental disorders. Sci Rep 6: 36566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Baez AC, Dajani DR, Voorhies W, Parladé MV, Alessandri M, Britton JC, et al. (2019): Parsing Heterogeneity of Executive Function in Typically and Atypically Developing Children: A Conceptual Replication and Exploration of Social Function. Journal of Autism and Developmental Disorders. 10.1007/s10803-019-04290-9 [DOI] [PubMed] [Google Scholar]
- 62.Brunsdon VEA, Happé F (2014): Exploring the “fractionation”of autism at the cognitive level. Autism 18: 17–30. [DOI] [PubMed] [Google Scholar]
- 63.Happe F, Booth R, Charlton R, Hughes C (2006): Executive function deficits in autism spectrum disorders and attention-deficit/hyperactivity disorder: examining profiles across domains and ages. Brain Cogn 61: 25–39. [DOI] [PubMed] [Google Scholar]
- 64.Lai CLE, Lau Z, Lui SSY, Lok E, Tam V, Chan Q, et al. (2017): Meta-analysis of neuropsychological measures of executive functioning in children and adolescents with high-functioning autism spectrum disorder. Autism Research, vol. 10 pp 911–939. [DOI] [PubMed] [Google Scholar]
- 65.Demetriou EA, Lampit A, Quintana DS, Naismith SL, Song YJC, Pye JE, et al. (2018): Autism spectrum disorders: a meta-analysis of executive function. Mol Psychiatry 23: 1198–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lopez BR, Lincoln AJ, Ozonoff S, Lai Z (2005): Examining the relationship between executive functions and restricted, repetitive symptoms of Autistic Disorder. J Autism Dev Disord 35: 445–460. [DOI] [PubMed] [Google Scholar]
- 67.Van Eylen L, Boets B, Steyaert J, Evers K, Wagemans J, Noens I (2011): Cognitive flexibility in autism spectrum disorder: Explaining the inconsistencies? Res Autism Spectr Disord 5: 1390–1401. [Google Scholar]
- 68.Yerys BE, Wallace GL, Harrison B, Celano MJ, Giedd JN, Kenworthy LE (2009): Set-shifting in children with autism spectrum disorders: reversal shifting deficits on the Intradimensional/Extradimensional Shift Test correlate with repetitive behaviors. Autism 13: 523–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Anderson KA, Shattuck PT, Cooper BP, Roux AM, Wagner M (2014): Prevalence and correlates of postsecondary residential status among young adults with an autism spectrum disorder. Autism 18: 562–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yerys BE, Wolff BC, Moody E, Pennington BF, Hepburn SL (2012): Brief Report: impaired Flexible Item Selection Task (FIST) in school-age children with autism spectrum disorders. J Autism Dev Disord 42: 2013–2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yeung MK, Han YMY, Sze SL, Chan AS (2016): Abnormal frontal theta oscillations underlie the cognitive flexibility deficits in children with high-functioning autism spectrum disorders. Neuropsychology, vol. 30 pp 281–295. [DOI] [PubMed] [Google Scholar]
- 72.van den Bergh SF, Scheeren AM, Begeer S, Koot HM, Geurts HM (2014): Age related differences of executive functioning problems in everyday life of children and adolescents in the autism spectrum. J Autism Dev Disord 44: 1959–1971. [DOI] [PubMed] [Google Scholar]
- 73.Russo N, Flanagan T, Iarocci G, Berringer D, Zelazo PD, Burack JA (2007): Deconstructing executive deficits among persons with autism: implications for cognitive neuroscience. Brain Cogn 65: 77–86. [DOI] [PubMed] [Google Scholar]
- 74.Geurts HM, Corbett B, Solomon M (2009): The paradox of cognitive flexibility in autism. Trends in Cognitive Sciences, vol. 13 pp 74–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ballaban-Gil K, Rapin I, Tuchman R, Shinnar S (1996): Longitudinal examination of the behavioral, language, and social changes in a population of adolescents and young adults with autistic disorder. Pediatr Neurol 15: 217–223. [DOI] [PubMed] [Google Scholar]
- 76.Gillberg C, Schaumann H (1982): Infantile autism and puberty. Journal of Autism and Developmental Disorders, vol. 11 pp 365–371. [DOI] [PubMed] [Google Scholar]
- 77.Bennett AE, Miller JS, Stollon N, Prasad R, Blum NJ (2018): Autism Spectrum Disorder and Transition-Aged Youth. Curr Psychiatry Rep 20: 103. [DOI] [PubMed] [Google Scholar]
