Abstract
Natural pyrethrin insecticides produced by Dalmatian pyrethrum (Tanacetum cinerariifolium) have low mammalian toxicity and short environmental persistence, providing an alternative to widely used synthetic agricultural insecticides that pose a threat to human health and the environment. A recent surge of interest in the use of pyrethrins as agricultural insecticides coincides with the discovery of several new genes in the pyrethrin biosynthetic pathway. Elucidation of this pathway facilitates efforts to breed improved pyrethrum varieties and to engineer plants with improved endogenous defenses or hosts for heterologous pyrethrin production. We describe the current state of knowledge related to global pyrethrum production, the pyrethrin biosynthetic pathway and its regulation, and recent efforts to engineer the pyrethrin pathway in diverse plant hosts.
Keywords: pyrethrins, Tanacetum cinerariifolium, trichomes, natural insecticides, specialized metabolism
EARLY USE AND DEVELOPMENT OF PYRETHRUM-BASED INSECTICIDES
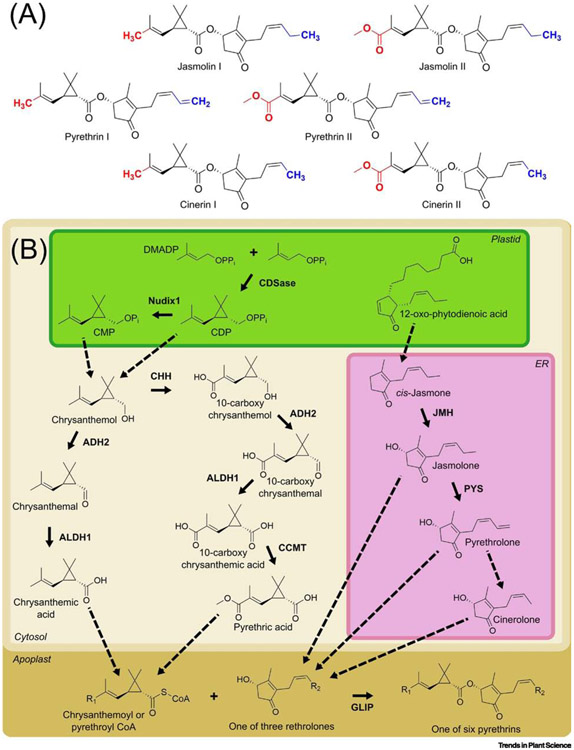
Pyrethrins constitute a small class of specialized metabolites (see Glossary) produced in Dalmatian pyrethrum (Tanacetum cinerariifolium) and provide the plant with an effective endogenous chemical defense against insect herbivores and fungal pathogens [1-4]. The insecticidal properties of pyrethrum have been known in western Europe and the United States since the 1840s but were likely discovered in eastern Europe as early as the late 17th century [5,6]. Pyrethrum products were used primarily as household insecticides in the 19th century [7]. By the early 20th century, they became tools for prevention of insect-borne diseases (e.g., malaria and yellow fever) [8,9] and alternatives to widely used agricultural pesticides with high mammalian toxicity (e.g., arsenic and cyanide) [10,11]. Identification of pyrethrins as the active components of pyrethrum in the early 1920s spurred research on their structure and mode of action [12]. This work eventually led to full structural elucidation of the six naturally occurring pyrethrins (Fig. 1A) and total syntheses for all six of these compounds [13-17] as well as the development of synthetic pyrethroid insecticides which exhibit increased environmental stability and toxicity toward insects relative to their natural counterparts (reviewed in [18]). When applied as a spray or powder, both natural pyrethrins and their synthetic pyrethroid derivatives cause knockdown and death of insects by binding to voltage-gated sodium channels in the insect nervous system, resulting in persistent channel activity [19-21].
Figure 1.
Pyrethrins and their biosynthesis. (A) Structures of the six natural pyrethrins found in Tanacetum cinerariifolium. Variable portions of the monoterpenoid moiety are highlighted in red, while variable portions of the rethrolone moiety are highlighted in blue. (B) The monoterpenoid skeleton of pyrethrins is plastidially derived while subsequent modifications of this skeleton such as oxidation, methylation, and conjugation to Coenzyme A occur in the cytosol and apoplast. The oxylipin skeleton of pyrethrins is produced in the plastid while conversion to rethrolones occurs in the endoplasmic reticulum. Final conjugation of the monoterpenoid and rethrolone moieties of pyrethrins occurs in the apoplast. Enzymes are indicated in bold text; solid arrows indicate known steps in the pathway; dashed arrows indicate steps not yet elucidated. Abbreviations: ADH2 – alcohol dehydrogenase 2; ALDH1 – aldehyde dehydrogenase 1; CCMT – 10-carboxychrysanthemic acid methyltransferase; CDP – chrysanthemyl diphosphate; CDSase – chrysanthemyl diphosphate synthase; CMP – chrysanthemyl monophosphate; CoA – coenzyme A; DMADP – dimethylallyl diphosphate; ER – endoplasmic reticulum; GLIP – GDSL lipase-like protein; JMH – jasmone hydroxylase; PYS – pyrethrolone synthase. R groups: R1 – CH3 or COOCH3; R2 – CH3, CH=CH2, or CH2CH3.
Nearly 100 years since their initial identification, scientists are again exploring natural pyrethrins as viable agricultural insecticides [22-32]. This renewed interest stems from unintended consequences of high pyrethroid stability. Pyrethroids were initially developed to solve the problem of rapid pyrethrin photodegradation [33]. While the natural pyrethrins exhibit half-lives of two hours to two days in agricultural settings [34-36]. synthetic pyrethroids exhibit half-lives of weeks to months [33,37]. The long half-lives of synthetic pyrethroids led to environmental persistence and ecological harm [38-40], as well as the development of knockdown resistance toward synthetic pyrethroids in agricultural insect pests and insect disease vectors [41-43]. In contrast, the natural pyrethrins remain effective against some insect pests that developed resistance to synthetic pyrethroids [44-47]. In the last several years, our understanding of the pyrethrin biosynthetic pathway expanded greatly with the number of characterized pyrethrin-specific biosynthetic genes increasing from two to nine [48-52]. Additionally, genetic engineering experiments demonstrated the promise of increasing commercial pyrethrin production via heterologous hosts or engineering crop plants with endogenous pyrethrin defenses [53,54]. These advances portend the beginning of a new era for use of pyrethrins as agricultural insecticides.
INDUSTRIAL PYRETHRINS PRODUCTION
Industrial production of the pyrethrin insecticides currently requires large-scale cultivation of Dalmatian pyrethrum. Pyrethrins accumulate to 1-2% of dry mass in the mature flower heads which are then harvested, dried, and powdered [1,55]. The powdered material may then be marketed directly or extracted with organic solvents for formulation into insecticidal soaps and sprays [56]. In addition to dry powders and liquid sprays used for small-scale ground-level treatment [57] or large-scale aerial application [58], pyrethrins are also formulated into other products such as lotions and mosquito coils for personal insect protection [59,60].