- 78.Picci G, Scherf KS (2015): A Two-Hit Model of Autism: Adolescence as the Second Hit. Clin Psychol Sci 3: 349–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bertollo JR, Strang JF, Anthony LG, Kenworthy L, Wallace GL, Yerys BE (2020): Adaptive Behavior in Youth with Autism Spectrum Disorder: The Role of Flexibility. J Autism Dev Disord 50: 42–50. [DOI] [PubMed] [Google Scholar]
- 80.Pugliese CE, Anthony LG, Strang JF, Dudley K, Wallace GL, Naiman DQ, Kenworthy L (2016): Longitudinal Examination of Adaptive Behavior in Autism Spectrum Disorders: Influence of Executive Function. J Autism Dev Disord 46: 467–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kenny L, Cribb SJ, Pellicano E (2019): Childhood Executive Function Predicts Later Autistic Features and Adaptive Behavior in Young Autistic People: a 12-Year Prospective Study. Journal of Abnormal Child Psychology, vol. 47 pp 1089–1099. [DOI] [PubMed] [Google Scholar]
- 82.Schmitz N, Rubia K, Daly E, Smith A, Williams S, Murphy DGM (2006): Neural correlates of executive function in autistic spectrum disorders. Biol Psychiatry 59: 7–16. [DOI] [PubMed] [Google Scholar]
- 83.Shafritz KM, Dichter GS, Baranek GT, Belger A (2008): The neural circuitry mediating shifts in behavioral response and cognitive set in autism. Biol Psychiatry 63: 974–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.D’Cruz A-M, Mosconi MW, Ragozzino ME, Cook EH, Sweeney JA (2016): Alterations in the functional neural circuitry supporting flexible choice behavior in autism spectrum disorders. Transl Psychiatry 6: e916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Taylor MJ, Donner EJ, Pang EW (2012): fMRI and MEG in the study of typical and atypical cognitive development. Neurophysiol Clin 42: 19–25. [DOI] [PubMed] [Google Scholar]
- 86.Uddin LQ, Menon V (2009): The anterior insula in autism: under-connected and under-examined. Neurosci Biobehav Rev 33: 1198–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Uddin LQ, Supekar K, Lynch CJ, Cheng KM, Odriozola P, Barth ME, et al. (2015): Brain State Differentiation and Behavioral Inflexibility in Autism. Cereb Cortex 25: 4740–4747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Uddin LQ, Supekar K, Lynch CJ, Khouzam A, Phillips J, Feinstein C, et al. (2013): Salience Network–Based Classification and Prediction of Symptom Severity in Children With Autism. JAMA Psychiatry 70: 869–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhang Z, Peng P, Zhang D (2020): Executive Function in High-Functioning Autism Spectrum Disorder: A Meta-analysis of fMRI Studies. Journal of Autism and Developmental Disorders. 10.1007/s10803-020-04461-z [DOI] [PubMed] [Google Scholar]
- 90.Casey BJ (2015): Beyond simple models of self-control to circuit-based accounts of adolescent behavior. Annu Rev Psychol 66: 295–319. [DOI] [PubMed] [Google Scholar]
- 91.Larsen B, Luna B (2018): Adolescence as a neurobiological critical period for the development of higher-order cognition. Neurosci Biobehav Rev 94: 179–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Solomon M, Ozonoff SJ, Ursu S, Ravizza S, Cummings N, Ly S, Carter CS (2009): The neural substrates of cognitive control deficits in autism spectrum disorders. Neuropsychologia 47: 2515–2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Solomon M, Ragland JD, Niendam TA, Lesh TA, Beck JS, Matter JC, et al. (2015): Atypical Learning in Autism Spectrum Disorders: A Functional Magnetic Resonance Imaging Study of Transitive Inference. J Am Acad Child Adolesc Psychiatry 54: 947–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Solomon M, Yoon JH, Ragland JD, Niendam TA, Lesh TA, Fairbrother W, Carter CS (2014): The development of the neural substrates of cognitive control in adolescents with autism spectrum disorders. Biol Psychiatry 76: 412–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kenworthy L, Wallace GL, Birn R, Milleville SC, Case LK, Bandettini PA, Martin A (2013): Aberrant neural mediation of verbal fluency in autism spectrum disorders. Brain Cogn 83: 218–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Uddin LQ, Supekar K, Menon V (2010): Typical and atypical development of functional human brain networks: insights from resting-state FMRI. Front Syst Neurosci 4: 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Di Martino A, Fair DA, Kelly C, Satterthwaite TD, Castellanos FX, Thomason ME, et al. (2014): Unraveling the miswired connectome: a developmental perspective. Neuron 83: 1335–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Reid AT, Headley DB, Mill RD, Sanchez-Romero R, Uddin LQ, Marinazzo D, et al. (2019): Advancing functional connectivity research from association to causation. Nat Neurosci. 10.1038/s41593-019-0510-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chang C, Glover GH (2010): Time–frequency dynamics of resting-state brain connectivity measured with fMRI. Neuroimage 50: 81–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hutchison RM, Womelsdorf T, Allen EA, Bandettini PA, Calhoun VD, Corbetta M, et al. (2013): Dynamic functional connectivity: promise, issues, and interpretations. Neuroimage 80: 360–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Calhoun VD, Miller R, Pearlson G, Adalı T (2014): The chronnectome: time-varying connectivity networks as the next frontier in fMRI data discovery. Neuron 84: 262–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Uddin LQ, Karlsgodt KH (2018): Future Directions for Examination of Brain Networks in Neurodevelopmental Disorders. J Clin Child Adolesc Psychol 47: 483–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Allen EA, Damaraju E, Plis SM, Erhardt EB, Eichele T, Calhoun VD (2014): Tracking whole-brain connectivity dynamics in the resting state. Cereb Cortex 24: 663–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Liu X, Duyn JH (2013): Time-varying functional network information extracted from brief instances of spontaneous brain activity. Proc Natl Acad Sci U S A 110: 4392–4397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chen JE, Chang C, Greicius MD, Glover GH (2015): Introducing co-activation pattern metrics to quantify spontaneous brain network dynamics. Neuroimage 111: 476–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Preti MG, Bolton TA, Van De Ville D (2016): The dynamic functional connectome: State-of-the-art and perspectives. Neuroimage. 10.1016/j.neuroimage.2016.12.061 [DOI] [PubMed] [Google Scholar]
- 107.Nomi JS, Vij SG, Dajani DR, Steimke R, Damaraju E, Rachakonda S, et al. (2017): Chronnectomic patterns and neural flexibility underlie executive function. Neuroimage 147: 861–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Cabral J, Vidaurre D, Marques P, Magalhães R, Silva Moreira P, Miguel Soares J, et al. (2017): Cognitive performance in healthy older adults relates to spontaneous switching between states of functional connectivity during rest. Sci Rep 7: 5135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Chen T, Cai W, Ryali S, Supekar K, Menon V (2016): Distinct Global Brain Dynamics and Spatiotemporal Organization of the Salience Network. PLoS Biol 14: e1002469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Douw L, Wakeman DG, Tanaka N, Liu H, Stufflebeam SM (2016): State-dependent variability of dynamic functional connectivity between frontoparietal and default networks relates to cognitive flexibility. Neuroscience, vol. 339 pp 12–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Cohen JR (2018): The behavioral and cognitive relevance of time-varying, dynamic changes in functional connectivity. Neuroimage 180: 515–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lurie DJ, Kessler D, Bassett DS, Betzel RF, Breakspear M, Keilholz S, et al. (2019): Questions and controversies in the study of time-varying functional connectivity in resting fMRI. Network Neuroscience. pp 1–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Falahpour M, Thompson WK, Abbott AE, Jahedi A, Mulvey ME, Datko M, et al. (2016): Underconnected, But Not Broken? Dynamic Functional Connectivity MRI Shows Underconnectivity in Autism Is Linked to Increased Intra-Individual Variability Across Time. Brain Connectivity, vol. 6 pp 403–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Di Martino A, Yan C-G, Li Q, Denio E, Castellanos FX, Alaerts K, et al. (2014): The autism brain imaging data exchange: towards a large-scale evaluation of the intrinsic brain architecture in autism. Mol Psychiatry 19: 