While T. cinerariifolium was originally harvested in its native Dalmatia in present-day Croatia, the crop was introduced into Japan in the late 19th century and by the 1930s, Japan produced most of the world’s supply [3,61,62]. More than a dozen countries have participated in industrial pyrethrum production with significant sources of pyrethrum coming from Africa, Asia, Europe, and South America (see: http://www.fao.org/faostat/). However, Japan maintained a virtual monopoly on the pyrethrum market until World War II, after which east African nations took over most production. By the mid-1980s, Japanese pyrethrum production was negligible. Kenya dominated the pyrethrum market for the second half of the 20th century but production dropped sharply in the mid-2000s. Currently, the major commercial pyrethrum producers are Rwanda, Tanzania, and the Australian state of Tasmania [63,64]. Total levels of global industrial pyrethrum production have also fluctuated widely during the past half-century. Since the Food and Agriculture Organization of the United Nations (FAO) began keeping records of pyrethrum production in 1961, production has ranged from a record high of more than 30,000 metric tons in 1983 to an apparent low of less than 5,000 tons in 2007, which marked the end of major production in Kenya. Production in Tanzania and Rwanda increased after this point, stabilizing global production. However, Australian production is not reported to the FAO and is thought to account for more than half of all global pyrethrum production [64], making reliable estimation of current global production difficult. FAO data indicates that global production excluding Australia totaled nearly 14,000 metric tons in 2017, the last year for which data are available. It is therefore likely that world production of pyrethrum approaches or exceeds the former 1983 record of 30,000 metric tons.
PYRETHRIN BIOSYNTHESIS
Natural pyrethrins comprise six esters, each consisting of a monoterpenoid acid moiety conjugated to a rethrolone-type oxylipin alcohol (Fig 1A) [13,15,65]. Their biosynthesis is known exclusively from T. cinerariifolium. Early feeding studies demonstrated that the pyrethrin biosynthetic pathway draws from two core plant metabolic pathways: the two monoterpenoids (chrysanthemic acid and pyrethric acid) are derived from the plastidial 1-deoxy-d-xylulose-5-phosphate (DXP) terpenoid pathway, while the three rethrolones (pyrethrolone, jasmolone, and cinerolone) are derived from the octadecanoid pathway (Fig. 1B) [66]. Pyrethrins containing chrysanthemic acid are termed ‘type I’ while those containing pyrethric acid are ‘type II’. The three rethrolones termed pyrethrolone, jasmolone, and cinerolone are found in pyrethrins I and II, jasmolins I and II, and cinerins I and II, respectively. Pyrethrin I is the most abundant of the six pyrethrins in T. cinerariifolium while pyrethrin II is the second most abundant (relative abundances of jasmolins and cinerins vary among T. cinerariifolium varieties) [1].
Monoterpenoid biosynthesis
The full monoterpenoid pathway to chrysanthemic and pyrethric acids was recently elucidated with the aid of T. cinerariifolium transcriptomic and genomic resources (Fig. 1B) [48,49,67-70]. Nearly two decades ago, it was shown that chrysanthemyl diphosphate synthase (CDSase) catalyzes the first step in biosynthesis of these monoterpenoids via an unusual head-to-middle condensation of two dimethylallyl diphosphate (DMADP) units in plastids (Fig. 1B) [4,68]. Further conversion of chrysanthemyl diphosphate (CDP) to the downstream acids requires dephosphorylation and oxidation; the action of one or more plastidial phosphatases was predicted to occur before oxidation of chrysanthemol in the cytosol. However, while initial characterization of CDSase identified CDP as the reaction product, results of subsequent investigations suggested that CDSase might perform both the condensation of the two DMADP molecule precursors to give the cyclic monoterpene skeleton and cleavage of the diphosphate group, yielding chrysanthemol [67]. This ‘chrysanthemol synthase’ model allowed for diffusion of the first monoterpenoid product directly into the cytosol without invoking as-of-yet undiscovered phosphatases. Recently, a Nudix-family phosphatase from T. cinerariifolium, Nudix1, was characterized that specifically dephosphorylates CDP yielding chrysanthemyl monophosphate (Fig. 1B) [52]. Therefore, the involvement of this and other phosphatases, perhaps in addition to the dephosphorylating activity of CDS, cannot yet be ruled out [49,52].
Regardless of how chrysanthemol is generated from CDP, all evidence shows this compound as the branch point for the synthesis of both chrysanthemic acid and pyrethric acid. Once in the cytosol, the chrysanthemol hydroxyl can be directly modified by two oxidoreductases validated in vitro and in planta, alcohol dehydrogenase 2 (ADH2) and aldehyde dehydrogenase 1 (ALDH1), which catalyze sequential oxidation of chrysanthemol to produce chrysanthemic acid (Fig. 1B) [49]. A portion of the chrysanthemol pool can be hydroxylated at the C10 position by the cytochrome P450 chrysanthemol 10-hydroxylase (CHH; CYP71BZ1), yielding the dihydroxylated compound 10-hydroxychrysanthemol [48]. The 10-hydroxyl group of this compound is converted to a carboxylic acid group by two additional oxidation steps catalyzed by CHH, yielding 10-carboxychrysanthemol, while the C1 hydroxyl group is oxidized to a carboxylic acid group by ADH2 and ALDH1, as in the biosynthesis of chrysanthemic acid described above. Transient expression of CDSase, ADH2, ALDH1, and CHH in Nicotiana benthamiana leaves indicated that oxidation of the 10-hydroxy group to the carboxylic acid by CHH precedes oxidation of the C1 hydroxyl by ADH2 and ALDH1. The combined result of the actions of these three enzymes is 10-carboxychrysanthemic acid [48,49]. The 10-carboxychrysanthemic acid molecule is then methylated by the SABATH-family 10-carboxychrysanthemic acid 10-methyltransferase (CCMT), yielding pyrethric acid (Fig. 1B) [49].
Current understanding of the pathway requires that both chrysanthemic and pyrethric acids are conjugated to coenzyme A (CoA) prior to incorporation of the monoterpenoid moiety into pyrethrins [71]. A chrysanthemic acid:CoA ligase from T. cinerariifolium (acyl activating enzyme 1; AAE1), was described (T. Yang, PhD Thesis, Wageningen University, 2013). However, this enzyme was plastid-localized while formation of chrysanthemic acid is likely cytosolic. Additionally, testing AAE1 enzyme activity in vitro or via transient expression in N. benthamiana with other pyrethrin pathway genes failed to demonstrate CoA-ligating activity with chrysanthemic acid (H. Xu and E. Pichersky, unpublished).
Rethrolone biosynthesis
In contrast to biosynthesis of pyrethrin monoterpenoid moieties, biosynthesis of rethrolone moieties is still less well understood. Structural similarities between rethrolones and octadecanoids such as jasmonic acid suggested a common origin. This hypothesis was supported by feeding of pyrethrin-producing T. cinerariifolium flowers with [1-13C]-d-glucose, which yielded a pyrethrin 13C labelling pattern consistent with a linolenic acid precursor for rethrolones [66,72]. Additionally, transcripts of octadecanoid pathway genes lipoxygenase 1 (LOX1), allene oxide synthase (AOS), and allene oxide cyclase (AOC) show co-expression with CDSase in T. cinerariifolium flowers [73,51]. However, enzymatic activities have not been confirmed for the AOS or AOC gene products. Recent labelling studies demonstrated that both 12-oxophytodienoic acid (OPDA) and cis-jasmone are precursors of rethrolones (Fig. 1B) while jasmonic acid is not [74]. This not only illuminates pyrethrin biosynthesis but also provides insight into the thus far incompletely resolved origins of cis-jasmone in plants [75,76,74].
While no enzymes from T. cinerariifolium have been identified that catalyze formation of the cis-jasmone intermediate, the cytochrome P450 jasmone hydroxylase (JMH; CYP71AT148) hydroxylates the 3-position of cis-jasmone, yielding jasmolone which may then be incorporated into jasmolins I and II (Fig. 1B) [51]. Alternatively, another cytochrome P450, pyrethrolone synthase (PYS; CYP82Q3), can desaturate the terminal bond of the jasmolone pentenyl tail, yielding pyrethrolone which may then be incorporated into pyrethrins I and II (Fig. 1B) [50]. The biosynthetic route to cinerolone, the third rethrolone and precursor for cinerins I and II, is still unknown but may proceed by further oxidation and decarboxylation of the terminal carbon of the side chain.
Condensation of monoterpenoids and rethrolones
Following formation of rethrolones and monoterpenoid acid CoA esters, the monoterpenoid moiety must be transferred from CoA to the rethrolone. This is catalyzed by the GDSL lipase-like protein (GLIP), which can accept either chrysanthemoyl-CoA or pyrethroyl-CoA as an acyl donor and any of jasmolone, pyrethrolone, or cinerolone as an acyl acceptor, thereby generating any of the six natural pyrethrins (Fig. 1A,B) [71]. In contrast to all other known steps in pyrethrin biosynthesis, this step occurs in the apoplast.
REGULATION OF PYRETHRIN BIOSYNTHESIS
Spatiotemporal specificity of pyrethrin biosynthesis
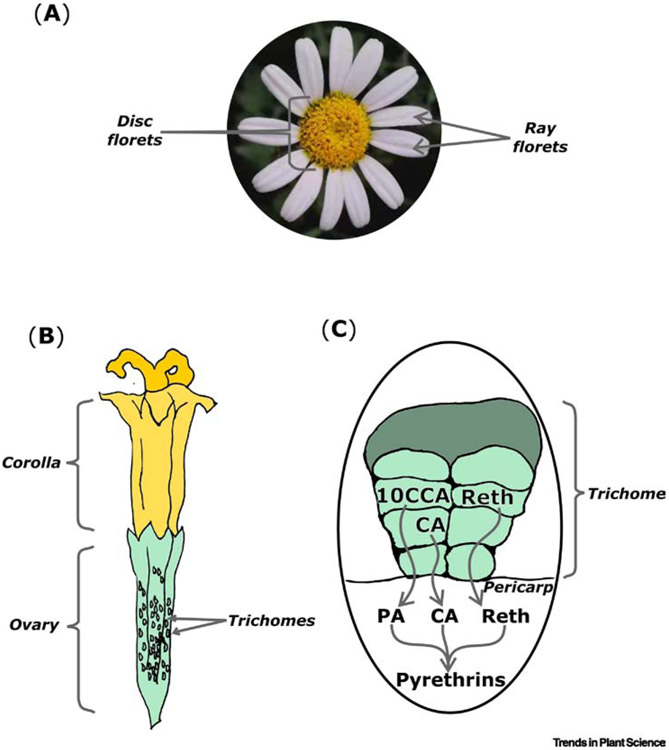
As with many specialized metabolites [77-79], production of pyrethrins is spatially restricted in T. cinerariifolium. Pyrethrins accumulate throughout above-ground parts of the plants but their levels in tissues are correlated with the presence of secretory glandular trichomes which are more abundant on the surface of disc floret ovaries of T. cinerariifolium than in any other plant tissue (Fig. 2A,B) [80]. Trichome density and pyrethrin levels increase as the flower buds develop, reaching their maximum in senescing flower heads [80,81]. Pyrethrin biosynthetic gene expression parallels pyrethrin accumulation: transcript analysis indicated that all genes associated with pyrethrin biosynthesis were expressed primarily in disc florets with relatively low levels of expression observed in the larger ray florets [48-51]. Just as overall levels of pyrethrin accumulation correlated with trichome density on the surface of disc florets [82], seven of nine presently known pyrethrin biosynthetic genes (CDSase, Nudix1, ADH2, ALDH1, CHH, JMH, and PYS) were primarily expressed in trichomes [48,50-52]. The CDSase promoter was also shown to drive expression of reporter genes specifically in trichome tip cells of Chrysanthemum x morifolium, a hybrid chrysanthemum closely related to T. cinerariifolium, and in the more phylogenetically distant Nicotiana tabacum [83]. CDSase enzyme activity was found at high levels in trichomes of developing seeds but at low levels in the ovaries and vegetative portions of the plant [4]. Expression of the oxylipin biosynthetic genes LOX1, AOS, and AOC were also primarily expressed in trichomes [51,73]. As the actions of LOX1, AOS, and AOC enzymes are also involved in jasmonic acid biosynthesis, restriction of the pyrethrin pathway to trichomes may facilitate high-level pyrethrin production without interfering with jasmonate signaling in adjacent tissues.
Figure 2.
Production of pyrethrins in flowers of Tanacetum cinerariifolium. (A) Pyrethrins accumulate primarily in disc florets of mature flowers while levels in ray florets are relatively low. (B) Pyrethrins production occurs in the trichome-laden ovaries of disc florets. (C) The rethrolone and chrysanthemic acid precursors of pyrethrins are biosynthesized in trichomes covering the disc floret ovary while pyrethric acid is produced in the ovary pericarp; mature pyrethrins accumulate in the ovary pericarp. Abbreviations: CA – chrysanthemic acid; PA – pyrethric acid; reth – rethrolone; 10CCA – 10-carboxychrysanthemic acid. Photograph in (A) by H. Xu.
In contrast to the trichome-localized expression of most pyrethrin biosynthetic genes, the genes encoding the final enzyme in pyrethric acid biosynthesis (CCMT) and the enzyme catalyzing the final esterification step in biosynthesis of all six pyrethrins (GLIP) are primarily expressed in ovary tissue [48,51]. This expression pattern, combined with the apoplastic localization of the GLIP enzyme, suggests that biosynthesis of pyrethrin monoterpenoid and rethrolone precursors occurs in the trichome followed by export of these components into the apoplast of underlying tissue and subsequent esterification to form the final pyrethrin products (Fig. 2C) [4,48].
Analysis of pyrethrin biosynthetic gene expression throughout floral development indicates that, in addition to tissue-level restriction, pyrethrin biosynthesis is also temporally restricted with the bulk of biosynthesis occurring in young developing flower buds. Accumulation of mRNAs from all pyrethrin biosynthetic genes was high during early stages of development but decreased as the flowers opened and matured, consistent with the gradual increase in pyrethrin content in early development that slows at later stages [48-51,81]. Paradoxically, young T. cinerariifolium seedlings also showed significant levels of pyrethrins but relatively low levels of CDSase transcript and possessed no CDSase activity [4], suggesting that transport of pyrethrins from surrounding ovary tissue into developing seeds occurs. Analysis of pyrethrin localization within the ovaries of mature flowers revealed that pyrethrin levels were high in pericarp tissue of developing achenes at late stages of floral development while pyrethrin levels in the developing embryo itself were low; however, ripe achenes contained little pyrethrin in the pericarp, instead accumulating the bulk of pyrethrins in the mature embryo [4]. Transfer of pyrethrins from floral tissue into developing achenes and the subsequent accumulation of pyrethrins in the embryo evidently provides young T. cinerariifolium seedlings with pre-synthesized chemical defenses, thus protecting the otherwise vulnerable seedlings from insect attack and fungal pathogens in the absence of pyrethrin biosynthetic gene expression.