659–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Yao Z, Hu B, Xie Y, Zheng F, Liu G, Chen X, Zheng W (2016): Resting-State Time-Varying Analysis Reveals Aberrant Variations of Functional Connectivity in Autism. Front Hum Neurosci 10: 463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lacy N de, de Lacy N, Doherty D, King BH, Rachakonda S, Calhoun VD (2017): Disruption to control network function correlates with altered dynamic connectivity in the wider autism spectrum. NeuroImage: Clinical, vol. 15 pp 513–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Watanabe T, Rees G (2017): Brain network dynamics in high-functioning individuals with autism. Nat Commun 8: 16048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Chen H, Nomi JS, Uddin LQ, Duan X, Chen H (2017): Intrinsic functional connectivity variance and state-specific under-connectivity in autism. Hum Brain Mapp 38: 5740–5755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Mash LE, Linke AC, Olson LA, Fishman I, Liu TT, Müller R-A (2019): Transient states of network connectivity are atypical in autism: A dynamic functional connectivity study. Hum Brain Mapp 40: 2377–2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Li Y, Zhu Y, Nguchu BA, Wang Y, Wang H, Qiu B, Wang X (2019): Dynamic Functional Connectivity Reveals Abnormal Variability and Hyper connected Pattern in Autism Spectrum Disorder. Autism Research. 10.1002/aur.2212 [DOI] [PubMed] [Google Scholar]
- 121.Harlalka V, Bapi RS, Vinod PK, Roy D (2019): Atypical Flexibility in Dynamic Functional Connectivity Quantifies the Severity in Autism Spectrum Disorder. Front Hum Neurosci 13: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Jaddoe VWV, van Duijn CM, Franco OH, van der Heijden AJ, van Iizendoorn MH, de Jongste JC, et al. (2012): The Generation R Study: design and cohort update 2012. Eur J Epidemiol 27: 739–756. [DOI] [PubMed] [Google Scholar]
- 123.Rashid B, Blanken LME, Muetzel RL, Miller R, Damaraju E, Arbabshirani MR, et al. (2018): Connectivity dynamics in typical development and its relationship to autistic traits and autism spectrum disorder. Hum Brain Mapp 39: 3127–3142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.White T, Calhoun VD (2019): Dissecting Static and Dynamic Functional Connectivity: Example From the Autism Spectrum. Journal of Experimental Neuroscience, vol. 13 p 117906951985180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Guo X, Duan X, Suckling J, Chen H, Liao W, Cui Q, Chen H (2019): Partially impaired functional connectivity states between right anterior insula and default mode network in autism spectrum disorder. Hum Brain Mapp 40: 1264–1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Guo X, Duan X, Chen H, He C, Xiao J, Han S, et al. (2020): Altered inter and intrahemispheric functional connectivity dynamics in autistic children. Human Brain Mapping, vol. 41 pp 419–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Fu Z, Tu Y, Di X, Du Y, Sui J, Biswal BB, et al. (2019): Transient increased thalamic-sensory connectivity and decreased whole-brain dynamism in autism. NeuroImage, vol. 190 pp 191–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Liu C, Xue J, Cheng X, Zhan W, Xiong X, Wang B (2019): Tracking the Brain State Transition Process of Dynamic Function Connectivity Based on Resting State fMRI. Computational Intelligence and Neuroscience, vol. 2019 pp 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.He Y, Byrge L, Kennedy DP (2020): Nonreplication of functional connectivity differences in autism spectrum disorder across multiple sites and denoising strategies. Hum Brain Mapp 41: 1334–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Saggar M, Uddin LQ (2019): Pushing the Boundaries of Psychiatric Neuroimaging to Ground Diagnosis in Biology. eNeuro 6 10.1523/ENEURO.0384-19.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Uddin LQ, Dajani DR, Voorhies W, Bednarz H, Kana RK (2017): Progress and roadblocks in the search for brain-based biomarkers of autism and attention-deficit/hyperactivity disorder. Transl Psychiatry 7: e1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hill EL (2004): Executive dysfunction in autism. Trends Cogn Sci 8: 26–32. [DOI] [PubMed] [Google Scholar]