Pyrethrin production in response to wounding and volatiles
While production of some plant defensive specialized metabolites is constitutive or developmentally regulated, other defensive compounds are induced (e.g., by herbivory, wounding, or abiotic stress) [84-86]. Pyrethrin production in floral tissues of T. cinerariifolium was constitutive but pathway gene expression and pyrethrin accumulation in vegetative tissues was inducible by mechanical wounding, application of methyl jasmonate, and volatile organic compounds (VOCs) [87-90]. While mechanical wounding of T. cinerariifolium plants grown under field conditions had no effect on total pyrethrin levels [87], wounding led to increased levels of pyrethrins in T. cinerariifolium plants grown in growth chambers [86,89]. There is evidence that VOCs emitted in response to wounding stimulate pyrethrin production in vegetative tissues. When T. cinerariifolium seedlings were grown near wounded conspecifics, levels of pyrethrins I and II increased [88]. In contrast, when portions of wounded plants were protected from exposure to their own VOCs, levels of pyrethrin II in protected tissues increased while levels of pyrethrin I remained unchanged, suggesting that some portions of the pyrethrin biosynthetic pathway were triggered via a systemic response while others responded only to an external volatile signal [86].
Analysis of volatile organic compounds (VOCs) emitted by wounded T. cinerariifolium plants revealed five major components: the green leaf volatiles (Z)-3-hexenal, (E)-2-hexenal, (Z)-3-hexen-1-ol, and (Z)-3-hexen-1-yl acetate, and the sesquiterpene (E)-β-farnesene which protects T. cinerariifolium from aphids by mimicking their natural alarm pheromones [91,92]. Consistent with reports from other species, exposure of T. cinerariifolium seedlings to a cocktail of VOCs similar to those observed in wounded conspecifics induced expression of several genes involved in specialized metabolism including genes involved in pyrethrin biosynthesis [88,92,93]. Specifically, genes from the DXP and oxylipin pathways as well as the pyrethrin-specific genes CDSase and GLIP showed increased expression at various time-points ranging from three to 12 hours after seedling exposure to VOCs [88,92].
ENGINEERING PYRETHRIN BIOSYNTHESIS
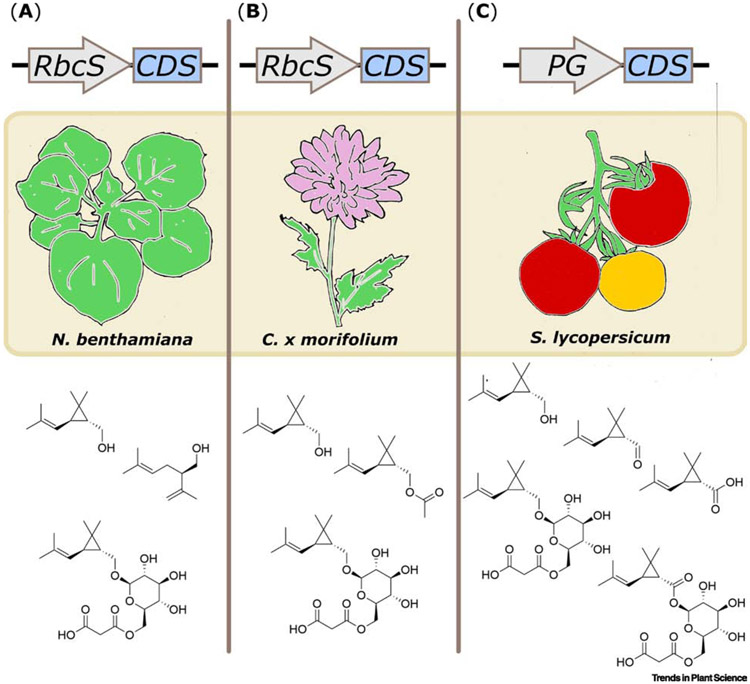
Metabolic engineering of pyrethrin pathway genes in heterologous plant hosts has the potential to increase industrial pyrethrin production or to augment endogenous chemical defenses in crop plants. Though T. cinerariifolium is currently the source of industrially produced pyrethrins, development of an alternative platform for large-scale production of pyrethrins could supplement the world’s current supply of these compounds for household or agricultural use, while engineering the pyrethrin pathway into crop plants could augment their endogenous defenses, thereby reducing crop loss or the need for external pesticide application. Portions of the pyrethrin pathway were engineered into several different plant species. Expression of the CDSase gene under tissue-specific or constitutive promoters in several plant species yielded production of chrysanthemol and a variety of volatile and non-volatile derivatives [53,54,67]. Transient expression of CDSase in Nicotiana benthamiana with the highly expressed RbcS promoter resulted in production of volatile chrysanthemol and lavandulol as well as non-volatile chrysanthemyl-6-O-malonyl-β-d-glucopyranoside (Fig. 3A) [67]. Use of a similar strategy in Chrysanthemum morifolium with the RbcS promoter achieved high levels of CDSase expression in photosynthetic tissues and also resulted in production of chrysanthemol and chrysanthemyl-6-O-malonyl-β-d-glucopyranoside but also yielded accumulation of volatile chrysanthemyl acetate (Fig. 3B). These transgenic lines producing chrysanthemol and its glucoside showed increased aphid resistance in greenhouse conditions [53]. This indicates that even engineering of an incomplete pyrethrin pathway into an insect-susceptible host can provide protective benefits like those conferred to T. cinerariifolium by pyrethrins [2,53]. It is also interesting to note that chrysanthemol, a volatile precursor of pyrethrins, acts as a feeding deterrent to insects by odor whereas non-volatile pyrethrins act on insects through contact or consumption. This may provide some insight into the evolutionary origins of pyrethrin biosynthesis as it is possible that the pyrethrin pathway evolved through co-option of genes initially involved in production of volatile anti-feedants.
Figure 3.
Engineering of chrysanthemyl diphosphate synthase (CDSase) into diverse plant hosts. (A) Transient expression of CDSase in Nicotiana benthamiana under control of a rubisco small subunit promoter (RbcS) yields the volatile compounds chrysanthemol and lavandulol as well as the non-volatile chrysanthemyl-6-O-malonyl-β-d-glucopyranoside. (B) Stable expression of CDSase in Chrysanthemum x morifolium under the control of the RbcS promoter yields the volatile compounds chrysanthemol and chrysanthemyl acetate and the non-volatile chrysanthemyl-6-O-malonyl-β-d-glucopyranoside. (C) Stable expression of CDSase in Solanum lycopersicum under the control of the fruit-specific polygalactonurase (PG) promoter yields accumulation of the volatile compounds chrysanthemol, chrysanthemal, and chrysanthemic acid as well as the non-volatile chrysanthemyl-6-O-malonyl-β-d-glucopyranoside and chrysanthemoyl-6-O-malonyl-β-d-glucopyranoside.