- 133.Strang JF, Anthony LG, Yerys BE, Hardy KK, Wallace GL, Armour AC, et al. (2017): The Flexibility Scale: Development and Preliminary Validation of a Cognitive Flexibility Measure in Children with Autism Spectrum Disorders. J Autism Dev Disord 47: 2502–2518. [DOI] [PubMed] [Google Scholar]
- 134.Rosenthal M, Wallace GL, Lawson R, Wills MC, Dixon E, Yerys BE, Kenworthy L (2013): Impairments in real-world executive function increase from childhood to adolescence in autism spectrum disorders. Neuropsychology 27: 13–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kenworthy L, Anthony LG, Naiman DQ, Cannon L, Wills MC, Luong-Tran C, et al. (2014): Randomized controlled effectiveness trial of executive function intervention for children on the autism spectrum. J Child Psychol Psychiatry 55: 374–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Vaidya CJ, You X, Mostofsky S (2020): Data driven identification of subtypes of executive function across typical development, attention deficit hyperactivity disorder, and autism spectrum disorders. Journal of Child. Retrieved from https://onlinelibrary.wiley.com/doi/abs/10.1111/jcpp.13114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Dajani DR, Burrows CA, Nebel MB, Mostofsky SH, Gates KM, Uddin LQ (2019): Parsing Heterogeneity in Autism Spectrum Disorder and Attention-Deficit/Hyperactivity Disorder with Individual Connectome Mapping. Brain Connectivity, vol. 9 pp 673–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Dajani DR, Burrows CA, Odriozola P, Baez A, Nebel MB, Mostofsky SH, Uddin LQ (2019): Investigating functional brain network integrity using a traditional and novel categorical scheme for neurodevelopmental disorders. Neuroimage Clin 21: 101678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Button KS, Ioannidis JPA, Mokrysz C, Nosek BA, Flint J, Robinson ESJ, Munafò MR (2013): Power failure: why small sample size undermines the reliability of neuroscience. Nat Rev Neurosci 14: 365–376. [DOI] [PubMed] [Google Scholar]
- 140.Galvan A, Van Leijenhorst L, McGlennen KM (2012): Considerations for imaging the adolescent brain. Dev Cogn Neurosci 2: 293–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Blakemore SJ, Burnett S, Dahl RE (2010): The role of puberty in the developing adolescent brain. Hum Brain Mapp 31: 926–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Bialystok E, Craik FIM, Luk G (2012): Bilingualism: consequences for mind and brain. Trends Cogn Sci 16: 240–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Dick AS, Garcia NL, Pruden SM, Thompson WK, Hawes SW, Sutherland MT, et al. (2019): No evidence for a bilingual executive function advantage in the ABCD study. Nature Human Behaviour 3: 692–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Drysdale H, van der Meer L, Kagohara D (2015): Children with Autism Spectrum Disorder from Bilingual Families: a Systematic Review. Review Journal of Autism and Developmental Disorders 2: 26–38. [Google Scholar]
- 145.Moore S, Pérez-Méndez C (2006): Working with Linguistically Diverse Families in Early Intervention: Misconceptions and Missed Opportunities. Seminars in Speech and Language, vol. 27 pp 187–198. [DOI] [PubMed] [Google Scholar]
- 146.Uljarević M, Katsos N, Hudry K, Gibson JL (2016): Practitioner Review: Multilingualism and neurodevelopmental disorders - an overview of recent research and discussion of clinical implications. Journal of Child Psychology and Psychiatry, vol. 57 pp 1205–1217. [DOI] [PubMed] [Google Scholar]
- 147.Soto G, Yu B (2014): Considerations for the Provision of Services to Bilingual Children Who Use Augmentative and Alternative Communication. Augment Altern Commun 30: 83–92. [DOI] [PubMed] [Google Scholar]
- 148.Iarocci G, Hutchison SM, O’Toole G (2017): Second Language Exposure, Functional Communication, and Executive Function in Children With and Without Autism Spectrum Disorder (ASD). J Autism Dev Disord 47: 1818–1829. [DOI] [PubMed] [Google Scholar]
- 149.Gonzalez-Barrero AM, Nadig AS (2019): Can Bilingualism Mitigate Set-Shifting Difficulties in Children With Autism Spectrum Disorders? Child Dev 90: 1043–1060. [DOI] [PubMed] [Google Scholar]