In contrast to strategies employing constitutive expression of pyrethrin biosynthetic genes, an alternative strategy is to produce large amounts of the desired compounds in tissues with a high biosynthetic capacity for the required pathways. Fruits of the cultivated tomato (Solanum lycopersicum) have high flux through the plastidial terpenoid pathway as evidenced by their high lycopene content and can be efficiently harvested by mechanical means [94].The CDSase gene was expressed in S. lycopersicum under the fruit-specific polygalacturonase (PG) promoter [54]. Like constitutive expression of the gene in N. benthamiana and C. morifolium, this strategy resulted in production of chrysanthemol and chrysanthemyl-6-O-malonyl-β-d-glucopyranoside, but also oxidized chrysanthemol derivatives including chrysanthemal, chrysanthemic acid, and chrysanthemoyl-6-O-malonyl-3-d-glucopyranoside (Fig. 3C) [53,54,67]; addition of two sesquiterpenoid oxidoreductase genes from Solanum habrochaites, alcohol dehydrogenase (ShADH) and aldehyde dehydrogenase (ShALDH), increased the ratio of oxidized chrysanthemol derivatives to chrysanthemol and increased the total yield of CDSase products [54]. Production of pyrethrins in tomato obviously requires the activity of additional pyrethrin pathway enzymes. Heterologous expression of additional enzymes from the rethrolone pathway and the final esterification enzyme, GLIP, in tomato fruit may yield intact pyrethrins. However, extensive experimentation is needed to determine whether incorporation of chrysanthemic and pyrethric acids into pyrethrins can outcompete glycosylation by endogenous fruit enzymes, and whether tomato fruit can support sufficient rethrolone biosynthesis to match high levels of monoterpenoid production.
In addition to showing promise for an alternative pyrethrin production platform, accumulation of pyrethrin precursors in tomato also provides an avenue for production of precursors useful for other insect control strategies. Several mealybug species known to damage fruit crops produce mating pheromones containing a chrysanthemol moiety: Ferrisia virgata, Phenacoccus madeirensis, and Pseudococcus calceolariae [95-97]. Traps baited with these chrysanthemol-based pheromones provide effective control of mealybug populations with some traps collecting hundreds of mealybugs per day [97,98]. Correct stereochemistry of the sex pheromone employed is essential for insect trapping efficacy but commercially available chrysanthemol contains a mixture of isomers, lowering the total yield of effective pheromone produced through a fully synthetic method. Pyrethrins were recently used as a source of trans-(1R,3R)-chrysanthemol for synthesis of trans-(1R,3R)-chrysanthemyl-(R)-2-acetoxy-3-methylbutanoate, the mating pheromone of P. calceolariae [99]; this semi-synthetic pheromone was then used to bait traps which successfully captured P. calceolariae under field conditions. While use of purified pyrethrins as a source of isomerically pure trans-(1R,3R)-chrysanthemol is effective at lab scale, the high commercial value of pyrethrins as an insecticide may make these compounds prohibitively expensive to use as starting materials on an industrial scale. Use of transgenic S. lycopersicum producing trans-(1R,3R)-chrysanthemol and its glucosylated derivative may provide a more economically feasible option [54,99].
CONCLUDING REMARKS AND FUTURE PERSPECTIVES
Pyrethrins and their derivatives have remained essential tools for pest control for over 100 years. With increased concerns about ecological harm caused by synthetic pesticides, natural solutions for pest control in agricultural and household settings are in greater demand than ever. The recent expansion of knowledge about biosynthesis and regulation of pyrethrins in T. cinerariifolium is timely and will help to improve industrial pyrethrin yields. Combined with modern advances in genetic engineering and genome editing, we can now look towards innovative crop protection strategies such as designer crops with improved endogenous chemical defenses against insect herbivores. Finally, there is the possibility of combining genetic engineering in planta with an in vitro process to optimize the commercial production of natural pyrethrins. The two moieties – the monoterpenoid acids and the rethrolones – are stereochemicals that are best produced in genetically engineered organisms rather than by organic synthesis. If production of pyrethrins in an engineered system falls short of projections based on precursor accumulation, precursors could be biosynthesized in different organisms, purified, and processed to remove non-specific conjugations such as glycosylation. Once stereospecific intermediates are obtained in large quantities by purification from the host organisms, the two moieties could be combined in vitro, possibly using GLIP or similar lipases.
While we are already well on our way to elucidating the full pyrethrin biosynthetic pathway, a few questions remain (see: Outstanding Questions). The route to the major pyrethrin constituents, pyrethrins and jasmolins I and II, has been elucidated but we lack an understanding of how cinerolone, a component of the minor pyrethrins cinerin I and II, is formed. Additionally, engineering of the full pathway into crop plants may benefit from a better understanding of pathway regulation in T. cinerariifolium. In the native producer, pyrethrin precursors are synthesized in trichomes but the mature pyrethrins are assembled in underlying tissue. This spatial separation of biosynthetic steps may be necessary for effective pyrethrin formation and may require transporters to shuttle pyrethrin precursors between tissues. If so, steps must be taken to ensure proper gene regulation or tissue-specific transporter expression when introducing the pathway into heterologous hosts.
OUTSTANDING QUESTIONS.
How is CDP converted to chrysanthemol? Does CDSase act directly as chrysanthemol synthase or are Nudix1 and an additional phosphatase involved? Can both pathways operate in parallel?
How is cinerolone formed? Is this an oxidation product of pyrethrolone or is there an alternative route from cis-jasmone to cinerolone?
How are pyrethrins and their precursors translocated between cellular compartments and tissues? Do transporters shuttle precursors between intracellular compartments and secrete them from the trichome to underlying pericarp tissue? Do transporters shuttle mature pyrethrins from the pericarp to the developing achene?
HIGHLIGHTS.
Pyrethrin insecticides produced by Tanacetum cinerariifolium provide a human-safe and ecologically sound alternative to widely used synthetic insecticides.
Development of transcriptomic and genomic resources for T. cinerariifolium facilitated the elucidation of numerous steps in the pyrethrin biosynthetic pathway.
Pyrethrin biosynthetic genes have been engineered into diverse plant hosts; these efforts demonstrate the promise of engineering plants with improved endogenous defenses or developing an alternative source to pyrethrins for use as insecticides.
ACKNOWLEDGEMENTS
The authors thank Dr. Thilani Anthony and Dr. Craig Schenck for providing helpful comments about the manuscript. This work was supported by the National Science Foundation collaborative research grants 1565355 to E.P. and 1565232 to R.L.L. as well as the National Institutes of Health predoctoral training grant T32-GM110523 to D.B.L.
GLOSSARY
- Achene
A dry, indehiscent fruit enclosing a single seed.
- Knockdown
Paralysis of insects upon exposure to toxic compounds such as pyrethrins; this may be followed by insect death depending on the compound and duration of exposure.
- Ovary
A part of the female reproductive structure of a plant housing the ovules; seeds develop within the ovary.
- Pericarp
The outer layer of a fruit or seed derived from the ovary wall.
- Pyrethrum
The common name of Tanacetum cinerariifolium and related species; pyrethrum also refers to a crude extract of said species in powder or liquid form used as an insecticide.
- Rethrolone
An oxylipin precursor of pyrethrins; a pyrethrin molecule incorporates one of three rethrolones.
- SABATH-family methyltransferase
A family of enzymes that methylate small molecules using S-adenosyl-L-methionine as a methyl donor.
- Specialized metabolite
A wide variety of compounds from diverse pathways that are involved in the interactions of the plant with its biotic or abiotic environment and that show restricted phylogenetic distribution.
- Trichome
A uni- or multicellular structure protruding from the surface of an aboveground plant tissue such as leaf or stem; trichomes that accumulate or secrete specialized metabolites are often referred to as secretory glandular trichomes.
- Volatile organic compounds (VOCs)
Small organic molecules with high vapor pressures; VOCs from plant sources often mediate plant-plant communication or plant-animal interactions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Grdisa M et al. (2013) Chemical Diversity of the Natural Populations of Dalmatian Pyrethrum (Tanacetum cinerariifolium (Trevir.) Sch.Bip.) in Croatia. Chem. Biodivers 10, 460–472 [DOI] [PubMed] [Google Scholar]
- 2.Yang T et al. (2012) Pyrethrins Protect Pyrethrum Leaves Against Attack by Western Flower Thrips, Frankliniella occidentalis. J. Chem. Ecol 38, 370–377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pares B et al. (1925) Economic Survey: The Economic Situation in Jugoslavia. Slav. Rev 4, 491–505 [Google Scholar]
- 4.Ramirez AM et al. (2012) Bidirectional Secretions from Glandular Trichomes of Pyrethrum Enable Immunization of Seedlings. Plant Cell 24, 4252–4265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McLaughlin GA (1973) History of Pyrethrum In Pyrethrum: The Natural Insecticide (Casida JE, ed), pp. 3–15, Academic Press [Google Scholar]
- 6.Clark JFM (2001) Bugs in the system: Insects, agricultural science, and professional aspirations in Britain, 1890-1920. Agric. Hist 75, 83–114 [DOI] [PubMed] [Google Scholar]
- 7.Lange HW and Akesson NB (1973) Pyrethrum for Control of Agricultural Insects In Pyrethrum: The Natural Insecticide (Casida JE, ed), pp. 261–279, Academic Press [Google Scholar]
- 8.Orenstein AJ (1913) Mosquito Catching in Dwellings in the Prophylaxis of Malaria. Am. J. Public Health 3, 106–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reed W and Carroll J (1901) The Prevention of Yellow Fever. Public Health Pap. Rep 27, 113–129 [PMC free article] [PubMed] [Google Scholar]
- 10.Fryer JCF et al. (1928) English-grown pyrethrum as an insecticide. I. Ann. Appl. Biol. 15, 423–445 [Google Scholar]
- 11.Richardson CH et al. (1937) The toxicity of certain insecticides to the chinch bug. J. Agric. Res 54, 0059–0078 [Google Scholar]
- 12.Staudinger H and Ruzicka L (1924) Substances for killing insects I. The isolation and constitution of effective parts of dalmatian insect powder. Helv. Chim. Acta 7, 177–201 [Google Scholar]
- 13.Laforge F and Barthel W (1947) Constituents of Pyrethrum Flowers .20. the Partial Synthesis of Pyrethrins and Cinerins and Their Relative Toxicities. J. Org. Chem 12, 199–202 [DOI] [PubMed] [Google Scholar]
- 14.Laforge FB and Barthel WF (1944) Constituents of pyrethrum flowers XVI Heterogeneous nature of pyrethrolone. J. Org. Chem 9, 242–249 [Google Scholar]
- 15.Godin P et al. (1965) Insecticidal Activity of Jasmolin 2 and Its Isolation from Pyrethrum (Chrysanthemum cinerariaefolium Vis). J. Econ. Entomol 58, 548– [Google Scholar]
- 16.Rugutt JK et al. (1999) NMR and Molecular Mechanics Study of Pyrethrins I and II. J. Agric. Food Chem 47, 3402–3410 [DOI] [PubMed] [Google Scholar]
- 17.Kawamoto M et al. (2020) Total Syntheses of All Six Chiral Natural Pyrethrins: Accurate Determination of the Physical Properties, Their Insecticidal Activities, and Evaluation of Synthetic Methods. J. Org. Chem 85, 2984–2999 [DOI] [PubMed] [Google Scholar]
- 18.Matsuo N (2019) Discovery and development of pyrethroid insecticides. Proc. Jpn. Acad. Ser. B-Phys. Biol. Sci 95, 378–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Amar M et al. (1992) Patch-Clamp Analysis of the Effects of the Insecticide Deltamethrin on Insect Neurons. J. Exp. Biol 163, 65–84 [DOI] [PubMed] [Google Scholar]
- 20.McCavera SJ and Soderlund DM (2012) Differential state-dependent modification of inactivation-deficient Na(v)1.6 sodium channels by the pyrethroid insecticides S-bioallethrin, tefluthrin and deltamethrin. Neurotoxicology 33, 384–390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen M et al. (2018) Action of six pyrethrins purified from the botanical insecticide pyrethrum on cockroach sodium channels expressed in Xenopus oocytes. Pest. Biochem. Physiol 151, 82–89 [DOI] [PubMed] [Google Scholar]
- 22.Van Timmeren S and Isaacs R (2013) Control of spotted wing drosophila, Drosophila suzukii, by specific insecticides and by conventional and organic crop protection programs. Crop Prot. 54, 126–133 [Google Scholar]
- 23.Wyss E and Daniel C (2004) Effects of autumn kaolin and pyrethrin treatments on the spring population of Dysaphis plantaginea in apple orchards. J. Appl. Entomol 128, 147–149 [Google Scholar]
- 24.Sial AA et al. (2019) Evaluation of organic insecticides for management of spotted-wing drosophila (Drosophila suzukii) in berry crops. J. Appl. Entomol 143, 593–608 [Google Scholar]
- 25.Joseph SV (2018) Lethal and Sublethal Effects of Organically-Approved Insecticides against Bagrada hilaris (Hemiptera: Pentatomidae). J. Entomol. Sci 53, 307–324 [Google Scholar]
- 26.Tacoli F et al. (2017) Control of Scapholdeus titanus with Natural Products in Organic Vineyards. Insects 8, 129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Razze JM et al. (2016) Evaluation of Bioinsecticides for Management of Bemisia tabaci (Hemiptera: Aleyrodidae) and the Effect on the Whitefly Predator Delphastus catalinae (Coleoptera: Coccinellidae) in Organic Squash. J. Econ. Entomol 109, 1766–1771 [DOI] [PubMed] [Google Scholar]
- 28.Oliveira CR et al. (2019) Nanopesticide based on botanical insecticide pyrethrum and its potential effects on honeybees. Chemosphere 236, 124282. [DOI] [PubMed] [Google Scholar]
- 29.Shrestha G et al. (2020) Spinosad and Mixtures of an Entomopathogenic Fungus and Pyrethrins for Control of Sitona lineatus (Coleoptera: Curculionidae) in Field Peas. J. Econ. Entomol 113, 669–678 [DOI] [PubMed] [Google Scholar]
- 30.Yao J et al. (2019) Differential susceptibilities of two closely-related stored product pests, the red flour beetle (Tribolium castaneum) and the confused flour beetle (Tribolium confusum), to five selected insecticides. J. Stored Prod. Res 84, 101524 [Google Scholar]
- 31.Fernandez-Grandon GM et al. (2020) Additive Effect of Botanical Insecticide and Entomopathogenic Fungi on Pest Mortality and the Behavioral Response of Its Natural Enemy. Plants 9, 173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Korunić Z et al. (2020) Evaluation of diatomaceous earth formulations enhanced with natural products against stored product insects. J. Stored Prod. Res 86, 101565 [Google Scholar]
- 33.Demoute J-P (1989) A brief review of the environmental fate and metabolism of pyrethroids. Pestic. Sci 27, 375–385 [Google Scholar]
- 34.Antonious GF (2004) Residues and Half-Lives of Pyrethrins on Field-Grown Pepper and Tomato. J. Environ. Sci. Heal. B 39, 491–503 [DOI] [PubMed] [Google Scholar]
- 35.Pan L et al. (2017) Dissipation and Residues of Pyrethrins in Leaf Lettuce under Greenhouse and Open Field Conditions. Int. J. Environ. Res. Public Health 14, 822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Feng X et al. (2018) Residue analysis and risk assessment of pyrethrins in open field and greenhouse turnips. Environ. Sci. Pollut. Res 25, 877–886 [DOI] [PubMed] [Google Scholar]
- 37.Katagi T (1991) Photodegradation of the pyrethroid insecticide esfenvalerate on soil, clay minerals, and humic acid surfaces. J. Agric. Food Chem 39, 1351–1356 [Google Scholar]
- 38.Hartz KEH et al. (2019) Survey of bioaccessible pyrethroid insecticides and sediment toxicity in urban streams of the northeast United States. Environ. Pollut 254 [DOI] [PubMed] [Google Scholar]
- 39.Li H et al. (2017) Global occurrence of pyrethroid insecticides in sediment and the associated toxicological effects on benthic invertebrates: An overview. J. Hazard. Mater 324, 258–271 [DOI] [PubMed] [Google Scholar]
- 40.Stehle S and Schulz R (2015) Agricultural insecticides threaten surface waters at the global scale. Proc. Natl. Acad. Sci 112, 5750–5755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maestre-Serrano R et al. (2019) Co-occurrence of V1016I and F1534C mutations in the voltage-gated sodium channel and resistance to pyrethroids in Aedes aegypti (L.) from the Colombian Caribbean region. Pest Manag. Sci 75, 1681–1688 [DOI] [PubMed] [Google Scholar]
- 42.Davila-Barboza J et al. (2019) Novel Kdr mutations (K964R and A943V) in pyrethroid-resistant populations of Triatoma mazzottii and Triatoma longipennis from Mexico and detoxifying enzymes. Insect Sci. 26, 809–820 [DOI] [PubMed] [Google Scholar]
- 43.Cheng X et al. (2019) Pyrethroid resistance in the pest mite, Halotydeus destructor: Dominance patterns and a new method for resistance screening. Pest. Biochem. Physiol 159, 9–16 [DOI] [PubMed] [Google Scholar]
- 44.Duchon S et al. (2009) Pyrethrum: A Mixture of Natural Pyrethrins Has Potential for Malaria Vector Control. J. Med. Entomol 46, 516–522 [DOI] [PubMed] [Google Scholar]
- 45.Anderson JF and Cowles RS (2012) Susceptibility of Cimex lectularlus (Hemiptera: Cimicidae) to Pyrethroid Insecticides and to Insecticidal Dusts With or Without Pyrethroid Insecticides. J. Econ. Entomol 105, 1789–1795 [DOI] [PubMed] [Google Scholar]
- 46.Scott JG et al. (2013) Insecticide resistance in house flies from the United States: Resistance levels and frequency of pyrethroid resistance alleles. Pest. Biochem. Physiol 107, 377–384 [DOI] [PubMed] [Google Scholar]
- 47.Kgoroebutswe TK et al. (2020) Distribution of Anopheles mosquito species, their vectorial role and profiling of knock-down resistance mutations in Botswana. Parasitol. Res DOI: 10.1007/s00436-020-06614-6 [DOI] [PubMed] [Google Scholar]
- 48.Xu H et al. (2019) Pyrethric acid of natural pyrethrin insecticide: complete pathway elucidation and reconstitution in Nicotiana benthamiana. New Phytol. 223, 751–765 [DOI] [PubMed] [Google Scholar]
- 49.Xu H et al. (2018) Coexpression Analysis Identifies Two Oxidoreductases Involved in the Biosynthesis of the Monoterpene Acid Moiety of Natural Pyrethrin Insecticides in Tanacetum cinerariifolium. Plant Physiol. 176, 524–537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li W et al. (2019) Pyrethrin Biosynthesis: The Cytochrome P450 Oxidoreductase CYP82Q3 Converts Jasmolone To Pyrethrolone. Plant Physiol. 181, 934–944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li W et al. (2018) Jasmone Hydroxylase, a Key Enzyme in the Synthesis of the Alcohol Moiety of Pyrethrin Insecticides. Plant Physiol. 177, 1498–1509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li W et al. (2020) A Trichome-Specific, Plastid-Localized Tanacetum cinerariifolium Nudix Protein Hydrolyzes the Natural Pyrethrin Pesticide Biosynthetic Intermediate trans-Chrysanthemyl Diphosphate. Front. Plant Sci 11, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hu H et al. (2018) Modification of chrysanthemum odour and taste with chrysanthemol synthase induces strong dual resistance against cotton aphids. Plant Biotechnol. J 16, 1434–1445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xu H et al. (2018) Production of trans-chrysanthemic acid, the monoterpene acid moiety of natural pyrethrin insecticides, in tomato fruit. Metab. Eng 47, 271–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li J et al. (2014) Comparative analysis of pyrethrin content improvement by mass selection, family selection and polycross in pyrethrum [Tanacetum cinerariifolium (Trevir.) Sch.Bip.] populations. Ind. Crop. Prod 53, 268–273 [Google Scholar]
- 56.Ginsburg JM and Kent C (1937) The Effect of Soap Sprays on Plants. J. N. Y. Entomol. Soc 45, 109–113 [Google Scholar]
- 57.Cilek JE et al. (2008) Evaluation of an Automatic-Timed Insecticide Application System for Backyard Mosquito Control. J. Am. Mosq. Control Assoc 24, 560–565 [DOI] [PubMed] [Google Scholar]
- 58.Elnaiem D-EA et al. (2008) Impact of aerial spraying of pyrethrin insecticide on Culex pipiens and Culex tarsalis (Diptera: Culicidae) abundance and West Nile virus infection rates in an urban/suburban area of Sacramento County, California. J. Med. Entomol 45, 751–757 [DOI] [PubMed] [Google Scholar]
- 59.John NA and John J (2015) Prolonged use of mosquito coil, mats, and liquidators: A review of its health implications. Int. J. Clin. Exp. Physiol 2, 209–213 [Google Scholar]
- 60.Barker SC and Altman PM (2010) A randomised, assessor blind, parallel group comparative efficacy trial of three products for the treatment of head lice in children - melaleuca oil and lavender oil, pyrethrins and piperonyl butoxide, and a “suffocation” product. BMC Dermatol. 10, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Glassford J (1930) The economics of pyrethrum. J. Econ. Entomol 23, 874–877 [Google Scholar]
- 62.Grunge WH (1939) Japan’s Pyrethrum Position Threatened. Far Eastern Survey 8, 109–110 [Google Scholar]
- 63.Pethybridge SJ et al. (2008) Diseases of pyrethrum in tasmania: Challenges and prospects for management. Plant Dis. 92, 1260–1272 [DOI] [PubMed] [Google Scholar]
- 64.Ryan RF et al. (2015) Pyrethrum: the Natural Choice in Pest Control In 1st International Symposium on Pyrethrum, the Natural Insecticide: Scientific and Industrial Developments in the Renewal of a Traditional Industry 1073 (Chung B, ed), pp. 131–135, Int. Soc. Horticultural Science [Google Scholar]
- 65.Crombie L and Holloway S (1985) Biosynthesis of the Pyrethrins - Unsaturated Fatty-Acids and the Origins of the Rethrolone Segment. J. Chem. Soc.-Perkln Trans 1 DOI: 10.1039/p19850001393 [DOI] [Google Scholar]
- 66.Matsuda K et al. (2005) Biosynthesis of pyrethrin I in seedlings of Chrysanthemum cinerariaefolium. Phytochemistry 66, 1529–1535 [DOI] [PubMed] [Google Scholar]
- 67.Yang T et al. (2014) Chrysanthemyl Diphosphate Synthase Operates In Planta as a Bifunctional Enzyme with Chrysanthemol Synthase Activity. J. Biol. Chem 289, 36325–36335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rivera SB et al. (2001) Chrysanthemyl diphosphate synthase: Isolation of the gene and characterization of the recombinant non-head-to-tail monoterpene synthase from Chrysanthemum cinerariaefolium. Proc. Natl. Acad. Sci 98, 4373–4378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yamashiro T et al. (2019) Draft genome of Tanacetum cinerariifolium, the natural source of mosquito coil. Sci. Rep 9, 18249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Khan S et al. (2017) Comparative transcriptome analysis reveals candidate genes for the biosynthesis of natural insecticide in Tanacetum cinerariifolium. BMC Genomics 18, 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kikuta Y et al. (2012) Identification and characterization of a GDSL lipase-like protein that catalyzes the ester-forming reaction for pyrethrin biosynthesis in Tanacetum cinerariifolium- a new target for plant protection. Plant J. 71, 183–193 [DOI] [PubMed] [Google Scholar]
- 72.Schaller A and Stintzi A (2009) Enzymes in jasmonate biosynthesis - Structure, function, regulation. Phytochemistry 70, 1532–1538 [DOI] [PubMed] [Google Scholar]
- 73.Ramirez AM et al. (2013) A Trichome-Specific Linoleate Lipoxygenase Expressed During Pyrethrin Biosynthesis in Pyrethrum. Lipids 48, 1005–1015 [DOI] [PubMed] [Google Scholar]
- 74.Matsui R et al. (2020) Jasmonic acid is not a biosynthetic intermediate to produce the pyrethrolone moiety in pyrethrin II. Sci. Rep 10, 6366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wasternack C and Strnad M (2018) Jasmonates: News on Occurrence, Biosynthesis, Metabolism and Action of an Ancient Group of Signaling Compounds, Int. J. Mol. Sci 19, 2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Matsui R et al. (2019) Feeding experiment using uniformly C-13-labeled alpha-linolenic acid supports the involvement of the decarboxylation mechanism to produce cis-jasmone in Lasiodiplodia theobromae. Biosci. Biotechnol. Biochem 83, 2190–2193 [DOI] [PubMed] [Google Scholar]
- 77.Dastmalchi M et al. (2019) Purine Permease-Type Benzylisoquinoline Alkaloid Transporters in Opium Poppy. Plant Physiol. 181, 916–933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nakashima T et al. (2016) Single-Cell Metabolite Profiling of Stalk and Glandular Cells of Intact Trichomes with Internal Electrode Capillary Pressure Probe Electrospray Ionization Mass Spectrometry. Anal. Chem. 88, 3049–3057 [DOI] [PubMed] [Google Scholar]
- 79.Yamamoto K et al. (2019) The complexity of intercellular localisation of alkaloids revealed by single-cell metabolomics. New Phytol. 224, 848–859 [DOI] [PubMed] [Google Scholar]
- 80.Zito S et al. (1983) Distribution of Pyrethrins in Oil Glands and Leaf Tissue of Chrysanthemum cinerariaefolium. Planta Med. 47, 205–207 [DOI] [PubMed] [Google Scholar]
- 81.Head SW (1973) Composition of Pyrethrum Extract and Analysis of Pyrethrins In Pyrethrum: The Natural Insecticide (Casida JE, ed), pp. 25–53, Academic Press [Google Scholar]
- 82.Suraweera DD et al. (2017) Dynamics of flower, achene and trichome development governs the accumulation of pyrethrins in pyrethrum (Tanacetum cinerariifolium) under irrigated and dryland conditions. Ind. Crops Prod 109, 123–133 [Google Scholar]
- 83.Sultana S et al. (2015) Molecular cloning and characterization of the trichome specific chrysanthemyl diphosphate/chrysanthemol synthase promoter from Tanacetum cinerariifolium. Sci. Horde 185, 193–199 [Google Scholar]
- 84.Van Geem M et al. (2015) Interactions Between a Belowground Herbivore and Primary and Secondary Root Metabolites in Wild Cabbage. J. Chem. Ecol 41, 696–707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Li D et al. (2015) Navigating natural variation in herbivory-induced secondary metabolism in coyote tobacco populations using MS/MS structural analysis. Proc. Natl. Acad. Sci 112, E4147–E4155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ueda H and Matsuda K (2011) VOC-mediated within-plant communications and nonvolatile systemic signals upregulate pyrethrin biosynthesis in wounded seedlings of Chrysanthemum cinerariaefolium. J. Plant Interact 6, 89–91 [Google Scholar]
- 87.Baldwin IT et al. (1993) Foliar and floral pyrethrins of Chrysanthemum cinerariaefolium are not induced by leaf damage. J. Chem. Ecol 19, 2081–2087 [DOI] [PubMed] [Google Scholar]
- 88.Kikuta Y et al. (2011) Specific Regulation of Pyrethrin Biosynthesis in Chrysanthemum cinerariaefolium by a Blend of Volatiles Emitted from Artificially Damaged Conspecific Plants. Plant Cell Physiol. 52, 588–596 [DOI] [PubMed] [Google Scholar]
- 89.Ueda H et al. (2012) Plant communication: Mediated by individual or blended VOCs? Plant Signal. Behav. 7, 222–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Dabiri M et al. (2020) Partial sequence isolation of DXS and AOS genes and gene expression analysis of terpenoids and pyrethrin biosynthetic pathway of Chrysanthemum cinerariaefolium under abiotic elicitation. Acta Physiol. Plant 42, 30 [Google Scholar]
- 91.Li J et al. (2019) Defense of pyrethrum flowers: repelling herbivores and recruiting carnivores by producing aphid alarm pheromone. New Phytol. 223, 1607–1620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sakamori K et al. (2016) Selective regulation of pyrethrin biosynthesis by the specific blend of wound induced volatiles in Tanacetum cinerariifolium. Plant Signal. Behav 11, e1149675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Dombrowski JE et al. (2019) Transcriptome analysis of the model grass Lolium temulentum exposed to green leaf volatiles. BMC Plant Biol. 19, 222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Garcia E and Barrett DM (2006) Assessing Lycopene Content in California Processing Tomatoes. J. Food Process Pres 30, 56–70 [Google Scholar]
- 95.Ho H-Y et al. (2009) Identification and Synthesis of the Sex Pheromone of the Madeira Mealybug, Phenacoccus madeirensis Green. J. Chem. Ecol 35, 724–732 [DOI] [PubMed] [Google Scholar]
- 96.Tabata J and Ichiki RT (2017) (1S,3R)-cis-Chrysanthemyl Tiglate: Sex Pheromone of the Striped Mealybug, Ferrisia virgata. J. Chem. Ecol 43, 745–752 [DOI] [PubMed] [Google Scholar]
- 97.Flores MF et al. (2015) Monitoring Pseudococcus calceolariae (Hemiptera: Pseudococcidae) in Fruit Crops Using Pheromone-Baited Traps. J. Econ. Entomol 108, 2397–2406 [DOI] [PubMed] [Google Scholar]
- 98.Sullivan NJ et al. (2019) Deployment of the sex pheromone of Pseudococcus calceolariae (Hemiptera: Pseudococcidae) as a potential new tool for mass trapping in citrus in South Australia. NZ Entomol. 42, 1–12 [Google Scholar]
- 99.Bergmann J et al. (2019) Synthesis of citrophilus mealybug sex pheromone using chrysanthemol extracted from Pyrethrum (Tanacetum cinerariifolium). Nat Prod. Res. 33, 303–308 [DOI] [PubMed] [Google Scholar]